Abstract
Purpose
To assess the prevalence of post-COVID symptoms in patients with recovered COVID-19 (nasopharyngeal RT PCR negative) who were discharged from an acute COVID care facility at a tertiary care teaching hospital in North India.
Methods
This study was an observational study with retrospective data collection, conducted in the COVID follow-up clinic, a combined clinic of medicine and endocrinology. Patients discharged from the acute COVID care facility were recruited after 14 days of discharge if they fulfilled inclusion and exclusion criteria. The retrospective data was collected from the hospital records/EMR and analysed by the SPSSv23.
Results
Fifty patients, who fulfilled the inclusion and exclusion criteria, were included in the study. The Mean age of patients was 53.4±13.8 years (range 28–77). Seventy six percent were male, and 38% had type 2 diabetes. Fever (94%), cough (78%) and breathlessness (68%), were the most common symptoms at presentation to acute care facility. Oxygen saturation at presentation had a negative correlation with D-Dimer, age, and C reactive protein. When patients were evaluated clinically, after 14 days (range 15 to 50 days) of the discharge, 82% of patients had at least one persistent symptom. Fatigue (74%) was the most common symptoms in follow-up followed by breathlessness (44%), and muscle weakness (36%). Two patients had persistent fever, even after negative RT PCR status.
Conclusion
Patients discharged from the acute COVID care facility had a high prevalence of post-COVID symptoms even after 14 days.
Keywords: COVID-19, diabetes, SARS-CoV-2, oxygen saturation, post-COVID
Introduction
The COVID-19 pandemic has challenged worldwide scientific power with mind-boggling economic and societal impact. Globally individuals and governments are engrossed in understanding the epidemiology and biology of this perilous virus. The causative agent of COVID-19, SARS-CoV-2 are classified under the coronaviridae family order nidovirales. This virus has a diameter of 0.1 micrometer. The spike glycoproteins of SARS-CoV-2 bind with angiotensin-converting enzyme and dipeptidyl peptidase-4 (DPP-4) enzymes to enter the human cells.1 After entry, the virus undergoes replication leading to viremia. In most patients, this phase lasts for 5 to 6 days.2 In inflammatory phase, our immune system recognizes the virus and mounts an immune response.
The immune response in COVID-19 has some characteristics (cytokine release syndrome, immunothrombosis) that are unusual in other viral fevers. The omnipresence of angiotensin-converting enzyme (ACE) receptors, which are the key receptors for viral attachment, leads to symptoms with broad clinical spectrum. Along with typical viral prodrome, patients can present with ST-elevation myocardial infarction (STEMI),3,4 stroke,5,6 acute liver failure,7 encephalitis,8,9 and sepsis with Multiple organ dysfunction syndrome (MODS).10 The loss of smell and taste are two symptoms that are characteristics of COVID-19.
The viral persistence is also the area of ongoing research. Although infectivity in upper airway secretions reduces after 10 days, viral ribonucleic acid (RNA) can persist in stool samples for weeks, sometimes at a remarkably high level. This raises the possibility of fecal-oral transmission in some patients. Additionally, low-level viremia was found for up to 4 weeks, which causes an illness to span for weeks. The continuous release of inflammatory mediators leads to a “cytokine storm,” a clinical state responsible for organ injury and MODS in COVID-19. Immunomodulators (Steroids/Tocilizumab), targeted to control cytokine release syndrome (CRS), are proven to curb morbidity and mortality.11,12
Patients with COVID-19 have microvascular complications including micro-thrombosis,13 and endotheliitis.14 These microvascular phenomena are due to activated clumps of neutrophils and platelets.15 The interaction of NETOsis and endothelial results in immunothrombosis, thrombosis mediated by immune cells. The resulting abnormal micro-circulation is responsible for the characteristics of ground glass opacities (GGO) and refractory hypoxia in COVID-19. Endothelial dysfunction and low-grade immune activation lead to a new inflammatory syndrome in children, resembling Kawasaki disease.16,17 The adult’s spectrum of this low-grade inflammation varies from MIS (multi-system inflammatory syndrome) as a most severe form to fatigue as a most benign form.18
Due to the persistence of organ dysfunction, mediated by microvascular phenomena, low grade viremia and immune dysregulation, patients may have persistence of symptoms even after negative nasopharyngeal RT PCR (real-time reverse transcription polymerase chain reaction test). The data regarding the persistence of symptoms in post-acute care in COVID patients are very scarce. There is no information regarding the effect of age, diabetes, and inflammatory markers on these symptoms. In this study, we tried to investigate the prevalence of different symptoms in the post-acute care phase in COVID-19. We also investigated the factors that were associated with these symptoms.
