Abstract
Background:
Cold storage of platelets (PLTs) has the potential advantage of prolonging storage time while reducing posttransfusion infection given the decreased likelihood of bacterial outgrowth during storage and possibly beneficial effects in treating bleeding patients. However, cold storage reduces PLT survival through the induction of complex storage lesions, which are more accentuated when storage is prolonged.
Study Design and Methods:
Whole blood–derived PLT-rich plasma concentrates from seven PLT pools (n = 5 donors per pool). PLT additive solution was added (67%/33% plasma) and the product was split into 50-mL bags. Split units were stored in the presence or absence of 1 mM of N-acetylcysteine (NAC) under agitation for up to 14 days at room temperature or in the cold and were analyzed for PLT activation, fibrinogen-dependent spreading, microparticle formation, mitochondrial respiratory activity, reactive oxygen species (ROS) generation, as well as in vivo survival and bleeding time correction in immunodeficient mice.
Results:
Cold storage of PLTs for 7 days or longer induces significant PLT activation, cytoskeletal damage, impaired fibrinogen spreading, enhances mitochondrial metabolic decoupling and ROS generation, and increases macrophage-dependent phagocytosis and macrophage-independent clearance. Addition of NAC prevents PLT clearance and allows a correction of the prolonged bleeding time in thrombocytopenic, aspirin-treated, immunodeficient mice.
Conclusions:
Long-term cold storage induces mitochondrial uncoupling and increased proton leak and ROS generation. The resulting ROS is a crucial contributor to the increased macrophage-dependent and -independent clearance of functional PLTs and can be prevented by the antioxidant NAC in a magnesium-containing additive solution.
Keywords: mitochondrial uncoupling, platelet cold storage, reactive oxygen species, survival and bleeding time correction
1 ∣. INTRODUCTION
Most blood centers in the world collect platelet (PLT) products by centrifugation of whole blood and store them at room temperature (RT) under gentle agitation with a maximum storage time of 3 to 7 days due to limitations related to bacterial contamination. This limited shelf life leads to frequent seasonal shortages or waste of outdated products.1 Such storage limitation persists despite recent advances in the development of PLT additive solutions (PASs) that potentially could allow RT storage of PLTs for 14 days or longer.2 Multiple PASs have been used clinically for the RT storage of PLTs,3 including magnesium in their composition, which is recognized as a major benefit to PLT long-term storage.4 Storage of PLTs in a PAS presents additional benefits to the blood centers, as more plasma may be recovered for fractionation and clinical use, and to the patient with reduced risk of PLT transfusion reactions, particularly allergic transfusion reactions and transfusion-related acute lung injury.5,6
Cold storage of PLTs has the potential advantage of prolonging storage time while reducing posttransfusion infection given the decreased likelihood of bacterial outgrowth during storage. However, previous studies have demonstrated that storing PLTs in the cold (1°C-6°C) resulted in a significant reduction in vivo recovery and survival compared with RT-stored PLTs when stored for the same period of time due to cold-elicited lesions.7 While the detailed reason for the cold lesion remains unclear, a clearance mechanism of cold-stored PLTs involving glycoprotein (GP) Ib clustering on the PLT surface, and in vivo binding to macrophage and hepatocyte receptors has been identified.8-11 In vitro studies suggest that cold-stored PLTs have superior hemostatic properties compared with RT-stored PLTs.12 Cold-stored PLTs corrected the bleeding time quicker than RT-stored PLTs in patients with thrombocytopenia when cold-stored PLTs have been maintained in the cold temperature for 72 hours or less in plasma.13
However, several pieces of evidence do not favor cold storage of PLTs. Slichter et al14 demonstrated that most bleeding time measurements in patients with thrombocytopenia transfused with cold-stored PLTs remained prolonged, whereas most bleeding times in subjects receiving RT-stored PLTs improved. In addition, Filip and Aster15 found that cold-stored PLTs were less effective after 72 hours of storage when compared to RT-stored PLTs in vivo. Multiple in vitro studies have shown that PLTs maintain good hemostatic properties when stored for periods of up to 21 days in cold conditions,16-19 but these studies have not yet identified the root cause of why long-term (>5 days) cold-stored PLTs do not function as well as those stored short term (<3 days).
Cold storage has been associated with increased reactive oxygen species (ROS) production correctable in vitro by the addition of 50 mM of N-acetylcysteine (NAC), which, at this concentration, affects PLTs through pH alkalinization.20
Here, we investigated the mechanism of long-term cold storage–induced damage of whole blood–derived PLTs stored in a magnesium-containing PAS. We identified the PLT mitochondrial dysfunction and its prooxidant consequences as a crucial source of PLT clearance and decreased hemostatic activity in long-term stored PLTs susceptible to amelioration by adding a stable antioxidant at concentrations amenable for translation into an improved PAS for up to 14-day storage.
2 ∣. MATERIAL AND METHODS
2.1 ∣. PLT products
Seven deidentified human, leukocyte-reduced PLT pools were purchased from Hoxworth Blood Center, University of Cincinnati. In brief, whole blood units from five donors per pool were collected in anticoagulant citrate phosphate double dextrose (Acrodose Plus System; Haemonetics, Braintree, Massachusetts) at RT and processed within 8 hours after collection following the manufacturer's instructions with some modifications. Briefly, PLT-rich plasma (PRP) was generated from each of the collected units through a first centrifugation at 1000g followed by separation of the PLT layer into separate bags. A second centrifugation at 2500g was performed to deplete plasma. A volume of approximately 15 mL of plasma was left for PLT resuspension in each unit. PRP units from five healthy donors were pooled and the additive solution PAS-E (PAS-3 M; Grifols Inc., Barcelona, Spain) was added at a final ratio of 67% PAS-E/33% plasma and leukoreduced through a leukoreduction filter (Acrodose PL Leukocyte Reduction Filter, Haemonetics). All units were ABO identical, pooled as pools of O or A blood groups. Pooled PLTs were stored overnight at RT until Day 1 and then split into 50-mL bags (CLX, Haemonetics) and stored thereafter at RT (20°C-24°C) or cold (1°C-4°C) under the same agitation conditions in horizontal agitators (PF900h or PF48h; Helmer Scientific, Noblesville, Indiana). Aliquots from the pooled PLT products were obtained at different time points for analysis. After sampling, the PLT pools were immediately returned to their storage conditions.
