Abstract
Schizophrenia is a severe neuropsychiatric disorder characterized by a diverse range of symptoms that can have profound impacts on the lives of patients. Currently available antipsychotics target dopamine receptors, and while they are useful for ameliorating the positive symptoms of the disorder, this approach often does not significantly improve negative and cognitive symptoms. Excitingly, preclinical and clinical research suggests that targeting specific muscarinic acetylcholine receptor subtypes could provide more comprehensive symptomatic relief with the potential to ameliorate numerous symptom domains. Mechanistic studies reveal that M1, M4, and M5 receptor subtypes can modulate the specific brain circuits and physiology that are disrupted in schizophrenia and are thought to underlie positive, negative, and cognitive symptoms. Novel therapeutic strategies for targeting these receptors are now advancing in clinical and preclinical development and expand upon the promise of these new treatment strategies to potentially provide more comprehensive relief than currently available antipsychotics.
Keywords: Muscarinic, Schizophrenia, Dopamine, Acetylcholine
1.1. Overview of schizophrenia and current treatments
Schizophrenia is a severe neurological disorder characterized by a complex and wide array of symptoms that can cause immense suffering for affected patients and their families. The term schizophrenia comes from the Greek for “splitting of the mind”; however, we have learned a lot about this disorder over the years with our current understanding reflecting that schizophrenia is a complex disorder that impacts multiple brain systems and has numerous genetic and environmental components [1]. Current antipsychotics differ in their pharmacological profiles but all share the common feature of reducing signaling of the neurotransmitter dopamine (DA) through the D2 subtype of DA receptor, a mechanism that may be necessary for antipsychotic efficacy of currently used therapeutics [2]. These medications broadly show efficacy in improving symptoms in the positive domain of the disorder, which includes hallucinations, disorganized speech, and delusions. However, these strategies often have profound side effect liabilities including undesirable metabolic and motor effects [3]. In addition to unwanted side effects, the inability of current therapies to treat negative and cognitive symptoms is another major drawback of both typical and atypical antipsychotics, as the anhedonia, lack of motivation, social withdrawal, and deficits in attention and working memory can be very difficult for patients to cope with [4].
Due to the wide array of symptoms, it should be no surprise that there are multiple theories and frameworks from which to view the development and treatment of schizophrenia. Views on how to best categorize schizophrenia patients have changed over the years but now many clinicians believe that schizophrenia is a syndrome spanning a spectrum of underlying etiologies [5]. The DA hypothesis of schizophrenia has been an important model for understanding how dysregulations of DA signaling relate to different symptoms. Briefly, this model posits that dysregulation of presynaptic DA release leads to 1.) DA hyperactivity in the striatum, which plays a key role in mediating positive symptoms and 2.) DA hypoactivity in cortical structures, which plays a key role in mediating negative and cognitive symptoms [6].
Typical (or first-generation antipsychotics, e.g. Haloperidol) are D2 receptor antagonists that can provide efficacy in treating positive symptoms. However, in some patients, the therapeutic window between antipsychotic efficacy and motor side effects is very narrow which greatly limits the utility of these treatments. The advent of atypical (or second-generation antipsychotics, e.g. Risperidone, and Clozapine) has provided relief for some of the patients that do not respond well to first-generation drugs [7]. Individual atypical antipsychotics have unique pharmacological profiles but are generally distinguished by less robust activity at the D2 receptor, as well as by their ability to modulate numerous other receptors including serotonin receptors (e.g. 5HT2A), adrenergic receptors, histamine receptors, and acetylcholine (ACh) receptors [8]. Some atypical antipsychotics even have weak agonist activity at the D2 receptor and this mechanism of action may offer advantages by providing some level of D2 receptor activity rather than complete blockade. However, these partial agonists are still thought to mediate their antipsychotic activity by reducing the ability of the full agonist DA to bind and signal through these receptors [9]. Due to these differences in pharmacology, atypical antipsychotics are generally characterized by reduced motor side effects, but they are also more likely to produce undesirable metabolic side effects [10, 11]. Similar to typical antipsychotics, the second-generation or atypical antipsychotics tend to show little to no efficacy in providing relief for negative and cognitive symptoms, and in some cases may even worsen symptoms [7, 12]. Given both the devastating consequences of negative and cognitive symptoms on patients’ quality of life, as well as the large number of patients that are resistant to currently available therapies, it is critical that we identify novel therapeutic strategies that move beyond D2 antagonism and provide more extensive relief that spans across multiple symptom domains.
