Abstract
Background
Treatment of multimorbid patients can be improved. Development of patient-centred care of high-quality requires context-bound understanding of the multimorbid population’s patterns of demographics, co-morbidities and medication use.
Objective
The aim of this study was to identify patterns of multimorbidity in the total population of Region Stockholm, Sweden, by exploring demographics, claimed prescription drugs, risk of mortality and non-random association of conditions.
Methods
In this cross-sectional descriptive population-based cohort study, we extracted data from the Swedish VAL database (N = 2 323 667) including all consultations in primary and specialized outpatient care, all inpatient care and all prescriptions claimed during 2017. We report number of chronic conditions and claimed prescription drugs, physical and mental co-morbidity, and 1-year mortality. We stratified the analyses by sex. We examined non-random associations between diseases using cluster analysis.
Results
In total, 21.6% had multimorbidity (two or more chronic conditions) and 24.1% had polypharmacy (more than five claimed prescription drugs). Number of claimed drugs, co-occurrence of mental and physical conditions, and 1-year mortality increased as multimorbidity increased. We identified seven multimorbidity clusters with clinically distinct characteristics. The smallest cluster (7% of individuals) had prominent cardiovascular disease, the highest 1-year mortality rate, high levels of multimorbidity and polypharmacy, and was much older. The largest cluster (27% of individuals) was younger and heterogenous, with primarily mental health problems.
Conclusions
Individuals with chronic conditions often show clinical complexity with both concordant and discordant conditions and polypharmacy. This study indicates that clinical guidelines addressing clustering of conditions may be one strategy for managing complexity.
Keywords: Chronic disease, frailty, multimorbidity, phamacology/drug reactions, population health, primary health care
Key Messages.
Multimorbidity present in 22% of individuals in the Stockholm region.
Increased multimorbidity associated with high prevalence of sedatives.
Clinical multimorbidity management guidelines should address clustering of conditions.
Introduction
As multimorbidity, defined as two or more chronic health conditions, becomes the norm, patients are increasingly complex in primary care where most consultations take place (1–4). Degree of multimorbidity has been shown to be highly correlated with health care resource use and costs in a variety of contexts (5–8). Multimorbidity leads to polypharmacy, increased hospitalization and non-adherence, and increased potentially inappropriate medication (9,10). Despite this, evidence-based management guidelines for common chronic conditions are usually based on a single-disease paradigm and seldom take into account co-morbidities or patient complexity (11,12). The NICE guidelines and comprehensive review of the literature published 2016 recommend a shift in primary care towards systematic identification of patients with multimorbidity who need individually tailored management (13).
High-quality patient-centred care requires context-bound understanding of the multimorbid population’s patterns of demographics, co-morbidities and medication use (14). In Sweden, there is a national impetus to improve care of individuals with multimorbidity (15). The Swedish Study on Aging and Care in Kungsholmen (SNAC-K), following a cohort of individuals aged 60+ in central Stockholm, indicated that some constellations of multimorbidity and trajectories of development are likely to lead to decreased function and cognitive ability (16–18). However, population-based patterns of multimorbidity, medication and increased risk for mortality have yet to be described.
The aim of this study was to identify patterns of multimorbidity in the total population of Region Stockholm, Sweden, by exploring demographics, claimed prescription drugs, risk of mortality and non-random association of conditions.
Material and methods
In this cross-sectional descriptive population-based cohort study, we used the Swedish VAL database to identify the entire population of Region Stockholm 31 December 2017 (N = 2 323 667).
Database and study sample
Region Stockholm (Stockholm city and surrounding suburban and rural areas) has 2.3 million residents, ~20% of the total population of Sweden. In Sweden, all necessary medical care is funded by public health insurance covering all legal residents. Services are provided by region, either at public facilities or by private providers under contractual agreement with the region. Providers are obligated to record diagnoses and file reports, including information on health care utilization, reasons for hospitalizations and consultations in primary and specialist care, all diagnosis codes, data on prescriptions and socio-demographics. In Region Stockholm, this information is automatically collated in the comprehensive health administration VAL database used for health care planning, practice remuneration and quality assessment. All living residents of Region Stockholm are registered in VAL. Date of death as well as migration in and out of the region are included in VAL. The VAL database is described in more detail elsewhere (19).
In this study, we included data from all consultations in primary care, all consultations in specialized outpatient care, all inpatient care and all prescriptions claimed during 2017. All extracted data were anonymized.
Variable identification
Chronic conditions and multimorbidity
As we wanted to investigate multimorbidity separately from pharmacotherapy, we revised a definition based on 40 chronic health conditions identified as internationally clinically important (2) using only ICD codes (Supplementary Table 1). We define multimorbidity as two or more chronic health conditions across the 40 listed chronic conditions. We report degree of multimorbidity using the intervals 0–1, 2–4, 5–9 and 10+ diagnoses, based on current literature indicating that 5+ and 10+ diagnoses reflect clinically relevant cut points (2).
Pharmacotherapy
We collected the Anatomical Therapeutic Chemical (ATC) Classification System codes for all the individual’s claimed prescription drugs during 2017. Each ATC code was counted as one claimed prescription in the analysis to avoid overcounting because of drug iteration. We report number of claimed prescription drugs in the intervals 0–4, 5–9, 10–14 and 15+, using accepted previous definitions of polypharmacy (20), and the current literature indicating that 15+ medications compared with less than five medications substantially increases risk for adverse events (21).