2.2 ∣. ROS generation analysis
It was quantified as described elsewhere.21 Briefly, washed PLTs were incubated with 2′7′-dichlorofluorescein diacetate (10 μM), a reporter for all forms of oxidative species including superoxide and hydrogen peroxide, for 15 minutes at 37°C, and washed in calcium-free phosphate-buffered saline (PBS). ROS level was determined by fluorescence-activated cell sorting at an emission of 525 nm. ROS level was expressed as mean florescence intensity. NAC (1 mM; Sigma, St. Louis, Missouri)22 or nothing (vehicle) was added to the stored PLTs (RT or cold) for the duration of the storage.
2.3 ∣. In vitro phagocytosis
In vitro phagocytosis was performed as previously described.8 Briefly, monocytic THP-1 cells (ATCC, Manassas, Virginia) were cultured in Dulbecco's Modified Eagle Medium (Invitrogen, Carlsbad, California) supplemented with 10% fetal calf serum (R&D, Minneapolis, Minnesota), 2 mM glutamine (Life Technologies, Waltham, Massachusetts), 100 IU/mL penicillin and 0.1 mg/mL streptomycin (Life Technologies), and differentiated using 1 ng/mL TGF-β1 (R&D) and 50 nM 1,25-(OH)2-vitamin D3 (Sigma) for 24 hours. The adherent macrophages were activated by the addition of 15 ng/mL phorbol-myristate acetate (Sigma) for 15 minutes. PLTs from each of the groups analyzed were incubated with carboxyfluorescein succinimidyl-ester (CFSE, 5 mM; Life Technologies) for 15 minutes at 37°C. Fluorescent PLTs were layered on top and incubated for 30 minutes at 37°C. Nonadherent and extracellular adhered PLTs were removed by three washes with calcium-free PBS. Monocytes were detached by treatment with 0.05% trypsin/0.53% ethylenediaminetetraacetic acid. Cells were spun down and pellets were fixed in 1% paraformaldehyde/PBS, stained with anti-CD61-PE (Becton-Dickinson, San Jose, California) and analyzed by dual-color flow cytometry. PLTs and macrophages were resolved by their light scatter characteristics and CFSE/CD61 staining. Carboxyfluorescein+/CD61-events with light scatter characteristics of macrophages were quantified in relation to the overall amount of the CD61-macrophage population. NAC was used at a final concentration of 1 mM.
2.4 ∣. Analysis of PLT recovery
Nonobese diabetic/severe combined immunodeficiency/γc−/− (NSG) mice were used for human PLT transfusion. NSG mice were sublethally irradiated (2.5 Gy) 7 days before the PLT infusion. Then, thrombocytopenic mice (reaching PLT counts of approx. 150, 000-350, 000/mm3) were administered clodronate liposomes (CLD-8901, Encapsula Nano Sciences, Brentwood, Tennessee) intravenously (retro-orbital)23 24 hours before their transfusion to deplete their macrophages.24Test PLTs were labeled with CFSE and infused to all experimental mice. A total of 3 ×108 human PLTs per mouse were infused intravenously (retro-orbital) in five NSG mice per group. Retro-orbital blood specimens were collected after the transfusion at different time points. PLT recovery was analyzed by flow cytometry analysis using anti-human CD61 (Becton-Dickinson) on a log-scale forward scatter/side scatter gate. Data were normalized to 100% for time zero. Time zero was analyzed at 3 minutes after the PLT transfusion.
2.5 ∣. Statistical analysis
Data are expressed as means ± SD as described in the figure legends. A minimum of three independent experiments with one to three pools per condition were analyzed. Linear regressions were calculated based on least square differences method and presented as R2. Statistical differences were identified by either one-way analysis of variance (ANOVA) or two-way ANOVA with Tukey's multiple comparison tests between cold and RT groups and between Day 1 of storage and subsequent days of storage. A P value of <.05 indicates statistically significant difference between the control and test samples. Analyses were performed by computer software (Prism version 8.0; GraphPad Software, San Diego, California).
The remaining methodological descriptions can be found in Appendix S1 (Supplemental Methods).
3 ∣. RESULTS
3.1 ∣. Cold storage of PRP pooled PLTs for 7 days or longer induces significant PLT activation, cytoskeletal damage, and agonistic response
PLT activation of cold-stored vs RT-stored whole blood (PRP)-derived PLTs was assessed by membrane expression analyses of P-selectin, activated GPIIb/IIIa and exposed phosphatidylserine.25 Cold PLTs stored for the first 5 days showed a similar level of membrane P-selectin expression compared with RT-stored PLTs. PRP pooled PLTs in plasma/PAS-E stored for 7 days or longer showed substantial increased expression of P-selectin compared with RT-stored pooled PLTs for Days 7, 10, and 14 of storage (Figure 1A). RT- and cold-stored PLTs maintained contained a low frequency of PLTs with activated GPIIb/IIIa when stored for up to 7 days (Figure 1B). In fact, RT-stored PLTs had no increase in activated GPIIb/IIIa when stored for up to 14 days in plasma/PAS-E (Figure 1B). However, similar to membrane P-selectin expression, PAC-1 binding to activated GPIIb/IIIa was significantly increased in pooled PLTs subject to cold storage for 10 or 14 days but not to pooled PLTs stored for up to 7 days (Figure 1B). Finally, we measured the exposure of phosphatidylserineassociated activation25, associated with apoptosis activation26 and procoagulant activity.27 Phosphatidylserine exposure was increased in stored PLTs as the storage time increased albeit at a faster pace and greater level in cold-stored PLTs (Figure 1C). While RT PLTs only had increased levels of exposed phosphatidylserine on Days 10 and 14 of storage, cold-stored PLTs had increased phosphatidylserine exposure on Day 7 of storage and onwards. Interestingly, plasma/PAS-E was optimal in maintaining the pH of cold-stored PLTs. The extracellular pH (at 22°C) of cold-stored PLTs remained basic, with a very modest decrease by Day 14. As expected, based on the known effect of time in RT stored PLTs, RT-stored PLTs did show a decrease in their pH with a minimum pH of 6.45 by Day 14 of storage. Cytoskeletal damage is a major hallmark of cold storage–induced PLT damage and a major mediator of decreased survival and function deficiency. Although multiple surrogate assays have been used to define the extent of such cytoskeletal damage, PLT fragmentation shedding microparticles as a result of activation and exocytosis28 and impaired integrin signaling resulting in deficient spreading on immobilized fibrinogen29 define two key aspects of impaired PLT integrity. To confirm whether the damage on Day 7 of cold storage–impaired key functional parameters of PLT activity, we performed analyses on the effects of 7-day cold storage on PLT microparticle formation and fibrinogen-mediated spreading. As shown in Figure S1A-B, microparticle formation was significantly increased (approx. 3-fold) in 7-day pooled, cold-stored PLTs than when stored at RT microparticles per transmission electron microscopy field) while PLTs also displayed the classical hallmarks of cold storage damage including dilation of the open canalicular system, formation of prominent pseudopods, and partial degranulation. Interestingly, despite increased expression of activated GPIIb/IIIa on the membrane surface, cold-stored, pooled PLTs had significant aggregation on fibrinogen, forming large clumps (Figure S1C). Analysis of individual PLTs spreading (excluding aggregates and microparticles) indicated that the area of spreading of PLTs on immobilized fibrinogen was significantly (approx. 80%) decreased over their RT-stored counterparts (Figure S1D). Together, these data strongly suggest that cold storage for 7 days or longer results in impaired PLT structure and function.