1.2. Clozapine efficacy in treatment-resistant psychosis and effects on muscarinic acetylcholine receptor (mAChR) signaling
Many schizophrenia patients have treatment-resistant psychosis as defined by being non-responsive to at least two antipsychotic medications. This treatment-resistant clinical phenotype is a large problem and represents up to 30% of the schizophrenia patient population [13]. Clozapine has shown superior efficacy as a therapy for treatment-refractory psychosis compared to other antipsychotics [14], and shows some degree of efficacy in treating 60–70% of patients who are refractory to other atypical antipsychotic drugs [15]. In addition, clozapine is somewhat uncommon in its ability to improve cognitive symptoms in some subsets of patients [16], although this effect is inconsistent as a lack of efficacy or deleterious effects are observed in other patients [17]. What pharmacologically distinguishes clozapine from other antipsychotics to create this particular clinical profile? Several theories, including the balance of activity at 5HT2A, 5HT6, D2, and D4 receptors have been proposed, but do not appear to be unique to clozapine [18–20]. As discussed below, one possible explanation that differentiates clozapine from olanzapine and other treatments with seemingly similar pharmacological profiles is through a complicated bidirectional modulation of mAChRs by clozapine and its metabolites.
Clozapine itself has a high affinity for all 5 mAChR subtypes and has been observed to have either weak partial agonist activity (6–25% of a full agonist) [21, 22] or competitive antagonist activity [23, 24] in over-expressing cell lines. However, in striatal tissue (with endogenous receptor expression that is lower than over-expressing cell lines) clozapine acts as a competitive mAChR antagonist [25]. Regardless of the ability of clozapine to act as a true mAChR antagonist, or as a weak partial agonist, the net effect of clozapine in physiological tissue is a reduction in ACh signaling through mAChRs. However, the properties of clozapine as an antagonist / weak agonist of mAChRs does not distinguish clozapine from other antipsychotic medications, as many first-generation and second-generation antipsychotics can also reduce mAChR signaling.
Interestingly, it may be not clozapine per se that distinguishes this therapeutic from other antipsychotics, but the primary metabolite N-Desmethylclozapine (norclozapine). Following systemic administration, clozapine is readily metabolized to norclozapine in the liver by cytochrome P450 1A2 (CYP1A2), CYP3A4, and to a lesser extent by CYP2C9 and CYP2D6 [26]. Surprisingly, norclozapine is a robust agonist of mAChRs with particularly strong activity at the M1 receptor subtype [24, 27]. This M1 agonist activity is mediated via binding at an allosteric site and is observed both in over-expressing cells, and in acute rodent brain slices, where it can induce mAChR-dependent increases in N-methyl-D-aspartate (NMDA) receptor-mediated currents and MAPK signaling in hippocampal tissue [24, 27]. No other currently used antipsychotics, or their metabolites, are known to act as mAChR agonists, which raises the possibility that this agonist activity of norclozapine may be one of the unique properties that distinguish the parent drug clozapine from other atypical antipsychotics.
Several lines of evidence suggest that activation of M1 receptors plays a key role in the ability of clozapine to modulate schizophrenia-related circuitry and mediate behavioral efficacy in rodents. The ability of clozapine and norclozapine to reverse PCP-mediated deficits in novel object recognition are blocked by the inclusion of either scopolamine or an M1-selective antagonist, whereas mAChR blockade did not affect the efficacy of the atypical antipsychotic lurasidone [28]. Interestingly, sub-efficacious doses of clozapine in reversing MK-801-induced deficits in sensorimotor gating are potentiated by co-administration of an M1 positive allosteric modulator (PAM), an effect that is not observed in M1 knockout (KO) mice [29]. On a circuit level, local administration of norclozapine can alter extracellular DA content as assessed via microdialysis in the PFC and hippocampus without altering DA in the nucleus accumbens. While clozapine has no effects on DA in the PFC alone, local co-administration of clozapine completely blocks the effects of norclozapine [30], consistent with these molecules having opposing effects on M1 activation.
Interestingly, lower clozapine:norclozapine ratios in schizophrenia patients (which would be associated with a greater mAChR agonist profile) are associated with improvements in working memory and executive function while higher ratios are associated with cognitive deficits [31, 32]. The ratio of clozapine:norclozapine appears to be much more predictive of cognitive effects than either measure alone, which could be attributable to the conflicting effects of these compounds on mAChR activity. In recent clinical studies, it was found that norclozapine is well tolerated in patients [33]; however, a phase 2b trial of norclozapine was discontinued due to lack of efficacy [34, 35]. While the lack of efficacy for norclozapine in the clinic is disappointing, it is consistent with results from preclinical studies showing that direct administration of norclozapine alone does not have efficacy in preclinical models predictive of antipsychotic-like activity [36]. However, it is still unclear if norclozapine would show effects in the subset of patients with treatment-resistant schizophrenia patients, as the enrollment for the clinical trial was not restricted to this patient sub-group. It is also possible that the presence of the parent drug clozapine may be required to observe the beneficial effects of norclozapine. In addition to mediating beneficial effects, it is also possible that norclozapine could be responsible for some of the side effects seen following clozapine administration such as hyper-salivation [37], which is consistent with mAChR activation. While there is currently limited enthusiasm for advancing norclozapine as a stand-alone therapy, it is not yet clear if co-dosing clozapine/norclozapine with M1 PAMs, or combining clozapine administration with modulators of CYP1A2 metabolism to obtain a more favorable norclozapine:clozapine ratio [38] could improve outcomes. However, these data collectively fit with the hypothesis that mAChR activation (and M1 activation in particular) could mediate beneficial effects in ameliorating cognitive deficits in schizophrenia and could be a key signaling pathway through which clozapine exerts its unique clinical profile.
