Abstract
COVID-19 affects a wide spectrum of organ systems. We report a 52-year-old man with hypertension and newly diagnosed diabetes mellitus who presented with hypoxic respiratory failure due to COVID-19 and developed severe brachial plexopathy. He was not treated with prone positioning respiratory therapy. Associated with the flaccid, painfully numb left upper extremity was a livedoid, purpuric rash on his left hand and forearm consistent with COVID-19-induced microangiopathy. Neuroimaging and electrophysiological data were consistent with near diffuse left brachial plexitis with selective sparing of axillary, suprascapular and pectoral fascicles. Given his microangiopathic rash, elevated D-dimers and paucifascicular plexopathy, we postulate a patchy microvascular thrombotic plexopathy. Providers should be aware of this significant and potentially under-recognised neurologic complication of COVID-19.
Keywords: neurological injury, neuromuscular disease, pain (neurology), peripheral nerve disease, infections
Background
The current COVID-19 pandemic has resulted in approximately 57.8 million cases and 1.3 million deaths.1 Although COVID-19 was originally seen as a predominantly respiratory illness, our understanding of its impact on multiple organ systems has evolved. It is often accompanied by a variety of neurologic symptoms such as headache, anosmia, confusion and altered mental status.2 Ischaemic vascular events have been frequently observed.3 4 Less commonly, neurologic presentations such as Guillain-Barre syndrome, necrotising haemorrhagic encephalopathy and focal status epilepticus have also been described.5–7 Here, we seek to add to our understanding of COVID-19 by presenting a case of brachial plexopathy.
Case presentation
A 52-year-old man with hypertension and newly diagnosed diabetes mellitus presented to our emergency department in March 2020 after 4 days of cough, shortness of breath, fatigue and myalgias. He had no sick contacts or recent travel. He was febrile (38.6°C), tachycardic, tachypneic and hypoxic (90% on room air). At the time, neurologic examination was unremarkable. Chest X-ray demonstrated extensive bilateral opacities consistent with multifocal pneumonia. Diagnosis of COVID-19 was made by detection of SARS-CoV-2 nucleic acid by reverse transcription PCR.
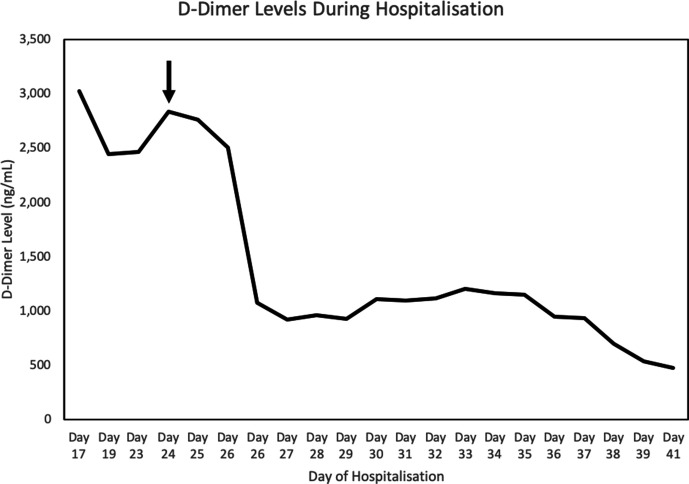
The patient’s oxygen requirements escalated, and he was intubated for respiratory failure within 24 hours of admission. For COVID-19-directed therapy, he received 5 days of hydroxychloroquine and two doses of a study drug (sarilumab vs placebo) on days 8 and 24. His course was complicated by transient renal failure without need for dialysis, Enterobacter pneumonia and Pseudomonas cystitis (both treated with piperacillin/tazobactam) and a hypercoagulable state with elevated D-dimers (figure 1). He was treated continuously with high-dose prophylactic enoxaparin, 30–40 mg two times per day. During intubation, he had multiple, uncomplicated central venous and arterial lines. There was no left axillary arterial catheterisation, and he never received prone positioning respiratory therapy. On day 22, he underwent percutaneous tracheostomy.
