Abstract
Purpose:
We sought to examine the association between shorter-term changes in markers of bone turnover and longer-term changes in bone mineral density (BMD) and microstructure in a cohort of frail elderly women with multiple comorbid conditions including osteoporosis.
Methods:
We performed a secondary analysis of a 2-year zoledronic acid trial for osteoporosis in 155 women residents of long-term care communities (mean age 86.9 years). We examined the association of the 6-month change in serum C-terminal crosslinking telopeptide of type I collagen (CTX) and serum intact procollagen type I N propeptide (PINP) with the 12- and 24-month changes in BMD at the spine and hip and the trabecular bone score (TBS), an indirect measure of bone microstructure.
Results:
For every 0.2 ng/ml 6-month CTX decrease, the corresponding increase in spine BMD at 12 and 24 months were 0.2% (p=0.7210) and 1.1% (p=0.0396), respectively; total hip BMD 1.1% (p=0.0279) and 0.9% (p=0.0716); and femoral neck BMD 1.7% (p=0.0079) and 0.9% (p=0.1698). Similarly, for every 20 ng/ml 6-month PINP decrease, the corresponding increase in spine BMD at 12 and 24 months were 0.9% (p=0.0286) and 1.4% (p=0.0012), respectively; total hip BMD 1.4% (p=0.0005) and 1.4% (p=0.0006); and femoral neck BMD 2.3% (p<0.0001) and 2.0% (p<0.0001). Bone marker changes were not consistently associated with TBS changes.
Conclusion:
Shorter-term 6-month changes in bone turnover markers are associated with the long-term changes in BMD over 1–2 years in the spine and hip but not with TBS.
Keywords: bone mineral density, bone turnover markers, osteoporosis, elderly, trabecular bone score
Mini Abstract:
Clinicians and patients want to know if therapy is working early in their course of treatment. We found that early changes in bone turnover markers at 6 months were associated with long-term changes in bone mineral density but not trabecular bone score at 12 and 24 months.
Introduction
Osteoporosis impacts 50% of women and 20% of men over age 50 years, and multiple antiresorptive and osteoanabolic therapies are available for treatment of patients at risk for fracture [1]. Once patients are on therapy, health care providers and patients would benefit by knowing early on if therapy is likely to be beneficial. Efficacy of therapy can be followed by surrogate assessments such as bone mineral density (BMD), but their changes, especially due to antiresportive therapy, are slow and may occur over 2–3 years. Alternatively, markers of bone turnover are more dynamic measures that respond in 3–6 months to osteoporosis therapy. These acute and short-term responses have the potential to help predict the longer changes in BMD and fracture reduction [2].
Bone turnover markers of resorption and formation have previously demonstrated significant changes with therapy for osteoporosis. Previous studies have demonstrated that bone turnover markers significantly decrease with antiresorptive therapy and increase with osteoanabolic therapy [3–6]. Furthermore, investigators have suggested that early changes in bone turnover markers may predict bone density response to therapy in middle-aged healthy women in the pivotal trials [7]. Early changes in bone turnover markers with antiresorptive therapy have also been associated with a reduction in fracture risk [8–11]. However, little data are available in fail older patients with multiple comorbid conditions who are at the highest risk of future fracture. Whether findings from previous studies conducted in younger, healthier cohorts translate to an older, sicker population remains to be seen. Moreover, there are little data that have examined the relationship between bone microarchitecture measured by trabecular bone score (TBS) and bone turnover [12], particularly in older women.
To examine the ability of early changes in bone turnover markers to predict longer term changes in BMD and TBS, we performed a secondary analysis of a zoledronic acid trial for osteoporosis in elderly women residents in long term care communities [13]. Our goal was to see if changes in markers of bone turnover after 6 months of therapy with a potent antiresorptive intravenous medication would be associated with the changes in bone density and TBS over the 2 years following infusion.
Methods
Study subjects
As previously described [13], the parent trial enrolled women age 65 and older, residing in a nursing home or assisted-living facility, who were not receiving a bisphosphonate but had a history of a vertebral or hip fracture or BMD in the osteoporosis range based on the 2003 guidelines of the National Osteoporosis Foundation [1]. The parent study had 181 women, of which only 158 completed the one-year DXA and 132 completed the 2-year DXA due to dropout, morbidity, and mortality [13]. The present secondary sub-analysis involved only 155 participants with both baseline and 6-month data for bone turnover markers.