Analyses
All descriptive data are reported as frequencies. For the 40 chronic conditions, we described median age, median number of co-morbidities, median number of medications and 1-year mortality. We identified the top 12 most common multimorbid conditions as those with the highest median number of co-morbidities and the largest number of individuals. For each degree of multimorbidity, we reported sex, age group (10-year intervals), number of claimed prescription drugs, frequency of physical–mental health co-morbidity, frequency for the top 12 conditions and 1-year mortality rate, calculated using mortality data from 1 January until 31 December 2018. For graphical display we defined age groups in 20-year intervals.
We stratified data for sex and repeated frequency calculations. Relative risk was calculated for women compared with men for age group (10-year intervals), number of claimed prescription drugs, frequency of physical–mental health co-morbidity, frequency of the top 12 conditions and 1-year mortality rate.
We use percentages and graphical display to describe the proportions of the top 25 co-morbidities and the proportions of the top 25 claimed medications for the top 12 conditions.
To examine non-random associations between diseases in individuals, we identified individuals with at least two conditions and conducted a cluster analysis. To find the optimum number of clusters the data was initially grouped into 50 clusters using the FASTCLUS k-means procedure with 100 iterations. We used the CLUSTER procedure using the centroid method to determine the optimal number of clusters. The Cubic Clustering Criterion, Pseudo F and Pseudo T-Squared statistics all suggested seven clusters which was deemed appropriate after clinical analysis. Last, individuals were grouped into seven clusters using the k-means procedure with 100 iterations.
Data analyses were performed with SAS EG 7.1. Due to the nature of the database, there was no missing data.
Results
Table 1 shows demographics, multimorbidity and pharmacotherapy characteristics, and 1-year mortality rate of all 2 323 667 individuals residing in the Stockholm region during 2017. 21.6% had multimorbidity. The top 12 conditions associated with multimorbidity were heart failure, chronic kidney disease, coronary heart disease, atrial fibrillation, COPD, stroke/TIA, dementia, peripheral vascular disease, hypertension, diabetes and cancer (Supplementary Table 2). One-year all-cause mortality increased as number of diagnoses increased (from 0.1% to 19.3%). There was 2.7%, absolute 1-year risk of mortality in multimorbid patients, but these deaths represented most deaths in the total population (>85%). In the total population, 24% had more than five claimed prescription drugs and number of drugs increased as the number of diagnoses increased. Of individuals with >10 diagnoses, 80% had >15 medications. Co-occurrence of mental and physical conditions increased with number of diagnoses (24.1–58.5%).
Table 1.
Demography, pharmacotherapy, common multimorbid diseases, physical–mental health co-morbidity and 1-year mortality by number of diagnoses
| Number of diagnoses | |||||
|---|---|---|---|---|---|
| All | 0–1 | 2–4 | 5–9 | 10+ | |
| N (%) | 2 323 667 (100%) | 1 822 056 (78.4%) | 424 489 (18.3%) | 75 305 (3.2%) | 1817 (0.1%) |
| Sex | |||||
| Female | 1 162 569 (50.0%) | 884 840 (48.6%) | 238 850 (56.3%) | 38 089 (50.6%) | 790 (43.5%) |
| Male | 1 161 098 (50.0%) | 937 216 (51.4%) | 185 639 (43.7%) | 37 216 (49.4%) | 1027 (56.5%) |
| Age | |||||
| 0–9 | 295 754 (12.7%) | 286 529 (15.7%) | 9204 (2.2%) | 21 (0.03%) | 0 (0%) |
| 10–19 | 258 750 (11.1%) | 241 006 (13.2%) | 17 538 (4.1%) | 206 (0.3%) | 0 (0%) |