Study Methodology
Study Design
Our study was a single-centre observational study conducted at COVID follow-up facility, NIMS hospital, Jaipur, Rajasthan, India. NIMS hospital is a tertiary-level university hospital attached to NIMS university. In this hospital’s follow-up OPD (Outpatient Department) operated by a joint collaboration of medicine and endocrine departments, we followed the patients after their discharge from the acute care facility. Patients who came to this OPD over a 1-month duration (October 1, 2020, to November 1, 2020), were screened for the study.
All the procedures performed in this study involving human participants were in adherence to the 1964 Helsinki declaration’s ethics and later amendments or comparable ethical standards. The research protocol was approved and supervised by the Institutional Ethics Committee, NIMS University Rajasthan, Jaipur, India (NIMSUR/IEC/2020/412-A). We conducted this study as per Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Written informed consent was taken from all subjects.
Establishing the Diagnosis of COVID-19 During Acute Care
All the doctors and hospitals, from where all the patients referred to us followed WHO/ICMR (World Health Organization/Indian Council of Medical Research) guidelines19,20 Patients who had symptoms suggestive of COVID-19 were tested with RT PCR to make a diagnosis of COVID-19. Patients who had negative reports but symptoms highly suggestive of COVID-19, again subjected to repeat RT PCR (reverse transcription polymerase chain reaction).
Management at the Acute Care Facility
After admission, they were triaged according to the severity of the disease. Patients who are having moderate to severe disease, were admitted into the high dependency units or intensive care units. At the time of admission, their symptoms, duration of symptoms, comorbidities, vitals including blood pressure, respiratory rate, oxygen saturation, and quick general physical examination were noted meticulously in the case records. They were treated as per the guidelines laid by ICMR (Indian council of medical research), council assigned to formulate the COVID-19 treatment in India. During their admission, we followed these patients as follows:
Clinical follow-up – All patients were seen daily by DAS and DES. They assessed the oxygenation status and clinical condition daily. The treatment was updated as per the requirement.
Biochemical follow-up – We used (interleukin-6) IL-6, c reactive protein (lactate dehydrogenase) LDH, ferritin, and D Dimer in serum as a marker of inflammation. These were repeated periodically to assess the disease status and to guide the treatment.
Radiological follow-up – We did an X-Ray or contrast tomography of the chest as per the treating physician’s discretion (DAS/DES).
Discharge Criteria (ICMR/MOHFW)
Mildly Symptomatic
Patients who had their symptoms ten days back and were afebrile for at least 72 hours were discharged from the acute care facility.
Moderate
These patients underwent monitoring of body temperature and oxygen saturation. If the fever resolved within three days and the patient-maintained saturation above 95% for the next four days (without oxygen support), such patients were discharged after ten days of symptom onset if they were fulfilling these criteria:
Absence of fever without antipyretics
Resolution of breathlessness
No oxygen requirement
Severe or Critical Cases
All patients who were critical at the time of admission were discharged after clinically recovered and had stable hemodynamic and oxygen status for at least 72 hours.
We advised mandatory seven days quarantine or isolation post-discharge to all patients. We followed the discharge policy as per MOHFW (Ministry of Health and Family Welfare)/govt of India guidelines.21
All the patients were discharged from the COVID-19 facility after they had a negative RT PCR report.
Follow-Up
Our COVID-19 response team contacted all the patients discharged from the acute care facility to track the recovery, assessed them for rebound CRS (Cytokine release syndrome) and deterioration in oxygenation status. These patients were called for follow-up after fourteen days. DMS and DAS saw these patients in the follow-up OPD. This follow-up was a focussed clinical follow-up, with re-assessment of inflammatory markers if required. All patients were evaluated for post-COVID sequelae, prevalent symptoms, oxygenation status, and psycho behavioural abnormalities. Their treatment was reviewed and modified as per the COVID rehabilitation protocol of our institute.