Study Protocol
The double-blind, placebo-controlled parent trial included women who were randomized to receive one dose of intravenous zoledronic acid (5 mg) or matching placebo at baseline [13]. Participants were followed for 2 years. All participants received 800 IU vitamin D and 1200 mg of calcium (supplement plus diet) daily.
Measurement
We measured BMD at the hip (total hip, femoral neck) and spine (posterior-anterior) at baseline, 12 and 24 months by dual x-ray absorptiometry (DXA) (Discovery densitometer, Hologic Inc., Marlborough, MA) as previously described [13]. Precision error of DXA scans in this population ranged from 1.2% – 1.9% [14]. Trabecular bone score (TBS) is derived from texture analysis of two-dimensional DXA images and is an indirect measure of bone microarchitecture [15–18]. TBS was extracted from the PA spine DXA measurements at baseline, 12 and 24 months using TBS iNsight software (Medimaps Group, Geneva, Switzerland). The 10-year fracture risk (FRAX) was calculated using baseline femoral neck BMD [19]. Non-fasting blood samples were collected at baseline and 6 months for assessment of serum C-terminal crosslinking telopeptide of type I collagen (CTX; Crosslaps, Osteometer Biotech, Herlev, Denmark) as a marker of bone resorption and serum intact procollagen type I N propeptide (PINP; Orion Diagnostica, Espoo, Finland) as a marker of bone formation. Inter- and intra-assay variability was 3.66% and 8.15% for CTX and 4.79% and 4.14% for PINP, respectively.
Functional assessments at baseline included the Katz Activities of Daily Living scale [20], Instrumental Activities of Daily Living scale [21], gait speed [22,23], Short Portable Mental Status Questionnaire [24], Comorbidity Index [25], modified Fried Frailty Index (categorized as frail, prefrail or robust) [26] and the Nursing Home Physical Performance Test [27].
Statistical Analysis
We used appropriate descriptive statistics to summarize participant characteristics at baseline. We used Pearson correlation coefficients to assess baseline associations among markers of bone turnover, measures of bone density/structure, fracture risk (by FRAX score), and function. We fitted a series of linear mixed models with each of the raw and percent change in skeletal measures as the dependent variable; baseline to 6-month change in each of the markers, follow-up time (12/24 months) and their interaction as fixed effects of interest; baseline values of markers and dependent variable, and the treatment group (zoledronic acid/placebo) as fixed effect covariates; and a participant random effect to account for repeated measurements. Model coefficients and standard errors for CTX and PINP change were re-scaled to 0.2 and 20 ng/ml, respectively, for a more intuitive interpretation without altering their statistical significance. Following methodology in similar prior work [5], bone marker changes were categorized into tertiles based on 6-month change in CTX (<−0.14, −0.14 to 0.03, >0.03 ng/ml) and PINP (<−17.2, −17.2 to −0.1, >−0.1 ng/ml). We also used the said categorical operational definitions instead of the continuous ones in the mixed models, and obtained between-tertile differences. All statistical analyses were performed using SAS® version 9.3 software (SAS Institute, Inc., Cary, North Carolina).
Results
Patients
The mean age ± standard deviation of the participants was 86.9 ± 5.1 years. The average functional assessments including gait speed, physical performance tests, IADL, ADL, Frailty Index and co-morbidity score are shown in Table 1. Forty-six percent of the women had at least one mild and 28% had at least one moderate vertebral fracture at baseline. The average FRAX score for a hip fracture was 8.8 ± 9.2% and for a major osteoporotic fracture was 21.6 ± 9.9%. The total hip BMD T-score was −2.1 ± 1.1 and lumbar spine −0.8 ± 1.7. Mean marker levels were CTX, 0.41 ± 0.22 ng/ml and PINP, 48.3 ± 26.2 ng/ml. Mean 6-month changes in bone turnover markers were −5.46 ± 49.26 ng/ml for CTX and −14.52 ± 45.72 ng/ml for PINP. After 12 months, mean changes in BMD were 1.9 ± 4.1% at the spine, 1.2 ± 4.2% at the total hip, and 0.6 ± 5.7% at the femoral neck. BMD changes after 24 months were 2.3 ± 5.3% at the spine, 0.5 ± 5.1% at the total hip, and −1.5 ± 5.3% at the femoral neck.