| 20–29 | 320 453 (13.8%) | 289 557 (15.9%) | 29 946 (7.1%) | 950 (1.3%) | 0 (0%) |
| 30–39 | 350 272 (15.1%) | 312 669 (17.2%) | 36 113 (8.5%) | 1481 (2.0%) | 9 (0.5%) |
| 40–49 | 327 025 (14.1%) | 2781 13 (15.3%) | 46 153 (10.9%) | 2742 (3.6%) | 17 (0.9%) |
| 50–59 | 289 043 (12.4%) | 215 439 (11.8%) | 66 978 (15.8%) | 6560 (8.7%) | 66 (3.6%) |
| 60–69 | 219 562 (9.5%) | 123 922 (6.8%) | 82 976 (19.6%) | 12 450 (16.5%) | 214 (11.78) |
| 70–79 | 174 220 (7.5%) | 60 800 (3.3%) | 89 295 (21.0%) | 23 563 (31.3%) | 562 (30.9%) |
| 80–89 | 70 159 (3.0%) | 11 903 (0.7%) | 37 139 (8.8%) | 20 383 (27.1%) | 734 (40.4%) |
| 90–99 | 17 963 (0.8%) | 2042 (0.1%) | 8904 (2.1%) | 6803 (9.0%) | 214 (11.8%) |
| 100+ | 466 (0.0%) | 76 (0.0%) | 243 (0.06%) | 146 (0.2%) | 1 (0.06%) |
| Number of medications | |||||
| 0–4 | 1 762 514 (75.9%) | 1 607 117 (88.2%) | 152 043 (35.8%) | 3337 (4.4%) | 17 (0.9%) |
| 5–9 | 365 566 (15.7%) | 180 121 (9.9%) | 167 089 (39.4%) | 18 288 (24.3%) | 68 (3.7%) |
| 10–14 | 125 794 (5.4%) | 27 816 (1.5%) | 72 501 (17.1%) | 25 200 (33.5%) | 277 (15.2%) |
| 15+ | 69 793 (3.0%) | 7002 (0.4%) | 32 856 (7.7%) | 28 480 (37.8%) | 1455 (80.1%) |
| Common multimorbid diseases | |||||
| Heart failure | 36 444 (1.6%) | 574 (0.03%) | 12 621 (3.0%) | 21 905 (29.1%) | 1344 (74.0%) |
| Chronic renal disease | 25 443 (1.1%) | 1303 (0.07%) | 10 417 (2.5%) | 12 816 (17.0%) | 907 (49.9%) |
| Coronary heart disease | 63 778 (2.7%) | 3292 (0.2%) | 32 842 (7.7%) | 26 382 (35.0%) | 1262 (69.5%) |
| Atrial fibrillation | 58 841 (2.5%) | 3997 (0.2%) | 28 064 (6.6%) | 25 582 (33.0%) | 1198 (65.9%) |
| COPD | 48 620 (2.1%) | 5406 (0.3%) | 25 644 (6.0%) | 16 613 (22.1%) | 957 (52.7%) |
| Stroke/TIA | 42 027 (1.8%) | 2826 (0.2%) | 21 260 (5.0%) | 17 067 (22.7%) | 874 (48.1%) |
| Dementia | 18 202 (0.8%) | 1329 (0.07%) | 9497 (2.2%) | 7050 (9.4%) | 326 (17.9%) |
| Peripheral vascular disease | 14 220 (0.6%) | 1896 (0.1%) | 6633 (1.6%) | 5326 (7.1%) | 365 (20.1%) |
| Bronchiectasis | 2411 (0.1%) | 282 (0.02%) | 1269 (0.3%) | 801 (1.1%) | 59 (3.3%) |
| Hypertension | 334 935 (14.4%) | 69 061 (3.8%) | 201 517 (47.5%) | 62 623 (83.2%) | 1734 (95.4%) |
| Diabetes | 105 534 (4.5%) | 13 141 (0.7%) | 63 300 (14.9%) | 27 997 (37.2%) | 1096 (60.3%) |
| Cancer | 98 369 (4.23%) | 20 068 (1.1%) | 54 844 (12.9%) | 22 590 (30.0%) | 867 (47.7%) |
| Physical–mental health co-morbidity | 133 942 (5.8%) | 0 (0%) | 102 069 (24.1%) | 30 810 (40.9%) | 1063 (58.5%) |
| Anxiety disorders | 131 088 (5.6%) | 41 659 (2.3%) | 75 458 (17.8%) | 13 516 (18.8%) | 455 (25.0%) |
| Depression | 72 286 (3.1%) | 14 165 (0.8%) | 46 662 (11.0%) | 11 061 (14.7%) | 398 (21.9%) |
| Alcohol problems | 60 671 (2.6%) | 17 441 (1.0%) | 33 347 (7.9%) | 9529 (12.7%) | 354 (19.5%) |
| Substance misuse | 30 591 (1.3%) | 7434 (0.4%) | 18 035 (4.3%) | 4888 (6.5%) | 234 (12.9%) |
| 1-Year mortality | 15 842 (0.7%) | 2315 (0.1%) | 7122 (1.7%) | 6054 (8.0%) | 351 (19.3%) |
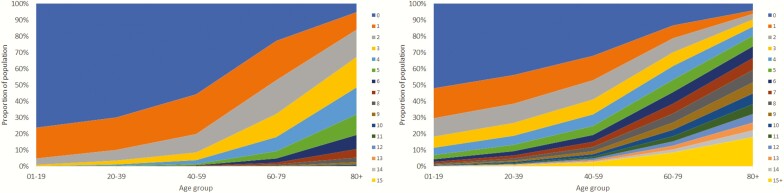
With age, the proportion of individuals with multimorbidity and number of co-morbidities increased (Fig. 1a), as did number of claimed prescription drugs (Fig. 1b). Multimorbidity increased after age 50, existed in more than half of the population at age 70, and in >80% over age 80. A similar age-related pattern was seen in the proportion of individuals with >5, 10 and 15 claimed prescription drugs. However, after the age of 90, the population showed decreased multimorbidity and polypharmacy.
Figure 1.
Proportion of the population in 2017 (N = 2 323 667) with (A) number of diagnoses per age group and (B) numbers of claimed prescription drugs per age group.