FIGURE 1.
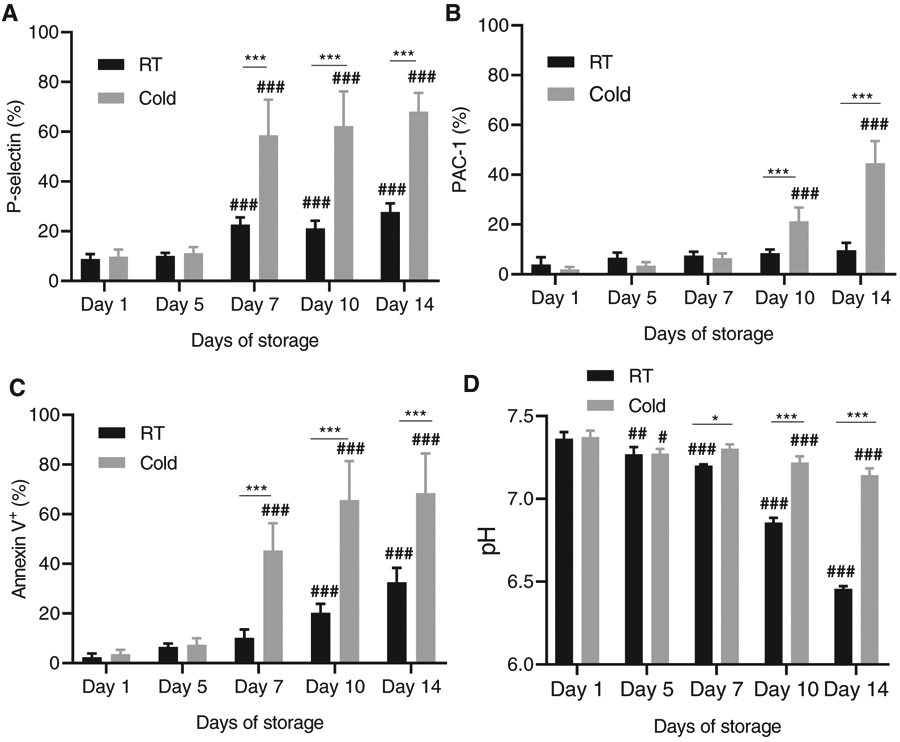
Long-term cold storage of human pooled PLTs in PAS-E induces PLT activation while maintaining pH. (A) P-Selectin expression analysis was measured by flow cytometry. (B) Activated GPIIb/IIIa (PAC-1 binding) analysis. (C) Phosphatidylserine exposure (annexin-V binding). (D) Extracellular pH (at 22°C). Values are represented as average ± SD. A minimum of three experiments were analyzed. Total platelet storage pools tested N = 3. *P < .05: ***P < .001 between RT and cold storage . ##, P < .01; ### P < .001 on different days of storage
3.2 ∣. Cold storage of PRP pooled PLTs for 7 days or longer induces growing mitochondrial metabolic decoupling
PLTs are highly dependent on mitochondrial activity and aerobic respiration.30 Acetate is the major source of energy in PAS-containing PLTs. The addition of magnesium in PAS-E or other similar PAS solutions is expected to improve the activity of adenosine triphosphate (ATP)- and guanosine triphosphate– dependent kinases.31 A key parameter defining mitochondrial activity is the oxygen consumption rate (OCR). Aerobic respiration is characterized by increased OCR, while alternative anaerobic respiration is independent of oxygen consumption.30 Intuitively, refrigeration should result in a decrease in the metabolic needs of stored PLTs. However, the aforementioned data strongly suggest that cold storage induces energydependent signals resulting in activation and dysfunction. To determine whether cold-stored PLT metabolism is decreased, we analyzed the OCR of PLTs cold stored or RT stored for up to 2 weeks. Basal OCR was assessed, and a mitochondrial stress test was performed upon the addition of ATPase inhibitors and decoupling agents to help determine the overall mitochondrial fitness. Functional metabolic analysis of PRP pooled PLTs stored at RT or in the cold for up to 14 days identified specific lesions in mitochondrial function that were not directly evident by analyzing the basal OCR (Figure S2A). Mitochondrial stress test assays, however, support that long-term cold storage, especially on Day 5 and thereafter, induces a decrease in ATP-linked OCR (Figure 2A) but no changes in the maximal OCR after complete mitochondrial uncoupling by Fluoromethoxy carbonylcyanide phenylhydrazone (FCCP) (Figure S2B). The decreased ATP-linked OCR does not associate with changes in nonmitochondrial OCR (Figure 2B) but associates with increased mitochondrial OCR as assessed by analysis of inducible proton-leak OCR, which is increased in PLTs stored for 7 days or longer (Figure 2C), suggesting mitochondrial uncoupling that shifts ATP-producing OCR into proton-leak OCR. Further length of storage does not result in further inducible proton-leak OCR (Figure 2C), suggesting that the mitochondrial dysfunction fundamentally occurs within Days 5 to 7 of cold storage. As a consequence, mitochondrial respiratory activity during the second week of storage, which is highly increased in RT-stored PLTs as expected by the predominant incorporation of acetate as an energy source to the Krebs cycle and subsequently to the electron transport chain, remains unresponsive in cold-stored PLTs, remaining at levels comparable to Day 1 of storage (Figure 2D). These data further confirm that cold-induced damage of PAS-containing, pooled PRP-derived PLTs accumulates during the second week of storage and identify a mitochondrial dysfunction unveiled only in stress tests as another hallmark of cold-storage lesion.
FIGURE 2.