Figure 1.
D-dimer levels in the patient over the course of his hospitalisation. The black arrow indicates the first appearance of the microthrombotic rash. Reference range: 0–229 ng/mL.
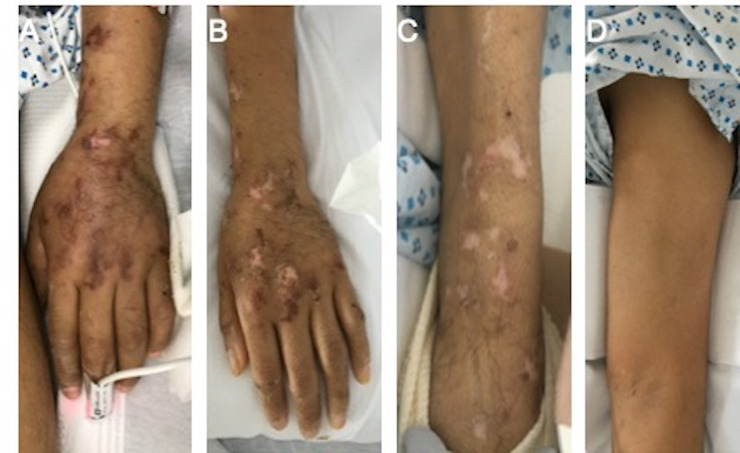
On day 24, a livedoid, retiform, purpuric rash was noted on the left hand and forearm (figure 2). Dermatology was consulted. The rash was deemed characteristic of COVID-19-induced thrombotic microvascular injury based on the department’s case series.8 A biopsy was therefore not performed.
Figure 2.
Livedoid, retiform purpura of the left hand and forearm (A) on day 24, (B) on day 45 (day of initial neurological consultation) and (C) on day 50 (day following EMG/NCS). (D) The rash did not involve the left upper arm, despite weakness of these muscle groups. EMG, electromyography; NCS nerve conduction study.
Within 4 days, the patient was noted to have severe weakness of the left upper extremity and neuropathic pain in the left hand and forearm. Neurology was consulted. On examination, tone was absent in the left hand, wrist and elbow. The left biceps reflex was reduced with absence of the left brachioradialis and triceps reflexes. Pin and thermal sensation were absent over the entire left forearm and hand, with presence of tactile allodynia. Strength was reduced as follows: biceps 2/5, triceps 0/5, wrist and finger extensors 0/5, wrist and finger flexors 3/5, forearm pronation 3/5, forearm supination 2/5, superficial and deep finger flexors 3/5 and intrinsic hand muscles 3/5. The left deltoid, infraspinatus and pectoral muscles were strong at 5/5. There was no scapular winging (table 1).
Table 1.
Neurologic examination of left upper extremity—inpatient examination and postdischarge follow-up
| Inpatient examination | Postdischarge follow-up | ||
| Tone | Hand | Absent | Reduced |
| Wrist | Absent | Reduced | |
| Elbow | Absent | Reduced | |
| Strength (MRC scale)† | Triceps | 0/5 | 4/5 |
| Biceps | 2/5 | * | |
| Supinator | 2/5 | 4/5 | |
| Wrist extensors | 0/5 | 0/5 | |
| Finger extensors | 0/5 | 0/5 | |
| Wrist flexors | 3/5 | 4/5 | |
| Finger flexors superficial | 3/5 | 4/5 | |
| Finger flexors deep | 3/5 | 4/5 | |
| Interossei | 3/5 | 4-/5 | |
| Abductor digiti minimi | * | 4-/5 | |
| Abductor pollicis brevis | * | 2/5 | |
| Reflexes | Biceps | Reduced | Absent |
| Brachioradialis | Absent | Absent | |
| Triceps | Absent | Absent | |
| Sensation | Pin | Reduced | Reduced |
| Vibration | * | Absent in the left elbow | |
| Proprioception | * | Absent in the left wrist |
*Not documented on examination.