Disease patterns differed between the sexes (Table 2, Supplementary Table 3). Under 60, women were more likely than men to have multimorbidity, with elevated risk of 10+ conditions in age groups 30–39 and 40–59 [relative risk (RR) 4.55 and 4.23, respectively]. After 60, there were more women than men that were healthy or had only one condition (RR 1.15) and this likelihood increased with increasing age (to RR 3.54 at age 90–99). Women were more likely than men to have polypharmacy. Men suffered multimorbidity due to cardiovascular disease, kidney disease and diabetes to a higher degree than women. Women had higher prevalence of multimorbidity including lung disease and dementia. Compared with men, women were more likely to have both physical and mental health conditions.
Table 2.
Relative risk proportion of women compared to men for demography, pharmacotherapy, common multimorbid diseases, physical–mental health co-morbidity and 1-year mortality by number of diagnoses
| All | 0–1 | 2–4 | 5–9 | 10+ | |
|---|---|---|---|---|---|
| Relative risk proportion of women versus men | N/A | 0.94 (0.94-0.94) | 1.29 (1.28–1.29) | 1.02 (1.01–1.04) | 0.77 (0.70–0.84) |
| Age | |||||
| 0–9 | 0.94 (0.93–0.94) | 1.00 (1.00–1.01) | 0.62 (0.60–0.65) | 1.59 (0.66–3.83) | N/A |
| 10–19 | 0.93 (0.93–0.94) | 0.96 (0.95–0.97) | 1.11 (1.08–1.14) | 3.31 (2.39–4.58) | N/A |
| 20–29 | 0.99 (0.98–1.00) | 0.99 (0.98–0.99) | 1.35 (1.32–1.38) | 3.36 (2.89–3.91) | N/A |
| 30–39 | 0.96 (0.95–0.96) | 0.94 (0.94–0.95) | 1.39 (1.36–1.42) | 2.64 (2.36–2.96) | 4.55 (0.95–21.8) |
| 40–49 | 0.97 (0.96–0.97) | 0.94 (0.94–0.95) | 1.21 (1.19–1.23) | 1.95 (1.80–2.11) | 4.23 (1.38–12.9) |
| 50–59 | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) | 0.89 (0.88–0.91) | 1.19 (1.13–1.24) | 1.30 (0.81–2.09) |
| 60–69 | 1.03 (1.02–1.04) | 1.15 (1.14–1.16) | 0.77 (0.77–0.78) | 0.79 (0.77–0.82) | 0.96 (0.75–1.24) |
| 70–79 | 1.13 (1.12–1.14) | 1.46 (1.44–1.49) | 0.86 (0.85–0.87) | 0.72 (0.71–0.74) | 0.87 (0.76–1.01) |
| 80–89 | 1.48 (1.46–1.50) | 2.15 (2.07–2.23) | 1.27 (1.25–1.30) | 1.05 (1.03–1.08) | 0.96 (0.85–1.07) |
| 90–99 | 2.46 (2.38–2.54) | 3.54 (3.20–3.93) | 2.34 (2.23–2.45) | 1.80 (1.72–1.89) | 1.32 (1.03–1.70) |
| 100+ | 5.95 (4.59–7.70) | 6.99 (3.59–13.6) | 5.52 (3.77–8.09) | 4.31 (2.84–6.54) | N/A |
| Number of medications | |||||
| 0–4 | 0.88 (0.87–0.88) | 0.92 (0.92-0.92) | 0.78 (0.78–0.79) | 0.67 (0.63–0.72) | 0.54 (0.19–1.53) |
| 5–9 | 1.43 (1.42–1.44) | 1.84 (1.82–1.85) | 0.98 (0.97–0.99) | 0.71 (0.69–0.72) | 0.28 (0.15–0.52) |
| 10–14 | 1.64 (1.62–1.66) | 2.66 (2.60–2.73) | 1.34 (1.32–1.36) | 0.93 (0.91–0.95) | 0.58 (0.45–0.73) |
| 15+ | 1.93 (1.90–1.96) | 3.05 (2.89–3.21) | 1.92 (1.87–1.96) | 1.40 (1.37–1.42) | 1.17 (1.12–1.22) |
| Common multimorbid diseases | |||||
| Heart failure | 0.87 (0.85–0.89) | 0.80 (0.68–0.94) | 0.69 (0.67–0.72) | 0.85 (0.83–0.87) | 0.98 (0.93–1.04) |
| Chronic renal disease | 0.70 (0.68–0.72) | 0.69 (0.62–0.78) | 0.57 (0.55–0.60) | 0.68 (0.65–0.70) | 0.72 (0.65–0.79) |
| Coronary heart disease | 0.63 (0.62–0.64) | 0.43 (0.40–0.46) | 0.47 (0.46–0.48) | 0.68 (0.67–0.70) | 0.93 (0.87–0.99) |
| Atrial fibrillation | 0.73 (0.72–0.74) | 0.42 (0.39–0.45) | 0.58 (0.56–0.59) | 0.77 (0.75–0.78) | 0.84 (0.78–0.90) |
| COPD | 1.34 (1.31–1.36) | 1.30 (1.23–1.37) | 1.18 (1.15–1.21) | 1.14 (1.11–1.17) | 1.13 (1.03–1.23) |
| Stroke/TIA | 0.91 (0.89–0.92) | 0.99 (0.92–1.06) | 0.74 (0.72–0.76) | 0.84 (0.82–0.86) | 0.94 (0.85–1.04) |