Patient Enrolment
All the patients who came in follow-up OPD over 1-month duration (October 1, 2020, to November 1, 2020) were screened for study. The study was explained in detail by DS and DH. Once patients gave informed consent, they were included in the study.
The following were the inclusion and exclusion criteria for this study.
Inclusion Criteria
Inclusion criteria were patient diagnosis with COVID-19 positive at admission, patients age ≥18 years, and patient discharge with negative RT PCR report.
Exclusion Criteria
All the confounders, which can affect the dependent and independent variables in the post-COVID-19 state symptoms were excluded from this study. Patients who had pre-existing underlying chronic organ dysfunction that may present with similar symptomatology as post-COVID-19 symptoms, were excluded. Hence, we excluded patients with pre-existing chronic liver disease, chronic kidney disease, or lung diseases.
Data Collection
We reviewed all the hospital medical case records to extract data using a standardized data collection form. All the data collected was reviewed by an expert team of physicians (DAS/DES) and then entered into the database. Socio-demographic details, disease onset, nature of the symptoms, duration of symptoms, comorbidities, and previous treatment were retrieved from the case files. We retrieved the blood pressure, oxygen saturation, respiratory rate, and pulse rate at the time of admission from the medical record. The investigations to assess the end-organ functions such as blood urea nitrogen (BUN) and serum creatinine, liver enzymes (aspartate aminotransferase/alanine aminotransferase) were also noted down from the case files and electronic medical records. The inflammatory markers, like serum IL-6 level, serum CRP, D dimer, and serum LDH levels, were noted from the hospital’s electronic medical records.
Laboratory and Investigations
RT PCR
As most of the patients had RT PCR outside our hospital, the analytical variables were not known. In our hospital we used the RT PCR (BIO-RAD, CFX96, real-time RT PCR, Hercules, California, USA) for the follow-up RT PCR of COVID-19. We collected the nasopharyngeal swab to detect the COVID-19 RNA in nasopharyngeal secretions. We used the E gene and the RdRp gene to detect the RNA.
Inflammatory Markers
The serum IL-6 level was assessed by ADVIA Centaur (Siemens Healthcare Diagnostics, Benedict Avenue, Tarrytown, New York, USA), a fully automated, one-step direct immunoassay using chemiluminescent technology. The assay utilized an acridinium ester-labelled monoclonal mouse anti-IL-6 antibody as the lite reagent. This serum IL6 assay had LoQ of ≤3.0 pg/mL, LoB of 1.3 pg/mL, and LoD of 2.7 pg/mL. The serum C reactive protein was assessed with turbidimetric assay (particle enhanced) (COBAS INTEGRA 400, Roche Diagnostics Ltd, Rotkreuz Switzerland). The lower limit of detection was 1.0 mg/L with a CV repeatability of 1.8%. The precipitate was determined at 552 nm.
Biochemistry
The glycosylated haemoglobin (HbA1C) was done with high-performance liquid chromatography (Variant II, Biorad laboratories, Alfred Nobel Drive, Hercules, California 94547, USA). The biochemical analysis was done with HUMAN (Gesellschaft für Biochemica und Diagnostica mbH Wiesbaden, Germany).
Statistics
Variables were entered from the proforma to the SPSS ver 23 (Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp). The Shapiro Wilk test checked the normality of the data. The distribution of parametric continuous variables was presented as mean and SD. Nonparametric continuous variable’s distribution was shown as a median, range and interquartile range. We analysed the nominal variables with the help of the chi-square test. An Independent Student’s t-test was used to compare the means of the parametric continuous variables. Mann–Whitney U-test was used to compare the means of the nonparametric continuous variables.
Results
This study was conducted between October 1 to October 30, 2020. The study was conducted at the COVID follow-up OPD. In this OPD, 150 patients discharged (interval between discharge and study recruitment was at least 14 days) and were not on oxygen therapy were screened for this study. We recruited fifty eligible patients who gave consent.
Demographics
The mean age of the patients was 53.4± 13.8 years (Range 28–77 years). Male patients were 76% and females were 24%. Most of the patients were of middle age, with Sixty-four percent of the patients had age more than 50 years. Thirty-eight percent of patients had type 2 diabetes at the time of presentation. This is shown in Table 1.
Table 1.