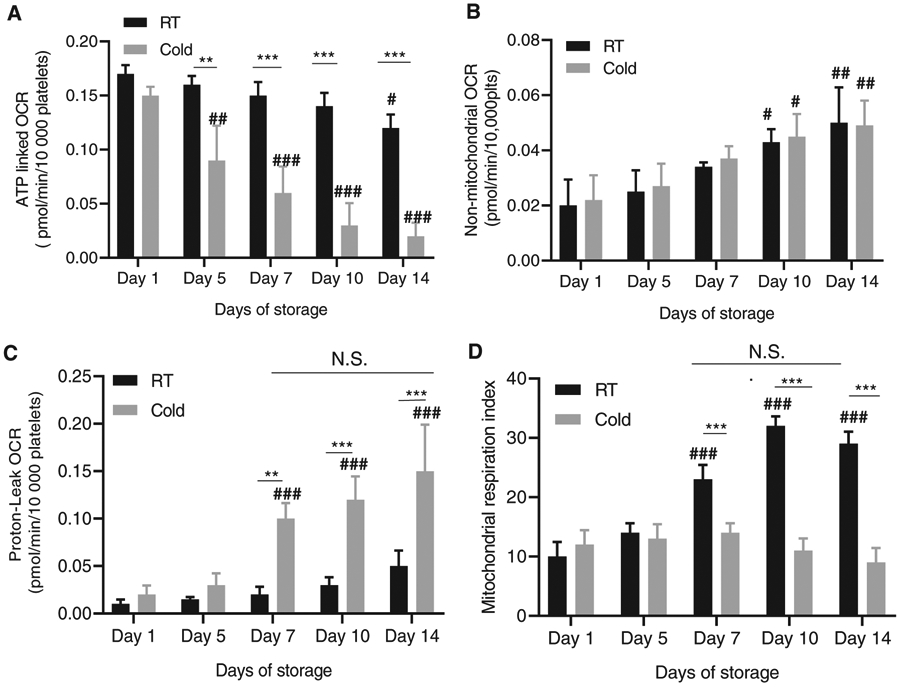
Uncoupling and shift of mitochondrial oxygen consumption to proton leak in long-term cold stored PLTs. (A) ATP linked OCR was measured with seahorse using oligomycin (1 μg/mL), Fluoromethoxy carbonylcyanide phenylhydrazone (FCCP) (0.6 μM) and Antimycin a (10 μM) (B) non-mitochondrial OCR. (C) Proton leak OCR. (D) Mitochondrial respiratory index. Total platelet storage pools tested N = 3. *P < .05; **P < .01; **P < .001; ***P < .001 between RT and cold storage on different days of storage. # P < .05; ##, P < .01; ### P < .001 compared with Day 1 of storage
3.3 ∣. Cold storage of PRP pooled PLTs for 7 days or longer increases ROS generation
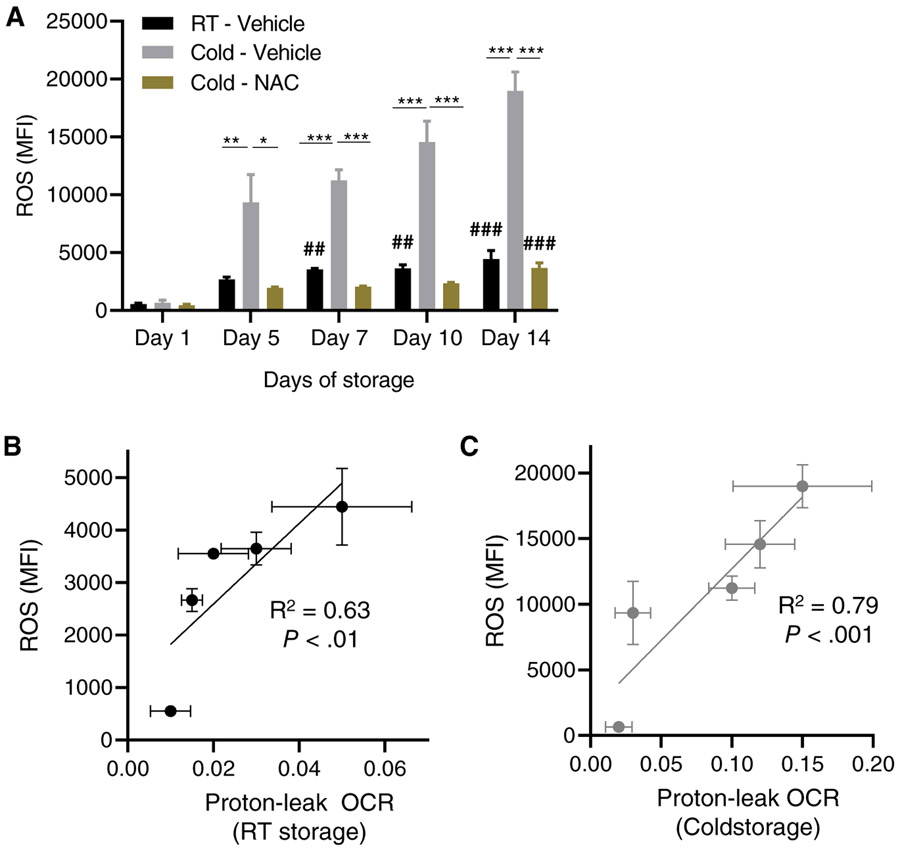
The inducible proton-leak OCR is a secondary event that is frequently associated with electron-leak–dependent mitochondrial superoxide production,32 the most prevalent type of ROS in PLTs.33 Increased levels of superoxide or the product of PLT superoxide dismutase (SOD), hydrogen peroxide, can be detrimental to overall viability of PLTs and results in cytoskeletal and signaling impairment through oxidative damage.34 To determine whether the increased inducible proton leak identified during the second week of cold PLT storage and the generation of ROS were associated, we first analyzed the overall levels of ROS generated by stored PLTs. As expected, the ROS levels of RT and especially cold-stored PLTs increased during storage (Figure 3A) and significantly correlated with the induced proton-leak OCR of RT-stored (Figure 3B) and especially cold-stored PLTs (Figure 3C). The increase in ROS levels of cold-stored PLTs was completely prevented by the addition of the antioxidant NAC (1 mM) to the PLT storage additive solution (Figure 3A). These data indicate that cold conditions of storage for 7 days or longer further enhance the generation of ROS in stored PLTs, which can be effectively prevented by an inclusion of the antioxidant NAC in the PAS.
FIGURE 3.
