Abstract
BACKGROUND/OBJECTIVE:
Medication discrepancies and adverse drug events are common following hospital discharge. This study evaluates whether a collaboration between community-based health coaches and primary care-based pharmacists was associated with a reduction in inpatient utilization following hospitalization.
DESIGN:
Retrospective cohort study using propensity score matching.
SETTING:
Urban, academic medical center and surrounding community.
PARTICIPANTS:
Intervention patients (n=494) were adults aged 65 or older admitted to the UCLA Ronald Reagan Medical Center during the study period and who met study inclusion criteria. A matched-control group was comprised of patients with similar demographic and clinical characteristics who were admitted to the study site during the study period, but who received usual care (n=2,470). A Greedy Algorithm approach was used to conduct the propensity score match.
INTERVENTION:
Following acute hospitalization, a health coach conducted a home visit and transmitted all medication-related information to a primary care practice-based pharmacist. The pharmacist compared this information to the patient’s electronic medical record medication list and consulted with the patient’s primary care provider to optimize medication management.
MEASUREMENTS:
30-day readmissions (primary outcome); 60 and 90-day readmissions and 30-day emergency department (ED) visits (secondary outcomes) to UCLA Health.
RESULTS:
Among 494 patients who received the intervention, 307 (62.1%) were female with a mean age of 83.0 [IQR 76–90] years. Among 2,470 matched control patients, 1541 (62.4%) were female with a mean age of 82.7 [IQR 74.9–89.5] years. For the propensity score match, standardized mean differences were <0.1 for 23 out of 25 variables, indicating good balance. Patients who received this intervention had a significantly lower predicted probability of being readmitted within 30 days compared with matched-control patients (10.6% [CI 7.9–13.2] versus 21.4 % [19.8–23.0], p-value <0.001).
CONCLUSION:
A home visit conducted by a health coach combined with medication review by a primary care-based pharmacist may prevent subsequent inpatient utilization.
Keywords: Care transitions, home-bound older adults, clinical pharmacists, medication management, hospital readmissions
INTRODUCTION
Among hospitalized patients 65 years of age and older, approximately 20% are readmitted within 30 days, and 34% are readmitted within 90 days1. Medication-related problems are common among older adults due to reasons such as complex medication regimens, polypharmacy, and altered pharmacokinetics2–4. These problems can be accentuated during care transitions for a variety of reasons, including inaccurate medication intake upon a patient’s admission to the hospital, changes to a patient’s medication regimen during hospitalization, and documentation errors that occur as patients move between settings and providers5–8. Each of these can contribute to the prescribing of inappropriate medications, patient confusion, and medication misuse post-discharge, which can in turn result in adverse drug events9–12. Several publications have demonstrated an association between adverse drug events, and emergency department visits and hospital readmissions.13–15 Identifying aways to improve medication safety has been recognized as an important component of discharge efforts and care transition programs.
While several care transition programs that employ multidisciplinary care team members have demonstrated effectiveness in reducing hospital readmissions and costs16–20, less is known about the impact of clinical pharmacist-anchored interventions that focus specifically on medication management for older, home-bound adults and that are rooted in the patient’s primary care setting21,22. Previous care transition programs have incorporated pharmacists, but they have typically done so at the time of hospital discharge to perform medication reconciliation.23 While beneficial, this approach does not allow the pharmacists to access a patient’s full list of medications, or provide knowledge as to how a patient is actually taking medications upon discharge to home.
This study evaluates a care transitions intervention that aimed to improve medication management and medication safety among Medicare patients following acute hospitalization. We hypothesized that patients who received the program would have a lower predicted probability of an unplanned hospital readmission or emergency department (ED) visit compared with similar patients who received usual care.
METHODS
This study is a retrospective cohort study that was conducted at University of California, Los Angeles (UCLA) Health in Los Angeles, California. This intervention was a collaboration between UCLA Health and a community-based partner, Partners in Care Foundation (Partners). Partners is a not-for-profit community-based organization that develops models of care for adults with complex needs in Los Angeles County. Patients were recruited for this intervention from the general medicine inpatient wards at UCLA Ronald Reagan Medical Center (RRMC). The intervention study period was July 1, 2014 to December 31, 2016. The study was approved by the institutional review board of the University of California, Los Angeles (UCLA).
Participants and Data
Patients were eligible for this program if they were hospitalized for a non-elective reason, had an assigned UCLA primary care provider (PCP), had Medicare fee-for-service insurance coverage, and were discharged to home after hospitalization. In addition, patients had to have two or more of the following risk factors, as identified using the UCLA Health electronic medical record (EMR): hospital readmission within the past 30 days and/or two or more admissions within the past 12 months; hospital length of stay greater than 10 days; eight or more outpatient prescription medications; depression as secondary diagnosis; mild cognitive impairment; two or more chronic conditions; and limited caregiver support, as determined by the referring source at the hospital such as the care manager. These risk factors used for inclusion in the program were derived from a root cause analysis conducted by UCLA Health researchers that aimed to identify risk factors for readmission.