Baseline Characteristics and Lab Investigations of the COVID-19 Patients
| Demographics | |
|---|---|
| Age [mean ± SD] | 53.2±13.8 (Range 28–77 years) |
| Sex (Male%/Female%) | 76%/24% |
| Duration of positive test to admission (Lag time) | 1 (Range 0–10) |
| Prevalence of diabetes [%] | 38% |
| Prevalence of kidney disease at presentation (eGFR <60 mL) (CKD EPI) [N%] | 8/44 (20%) |
| Prevalence of liver injury (AST and or ALT >ULN) [N%] | 27/44 (61.3%) |
| Prevalence of liver injury (AST and or ALT > 3x ULN) [N%] | 6/44 (14%) |
| Investigations | Results |
| Haematology | |
| Haemoglobin (g/dl) | 12.68 (8.9–17.3) |
| TLC (/ul) | 8820 (2700–18200) |
| Neutrophil (%) | 70 (16–91) |
| Lymphocyte (%) | 23.1 (3–90) |
| Biochemistry | |
| BUN (mg/dl) | 18 (7–50) |
| Serum creatinine (mg/dl) | 1.0 (0.6–1.95) |
| AST (IU/L) | 67 (18–383) |
| ALT (IU/L) | 61 (19–379) |
| Inflammatory markers | |
| Interleukin 6 (pg/mL) | 259 (6–2270) |
| C reactive protein | 34 (3.0–195) |
| D Dimer (ng/mL) | 1673 (100–10000) |
| Vitals at presentation | |
| Blood pressure | |
| Systolic (mmHg) | 126±15 (80–168) |
| Diastolic (mmHg) | 80±7 (62–95) |
| Pulse rate (Per minute) | 89±15 (63–100) |
| Respiratory rate (Per/Minute) | 22±4 (18–34) |
| SpO2 (%) | 93±6 (68–100) |
| Saturation <94% on the time of admission | 16/50 (32%) |
Disease Characteristics
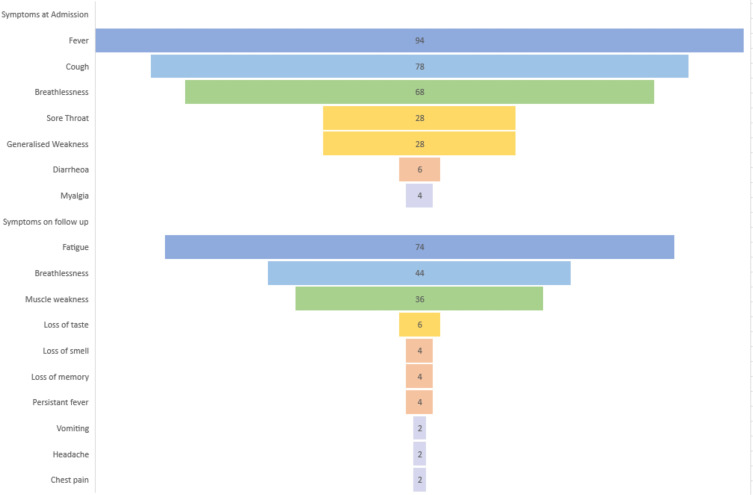
Fever was the most common symptom (94%) at presentation. The other common symptoms were cough, breathlessness, fatigue, myalgia, diarrhoea, and sore throat. As this is a retrospective data collection, the frequency of abnormal smell and taste was noted documented in the case files, leading to the omission of these two symptoms from the study. The other symptoms were as follows-Cough (78%), breathlessness (68%), generalized weakness (28%), and sore throat (28%). The mean interval from the symptoms onset to contact with a medical facility was 4 days for most patients. This symptomatology at presentation is illustrated in Figure 1.
Figure 1.
The characterization of symptoms at admission and follow-up to the acute care facility of COVID-19.
Inflammatory Markers
Almost all patients had raised inflammatory markers at the time of admission. The median serum IL-6 level was 259 (range 6–2270) pg/mL. Baseline investigations of the patients are shown in Table 2.
Table 2.