Cold storage induces ROS which can be prevented by addition of NAC to the PAS-E additive solution. (A) ROS generation of PRP pooled PLTs by flow cytometry (2′7′-dichlorofluorescein). Mean fluorescence intensity (MFI) is plotted. (B) Correlation and linear regression between increasing proton leak during RT storage and ROS generation. (C) Correlation and linear regression between increasing proton leak during cold storage and ROS generation. Total platelet storage bags tested N = 3. * P < .05; **P < .01; **P < .001 between RT/vehicle and cold/vehicle storage or between cold/vehicle and cold/NAC groups. ##, P < .01; ### P < .001 between cold - vehicle and cold - NAC.
N.S. = nonsignificant when compared to RT or cold by itself for storage between Days 7 and 14
3.4 ∣. Cold storage of PRP pooled PLTs for 7 to 14 days results in increased macrophage-dependent phagocytosis partly preventable by NAC
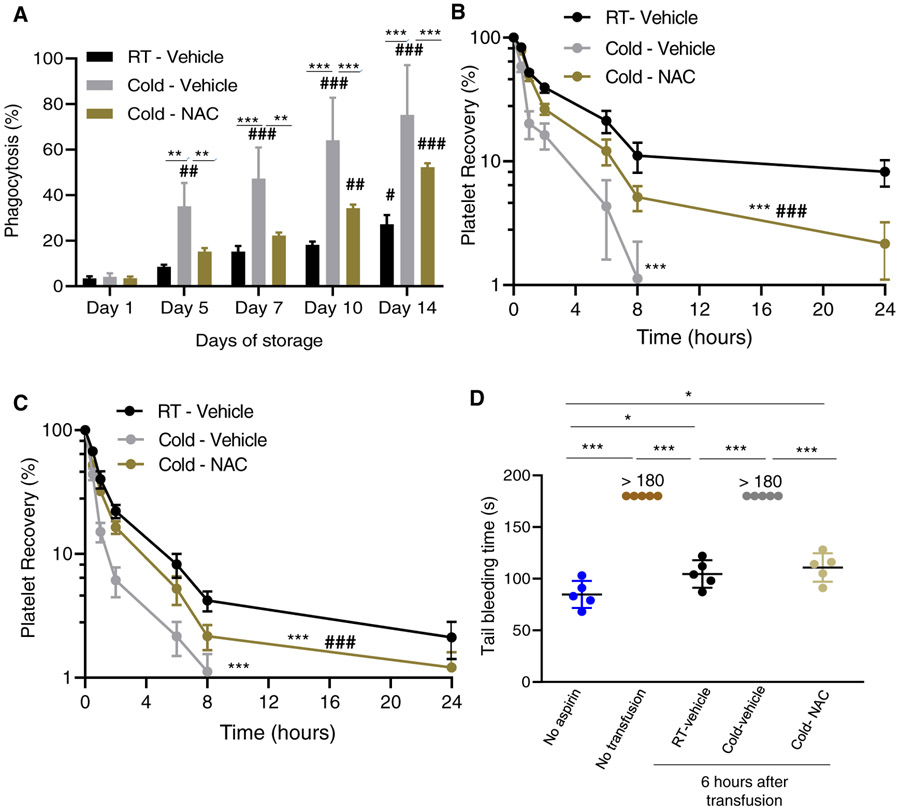
Cold-induced damaged PLT lesions include reorganization of membrane GPs (eg, GPIa, GPIIb/IIIa,9-11 which result in recognition and clearance by internalization (phagocytosis) mediated by macrophage integrins (αMβ2 integrin,35 or hepatocyte Ashwell-Morell receptors.36To determine the status of the macrophage-dependent phagocytosis, we analyzed macrophage-dependent PLT phagocytosis by activated THP-1 cells.9 Length of storage resulted in a modest increase of the macrophage-dependent phagocytosis of 14-day RT-stored PLTs compared with Day 1 stored PLTs (Figure 4A). Cold storage induced a severe PLT damage that resulted in storage length–dependent macrophage-dependent phagocytosis compared with Day 1 macrophage-dependent phagocytosis of cold-stored damaged PLTs was prevented by the addition of the antioxidant NAC to the PAS during the first week of storage and partly prevented for PLTs stored for over 7 days. These data support the concept that long-term oxidative stress is the main mediator of PLT damage resulting in macrophage-dependent phagocytosis. The antioxidant NAC can significantly ameliorate the cold-induced storage damage.
FIGURE 4.
Addition of the antioxidant NAC (1 mM) to the PAS-E additive solution partially prevents the storage damage of PLTs as assessed functionally in vitro and in vivo. (A) Macrophage-dependent phagocytosis analysis using phorbol-myristate acetate induced THP-1 cells. Total PLT pools tested N = 3. **P < .01; ***P < .001. (B) Recovery of 7-day cold stored in xenotransfused, clodronate treated, sublethally irradiated thrombocytopenic NSG immunodeficient mice (n = 8 mice/group). (C) Same as in C) but tested after 14 days of storage (n = 8 mice/group). * P < .05; **P < .01; ***P < .001; between RT/vehicle and cold/vehicle storage or between cold/vehicle and cold/NAC groups. # P < .05; ##, P < .01; ### P < .001 between cold/vehicle and cold/NAC. (D) Representative experiment (out of two experiments with similar results) on bleeding time analysis in aspirin-treated mice after infusion of indicated groups of PLTs (n = 5 mice/group).* P < .05; **P < .01, ***P < .001
3.5 ∣. Cold storage of PRP pooled PLTs for 7 to 14 days results in increased macrophage-independent in vivo clearance that is partly preventable by NAC
We used xenogeneic transfusion of carboxyfluoresceinlabeled stored PLTs in NSG immunodeficient mice37,38 with some modifications. Mice were sublethally irradiated (2.5 Gy) to induce moderate thrombocytopenia and macrophage depletion through the administration of clodronate liposomes. We opted to use this approach to complement our macrophage-dependent phagocytosis studies and use this in vivo model as an approach to measure hepatocytemediated clearance. PLTs that were cold stored for 7 (Figures 4B and S3A) or 14 days (Figures 4C and S3B) were cleared faster and more efficiently by macrophage-depleted NSG mice, with 1% or fewer circulating PLTs by 24 hours after administration (Figure 4B-C). Addition of 1 mM of NAC to the PAS resulted in partial extension of the survival of cold-stored human PLTs with levels of circulating PLTs higher than 1% for as long as 24 hours after administration (P < .001; Figure 4B-C) but did not reach the same levels as the RT-stored PLTs used as control (P < .001; Figure 4B-C). We chose the concentration of 1 mM because it does not modify the pH of the additive solution (data not shown) and has been shown to suffice to help the storage of RT-stored PLTs suspended in plasma.22 These changes were also evidenced by PLT counts in peripheral blood of recipient mice by 6 hours after transfusion (Figure S3C) and the area under the curve derived from the survival analyses (Figure S3A-B). These data demonstrate that ROS is a major mediator of the cold-storage damage of PLTs and that addition of a stable antioxidant can significantly prevent such damage during storage to extend the life span of transfused PLTs.