Table 1:
Participant Characteristics (n=155)
| Age (years) | 86.9 ± 5.1 |
| BMI (kg/m2) | 27.6 ± 5.3 |
| IADL(0–14)a | 6.4 ± 4.2 |
| ADL (0–14)a | 10.4 ± 3.3 |
| Gait speed (m/s) | 0.53 ± 0.45 |
| CTX (ng/ml) | 0.41 ± 0.22 |
| PINP (ng/ml) | 48.3 ± 26.2 |
| Total hip | |
| BMD (g/cm2) | 0.688 ± 0.132 |
| T-score | −2.1 ± 1.1 |
| Lumbar spine | |
| BMD (g/cm2) | 0.962 ± 0.185 |
| T-score | −0.8 ± 1.7 |
| TBS | 1.305 ± 0.294 |
| FRAX major fracture risk (%) | 21.6 ± 9.9 |
| FRAX hip fracture risk (%) | 8.8 ± 9.2 |
| Comorbidity index (0–8 domains)b | 3.7 ± 1.3 |
| Cardiac problems | 53 (34.2) |
| Neurological problems | 5 (3.2) |
| Musculoskeletal problems | 152 (98.1) |
| Sleep/pain/depression | 106 (68.4) |
| Visual/hearing problems | 114 (73.5) |
| Diabetes | 28 (18.1) |
| Cancer | 42 (27.1) |
| Lung problems | 58 (37.4) |
| Frailty, n(%) | |
| Frail | 100 (64.5) |
| Prefrail | 44 (28.4) |
| Robust | 11 (7.1) |
Mean ± standard deviation or n(%)
Lower score is worse,
Higher score is worse
BMI: body mass index; IADL: instrumental activities of daily living; ADL: activity of daily living; CTX: C-terminal crosslinking telopeptide of type I collagen; PINP: procollagen type I N propeptide; BMD: bone mineral density; TBS: trabecular bone score
Biochemical markers of bone turnover
At baseline there was a statistically significant but modest magnitude association between the markers of bone turnover and BMD at total hip and femoral neck (Table 2) but not with spine BMD or functional status. Baseline FRAX scores were not associated with baseline functional status scores (not shown in table).
Table 2:
Pearson Correlation Coefficients between Baseline Measures and Bone Turnover Markers
| Measures | CTX | PINP |
|---|---|---|
| Spine BMD | −0.031 | −0.066 |
| Total Hip BMD | −0.298* | −0.178* |
| Femoral Neck BMD | −0.185* | −0.153 |
| TBS | −0.033 | 0.007 |
| IADL | −0.049 | −0.081 |
| ADL | −0.112 | −0.102 |
| Gait speed | −0.157 | −0.098 |
p<0.05;
CTX: C-terminal crosslinking telopeptide of type I collagen; PINP: procollagen type I N propeptide; BMD: bone mineral density; TBS: trabecular bone score; IADL: instrumental activities of daily living; ADL: activity of daily livin
For every 0.2 ng/ml 6-month CTX decrease, the corresponding increase in spine BMD at 12 and 24 months were 0.2% (p=0.7210) and 1.1% (p=0.0396), respectively; total hip BMD 1.1% (p=0.0279) and 0.9% (p=0.0716); and femoral neck BMD 1.7% (p=0.0079) and 0.9% (p=0.1698) (see Table 3). Those in the tertile that had the greatest 6-month CTX decrease (<−0.14 ng/ml) had 2.3% (p=0.0488) greater gains in total hip BMD over 24 months and 3.3% (p=0.0218) greater gains in femoral neck BMD over 12 months compared to those in the tertile with the least 6-month CTX decrease (>0.04 ng/ml). The same information is graphically depicted in Figure 1. Additional data are provided in Online Resource 1. Similarly, for every 20 ng/ml 6-month PINP decrease, the corresponding increase in spine BMD at 12 and 24 months were 0.9% (p=0.0286) and 1.4% (p=0.0012), respectively; total hip BMD 1.4% (p=0.0005) and 1.4% (p=0.0006); and femoral neck BMD 2.3% (p<0.0001) and 2.0% (p<0.0001). Those in the tertile that had the greatest 6-month PINP decrease (<−17.2 ng/ml) had 2.6% (p=0.0391) greater gains in spine BMD over 24 months compared to those in the tertile with the least 6-month PINP decrease (>−0.1 ng/ml). Bone markers changes did not show a consistent association with changes in TBS.