| Dementia | 1.72 (1.67–1.77) | 2.65 (2.35–2.98) | 1.66 (1.59–1.73) | 1.24 (1.19–1.30) | 1.11 (0.91–1.35) |
| Peripheral vascular disease | 1.33 (1.28–1.37) | 2.39 (2.16–2.63) | 1.26 (1.20–1.32) | 0.89 (0.85–0.94) | 0.96 (0.80–1.16) |
| Bronchiectasis | 2.16 (1.98–2.36) | 2.34 (1.82–3.00) | 1.87 (1.66–2.11) | 1.85 (1.60–2.14) | 1.77 (1.06–2.94) |
| Hypertension | 1.07 (1.06–1.07) | 1.18 (1.16–1.20) | 0.85 (0.84–0.86) | 0.93 (0.92–0.93) | 0.98 (0.96–1.00) |
| Diabetes | 0.75 (0.74–0.76) | 0.59 (0.57–0.61) | 0.60 (0.60–0.61) | 0.77 (0.75–0.78) | 0.91 (0.84–0.98) |
| Cancer | 1.00 (0.99–1.02) | 1.29 (1.26–1.33) | 0.82 (0.81–0.84) | 0.74 (0.72–0.75) | 0.75 (0.68–0.83) |
| Physical–mental health co-morbidity | 1.56 (1.54–1.57) | N/A | 1.28 (1.26–1.29) | 1.31 (1.29–1.33) | 1.16 (1.07–1.25) |
| 1-Year mortality | 1.07 (1.03–1.10) | 0.97 (0.89–1.05) | 0.97 (0.92–1.01) | 0.95 (0.91–1.00) | 0.90 (0.75–1.10) |
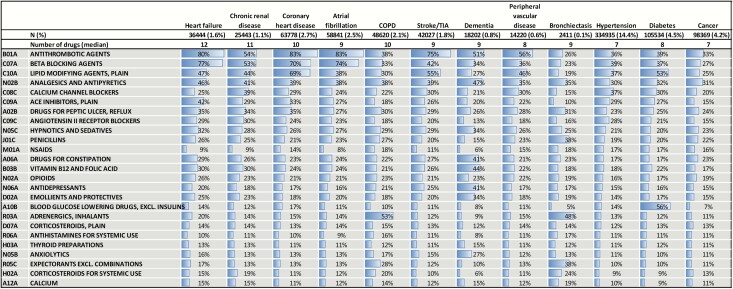
For individuals with the top 12 conditions, 6 of the top 10 drugs were medications aimed at improvement of cardiovascular health (Table 3). There was high prevalence of potentially inappropriate medications: hypnotics and sedatives were prescribed to between 19.8% (diabetes) and 33.5% (dementia); anxiolytics to between 10% (diabetes) and 27% (dementia); proton-pump inhibitors to between 23.3% (hypertension) and 35.2% (heart failure). Additionally, opioids were the 14th most prescribed drug and were prescribed to between 16.5% (hypertension) and 25.6% (heart failure). Anti-depressants were claimed by between 15% (hypertension, cancer) and 41% (dementia).
Table 3.
The 12 most common conditions with prevalence of the top 25 claimed medication groups (N = 469 778)
In the top 12 conditions, we identified the 25 most common co-morbidities (Supplementary Table 4). Hypertension was the most common, found in 44–81%, followed by diabetes (11–32%), hearing loss (10–16%) and thyroid disorders (12–16%). Anxiety disorders and depression were associated with all 12 common conditions, but at low rates (5–8% and 5–7%, respectively).
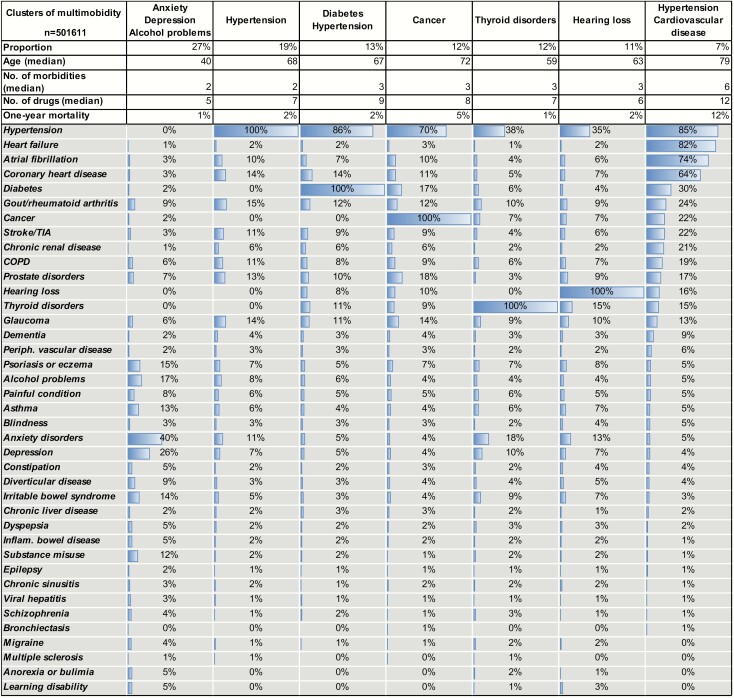
Cluster analysis examining non-random associations between diseases in the 501 611 individuals with at least two conditions, resulted in seven clusters (Table 4). The largest cluster included the 27% of individuals with multimorbidity not in other clusters. This cluster was characterized by lower median age, number of co-morbidities and number of drugs compared to the other clusters. No single disease was dominant, but in this group, 40% had anxiety, 26% depression, 17% alcohol problems and 14% had irritable bowel syndrome (IBS). The second largest cluster consisted of individuals with hypertension but without diabetes, cancer, thyroid disorders or hearing loss, and with a low degree of other cardiovascular disease, followed by a cluster of individuals with hypertension and diabetes. The fourth cluster consisted of individuals with cancer and hypertension. In the fifth cluster, thyroid disorders, 18% had anxiety, 10% had depression, 38% had hypertension and 9% had IBS. In the sixth cluster, hearing loss, there was a broad spectrum of conditions with hypertension and anxiety disorders most common. The smallest cluster, 7% of individuals, was characterized by hypertension in combination with one or several other cardiovascular diseases and to some extent diabetes. This cluster had a higher median age, number of co-morbidities and number of drugs, and higher 1-year mortality rate compared with the other clusters.
