Abstract
Monocytes and monocyte-derived cells, including macrophages and dendritic cells, exhibit a diverse array of phenotypic states that are dictated by their surrounding microenvironment. These cells provide cues ranging from immunosuppressive to immunostimulatory cues to T cells, directing their activation and function. Solid tumors and atherosclerotic plaques represent two pathological niches with distinct immune microenvironments. While monocytes and their progeny possess a phenotypic spectrum found within both disease contexts, most within tumors are pro-tumoral and support evasion of host immune responses by tumor cells. In contrast, monocyte-derived cells within atherosclerotic plaques are usually pro-atherogenic, pro-inflammatory, and predominantly directed against self-antigens. Consequently, cancer immunotherapies strive to enhance the immune response against tumor antigens, whereas atherosclerosis treatments seek to dampen the immune response against lipid antigens. Insights into monocyte-T cell interactions within these niches could thus inform therapeutic strategies for two immunologically-distinct diseases. Here, we review monocyte diversity, interactions between monocytes and T cells within tumor and plaque microenvironments, how certain therapies have leveraged these interactions, and novel strategies to assay such associations.
Keywords: atherosclerosis, cancer, T cells, monocytes
Graphical Abstract
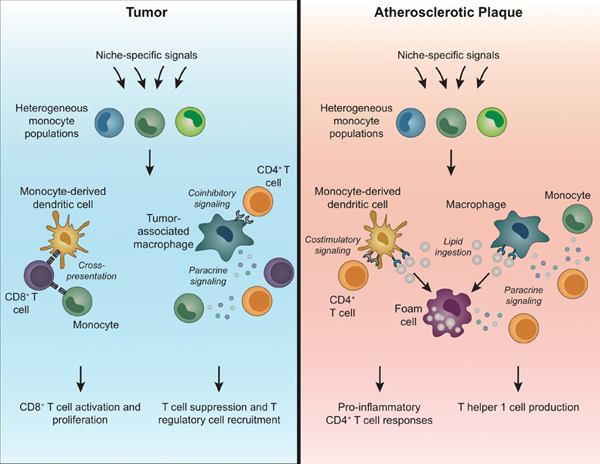
Summary Sentence:
This review summarizes our current understanding of monocyte heterogeneity and how monocytes differentially influence T cell responses within the tumor and plaque microenvironments.
Introduction
Monocytes and monocyte-derived cells, including macrophages and dendritic cells (DCs), display a wide range of phenotypic and functional diversity. This plasticity makes myeloid cells particularly responsive to their surrounding microenvironments and ideal for integrating and relaying complex signals [1]. Indeed, one of the primary functions of these cells is to instruct adaptive immune responses, with cues ranging from immunostimulatory to immunosuppressive. Here, we examine interactions between monocytes and T cells in two distinct pathologic niches that broadly represent opposite ends of the spectrum of immune activation: atherosclerotic plaques and tumors. Further, cancer and cardiovascular disease (CVD) are the two leading causes of mortality worldwide [2,3], highlighting the importance of understanding the immunobiology of these disease states.
In solid tumor cancers, mutations and epigenetic reprogramming dysregulate the expression of genes controlling normal cell growth, resulting in unrestrained cellular expansion. This is followed by malignant transformation and eventual metastasis of these cells to distal sites. Monocytes contribute to anti-tumoral immunity by recognizing and infiltrating established tumors, as well as preventing tumor metastasis [4–7]. However, cues from the tumor microenvironment can convert monocytes and their progeny into macrophages that aid tumor cells in evading cytotoxic T cells [8]. Increased numbers of CD8+ T cells in tumors are associated with positive outcomes in cancer [9]. Nevertheless, the events controlling monocyte recruitment and modulation of T cells within the tumor immune microenvironment (TIME) are still under investigation.
Conversely, the initiation of atherosclerotic plaque formation that frequently underlies CVD is characterized by deposition of lipoproteins within the vascular intima of arterial walls [10]. Subsequently, these lipoproteins undergo oxidation in the subendothelial space, which recruits inflammatory monocytes, macrophages, and DCs into the plaque via damage-associated molecular patterns [11,12]. Monocytes are one of the first immune cells recruited to the atherosclerotic plaque and they differentiate into either monocyte-derived macrophages or DCs once residing in this environment. In response to the inflammatory signaling initiated by monocyte-derived cells, CD4+ and CD8+ T cells infiltrate the plaque, where they further agonize plaque destabilization and increase the risk of myocardial infarction [12].
Monocytes are challenging to isolate from their macrophage/DC progeny because of their overlapping phenotypes, heterogeneity, and highly interdependent functions. These challenges are compounded in pathological settings, such as atherosclerosis and cancer, where new activation and differentiation states emerge [1]. For example, upon entry into lymph nodes and tissue, monocytes upregulate CCR7 and MHCII [13], which are also highly expressed on DCs and certain macrophages. While undifferentiated monocytes are abundant in lymph nodes and can present antigen, their relative contributions to T cell activation compared to macrophages and DCs remain unclear [13–18]. For example, compared to conventional DCs (cDCs), antigen-expressing monocytes elicit more robust T helper (Th) 1 and Th2 cell responses, during viral infection and airway inflammation, respectively [16,19]. Conversely, cDCs play dominant roles in activating both CD4+ and CD8+ T cells in murine tumor models [20,21]. Understanding the unique functions of monocytes in controlling T cell responses within diverse niches will help identify novel therapeutic targets for a range of diseases. In this review, we provide an overview of monocyte heterogeneity, the mechanisms coordinating interactions between particular subsets of monocytes and T cells, and how the distinctive plaque and tumor microenvironments impact these interactions. Understanding this crosstalk may be critical for improving treatment of cancer, CVD, and other diseases.
Monocyte Heterogeneity
Monocytes (Table 1) derive from a lineage-committed monocyte progenitor (cMoP; CD117+CD115+CD135−Ly6C+CD11b− in mice [22] and CD34+CD135+CD64+CLEC12A+ in humans [23]) that resides in the bone marrow (BM). Human monocytes universally express CD11b, the major histocompatibility class II (MHC-II) receptor HLA-DR, and CD86, whereas murine monocytes universally express CD115, CD11b, and CD64. Monocytes have traditionally been classified into classical, nonclassical, and intermediate subsets, and this framework has provided the basis for functional studies to date. Classical monocytes (CD14+CD16− in humans, Ly6ChiCCR2hiCX3CR1lo in mice) rapidly extravasate into tissues during infection and inflammation [24] and are required for replenishment of macrophage populations in tissues such as the skin, intestines, heart, and liver [25]. Nonclassical monocytes (CD14loCD16+ in humans, Ly6CloCCR2loCX3CR1hi in mice) patrol the endothelium during homeostasis and extravasate into tissues less frequently than classical monocytes [26], although they can be detected in the lungs [27] and kidneys [28]. Intermediate monocytes (CD14+CD16+ in humans, Ly6CintCCR2hiCX3CR1hi in mice) appear to represent a transition between classical and nonclassical subsets, contributing to the frequent contamination seen in monocytes during conventional flow cytometry [29,30]. Still, the contribution of intermediate monocytes to immune responses remains unclear, despite their increased frequency in patients with advanced CVD [31].
Table 1.
Monocyte Subsets and Potential T cell interactions in Tumor and Atherosclerotic Plaque Niches
| Monocyte Subset | Mouse Markers | Human Markers |
|---|---|---|
| Classical monocytes | Ly6ChiCCR2hiCX3CR1lo; (CD115+CD11b+CD64+) |
CD14+CD16−, (CD11b+HLA-DR+ CD86+) |
| Nonclassical monocytes | Ly6CloCCR2loCX3CR1hi (CD115+CD11b+CD64+) |
CD14loCD16hi, (CD11b+HLA-DR+ CD86+) |
| Tumors | ||
| Monocyte | Function | T cell interaction |
| Classical monocytes | Ly6ChiCD103+ monocyte-derived cells cross-present antigens with B16 [70]. | Antigen Presentation |
| F4/80hiCD24+ monocyte-derived cells cross-present antigens with B16 [69]. | ||
| Upregulate macrophage-associated genes at 5 days post injection into mammary tumor-bearing PyMT mice [47]. | ||
| Monocyte-derived TAMs from mammary tumors display increased antigen presentation transcripts [56]. | ||
| Monocytes from renal carcinoma, breast cancer, and endometrial cancer individuals display increased TNF, IL1B, IL6, and CCL3 [55]. | Paracrine Signaling | |
| Recruit Tregs via CCR5 in SubQ RMA-S lymphoma tumors [59]. | Treg Recruitment | |
| Employ CD40 [60], CD86 [61], and arginase-1 [62] to expand Treg numbers | ||
| Nonclassical monocytes | Display increased CCL3, CCL4, and CCL5 within lung tumor metastases [7]. | Lymphocyte Recruitment |
| Atherosclerotic plaques | ||
| Monocyte | Function | T cell interaction |
| Classical monocytes | Differentiate into CD11bhiCD11hi cells expressing CD80 and CD86; Some express F4/80 [86–90]. |
Antigen Presentation |
| Macrophages secrete TNF-α, IL-12, iNOS, and IL-6. [98,99] | Paracrine Signaling | |
| Macrophages and DCs take up cholesterol and become foam cells [103,104,105]. | DC-derived foam cells elicit Th1 CD4 T cells [106,107], potentially contributing to plaque rupture. | |
| Nonclassical monocytes | Accumulate in vessel wall and under hypercholesterolemic conditions, display increased CD11c positivity [91–93]. | Antigen Presentation (Possible acquisition of DC-like phenotype) |
Work in both humans and mice suggests that classical monocytes develop in the BM and egress into peripheral blood in a CCR2-dependent manner [24]. Classical monocytes circulate in the periphery for approximately 1 day before entering tissues and then either differentiate or undergo apoptosis [32,33]. In contrast, nonclassical monocytes circulate for at least 2 days in mice (with a half-life of 2.2 days) and 7 days in humans [32,33]. Most nonclassical monocytes are thought to derive directly from classical monocytes [32,34]. However, the cues regulating the conversion of classical monocytes into nonclassical monocytes remain only partially characterized. Our laboratory has demonstrated that development of nonclassical monocytes requires the orphan nuclear receptor NR4A1, which is regulated by a superenhancer region specifically active in these cells [5,6]. NR4A1 expression is controlled by the transcription factors KLF2 and C/EBPβ [5], but additional studies are needed to identify the signals driving this genetic program.
