Abstract
Background:
Binge drinking, characterized by brief periods of high intoxication interspersed with periods of abstinence, appears to be particularly damaging to the brain. Binge drinking is increasing among American women, yet few preclinical studies have assessed sex differences in the neurobehavioral effects of binge alcohol.
Methods:
Adult Long–Evans rats were administered 4 g/kg ethanol (EtOH; or an isocaloric control dose) via intragastric gavage once-weekly. Brains were collected after 3 or 8 binge doses, and immunohistochemistry for mature neurons (NeuN), microglia (Iba1), neurogenesis (DCX), and reactive astrogliosis (vimentin) performed. Stereology was used to quantify target cell populations in the hippocampus and medial prefrontal cortex (mPFC). In a separate cohort of animals, cognition (spatial navigation and reversal learning), affect (tickling-evoked ultrasonic vocalizations), and task-induced c-fos activation were assessed after 3 or 8 binge doses.
Results:
Blood EtOH concentration did not differ significantly between females (175 ± 3.6 mg/dl) and males (180 ± 3.7 mg/dl) and did not change significantly over time, indicating that tolerance did not develop. After 3 or 8 binge doses, the number of granule neurons in the hippocampal dentate gyrus of both sexes was significantly reduced in comparison with controls, although there was no binge effect on newly generated neurons. Moreover, 8 (but not 3) binge doses significantly increased the total number of microglia and the number of partially activated microglia in the hippocampus and mPFC in both sexes. There was no detectable reactive astrogliosis (vimentin) in either region at any timepoint. There was no effect of binge alcohol on behavior outcomes in either sex, but binged rats showed increased cellular activation in the mPFC following reversal learning.
Conclusions:
Our data indicate that recurrent binge alcohol results in similar neural damage and neuroimmune activation in alcohol-vulnerable corticolimbic brain regions in males and females.
Keywords: Hippocampus, Medial Prefrontal Cortex, Microglia, Sex Differences, Ethanol
BINGE DRINKING, DEFINED by the National Institute on Alcohol Abuse and Alcoholism (NIAAA, 2004) as drinking enough to bring an individual’s blood alcohol concentration (BAC) to 0.08 g/dl or above, may be particularly capable of damaging the brain. To illustrate, examining the volume of alcohol consumed during drinking episodes is more predictive of brain damage than examining the total lifetime alcohol consumption (Bobak et al., 2004; Hunt, 1993). Binge drinking differs from chronic heavy drinking in the pattern of intoxication—short episodes of heavy intake producing high BACs, followed by periods of abstinence. Recent data indicate that binge drinking American adults do so weekly, with an average intensity of 7 drinks per binge (Kanny et al., 2018).
Binge alcohol has been linked to brain damage and cognitive impairment in both human and animal studies (Hunt, 1993). For example, binge alcohol is associated with impaired cognitive abilities (Duka et al., 2003), microglial activation (McClain et al., 2011), disrupted cortical functioning (Campanella et al., 2013), and widespread neurodegeneration, indicated by vimentin-positive reactive astrocytes (Kelso et al., 2011). Two brain areas particularly susceptible to alcohol’s damaging effects are the hippocampus (Wilson et al., 2017) and prefrontal cortex (Kubota et al., 2001). These areas are of particular interest because of their critical role in cognition and implication in addiction.
Women disproportionately experience alcohol-related organ damage (Erol and Karpyak, 2015), but the extent to which this includes the brain has been a long-standing question. Human neuroimaging studies have addressed this question, but produced a variety of results: Women develop brain damage after a shorter drinking history compared to men (known as “telescoping”; Mann et al., 1992), women have more brain damage regardless of length of drinking (Hommer et al., 2001), or no difference between the sexes (Demirakca et al., 2011; Sullivan et al., 2010). All of these studies have focused on chronic alcohol consumption, however, and it remains unknown whether the female brain is selectively vulnerable to binge alcohol. Investigation of potential sex-dependent effects of binge alcohol is crucial, as binge drinking is becoming increasingly common in American women, even in middle-aged and older age groups (Keyes et al., 2019).
We have previously shown that female rats are more susceptible than males to hippocampal damage and cognitive impairment after a 4-day alcohol binge (Maynard et al., 2018). Four days of continuous exposure, however, does not well represent the apparent pattern of binge consumption of most Americans, which has been reported as 6 to 8 drinks once-weekly (Kanny et al., 2018). We therefore assessed hippocampal cell loss and neuroimmune activation in female rats administered a once-weekly dose of binge alcohol that brought the blood ethanol concentration (BEC) to ~0.175 mg/dl, a level that could be easily achieved by a person consuming 6 to 8 drinks during a binge. After 11 weeks, there was a near 20% decrease in dentate gyrus (DG) granule neurons and significantly increased neuroimmune activation, as evidenced by microglial proliferation (West et al., 2019). Thus, recurrent binge exposure damages the female brain, although it remains unknown whether the male brain is similarly affected, and whether binge damage gradually increases in magnitude with increasing number of exposures.
In the present study, we determined whether the emergence and magnitude of binge alcohol-induced brain damage is sex-dependent (Experiment 1). Male and female rats were administered once-weekly doses of binge alcohol via oral gavage and sacrificed after 3 or 8 weeks. We hypothesized that 8 binge exposures would result in more severe neural and behavioral effects compared to 3 exposures. Given our previous findings of increased female brain susceptibility to a 4-day binge, we hypothesized that cell loss, reactive astrogliosis, and neuroimmune activation would occur earlier and be more severe in females. In a separate set of animals (Experiment 2), we assessed binge alcohol effects on hippocampus-associated cognition (spatial navigation in the Morris water maze (MWM)) and affect (tickling-induced ultrasonic vocalizations (USV)). Again, given our prior findings using a 4-day binge, we hypothesized that impairments would emerge sooner and be more prominent in females. Finally, we quantified c-fos in the medial prefrontal cortex (mPFC) after reversal trials in the MWM, as we have previously shown that binged rats of both sexes show increased c-fos after a cognitive task (West et al., 2018).
MATERIALS AND METHODS
Experiment 1
Animals.
All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of health. The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Houston. All rats were housed in groups of 2 to 3 per cage on a reversed light–dark cycle (9 am to 9 pm). Upon arrival from the supplier (Envigo, IN) at 8 weeks of age, adult rats spent 10 days acclimating to their new housing while also being handled daily to become accustomed to the experimenters. Cellular analysis of binge alcohol effects involved the use of 100 male and female adult Long–Evans rats that were randomly assigned to 1 of 5 groups within sex (3-week binge, 3-week control, 8-week binge, 8-week control, cage control). Cage control rats were group housed in the same room, and all other conditions (apart from oral gavage) were matched to the experiences of the standard control rats. Tissue was collected 4 days following the final gavage for control and binged rats. Cage control rats were sacrificed 2 days following the rats that received 8 weeks of binge (therefore, they were age-matched to the 8-week groups).