†Medical Research Council.
Investigations
Subsequent chest X-ray showed resolving multifocal pneumonia without elevation of the left hemidiaphragm.
MRI of the left brachial plexus with and without intravenous contrast revealed T2 hyperintensity and thickening of the entire brachial plexus without contrast enhancement (figure 3A). There was enhancement and T2 hyperintensity of the left serratus anterior reflecting acute denervation oedema (figure 3B).
Figure 3.

MRI reveals diffuse left brachial plexitis (A: coronal T2 STIR 20 mm reformat) with denervation enhancement and oedema of the left serratus anterior (B: coronal T1 with gadolinium). STIR, short tau inversion recovery.
Nerve conduction studies (NCSs) and needle electromyography (EMG) were performed on day 49 (online supplemental tables 1A, 2A, 3A). NCS revealed markedly reduced left median and ulnar compound muscle action potential response amplitudes with slowing of motor conduction velocities due to axon loss. The left-sided median, ulnar, radial, musculocutaneous and medial antebrachial cutaneous responses were all absent. Needle EMG of muscles in the left upper extremity showed fibrillations and positive sharp waves in all muscles except the deltoid, where examination was normal. Recruitment was absent in the left triceps, brachioradialis, extensor carpi radialis, extensor digitorum communis and extensor indicis. Recruitment was reduced in the remaining assessed muscles. A selective fascicular biopsy of the brachial plexus was felt to be against the best interests of the patient.
bcr-2020-237459supp001.pdf (225.3KB, pdf)
Laboratory studies were sent to evaluate vasculitides and coagulation disorders. Factor VIII activity was elevated (184%; reference 50%–150%) and protein S activity was mildly reduced (65%; reference 75%–125%). Work-up was otherwise unremarkable (online supplemental table 4).
Differential diagnosis
Injury to the brachial plexus can be caused by a variety of mechanisms including trauma/compression, autoimmune reactions, direct viral infection and microvascular ischaemia.9 Traumatic plexopathies are occasionally seen secondary to stretch injury, often from intraoperative positioning and typically with good recovery of symptoms.10 Prone positioning, frequently employed in the management of acute respiratory distress syndrome, has been reported to cause neurapraxic brachial plexopathy from pressure injury.11 12 Brachial plexus injury has also been reported in conjunction with trauma to the axillary artery, including complications of axillary arterial lines (eg, local thromboses, haematomas and pseudoaneurysms).13 14 In our case, traumatic and compressive aetiologies were ruled out. The patient was never proned, nor his left arm was ever hyperabducted or cannulated by an axillary line. Additionally, there was no evidence of focal trauma or compression on MRI. The severe weakness and numbness with axonotmetic electrodiagnostic findings eliminate a stretching neurapraxic injury.
Given his concurrent rash, vasculitis of the brachial plexus was considered. Medium and small vessel vasculitides can cause inflammation of the epineural arteries of the vasa nervorum with subsequent thrombosis and ischaemia.15–18 However, most vasculitides present with mononeuritis or mononeuritis multiplex; vasculitis-associated plexopathies are rare.19 In our patient, autoimmune and vasculitis labs were unremarkable. His new diagnosis of diabetes mellitus prompted consideration of diabetic radiculoplexus neuropathy. Most cases, however, progress to involve the contralateral side or are associated with lumbosacral plexus disease.20
Direct viral invasion of the plexus, as with varicella-zoster virus (VZV)-associated plexopathy, was also plausible. VZV can rarely infect the plexus with a radial nerve-predominant palsy, however the distribution of weakness and rash would be expected to colocalise.21 22 In this case, weakness was found in muscle groups beyond the distribution of the rash.