1.3. Cholinergic signaling and schizophrenia
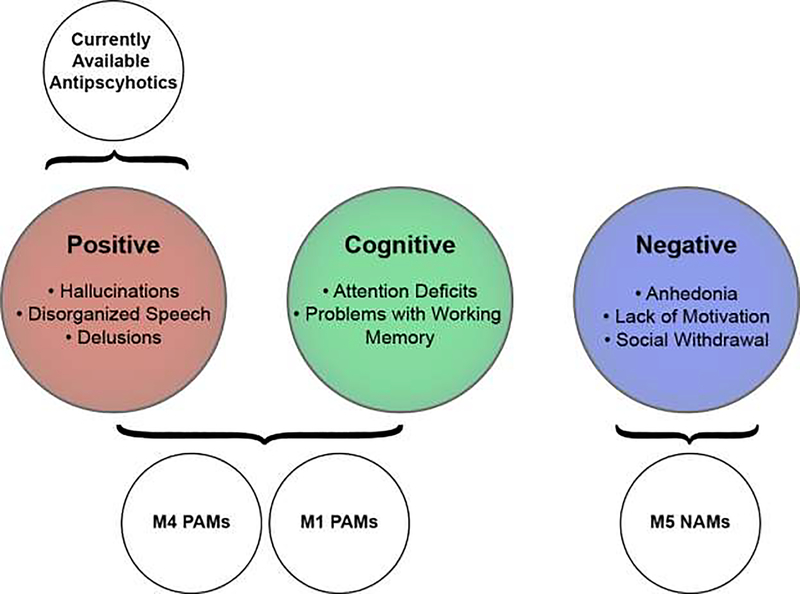
While the link between DA and schizophrenia has been extensively studied, it is clear that many other neurotransmitter systems are dysregulated in this disease. The psychotomimetic effects of NMDA receptor antagonists, as well as postmortem studies in schizophrenia patients [39], have led to a glutamatergic hypothesis of schizophrenia that highlights the importance of glutamatergic signaling, especially through NMDA receptors, as a key regulator of schizophrenia-related circuitry [40]. There is also growing evidence that inhibitory γ-aminobutyric acid (GABA) signaling is dysregulated in schizophrenia, particularly in the cortex [41]. Dopaminergic, GABAergic, and glutamatergic signaling can all be strongly modulated by the activation of the cholinergic system, and below we review clinical and preclinical data suggesting that modulation of mAChRs represents an exciting new strategy with the potential of providing broad symptomatic relief to schizophrenia patients that is not currently provided by available treatment strategies (Figure 1).
Figure 1: Muscarinic receptor based therapies have the potential to provide more comprehensive relief to schizophrenia patients than currently available dopamine receptor-based approaches.
Positive allosteric modulators (PAMs) targeting M4 and M1 receptor subtypes can mediate pro-cognitive and antipsychotic-like activity in preclinical animal models, while M5-selective negative allosteric modulators (NAMs) have potential in treating antihedonic and depressive symptoms. Collectively, targeting muscarinic receptors has the potential to alleviate all three symptom clusters in schizophrenia patients including cognitive and negative symptom clusters that are currently not alleviated by currently used antipsychotics.
The cholinergic system is comprised of two families of receptors that are activated by binding of the neurotransmitter acetylcholine (ACh). This includes a family of ion channels called nicotinic acetylcholine receptors (nAChRs), and a family of G-protein coupled receptors called muscarinic acetylcholine receptors (mAChRs). Both nAChRs and mAChRs have been found to modulate brain circuitry dysregulated in schizophrenia [42]. While nAChRs are primarily targeted for cognitive enhancement, the effects of mAChRs appear to have greater potential for providing relief for a wide range of schizophrenia symptoms. There are five subtypes of muscarinic receptors (M1-M5). M1, M3, and M5 typically couple to the G-protein Gαq leading to activation of phospholipase C, IP3 production, and Ca2+ mobilization. In contrast, M2 and M4 receptors couple to the G-protein Gαi resulting in a decrease in adenylyl cyclase function and reduced cAMP formation. It should be noted that these receptors are more complex than simply being an on/off switch for these canonical signaling pathways and can engender important physiological effects through numerous signal transduction pathways [43]. mAChR antagonists showing little to no selectivity can induce schizophrenia-like symptoms in healthy controls [44], and worsen positive symptoms while alleviating negative symptoms in schizophrenia patients[45]. Collectively, these early studies suggesting that targeted activation of specific mAChRs could be a novel therapeutic strategy for treating schizophrenia. As discussed in detail below and highlighted in Figure 1, the M1, M4, and M5 mAChR subtypes can all robustly modulate schizophrenia-related circuitry and are intriguing novel targets for moving schizophrenia treatments beyond the currently available dopamine receptor based approaches.