Binge EtOH Administration.
All food was removed from both the alcohol and control group 8 hours prior to alcohol administration; however, water was always accessible. A single EtOH dose of 4 g/kg of animal body weight was given via intragastric gavage every 7 days at the end of the animals dark (active) cycle. The control group received an isocaloric solution and were given the average volume of fluid that the EtOH group received. To determine BEC, blood was drawn via the lateral saphenous vein using heparinized capillary tubes, 90 minutes following each alcohol binge. This time-point has been cited as the peak level of intoxication in rats following gavage alcohol dosing (Crews et al., 2006). Plasma was analyzed using a 5 μl aliquot in a GM7 Analyzer (Analox, MA).
Food and water intake was measured in a subset of animals. Food and water intake for each cage was monitored in the 14 hours following gavage by removing access to the automatic watering system and replacing it with 2 water bottles in each cage. Bottles were weighed before and after, to determine amount consumed. Water consumption was measured only after the first 3 binges. Food intake was measured after all binges by weighing food hoppers prior to replacing them after blood collection and again the following morning. Total (cage) consumption of food and water was then divided by the number of rats in the cage.
Tissue Collection and Processing.
Rats were given an overdose of anesthetic (ketamine, xylazine, and acepromazine) followed by intracardial perfusion with cold saline and then 4% paraformaldehyde in 0.2 M PBS (pH 7.2) until the limbs were stiff. Brains were extracted, postfixed overnight, and then refrigerated in 30% sucrose until they sank in the solution. Brains were sectioned coronally at 50-μm intervals on freezing a microtome (Leica SM 2000R, Bannockburn, IL) and sections stored in cryoprotectant at −20°C.
The IHC protocol used was identical to that outlined previously (West et al., 2018). Sections underwent 72-hour incubations at 4°C in primary antibody solutions to mark mature neurons (guinea pig anti-NeuN, EMD Millipore, Billerica, MA, #ABN90P; 1:1,000), immature neurons (goat anti-DCX polyclonal, Santa Cruz Biotechnology, Dallas, TX, #sc-8066 [discontinued]; 1:100), and microglia (rabbit anti-Iba1, Wako USA, Richmond, VA, #019-19741; 1:10,000). This was followed by 24-hour incubations at room temperature in corresponding secondary antibody solutions (Jackson ImmunoResearch, West Grove, PA; 1:250) with subsequent Avidin-Biotin Complex (ABC, Vector Laboratories, Burlingame, CA, #PK-4000) treatment and diaminobenzidine (DAB, Vector Laboratories, #SK-4000) visualization. Tissue was mounted on gelatin-coated slides and dried overnight. Slides were then either counterstained and coverslipped (Iba1, DCX) or cleared in xylene for 5 minutes before cover slipping (NeuN).
Vimentin staining was used for qualitative analysis of reactive astrocytes and followed a slightly modified IHC protocol from that outlined in Kelso and colleagues (2011). It consisted of 3 × 5-minute rinses in tris buffer solution (TBS), followed by incubation in 0.6% hydrogen peroxide in TBS for 30 minutes, 2 × 5-minute TBS rinses, 30-minute incubation in 3% normal horse serum (Sigma-Aldrich, St Louis, MO)/0.1% Triton X/TBS blocking buffer, and overnight incubation in primary antibody solution (mouse anti-vimentin, Millapore Sigma, St Louis, MO, #MAB3400; 1:750) at 4°C. Next, sections underwent 3 × 10-minute washes in blocking buffer, 1-hour incubation in secondary antibody solution (horse anti-mouse, rat adsorbed, Vector Laboratories, Burlingame, CA; 1:200) at room temperature, 3 × 10-minute TBS rinses, 1-hour incubation in ABC solution at room temperature, and 3 × 10-minute TBS rinses. Following the last rinse, all sections were incubated in DAB and rinsed 3 × 10 minutes in TBS before being mounted on gelatin-coated slides, dried overnight, cleared in xylene for 5 minutes, and then coverslipped.
Cellular Quantification.
Stereology was used for unbiased quantification of cellular markers under an Olympus BX51WI upright microscope, by an experimenter blind to condition. For the hippocampus, every 12th section (600 μm apart) between Bregma −1.88 and −6.04 mm and for the mPFC, every sixth section (300 μm apart) between Bregma 3.7 and 1.7 mm (Paxinos and Watson, 2005) was analyzed. The optical fractionator (Stereoinvestigator, MicroBright-Field, VT) was used to quantify NeuN + cells (20 × 20 μm counting frame, 200 × 200 μm grid) and DCX + cells (40 × 40 μm counting frame, 200 × 200 μm grid) in the DG. Iba1 + cells were quantified in the entire hippocampus and mPFC, using 100 × 100 μm counting frames and a 400 × 400 μm grid. Iba + microglial cells were categorized as either partially activated or resting based on visible morphology, such as larger, dark cell bodies, and thicker, bushy processes in partially primed microglia (Barton et al., 2017; Kreutzberg, 1996; Raivich et al., 1999; see Fig. 2). Average Gunderson coefficient of error was maintained at less than 0.10. Qualitative vimentin analysis was accomplished by visual comparison between control and binged groups. Tissue from a previous study using a 4-day model of binge EtOH (West et al., 2018) was used as a positive control, as that binge model has been shown to cause a significant increase in reactive astrogliosis (Kelso et al., 2011).
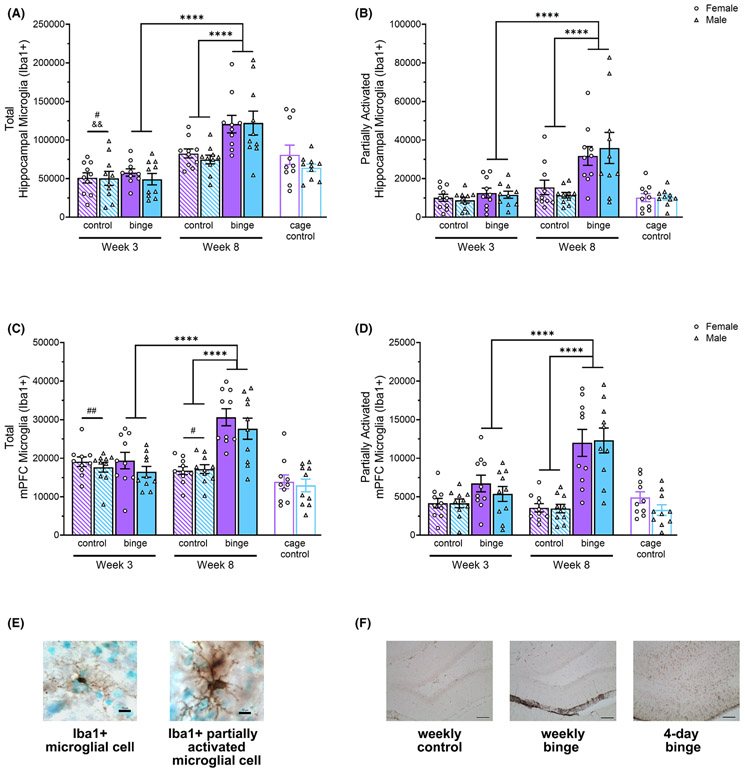
Fig. 2.