Table 3:
Associations between 6-month change in bone turnover markers and 12- to 24-month percent change in skeletal measuresa
| Shorter-Term 6-Month Change in Marker of Bone Turnover | Longer-Term Percent Change in Skeletal Measures | |||||||
|---|---|---|---|---|---|---|---|---|
| Spine BMD | Total Hip BMD | Femoral Neck BMD | TBS | |||||
| 12 Months | 24 Months | 12 Months | 24 Months | 12 Months | 24 Months | 12 Months | 24 Months | |
| CTX Change: | ||||||||
| Continuous (per 0.2 ng/ml decrease) | 0.2±0.5 (0.7210) |
1.1±0.5 (0.0396) |
1.1±0.5 (0.0279) |
0.9±0.5 (0.0716) |
1.7±0.6 (0.0079) |
0.9±0.6 (0.1698) |
−1.3±0.7 (0.0718) |
−1.5±0.7 (0.0386) |
| Tertile 1 (<−0.14 ng/ml) | 0.6±1.2 (0.6376) |
2.5±1.2 (0.1040) |
1.7±1.1 (0.1392) |
2.3±1.2 (0.0488) |
3.3±1.4 (0.0218) |
1.6±1.5 (0.2947) |
−1.9±1.6 (0.2430) |
−2.0±1.7 (0.2495) |
| Tertile 2 (−0.14 to 0.03 ng/ml) | 0.1±1.0 (0.9195) |
0.6±1.0 (0.5565) |
0.8±0.9 (0.3623) |
1.7±1.0 (0.0946) |
1.5±1.2 (0.2039) |
0.6±1.3 (0.6414) |
−2.7±1.3 (0.0433) |
−1.9±1.5 (0.2029) |
| Tertile 3 (>0.03 ng/ml) | 0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
| PINP Change: | ||||||||
| Continuous (per 20 ng/ml decrease) | 0.9±0.4 (0.0286) |
1.4±0.4 (0.0012) |
1.4±0.4 (0.0005) |
1.4±0.4 (0.0006) |
2.3±0.5 (<0.0001) |
2.0±0.5 (<0.0001) |
−0.0±0.6 (0.9882) |
−0.7±0.6 (0.2489) |
| Tertile 1 (<−17.2 ng/ml) | 0.7±1.2 (0.5549) |
2.6±1.2 (0.0391) |
−0.7±1.2 (0.5335) |
−0.1±1.2 (0.9608) |
0.7±1.5 (0.6167) |
1.4±1.6 (0.3709) |
2.0±1.7 (0.2270) |
−0.7±1.8 (0.6931) |
| Tertile 2 (−17.2 to −0.1 ng/ml) | 0.1±1.0 (0.8838) |
1.1±1.1 (0.2861) |
−0.1±1.0 (0.9065) |
1.5±1.1 (0.1441) |
−0.6±1.2 (0.6148) |
1.9±1.3 (0.1570) |
1.1±1.4 (0.4312) |
−0.8±1.5 (0.6002) |
| Tertile 3 (>−0.1 ng/ml) | 0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
0.00 (reference) |
Rescaled model coefficient ± standard error (p-value) adjusting for baseline values of marker and skeletal measure and treatment arm.
CTX: C-terminal crosslinking telopeptide of type I collagen; PINP: procollagen type I N propeptide; BMD: bone mineral density; TBS: trabecular bone score P-value for continuous change in markers indicate the statistical significance, model coefficient for continuous change in markers indicate rate of association, and model coefficients for tertiles indicate a more intuitive means difference between tertiles.
Figure 1.