1.4. The mAChR agonist xanomeline shows efficacy in treating positive, negative, and cognitive symptoms
Pharmaceutical companies have been targeting mAChRs as potential cognitive enhancers for decades, owing to the robust pro-cognitive effects seen in both clinical and preclinical settings with compounds that either directly activate mAChRs or indirectly activate mAChRs by increasing cholinergic signaling (ie acetylcholinesterase inhibitors). Using traditional therapies that lack specificity among mAChR subtypes requires tailoring a therapy to hit a therapeutic window between beneficial effects (e.g. pro-cognitive efficacy) and classical cholinergic side effects such as salivation, lacrimation, urination, defecation, and emesis. Many of these side effects are mediated by peripherally expressed M2 and M3 receptors [46], making direct targeting of M1, M4, and M5 therapeutically attractive. The orthosteric binding pocket, or site where mAChRs bind ACh, is a highly conserved region of the different mAChR subtypes. This high level of conservation makes it challenging to design molecules targeting this site with appreciable subtype-selectivity. However, some agonists have been developed that, while lacking complete subtype-selectivity, show profiles of preferential activation of certain subtypes over others. One of the best-studied mAChR agonists in clinical studies of CNS disorders is the M1- and M4-preferring orthosteric agonist Xanomeline. When xanomeline was given to Alzheimer’s patients, there was a trend towards improving cognitive measures, but also an unexpected reduction in behavioral disturbances such as hallucinations, vocal outbursts, and agitation [47, 48]. This promising efficacy at reducing behavioral disturbances and improving cognitive deficits lead to a Phase II clinical trial in schizophrenia patients, where efficacy was examined in all symptom clusters by evaluating the Positive and Negative Syndrome Scale (PANSS) and the Brief Psychiatric Rating Scale (BPRS), Clinical Global Impression Scale (CGI), and a wide battery of tests to assess cognitive function. The 4-week, double-blind and placebo-controlled study discovered that Xanomeline significantly improved BRPS and PANSS scores in schizophrenia patients, as well as facilitated improvement in verbal learning and short-term memory function [49], which provides clinical evidence that targeting mAChR activation can provide broad symptomatic relief to schizophrenia patients. Unfortunately, in both trials xanomeline treatment also resulted in gastrointestinal distress in the form of nausea, indigestion, vomiting, sweating, and salivation, which led to a large discontinuation rate and ultimately caused the removal of this treatment from clinical consideration.
The gastrointestinal side effects seen with xanomeline fit with symptoms of classical cholinergic toxicity and are likely due to the activity of xanomeline at M2 and M3 receptors [46]. Recently, Karuna pharmaceuticals have adopted a novel clinically strategy for maintaining the clinical efficacy of xanomeline while mitigating the side effect profile. Their candidate, KarXT, is a co-formulation of xanomeline along with trospium, a non-selective mAChR antagonist. Trospium has been clinically used for decades in the treatment of overactive bladder syndrome and the safety and tolerability of this compound are well-documented [50]. Importantly, trospium has a quaternary amine in its structure that results in this drug being peripherally-restricted following oral dosing since it does not readily cross the blood-brain barrier [51]. By blocking the peripheral receptors with trospium, and activating the central M1 and M4 receptors with xanomeline, this strategy has the potential to activate the receptors mediating antipsychotic efficacy while avoiding activation of the peripheral receptors that mediate undesirable side effects. Critical to the success of this strategy is finding a therapeutic window of trospium that is below the level at which this compound can induce anticholinergic side effects [50], while simultaneously achieving an exposure that can competitively displace efficacious doses of xanomeline from peripheral mAChRs. Excitingly, Karuna announced results from a Phase II trial showing that KarXT was well tolerated and significantly reduced PANSS scores in schizophrenia patients [52]. While additional large-scale clinical studies are needed to further determine the efficacy of KarXT in schizophrenia patients, this is a very exciting and novel therapeutic strategy with great promise.
2.1. Allosteric modulators target specific mAChR subtypes
Xanomeline is referred to as “M1- and M4-preferring agonist” due to the increased potency and efficacy of this compound at M1 and M4 receptors compared to other mAChR subtypes. However, this compound can bind and activate all mAChR subtypes at higher concentrations [53, 54]. While recent rodent studies indicate that consequences of M4 receptor activation in vivo can be observed prior to activation of other mAChR subtypes [55], the therapeutic window between antipsychotic activity at M1 / M4 and gastrointestinal side effects seen with M2 / M3 activation is not sufficient for xanomeline to be considered as a standalone therapy. While the clinical efficacy of xanomeline serves as a proof-of-concept for targeting mAChRs for the treatment of schizophrenia, there is still a great need for developing compounds that are highly selective for M1 and/or M4 receptors and avoid activity at other mAChR subtypes. Several efforts have been made and have resulted in agonists that have slightly more favorable selectivity profiles than xanomeline [56]; however, finding highly selective agonists by targeting the orthosteric binding pocket has proved challenging. Recent advances in allosteric pharmacology (targeting binding pockets that are outside of the orthosteric binding pocket that binds to ACh) have provided great advances in achieving subtype-selectivity and are showing great promise potential as novel therapeutics for treating schizophrenia.