Effect of Diabetes on Inflammatory Markers at the Time of Admission
| Inflammatory Marker | Diabetic | Nondiabetic | p value |
|---|---|---|---|
| Serum CRP | 17.5 (6–30.2) (n-24) | 46 (20–60) (n-15) | 0.017 |
| Serum IL-6 level | 239 (210–260) | 246 (105.7–259.2) | 0.87 |
| Serum D dimer | 820 (275–2055) | 810 (372–1975) | 0.77 |
Serum IL-6 levels did not decrease when the patients converted from positive to negative RT PCR status (241 pg/mL (IQR 56) v/s 218 pg/mL (IQR-125). The levels of C reactive protein decreased and showed a trend towards significance at this point of time (28 (IQR 42) v/s 23 (IQR 24), p-0.09). Blood urea nitrogen was more elevated in diabetic patients than in those who did not have it (p 0.015).
Determinants of Inflammation
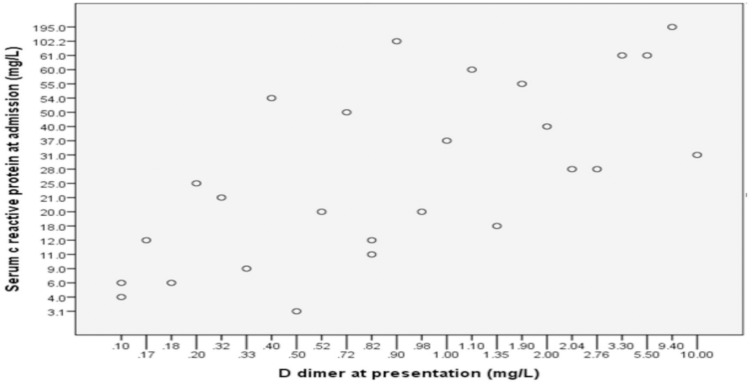
Patients who had diabetes had higher levels of c reactive protein (46 (n-15) vs 17.5 (n-24), p value 0.017). The serum levels of IL-6 were not different between diabetes and non-diabetic patients (p-value 0.8) (Table 2). The C reactive protein correlated positively with age (r-0.34, p 0.03, Spearman test). The D dimer also correlated positively with the CRP (r-0.67, p 0.000, Spearman test). This is shown in Figure 2.
Figure 2.
Correlation between serum C reactive protein and D dimer.
Oxygen Status and Its Determinants
The mean oxygen saturation of the patients was 93±6% (Range-68-100%) at the time of admission. Thirty-two percent of the patients had saturation <94%, and 22% had saturation ninety percent or less. The mean respiratory rate of the patients was 22±4 (18–34) per minute. Twelve percent of the patients did not have elevated respiratory rate (RR >24) despite having a saturation of less than 94%. Two patients had a saturation of less than 90% with no tachypnoea (RR <20/min).
Oxygen saturation at the time of admission, negatively correlated with age (r-0.315, p 0.03) and D dimer (r −0.448, p 0.015). The CRP also showed a trend of negative correlation with oxygen status (r-0.314, p 0.06). Patients with diabetes had higher respiratory rates than the patients who did not have (24 v/s 21, p 0.03).
Post-Acute Care Symptoms of COVID-19 (Long COVID-19)
In this study, the mean interval between the discharge and follow-up visit was 31 days (range 14 to 50 days). Despite negative RT PCR at the time of discharge, when assessed after 14 days after their discharge from an acute care facility, a high proportion of patients (82%) had symptoms of long COVID/post-COVID sequelae. Fatigue (74%) was the most common symptom reported, followed by breathlessness (44%). Four percent of the patients had fever spikes persisting even after 14 days of negative nasopharyngeal RT PCR. The symptomatology of long COVID is demonstrated in Figure 1. Symptoms were not different in the patients who had diabetes than who did not have it. Persistent muscle weakness was more common in patients who had aged more than 50 years.
Discussion
This study is one of the few that focussed on the symptoms prevalent in patients discharged from the acute care facility. There are some facts which came out of our study and will improve the future insight into the disease. The serum IL-6 did not improve despite nasopharyngeal negative RT PCR status and were elevated at the time of discharge. We also found that even after 14 days of the negative nasopharyngeal RT PCR status, patients had symptoms in the form of long COVID. The elderly patients had more microcirculation abnormalities. The diabetic patients had more severe inflammation at the time of admission to the acute care facility but had similar prevalence of symptoms in the follow-up.