Another possibility was Parsonage-Turner syndrome (or neuralgic amyotrophy) in a postviral setting. Classically, patients report sudden-onset, severe but self-limited unilateral shoulder pain. Weakness, generally localised to the upper proximal limb, is noted 1–2 weeks after onset of pain. Neuralgic amyotrophy of the brachial plexus has been described in association with COVID-19, with the proposed mechanism of action being a COVID-19 immune-mediated inflammation.23 However, the focal demyelination that would be seen with this process differs from the patchy pattern of axonotmesis seen in our patient with complete sparing of axillary, pectoral and suprascapular fascicles.24 25
The complete sparing of some fascicles and severe denervation of other fascicles that run through the same trunks and cords suggest a microvascular paucifascicular infarction due to thrombosis of the vasa nervorum in the setting of COVID-19-induced hypercoagulability. The pattern of brachial plexus injury with selective fascicular involvement, along with the microthrombotic rash on the ipsilateral arm, supports this mechanism of disease.
Treatment
His wrist was splinted to prevent contractures. He received physical therapy, and his pain improved with gabapentin, oxycodone and acetaminophen.
Outcome and follow-up
The patient was transferred to a rehabilitation unit on day 69 and was discharged home on day 106 of his hospitalisation. A neurological follow-up examination and EMG were conducted 3 months after discharge. His neuropathic pain had resolved. On examination of the left upper extremity, tone was no longer absent but was reduced across the left wrist. The left triceps and forearm showed severe muscle atrophy without fasciculations. Tendon reflexes were absent in the left arm. There was reduced pin and thermal sensation in the left C5-T1 dermatomes with absent vibration to the left elbow and absent proprioception to the left wrist. Strength was reduced as follows: triceps 4/5, wrist and finger extensors 0/5, wrist and finger flexors 4/5, forearm supination 4/5 and intrinsic hand muscles 4-/5 (table 1). NCSs were unchanged, but needle EMG of the left upper extremity showed complete reinnervation of the biceps, flexor carpi radialis, first dorsal interosseous, superficial and deep finger flexors, partial reinnervation of the triceps, abductor digiti minimi and abductor pollicis brevis and persisting, complete denervation of the wrist and finger extensors (online supplemental table 1B, 2B, 3B).
Discussion
To our knowledge, this is the first reported case of brachial plexopathy associated with COVID-19 in a patient never placed in the prone position. We propose that a COVID-19-induced hypercoagulable state resulted in microthrombi which occluded portions of the vasa nervorum supplying the brachial plexus, thereby causing nerve ischaemia and infarction.
COVID-19 can cause a hypercoagulable state due to activation of the coagulation cascade and complement system.8 26 27 Laboratory abnormalities include elevated fibrinogen, elevated D-dimer and changes to prothrombin time (PT), activated partial thromboplastin time (aPTT), and international normalized ratio (INR).27 28 Multiple reports have described the high likelihood of pulmonary embolism and deep venous thrombosis in COVID-19, with Klok et al reporting a 49% incidence of large-vessel thrombotic complications in critically ill patients with COVID-19.29–31 Additionally, multiple reports have described the formation of microthrombi in small vessels affecting the lungs, heart and skin.8 32–34 Magro et al described COVID-19 patients with retiform, purpuric lesions identical to those in this case. These skin lesions, on immunohistochemical staining, showed thrombotic vasculopathy with significant deposition of C5b-9 in the microvasculature.8
In this patient, prior to the onset of his rash, D-dimers were elevated between 2400 ng/mL and 3000 ng/mL, with abnormal factor VIII and protein S levels supporting the presence of an acquired prothrombotic state during acute viral infection.35 Electrophysiology showed a combination of complete or partial axonotmesis of most but not all of the motor fascicles, supporting a patchy infarction pattern of the brachial plexus. For example, though both radial and axillary nerves are branches of the posterior cord of the brachial plexus, muscles innervated by the radial nerve were affected, while those by the axillary nerve were spared. Considering the prothrombotic state, these findings support a variable microthrombosis of the vasa nervorum as has also been hypothesised by Needham et al in a recent case series of patients with COVID-19 and mononeuritis multiplex.36
Further investigations should examine microthrombi formation as an important mechanism by which COVID-19 affects the peripheral nervous system and also explore more effective therapy against microthrombi formation in COVID-19 infection.