In the hippocampus, 8 once-weekly EtOH binges significantly increased total (A) and partially activated (B) microglia compared to 3 weeks of EtOH binges and 8 weeks of control diet (note change in y-axis scale for panels B and D). In the mPFC, 8 once-weekly EtOH binges significantly increased total (C) and partially activated (D) microglia compared to 3 weeks of EtOH binges and 8 weeks of control diet. Representative images of Iba1 + cells (10 μm scale bar) (E). There was no indication of ongoing neurodegeneration after 8 weeks of binge exposure, as evidenced by a lack of reactive (vimentin+; 100 μm scale bar) astrogliosis. In contrast, 4-day binge tissue used as a positive control showed abundant vimentin (F). # p < 0.05, ## p < 0.01 relative to cage controls; &&p < 0.01 relative to 8-week controls; ****p < 0.0001.
Statistical Analyses.
BEC data for 3 binges and 8 binges were analyzed separately by repeated measures analysis of variance (ANOVA) with Sex as the between-subjects factor and Time (3 or 8 timepoints) as the within-subjects factor. Given baseline sex differences in body weight and food intake, these data were analyzed separately for each sex with Diet (Binge, Control) as the between-subjects factor and Time (3 or 8 timepoints) as the within-subjects factor. Also, since 3- and 8-week rats were often housed together, these groups were collapsed to analyze food and water consumption using repeated measures ANOVA. The Greenhouse–Geisser correction was applied for any repeated measures analysis wherein Mauchly’s test of sphericity indicated that the assumption of sphericity was violated. For each cellular outcome measure, 2 × 2 × 2 ANOVAs were used to test the effects of Sex, Diet, Week (total number of weekly binge/control diet exposures: 3 or 8), and their interactions. Statistically significant 2-way interactions were followed up with Bonferroni-corrected F-tests of simple effects. To evaluate the impact of gavage experience on cellular outcome measures, 2-way ANOVAs with Sex and Experience (cage control, 3-week control, 8-week control) as factors were conducted. Statistically significant Experience effects were followed up with Bonferroni-corrected t-tests (cage controls vs. 3-week controls and cage controls vs. 8-week controls). For both Experiments 1 and 2, results that reached statistical significance at p < 0.05 are reported along with effect sizes—partial eta-squared (η2) for F-tests and Cohen’s d for t-tests. Statistical analyses were performed using JASP version 0.12.2 and GraphPad Prism 9 (Graphpad Software, San Diego, CA). Data are expressed as group mean ± standard error of the mean (SEM) in each graph.
Experiment 2
Animals and EtOH Administration.
Housing and EtOH administration procedures were identical to those outlined in Experiment 1. This experiment involved 80 male and female adult Long–Evans rats. These rats were randomly assigned into 1 of 4 groups within sex (3-week binge, 3-week control, 8-week binge, 8-week control). USV testing began the day after the final gavage, and MWM testing began the morning of the second day following the final gavage. Tissue was collected 6 days following the final gavage and 60 to 90 minutes following reversal learning in the MWM. All testing occurred during the rat’s active (dark) cycle, under red-light illumination. Rats were transported in homecages to testing rooms and allowed to acclimate for at least 15 minutes prior to testing.
Tickling-Induced USV.
The hippocampus has a significant role in emotional regulation and mood; therefore, we investigated whether cellular damage resulting from weekly binge exposure would impact USV. To examine affective state in rats, 2 USV frequencies are typically examined. The higher frequency, 50-kHz USV are typically emitted in pro-social interactions, such as during play or mating (Burgdorf et al., 2008). In contrast to 50-kHz USV, the emission of 22-kHz USV is associated with negative affect resulting from states such as fear, pain, anxiety, or stress (Cuomo et al., 1988). The 50-kHz vocalizations commonly associated with juvenile play behavior can be induced in adults through “playful handling” or “tickling” by a human hand (Schwarting et al., 2007). All rats were habituated to the USV apparatus for 5 days prior to testing, and USVs were collected individually for each rat, for 5 consecutive days following the final gavage. The apparatus consisted of a sound box (measuring 60 H × 80 W × 60 D cm) with a large rectangular bin (measuring 13 H × 48 W × 35 D cm) lined with an absorbent pad and clean cage bedding, situated 27 cm below a Nutrick NC MX-HD microphone. The box was spot cleaned between rats, and bedding was replaced between the testing of male and female rats and at the end of each day. Tickling sessions consisted of rapid finger movements across the back and neck and flipping the animal on the back for quick “wrestling” sessions, combined with rapid finger movements across the animal’s ventral side. Play chasing was also done, in which animals could chase or were gently chased by the experimenter’s hand, to mimic the play chasing behavior seen in juvenile rats. The testing sessions were comprised of alternating 15 seconds of experimenter interaction (“play”), with 15 seconds of rest, repeated 6 times for a total of 3 minutes. USV were recorded with Avisoft SASLab Pro v5.1 software (Avisoft Bioacoustics, Berlin, Germany). Data from each animal over the 3-minute period were visualized as a spectrogram, which was then examined and manually counted by the experimenter. The 22-kHz calls spanned a frequency range of 20 to 32 kHz, and vocalizations of 35 to 96 kHz were recorded as 50-kHz (Schwarting et al., 2007).
Spatial Memory Testing and Reversal Learning.
The hidden-platform MWM task is a common spatial learning and memory task that has been shown to be sensitive to cortical and hippocampal damage (D’Hooge and De Deyn, 2001). Testing took place in a circular pool (183 cm dia.; 75 cm deep) surrounded by extramaze cues and using an automated animal behavior tracking system, EthoVision XT (Noldus, Amsterdam, Netherlands). Acquisition consisted of 4 60-second trials/d × 4 days. The platform location remained constant throughout all 16 acquisition trials but the release quadrant changed each trial in a pseudorandom order. After the last acquisition trial, a 60-second probe trial (platform removed) was given, followed by a ‘reminder’ trial (platform returned) to reinforce the location of the platform prior to reversal learning the following day. If necessary, rats repeated this reminder trial until they had located the platform within 30 seconds. Outcome measures were % time in platform quadrant, # of platform crossings, and % time in platform zone. Next day, 4 60-second reversal learning trials were given, in which the location of the platform was moved from its original location to the opposite side of the tank. The outcome measures were latency to platform and swim speed for each trial.
Tissue Processing and Cellular Quantification.