Patients were ineligible for the intervention if they were homeless; sent to hospice on the day of discharge; in an observation unit; had a primary admission diagnosis of mental disease and/or substance abuse; or were admitted for scheduled or recurring chemotherapy, immunotherapy, radiation therapy, rehabilitation, or dialysis. All study data were obtained from the UCLA EMR. In total, 494 patients were included in the intervention arm of this study.
This was not a randomized controlled trial (RCT). Therefore, we constructed a control group comprised of patients who were admitted to the study site during the study period, but who received usual care. We first identified all patients admitted to UCLA’s RRMC during the study period (n=725,874). All patients had an assigned UCLA PCP. We then applied the study inclusion and exclusion criteria to these patients using UCLA EMR data. To apply the inclusion criterion of having two or more risk factors, we created a risk factor count variable where a patient was assigned one point for each risk factor described above. We applied this to both the intervention and control group. Patients with zero risk factors were excluded.
After applying the inclusion and exclusion criteria to all non-intervention hospitalized patients during the study period, the total number of hospitalizations (i.e., one patient could be hospitalized numerous times during the 17-month study period) decreased from 725,874 to 20,537. To derive a usual care comparator group at the patient level as opposed to the encounter level, and to achieve balance between the intervention and control groups, we used propensity score matching. To obtain the predicted probability of receiving the intervention (i.e., propensity score) for each patient, we used a logistic regression model using the following covariates, which we determined were likely to influence receipt of the intervention: gender, race, age, presence of hypertension, coronary artery disease (CAD), mental health diagnosis, dementia, congestive heart failure (CHF), atrial fibrillation, acute kidney injury (AKI), stroke, peripheral vascular disease (PVD), diabetes, schizophrenia, depression, MCI, use of warfarin, total number of prescription medications, number of hospital visits in year prior to index visit, hospital visit 30 days prior to index hospitalization (yes/no), number of days between study period start date to index hospitalization admission, index hospitalization length of stay, and number of emergency room visits in year prior to index hospitalization. Five control patient encounters were matched to each intervention patient. A Greedy Search approach was used to conduct the match. Specifically, after a control patient was matched, the patient’s remaining encounters were removed from the pool of possible control encounters to mitigate within patient correlation. Unmatched patients had propensity scores with median 0.018 (IQR 0.010, 0.030) for control patients and median 0.034 (IQR 0.020, 0.052) for intervention patients, p < 0.001. After matching, median propensity scores were 0.034 (IQR 0.020, 0.052) for control patients and 0.034 (IQR 0.020, 0.052) for intervention patients, p = 0.95, indicating balance between the covariates. Figure 1 shows the study flow diagram for the construction of the intervention and control groups.
Figure 1.
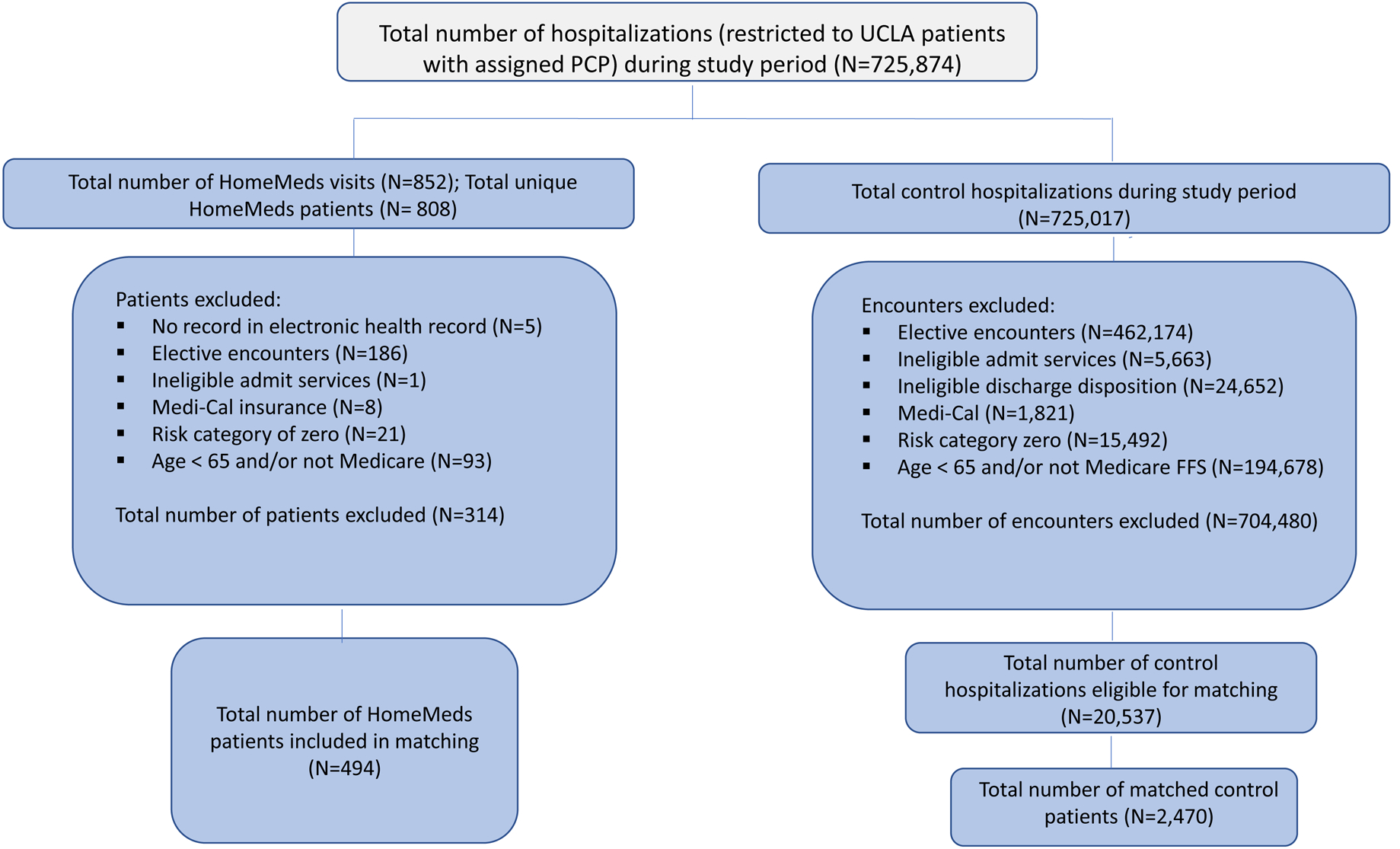
Study flow diagram, intervention and control groups. PCP, primary care provider; UCLA, University of California, Los Angeles.