2.2. Allosteric modulation of M4 receptors for treatment of schizophrenia
One of the best-studied mAChR targets for schizophrenia is the M4 receptor subtype. In preclinical studies, the antipsychotic-like efficacy of xanomeline is lost in M4 KO mice [57], suggesting a key role for this subtype in mediating xanomeline efficacy related to positive symptoms. Several groups have had great success in discovering highly selective M4 compounds by targeting allosteric sites that are distinct from the ACh binding pocket [43]. This has led to the discovery of several PAMs from numerous chemical scaffolds that can robustly modulate M4 receptor signaling with little to no activity at other mAChR subtypes. M4 PAMs have been found to have robust efficacy in numerous preclinical assays that are predictive of antipsychotic efficacy including amphetamine- and apomorphine-disrupted prepulse inhibition, amphetamine- and MK-801-induced hyperlocomotion, and conditioned avoidance responding [58–62]. Importantly, the antipsychotic-like efficacy of M4 PAMs are absent in M4 KO mice and are not accompanied by side effect profiles in rodents that are seen with non-selective mAChR agonists [58]. Collectively, these studies suggest that selectively targeting M4 may confer the beneficial effects of xanomeline without the dose-limiting side effects.
An abundance of preclinical and clinical evidence supports the hypothesis that hyperactive DA signaling in the striatum is associated with positive symptoms of schizophrenia [6], and the cholinergic system can robustly modulate striatal DA signaling through the activation of both mAChRs and nAChRs [63]. Studying the contribution of different mAChRs to DA signaling is complicated by the fact that all 5 mAChR subtypes are expressed in the striatum [64–69], and that cholinergic effects are highly dependent on the receptor subtype being activated as well as by the mode of cholinergic signaling [63, 70, 71]. In the striatum, the M4 subtype is primarily expressed postsynaptically on direct pathway spiny projection neurons [65], on cholinergic interneurons where it acts as an autoreceptor [66], as well as on glutamatergic inputs where it acts as a heteroreceptor [72]. The M4 receptor populations on cholinergic interneurons and spiny projection neurons can both robustly modulate DA signaling, although through very different mechanisms. Activation of M4 autoreceptors on cholinergic interneurons can reduce ACh release and subsequent nAChR-dependent effects on DA [69], while activation of M4 receptors expressed on spiny projection neurons leads to an endocannabinoid-dependent inhibition of DA release that is nAChR-independent [60]. Selective deletion of M4 receptors from D1-expressing neurons (D1-M4 knockout mice) [73] results in the loss of functional M4 receptors from direct pathway SPNs and endocannabinoid-mediated reductions in DA release [60]. Interestingly, the antipsychotic-like efficacy of both xanomeline and M4 PAMs are absent in these mice [60, 74], suggesting that this receptor subpopulation is critical to the behavioral efficacy of these compounds. The ability of M4 PAMs to reduce DA release in the striatum via locally mobilized endocannabinoid signaling provides a mechanism whereby striatal DA signaling can be reduced without altering DA release in other brain regions such as the cortex and hippocampus. This provides M4 PAMs with a key advantage over currently utilized antipsychotics that antagonize D2 receptors throughout the brain.
In addition to exerting important modulator effects on striatal signaling, the M4 receptor can also robustly modulate hippocampal circuitry involved in cognitive processes. Activation of the M4 receptor with numerous highly selective M4 PAMs from different chemical scaffolds can robustly inhibit excitatory potentials at the Schaffer collateral inputs to CA1 without affecting either temporoammonic inputs or inhibitory inputs in ex vivo slices [75, 76]. Further in vivo studies have shown that M4 PAMs can reduce CA1 pyramidal activity in vivo and reverse deficits in spatial learning and memory as assessed using the Morris Water maze [77]. Imaging studies have shown that M4 PAM administration can normalize amphetamine-induced changes in hippocampal activity [78], and M4 PAM administration can dose-dependently reverse MK-801-induced deficits in both a visual pairwise discrimination task and contextual fear conditioning [58]. Interestingly, chronic administration of M4 PAMs can also enhance the rate of acquisition in pairwise discrimination in WT, but not M4 KO mice, suggesting that M4 PAMs can enhance cognition under both basal conditions and MK-801 disrupted conditions [79]. These exciting findings suggest that M4 PAMs have the potential to not only treat positive symptoms in schizophrenia, but to alleviate cognitive deficits as well.