High-dimensional technologies, such as single-cell RNA sequencing (scRNA-Seq) and mass cytometry by time-of-flight (CyTOF), have largely confirmed established monocyte subsets in humans and mice [34–36], while also identifying novel populations. For example, our laboratory recently employed CyTOF to identify a subset of nonclassical CD16+ monocytes that expresses the 6-Sulfo LacNAc (Slan) carbohydrate modification of P-selectin glycoprotein ligand-1 (PSGL-1) in humans [29]. CD16+Slan+ monocytes have an increased capacity for efferocytosis compared to CD16+Slan− monocytes and are positively correlated with CVD severity [29]. In mice, scRNA-Seq has identified a subset of intermediate monocytes enriched for DC-related genes, including CD209a [34]. Whether this subset is related to the previously-identified CD209a-expressing Ly6C+ monocytes that differentiate into DCs following exposure to GM-CSF [37], or the CD74-expressing “monoDC” population detected in lung tumors [36], requires further investigation. High-dimensional technologies have revolutionized the profiling of immune cells, including simultaneous measurement of transcriptomes and epitopes [38], single cell assessment of chromatin accessibility [39], and in-depth metabolomic surveys [40]. Moreover, multiplexed spatial cytometry [41] and novel bioinformatics approaches to identify putative cellular interactions [42] have revealed new insight into the distribution of immune cells in tissues and revealed cellular networks. These and other high-dimensional approaches will likely continue to reveal new monocyte subsets and their interactions with cells in the surrounding microenvironment.
Monocyte-T Cell Interactions in Cancer
Monocytes and their progeny regulate the TIME by directly interacting with tumor cells, fibroblasts, endothelial cells, and other leukocytes, including T cells [43]. In a murine model of lung metastasis, both classical and nonclassical monocytes extravasate towards metastatic sites within hours of tumor cell seeding [7,44]. While monocytes initially contribute anti-tumoral functions [45], the TIME often promotes the differentiation of these cells into pro-tumoral tumor-associated macrophages (TAMs) [46]. Classical monocytes are recruited to mammary tumors and upregulate their expression of macrophage-associated markers such as F4/80, MHCII, and CD11c within 5 days after intravascular transfer [47]. Recruitment of Ly6Chi monocytes from peripheral blood into the tumor, and the subsequent generation of TAMs, depends on the CCR2/CCL2 signaling axis [48], with M-CSF (CSF-1) also contributing to this process [49].
Tumor expression of CCL2 negatively correlates with intratumoral infiltration of CD8+ T cells in hepatocellular carcinoma patients [50] and, in combination with CD8, stratifies survival outcomes in pancreatic cancer patients [9]. Inhibition of CCR2 or CSF1R reduces TAM accumulation, increases CD8+ T cell abundance, and reduces tumor burden in mouse models of pancreatic and liver cancer [50,51]. Furthermore, the anti-tumoral activity of CCR2 inhibition in mice bearing Hepa1–6 liver tumors requires the presence of CD8+ T cells [50]. Interestingly, a higher frequency of classical CD14+ monocytes in peripheral blood is associated with increased T cell activation and improved survival in melanoma patients [52]. The precise relationship between monocytes and T cells likely depends on the immunostimulatory versus immunosuppressive capacity of monocytic cells within a specific TIME. Monocytic myeloid-derived suppressor cells (M-MDSCs) are defined by their ability to suppress T cell activation and proliferation [53]. While M-MDSCs share expression of markers such as CD14, CD33, and CD11b with monocytes, whether monocytes and M-MDSCs are distinct cell types remains unclear [54]. A better understanding of the relationship between monocytes, M-MDSCs, and other TIME myeloid cells
Paracrine Interactions
Monocytes isolated from the blood of renal cell carcinoma [55], breast cancer [56], and endometrial cancer patients [56] exhibit distinct transcriptional signatures, compared to monocytes from healthy individuals. In renal cell carcinoma specifically, transcripts associated with downstream activation of T cells, such as TNF, IL1B, IL6, and CCL3, are elevated [55]. TAMs derived from mammary tumors display increased expression of transcripts associated with antigen presentation, immune activation, and T cell costimulation [56]. Given that these transcripts are not similarly upregulated in cancer-derived monocytes, these gene expression programs may only be imprinted once monocytes begin maturation within the TIME. Blockade of monocyte recruitment via CCR2 inhibition reduces macrophage-based production of the cytokines IL-6, IL-13, IL-15, and TNFɑ that can differentially impact T cell responses [50,57,58]. Nonclassical monocytes within lung tumor metastases generate chemokines such as CCL3, CCL4, and CCL5, which are involved in lymphocyte recruitment to tumor sites [7] (Table 1). How T cells integrate and respond to diverse monocyte-derived signals will be important to understanding how to therapeutically target these interactions.
M-MDSCs display a monocyte-like phenotype and recruit T regulatory (Tregs) cells to lymphoma tumors in a CCL5/CCR5-dependent manner, which facilitates tumor growth [59] (Figure 1, Table 1). Consequently, monocyte-derived chemokines such as CCL5 may recruit both pro- and anti-tumoral T cell populations in a context-specific manner. Immunosuppressive M-MDSCs employ CD40 [60], CD80 [61], and arginase-1 [62] to expand Treg numbers and suppress anti-tumoral T cells at the tumor site, with accumulation of Tregs predicting poor prognosis in non-Hodgkin’s lymphoma and fibrosarcoma [63,64]. Targeting arginase has been explored in early phase clinical trials that demonstrated a small molecule inhibitor is well-tolerated and displays efficacy in inhibiting arginase in solid tumor patients [65]. Interestingly, Tregs also influence the fate of intratumoral monocytes by inhibiting their migration and/or differentiation and by eliciting an alternatively-activated phenotype via the anti-inflammatory cytokine IL-10 [66,67]. These observations warrant the continued exploration of Treg-monocyte interactions and potential for therapeutic targeting within the TIME.
Figure 1. Crosstalk between monocytes and T cells in the tumor niche.
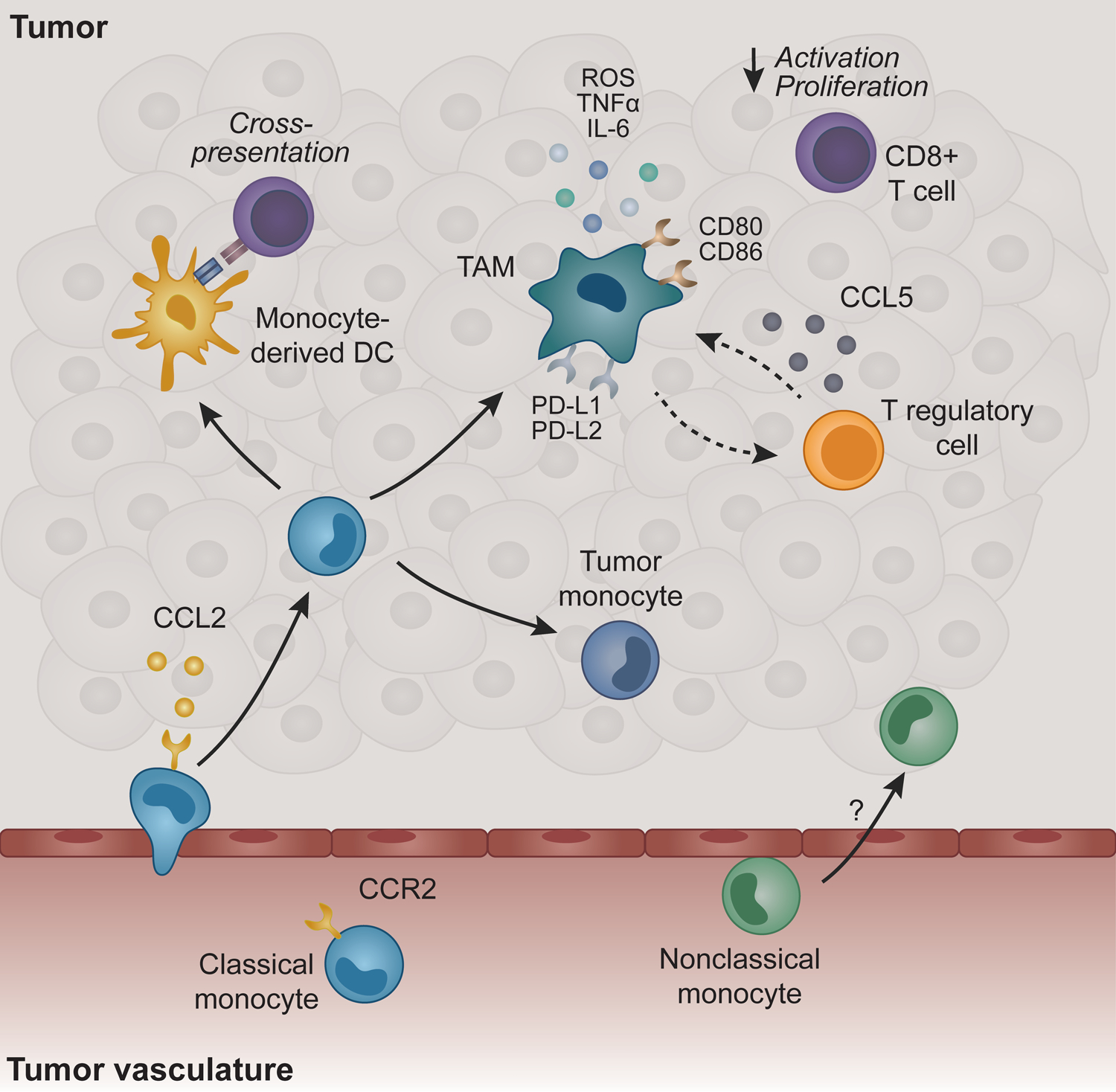
Classical Ly6Chi monocytes recruited through CCR2/CCL2 are a primary source of tumor-associated macrophages (TAMs) and dendritic cells (DCs). TAMs are primarily pro-tumoral in part through local immune suppression characterized by the production of cytokines that inhibit CD8+ T cell activation/proliferation and recruit T regulatory cells that further dampen innate and adaptive immune responses. Costimulatory and coinhibitory molecules are upregulated on TAMs compared to monocytes, which may play a role in modulating T cell responses. Monocytes and monocyte-derived DCs cross-present antigen to activate CD8+ T cells, particularly in the absence of conventional DCs. Nonclassical Ly6Chi monocytes are important for anti-tumoral immunity in metastatic lesions, but their function in solid tumors remains largely unknown.