Description of the Program
This intervention leveraged the core components of two widely adopted, evidence-based programs: HomeMeds and the Coleman Care Transitions Intervention™ (CTI)20,24. The HomeMeds program uses community-based organizations to arrange home visits conducted by health coaches in partnership with independent clinical pharmacists to address medication problems common among older, home-bound adults. The CTI program is a comprehensive care transitions intervention that is initiated while a patient is still hospitalized, and aims to improve outcomes for patients who transition between the hospital and home by using a coaching model that includes four “pillars”: Medication Self-Management, Dynamic Patient-Centered Record, Follow-Up, and Red Flags. In the intervention studied here, health coaches who introduced patients to the intervention while the patient was still hospitalized, and who conducted the home visits were trained by members of Dr. Eric Coleman’s team on all four pillars of the model. The typical educational background for health coaches was a bachelor’s degree in social work, gerontology, or public health. Partners and UCLA Health formed a collaboration in 2015 that leveraged both the HomeMeds program and the UCLA UCMyRx program. UCLA Health initiated UCMyRx in 2012 to improve medication adherence and medication safety, and to fully enfranchise clinical pharmacists embedded in primary care practices at UCLA Health.
The intervention evaluated here was initiated at the hospital before a patient was discharged to home. A study coordinator invited eligible patients to participate. If a patient agreed, the Partners health coach visited the patient at the bedside to describe the program and to schedule a home visit, which took place 11 days, on average, after a patient was discharged. The majority of home visits (94%) occurred within 30 days of a patient’s hospital discharge.
During the home visit, health coaches followed the protocol outlined in the HomeMeds and CTI™ models. Specifically, the health coach recorded all prescribed and over-the-counter medications or supplements; interviewed patients and caregivers to determine how medications were being used; and documented any patient self-reported incidents such as falls, as well as health-related habits, symptoms, and vital signs. The health coach also worked with patients to set a personal goal, used techniques like role playing to promote patient self-management, and assisted with scheduling follow-up appointments for the patient. The home visit lasted 1.5–2 hours. The health coach also called patients at 7, 14, and 30 days following the home visit to reinforce goals and coaching content and to identify emerging issues.
The information collected by the health coach was electronically transmitted using a tablet computer to UCLA clinical pharmacists who had full access to the UCLA Health EMR. The clinical pharmacist conducted medication reconciliation and focused especially on identifying potential dangers that could result from the patient’s medication regimen (e.g., Beers Criteria medications, duplications, and drug-drug interactions). To document problem areas or discrepancies, the clinical pharmacists used a detailed template, which they used to communicate (1) with the patient directly if there were any items that were unclear or of immediate concern, and (2) with the patient’s PCP. In communication with the patient’s PCP, the clinical pharmacist made a set of recommendations via the EMR in-basket with suggested changes to improve the safety and effectiveness of the medications and, with PCP approval, operationalized the changes in real time. This documentation then became part of the patient’s medical record. If the clinical pharmacist identified a potentially life-threatening issue during their review, they called the PCP so that changes could be made immediately. This review by the pharmacist was completed within 72 hours of the home visit. Figure 2 summarizes this intervention workflow.
Figure 2.