Collectively, these preclinical studies have generated great enthusiasm about targeting the M4 receptor for the treatment of schizophrenia. The recent development of a radiolabeled M4 PAM PET tracer has opened up the possibility of assessing receptor occupancy in both non-human primates and patients [80], which will greatly facilitate efforts to determine relevant dose ranges. In addition, a phase 1b study of the M4 PAM CVL-231 was recently initiated in schizophrenia patients [35], which will provide important information on the safety and tolerability of M4 PAMs. The next few years promise to be an exciting time for M4 PAM research as we continue to learn more about M4 receptor function in relation to schizophrenia as well as the safety and utility of M4 PAMs in the clinic.
2.2. Allosteric modulation of M1 receptors for treatment of schizophrenia
The M1 receptor subtype is a well-studied therapeutic target for providing cognition-enhancing effects, particularly via modulation of memory and attentional processes. One of the first M1-selective PAMs discovered was BQCA, which has been demonstrated to have robust pro-cognitive efficacy in rodents where it can improve the rate of acquisition in a pairwise discrimination task, reverse deficits in memory tasks and contextual fear conditioning, and modulate sleep-wake architecture in rodents and nonhuman primates [81, 82]. Several M1-selective PAMs and agonists have been discovered that possess more desirable physiochemical properties than BQCA and can robustly facilitate novel object recognition [83–85] and improve performance in sustained attention tasks [86] in rodents. In non-human primates M1 PAM administration has been shown to improve paired associates learning, continuous-performance task performance, and working memory [87, 88], further demonstrating the pro-cognitive effects of M1 receptor potentiation. M1 agonists and PAMs can also reverse deficits in reversal learning, social interaction, novel object recognition, and fear conditioning in rodents [84, 89, 90], providing exciting evidence that targeting M1 may be able to provide efficacy in treating both cognitive and negative symptoms.
The behavioral efficacy of M1-selective compounds in the preclinical studies described above are thought in large part to be mediated via M1 receptors that are expressed in the cortex and hippocampus. Both of these brain regions are highly involved in memory processing and top-down cognitive processing and express M1 receptors that can robustly modulate neuronal function. In the hippocampus, M1 receptor activation can increase the excitability of CA1 pyramidal neurons [75, 91], and can potentiate NMDA receptor-dependent long-term potentiation of glutamatergic signaling onto CA1 neurons [92, 93]. In the prefrontal cortex (PFC), M1 activation can induce long-term depression (LTD) of glutamatergic signaling [89] via a mechanism that depends on endocannabinoid signaling [94] and phospholipase D signaling [95]. M1-mediated plasticity of inputs to PFC pyramidal neurons is input-specific with robust LTD observed on hippocampal and amygdala inputs, with no LTD observed at thalamic terminals [96]. Interestingly, M1 mediated LTD can be observed with M1 PAMs but not with M1 agonists [83], suggesting that avoiding agonist activity may be key to modulating critical cognitive circuitry as well as in avoiding undesirable side effects [84]. Importantly, M1 PAMs can reverse deficits in mAChR-LTD induced by subchronic administration of NMDA receptor antagonist [89] and deficits in mAChR-LTD observed in a model of NMDA receptor hypofunction [97], suggesting that normalization of disrupted PFC plasticity may play a key role in the ability of M1 PAMs to reverse cognitive and social deficits in these models.
While the M1 receptor can robustly modulate cognitive related circuits, accumulating data suggests that M1 receptor expression is reduced in a subset of schizophrenia patients. Post-mortem studies have shown decreased M1 protein, RNA, and radioligand binding in the cortex of schizophrenia patients compared to controls [98], with no significant changes observed with other mAChR subtypes [99]. This downregulation of M1 receptors is only observed in ~25% of schizophrenia patients, who are collectively referred to as a muscarinic receptor deficit sub-group (MRDS; for review see [100]). The deficits in M1 receptor expression are brain region-specific as M1 receptor-expressing neurons are reduced in the cortex, but not thalamus or hippocampus, of schizophrenia patients compared to controls [101]. In particular, cortical pyramidal neurons in laminae III and V appear to be vulnerable to changes in M1 expression, as the number of M1 receptor-positive neurons in these laminae were reduced in MRDS patients, and to a lesser, but still significant, effect reduced in non-MRDS patients [101]. Changes in M1 receptor expression on specific neuronal populations could profoundly impact the efficacy of compounds targeting this receptor as agonist and allosteric modulators require the receptor to be present in order to mediate their effects. However, a recently developed M1-selective PET-ligand suitable for use in non-human primates [102] is now under evaluation in human studies and has great potential to assist in identifying both MRDS patients and populations of patients who may be most likely to respond to M1-targeted therapies.