Perfusions were performed and brain tissue sections prepared as described in Experiment 1. IHC for c-fos staining in mPFC sections was performed as we have previously described (West et al., 2018), using a mouse anti-c-fos monoclonal primary antibody (Santa Cruz Biotechnology, # sc-166940; 1:100). Cellular activation induced by behavioral testing (c-fos + cells) was examined using stereology in every sixth section (300 μm apart) of the mPFC (40 × 40 μm counting frame, 400 × 400 μm grid).
Statistical Analyses.
BEC and body weight analyses were the same as in Experiment 1. Since females vocalize more than males, USV data (average daily number of 50- and 22-kHz vocalizations as well as proportion of USVs that were 50-kHz) were analyzed separately for each sex. Furthermore, as all outcome measures with the exception of average daily number of 50-kHz USVs for females did not pass the Shapiro–Wilk test of normality, they were analyzed by nonparametric Kruskal–Wallis tests. MWM data were analyzed by repeated measures ANOVA with Sex, Diet, and Week as between-subjects factors and Day (1, 2, 3, 4) and Trial (1, 2, 3, 4) as the repeated measure for acquisition and reversal learning, respectively. Probe trial data and number of c-fos + cells in the mPFC were analyzed by Sex × Diet × Week ANOVA. For c-fos cellular analysis, we used cage controls from Experiment 1 and performed a Sex × Experience ANOVA to determine whether gavage experience alone had a significant effect. Follow-up analyses, when appropriate, were conducted as described in Experiment 1.
RESULTS
Experiment 1
Effect of Weekly Binge Exposure on BEC, Body Weight, Food, and Water Consumption.
There were no statistically significant Sex, Time, or Sex × Time effects on BEC regardless of whether animals underwent 3 binges or 8, indicating that BEC did not vary with repeated binge exposure in this once-weekly binge model. Overall, females had a mean BEC of 171 ± 4.9 mg/dl and males, 185 ± 5.1 mg/dl (mean ± SEM). There was a significant effect of Time on body weight within both sexes in the 3-week, Ffemales(2, 36) = 75.27, p < 0.0001, η2 = 0.81; Fmales(1.28, 23.11) = 97.76, p < 0.0001, η2 = 0.84, and 8-week, Ffemales(3.39, 61.08) = 174.94, p < 0.0001, η2 = 0.91; Fmales(2.13, 38.38) = 368.47, p < 0.0001, η2 = 0.95, groups, such that all groups gained weight across weeks (see Table 1). There was also a significant Diet × Time interaction for 8-week males, F(2.13, 38.38) = 4.11, p = 0.022, η2 = 0.19, but simple effects tests of Diet for each week were not significant. There was no significant Diet, Time, or Diet × Time effects on food intake in either sex. There was a significant effect of Time on water intake, F(2, 16) = 4.26, p = 0.033, η2 = 0.35, with increased consumption across weeks. Effects of Sex and Sex × Time on water intake were not significant.
Table 1.
Body Weights (g) Across 8 Weeks of Exposure to EtOH Binge or Control Diet for Females (F) and Males (M)
| Sex | Diet | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment1 | F | Binge | 210 ± 3.6 | 222 ± 4.1 | 233 ± 4.4 | 238 ± 4.8 | 246 ± 5.7 | 249 ± 5.1 | 255 ± 5.7 | 257 ± 6.4 |
| Control | 208 ± 4.2 | 221 ± 4.2 | 225 ± 4.2 | 231 ± 5.4 | 239 ± 5.2 | 239 ± 4.4 | 244 ± 5.1 | 248 ± 5.4 | ||
| M | Binge | 292 ± 11.3 | 323 ± 5.9 | 342 ± 7.2 | 360 ± 7.7 | 376 ± 7.6 | 386 ± 8.3 | 402 ± 8.7 | 413 ± 9.9 | |
| Control | 293 ± 5.6 | 319 ± 6.3 | 343 ± 5.7 | 364 ± 5.8 | 380 ± 6.0 | 404 ± 7.4 | 420 ± 7.4 | 433 ± 6.9 | ||
| Experiment 2 | F | Binge | 201 ± 5.0 | 212 ± 5.2 | 224 ± 3.4 | 233 ± 3.6 | 242 ± 4.5 | 249 ± 4.0 | 254 ± 3.9 | 250 ± 4.7 |
| Control | 199 ± 6.1 | 211 ± 5.9 | 219 ± 5.9 | 230 ± 4.8 | 240 ± 5.0 | 247 ± 5.2 | 252 ± 5.5 | 238 ± 9.1 | ||
| M | Binge | 266 ± 6.8 | 294 ± 6.6 | 311 ± 8.0 | 330 ± 8.2 | 351 ± 9.6 | 366 ± 10.7 | 381 ± 9.7 | 366 ± 9.7 | |
| Control | 257 ± 3.3 | 286 ± 4.3 | 309 ± 5.2 | 333 ± 4.9 | 355 ± 5.7 | 369 ± 7.2 | 390 ± 7.9 | 377 ± 14.0 |
Data expressed as mean ± SEM.
Effect of Weekly Binge Exposure on the Hippocampal DG.
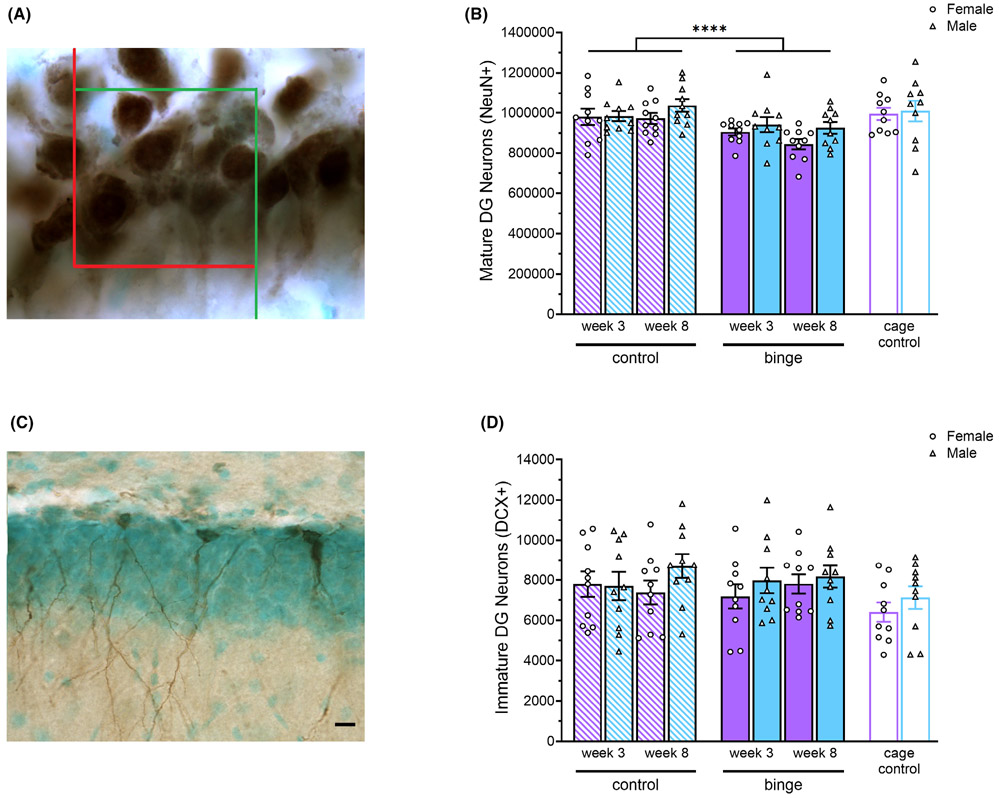
There were statistically significant effects of Diet, F(1, 72) = 17.61, p < 0.0001, η2 = 0.20, and Sex, F(1, 72) = 4.82, p = 0.031, η2 = 0.06, on the number of NeuN + cells in the DG (see Fig. 1A,B). Binged rats had fewer NeuN + cells compared to controls, and females had fewer NeuN + cells compared to males. For the number of DCX + cells in the DG, there were no significant main effects of Sex, Diet, Week, or significant interaction effects (see Fig. 1C,D). There were also no significant Sex, Experience, or Sex × Experience effects either the number of NeuN + cells or DCX + cells in the DG, indicating that there were no significant baseline sex differences in the number of mature or immature neurons in the DG and that gavage exposure alone had no effect on them.