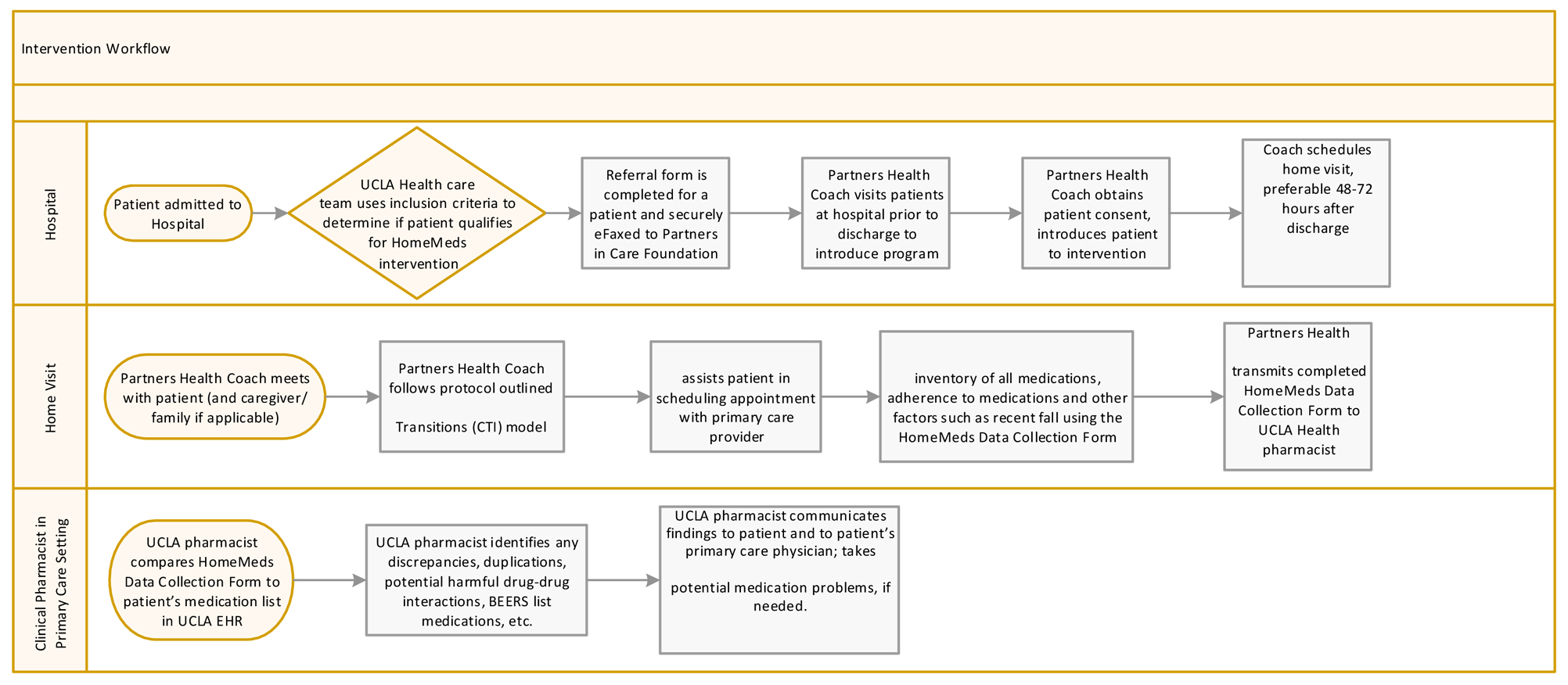
Intervention workflow. EMR, electronic health record; UCLA, University of California, Los Angeles.
Measures
The primary outcome measure was 30-day all cause hospital readmissions. The secondary outcome measures were 60 and 90-day all-cause hospital readmissions, and 30-day ED visits. All data were obtained from the UCLA EMR. Readmissions and ED visits reflect only those that occurred at UCLA RRMC or UCLA Santa Monica Hospital. The outcome variables for readmissions and ED visit 30 days post-intervention were formatted from the 30 days post-discharge from the index hospitalization. Planned re-hospitalizations and elective hospitalizations were removed from both the intervention and control groups (detailed in Figure 1).
Statistical Analysis
We used logistic regression models to adjust for observable patient characteristics and obtain the predicted probability of experiencing a readmission or ED visit, intervention versus control. We selected variables for each model (e.g., 30, 60, 90-day readmissions) using bivariate analyses of the individual predictors and the outcome of interest. Variables with a p-value below an alpha of 0.2 were included. All hypothesis tests were two-sided and a p-value below 0.05 was considered statistically significant. For sensitivity analyses, we investigated reasons for readmissions (using primary diagnosis codes) among the intervention and control groups. We also performed the propensity score match using a 1:1 and 1:2 match. We also explored differences in the adjusted multivariate models by removing any variable from the logistic regressions that was included in the match. Stata (IC-12; StataCorp LP, College Park, TX, USA) and R (R Core Team, Vienna, Austria) were used to conduct the statistical analyses.
In addition to this quantitative analysis, and in recognition of the limitations of observational studies, we reviewed 100 randomly selected patient charts (equally spaced over the study period) for patients who received the intervention to explore the potential mechanisms by which this intervention influenced readmissions. We developed a template with 13 categories to code progress notes completed by clinical pharmacists to understand what the pharmacist discovered in their review process, and what actions were taken. Two independent reviewers completed the template, and discrepancies between reviewers were discussed and resolved.