Patient’s perspective.
I have spent a lot of time in the hospital, so I feel anxious about what comes next. I worry about my ability to return to work as I did before. But my family waits for me, and I miss them as well; I want to see them again.
Specific to my hand, I still have some swelling, pain and numbness, but I have a little more strength and range of motion than before. I feel like I have received good care here in the hospital. Though I need assistance, I am now able to walk. I hope that I get better, and that I am able to leave here soon. I hope to get back mobility in my hand, to be able to lift myself up and to be able to eat by myself.
If I were to give advice to someone in my shoes, I would advise them to have patience and to always hope for recovery. The main thing that brought me hope during my own hospital stay was God. (Translated from Spanish, interviewed before patient’s discharge.)
Learning points.
Injury to the brachial plexus can be caused by a variety of mechanisms including trauma/compression, autoimmune responses, direct viral infection or microvascular ischaemia.
Clinicians should be aware that COVID-19-induced hypercoagulability may present with microthrombotic complications in multiple organ systems.
Early splinting and physiotherapy should be considered in patients who show evidence of brachial plexus injury.
Footnotes
Contributors: CYH contributed to writing/manuscript preparation, writing the initial draft, making critical revisions and creating figures/tables. She aided with translation and patient communication. AMT contributed to writing/manuscript preparation, making critical revisions and creating figures/supplemental tables. He wrote portions of the paper including the summary. He was part of the neurology consult team that was involved in the patient’s care. ANG contributed to writing/manuscript preparation, making critical revisions and writing portions of the paper including the differential diagnoses. She was part of the neurology consult team that was involved in the patient’s care. UWK contributed to project conception, writing/manuscript preparation and critical revisions. She was part of the neurology consult team that was involved in the patient’s care. PRB contributed to writing/manuscript preparation, making critical revisions and finalising manuscript content. She contributed to direct patient care as the attending physician taking care of the patient. She also obtained final patient’s consent and patient’s quotations. DJLM contributed to analysis and interpretation of vital data as the consulting electromyographer and also made critical revisions of the manuscript. TC contributed to project conception, as project supervisor and as the attending physician taking care of the patient when the brachial plexopathy was initially investigated. He also made contributions to writing and critical revisions. All authors gave final approval of the case report before publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Weekly epidemiological update – 24 November 2020. World Health Organization 2020.
- 2.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci 2020;413:116832. 10.1016/j.jns.2020.116832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 2020;382:e60. 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol 2020;77:1366–72. 10.1001/jamaneurol.2020.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci 2020;76:233–5. 10.1016/j.jocn.2020.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vollono C, Rollo E, Romozzi M, et al. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure 2020;78:109–12. 10.1016/j.seizure.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology 2020;296:E119–20. 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020;220:1–13. 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakrasek EM, Karandikar NSM. Brachial plexopathy: differential diagnosis and treatment. American Academy of Physical Medicine and Rehabilitation. [Google Scholar]
- 10.Ben-David B, Stahl S. Prognosis of intraoperative brachial plexus injury: a review of 22 cases. Br J Anaesth 1997;79:440–5. 10.1093/bja/79.4.440 [DOI] [PubMed] [Google Scholar]