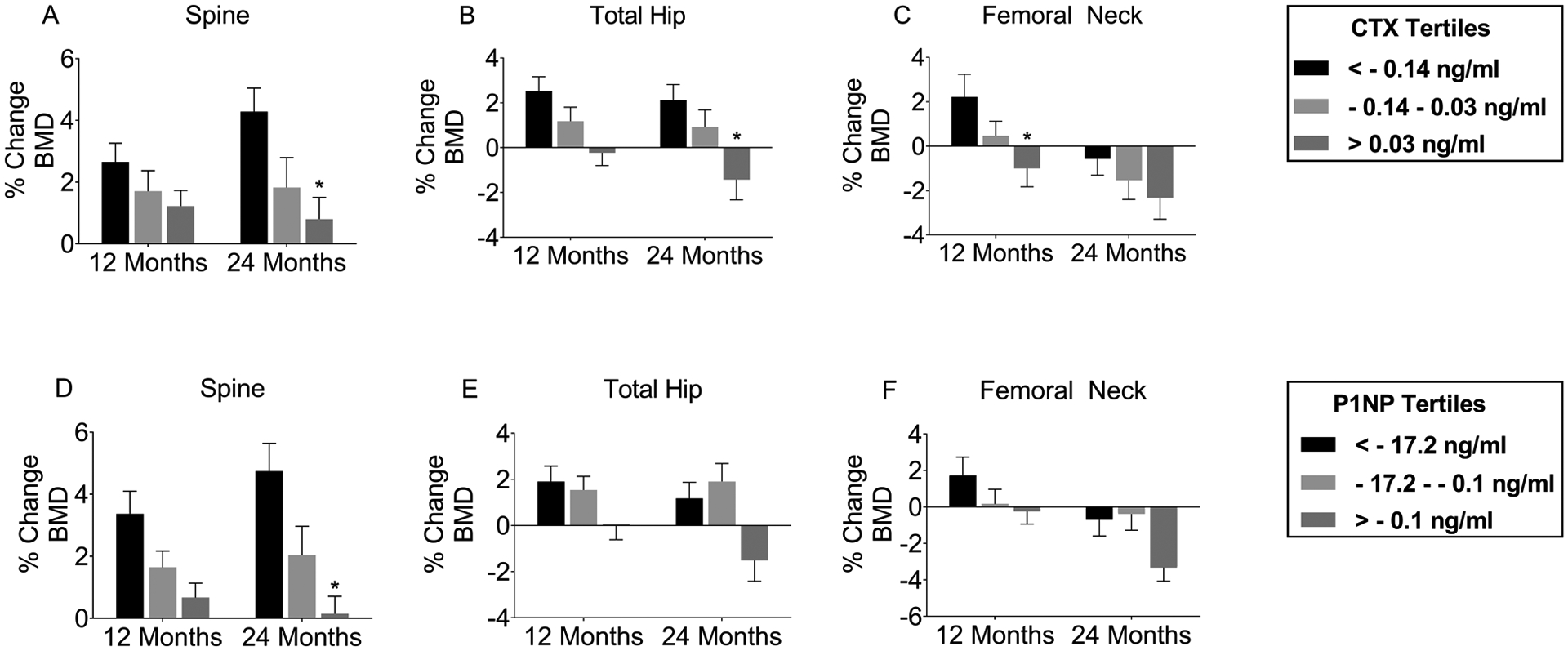
Percent change in bone mineral density by bone marker tertiles
A-C.Percent change in BMD at the lumbar spine (A), total hip (B), and femoral neck (C) at 12 and 24 months among tertiles grouped by 6-month change in CTX. D-F. Percent change in BMD at the lumbar spine (D), total hip (E), and femoral neck (F) at 12 and 24 months among tertiles grouped by 6-month change in PINP. *p<0.05 compared to lowest tertile (<−0.14 ng/ml for CTX and <−17.2 ng/ml for PINP). CTX: C-terminal crosslinking telopeptide of type I collagen; PINP: procollagen type I N propeptide; BMD: bone mineral density
Discussion
In our secondary analysis, we found that shorter term 6-month changes in markers of bone resorption and formation are associated with longer-term changes over 1–2 years in lumbar spine and total hip BMD but not TBS. Previous studies have examined the relationship of bone turnover markers with bone density following oral bisphosphonates. Burnette-Bowie and colleagues performed a post-hoc secondary analysis of women treated with oral alendronate [7]. Patients in the tertile with the greatest decrease in bone turnover markers had the greatest increase in BMD. Similar to our study, they observed that the bone turnover and BMD response over time were greater for sites rich in trabecular bone (spine) than sites with more cortical bone (hip and femoral neck). They also noted that characteristics of those that did not respond to therapy included younger age of menopause, family history of osteoporosis, and a higher baseline trochanteric hip BMD [7]. The TRIO study examined women on 3 different types of oral bisphosphonates (alendronate, risedronate and ibandronate) and found that the change in markers at 4, 12, and 48 weeks was inversely related to change in hip and spine BMD at 48 and 96 weeks [28]. We have also previously shown that early 6-month changes in bone turnover markers were associated with changes in BMD at three years in women receiving alendronate, hormone replacement therapy, or the combination of the both therapies [29,5] or alendronate alone over 2.5 years [29,5]. Others have also demonstrated that bone turnover markers predict hip fracture in women not on therapy [30] and can predict fractures in women on bisphosphonate therapy [31].