RESULTS
Our study population included 494 intervention patients and 2,470 matched-control patients. Table 1 shows pre and post-match descriptive statistics for these two groups. The baseline characteristics show that our study population was comprised of older patients who experienced high levels of inpatient utilization in the period preceding this intervention. After the propensity score matching, baseline characteristics were similar among intervention and control patients with regard to gender-female (62.1% versus 62.4% p-value=0.919), mean age (83.0 versus 82.7, p-value=0.476), race-White (66.0% vs. 67.8%, p-value=0.461), race-Black (15.2% versus 13.4%, p-value=0.316), race-Asian (7.5% versus 7.0%, p-value=0.701), ethnicity-Hispanic (14.0% versus 12.9%, p-value=0.512), primary language-English (86.0% vs. 82.8%, p-value=0.085), and partnership status-Married/Partner (43.9% vs. 44.6%, p-value=0.804). Intervention and control patients were also similar with regard to comorbidities: patients in both groups did not show statistically or clinically significant differences in having hypertension (57.5% versus 55.4%, p-value=0.399), coronary artery disease (23.5% versus 21.0%, p-value=0.23), mental health diagnosis (16.0% versus 14.4%, p-value=0.366), dementia (10.3% versus 8.9%, p-value=0.304), or diabetes (21.1% vs. 20.2%, p=0.669).
Table 1:
Baseline Characteristics, Intervention and Matched Control
| Variable | HomeMeds – Intervention (n=494) | Control encounters, pre-match (n=20537) | p-value | Matched control patients (n = 2470) | p-value |
|---|---|---|---|---|---|
| Female | 307 (62.1%) | 10534 (51.3%) | <0.001 | 1541 (62.4%) | 0.919 |
| Race | |||||
| White | 326 (66%) | 14603 (71.1%) | 0.014 | 1674 (67.8%) | 0.461 |
| Black | 75 (15.2%) | 1958 (9.5%) | <0.001 | 332 (13.4%) | 0.316 |
| Asian | 37 (7.5%) | 1515 (7.4%) | 0.931 | 173 (7%) | 0.701 |
| Other/Unknown | 56 (11.3%) | 2461 (12%) | 0.726 | 291 (11.8%) | 0.818 |
| Ethnicity - Hispanic | 69 (14%) | 2326 (11.3%) | 0.073 | 319 (12.9%) | 0.512 |
| Age | 83 (76–90) | 76.5 (70.1–84.7) | <0.001 | 82.7 (74.9–89.5) | 0.476 |
| Age - categorical | <0.001 | 0.166 | |||
| 65–74 | 112 (22.7%) | 9149 (44.5%) | 630 (25.5%) | ||
| 74–84 | 155 (31.4%) | 6400 (31.2%) | 814 (33%) | ||
| >=85 | 227 (46%) | 4988 (24.3%) | 1026 (41.5%) | ||
| Primary Language - English | 425 (86%) | 17306 (84.3%) | 0.316 | 2045 (82.8%) | 0.085 |
| Partnership Status - Married/Partner | 217 (43.9%) | 9821 (47.8%) | 0.092 | 1102 (44.6%) | 0.804 |
| Comorbidities | |||||
| Hypertension | 284 (57.5%) | 9819 (47.8%) | <0.001 | 1368 (55.4%) | 0.399 |
| Coronary artery disease | 116 (23.5%) | 4849 (23.6%) | 1 | 519 (21%) | 0.23 |
| Mental Health diagnosis | 79 (16%) | 2918 (14.2%) | 0.268 | 356 (14.4%) | 0.366 |
| Dementia | 51 (10.3%) | 1312 (6.4%) | 0.001 | 219 (8.9%) | 0.304 |
| Congested heart failure | 98 (19.8%) | 3421 (16.7%) | 0.067 | 462 (18.7%) | 0.571 |
| Atrial fibrillation | 159 (32.2%) | 6011 (29.3%) | 0.162 | 784 (31.7%) | 0.874 |
| Acute kidney injury | 99 (20%) | 3482 (17%) | 0.079 | 410 (16.6%) | 0.067 |
| Stroke | 69 (14%) | 2561 (12.5%) | 0.335 | 336 (13.6%) | 0.83 |
| Pulmonary vascular disease | 30 (6.1%) | 1156 (5.6%) | 0.622 | 131 (5.3%) | 0.514 |
| Diabetes Mellitus | 104 (21.1%) | 4140 (20.2%) | 0.61 | 500 (20.2%) | 0.669 |
| Schizophrenia | 8 (1.6%) | 375 (1.8%) | 0.865 | 36 (1.5%) | 0.838 |
| Mild cognitive impairment | 33 (6.7%) | 917 (4.5%) | 0.027 | 146 (5.9%) | 0.534 |
| Number of medications | 15.5 (10–21) | 10 (1–18) | <0.001 | 14 (7–22) | 0.008 |
| Hospital Visits 1 year prior to index visit | <0.001 | 0.047 | |||
| 0 | 224 (45.3%) | 6528 (31.8%) | 1235 (50%) | ||
| 1–5 | 253 (51.2%) | 11859 (57.7%) | 1185 (48%) | ||
| >5 | 17 (3.4%) | 2150 (10.5%) | 50 (2%) | ||
| Any hospital visit 30 day prior to index visit | 104 (21.1%) | 6626 (32.3%) | <0.001 | 443 (17.9%) | 0.112 |
| ED Visits 1 year prior to index visit | <0.001 | 0.039 | |||
| 0 | 231 (46.8%) | 7234 (35.2%) | 1292 (52.3%) | ||
| 1–5 | 250 (50.6%) | 11592 (56.4%) | 1138 (46.1%) | ||
| >5 | 13 (2.6%) | 1711 (8.3%) | 40 (1.6%) | ||
| Length of stay of index visit, mean (SD) | 4.2 (4.4) | 2.9 (5.7) | <0.001 | 4.1 (8) | 0.70 |