This study showed that eighty two percent patients had at least one symptom in the post-acute care phase of COVID-19. Fatigue (74%) was the most common symptom reported, followed by breathlessness (44%). Few studies describe the prevalence of symptoms in the post-acute care phase. In a social media-based study, when they analyzed the Twitter hashtags “#longcovid” and “#chroniccovid,” malaise and fatigue (62%) were the most common symptoms followed by dyspnoea (19%).22 This study reflects the same prevalence of fatigue, but prevalence of dyspnoea was more than this social media-based study. This discrepancy is because we asked the patients in person and took a detailed history of dyspnoea. This high prevalence of dyspnoea is corroborating with another study from Italy, where the prevalence of dyspnoea was 43.4.23 In a letter to the editor Perrin Ray et al mentioned Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.24 Similarly, a high prevalence of fatigue was also noted in the current study. Persistent post-COVID symptoms lead to a low quality of life, highlighted in multiple studies.25
The high prevalence of fatigue in these patients is due to multiple aetiologies. In COVID-19, it was found that low-grade viremia may last for weeks even after a negative nasopharyngeal RT PCR report.26 This low-level viremia maintains a low level of inflammation that causes either increased cytokines or persistent end-organ dysfunction. Lung injury, cardiac injury, vascular dysfunction, muscle injury and low-grade inflammation, critical care neuropathy, critical care myopathy and steroid-induced physical deconditioning are some of the reasons that may also cause fatigue in these patients. The lung injuries in the patients with moderate to severe COVID-19, are extensive, slow to recover and leave residual fibrosis in some patients. Some of the patients reported fatigue even after two months after discharge. SARS Cov2 virus can directly involve the muscle, leading to myopathy, myalgia, or even frank myositis.27–29 This muscle dysfunction may be the cause of easy fatigability and breathlessness. The prevalence of subclinical or clinical cardiac dysfunction in these patients is remarkably high.30–34 These patients may have cytokines induced functional cardiac dysfunction, without elevation of cardiac biomarkers. Some of the patients were found to have the virus in the myocardium without fulminant myocarditis.35 The cardiac changes may persist even after recovery from COVID-19.36 The persistent cardiac dysfunction may also cause breathlessness and easy fatigability. All these above-mentioned aetiologies explain the persistence of fatigue in these patients.
In our study, patients with diabetes had a high c reactive protein level at the time of admission in an acute care facility, when compared with the patients who did not have diabetes. The C reactive protein is a marker of inflammation, and this showed that diabetic patients had more inflammation in COVID-19. Previous studies had shown that diabetes is a risk factor for the severe disease at onset, along with progression from mild to severe disease.37,38 Elevated glucose level acting on HIF-1α/glycolysis-dependent axis favours the SARS-CoV-2 replication through exaggerated monocyte response.39 Patients with diabetes have a high risk of immune dysregulation noted previously during the viral epidemic of MERS (middle east respiratory syndrome).40 Similarly, also in COVID-19, when the inflammatory markers were studied in two sub cohorts of sex- and age-matched participants, diabetic patients (n-33) had higher CRP levels than non-diabetics patients41(n-37). In PROSPERO-based pooled analysis, the diabetic patients had higher CRP levels than non-diabetic.42
Elevated C reactive protein was found to be a marker of mortality in COVID-19 patients. In a retrospective study of 904 patients with COVID-19 (136 with diabetes, mostly type 2 diabetes), C reactive protein was associated with increased risk of mortality (OR-1.12).43 In our study, patients who had a high C reactive protein also had high D dimer values. This may be due to increased immunothrombosis in the patients who had high levels of inflammation, connecting the link between inflammation (CRP) to thrombosis (D dimer). Occlusion of the micro vessels is the leading cause of morbidity and mortality in severe COVID-19 infection. The initiator of the immune microthrombi, the NETOsis, is induced by C reactive protein.44–46
Aging was associated with a worse prognosis marker in our study. Older patients had a high D Dimer level and low level of oxygen saturation at the time of admission to the acute care facility. Endothelial dysfunction is one of the hallmarks of the aging process.47 In older adults, the endothelium has less capacity to secrete the anti-inflammatory and antithrombotic molecule nitrous oxide. The expression of the inflammatory markers is more robust and dysregulated. The micro-occlusion of pulmonary vasculature by immune thrombosis caused desaturation, and increased D dimer due to clot lysis.