M1-selective compounds can be divided in ago-PAMs (compounds that show both agonist activity and allosteric modulator activity) and pure PAMs that do not directly activate the M1 receptor in the absence of acetylcholine. This distinction is especially important when considering the important temporal nature of cholinergic signaling with regards to cue detection in the prefrontal cortex and how this timing regulates PFC synchrony and cognitive processing [103, 104]. Pure PAMs would retain the pattern and timing of receptor activation to coincide with the activity of cholinergic projections to the PFC, while Ago-PAMs will activate the receptors even when cholinergic activity is silent [43]. Consistent with the idea that the temporal nature of cholinergic signaling must be preserved for optimal cognitive enhancement, it was found that ago-PAMs did not have the robust cognitive enhancing efficacy in rodent models that was observed with pure PAMs [83]. Compound with ago-PAM activity such as MK-7622 have also failed to demonstrate efficacy in the clinic [105], further supporting the idea that pure PAMs may provide an optimal profile for cognition enhancing efficacy and avoiding adverse side effects [83].
In addition to the cognitive enhancing properties, some M1-selective modulators have also shown antipsychotic-like efficacy in rodents. Administration of the M1 PAM TAK-071 can reverse MK-801-induced hyperlocomotion but does not reduce methamphetamine-induced hyperlocomotion [106], whereas the M1 ago-PAM PF-06767832 can reverse both amphetamine-induced hyperlocomotion and amphetamine-induced deficits in sensorimotor gating [107]. While the M1 PAM BQCA shows little to no efficacy in reversing MK-801-induced disruptions in sensorimotor gating, it can potentiate submaximal effects seen with atypical antipsychotics in an M1-dependent manner [29]. While M1 is still primarily considered as a target for enhancing cognition, the antipsychotic-like activity seen with some M1 PAMs and the possible importance of M1 modulation to the clinical efficacy of clozapine / norclozapine discussed above, raise the possibility that M1 receptors may be able to provide symptomatic relief across positive, negative, and cognitive symptom domains of schizophrenia.
2.3. Allosteric modulation of M5 receptors for treatment of schizophrenia
The M5 receptor is a Gαq coupled receptor with a unique expression pattern in the brain that makes it an interesting target for treating dopaminergic disorders including schizophrenia. While M5 expression in the brain accounts for less than 2% of total mAChR expression [108], it is the only mAChR that is expressed on DA neurons in both the substania nigra pars compacta and ventral tegmental area [67]. Accordingly, targeting of M5 could provide a strategy for targeting dopaminergic neuron activity that is devoid of direct effects in other brain regions.
The majority of studies using M5-selective compounds to date have identified the M5 receptor as a promising target for treating substance abuse [109, 110]. However, several lines of evidence suggest that M5 expressed in the midbrain DA could also play a key role in modulating schizophrenia symptom clusters. The mAChR subtypes expressed in the midbrain include the M5 subtype that is expressed on midbrain DA neurons [67], mAChR autoreceptors expressed on cholinergic inputs arising from the pedunculopontine nucleus and lateral dorsal tegmental nucleus [111], and M4 heteroreceptors expressed on GABAergic projections from the striatum [112]. Administration of non-selective mAChR antagonists such as scopolamine can induce psychosis in humans and exacerbate positive, negative, and cognitive symptoms in schizophrenia patients [44, 113]. In rodents, local injections of mAChR agonists and antagonists into the midbrain is sufficient to induce bidirectional changes in locomotion, stereotypy, reward, and immobility in the forced swim test [114–116], suggesting that this brain region plays a key role in the mediating many of the schizophrenia-like behavioral effects induced by systemic mAChR antagonist administration. Activation of somatic M5 receptors on DA neurons can induce robust physiological changes including the generation of a large inward current, mobilization of Ca2+ release, and increased excitability [68]. Interestingly, local application of an M5-selective negative allosteric modulator (NAM) into the ventral tegmental area can normalize disruptions in the forced swim test induced by a hyper-cholinergic state, suggesting that M5 neurons on DA neurons can robustly modulate depressive-like behaviors [117]. These exciting findings suggesting that M5 could be an interesting target for treating negative symptoms in schizophrenia.
In addition to effects at the level of DA cell bodies, activation of M5 receptors can also modulate DA release at the level of DA neuron terminals. Potentiation of M5 signaling with the M5 PAM VU0238429 can lead to an inhibition of DA release in the dorsal striatum, an effect that is paradoxically opposite to the excitatory effects seen with M5 expressed in DA cell bodies [68]. Interestingly, M5 activation can lead to an enhancement of DA release in the ventral striatum [69], suggesting that M5 effects on DA release are region-dependent. While the mechanism(s) whereby M5 activation can lead to different effects in different striatal sub-regions still need to be dissected, the ability of M5 to robustly modulate DA signaling via actions at both DA terminals and DA cell bodies makes it an interesting target in the context of positive symptoms in schizophrenia. One major drawback has been a lack of systemically available PAMs and NAMs with high selectivity for the M5 receptor. However, with the advent of highly M5-selective NAMs with good CNS penetration, we are starting to learn a lot of about this receptor and its potential to regulate substance abuse disorders [109, 118]. As medicinal chemistry efforts continue to discover new improved compounds targeting M5 [119, 120], these tools will be critical in elucidating the potential efficacy for M5-selective NAMs and PAMs in rodent models of positive and negative symptoms of schizophrenia.