Table 4.
Clusters of co-morbidities in 501 611 individuals in the Stockholm Region in 2017
Discussion
Summary of the findings
In this total population study, the proportion of individuals with multimorbidity and polypharmacy increased with age until age 90, after which multimorbidity and polypharmacy decreased drastically. Multimorbid individuals had a low absolute 1-year risk of mortality but represented most deaths in the total population. Increased number of conditions was associated with increased risk for concomitant physical–mental disease. The top 12 conditions associated with multimorbidity were heart failure, chronic kidney disease, coronary heart disease, atrial fibrillation, COPD, stroke/TIA, dementia, peripheral vascular disease, hypertension, diabetes and cancer, all associated with high prevalence of multiple co-morbid conditions and high prevalence of prescribed medication, including potentially inappropriate medications. Women were more likely than men to have multimorbidity before age 60, less likely to have multimorbidity after age 80 and more likely than men to have lung disease, dementia and co-morbid physical and mental conditions. We identified seven clinically distinct non-randomly clustered conditions: a mental health cluster; a thyroid disease cluster; a cancer cluster; a hearing loss cluster; and a hypertension cluster, a hypertension-metabolic cluster and a cardiovascular cluster.
Relationship with previous research
Our study population resembles other cohorts. In a cross-sectional study of individuals registered at medical practices in Scotland, 23.2% had multimorbidity (2). As in the current study, prevalence increased with increasing age and prevalence of mental health disorders increased as the number of physical co-morbidities increased (2). In a retrospective cohort study of a random sample of primary care attenders in England, 16% of individuals had multimorbidity (22). Prevalence of multimorbidity in a population-based study in Ontario, Canada was 24.3% and patterns of concurrent disease were complex, similar to our study (3). No previous study has reported both the patterns of multimorbidity and polypharmacy on a population level.
The top 12 conditions identified in our study population overlap with clinically relevant conditions collated in a systematic review (23), but also include chronic kidney disease, dementia and peripheral vascular disease, while depression and arthritis do not make our top 12. Our list overlaps with the common conditions identified in multimorbid populations by several other large population-based studies (3,22,24).
Three general patterns of non-random associations between conditions have been identified by systematic review: cardiovascular and metabolic diseases, mental health problems and musculoskeletal disorders (25). In our study, the mental health cluster and the thyroid disease cluster are both younger than the other clusters and have a very low 1-year mortality rate. We identified three cardiovascular/metabolic disease clusters that seemed to represent different phases and/or possibly different health trajectories. Individuals in the hypertension cluster had a low level of associated cardiovascular conditions, no diabetes, and few drugs while individuals in the hypertension-metabolic cluster had diabetes, more co-morbidities and more medication. Individuals in the cardiovascular cluster were older, had many concurrent conditions and drugs, and had a high one-year mortality rate. Hypertension was highly prevalent in the multimorbid population, not specific to any one cluster. Hypertension may be best understood as a disease marker or a predictive factor for multimorbidity, and it would be interesting to further evaluate differences between individuals in the hypertension cluster compared to other clusters.
A cohort study of a population aged 77 years or older in Region Stockholm identified similar clusters: two clusters of cardiovascular conditions, one mental illness and musculoskeletal cluster, a diabetes mellitus and malignancy cluster, and a visual impairment and anaemia cluster (16). The clusters in the current study included all age groups, explaining some of the differences between studies. The mental health and thyroid clusters as well as the three cardiovascular/metabolic clusters may represent causal associative multimorbidity, or common pathophysiological pathways within these clusters (26).
Our study aligns with other cross-sectional analyses showing an increase in prescribing with increased age, for example in Scotland (27), Italy (28) and Sweden (27). However, this is the first study of its size to identify the frequencies of claimed prescription for the most common multimorbid conditions. A recent Swedish study of polypharmacy in the elderly found similar trends for potentially inappropriate prescribing, including high frequencies of anxiolytics and hypnotics (29). Our study may indicate deficiencies in appropriate prescribing for atrial fibrillation (30) and heart failure (31), similar to a recent Irish study (32).