Fig. 1.
Representative image of NeuN + cells in the densely packed granule neuron layer of the hippocampal DG, with counting frame for stereology overlaid at 100× (A) There were significant main effects of Diet and Sex on number of mature DG neurons with fewer NeuN + cells in rats exposed to binge EtOH versus control diet and also in females versus males (B). Representative image of DCX + cells at 40× (10 μm scale bar) in the DG of the hippocampus (C). There were no significant main or interaction effects on number of DCX + immature neurons (D). ****p < 0.0001.
Effect of Weekly Binge Exposure on Microglia in the Hippocampus.
Analysis of the total number of Iba1 + cells in the hippocampus showed that there were statistically significant Diet, F(1, 72) = 13.13, p = 0.0005, η2 = 0.15, Week, F(1, 72) = 57.88, p < 0.0001, η2 = 0.45, and Diet × Week, F(1, 72) = 9.94, p = 0.002, η2 = 0.12, effects (see Fig. 2A). Follow-up tests of simple effects showed that 8 weeks of binge exposure significantly increased number of Iba1 + cells compared to 3 weeks, F(1, 72) = 57.89, p < 0.0001, η2 = 0.45, and compared to 8 weeks of control diet, F(1, 72) = 22.96, p < 0.0001, η2 = 0.24. Also, 3-week controls had significantly fewer Iba1 + cells than 8-week controls, F(1, 72) = 9.93, p = 0.002, η2 = 0.12. Sex × Experience ANOVA revealed a significant effect of Experience, F(2, 54) = 6.96, p = 0.002, η2 = 0.20, and post hoc pairwise comparisons showed that 3-week controls had significantly fewer Iba1 + cells than cage controls [t(54) = 2.76, p = 0.016, d = 0.79].
Analysis of partially activated Iba1 + cells revealed significant Diet, F(1, 72) = 17.75, p < 0.0001, η2 = 0.20, Week, F(1, 72) = 22.10, p < 0.0001, η2 = 0.23, and Diet × Week, F(1, 72) = 10.55, p = 0.002, η2 = 0.13, effects (see Fig. 2B). Simple effects analyses showed that binge exposure for 8 weeks significantly increased number of Iba1 + cells compared to 3 weeks, F(1, 72) = 31.59, p < 0.0001, η2 = 0.30, and compared to 8 weeks of control diet, F(1, 72) = 27.84, p < 0.0001, η2 = 0.28. Sex × Experience ANOVA, however, showed no significant effects, indicating no baseline sex differences, and no influence of gavage exposure.
Effect of Weekly Binge Exposure on Microglia in the mPFC.
Analysis of the total number of Iba1 + cells in the mPFC revealed statistically significant Diet, F(1, 72) = 22.57, p < 0.0001, η2 = 0.24, Week, F(1, 72) = 15.77, p = 0.0002, η2 = 0.18, and Diet × Week, F(1, 72) = 26.11, p < 0.0001, η2 = 0.27, effects (see Fig. 2C). Follow-up simple effects tests showed that rats given 8 binges had significantly more Iba1 + cells than 8-week controls, F(1, 72) = 48.62, p < 0.0001, η2 = 0.40, and those given 3 binges, F(1, 72) = 41.23, p < 0.0001, η2 = 0.36. Sex × Experience ANOVA revealed a significant effect of Experience, F(2, 54) = 6.89, p = 0.002, η2 = 0.20, and post hoc pairwise comparisons showed that cage controls had significantly fewer Iba1 + cells than 3-week controls [t(54) = 3.60 p = 0.001, d = 1.06] and 8-week controls [t(54) = 2.58, p = 0.025, d = 0.8] indicating that gavage experience was associated with increased total microglia in the mPFC of both sexes.
Similarly, analysis of partially activated Iba1 + cells revealed significant Diet, F(1, 72) = 48.74, p < 0.0001, η2 = 0.40, Week, F(1, 72) = 13.21, p = 0.0005, η2 = 0.15, and Diet × Week, F(1, 72) = 20.07, p < 0.0001, η2 = 0.22, effects (see Fig. 2D). Simple effects analyses showed that binge exposure for 8 weeks significantly increased Iba1 + cells compared to 3 weeks, F(1, 72) = 32.92, p < 0.0001, η2 = 0.31, and compared to 8 weeks of control diet, F(1, 72) = 65.68, p < 0.0001, η2 = 0.48. Sex × Experience ANOVA, however, showed no significant effects, indicating the absence of baseline sex differences or gavage effects.
No Evidence for Reactive Astrogliosis.
Notably, despite the increase in microgliosis, we saw no evidence of reactive astrogliosis, indicated by the absence of vimentin immunoreactivity in binged animals of either sex, at either timepoint. This was true for both the hippocampus (see Fig. 2F) and mPFC. In contrast, tissue from a 4-day binge-exposed animal (used as a positive control) showed robust vimentin immunoreactivity.
Experiment 2
Effect of Weekly Binge Exposure on BEC and Body Weight.