3.6 ∣. In vivo circulating PLTs derived from long-term, cold-stored PRP pooled PLTs can restore the bleeding time of thrombocytopenic, aspirin-treated mice
To determine whether cold-stored PLTs will affect the hemostatic function in vivo, we measured the bleeding time of transfused, macrophage-depleted NSG mice. Sublethally irradiated mice were subsequently aspirin treated and subsequently transfused with 7-day stored PLTs that had been stored at RT, in the cold, or in the cold with the addition of NAC to the PAS. PLT counts at 6 hours after transfusion were higher than the basal, pretransfusion counts for the groups who received RT-stored PLTs) and cold-stored with NAC compared with pretransfusion PLT counts or after transfusion of cold-stored PLTs (Figure S1D). RT-stored PLTs were able to correct the bleeding time at 6 hours after transfusion to levels similar to non–aspirin-treated mice (Figure 4D). Aspirin-treated mice that received RT-stored PLTs or PLTs stored in the cold with the addition of NAC to the PAS were able to correct the bleeding time significantly better than those mice that received 7-day cold-stored PLTs with no NAC (Figure 4D) and at close to the levels of mice that did not receive aspirin (Figure 4D). These data indicate that the addition of the antioxidant NAC results in the survival of functional PLTs that are able to substantially correct the bleeding time of aspirin-treated mice.
4 ∣. DISCUSSION
To our knowledge, this study identifies for the first time the role of mitochondrial metabolic dysfunction in which proton-leak– and electron-leak–dependent ROS generation upon >5 to 7 days of cold storage induces PLT oxidative stress. This oxidative stress results in in vitro macrophage-dependent and in vivo macrophage-independent PLT clearance, outcomes of cold-induced PLT lesions. The deteriorating effect of long-term cold storage is significantly dependent on oxidative damage since it can be prevented by the addition of a low but effective concentration (1 mM) of NAC,22 a US Food and Drug Administration (FDA)-approved drug, to the storage additive solution.
Storing PLTs in the cold results in an irreversible reduction in the survival of PLTs compared with RT-stored PLTs.7 The cytologic consequences of the cold storage damage have been a subject of study for decades.39-43 These consequences include microtubule disintegration and actin-based cytoskeleton rearrangements, channels of the open canalicular system become dilated, partial degranulation, formation of prominent pseudopods, increased cytosolic calcium concentration, and impaired αIIbβ3 integrin signaling.
Stolla et al44 found that cold-stored PLTs have a progressive decline in their survival upon autologous transfusion in humans within the first 10 days of storage with even further decline in the first 24-hour recovery when they are cold stored for up to 20 days. Clustering of GPIbα on the PLT surface was noticed following refrigeration of PLTs.8 The clustered GPIbα may contribute to the recognition of refrigerated PLTs by the lectin domain of the integrin αMβ2 on hepatic macrophages45 and when exposed to long-term cold storage by von Willebrand factor–mediated lectin binding to the Ashwell-Morell receptor of hepatocytes.36 Interestingly, refrigeration-induced GPIbα clustering is thought to induce PLT apoptosis.46
Studies on long-term RT-stored PLTs have shown increased ROS production associated with apoptotic events, including the cytochrome C release, caspase activation, and a loss of overall mitochondrial respiration capacity.47
Cold storage of PLTs in plasma has been associated with the formation of micro- and macroaggregates during cold storage, resulting in up to 18% of wasted products.48 Our experience (data not shown) suggests that agitation identical to RT storage eliminates the presence of visible PLT aggregates, and given the choice, we incorporated agitation in the cold-stored PLT group. The aggregate problem is also significantly mitigated with partial replacement of plasma for PAS, presumably because of decreased crosslinking between preactivated PLTs and fibrinogen or other plasma proteins.19
Increased ROS generation in cold-stored PLTs has been observed by several groups but not by others. The difference seems to be related to the type of ROS reporter used. When a global ROS reporter was used, cold storage was identified as a process that increases ROS generation,20,22 while there was no increase in the level of superoxide species,49 possibly due to SOD2 expressed by human PLT mitochondria.50 Our study confirms the observation that long-term cold storage results in dysmetabolic mitochondrial activity and ROS production, which may associate with increased inflammatory activation in vivo.51
Our study highlights the importance of using preclinical animal models to determine the outcomes of interventions that impact on PLT survival and function. NSG mice were used and validated for preclinical PLT survival studies to determine the effect of temperature cycling during cold storage.37,38,52,53 We have modified this animal model by adding sublethal irradiation, which results in thrombocytopenia (drop from approx. 1 100 000 PLTs/mm3 in untreated mice to approx. 185 000 PLTs/mm3) and macrophage depletion to further extend the life span of human PLTs, increase the sensitivity of the assay and determine the role of other than macrophages in PLT clearance. The fact that human GPIb is recognized by mouse von Willebrand factor54,55 allows the study of bleeding time in immunodeficient mice. Taking advantage of this fact, we have also used the same mouse model to determine the ability of human PLTs to correct the bleeding time of aspirin-treated mice and detected a good correlation between PLT counts at 6 hours after transfusion and the ability of transfused PLTs in correcting the murine bleeding time. Our data suggest that the modified NSG mouse model presented in this report may become a useful tool for studies in which new PAS can be tested preclinically.
Limitations to this study include the limited number of replicates for some of the experiments performed and the use of a pool-split design in which the products analyzed are not the pools themselves but fractions of the pools. The use of a paired (controlled) analysis side by side allows a fair comparison with a small number of pools analyzed.
Recently, the US Food and Drug Administration (FDA) and the US Department of Defense have indicated their interest in promoting research in cold storage of PLTs for transfusion.55 Undoubtedly, more studies are expected to result in better methods of cold storage that prevent refrigeration-induced damage while preserving the beneficial profile of cold-stored PLTs.
Our study here indicates that long-term cold storage induces mitochondrial uncoupling and increased proton leak and ROS generation, which is a crucial contributor to the increased macrophage-dependent and -independent clearance of functional PLTs. This can be prevented by the addition of the antioxidant NAC to a magnesium-containing PAS.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Judith Gonzalez, Michelle Bailey, Neeta Rugg, and Shawnagay Nestheide for the collection and manufacturing of whole blood–derived pooled PLT products. The authors also thank the mouse and flow cytometry cores of the Division of Experimental Hematology and Cancer Biology at CCHMC.