Strengths and weaknesses
This study used the total population of Stockholm County, >2 million individuals and is one of the largest population-based studies of multimorbidity to date. This is a complete data set comprising all health care visits, diagnoses and claimed prescription medications for this population. This data set is representative for Sweden but may have limited generalizability in other settings.
Registry data relies on reporting and therefore risks misclassification of diagnoses. Previous studies have shown much higher prevalence of anxiety (33) and depression (34) than in the VAL database, and have identified underreporting of these diagnoses (35). In our data, individuals with multimorbidity more often had a prescription for anti-depressants than a diagnosis of mental disorder, indicating underdiagnosing in this group. Mental disorders are often underdiagnosed in the elderly population. Our ‘mental health’ cluster is younger than the other clusters. Using medication data in our clustering model as proxy for mental health diagnoses might yield different clusters. Our data likely reflects underdiagnosing of chronic kidney disease. A study of the total population of Stockholm, reported only 12 % of individuals with a glomerular filtration rate below 60 ml/min/1.73 m2 ICD-coded for chronic kidney disease (36). Accurate prevalence figures could result in these conditions being much higher. In the VAL database, inpatient and outpatient data are reported to the Swedish National Inpatient Register, which has been validated (37). However, data from primary care have not been validated. An individual’s age and sex are identifiable from the national identification number, and registered visits are directly relayed to VAL, so these variables are unlikely to be misclassified. Residual confounding by variables not recorded in VAL could not be evaluated. Data includes only claimed prescription drugs, not written prescriptions, adherence to treatment, nor over the counter drugs.
Implications for practice
Patient-centred primary care should take account of patient complexity and multimorbidity (38). The Ariadne principles of multimorbidity management in primary care advise patients and providers to set realistic treatment goals based on assessment of interactions between conditions and treatment, patient priorities, and individualized plans and follow-up (39). This requires the physician to identify serious or debilitating dominant conditions for prioritization and to distinguish between concordant conditions (with shared pathophysiology profile and similar management strategies), and discordant conditions (that may require separate or competing management strategies) (40). In this study, the identified clusters include concordant conditions, but also discordance, potentially complicating individual management (25). For example, while clustering of cancer and hypertension cluster may not change cancer management per se, it points out that even management of cancer patients needs to take account of multimorbidity. Better understanding of disease clustering and development of clinical management guidelines for common clusters could help clinicians with the difficulties of managing the complexity of multimorbidity.
Future research should explore understanding why many patients with multimorbidity are prescribed potentially inappropriate drugs such as sedatives, opioids and anxiolytics, and if there are potential omissions in prescribing. Finally, the findings from this study should inform development and testing of interventions for improving the health care of the steadily growing multimorbid population.
Conclusions
Individuals with chronic conditions often show clinical complexity with both concordant and discordant conditions and polypharmacy. This study indicates that clinical guidelines addressing clustering of conditions may be one strategy for managing complexity.
Declaration
Funding: This project was supported by the Karolinska Institute and the Stockholm Region. Funders of this study had no role in study design, data collection, analysis or write-up.
Ethical approval: This study was approved by the Regional Ethics Committee in Stockholm (2017/1690-32).
Conflict of interest: none.
Supplementary Material
References
- 1. Uijen AA, van de Lisdonk EH. Multimorbidity in primary care: prevalence and trend over the last 20 years. Eur J Gen Pract 2008; 14 (suppl 1): 28–32. [DOI] [PubMed] [Google Scholar]
- 2. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380: 37–43. [DOI] [PubMed] [Google Scholar]
- 3. Pefoyo AJ, Bronskill SE, Gruneir Aet al. . The increasing burden and complexity of multimorbidity. BMC Public Health 2015; 15: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Violan C, Foguet-Boreu Q, Flores-Mateo Get al. . Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One 2014; 9: e102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perkins AJ, Kroenke K, Unützer Jet al. . Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol 2004; 57: 1040–8. [DOI] [PubMed] [Google Scholar]
- 6. McPhail SM. Multimorbidity in chronic disease: impact on health care resources and costs. Risk Manag Healthc Policy 2016; 9: 143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thavorn K, Maxwell CJ, Gruneir Aet al. . Effect of socio-demographic factors on the association between multimorbidity and healthcare costs: a population-based, retrospective cohort study. BMJ Open 2017; 7: e017264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Violán C, Foguet-Boreu Q, Roso-Llorach Aet al. . Burden of multimorbidity, socioeconomic status and use of health services across stages of life in urban areas: a cross-sectional study. BMC Public Health 2014; 14: 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wimmer BC, Cross AJ, Jokanovic Net al. . Clinical outcomes associated with medication regimen complexity in older people: a systematic review. J Am Geriatr Soc 2017; 65: 747–53. [DOI] [PubMed] [Google Scholar]