Like in Experiment 1, there were no statistically significant Sex, Time, or Sex × Time effects on BEC regardless of whether animals underwent 3 binges or 8. Overall, females had a mean BEC of 178 ± 5.2 mg/dl and males, 174 ± 5.2 mg/dl (mean ± SEM). There was a significant effect of Time on body weight within both sexes in the 3-week, Ffemales(1.47, 26.43) = 90.92, p < 0.0001, η2 = 0.83; Fmales(1.30, 23.40) = 280.67, p < 0.0001, η2 = 0.94, and 8-week groups, Ffemales(3.43, 61.82) = 191.14, p < 0.0001, η2 = 0.91; Fmales(1.49, 26.79) = 285.45, p < 0.0001, η2 = 0.94, such that all groups gained weight across weeks (see Table 1).
Effect of Weekly Binge on USV and Spatial Performance.
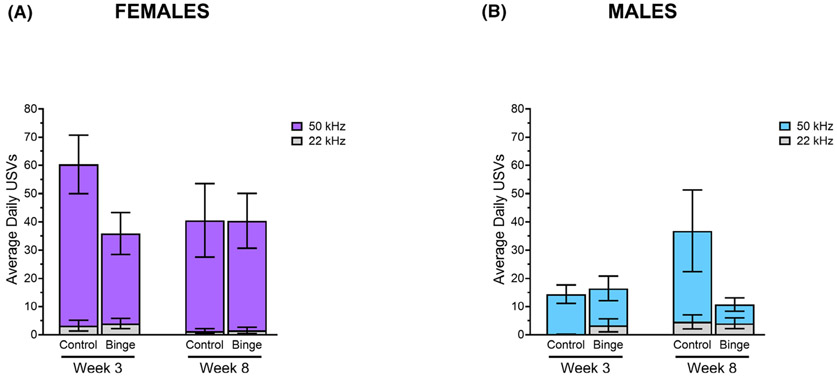
Playful tickling elicited USV from all animals, with the exception of 1 binged male and 1 binged female. Analyses of USV data revealed no statistically significant group differences for either sex in average number of daily 50-kHz USVs, 22-kHz USVs, or proportion of 50-kHz calls. After 8 weeks, control males did make more 50-kHz USV compared to binged males, but this did not reach statistical significance (see Fig. 3).
Fig. 3.
There were no significant differences between groups in the average number of daily 50-kHz or 22-kHz USVs within females (A) or males (B).
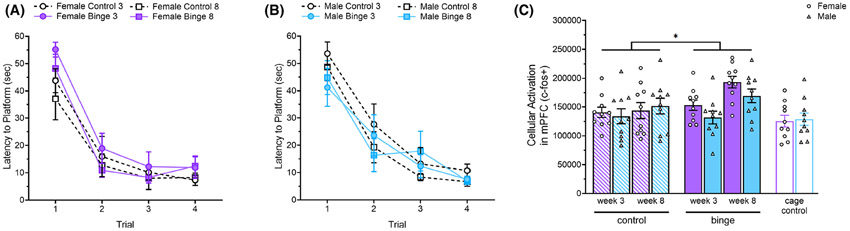
Analysis of MWM acquisition data showed a significant effect of Day on latency to escape, F(3, 216) = 175.96, p < 0.0001, η2 = 0.71, and swim speed, F(2.67, 192.35) = 104.55, p < 0.0001, η2 = 0.59. As expected, average latency and swim speed decreased across days (data not shown). There were also significant Day × Sex × Week, F(3, 216) = 3.43, p = 0.018, η2 = 0.05, and Day × Diet × Week, F(3, 216) = 3.20, p = 0.024, η2 = 0.04, effects on latency to platform. Follow-up analyses of simple interaction effects at the level of Day revealed a significant Diet × Week effect on the third day of acquisition, F(1, 216) = 6.54, p = 0.011, η2 = 0.08, and further simple effects tests were not significant. All groups showed a significantly greater than chance preference for the platform quadrant during the probe trial which suggests they all formed a spatial memory for the platform location (data not shown). There were significant effects of Week on % time in platform quadrant, F(1, 72) = 4.46, p = 0.038, η2 = 0.06, and of Sex on # of platform crossings, F(1, 72) = 11.78, p = 0.0001, η2 = 0.14, and % time in platform zone, F(1, 72) = 14.78, p = 0.0003, η2 = 0.17. There were also significant Sex × Diet × Week effects on % time in platform quadrant, F(1, 72) = 4.82, p = 0.031, η2 = 0.06, # of platform crossings, F(1, 72) = 6.58, p = 0.012, η2 = 0.08, and % time in platform zone, F(1, 72) = 5.19, p = 0.026, η2 = 0.07 (data not shown). Follow-up analyses of simple interaction effects at the level of Sex revealed a significant Diet × Week effect on % time spent in platform zone, F(1, 72) = 6.26, p = 0.015, η2 = 0.10, in males. Follow-up tests of simple effects were not significant. There were no significant differences between groups on average swim speed during the probe. For reversal learning, some rats were excluded from analyses because they spent 1 or all 4 trials floating. There was a significant effect of Trial on latency to platform, F(2.56, 171.45) = 109.05, p < 0.0001, η2 = 0.62, (see Fig. 4A,B) and swim speed, F(2.36, 156.12) = 17.85, p < 0.0001, η2 = 0.21. Average latency and swim speed decreased across trials, as expected.
Fig. 4.
All groups successfully learned the new location of the platform during 4 reversal learning trials (A, B). Data are presented separately for females and males for clarity. There were significant Diet and Week effects on the number of c-fos + cells in the mPFC with binged rats having more than controls and 8-week rats having more than 3-week rats (C). Behavioral testing did not significantly increase cellular activation in the mPFC in control animals. *p < 0.05.
Effect of Weekly Binge on Task-Induced c-fos in the mPFC.
One female and 1 male rat from the 8-week control diet group were excluded from c-fos analysis as they spent all 4 trials during reversal learning floating. There were statistically significant main effects of Diet, F(1, 70) = 5.54, p = 0.027, η2 = 0.06, and Week, F(1, 70) = 9.04, p = 0.003, η2 = 0.10, on the number of c-fos + cells in the mPFC during reversal learning (see Fig. 4C). Binged rats had more c-fos + cells than controls and 8-week rats had more than 3-week rats. There were no significant Sex, Experience, or Sex × Experience effects on number of c-fos + cells in the mPFC. Because cage controls did not undergo behavioral testing, this indicates that behavioral testing did not significantly increase cellular activation in the mPFC of control rats.
DISCUSSION
Studies of sex differences in alcohol-induced brain damage have historically focused on chronic alcohol consumption, and preclinical studies of sex-dependent effects of binge alcohol are scarce. Animal models provide a unique opportunity to standardize the amount, timing, and duration of binge alcohol between the sexes, thereby circumventing the difficulty inherent to matching drinking histories between men and women in clinical studies. Moreover, the recurring once-weekly binge model we used allows investigation of cumulative impact, enabling us to examine whether the magnitude of effects of binge alcohol increases as the number of binge exposures increases. Accordingly, this study examined brain and behavior of male and female rats after 3 and 8 once-weekly binge alcohol exposures.