- 11.Goettler CE, Pryor JP, Reilly PM. Brachial plexopathy after prone positioning. Crit Care 2002;6:540–2. 10.1186/cc1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diprose WK, et al. Bilateral upper limb neuropathies following prone ventilation for COVID-19 pneumonia. Neurology: Clinical Practice 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo F, Park J, Chow K, et al. Avoiding peripheral nerve injury in arterial interventions. Diagn Interv Radiol 2019;25:380–91. 10.5152/dir.2019.18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham JM, Mattox KL, Feliciano DV, et al. Vascular injuries of the axilla. Ann Surg 1982;195:232–8. 10.1097/00000658-198202000-00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaes F. Diagnosis and therapeutic options for peripheral vasculitic neuropathy. Ther Adv Musculoskelet Dis 2015;7:45–55. 10.1177/1759720X14566617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naseri Alavi SA, Meshkini M, Pourlak T, et al. Microscopic polyangiitis complicated with bilateral brachial plexopathy: a case report and review of the literature. Rheumatol Int 2016;36:997–1001. 10.1007/s00296-016-3424-4 [DOI] [PubMed] [Google Scholar]
- 17.Raz I, Leitersdorf E, Kleinman Y. Acute bilateral brachial plexus neuritis associated with hypersensitivity vasculitis. A case report and review of literature. Klin Wochenschr 1985;63:643–5. 10.1007/BF01732860 [DOI] [PubMed] [Google Scholar]
- 18.Aralasmak A, Karaali K, Cevikol C, et al. Mr imaging findings in brachial plexopathy with thoracic outlet syndrome. AJNR Am J Neuroradiol 2010;31:410–7. 10.3174/ajnr.A1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imboden JB. Involvement of the peripheral nervous system in polyarteritis nodosa and antineutrophil cytoplasmic Antibodies-Associated vasculitis. Rheum Dis Clin North Am 2017;43:633–9. 10.1016/j.rdc.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 20.Massie R, Mauermann ML, Staff NP, et al. Diabetic cervical radiculoplexus neuropathy: a distinct syndrome expanding the spectrum of diabetic radiculoplexus neuropathies. Brain 2012;135:3074–88. 10.1093/brain/aws244 [DOI] [PubMed] [Google Scholar]
- 21.Jeevarethinam A, Ihuoma A, Ahmad N. Herpes zoster brachial plexopathy with predominant radial nerve palsy. Clin Med 2009;9:500–1. 10.7861/clinmedicine.9-5-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Wu B-Y, Ma Z-S, et al. A retrospective case series of segmental zoster paresis of limbs: clinical, electrophysiological and imaging characteristics. BMC Neurol 2018;18:121. 10.1186/s12883-018-1130-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siepmann T, Kitzler HH, Lueck C, et al. Neuralgic amyotrophy following infection with SARS-CoV-2. Muscle Nerve 2020;62): :E68–70. 10.1002/mus.27035 [DOI] [PubMed] [Google Scholar]
- 24.Feinberg JH, Radecki J. Parsonage-turner syndrome. Hss J 2010;6:199–205. 10.1007/s11420-010-9176-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz Torres M, Gudlavalleti A, Mesfin FB. Brachial plexitis (Parsonage Turner syndrome, brachial neuropathy, brachial radiculitis). StatPearls 2020. [Google Scholar]
- 26.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020;18:1747–51. 10.1111/jth.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020;18:1023–6. 10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klok FA, Kruip M, van der Meer NJM. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020;191:148–50. 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–98. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo W, Yu H. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID‐19). Preprints. [Google Scholar]
- 33.Liu PP, Blet A, Smyth D, et al. The science underlying COVID-19: implications for the cardiovascular system. Circulation 2020;142:68–78. 10.1161/CIRCULATIONAHA.120.047549 [DOI] [PubMed] [Google Scholar]
- 34.Ackermann M, Verleden SE, Kuehnel M. Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goeijenbier M, van Wissen M, van de Weg C, et al. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol 2012;84:1680–96. 10.1002/jmv.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Needham E, Newcombe V, Michell A. Mononeuritis multiplex: an unexpectedly common feature of severe COVID-19. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2020-237459supp001.pdf (225.3KB, pdf)