Antigen Presentation
Cross-presentation is particularly important for immune responses against tumor cells that express a high number of mutations but lack the machinery necessary for priming T cells directly. While cDCs are believed to be primarily responsible for both cross-presenting tumor antigens to CD8+ T cells and MHCII antigen presentation to CD4+ T cells [21,68], evidence suggests that other monocyte-derived cells also process and present tumor-derived antigens [69,70]. For example, F4/80hiCD24+ cells within B16 melanoma and intestinal tumors cross-present tumor antigens and are CCR2-dependent, suggesting they derive from a monocytic origin [69]. In Pten−/−Foxp3-Cre mice bearing B16 tumors, a population of Ly6C+CD103+ monocyte-derived cells cross-present antigens and re-activate anergic CD8+ T cells [70] (Figure 1, Table 1).
Maturation into macrophages/DCs is likely required for cross-presentation, as monocytes derived from human lung tumors are unable to present tumor antigens, while macrophages from the same tumors can cross-present and stimulate IFNɣ production by antigen-specific effector T cells [71]. Interestingly, tumor antigen in metastatic lung sites is redirected from macrophages to cDCs in CCR2-deficient mice, indicating that different APCs may compete for tumor antigen [44]. Additionally, monocytes may most effectively contribute to anti-tumoral immunity, especially in TIMEs with sufficient numbers of cDCs, by transporting antigen to lymphoid organs before transfer to APCs [72].
Costimulatory and Coinhibitory Molecules
Myeloid cells impact the strength of T cell receptor signaling and downstream T cell responses by surface expression of costimulatory and coinhibitory molecules [73]. In peripheral blood, the costimulatory molecule CD86 is universally expressed across monocyte subsets, while CD80 is lowly expressed at homeostasis [29]. In mice, expression of the coinhibitory molecule programmed death ligand 1 (PD-L1) is restricted to nonclassical Ly6Clo monocytes at homeostasis [74], but appears to be broadly induced in both classical Ly6Chi monocytes and myeloid progenitors in mice bearing B16 melanoma tumors [75]. Monocytes upregulate both the PD-L1/2 and CD80/CD86 pathways as they enter the TIME and differentiate into TAMs [76]. Tumor-derived RNA may serve as one of the signals regulating expression of coinhibitory molecules in monocytes, as RNA-loaded exosomes derived from leukemic cells increase PD-L1 expression in human monocytes [77]. Interestingly, the receptor for PD-L1/2, programmed cell death protein-1 (PD-1), is also absent from monocytes during homeostasis, but induced in tumor-bearing mice [75]
CD28 expressed on naive T cells binds to CD80 and CD86 expressed on APCs, and interactions between CD28 with CD80/CD86 are critical for facilitating memory and effector T cell formation [78]. Costimulation by CD86 generally promotes T cell activation, but CD86 can also inhibit this process through interaction with CTLA-4. In monocyte-derived TAM precursors recruited to lung metastases, CD86 suppresses CD8+ T cell-mediated tumor cell cytotoxicity through CTLA-4 [76]. Consequently, anti-CTLA-4 immunotherapy (currently approved for treatment of metastatic melanoma and renal cell carcinoma [79]) may act in part by interfering with interactions between immunosuppressive monocyte-derived cells and T cells, although this requires further investigation.
Recent work demonstrated that increased PD-1 expression on myeloid cells in tumor-bearing mice leads to enhanced production of myeloid progenitors and MDSCs that suppress T cell responses [75]. Immune checkpoint inhibitors targeting PD-1 and PD-L1/2 have been highly successful in subsets of non-small cell lung cancer, renal cell carcinoma, melanoma, and other solid tumor patients [79], but whether these therapies inhibit monocyte-T cell interactions remains unclear. Melanoma patients with higher baseline levels of classical monocytes display superior clinical responses and survival following anti-PD-1 treatment [52], providing evidence that monocyte-T cell interactions may contribute to therapies targeting PD-1:PD-L1/2 signaling. Additionally, monocytes can express OX40L, CD137L, and CD40 [80–82], which are currently under investigation as drug targets for cancer immunotherapy. Multiple Phase I and Phase II clinical trials are underway to examine the safety and efficacy of CD40 monoclonal antibodies in solid tumors [83]. Whether these molecules regulate crosstalk between monocytes and T cells in cancer, and the extent to which these interactions may be targeted clinically to increase anti-tumoral immunity will be of interest as further research is performed in this area.
Monocyte-T Cell Interactions in Atherosclerosis
Classical Ly6Chi monocytes (Table 1, Figure 2) represent the first immune cell population to arrive at the atherosclerotic plaque via recruitment by CCR2-CCL2, CX3CR1-CX3CL1, and CCR5-CCL5 signaling [84,85]. Ly6Chi monocyte frequencies double every month in atherosclerotic Apolipoprotein E-deficient (ApoE−/−) mice fed a Western (high-cholesterol) diet [84]. Once recruited to the vessel wall, Ly6Chi monocytes differentiate into CD11bhiCD11chi cells and upregulate the costimulatory molecules CD80 and CD86 [86–89], suggesting acquisition of APC capacity [90]. Many of these cells also express F4/80, indicating that plaque monocytes may differentiate into both DCs and macrophages.
Figure 2. Crosstalk between monocytes and T cells in the atherosclerotic plaque niche.
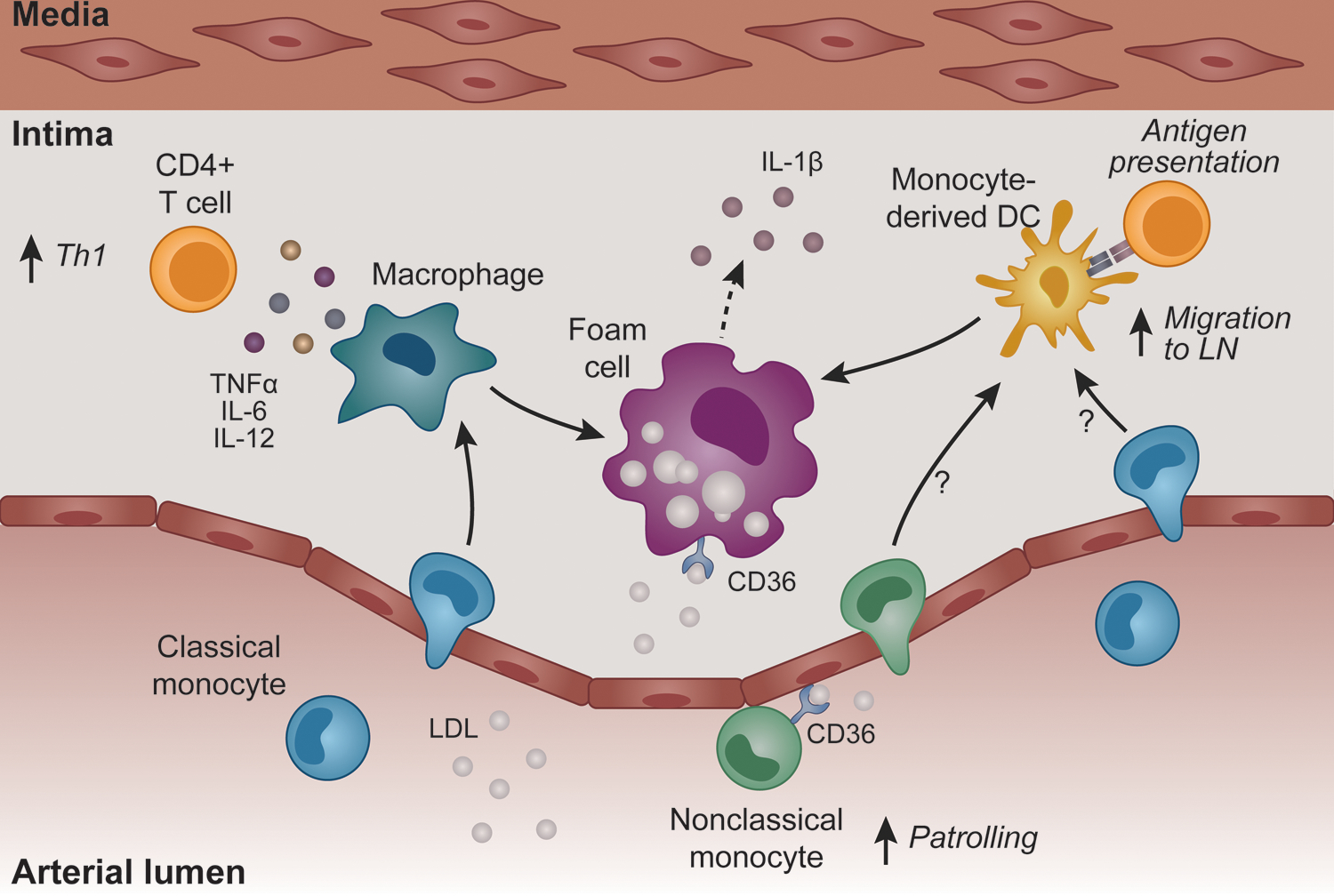
Monocytes are the first immune cell type recruited to the atherosclerotic plaque. Ly6Clo monocytes patrol the endothelium, yet whether these monocytes influence T cell responses in atherosclerosis is unknown. Ly6Chi monocytes differentiate into macrophages and DCs that are CD11b+CD11c+, characterized by high expression of the costimulatory molecules. DCs, and likely monocytes, can egress from the plaque to the lymph nodes and present antigenic peptides to CD4+ T cells. However, as the atherosclerotic process progresses, DCs are maintained within the plaque. Macrophages secrete pro-inflammatory cytokines that increase Th1 differentiation. Macrophages and DCs also accumulate lipids and become foam cells that comprise the necrotic core and secrete IL-1β. DC-derived foam cells retain antigen presentation capabilities and drive pro-atherogenic CD4+ T cell responses.