Other investigators have looked at a similar association of bone turnover markers and changes in BMD following anabolic therapy with teriparatide and antiresorptive therapy with denosumab. Tsai and colleagues reported that when women were treated with teriparatide or denosumab, the early changes in bone turnover markers (increases with teriparatide and decreases with denosumab) predicted the 2-year gains in BMD at the spine and hip [32]. Bauer and colleagues also examined bone turnover markers and the BMD response to therapy in patients treated with parathyroid hormone (PTH), an osteoanabolic agent [4]. They reported that higher levels of baseline PINP were associated with greater increases in BMD, and the short-term increases in turnover markers were associated with greater one-year increases in spine and hip BMD. Other investigators have also reported similar findings with abaloparatide [33] and PTH in women on glucocorticoids [34]. Because the parent trial used an antiresorptive therapy, we would not expect to observe such increases in bone turnover markers.
We did not find a consistent significant association with bone turnover markers and TBS. TBS is an indirect measure of trabecular connectivity that has demonstrated significant correlations with 3-D bone microarchitecture assessments [16–18]. However, changes in TBS with treatment are small in comparison to gains in BMD [35]. In our study, changes in TBS after one dose of zoledronic acid may not have been of sufficient magnitude to detect differences between tertiles of participants based on early bone marker changes. Additionally, the impact of zoledronic acid on bone microarchitecture may be difficult to discern using TBS measurements derived from DXA images. Previous investigations using higher resolution imaging techniques such as micro computed tomography have demonstrated mixed effects of zoledronic acid treatment on trabecular density and connectivity [36,37].
Our study had several limitations. It was short-term and neither intended nor was sufficiently large to examine fractures. Our cohort of older women had multiple comorbid conditions and lower gait speed; the results may not translate to men or younger and healthier women. Furthermore, although our samples were obtained in the morning, they were not fasting and the assays and markers may be altered with food [38]. Finally, clinically significant dichotomous indicators such as a BMD improvement above a certain threshold are most meaningful. However, they also require larger sample sizes to have adequate statistical power which we could not ensure within the confines of the present unplanned secondary analysis.
Our study does have several strengths despite these limitations. Other larger studies with healthier women have examined short-term changes in bone turnover markers with zoledronic acid and looked at long-term changes in BMD [11]. However, we enrolled a more frail cohort of women who would have likely not been included in these pivotal trials and who may have varying responses to zoledronic acid therapy. All participants were enrolled by a single team from an academic university hospital mobile unit using a Hologic bone densitometer that reduces the inter-site and inter-instrument variability in bone mass measurements that often requires statistical adjustment. Because we performed all assessments on site at the long-term care facility, our cohort is more generalizable to the target population of frail older adults that have the highest risk of fracture.
In summary, early changes in bone turnover markers in frail older women with osteoporosis were associated with longer-term changes in lumbar spine, total hip and femoral neck BMD. There was no evidence that changes in these markers predict changes in TBS in this population. Future studies are needed to determine if markers are helpful in predicting the long-term outcome following therapy on an individualized basis in the senior community population, and validating any such predictive rules.
Supplementary Material
Acknowledgements:
This work was supported by National Institutes of Health, National Institute on Aging grants R01AG028086, T32AG021885, and the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30AG024827).
Footnotes
Conflict of Interest: Mary P. Kotlarczyk, Subashan Perera, Neil M. Resnick, and David A. Nace declare that they have no conflict of interest. Susan L. Greenspan has received research grant support from Amgen.
Ethical approval: All procedures involving human participants were in accordance with the ethical standards of the University of Pittsburgh institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained for all participants in the main study as previously indicated [13].