Patients with COVID-19, are having long-term pulmonary sequalae, as a complication of SARS CoV2 associated lung injury. These are lung fibrosis, secondary pulmonary hypertension and hypoxia. Due to the magnitude of the infection, we may need a dedicated system to tackle these patients. Ongoing deterioration of the lung function and frequent need of hospitalization, may strain the resource-limited health care of developing countries like India.47 Also, in the developed world, due to wide clinical spectrum of the post-COVID-19 sequelae, a dedicated multi-disciplinary post-COVID team needed to form which include pulmonologists, intensivists, cardiologists, and physiotherapists may be needed to take care of these patients. There is one more hypothesis, that has to be studied further that the endothelial dysfunction precipitated by the COVID-19, may lead to long term increased risk of atherosclerotic events.48 If this happens, we will see pandemic of cardiovascular disease, evolving after a lead time. So, we may have to strengthen our healthcare systems to deal with these complications. The loss of the work days, due to these post-COVID-19 symptoms, may also negatively affect economy in both developing and developed countries.
This study has some limitations. First, this is an observational study with retrospective data collection. Second, the sample size is small, having only fifty patients. Third, as we do not have the quality-of-life assessment, how these symptoms affect the quality of life still needs to be investigated.
Our study has some strengths. To our knowledge, this is the first study exploring the symptoms in COVID-19 patients once they are discharged from an acute care facility. This study also highlights that although the disease may be mild on presentation; however, the sequelae are very severe and prolonged.
Conclusion
The prevalence of symptoms in patients who had negative RT PCR is very high. The mere negative nasopharyngeal RT PCR may not mean clinical recovery in patients with COVID-19. Immunothrombosis induced microcirculation dysfunction may be the cause of low saturation and poor prognosis, in the elderly patients.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Davidson AM, Wysocki J, Batlle D. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: therapeutic implications. Hypertension. 2020;76(5):1339–1349. doi: 10.1161/HYPERTENSIONAHA.120.15256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roe K. High COVID-19 virus replication rates, the creation of antigen-antibody immune complexes and indirect haemagglutination resulting in thrombosis. Transboundary emerging dis. 2020;67:1418–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalid N, Chen Y, Case BC, et al. COVID-19 (SARS-CoV-2) and the Heart – an Ominous Association. Cardiovasc Revascularization Med. 2020;21(8):946–949. doi: 10.1016/j.carrev.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yousefzai R, Bhimaraj A. Misdiagnosis in the COVID-19 Era: when Zebras Are Everywhere, Don’t Forget the Horses. JACC Case Rep. 2020;10:1614–1619. doi: 10.1016/j.jaccas.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020;51(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avula A, Nalleballe K, Narula N, et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: a Retrospective Observational Cohort Study of 1827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72(4):1169–1176. doi: 10.1002/hep.31487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;88:945–946. doi: 10.1016/j.bbi.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efe IE, Aydin OU, Alabulut A, Celik O, Aydin K. COVID-19−Associated Encephalitis Mimicking Glial Tumor. World Neurosurg. 2020;140:46–48. doi: 10.1016/j.wneu.2020.05.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Analysis of 92 deceased patients with COVID-19. J Med Virol. 2020;92:2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized patients with coronavirus disease 2019: survival and Clinical Outcomes. Chest. 2020;158(4):1397–1408. doi: 10.1016/j.chest.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharun K, Tiwari R, Dhama J, Dhama K. Dexamethasone to combat cytokine storm in COVID-19: clinical trials and preliminary evidence [Internet]. Int J Surg. 2020;82:179–181. [Elsevier Ltd]. doi: 10.1016/j.ijsu.2020.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2(7):E437–E445. doi: 10.1016/S2665-9913(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24(1):353. doi: 10.1186/s13054-020-03062-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skendros P, Mitsios A, Chrysanthopoulou A, et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130(11):6151–6157. doi: 10.1172/JCI141374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki Disease: novel Virus and Novel Case. Hosp Pediatr. 2020;6:537–540. doi: 10.1542/hpeds.2020-0123 [DOI] [PubMed] [Google Scholar]
- 17.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395(10239):1741–1743. doi: 10.1016/S0140-6736(20)31129-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ministry of Health & Family Welfare, Government of India. Guidelines on Clinical Management of COVID – 19; 2020. Available from: https://www.mohfw.gov.in/pdf/GuidelinesonClinicalManagementofCOVID1912020.pdf. Accessed Nov 20, 2020. [Google Scholar]
- 19.Pan American Health Organization and World health Organisation database.Laboratory Guidelines for the Detection and Diagnosis of COVID-19 Virus Infection; 2020. Available from: https://iris.paho.org/handle/10665.2/52458. Accessed Accessed Nov 20, 2020. [Google Scholar]
- 20.Ministry of Health & Family Welfare, Government of India. Revised Discharge Policy for COVID-19; 2020.Available from: http://health.delhigovt.nic.in/wps/wcm/connect/b6af6e004e9fefb89ce8bd5dc9149193/Order11620.pdf?MOD=AJPERES&lmod=-1471732290&CACHEID=b6af6e004e9fefb89ce8bd5dc9149193. Accessed Nov 20, 2020.