| Index discharge on a weekend | 75 (15.2%) | 4999 (24.3%) | <0.001 | 614 (24.9%) | <0.001 |
| Count of risk factors | <0.001 | <0.001 | |||
| 1 | 149 (30.2%) | 9136 (44.5%) | 1031 (41.7%) | ||
| 2 | 203 (41.1%) | 6797 (33.1%) | 887 (35.9%) | ||
| 3 | 99 (20%) | 3623 (17.6%) | 420 (17%) | ||
| 4 | 40 (8.1%) | 865 (4.2%) | 117 (4.7%) | ||
| 5 | 3 (0.6%) | 109 (0.5%) | 13 (0.5%) | ||
| 6 | 0 (0%) | 7 (0%) | 2 (0.1%) |
Matched ratio of 5-to-1 on the following variables: gender, race, age, hypertension, coronary artery disease, mental health diagnosis, dementia, congestive heart failure, atrial fibrillation, acute kidney injury, stroke, peripheral vascular disease, diabetes, schizophrenia, mild cognitive impairment, warfarin, number of medications, number of hospital visits in year prior to index visit, whether or not hospital visit 30 days prior to index hospitalization, days from study period start to index hospitalization admission, length of stay of index hospitalization, and number of emergency room visits one year prior to index hospitalization.
Variables that remained statistically significant after the propensity score match included experiencing between one and five hospitalizations in the 12 months prior to the index hospitalization (51.2% for intervention patients versus 48.0% for control patients, p=0.047); experiencing between one and five ED visits in the 12 months prior to the intervention date (50.6% for intervention patients versus 46.1% for control patients, p=0.039), average number of outpatient medications prescribed (15.5 for intervention patients versus 14.0 for control patients, p=0.008), and number of risk factors where the p-value reflects one test for all levels of the factor (1 risk factor: 30.2% versus 41.7%; 2 risk factors: 41.1% versus 35.9%; 3 risk factors: 20% versus 17%; 4 risk factors: 8.1% versus 4.7%; 5 risk factors: 0.6% versus 0.5%; 6 or more risk factors: 0% versus 0.1% for control patients, p<0.001.) These observed differences for intervention versus propensity score-matched control patients, specifically with regard to utilization and the number of medications prescribed, suggest that the intervention group was in worse health compared with the control group, which would bias our analysis toward the null hypothesis.
Unadjusted and Adjusted Outcomes
Table 2 shows the unadjusted outcomes of interest for intervention and matched-control patients. Intervention patients had a significantly lower unadjusted rate of 30-day hospital readmissions (11.1% vs. 21.2%, p-value<0.001), 60-day readmissions (22.9% vs. 28.6%, p-value<0.001), and 30-day ED visits (10.9% vs. 18.8%, p-value<0.001). The intervention and matched-control groups were not significantly different for 90-day readmissions (31.4% vs. 33.6%, p-value=0.347).
Table 2:
Unadjusted outcome model
| Intervention | Matched Control | P-value | |
|---|---|---|---|
| 30-day hospital readmission | 55 (11.1%) | 524 (21.2%) | <0.001 |
| 60-day hospital readmission | 113 (22.9% | 706 (28.6%) | 0.010 |
| 90-day hospital readmission | 155 (31.4%) | 830 (33.6%) | 0.347 |
| 30-day ED visit | 54 (10.9%) | 464 (18.8%) | <0.001 |
Table 3 shows the adjusted outcomes of interest, expressed as predicted probabilities for all outcomes for intervention and matched-control patients. After adjusting for patient-level demographic and clinical covariates, patients who received the intervention had a significantly lower predicted probability for experiencing all outcomes. Patients who received the intervention had a 10.6% predicted probability (95% CI 7.9–13.2) of readmission within 30 days while patients who received usual care had a 21.4% predicted probability (95% CI 19.8–23.0, p-value<0.001). The effect size was attenuated for 60 and 90-day hospital readmissions, and remained statistically significant (p-value=0.001) for the 60-day readmission outcome, though not for the 90-day readmission outcome. For ED visits, patients who received the intervention had a 10.4% predicted probability (95% CI 7.8–13.0) of experiencing an ED visit within 30 days of discharge while patients who received usual care had an 18.9% predicted probability (95% CI 17.4–20.5, p-value <0.001).