References:
- 1.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R, National Osteoporosis F (2014) Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int 25 (10):2359–2381. doi: 10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnick SL, Shulman L (2006) Monitoring osteoporosis therapy: bone mineral density, bone turnover markers, or both? Am J Med 119 (4 Suppl 1):S25–31. doi: 10.1016/j.amjmed.2005.12.020 [DOI] [PubMed] [Google Scholar]
- 3.Bauer DC (2019) Clinical Use of Bone Turnover Markers. JAMA. doi: 10.1001/jama.2019.9372 [DOI] [PubMed] [Google Scholar]
- 4.Bauer DC, Garnero P, Bilezikian JP, Greenspan SL, Ensrud KE, Rosen CJ, Palermo L, Black DM (2006) Short-term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 91 (4):1370–1375. doi: 10.1210/jc.2005-1712 [DOI] [PubMed] [Google Scholar]
- 5.Greenspan SL, Resnick NM, Parker RA (2005) Early changes in biochemical markers of bone turnover are associated with long-term changes in bone mineral density in elderly women on alendronate, hormone replacement therapy, or combination therapy: a three-year, double-blind, placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab 90 (5):2762–2767. doi: 10.1210/jc.2004-1091 [DOI] [PubMed] [Google Scholar]
- 6.Niimi R, Kono T, Nishihara A, Hasegawa M, Matsumine A, Nakamura T, Kono T, Sudo A (2014) An algorithm using the early changes in PINP to predict the future BMD response for patients treated with daily teriparatide. Osteoporos Int 25 (1):377–384. doi: 10.1007/s00198-013-2426-2 [DOI] [PubMed] [Google Scholar]
- 7.Burnett-Bowie SM, Saag K, Sebba A, de Papp AE, Chen E, Rosenberg E, Greenspan SL (2009) Prediction of changes in bone mineral density in postmenopausal women treated with once-weekly bisphosphonates. J Clin Endocrinol Metab 94 (4):1097–1103. doi: 10.1210/jc.2008-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, Thompson DE, Ewing SK, Delmas PD, Fracture Intervention Trial Study G (2004) Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res 19 (8):1250–1258. doi: 10.1359/JBMR.040512 [DOI] [PubMed] [Google Scholar]
- 9.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18 (6):1051–1056. doi: 10.1359/jbmr.2003.18.6.1051 [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD (2002) Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 87 (4):1586–1592. doi: 10.1210/jcem.87.4.8415 [DOI] [PubMed] [Google Scholar]
- 11.Jacques RM, Boonen S, Cosman F, Reid IR, Bauer DC, Black DM, Eastell R (2012) Relationship of changes in total hip bone mineral density to vertebral and nonvertebral fracture risk in women with postmenopausal osteoporosis treated with once-yearly zoledronic acid 5 mg: the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27 (8):1627–1634. doi: 10.1002/jbmr.1644 [DOI] [PubMed] [Google Scholar]
- 12.Aleksova J, Kurniawan S, Elder GJ (2018) The trabecular bone score is associated with bone mineral density, markers of bone turnover and prevalent fracture in patients with end stage kidney disease. Osteoporos Int 29 (6):1447–1455. doi: 10.1007/s00198-018-4468-y [DOI] [PubMed] [Google Scholar]
- 13.Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM (2015) Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Intern Med 175 (6):913–921. doi: 10.1001/jamainternmed.2015.0747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varney LF, Parker RA, Vincelette A, Greenspan SL (1999) Classification of osteoporosis and osteopenia in postmenopausal women is dependent on site-specific analysis. J Clin Densitom 2 (3):275–283. doi: 10.1385/jcd:2:3:275 [DOI] [PubMed] [Google Scholar]
- 15.Binkley N, Leslie WD (2016) Clinical Application of Spine Trabecular Bone Score (TBS). Clinical Reviews in Bone and Mineral Metabolism 14 (1):14–25. doi: 10.1007/s12018-016-9203-7 [DOI] [Google Scholar]
- 16.Shevroja E, Lamy O, Kohlmeier L, Koromani F, Rivadeneira F, Hans D (2017) Use of Trabecular Bone Score (TBS) as a Complementary Approach to Dual-energy X-ray Absorptiometry (DXA) for Fracture Risk Assessment in Clinical Practice. J Clin Densitom 20 (3):334–345. doi: 10.1016/j.jocd.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 17.Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14 (3):302–312. doi: 10.1016/j.jocd.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 18.Winzenrieth R, Michelet F, Hans D (2013) Three-dimensional (3D) microarchitecture correlations with 2D projection image gray-level variations assessed by trabecular bone score using high-resolution computed tomographic acquisitions: effects of resolution and noise. J Clin Densitom 16 (3):287–296. doi: 10.1016/j.jocd.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 19.Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359 (9321):1929–1936. doi: 10.1016/S0140-6736(02)08761-5 [DOI] [PubMed] [Google Scholar]