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Numbers: R01 HL147536, R43 HL123103; NIH Center for Accelerated Innovations at Cleveland Clinic; State of Ohio Third Frontier Program
Abbreviations:
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- CFSE
carboxyfluorescein succinimidyl-ester
- FDA
US Food and Drug Administration
- GP
glycoprotein
- NAC
N-acetylcysteine
- NSG
nonobese diabetic/severe combined immunodeficiency/γc−/−
- OCR
oxygen consumption rate
- PAS
platelet additive solution
- PBS
phosphate-buffered saline
- PLT
platelet
- PRP
platelet-rich plasma
- ROS
reactive oxygen species
- RT
room temperature
- SOD
superoxide dismutase
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Guan L, Tian X, Gombar S, et al. Big data modeling to predict platelet usage and minimize wastage in a tertiary care system. Proc Natl Acad Sci U S A. 2017;114(43):11368–11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slichter SJ, Corson J, Jones MK, et al. Exploratory studies of extended storage of apheresis platelets in a platelet additive solution (PAS). Blood. 2014;123(2):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumont LJ, Cancelas JA, Graminske S, et al. In vitro and in vivo quality of leukoreduced apheresis platelets stored in a new platelet additive solution. Transfusion. 2013;53(5): 972–980. [DOI] [PubMed] [Google Scholar]
- 4.van der Meer PF, de Korte D. Platelet additive solutions: a review of the latest developments and their clinical implications. Transfus Med Hemother. 2018;45(2):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerkhoffs JL, Eikenboom JC, Schipperus MS, et al. A multicenter randomized study of the efficacy of transfusions with platelets stored in platelet additive solution II versus plasma. Blood. 2006;108(9):3210–3215. [DOI] [PubMed] [Google Scholar]
- 6.Silliman CC, Fung YL, Ball JB, Khan SY. Transfusion-related acute lung injury (TRALI): current concepts and misconceptions. Blood Rev. 2009;23(6):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability–deleterious effect of refrigerated storage. N Engl J Med. 1969;280(20):1094–1098. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmeister KM, Felbinger TW, Falet H, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;112(1):87–97. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmeister KM, Josefsson EC, Isaac NA, Clausen H, Hartwig JH, Stossel TP. Glycosylation restores survival of chilled blood platelets. Science. 2003;301(5639):1531–1534. [DOI] [PubMed] [Google Scholar]
- 10.Babic AM, Josefsson EC, Bergmeier W, et al. In vitro function and phagocytosis of galactosylated platelet concentrates after long-term refrigeration. Transfusion. 2007;47(3):442–451. [DOI] [PubMed] [Google Scholar]
- 11.Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. Transfus Apher Sci. 2010; 42(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair PM, Pandya SG, Dallo SF, et al. Platelets stored at 4 degrees C contribute to superior clot properties compared to current standard-of-care through fibrin-crosslinking. Br J Haematol. 2017;178(1):119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker GA, Tuccelli M, Kunicki T, Chalos MK, Aster RH. Studies of platelet concentrates stored at 22 C nad 4 C. Transfusion. 1973;13(2):61–68. [DOI] [PubMed] [Google Scholar]
- 14.Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. II. Storage variables influencing platelet viability and function. Br J Haematol. 1976;34(3):403–419. [DOI] [PubMed] [Google Scholar]
- 15.Filip DJ, Aster RH. Relative hemostatic effectiveness of human platelets stored at 4 degrees and 22 degrees C. J Lab Clin Med. 1978;91(4):618–624. [PubMed] [Google Scholar]
- 16.Opheim EN, Apelseth TO, Stanworth SJ, Eide GE, Hervig T. Thromboelastography may predict risk of grade 2 bleeding in thrombocytopenic patients. Vox Sang. 2017;112(6):578–585. [DOI] [PubMed] [Google Scholar]
- 17.Reddoch-Cardenas KM, Sharma U, Salgado CL, et al. An in vitro pilot study of apheresis platelets collected on trima accel system and stored in T-PAS+ solution at refrigeration temperature (1-6 degrees C). Transfusion. 2019;59(5): 1789–1798. [DOI] [PubMed] [Google Scholar]
- 18.Reddoch-Cardenas KM, Montgomery RK, Lafleur CB, Peltier GC, Bynum JA, Cap AP. Cold storage of platelets in platelet additive solution: an in vitro comparison of two Food and drug administration-approved collection and storage systems. Transfusion. 2018;58(7):1682–1688. [DOI] [PubMed] [Google Scholar]
- 19.Getz TM, Montgomery RK, Bynum JA, Aden JK, Pidcoke HF, Cap AP. Storage of platelets at 4 degrees C in platelet additive solutions prevents aggregate formation and preserves platelet functional responses. Transfusion. 2016;56(6):1320–1328. [DOI] [PubMed] [Google Scholar]
- 20.Handigund M, Bae TW, Lee J, Cho YG. Evaluation of in vitro storage characteristics of cold stored platelet concentrates with N acetylcysteine (NAC). Transfus Apher Sci. 2016;54(1): 127–138. [DOI] [PubMed] [Google Scholar]
- 21.Akbar H, Duan X, Saleem S, Davis AK, Zheng Y. RhoA and Rac1 GTPases differentially regulate agonist-receptor mediated reactive oxygen species generation in platelets. PLoS One. 2016;11(9):e0163227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosseini E, Ghasemzadeh M, Atashibarg M, Haghshenas M. ROS scavenger, N-acetyl-l-cysteine and NOX specific inhibitor, VAS2870 reduce platelets apoptosis while enhancing their viability during storage. Transfusion. 2019;59(4):1333–1343. [DOI] [PubMed] [Google Scholar]
- 23.Hu Z, Yang YG. Full reconstitution of human platelets in humanized mice after macrophage depletion. Blood. 2012; 120(8):1713–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang KH, Sengupta A, Nayak RC, et al. p62 is required for stem cell/progenitor retention through inhibition of IKK/NF-kappaB/Ccl4 signaling at the bone marrow macrophageosteoblast niche. Cell Rep. 2014;9(6):2084–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma R, Xie R, Yu C, et al. Phosphatidylserine-mediated platelet clearance by endothelium decreases platelet aggregates and procoagulant activity in sepsis. Sci Rep. 2017;7(1):4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.del Conde I, Nabi F, Tonda R, Thiagarajan P, Lopez JA, Kleiman NS. Effect of P-selectin on phosphatidylserine exposure and surface-dependent thrombin generation on monocytes. Arterioscler Thromb Vasc Biol. 2005;25(5):1065–1070. [DOI] [PubMed] [Google Scholar]