In Experiment 1, we compared the effects of 3 or 8 weekly binge exposures on hippocampal cell loss and cell genesis, as well as reactive astrogliosis and neuroimmune activation in the hippocampal formation and mPFC. Our initial hypothesis of selective vulnerability of the female brain was not supported. Binge-induced cell loss and microglial numbers were similar in both sexes, and neither sex showed any evidence of reactive astrogliosis. These data are in contrast to our prior finding that hippocampal cell loss and impairment in a hippocampal-dependent task was more severe in females following a 4-day continuous binge exposure (Maynard et al., 2018). There are major differences between the 4-day binge exposure model and the model used in the present study, however. First, the 4-day binge model involves 96 hours of continuous intoxication, whereas the present model causes mild behavioral intoxication symptoms for 4 to 6 hours in young rats. Second, the 4-day binge model produces very high BEC (>250 mg/dl), whereas those in the present study averaged 185 mg/dl or less. Third, the 4-day binge model induces dependence and visibly apparent withdrawal symptoms. In contrast, in the present study, BEC did not change across weeks, indicating that physiological adaptations did not develop. Also, we observed no withdrawal signs (sensitivity to light/sound, tail tremors, splayed limbs) the day after binge, and there was no apparent effect of binge on food and water intake. Moreover, the 4-day binge model causes weight loss, whereas there were no significant effects of binge on body weight in the present study. In summary, past studies showing selective vulnerability of the female brain to alcohol-induced damage have focused on chronic heavy alcohol use, which is characterized by neural adaptation and metabolic tolerance. In the present study, we observed no sex differences after intermittent binge exposure. Thus, our results raise the possibility that the male brain is less vulnerable to alcohol compared to the female only if physiological adaptations occur or when BEC are very high (>250 mg/dl).
Our hypothesis that the damaging effects of binge exposure on brain and behavior would be cumulative was partially supported. Our result showing that binge exposure significantly reduced the number of remaining DG granule neurons regardless of the number of binges did not support the hypothesis of cumulative damage. Neuroimmune activation (total microglia and partially activated microglia), however, was more pronounced after 8 binges compared to 3, consistent with prior findings of a potentiated response after repeated binge (Marshall et al., 2016). That alcohol increases microglial numbers is well-established (see Crews et al., 2015 for review). Although the significance of increased numbers of microglia (including partially activated microglia) remains unclear (Cherry et al., 2014a, 2014b), evidence suggests that they play a supportive, healing role in the binge-exposed adult brain (Marshall et al., 2013). Indeed, the increased microglia in the present study were observed in the absence of reactive astrogliosis, upholding the idea that microglial activation in the binge-exposed brain is neuroprotective (Melbourne et al., 2019). The progressive increase with ongoing binge exposure is especially interesting in light of recent evidence that microglia may be causal in the escalation of drinking behavior (Warden et al., 2020), potentially contributing to the link between binge drinking and future AUD (Gowin et al., 2017).
The significant binge-induced decrease in DG granule neurons was not accompanied by reactive astrogliosis, supporting the idea that cell loss was the result of impaired adult neurogenesis, and not merely alcohol-induced cell death, as has been previously suggested (Morris et al., 2010). Although binge alcohol suppresses neurogenesis (Nixon and Crews, 2002, 2004), a compensatory increase occurs with abstinence (Nixon and Crews, 2004). We have previously observed an increase in DCX + cells during abstinence after weekly repeated binge exposure (West et al., 2019), but we did not in the present study. There are several key differences between our prior study and the present one, however, including the number of binges (eleven in the previous study). Moreover, rats in the prior study were repeatedly tested in hippocampal-dependent tasks, whereas in the present study we performed behavior on a separate cohort of rats (Experiment 2), to avoid potential contribution of behavioral testing experience to cellular outcome measures.
Due to concern that gavage exposure is stressful and could thus impact cellular outcomes, we included cage controls in our experimental design and statistical analyses. For DCX + cells and number of remaining granule neurons, rats gavaged with control solution were not different from gavage-naïve cage controls. The same was true for partially activated microglia in the hippocampus and mPFC. For total microglia, gavaged animals had fewer in the hippocampus, but more in the mPFC compared to cage controls. Collectively, these results do not indicate a consistent effect of gavage experience on our cellular outcome measures.
In Experiment 2, we assessed spatial navigation using the MWM and tickling-evoked USV, both of which are associated with the integrity of the hippocampal DG (D’Hooge and De Deyn, 2001; Wohr et al., 2009). We hypothesized that behavioral impairments due to binge alcohol would be more pronounced in females and that they would progressively worsen. Neither hypothesis was supported. We found no evidence for alteration of tickling-evoked USV. Generally, 50-kHz USV in rats are associated with positive affective states and 22-kHz USV are indicative of the opposite (Burgdorf et al., 2008; Cuomo et al., 1988). Neither sex showed a binge-induced decrease in 50-kHz or an increase in 22-kHz USV in response to tickling by the experimenter.
We also did not find impairments in the MWM in either sex. This is in contrast to our prior findings from the 4-day binge model, in which females were more affected (Maynard et al., 2018). As noted above, however, there are many important differences between the 4-day binge model and weekly binge exposures. Our future studies will utilize cognitive tests that are dependent on frontal lobe integrity, as we observed an effect of binge exposure on c-fos induction in the mPFC due to reversal learning. This is consistent with our previous findings following 4-day binge exposure, in which binged rats showed increased c-fos activation in the mPFC despite control-level performance on a rewarded alternation task (West et al., 2018). In the present study, we also found that rats gavaged with control solution were not different from cage controls, indicating that the task either was not challenging enough to require the recruitment of mPFC neurons or that this task relies primarily on other brain regions in rats. Indeed, in rodents, mPFC damage does not always affect reversal learning (Bissonette et al., 2008). Although reversal learning may not have been challenging enough to activate the mPFC in controls, the fact that it did in binged rats suggests that they had to employ an additional brain region to achieve the same performance as controls. Recruitment of additional brain regions during cognitive testing has also been observed in those with chronic AUD (Parks et al., 2010). Additionally, EEG evaluation showed similar results, with human binge drinkers showing increased brain activity despite similar working memory task performance (Campanella et al., 2013). Our findings support this compensatory hypothesis and suggest that the brains of binged rats may employ more resources to perform at control levels.
In summary, this study assessed progressive damage and sex-specific effects of once-weekly binge exposure. We found evidence that binge effects on neuroimmune activation are cumulative, as total and partially activated microglia were more prevalent in the hippocampus and mPFC after 8 binges compared to 3. We found no evidence of sex-dependent effects, as both sexes showed binge-induced granule neuron loss, neuroimmune activation, and increased task-induced neuronal activation despite control-level performance in a reversal learning task. The cumulative increase in microglial presence upholds the idea that repeated binge exposure impacts brain health gradually over time. The absence of sex-dependent effects in this once-weekly binge model contrasts with evidence from models that produce dependence, suggesting that male and female brains are similarly vulnerable to alcohol if physiological adaptations to it do not occur. Given the prevalence of binge drinking in the general population, it important to promote awareness in both genders of how this pattern of alcohol consumption can negatively impact brain health.