- 20.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA 185:914–919 [DOI] [PubMed] [Google Scholar]
- 21.Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9 (3):179–186 [PubMed] [Google Scholar]
- 22.Abellan van Kan G, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B (2010) The assessment of frailty in older adults. Clin Geriatr Med 26 (2):275–286. doi: 10.1016/j.cger.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 23.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J (2011) Gait speed and survival in older adults. JAMA 305 (1):50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeiffer E (1975) A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 23 (10):433–441 [DOI] [PubMed] [Google Scholar]
- 25.Rigler SK, Studenski S, Wallace D, Reker DM, Duncan PW (2002) Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil 16 (4):420–428 [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research G (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56 (3):M146–156 [DOI] [PubMed] [Google Scholar]
- 27.Binder EF, Miller JP, Ball LJ (2001) Development of a test of physical performance for the nursing home setting. Gerontologist 41 (5):671–679 [DOI] [PubMed] [Google Scholar]
- 28.Gossiel F, Paggiosi MA, Naylor KE, McCloskey EV, Walsh J, Peel N, Eastell R (2019) The effect of bisphosphosphonates on bone turnover and bone balance in postmenopausal women with osteoporosis: The T-score bone marker approach in the TRIO study. Bone 131:115158. doi: 10.1016/j.bone.2019.115158 [DOI] [PubMed] [Google Scholar]
- 29.Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB (1998) Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res 13 (9):1431–1438. doi: 10.1359/jbmr.1998.13.9.1431 [DOI] [PubMed] [Google Scholar]
- 30.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD (1996) Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11 (10):1531–1538. doi: 10.1002/jbmr.5650111021 [DOI] [PubMed] [Google Scholar]
- 31.Bell KJ, Hayen A, Glasziou P, Irwig L, Eastell R, Harrison SL, Black DM, Bauer DC (2016) Potential Usefulness of BMD and Bone Turnover Monitoring of Zoledronic Acid Therapy Among Women With Osteoporosis: Secondary Analysis of Randomized Controlled Trial Data. J Bone Miner Res 31 (9):1767–1773. doi: 10.1002/jbmr.2847 [DOI] [PubMed] [Google Scholar]
- 32.Tsai JN, Burnett-Bowie SM, Lee H, Leder BZ (2017) Relationship between bone turnover and density with teriparatide, denosumab or both in women in the DATA study. Bone 95:20–25. doi: 10.1016/j.bone.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eastell R, Mitlak BH, Wang Y, Hu M, Fitzpatrick LA, Black DM (2019) Bone turnover markers to explain changes in lumbar spine BMD with abaloparatide and teriparatide: results from ACTIVE. Osteoporos Int 30 (3):667–673. doi: 10.1007/s00198-018-04819-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD (2000) Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int 11 (5):434–442. doi: 10.1007/s001980070111 [DOI] [PubMed] [Google Scholar]
- 35.Krieg MA, Aubry-Rozier B, Hans D, Leslie WD, Manitoba Bone Density P (2013) Effects of antiresorptive agents on trabecular bone score (TBS) in older women. Osteoporos Int 24 (3):1073–1078. doi: 10.1007/s00198-012-2155-y [DOI] [PubMed] [Google Scholar]
- 36.Marques IDB, Araujo M, Graciolli FG, Dos Reis LM, Pereira RMR, Alvarenga JC, Custodio MR, Jorgetti V, Elias RM, Moyses RMA, David-Neto E (2019) A Randomized Trial of Zoledronic Acid to Prevent Bone Loss in the First Year after Kidney Transplantation. J Am Soc Nephrol 30 (2):355–365. doi: 10.1681/ASN.2018060656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Recker RR, Delmas PD, Halse J, Reid IR, Boonen S, Garcia-Hernandez PA, Supronik J, Lewiecki EM, Ochoa L, Miller P, Hu H, Mesenbrink P, Hartl F, Gasser J, Eriksen EF (2008) Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J Bone Miner Res 23 (1):6–16. doi: 10.1359/jbmr.070906 [DOI] [PubMed] [Google Scholar]
- 38.Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R (2002) Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone 30 (6):886–890. doi: 10.1016/s8756-3282(02)00728-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