In contrast to Ly6Chi monocytes, nonclassical Ly6Clo monocytes (Table 1, Figure 2) accumulate within the vessel wall via CCR5, but at a considerably slower rate [84,91]. Under hypercholesterolemic conditions, Ly6Clo monocytes display increased CD11c surface expression, indicating potential acquisition of a DC-like phenotype [91–93]. Global knockout of Nr4a1 within ApoE−/− mice results in loss of nonclassical Ly6Clo monocytes and enhances atherosclerosis disease severity, which is accompanied by an increase in pro-inflammatory macrophages [94]. Western-diet feeding also increases the patrolling behavior of Ly6Clo monocytes along the vascular endothelium in a CD36− and oxidized low-density lipoprotein (OxLDL)-dependent manner [95]. Unpublished data (Marcovecchio P.M.) from our laboratory suggests that disrupting patrolling behavior may impact endothelial cell homeostasis, which could have broad implications for leukocyte recruitment into the plaque [95]. Whether nonclassical monocytes interact with and influence atherogenic T cell responses remains unclear; however, recent evidence indicates that patrolling monocytes are able to present antigens within the vasculature to effector CD4+ T cells [96].
The persistence of undifferentiated monocytes within the plaque has yet to be determined, in part because the aforementioned studies have not included markers for distinguishing monocytes from macrophages and DCs. Thus, we now also focus on the interactions of T cells with monocyte-derived macrophages and DCs, as this constitutes the majority of atherosclerosis research.
Paracrine Interactions
Monocyte-derived macrophages (F4/80hi CD11b+CD11c+; Table 1, Figure 2) represent the most abundant immune cell type within atherosclerotic plaques [97]. Following differentiation from monocytes, macrophages secrete pro-inflammatory cytokines, such as TNFɑ, IL-12, IL-6, and iNOS, which promote differentiation of naive CD4+ T cells into Th1 cells [98,99]. Th1 CD4+ T cells cell are pro-atherogenic, as deletion of the genes encoding Th1-associated factors IFNɣ or T-bet protects against atherosclerosis [100]. Plaque-associated macrophages instruct T cells to secrete pro-atherogenic cytokines and recruit additional leukocytes into the atherosclerotic lesion [101,102].
As atherosclerosis progresses, monocyte-derived macrophages ingest excess lipids and eventually become foam cells (reviewed in [103]). Foam cells contribute to the mass of the atherosclerotic plaque and are directly implicated in the development of the necrotic core, a hallmark of plaques vulnerable to rupture [97]. In addition to macrophages, monocyte-derived DCs can ingest lipids and differentiate into foam cells that retain their ability to prime T cell responses [104,105] (Figure 2). Cholesterol uptake by macrophages and DCs is characterized by activation of the inflammasome, concomitant with increased expression of IL-1β, and DC-derived foam cells have been implicated in the generation of pro-atherogenic Th1 CD4+ T cells [106,107]. Whether macrophage-derived foam cells retain their antigen presentation capabilities in a similar fashion to DCs and influence T cell responses is an unanswered question that will elucidate interactions between monocyte-derived cells and T cells within the plaque.
Monocyte-T cell interactions are not unidirectional, as T cells have a reciprocal impact on monocyte development in cardiovascular disease. A recent study demonstrated that CD8+ T cells promote medullary monocyte production and CD8+ T cell depletion reduces atherosclerosis and is accompanied by attenuated Ly6Chi monocytes in blood, BM, and spleen, and lesional macrophages with atherosclerotic plaques [108]. These results indicate that CD8+ T cells modulate atherosclerosis severity by controlling myelopoiesis, similar to acute viral infection, and implicate GM-CSF as a key factor [108,109].
Paracrine signaling that impacts monocyte-T cell interactions has not been explicitly targeted in CVD. Instead, targets for CVD therapeutics have largely focused on lipids, as free and esterified cholesterol are robustly identified in plaques, and elevated blood cholesterol levels, particularly LDL-C, predict cardiovascular events [110,111]. Indeed, the inflammatory response was originally considered a byproduct of cholesterol accumulation in vessels and attributed to smooth muscle cell proliferation [110,112,113]. IL-1β, a cytokine synthesized by blood-derived monocytes, macrophages, and DCs, which provides essential cues for T cell activation, is the focus of a recent clinical trial to lessen atherosclerotic disease [114,115]. The double-blind, placebo Canakunimab Anti-inflammatory Thrombosis Study (CANTOS) treated individuals with high risk for myocardial infarction with the IL-1β monoclonal antibody canakinumab [116]. Canakinumab produces a 15% decrease in the primary composite endpoint (non-fatal myocardial infarction, stroke, CVD death) compared to placebo-treated individuals, which is accompanied by 60% reductions in C-Reactive Protein, a risk factor for future CVD events [116]. Interestingly, IL-1β inhibition is especially effective within individuals with lung cancer comorbidity, with a 77% decrease in fatality [117–119], highlighting the need to explore integrated therapeutic strategies.
Antigen Presentation
Interactions between T cells and APCs occur in the mouse aorta and elicit production of TNFɑ and IFNɣ [120–126]. Monocyte-derived DCs capture antigens in the vessel wall and traffic to draining lymph nodes for antigen presentation to T cells [127–129]. However, as atherosclerosis progresses, DCs are retained within the atherosclerotic plaque, where they prime effector T cells [130,131]. This is purportedly due to increased expression of chemokines such as CCL19, CCL21, P-Selectin, and V-CAM1 [132–134]. The cellular mechanisms of antigen presentation have primarily focused on DCs in the context of atherosclerosis, and whether these interactions occur between monocytes and T cells remains an open question.
The peptide antigens presented by APCs to T cells in atherosclerosis remain largely unknown, but numerous candidates have been proposed [135]. The best studied of these is LDL and its core protein ApoB [136]. CD4+ T cells displaying reactivity to oxLDL are located within human plaques [137,138]. In mice immunized with human oxLDL, MHC class II-restricted T cell clones reactive to native LDL possess a T cell receptor with the β chain TRBV31 and blockade of this receptor protects against atherosclerosis [139]. In addition to oxLDL, HSP60 [140] and ApoB100 [139] are also essential antigens for T cell activation during atheroprogression.
There are conflicting reports concerning the importance of cross-presentation by DCs in the development of atherosclerosis. Depletion of CD8ɑ+ and CD103+ cDCs via Batf3 deficiency has no effect on atheroprogression, despite inhibiting cross-presentation [141]. Moreover, atherosclerosis remains unchanged with deficiency of Antigen Peptide Transporter 1 (TAP-1), an essential component of the MHC class I presentation complex that participates in cross-presentation [142]. While these reports indicate that cross-presentation is dispensable for atherosclerosis, these studies do not rule out compensatory cross-presentation by monocytes, which occurs in cancer [70]. Therefore, further efforts should examine whether cross-presentation by monocytes occurs in atherosclerosis.
Costimulatory/Coinhibitory Molecules
Interactions between costimulatory molecules expressed on monocyte-derived cells and T cells are essential for atherosclerotic progression [143–146] and have been extensively reviewed elsewhere [147]. Mice deficient in CD80 and CD86 show marked reduction of atherosclerotic severity, but increased synthesis of the pro-atherogenic cytokine IFNɣ [148]. Expression of CD80 and CD86 is enhanced on monocyte derived-DCs from individuals with CVD [149]. Additionally, while the CD80/CD86 receptor CD28 was initially proposed as a promising therapeutic target, a CD28 superagonist antibody elicits cytokine release syndrome due to increased memory and effector T cell formation in healthy individuals [150].
PD-1 is upregulated in aorta-infiltrating T cells of Ldlr−/− mice fed a cholesterol diet and expressed in human carotid plaque-infiltrating T cells. [151,152]. Interestingly, this transcriptional signature is similar to that of exhausted T cells in the TIME [152]. Myocardial infarction elicits an influx of PD-L1+ nonclassical monocytes into pericardial tertiary lymphoid organs within 5 days [74]. PD-L1 is localized at the interface of contacting nonclassical monocytes and T cells, and promotes T cell survival [74]. Whether PD-L1-expressing nonclassical monocytes are recruited to periaortic lymph nodes during atherosclerosis progression and impact atherosclerosis severity remains unknown. However, high fat diet-feeding of PD-L1- or PD-L2-deficient Ldlr−/− mice increases atherosclerosis and aggravates effector CD8+ T cell responses, purportedly due to enhanced antigen presentation by DCs [153]. Thus, immune checkpoint blockade may have unintended consequences in treating individuals with CVD and cancer, and conversely, PD-1 agonists may prove beneficial in CVD [154].