- 10. Morin L, Vetrano DL, Rizzuto D, Calderón-Larrañaga A, Fastbom J, Johnell K. Choosing Wisely? Measuring the burden of medications in older adults near the end of life: nationwide, longitudinal cohort study. Am J Med 2017; 130: 927–36.e9. [DOI] [PubMed] [Google Scholar]
- 11. Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition–multimorbidity. JAMA 2012; 307: 2493–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wyatt KD, Stuart LM, Brito JPet al. . Out of context: clinical practice guidelines and patients with multiple chronic conditions: a systematic review. Med Care 2014; 52(Suppl 3): S92–S100. [DOI] [PubMed] [Google Scholar]
- 13. National Guideline Centre. National Institute for Health and Care Excellence: Clinical Guidelines. Multimorbidity: Assessment, Prioritisation and Management of Care for People with Commonly Occurring Multimorbidity. London, UK: National Institute for Health and Care Excellence (UK) Copyright (c) National Institute for Health and Care Excellence; 2016. [PubMed] [Google Scholar]
- 14. Navickas R, Petric VK, Feigl AB, Seychell M. Multimorbidity: what do we know? What should we do? J Comorb 2016; 6: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Effektiv vård GS. Slutbetänkande av En nationell samordnare för effektivare resursutnyttjande inom hälso och sjukvården (Effective Healthcare: Conclusions from the National Coordinator for More Effective Use of Resources in Healthcare). In: SOU (ed). Stockholm, Sweden: Wolters Kluwer Sverige AB, 2016. [Google Scholar]
- 16. Marengoni A, Roso-Llorach A, Vetrano DLet al. . Patterns of multimorbidity in a population-based cohort of older people: sociodemographic, lifestyle, clinical, and functional differences. J Gerontol A Biol Sci Med Sci 2020; 75: 798–805. [DOI] [PubMed] [Google Scholar]
- 17. Calderón-Larrañaga A, Santoni G, Wang HXet al. . Rapidly developing multimorbidity and disability in older adults: does social background matter? J Intern Med 2018; 283: 489–99. [DOI] [PubMed] [Google Scholar]
- 18. Vetrano DL, Rizzuto D, Calderón-Larrañaga Aet al. . Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: a Swedish cohort study. PLoS Med 2018; 15: e1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carlsson AC, Wändell P, Ösby U, Zarrinkoub R, Wettermark B, Ljunggren G. High prevalence of diagnosis of diabetes, depression, anxiety, hypertension, asthma and COPD in the total population of Stockholm, Sweden – a challenge for public health. BMC Public Health 2013; 13: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cadogan CA, Ryan C, Hughes CM. Appropriate polypharmacy and medicine safety: when many is not too many. Drug Saf 2016; 39: 109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995–2010. BMC Med 2015; 13: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2011; 61: e12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases–a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci 2011; 66: 301–11. [DOI] [PubMed] [Google Scholar]
- 24. Nunes BP, Flores TR, Mielke GI, Thumé E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2016; 67: 130–8. [DOI] [PubMed] [Google Scholar]
- 25. Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol 2014; 67: 254–66. [DOI] [PubMed] [Google Scholar]
- 26. van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol 2001; 54: 675–9. [DOI] [PubMed] [Google Scholar]
- 27. Hovstadius B, Hovstadius K, Astrand B, Petersson G. Increasing polypharmacy – an individual-based study of the Swedish population 2005-2008. BMC Clin Pharmacol 2010; 10: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franchi C, Tettamanti M, Pasina Let al. . Changes in drug prescribing to Italian community-dwelling elderly people: the EPIFARM-Elderly Project 2000-2010. Eur J Clin Pharmacol 2014; 70: 437–43. [DOI] [PubMed] [Google Scholar]
- 29. Hovstadius B, Petersson G, Hellström L, Ericson L. Trends in inappropriate drug therapy prescription in the elderly in Sweden from 2006 to 2013: assessment using national indicators. Drugs Aging 2014; 31: 379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012; 125: 2298–307. [DOI] [PubMed] [Google Scholar]
- 31. McMurray JJ, Adamopoulos S, Anker SDet al. ; Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–69. [DOI] [PubMed] [Google Scholar]
- 32. Galvin R, Moriarty F, Cousins Get al. . Prevalence of potentially inappropriate prescribing and prescribing omissions in older Irish adults: findings from the Irish LongituDinal Study on Ageing study (TILDA). Eur J Clin Pharmacol 2014; 70: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lotfi L, Flyckt L, Krakau I, Mårtensson B, Nilsson GH. Undetected depression in primary healthcare: occurrence, severity and co-morbidity in a two-stage procedure of opportunistic screening. Nord J Psychiatry 2010; 64: 421–7. [DOI] [PubMed] [Google Scholar]
- 34. Puyat JH, Marhin WW, Etches Det al. . Estimating the prevalence of depression from EMRs. Can Fam Physician 2013; 59: 445. [PMC free article] [PubMed] [Google Scholar]
- 35. Cepoiu M, McCusker J, Cole MG, Sewitch M, Belzile E, Ciampi A. Recognition of depression by non-psychiatric physicians–a systematic literature review and meta-analysis. J Gen Intern Med 2008; 23: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gasparini A, Evans M, Coresh Jet al. . Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 2016; 31: 2086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ludvigsson JF, Andersson E, Ekbom Aet al. . External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guthrie B, Payne K, Alderson P, McMurdo ME, Mercer SW. Adapting clinical guidelines to take account of multimorbidity. BMJ 2012; 345: e6341. [DOI] [PubMed] [Google Scholar]
- 39. Muth C, van den Akker M, Blom JWet al. . The Ariadne principles: how to handle multimorbidity in primary care consultations. BMC Med 2014; 12: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care 2006; 29: 725–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.