- 27.Fager AM, Wood JP, Bouchard BA, Feng P, Tracy PB. Properties of procoagulant platelets: defining and characterizing the subpopulation binding a functional prothrombinase. Arterioscler Thromb Vasc Biol. 2010;30(12):2400–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosseini E, Mohtashami M, Ghasemzadeh M. Downregulation of platelet adhesion receptors is a controlling mechanism of thrombosis, while also affecting post-transfusion efficacy of stored platelets. Thromb J. 2019;17:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akbar H, Shang X, Perveen R, et al. Gene targeting implicates Cdc42 GTPase in GPVI and non-GPVI mediated platelet filopodia formation, secretion and aggregation. PLoS One. 2011;6(7):e22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kholmukhamedov A, Jobe S. Platelet respiration. Blood Adv. 2019;3(4):599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringwald J, Zimmermann R, Eckstein R. The new generation of platelet additive solution for storage at 22 degrees C: development and current experience. Transfus Med Rev. 2006;20(2): 158–164. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J, Nanayakkara G, Shao Y, et al. Mitochondrial proton leak plays a critical role in pathogenesis of cardiovascular diseases. Adv Exp Med Biol. 2017;982:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masselli E, Pozzi G, Vaccarezza M, et al. ROS in platelet biology: functional aspects and methodological insights. Int J Mol Sci. 2020;21, 4866:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbar H, Duan X, Piatt R, et al. Small molecule targeting the Rac1-NOX2 interaction prevents collagen-related peptide and thrombin-induced reactive oxygen species generation and platelet activation. J Thrombos Haemost. 2018;16(10): 2083–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torres-Gomez A, Cabanas C, Lafuente EM. Phagocytic Integrins: activation and signaling. Front Immunol. 2020;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rumjantseva V, Grewal PK, Wandall HH, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15(11):1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piper JT, Gelderman MP, Vostal JG. In vivo recovery of human platelets in severe combined immunodeficient mice as a measure of platelet damage. Transfusion. 2007;47(8):1540–1549. [DOI] [PubMed] [Google Scholar]
- 38.Gelderman MP, Cheng C, Xu F, et al. Validation of a SCID mouse model for transfusion by concurrent comparison of circulation kinetics of human platelets, stored under various temperature conditions, between human volunteers and mice. Transfusion. 2020;60:2379–2388. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmeister KM, Falet H, Toker A, Barkalow KL, Stossel TP, Hartwig JH. Mechanisms of cold-induced platelet actin assembly. J Biol Chem. 2001;276(27):24751–24759. [DOI] [PubMed] [Google Scholar]
- 40.Winokur R, Hartwig JH. Mechanism of shape change in chilled human platelets. Blood. 1995;85(7):1796–1804. [PubMed] [Google Scholar]
- 41.White JG, Krumwiede M. Influence of cytochalasin B on the shape change induced in platelets by cold. Blood. 1973;41(6): 823–832. [PubMed] [Google Scholar]
- 42.Kattlove HE, Alexander B, White F. The effect of cold on platelets. II. Platelet function after short-term storage at cold temperatures. Blood. 1972;40(5):688–696. [PubMed] [Google Scholar]
- 43.White JG, Krivit W. An ultrastructural basis for the shape changes induced in platelets by chilling. Blood. 1967;30(5):625–635. [PubMed] [Google Scholar]
- 44.Stolla M, Bailey SL, Fang L, et al. Effects of storage time prolongation on in vivo and in vitro characteristics of 4 degrees C-stored platelets. Transfusion. 2020;60(3):613–621. [DOI] [PubMed] [Google Scholar]
- 45.Josefsson EC, Gebhard HH, Stossel TP, Hartwig JH, Hoffmeister KM. The macrophage alphaMbeta2 integrin alphaM lectin domain mediates the phagocytosis of chilled platelets. J Biol Chem. 2005;280(18):18025–18032. [DOI] [PubMed] [Google Scholar]
- 46.van der Wal DE, Du VX, Lo KS, Rasmussen JT, Verhoef S, Akkerman JW. Platelet apoptosis by cold-induced glycoprotein Ibalpha clustering. J Thrombos Haemost. 2010;8(11):2554–2562. [DOI] [PubMed] [Google Scholar]
- 47.Skripchenko A, Myrup A, Thompson-Montgomery D, Awatefe H, Moroff G, Wagner SJ. Periods without agitation diminish platelet mitochondrial function during storage. Transfusion. 2010;50(2):390–399. [DOI] [PubMed] [Google Scholar]
- 48.Stubbs JR, Tran SA, Emery RL, et al. Cold platelets for traumaassociated bleeding: regulatory approval, accreditation approval, and practice implementation-just the "tip of the iceberg.". Transfusion. 2017;57(12):2836–2844. [DOI] [PubMed] [Google Scholar]
- 49.Bynum JA, Meledeo MA, Getz TM, et al. Bioenergetic profiling of platelet mitochondria during storage: 4 degrees C storage extends platelet mitochondrial function and viability. Transfusion. 2016;56(Suppl 1):S76–S84. [DOI] [PubMed] [Google Scholar]
- 50.Fidler TP, Rowley JW, Araujo C, et al. Superoxide dismutase 2 is dispensable for platelet function. Thromb Haemost. 2017; 117(10):1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen BZ, Xia R. Pro-inflammatory effects after platelet transfusion: a review. Vox Sang. 2020;115(5):349–357. [DOI] [PubMed] [Google Scholar]
- 52.Vostal JG, Gelderman MP, Skripchenko A, et al. Temperature cycling during platelet cold storage improves in vivo recovery and survival in healthy volunteers. Transfusion. 2018;58(1): 25–33. [DOI] [PubMed] [Google Scholar]
- 53.Skripchenko A, Gelderman MP, Awatefe H, et al. Automated cold temperature cycling improves in vitro platelet properties and in vivo recovery in a mouse model compared to continuous cold storage. Transfusion. 2016;56(1):24–32. [DOI] [PubMed] [Google Scholar]
- 54.Ware J, Russell S, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc Natl Acad Sci U S A. 2000;97(6):2803–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ware J, Russell SR, Marchese P, Ruggeri ZM. Expression of human platelet glycoprotein Ib alpha in transgenic mice. J Biol Chem. 1993;268(11):8376–8382. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.