Additional interactions between costimulatory molecules, such as CD40/CD40L and OX40/OX40L may mediate interactions between antigen-presenting monocytes and T cells. CD40 upregulation on APCs activates CD40L (CD154)-expressing CD4+ T cells and increases production of pro-atherogenic cytokines [155–157]. CD40/CD40L interaction also increases CD80 and CD86 expression on APCs and vice-versa [144]. Clinical trials targeting interactions between CD40 and CD40L in atherosclerosis were discontinued due to thrombotic complications; nevertheless, an antisense oligonucleotide approach targeting CD40 may represent a promising approach [149,158]. Signaling by OX40L expressed on myeloid cells is essential for memory T cell survival and implicated in atheroprogression [145]. Furthermore, global OX40L overexpression and deletion increases and reduces fatty streak formation, respectively, in high-fat diet-fed mice [145,146]. Plaque-resident CD4+ T cells and macrophages express OX40 and OX40L, respectively [145], and accumulation of TNFRSF4 and TNFSF4 transcripts, which encode OX40 and OX40L, correlate with CVD risk [159]. While there have been no clinical trials on these targets to date, an anti-OX40 antagonistic antibody that elicits increased OX40+ T cells is currently under trial for individuals with solid tumors [160].
Concluding Remarks
This review highlights the spectrum of monocyte and monocyte-derived macrophage phenotypes that are present within tumors and the atherosclerotic plaque. Monocytes are uniquely influenced by these niche microenvironments, with reparative and pro-inflammatory phenotypes prevailing in the tumor and plaque, respectively. Although T cells are influenced by diverse cell types other than monocytes, including endothelial cells, NK cells, smooth muscle cells, and neutrophils [135,161], monocytes and their progeny provide non-redundant cues that impact T cell-mediated immunity. Comparing the monocyte-T cell interactions within these divergent microenvironment niches in cancer and atherosclerosis provide essential insight regarding the dysregulated immune response in these diseases.
High-dimensional immunophenotyping via CyTOF and scRNA-Seq has uncovered striking heterogeneity in monocytes and T cells [34–36], paving the way for functional studies to examine interactions between new subsets. These methods have been championed in the cancer field, revealing novel insight into potential immunotherapeutic targets in the cancer setting [36,52,162]. Still, further study is needed in atherosclerosis to interrogate potential interactions between monocytes and T cells and the outputs of these interactions in atherosclerosis [152,163–166]. Recently, the algorithm Clustergrammer [162] was employed to predict the different potential macrophage-T cell interactions within atherosclerotic plaques that bring about either symptomatic or asymptomatic states in CVD patients [152]. Plaque-resident macrophages from asymptomatic individuals express IL1B, predicted to bind to IL1RAP, which encodes a component of the IL-1 receptor complex expressed by T cells [152]. In contrast, T cells within plaques from symptomatic patients express ligand-encoding transcripts predicted to elicit reparative macrophages [152]. Bioinformatic tools such as this, employed alongside multiplexed spatial immune profiling of tumors and atherosclerotic plaques, will be powerful tools to shed light on the monocyte-T cell interactions.
Improved fate-mapping studies to pinpoint the specific effects of monocyte-derived APCs on T cells versus DC subsets from independent lineages could greatly benefit atherosclerosis research [32,34,167]. However, fluorescence-based mouse transgenics, such as the Cx3cr1gfp/+ strain, do not allow monocytes to be clearly separated from macrophages and DCs [167]. These technical limitations have contributed to the limited knowledge regarding whether monocyte-derived cells present lipid antigens within the atherosclerostic plaque.
Ultimately, insights on the influence of monocytes and their progeny on T cell responses in the setting of cancer and atherosclerosis are of great interest, as they will likely reveal novel therapeutic targets for a range of diseases. By comparing and contrasting the monocyte-T cell interactions within these distinct niches in cancer and atherosclerosis, we gain essential insights regarding the dysregulated immune response in these diseases. Importantly, these insights will identify new targets of innate and adaptive immunity that can improve current therapies for atherosclerosis and cancer.
Acknowledgements
The authors thank the members of the Hedrick laboratory for thoughtful discussions on this review. This work is supported by National Institutes of Health R01 CA202987, R01 HL134236, P01 HL136275, and U01 CA224766 (all to C.C.H.), T32 AI125279–01, F32 HL146069–01A1 and AHA 19POST34450020 ( to L.E.P).
Abbreviations
- APC
Antigen-presenting cell
- BM
Bone marrow
- CANTOS
Canakunimab Anti-inflammatory Thrombosis Study
- cDC
Conventional dendritic cell
- CITE-Seq
Cellular indexing of transcriptome and epitopes by sequencing
- CyTOF
Cytometry by Time-Of-Flight
- CVD
Cardiovascular disease
- DC
Dendritic cell
- LDL
Low-density lipoprotein
- MHC
Major histocompatibility complex
- MDSC
Myeloid-derived suppressor cell
- oxLDL
Oxidized low-density lipoprotein
- PD-1
Programmed cell death protein-1
- PD-L1/2
Programmed cell death ligand-1/2
- PSGL1
P-selectin glycoprotein ligand-1
- scRNA-Seq
Single-cell RNA-sequencing
- TAM
Tumor-associated macrophage
- TIME
Tumor immune microenvironment
- Slan
6-Sulfo LacNac
Footnotes
Conflict of Interest Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17: 34–40. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141: e139–e596. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70: 7–30. [DOI] [PubMed] [Google Scholar]
- 4.Narasimhan PB, Marcovecchio P, Hamers AAJ, Hedrick CC. Nonclassical Monocytes in Health and Disease. Annual Review of Immunology. 2019. pp. 439–456. doi: 10.1146/annurev-immunol-042617-053119 [DOI] [PubMed]
- 5.Thomas GD, Hanna RN, Vasudevan NT, Hamers AA, Romanoski CE, McArdle S, et al. Deleting an Nr4a1 Super-Enhancer Subdomain Ablates Ly6C Monocytes while Preserving Macrophage Gene Function. Immunity. 2016;45: 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350: 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19: 3404–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5: 56. [DOI] [PubMed] [Google Scholar]
- 11.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104: 503–516. [DOI] [PubMed] [Google Scholar]
- 12.Ketelhuth DFJ, Hansson GK. Adaptive Response of T and B Cells in Atherosclerosis. Circulation Research. 2016. pp. 668–678. doi: 10.1161/circresaha.115.306427 [DOI] [PubMed]
- 13.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, et al. Minimal Differentiation of Classical Monocytes as They Survey Steady-State Tissues and Transport Antigen to Lymph Nodes. Immunity. 2013. pp. 599–610. doi: 10.1016/j.immuni.2013.08.007 [DOI] [PMC free article] [PubMed]
- 14.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4: e4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, et al. Inflammatory Monocytes Facilitate Adaptive CD4 T Cell Responses during Respiratory Fungal Infection. Cell Host & Microbe. 2009. pp. 470–481. doi: 10.1016/j.chom.2009.10.007 [DOI] [PMC free article] [PubMed]
- 16.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and Monocyte-Derived CD11b Dendritic Cells Initiate and Maintain T Helper 2 Cell-Mediated Immunity to House Dust Mite Allergen. Immunity. 2013. pp. 322–335. doi: 10.1016/j.immuni.2012.10.016 [DOI] [PubMed]
- 17.Randolph GJ. Differentiation of Monocytes into Dendritic Cells in a Model of Transendothelial Trafficking. Science. 1998. pp. 480–483. doi: 10.1126/science.282.5388.480 [DOI] [PubMed]
- 18.León B, López-Bravo M, Ardavín C. Monocyte-Derived Dendritic Cells Formed at the Infection Site Control the Induction of Protective T Helper 1 Responses against Leishmania. Immunity. 2007. pp. 519–531. doi: 10.1016/j.immuni.2007.01.017 [DOI] [PubMed]
- 19.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nature Immunology. 2009. pp. 394–402. doi: 10.1038/ni.1707 [DOI] [PMC free article] [PubMed]
- 20.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the Tumor Myeloid Compartment Reveals Rare Activating Antigen-Presenting Cells Critical for T Cell Immunity. Cancer Cell. 2014. pp. 638–652. doi: 10.1016/j.ccell.2014.09.007 [DOI] [PMC free article] [PubMed]
- 21.Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4 T Cell Immunity. Cell. 2019;177: 556–571.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko A-C, Krijgsveld J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14: 821–830. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura S, Onai N, Miya F, Sato T, Tsunoda T, Kurabayashi K, et al. Identification of a Human Clonogenic Progenitor with Strict Monocyte Differentiation Potential: A Counterpart of Mouse cMoPs. Immunity. 2017. pp. 835–848.e4. doi: 10.1016/j.immuni.2017.04.019 [DOI] [PubMed]
- 24.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7: 311–317. [DOI] [PubMed] [Google Scholar]
- 25.Guilliams M, Scott CL. Does niche competition determine the origin of tissue-resident macrophages? Nat Rev Immunol. 2017;17: 451–460. [DOI] [PubMed] [Google Scholar]