ACKNOWLEDGMENTS
We thank Kimberly Nixon, PhD for a critical review of the manuscript. This study was supported by NIAAA R01AA025380.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Contributor Information
Rebecca K. West, Department of Psychology, University of Houston, Houston, TX
Shaefali P. Rodgers, Department of Psychology, University of Houston, Houston, TX
J. Leigh Leasure, Department of Psychology, University of Houston, Houston, TX; Department of Biology and Biochemistry, University of Houston, Houston, TX..
REFERENCES
- Barton EA, Baker C, Leasure JL (2017) Investigation of sex differences in the microglial response to binge ethanol and exercise. Brain Sci 7(10), 139. 10.3390/brainsci7100139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM (2008) Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci 28:11124–11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak M, Room R, Pikhart H, Kubinova R, Malyutina S, Pajak A, Kurilovitch S, Topor R, Nikitin Y, Marmot M (2004) Contribution of drinking patterns to differences in rates of alcohol related problems between three urban populations. J Epidemiol Community Health 58:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J (2008) Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol 122:357–367. [DOI] [PubMed] [Google Scholar]
- Campanella S, Peigneux P, Petit G, Lallemand F, Saeremans M, Noel X, Metens T, Nouali M, De Tiège X, De Witte P, Ward R, Verbanck P (2013) Increased cortical activity in binge drinkers during working memory task: a preliminary assessment through a functional magnetic resonance imaging study. PLoS One 8:e62260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK (2014a) Are “resting” microglia more “m2”? Front Immunol 5:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK (2014b) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. 10.1186/1742-2094-11-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K (2006) Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience 137:437–445. [DOI] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, Vetreno RP (2015) Neuroimmune function and the consequences of alcohol exposure. Alcohol Res 37:331–341, 344–351. [PMC free article] [PubMed] [Google Scholar]
- Cuomo V, Cagiano R, De Salvia MA, Maselli MA, Renna G, Racagni G (1988) Ultrasonic vocalization in response to unavoidable aversive stimuli in rats: effects of benzodiazepines. Life Sci 43:485–491. [DOI] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K (2011) Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res 35:1678–1685. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36:60–90. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN (2003) Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res 27:1563–1572. [DOI] [PubMed] [Google Scholar]
- Erol A, Karpyak VM (2015) Sex and gender-related differences in alcohol use and its consequences: contemporary knowledge and future research considerations. Drug Alcohol Depend 156:1–13. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA (2017) Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry 174:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R (2001) Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry 158:198–204. [DOI] [PubMed] [Google Scholar]
- Hunt WA (1993) Are binge drinkers more at risk of developing brain damage? Alcohol 10:559–561. [DOI] [PubMed] [Google Scholar]
- Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD (2018) Annual total binge drinks consumed by U.S. Adults, 2015. Am J Prev Med 54:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Liput DJ, Eaves DW, Nixon K (2011) Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience 197:381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Jager J, Mal-Sarkar T, Patrick ME, Rutherford C, Hasin D (2019) Is there a recent epidemic of women’s drinking? A critical review of national studies. Alcohol Clin Exp Res 43:1344–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318. [DOI] [PubMed] [Google Scholar]
- Kubota M, Nakazaki S, Hirai S, Saeki N, Yamaura A, Kusaka T (2001) Alcohol consumption and frontal lobe shrinkage: study of 1432 non-alcoholic subjects. J Neurol Neurosurg Psychiatry 71:104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Batra A, Gunthner A, Schroth G (1992) Do women develop alcoholic brain damage more readily than men? Alcohol Clin Exp Res 16:1052–1056. [DOI] [PubMed] [Google Scholar]
- Marshall SA, Nixon K (2016) Prior binge ethanol exposure potentiates the microglial response in a model of alcohol-induced neurodegeneration. Brain Sci 6(2), 16. 10.3390/brainsci6020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K (2013) Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: the importance of microglia phenotype. Neurobiol Dis 54:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard ME, Barton EA, Robinson CR, Wooden JI, Leasure JL (2018) Sex differences in hippocampal damage, cognitive impairment, and trophic factor expression in an animal model of an alcohol use disorder. Brain Struct Funct 223:195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K (2011) Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun 25(Suppl. 1):S120–S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melbourne JK, Thompson KR, Peng H, Nixon K (2019) Its complicated: the relationship between alcohol and microglia in the search for novel pharmacotherapeutic targets for alcohol use disorders. Prog Mol Biol Transl Sci 167:179–221. [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K (2010) Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus 20:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA (2004) NIAAA Council approves definition of binge drinking. NIAAA Newsl; 3. https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf [Google Scholar]
- Nixon K, Crews FT (2002) Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem 83:1087–1093. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT (2004) Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci 24:9714–9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks MH, Greenberg DS, Nickel MK, Dietrich MS, Rogers BP, Martin PR (2010) Recruitment of additional brain regions to accomplish simple motor tasks in chronic alcohol-dependent patients. Alcohol Clin Exp Res 34:1098–1109. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates. 5th ed, San Diego, CA: Elsevier. [Google Scholar]
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW (1999) Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev 30:77–105. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Jegan N, Wohr M (2007) Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav Brain Res 182:208–222. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A (2010) Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “telescoping”. Psychopharmacology 208:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden AS, Triplett TA, Lyu A, Grantham EK, Azzam MM, DaCosta A, Mason S, Blednov YA, Ehrlich LIR, Mayfield RD, Harris RA (2020) Microglia depletion and alcohol: transcriptome and behavioral profiles. Addict Biol e12889. 10.1111/adb.12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RK, Maynard ME, Leasure JL (2018) Binge ethanol effects on prefrontal cortex neurons, spatial working memory and task-induced neuronal activation in male and female rats. Physiol Behav 188:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RK, Wooden JI, Barton EA, Leasure JL (2019) Recurrent binge ethanol is associated with significant loss of dentate gyrus granule neurons in female rats despite concomitant increase in neurogenesis. Neuropharmacology 148:272–283. [DOI] [PubMed] [Google Scholar]
- Wilson S, Bair JL, Thomas KM, Iacono WG (2017) Problematic alcohol use and reduced hippocampal volume: a meta-analytic review. Psychol Med 47:2288–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Kehl M, Borta A, Schanzer A, Schwarting RK, Hoglinger GU (2009) New insights into the relationship of neurogenesis and affect: tickling induces hippocampal cell proliferation in rats emitting appetitive 50-kHz ultrasonic vocalizations. Neuroscience 163:1024–1030. [DOI] [PubMed] [Google Scholar]