Table 3:
Adjusted outcome model
| Predicted Probability | P-value | ||
|---|---|---|---|
| Intervention (95% CI) | Matched Control (95% CI) | ||
| Adjusted 30-day hospital readmission* | 10.6 (7.9–13.2) | 21.4 (19.8–23.0) | <0.001 |
| Adjusted 60-day hospital readmission** | 21.8 (18.3–25.3) | 28.8 (27.1–30.6) | 0.001 |
| Adjusted 90-day hospital readmission*** | 29.9 (26.0–33.8) | 34.0 (32.1–35.7) | 0.072 |
| Adjusted 30-day ED visit**** | 10.4 (7.8–13.0) | 18.9 (17.4–20.5) | <0.001 |
Control variables include: Female, ethnicity-Hispanic, hypertension, coronary artery disease, mental health diagnosis, dementia, congestive heart failure, atrial fibrillation, stroke, schizophrenia, depression, mild cognitive impairment, hospital visit 1 year prior, ED visit 30 days prior, and count of risk factors.
Control variables include: Female, ethnicity-Hispanic, age, hypertension, coronary artery disease, mental health diagnosis, dementia, congestive heart failure, atrial fibrillation, acute kidney injury, stroke, diabetes, schizophrenia, depression, mild cognitive impairment, number of medications, hospital visits 1 year prior, hospital visit 30 days prior, and count of risk factors.
Control variables include: Female, ethnicity-Hispanic, age, primary language-English, partnership status-married/partner, hypertension, coronary artery disease, mental health dx, dementia, congestive heart failure, , atrial fibrillation, acute kidney injury, stroke, peripheral vascular disease, diabetes, schizophrenia, depression, mild cognitive impairment, hospital visit 1 year prior, hospital visit 30 days prior, ED visit 1 year prior, and count of risk factors.
Control variables include: Female, ethnicity-Hispanic, age, partnership status-married/partner, hypertension, coronary artery disease, mental health diagnosis, dementia, congestive heart failure, atrial fibrillation, acute kidney injury, stroke, peripheral vascular disease, diabetes, schizophrenia, depression, mild cognitive impairment, hospital visit 1 year prior, hospital visit 30 days prior, and count of risk factors.
Using a 1:1 and 1:2 propensity score match did not significantly change any of the outcomes, nor did removing any variable from the regression models that were used in the match. Our investigation of the most common reasons for readmissions among the intervention and control groups revealed that the most common reasons for readmission were similar in both groups. Specifically, hypertension, other symptoms involving abdomen and pelvis, and other disorders of urethra and urinary tract were among the top five most common reasons in both groups. It is important to note that these top 10 readmission diagnoses comprised only approximately 20% of all readmissions for both groups.
The review of 100 randomly selected intervention patient charts revealed that the most common issue identified by the clinical pharmacists were discrepancies between the EMR and the home visit medication list (83/100). The next most frequently identified issues were that patients were taking medications differently than prescribed (e.g., dose, timing) (52/100), that the patient experienced recent dizziness or falls in the past three months (46/100), that Beers Criteria medications were present on the patient’s medication list and that the pharmacist made a note to alter the use of the medication (29/100), and that potential drug-drug interactions were identified (27/100).
DISCUSSION
We found that this health coach and clinical pharmacist-driven intervention for older patients transitioning from acute hospitalization to home was associated with significantly lower predicted probabilities of being readmitted after 30 and 60 days, and a significantly lower predicted probability of experiencing an ED visit within 30 days. Based on our detailed review of the home visit and clinical pharmacist notes for a random sample of intervention patients, we hypothesize that the mechanisms by which this intervention reduced utilization included the clinical pharmacist’s ability to identify and address (1) discrepancies between medication lists, (2) patients taking medications differently than prescribed, (3) inappropriate prescribing and use of Beers Criteria medications, and (4) adjusting the dosage of or discontinuing medications that contribute to dizziness and falls. The pharmacist’s access to these multiple sources of information (i.e., those captured during a home visit in combination with a patient’s EMR medication list) potentially allowed for a more complete picture of the medication-related problems that commonly occur post-discharge. We believe the pharmacist’s ability to make recommendations to the PCP and operationalize approved changes to the patient’s medication lists played an important role in improving medication safety and bringing awareness of potential problems in a timely manner to both the patient and the PCP so as to prevent future complications.
To our knowledge, our study is the first evaluation of a care transitions program that modified the HomeMeds and CTI™ models by incorporating into the workflow clinical pharmacists who were embedded in primary care practices. Embedding clinical pharmacists in the primary care team where they have full access to the EMR can provide the crucially needed bridge between care at home and care in the health system. Improving the linkage between the hospital, home, and primary care setting following a care transition has been identified as an important component of the care transition process that is often missing25.
The results we observed compare similarly to the results reported in comprehensive care transition studies that use multiple care team members and that include numerous components. The heterogeneity in primary outcome measures and methods undertaken in these studies (e.g., composite measure for readmissions and ED visits, measuring outcomes using a time to event approach) makes it difficult to draw exact comparisons to our findings. Broadly, though, the direction and magnitude of our results are similar to these previous care transition studies. An evaluation of Project RED showed that the program was associated with a 30% reduction in a combined measure of hospital readmissions and ED visits16. Results from an RCT of the Transitional Care Model showed that intervention patients had a lower likelihood of readmission at 24 weeks compared with control patients (20.3% vs. 37.1%), and that intervention patients had a significantly longer time to first re-hospitalization18. In an RCT of the Coleman CTI™ model, the 30-day readmission rate for patients who received this intervention was 30% lower compared with patients who received usual care; at 180 days, the readmission rate for intervention patients was 17% lower compared with patients who received usual care20. The results from our study suggest that a program focused on medication management that is linked with a patient’s primary care team can achieve similar outcomes as more comprehensive and potentially more resource-intensive care transition programs.