- 26.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317: 666–670. [DOI] [PubMed] [Google Scholar]
- 27.Schyns J, Bai Q, Ruscitti C, Radermecker C, De Schepper S, Chakarov S, et al. Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat Commun. 2019;10: 3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. Nr4a1-Dependent Ly6Clow Monocytes Monitor Endothelial Cells and Orchestrate Their Disposal. Cell. 2013. pp. 362–375. doi: 10.1016/j.cell.2013.03.010 [DOI] [PMC free article] [PubMed]
- 29.Hamers AAJ, Dinh HQ, Thomas GD, Marcovecchio P, Blatchley A, Nakao CS, et al. Human Monocyte Heterogeneity as Revealed by High-Dimensional Mass Cytometry. Arterioscler Thromb Vasc Biol. 2019;39: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas GD, Hamers AAJ, Nakao C, Marcovecchio P, Taylor AM, McSkimming C, et al. Human Blood Monocyte Subsets: A New Gating Strategy Defined Using Cell Surface Markers Identified by Mass Cytometry. Arterioscler Thromb Vasc Biol. 2017;37: 1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wildgruber M, Aschenbrenner T, Wendorff H, Czubba M, Glinzer A, Haller B, et al. The “Intermediate” CD14 CD16 monocyte subset increases in severe peripheral artery disease in humans. Scientific Reports. 2016. doi: 10.1038/srep39483 [DOI] [PMC free article] [PubMed]
- 32.Yona S, Kim K-W, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. 2017;214: 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mildner A, Schönheit J, Giladi A, David E, Lara-Astiaso D, Lorenzo-Vivas E, et al. Genomic Characterization of Murine Monocytes Reveals C/EBPβ Transcription Factor Dependence of Ly6C Cells. Immunity. 2017;46: 849–862.e7. [DOI] [PubMed] [Google Scholar]
- 35.Villani A-C, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356. doi: 10.1126/science.aah4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity. 2019;50: 1317–1334.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menezes S, Melandri D, Anselmi G, Perchet T, Loschko J, Dubrot J, et al. The Heterogeneity of Ly6Chi Monocytes Controls Their Differentiation into iNOS Macrophages or Monocyte-Derived Dendritic Cells. Immunity. 2016. pp. 1205–1218. doi: 10.1016/j.immuni.2016.12.001 [DOI] [PMC free article] [PubMed]
- 38.Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, et al. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods. 2017;14: 865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satpathy AT, Saligrama N, Buenrostro JD, Wei Y, Wu B, Rubin AJ, et al. Transcript-indexed ATAC-seq for precision immune profiling. Nat Med. 2018;24: 580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Gioia M, Spreafico R, Springstead JR, Mendelson MM, Joehanes R, Levy D, et al. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat Immunol. 2020;21: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Efremova M, Vento-Tormo M, Teichmann SA, Vento-Tormo R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nature Protocols. 2020. doi: 10.1038/s41596-020-0292-x [DOI] [PubMed]
- 42.Goltsev Y, Samusik N, Kennedy-Darling J, Bhate S, Hale M, Vazquez G, et al. Deep Profiling of Mouse Splenic Architecture with CODEX Multiplexed Imaging. Cell. 2018;174: 968–981.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106: 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. 2016;531: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated Tumoricidal Activity via the Tumor Necrosis Factor–related Cytokine, TRAIL. The Journal of Experimental Medicine. 1999. pp. 1343–1354. doi: 10.1084/jem.189.8.1343 [DOI] [PMC free article] [PubMed]
- 46.Richards DM, Hettinger J, Feuerer M. Monocytes and Macrophages in Cancer: Development and Functions. Cancer Microenvironment. 2013. pp. 179–191. doi: 10.1007/s12307-012-0123-x [DOI] [PMC free article] [PubMed]
- 47.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344: 921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475: 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Overmeire E, Stijlemans B, Heymann F, Keirsse J, Morias Y, Elkrim Y, et al. M-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment. Cancer Res. 2016;76: 35–42. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Yao W, Yuan Y, Chen P, Li B, Li J, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66: 157–167. [DOI] [PubMed] [Google Scholar]
- 51.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73: 1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nature medicine. 2018. pp. 144–153. [DOI] [PubMed]
- 53.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19: 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bronte V, Brandau S, Chen S-H, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7: 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41: 815–829. [DOI] [PubMed] [Google Scholar]
- 56.Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell. 2019;35: 588–602.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okada M, Kitahara M, Kishimoto S, Matsuda T, Hirano T, Kishimoto T. IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J Immunol. 1988;141: 1543–1549. [PubMed] [Google Scholar]
- 58.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8: 591–599. [DOI] [PubMed] [Google Scholar]
- 59.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189: 5602–5611. [DOI] [PubMed] [Google Scholar]
- 60.Pan P-Y, -Y. Pan P, Ma G, Weber KJ, Ozao-Choy J, Wang G, et al. Immune Stimulatory Receptor CD40 Is Required for T-Cell Suppression and T Regulatory Cell Activation Mediated by Myeloid-Derived Suppressor Cells in Cancer. Cancer Research. 2010. pp. 99–108. doi: 10.1158/0008-5472.can-09-1882 [DOI] [PMC free article] [PubMed]
- 61.Yang R, Cai Z, Zhang Y, Yutzy WH, Roby KF, Roden RBS. CD80 in Immune Suppression by Mouse Ovarian Carcinoma–Associated Gr-1 CD11b Myeloid Cells. Cancer Research. 2006. pp. 6807–6815. doi: 10.1158/0008-5472.can-05-3755 [DOI] [PubMed]
- 62.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-Derived Suppressor Cells Promote Cross-Tolerance in B-Cell Lymphoma by Expanding Regulatory T Cells. Cancer Research. 2008. pp. 5439–5449. doi: 10.1158/0008-5472.can-07-6621 [DOI] [PMC free article] [PubMed]
- 63.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4 CD25 regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008. pp. 5359–5370. doi: 10.1182/blood-2007-08-105395 [DOI] [PubMed]
- 64.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, et al. Intratumor depletion of CD4 cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. Journal of Experimental Medicine. 2005. pp. 779–791. doi: 10.1084/jem.20041684 [DOI] [PMC free article] [PubMed]
- 65.Papadopoulos KP, Tsai FY-C, Bauer TM, Muigai L, Liang Y, Bennett MK, et al. CX-1158–101: A first-in-human phase 1 study of CB-1158, a small molecule inhibitor of arginase, as monotherapy and in combination with an anti-PD-1 checkpoint inhibitor in patients (pts) with solid tumors. Journal of Clinical Oncology. 2017. pp. 3005–3005. doi: 10.1200/jco.2017.35.15_suppl.3005 [DOI]
- 66.Pommier A, Audemard A, Durand A, Lengagne R, Delpoux A, Martin B, et al. Inflammatory monocytes are potent antitumor effectors controlled by regulatory CD4+ T cells. Proc Natl Acad Sci U S A. 2013;110: 13085–13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJC, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104: 19446–19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, et al. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell. 2016;30: 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheng J, Chen Q, Soncin I, Ng SL, Karjalainen K, Ruedl C. A Discrete Subset of Monocyte-Derived Cells among Typical Conventional Type 2 Dendritic Cells Can Efficiently Cross-Present. Cell Rep. 2017;21: 1203–1214. [DOI] [PubMed] [Google Scholar]
- 70.Sharma MD, Rodriguez PC, Koehn BH, Baban B, Cui Y, Guo G, et al. Activation of p53 in Immature Myeloid Precursor Cells Controls Differentiation into Ly6cCD103 Monocytic Antigen-Presenting Cells in Tumors. Immunity. 2018;48: 91–106.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singhal S, Stadanlick J, Annunziata MJ, Rao AS, Bhojnagarwala PS, O’Brien S, et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med. 2019;11. doi: 10.1126/scitranslmed.aat1500 [DOI] [PMC free article] [PubMed]
- 72.Huang M-N, Nicholson LT, Batich KA, Swartz AM, Kopin D, Wellford S, et al. Antigen-loaded monocyte administration induces potent therapeutic antitumor T cell responses. J Clin Invest. 2020. doi: 10.1172/JCI128267 [DOI] [PMC free article] [PubMed]
- 73.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13: 227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bianchini M, Duchêne J, Santovito D, Schloss MJ, Evrard M, Winkels H, et al. PD-L1 expression on nonclassical monocytes reveals their origin and immunoregulatory function. Sci Immunol. 2019;4. doi: 10.1126/sciimmunol.aar3054 [DOI] [PubMed] [Google Scholar]
- 75.Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol. 2020;5. doi: 10.1126/sciimmunol.aay1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kitamura T, Doughty-Shenton D, Cassetta L, Fragkogianni S, Brownlie D, Kato Y, et al. Monocytes Differentiate to Immune Suppressive Precursors of Metastasis-Associated Macrophages in Mouse Models of Metastatic Breast Cancer. Front Immunol. 2017;8: 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haderk F, Schulz R, Iskar M, Cid LL, Worst T, Willmund KV, et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci Immunol. 2017;2. doi: 10.1126/sciimmunol.aah5509 [DOI] [PubMed] [Google Scholar]