- 21.Banda JM, Singh GV, Alser O, Prieto-alhambra D. Long-term patient-reported symptoms of COVID-19: an analysis of social media data. medRxiv. 2020. [Google Scholar]
- 22.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19 [Internet]. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrin R, Riste L, Hann M, Walther A, Mukherjee A, Heald A. Into the looking glass: post-viral syndrome post COVID-19. Med Hypotheses. 2020;144:110055. doi: 10.1016/j.mehy.2020.110055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2020;93(2):1013–1022. doi: 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 25.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19 [Internet]. Lancet Neurol. 2020;19(9):767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsueh SJ, Lee MJ, Chen HS, Chang KC. Myopathy associated with COVID-19. J Formos Med Assoc. 2021;120(3):1022-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19 article metrics metric details related articles early introduction of SARS-CoV-2 into Europe Case-Fatality Risk Estimates for COVID-19 Calculated by Using a Lag Time for Fatality Co-infection with SARS-CoV-2 and Influenza A Virus in Patient with. Emerg Infect Dis j. 2020;26(7):1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: a Review [Internet]. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286 [DOI] [PubMed] [Google Scholar]
- 30.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 Pandemic [Internet]. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system [Internet]. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindner D, Fitzek A, Bräuninger H, et al. Association of Cardiac Infection with SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Codo AC, Davanzo GG, Monteiro LDB, et al. Elevated glucose levels favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020;32(3):437–446. doi: 10.1016/j.cmet.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao R, Sun Y, Zhang Y, et al. Distinguishable immunologic characteristics of COVID-19 patients with comorbid type 2 diabetes compared with non-diabetic individuals. Mediators Inflamm. 2020;2020:1–10. doi: 10.1155/2020/6914878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varikasuvu SR, Varshney S, Dutt N. Markers of coagulation dysfunction and inflammation in diabetic and non-diabetic COVID-19. J Thromb Thrombolysis. 2020;1. doi: 10.1007/s11239-020-02270-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660 [DOI] [PubMed] [Google Scholar]
- 43.Middleton EA, He XY, Delorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnekoh H, Scheffel J, Wu J, Hoffmann S, Maurer M, Krause K. Skin and systemic inflammation in schnitzler’s syndrome are associated with neutrophil extracellular trap formation. Front Immunol. 2019;1:546. doi: 10.3389/fimmu.2019.00546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, García-Orozco A, Alvarado-Navarro A, Fafutis-Morris M. Neutrophil extracellular traps and its implications in inflammation: an overview [Internet]. Front Immunol. 2017;8:81. doi: 10.3389/fimmu.2017.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yavuz BB, Yavuz B, Sener DD, et al. Advanced Age Is Associated with EndothelialDysfunction in Healthy Elderly Subjects. Gerontology. 2008;54:153–156. doi: 10.1159/000129064 [DOI] [PubMed] [Google Scholar]
- 47.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: a Review [Internet]. JAMA Cardiology. 2020;5(7):831–840. [DOI] [PubMed] [Google Scholar]
- 48.Becker RC. Anticipating the long-term cardiovascular effects of COVID-19 [Internet]. J Thromb Thrombolysis. 2020;50(3):512–524. [Springer]. doi: 10.1007/s11239-020-02266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]