Our study has several limitations. First, this study was conducted at a single urban health system and therefore may not be generalizable to all settings and patient populations. Second, due to the non-randomized nature of the study and despite our use of propensity score matching and regression techniques, we could not control for unobservable characteristics that may have influenced our primary outcome. Third, the data used do not allow us to know whether a patient was readmitted to a hospital outside of UCLA Health, though we suspect this occurred at a comparable rate for both intervention and control patients and therefore should not bias the differences in utilization observed.
In conclusion, this study demonstrates the potential benefits that health coaches and clinical pharmacists who have a full view of the patient’s medical history and medication list can offer to patients who transition between acute hospitalization and home. Further study of this type of intervention that links community-based team members to clinicians such as clinical pharmacists embedded within primary care teams, as well as investigation of the potential cost savings, will be important for continuing to improve outcomes following care transitions.
ACKNOWLEDGMENTS
Role of the Sponsor:
Dr. Andrea Sorensen was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number TL1TR001883 and TL1TR000121.
Dr. Carol M. Mangione received support from the University of California at Los Angeles (UCLA), Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly under National Institutes of Health (NIH)/NIA under Grant P30AG021684, by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH under Grant R18DK105464, the Centers for Disease Control and Prevention (CDC) under Grant U18DP006140 and from NIH/National Center for Advancing Translational Sciences UCLA Clinical and Translational Science Institute under Grant UL1TR001881. Dr. Mangione holds the Barbara A. Levey and Gerald S. Levey Endowed Chair in Medicine, which partially supported her work. Dr. Carol M. Mangione is a member of the United States Preventive Services Task Force (USPSTF). This article does not necessarily represent the views and policies of the USPSTF.
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review of approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: The authors report no conflicts of interest to this work.
Related presentations:
Transitional Care Between Hospital and Home: Using Health Coaches and Primary Care-based Clinical Pharmacists to Reduce Medication Errors, Improve Medication Management, and Reduce Utilization. Podium presentation at American Society of Aging Conference, April 2019.
REFERENCES
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. [DOI] [PubMed] [Google Scholar]
- 2.McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56(2):163–184. [DOI] [PubMed] [Google Scholar]
- 3.ElDesoky ES. Pharmacokinetic-pharmacodynamic crisis in the elderly. Am J Ther. 2007;14(5):488–498. [DOI] [PubMed] [Google Scholar]
- 4.Patterns of Medication Use in the United States, 2006: A Report from the Slone Survey. Slone Epidemiology Center at Boston University 2006. [Google Scholar]
- 5.Beers MH, Dang J, Hasegawa J, Tamai IY. Influence of hospitalization on drug therapy in the elderly. J Am Geriatr Soc. 1989;37(8):679–683. [DOI] [PubMed] [Google Scholar]
- 6.Tjia J, Bonner A, Briesacher BA, McGee S, Terrill E, Miller K. Medication discrepancies upon hospital to skilled nursing facility transitions. J Gen Intern Med. 2009;24(5):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau HS, Florax C, Porsius AJ, De Boer A. The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. Br J Clin Pharmacol. 2000;49(6):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165(16):1842–1847. [DOI] [PubMed] [Google Scholar]
- 9.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. [DOI] [PubMed] [Google Scholar]
- 10.Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323. [DOI] [PubMed] [Google Scholar]
- 11.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167. [DOI] [PubMed] [Google Scholar]
- 12.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. [DOI] [PubMed] [Google Scholar]
- 13.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147(11):755–765. [DOI] [PubMed] [Google Scholar]
- 14.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and Preventability of Adverse Drug Events Among Older Persons in the Ambulatory Setting. JAMA. 2003;289(9):1107–1116. [DOI] [PubMed] [Google Scholar]
- 15.Stone J, Hoffman G. Medicare Hospital Readmissions: Issues, Policy Options and PPACA. 2010. [Google Scholar]
- 16.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: The importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746–754. [DOI] [PubMed] [Google Scholar]
- 18.Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. Jama. 1999;281(7):613–620. [DOI] [PubMed] [Google Scholar]
- 19.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675–684. [DOI] [PubMed] [Google Scholar]
- 20.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828. [DOI] [PubMed] [Google Scholar]
- 21.Kwan JL, Lo L, Sampson M, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):397–403. [DOI] [PubMed] [Google Scholar]
- 22.Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy-led medication reconciliation programmes at hospital transitions: a systematic review and meta-analysis. J Clin Pharm Ther. 2016;41(2):128–144. [DOI] [PubMed] [Google Scholar]
- 24.Foundation PiC. HomeMeds Medication Safety Program. https://www.picf.org/homemeds/. [Google Scholar]
- 25.Huckfeldt P, Neprash H, Nuckols T. Transitional Care Management Services for Medicare Beneficiaries-Better Quality and Lower Cost but Rarely Used. JAMA internal medicine. 2018. [DOI] [PubMed] [Google Scholar]