- 78.Wells AD, Walsh MC, Bluestone JA, Turka LA. Signaling through CD28 and CTLA-4 controls two distinct forms of T cell anergy. J Clin Invest. 2001;108: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8: 1069–1086. [DOI] [PubMed] [Google Scholar]
- 80.Karulf M, Kelly A, Weinberg AD, Gold JA. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J Immunol. 2010;185: 4856–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laderach D, Wesa A, Galy A. 4–1BB-ligand is regulated on human dendritic cells and induces the production of IL-12. Cell Immunol. 2003;226: 37–44. [DOI] [PubMed] [Google Scholar]
- 82.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. The Journal of Experimental Medicine. 1993. pp. 669–674. doi: 10.1084/jem.178.2.669 [DOI] [PMC free article] [PubMed]
- 83.Jahchan NS, Mujal AM, Pollack JL, Binnewies M, Sriram V, Reyno L, et al. Tuning the Tumor Myeloid Microenvironment to Fight Cancer. Front Immunol. 2019;10: 1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swirski FK, Libby P, Aikawa E, Alcaide P, William Luscinskas F, Weissleder R, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. Journal of Clinical Investigation. 2007. pp. 195–205. doi: 10.1172/jci29950 [DOI] [PMC free article] [PubMed]
- 85.Auffray C, Sieweke MH, Geissmann F. Blood Monocytes: Development, Heterogeneity, and Relationship with Dendritic Cells. Annual Review of Immunology. 2009. pp. 669–692. doi: 10.1146/annurev.immunol.021908.132557 [DOI] [PubMed]
- 86.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of Monocytes, Macrophages, and Dendritic Cells. Science. 2010. pp. 656–661. doi: 10.1126/science.1178331 [DOI] [PMC free article] [PubMed]
- 87.Merad M, Sathe P, Helft J, Miller J, Mortha A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annual Review of Immunology. 2013. pp. 563–604. doi: 10.1146/annurev-immunol-020711-074950 [DOI] [PMC free article] [PubMed]
- 88.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 89.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine Deficiency Markedly Reduces Macrophage Accumulation and Atherosclerotic Lesion Formation in CCR2 −/− Mice. Circulation. 2008. pp. 1642–1648. doi: 10.1161/circulationaha.107.743872 [DOI] [PMC free article] [PubMed]
- 90.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, et al. Dynamic T cell–APC interactions sustain chronic inflammation in atherosclerosis. Journal of Clinical Investigation. 2012. pp. 3114–3126. doi: 10.1172/jci61758 [DOI] [PMC free article] [PubMed]
- 91.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. Journal of Clinical Investigation. 2007. pp. 185–194. doi: 10.1172/jci28549 [DOI] [PMC free article] [PubMed]
- 92.Wu H, Michael Gower R, Wang H, Perrard X-YD, Ma R, Bullard DC, et al. Functional Role of CD11c Monocytes in Atherogenesis Associated With Hypercholesterolemia. Circulation. 2009. pp. 2708–2717. doi: 10.1161/circulationaha.108.823740 [DOI] [PMC free article] [PubMed]
- 93.Gower RM, Michael Gower R, Wu H, Foster GA, Devaraj S, Jialal I, et al. CD11c/CD18 Expression Is Upregulated on Blood Monocytes During Hypertriglyceridemia and Enhances Adhesion to Vascular Cell Adhesion Molecule-1. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011. pp. 160–166. doi: 10.1161/atvbaha.110.215434 [DOI] [PMC free article] [PubMed]
- 94.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110: 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marcovecchio PM, Thomas GD, Mikulski Z, Ehinger E, Mueller KAL, Blatchley A, et al. Scavenger Receptor CD36 Directs Nonclassical Monocyte Patrolling Along the Endothelium During Early Atherogenesis. Arterioscler Thromb Vasc Biol. 2017;37: 2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Westhorpe CLV, Norman MU, Hall P, Snelgrove SL, Finsterbusch M, Li A, et al. Effector CD4 T cells recognize intravascular antigen presented by patrolling monocytes. Nat Commun. 2018;9: 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nature Reviews Immunology. 2006. pp. 508–519. doi: 10.1038/nri1882 [DOI] [PubMed]
- 98.Davenport P, Tipping PG. The Role of Interleukin-4 and Interleukin-12 in the Progression of Atherosclerosis in Apolipoprotein E-Deficient Mice. The American Journal of Pathology. 2003. pp. 1117–1125. doi: 10.1016/s0002-9440(10)63471-2 [DOI] [PMC free article] [PubMed]
- 99.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13: 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robertson A-KL, Hansson GK. T Cells in Atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006. pp. 2421–2432. doi: 10.1161/01.atv.0000245830.29764.84 [DOI] [PubMed]
- 101.Robertson A-KL, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006;26: 2421–2432. [DOI] [PubMed] [Google Scholar]
- 102.Tedgui A, Mallat Z. Cytokines in Atherosclerosis: Pathogenic and Regulatory Pathways. Physiological Reviews. 2006. pp. 515–581. doi: 10.1152/physrev.00024.2005 [DOI] [PubMed]
- 103.Moore KJ, Freeman MW. Scavenger Receptors in Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006. pp. 1702–1711. doi: 10.1161/01.atv.0000229218.97976.43 [DOI] [PubMed]
- 104.Packard RRS, Maganto-García E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res. 2008;103: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Paulson KE, Zhu S-N, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106: 383–390. [DOI] [PubMed] [Google Scholar]
- 106.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010. pp. 1357–1361. doi: 10.1038/nature08938 [DOI] [PMC free article] [PubMed]
- 107.Westerterp M, Gautier EL, Ganda A, Molusky MM, Wang W, Fotakis P, et al. Cholesterol Accumulation in Dendritic Cells Links the Inflammasome to Acquired Immunity. Cell Metab. 2017;25: 1294–1304.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cochain C, Koch M, Chaudhari SM, Busch M, Pelisek J, Boon L, et al. CD8 T Cells Regulate Monopoiesis and Circulating Ly6C high Monocyte Levels in Atherosclerosis in Mice. Circulation Research. 2015. pp. 244–253. doi: 10.1161/circresaha.117.304611 [DOI] [PubMed]
- 109.Schürch CM, Riether C, Ochsenbein AF. Cytotoxic CD8 T Cells Stimulate Hematopoietic Progenitors by Promoting Cytokine Release from Bone Marrow Mesenchymal Stromal Cells. Cell Stem Cell. 2014. pp. 460–472. doi: 10.1016/j.stem.2014.01.002 [DOI] [PubMed]
- 110.Kannel WB, Castelli WP, Gordon T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann Intern Med. 1979;90: 85–91. [DOI] [PubMed] [Google Scholar]
- 111.Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA: the journal of the American Medical Association. 1993. pp. 3015–3023. [PubMed]
- 112.Classics in arteriosclerosis research: On experimental cholesterin steatosis and its significance in the origin of some pathological processes by N. Anitschkow and S. Chalatow, translated by Mary Z. Pelias, 1913. Arteriosclerosis: An Official Journal of the American Heart Association, Inc. 1983. pp. 178–182. doi: 10.1161/01.atv.3.2.178 [DOI] [PubMed]
- 113.Grundy SM. Summary of the Second Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA: The Journal of the American Medical Association. 1993. p. 3015. doi: 10.1001/jama.1993.03500230097036 [DOI] [PubMed]
- 114.Warner SJ, Auger KR, Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. The Journal of Experimental Medicine. 1987. pp. 1316–1331. doi: 10.1084/jem.165.5.1316 [DOI] [PMC free article] [PubMed]
- 115.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Current Opinion in Immunology. 2010. pp. 333–340. doi: 10.1016/j.coi.2010.02.013 [DOI] [PMC free article] [PubMed]
- 116.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 117.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390: 1833–1842. [DOI] [PubMed] [Google Scholar]
- 118.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25: 387–408. [DOI] [PubMed] [Google Scholar]
- 119.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010. pp. 883–899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed]
- 120.Choi J-H, Do Y, Cheong C, Koh H, Boscardin SB, Oh Y-S, et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. The Journal of Experimental Medicine. 2009. pp. 497–505. doi: 10.1084/jem.20082129 [DOI] [PMC free article] [PubMed]
- 121.Han JW, Shimada K, Ma-Krupa W, Johnson TL, Nerem RM, Goronzy JJ, et al. Vessel Wall–Embedded Dendritic Cells Induce T-Cell Autoreactivity and Initiate Vascular Inflammation. Circulation Research. 2008. pp. 546–553. doi: 10.1161/circresaha.107.161653 [DOI] [PubMed]
- 122.Weber C, Meiler S, Döring Y, Koch M, Drechsler M, Megens RTA, et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. Journal of Clinical Investigation. 2011. pp. 2898–2910. doi: 10.1172/jci44925 [DOI] [PMC free article] [PubMed]
- 123.Erbel C, Sato K, Meyer FB, Kopecky SL, Frye RL, Goronzy JJ, et al. Functional profile of activated dendritic cells in unstable atherosclerotic plaque. Basic Research in Cardiology. 2007. pp. 123–132. doi: 10.1007/s00395-006-0636-x [DOI] [PubMed]
- 124.van Zandvoort M, Engels W, Douma K, Beckers L, Egbrink MO, Daemen M, et al. Two-Photon Microscopy for Imaging of the (Atherosclerotic) Vascular Wall: A Proof of Concept Study. Journal of Vascular Research. 2004. pp. 54–63. doi: 10.1159/000076246 [DOI] [PubMed]
- 125.Boulesteix T, -M. Pena A, Pagès N, -P. Godeau G, Sauviat M, Beaurepaire E, et al. Micrometer scaleEx Vivo multiphoton imaging of unstained arterial wall structure. Cytometry Part A. 2006. pp. 20–26. doi: 10.1002/cyto.a.20196 [DOI] [PubMed]
- 126.Maffia P, Zinselmeyer BH, Ialenti A, Kennedy S, Baker AH, McInnes IB, et al. Multiphoton Microscopy for 3-Dimensional Imaging of Lymphocyte Recruitment Into Apolipoprotein-E–Deficient Mouse Carotid Artery. Circulation. 2007. doi: 10.1161/circulationaha.106.658492 [DOI] [PubMed]
- 127.Steinman RM, Inaba K, Turley S, Pierre P, Mellman I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Human Immunology. 1999. pp. 562–567. doi: 10.1016/s0198-8859(99)00030-0 [DOI] [PubMed]
- 128.Steinman RM, Bonifaz L, Fujii S-I, Liu K, Bonnyay D, Yamazaki S, et al. The Innate Functions of Dendritic Cells in Peripheral Lymphoid Tissues. Mechanisms of Lymphocyte Activation and Immune Regulation X. pp. 83–97. doi: 10.1007/0-387-24180-9_12 [DOI] [PubMed]
- 129.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. Proceedings of the National Academy of Sciences. 2002. pp. 351–358. doi: 10.1073/pnas.231606698 [DOI] [PMC free article] [PubMed]
- 130.Packard RRS, Maganto-García E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c Dendritic Cells Maintain Antigen Processing, Presentation Capabilities, and CD4 T-Cell Priming Efficacy Under Hypercholesterolemic Conditions Associated With Atherosclerosis. Circulation Research. 2008. pp. 965–973. doi: 10.1161/circresaha.108.185793 [DOI] [PMC free article] [PubMed]
- 131.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proceedings of the National Academy of Sciences. 2004. pp. 11779–11784. doi: 10.1073/pnas.0403259101 [DOI] [PMC free article] [PubMed]
- 132.Gräbner R, Lötzer K, Döpping S, Hildner M, Radke D, Beer M, et al. Lymphotoxin β receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. The Journal of Experimental Medicine. 2009. pp. 233–248. doi: 10.1084/jem.20080752 [DOI] [PMC free article] [PubMed]
- 133.Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, et al. Direct Demonstration of P-Selectin– and VCAM-1–Dependent Mononuclear Cell Rolling in Early Atherosclerotic Lesions of Apolipoprotein E–Deficient Mice. Circulation Research. 1999. pp. 1237–1244. doi: 10.1161/01.res.84.11.1237 [DOI] [PubMed]
- 134.Huo Y, Hafezi-Moghadam A, Ley K. Role of Vascular Cell Adhesion Molecule-1 and Fibronectin Connecting Segment-1 in Monocyte Rolling and Adhesion on Early Atherosclerotic Lesions. Circulation Research. 2000. pp. 153–159. doi: 10.1161/01.res.87.2.153 [DOI] [PubMed]
- 135.Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13: 368–380. [DOI] [PubMed] [Google Scholar]
- 136.Colantonio LD, Bittner V, Reynolds K, Levitan EB, Rosenson RS, Banach M, et al. Association of Serum Lipids and Coronary Heart Disease in Contemporary Observational Studies. Circulation. 2016;133: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ylä-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14: 32–40. [DOI] [PubMed] [Google Scholar]
- 138.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proceedings of the National Academy of Sciences. 1995. pp. 3893–3897. doi: 10.1073/pnas.92.9.3893 [DOI] [PMC free article] [PubMed]
- 139.Hermansson A, Ketelhuth DFJ, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, et al. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. The Journal of Experimental Medicine. 2010. pp. 1081–1093. doi: 10.1084/jem.20092243 [DOI] [PMC free article] [PubMed]
- 140.Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, et al. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. Journal of Clinical Investigation. 1995. pp. 2569–2577. doi: 10.1172/jci118320 [DOI] [PMC free article] [PubMed]
- 141.Legein B, Janssen EM, Theelen TL, Gijbels MJ, Walraven J, Klarquist JS, et al. Ablation of CD8α dendritic cell mediated cross-presentation does not impact atherosclerosis in hyperlipidemic mice. Scientific Reports. 2015. doi: 10.1038/srep15414 [DOI] [PMC free article] [PubMed]
- 142.Kolbus D, Ljungcrantz I, Söderberg I, Alm R, Björkbacka H, Nilsson J, et al. TAP1-deficiency does not alter atherosclerosis development in Apoe−/− mice. PLoS One. 2012;7: e33932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH. B7–1/B7–2 Costimulation Regulates Plaque Antigen–Specific T-Cell Responses and Atherogenesis in Low-Density Lipoprotein Receptor–Deficient Mice. Circulation. 2004. pp. 2009–2015. doi: 10.1161/01.cir.0000127121.16815.f1 [DOI] [PubMed]
- 144.Buono C Co-Stimulation and Plaque-Antigen-Specific T-Cell Responses in Atherosclerosis. Trends in Cardiovascular Medicine. 2004. pp. 166–172. doi: 10.1016/j.tcm.2004.03.001 [DOI] [PubMed]
- 145.Wang X, Ria M, Kelmenson PM, Eriksson P, Higgins DC, Samnegård A, et al. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nature Genetics. 2005. pp. 365–372. doi: 10.1038/ng1524 [DOI] [PubMed]
- 146.Nakano M, Fukumoto Y, Satoh K, Ito Y, Kagaya Y, Ishii N, et al. OX40 ligand plays an important role in the development of atherosclerosis through vasa vasorum neovascularization. Cardiovasc Res. 2010;88: 539–546. [DOI] [PubMed] [Google Scholar]
- 147.Simons KH, de Jong A, Wouter Jukema J, de Vries MR, Arens R, Quax PHA. T cell co-stimulation and co-inhibition in cardiovascular disease: a double-edged sword. Nature Reviews Cardiology. 2019. pp. 325–343. doi: 10.1038/s41569-019-0164-7 [DOI] [PubMed]
- 148.Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH. B7–1/B7–2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109: 2009–2015. [DOI] [PubMed] [Google Scholar]
- 149.Dopheide JF, Sester U, Schlitt A, Horstick G, Rupprecht HJ, Münzel T, et al. Monocyte-derived dendritic cells of patients with coronary artery disease show an increased expression of costimulatory molecules CD40, CD80 and CD86 in vitro. Coronary Artery Disease. 2007. pp. 523–531. doi: 10.1097/mca.0b013e3282eff1ad [DOI] [PubMed]
- 150.Galbraith GMP. Cytokine Storm in a Phase 1 Trial of the Anti-CD28 Monoclonal Antibody TGN1412. Yearbook of Dermatology and Dermatologic Surgery. 2007. pp. 257–258. doi: 10.1016/s0093-3619(08)70546-x [DOI]
- 151.Bu D-X, Tarrio M, Maganto-Garcia E, Stavrakis G, Tajima G, Lederer J, et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol. 2011;31: 1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir E-AD, Amadori L, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25: 1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. Journal of Clinical Investigation. 2007. pp. 2974–2982. doi: 10.1172/jci31344 [DOI] [PMC free article] [PubMed]
- 154.Kusters PJH, Lutgens E, Seijkens TTP. Exploring immune checkpoints as potential therapeutic targets in atherosclerosis. Cardiovasc Res. 2018;114: 368–377. [DOI] [PubMed] [Google Scholar]
- 155.Hollenbaugh D, Mischel-Petty N, Edwards CP, Simon JC, Denfeld RW, Kiener PA, et al. Expression of functional CD40 by vascular endothelial cells. The Journal of Experimental Medicine. 1995. pp. 33–40. doi: 10.1084/jem.182.1.33 [DOI] [PMC free article] [PubMed]
- 156.Karmann K, Hughes CC, Schechner J, Fanslow WC, Pober JS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proceedings of the National Academy of Sciences. 1995. pp. 4342–4346. doi: 10.1073/pnas.92.10.4342 [DOI] [PMC free article] [PubMed]
- 157.Yellin MJ, Brett J, Baum D, Matsushima A, Szabolcs M, Stern D, et al. Functional interactions of T cells with endothelial cells: the role of CD40L-CD40-mediated signals. The Journal of Experimental Medicine. 1995. pp. 1857–1864. doi: 10.1084/jem.182.6.1857 [DOI] [PMC free article] [PubMed]
- 158.Donner AJ, Yeh ST, Hung G, Graham MJ, Crooke RM, Mullick AE. CD40 Generation 2.5 Antisense Oligonucleotide Treatment Attenuates Doxorubicin-induced Nephropathy and Kidney Inflammation. Mol Ther Nucleic Acids. 2015;4: e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen J, Li J-H, Zhao S-J, Wang D-Y, Zhang W-Z, Liang W-J. Clinical significance of costimulatory molecules CD40/CD40L and CD134/CD134L in coronary heart disease: A case-control study. Medicine . 2017;96: e7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Infante JR, Ahlers CM, Stephen Hodi F, Postel-Vinay S, Schellens JHM, Heymach J, et al. ENGAGE-1: A first in human study of the OX40 agonist GSK3174998 alone and in combination with pembrolizumab in patients with advanced solid tumors. Journal of Clinical Oncology. 2016. pp. TPS3107–TPS3107. doi: 10.1200/jco.2016.34.15_suppl.tps3107 [DOI]
- 161.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kumar MP, Du J, Lagoudas G, Jiao Y, Sawyer A, Drummond DC, et al. Analysis of Single-Cell RNA-Seq Identifies Cell-Cell Communication Associated with Tumor Characteristics. Cell Reports. 2018. pp. 1458–1468.e4. doi: 10.1016/j.celrep.2018.10.047 [DOI] [PMC free article] [PubMed]
- 163.Winkels H, Ehinger E, Ghosheh Y, Wolf D, Ley K. Atherosclerosis in the single-cell era. Curr Opin Lipidol. 2018;29: 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res. 2018;122: 1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, et al. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res. 2018;122: 1661–1674. [DOI] [PubMed] [Google Scholar]
- 166.Paik DT, Cho S, Tian L, Chang HY, Wu JC. Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat Rev Cardiol. 2020. doi: 10.1038/s41569-020-0359-y [DOI] [PMC free article] [PubMed]
- 167.Gamrekelashvili J, Giagnorio R, Jussofie J, Soehnlein O, Duchene J, Briseño CG, et al. Regulation of monocyte cell fate by blood vessels mediated by Notch signalling. Nature Communications. 2016. doi: 10.1038/ncomms12597 [DOI] [PMC free article] [PubMed]