3.1. Summary
Cholinergic signaling through mAChRs can robustly modulate circuitry that is involved in mediating positive, negative, and cognitive symptoms of schizophrenia. Several important landmarks have recently been passed as compounds targeting these receptors move into the clinic with the potential to provide desperately needed therapeutic strategies capable of providing more comprehensive relief from schizophrenia symptoms. One interesting area that has received relatively little attention has been potential sex differences with regards to mAChR regulation of schizophrenia related circuitry. Recent studies have highlighted how schizophrenia associated circuitry such as dopaminergic signaling is regulated in a sex-specific manner [121], and these changes could play a key role in influencing symptom severity, adverse effect liability, and potential therapeutic efficacy between male and female patients [11, 122]. While no studies to our knowledge have directly looked at sex differences of mAChR-specific modulators in schizophrenia patients, it has been reported that females respond more poorly to clozapine [123], a finding that could potentially be explained by differences in clozapine to norclozapine ratio in females [124] which could effect mAChR activity as discussed above. Collectively, these findings point to the need for more pre-clinical and clinical research into potential sex-specific differences that could be observed with different mAChR targeted therapies.
As discussed above, compounds targeting M1 have shown great efficacy in improving cognition in rodent models and some efficacy in mediating antipsychotic-like effects. As M1 allosteric modulators move into the clinic for the first time [125], we hope to learn more about the therapeutic potential of selectively targeting M1 for mediating cognitive enhancement in numerous disorders. Given the potential involvement of M1 receptors in the etiology of schizophrenia [100], it will be important to develop strategies to help classify patients and identify those that are most likely to benefit from M1-targeted therapies. As discussed above, the M4 receptor also shows great promise in modulating both the positive and cognitive symptoms in preclinical studies. The movement of allosteric compounds targeting this receptor into the clinic [35] represents an exciting opportunity to learn about the therapeutic efficacy of targeting M4. In addition, targeting M1 and M4 with xanomeline has already shown efficacy in ameliorating a diverse array of schizophrenia symptoms but has been limited by adverse side effects. Current trials pairing xanomeline with a peripherally restricted antagonist could provide an exciting new strategy for maintaining the efficacy of xanomeline without the dose-limiting peripheral side effects [52].Collectively, the studies described above highlight several new avenues to unlocking the exciting therapeutic potential of targeting mAChRs. Advancing beyond DA-centric schizophrenia therapies is a critical need and there is hope that targeting mAChRs may provide a path to improved clinical outcomes.
Acknowledgements:
The authors would like to thank the following funding sources for their generous support of this work: Vanderbilt Faculty Research Scholar award, NARSAD Young Investigator Award, and NIH R01MH122545 to D.J.F., and NIH R01MH073676 to P.J.C.
Abbreviations
- (DA)
Dopamine
- (mAChRs)
Muscarinic acetylcholine receptors
- (Ach)
Acetylcholine
- (nAChRs)
Nicotinic acetylcholine receptors
- (PAM)
Positive allosteric modulator
- (PANSS)
Positive and Negative Syndrome Scale
- (BPRS)
Brief Psychiatric Rating Scale
- (CGI)
Clinical Global Impression Scale
- (PFC)
Prefrontal cortex
- (LTD)
Long-term depression
- (GABA)
γ-aminobutyric acid
- (NMDA)
N-methyl-D-aspartate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Tandon R, Nasrallah HA, Keshavan MS, Schizophrenia, “just the facts” 4. Clinical features and conceptualization, Schizophr Res 110(1–3) (2009) 1–23. [DOI] [PubMed] [Google Scholar]
- [2].Kapur S, Remington G, Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient, Biol Psychiatry 50(11) (2001) 873–83. [DOI] [PubMed] [Google Scholar]
- [3].Sawa A, Snyder SH, Schizophrenia: neural mechanisms for novel therapies, Mol Med 9(1–2) (2003) 3–9. [PMC free article] [PubMed] [Google Scholar]
- [4].Galderisi S, Mucci A, Buchanan RW, Arango C, Negative symptoms of schizophrenia: new developments and unanswered research questions, Lancet Psychiatry 5(8) (2018) 664–677. [DOI] [PubMed] [Google Scholar]
- [5].Guloksuz S, van Os J, The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum, Psychol Med 48(2) (2018) 229–244. [DOI] [PubMed] [Google Scholar]
- [6].Howes OD, Kapur S, The dopamine hypothesis of schizophrenia: version III--the final common pathway, Schizophr Bull 35(3) (2009) 549–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McCutcheon RA, Reis Marques T, Howes OD, Schizophrenia-An Overview, JAMA Psychiatry (2019) 1–10. [DOI] [PubMed] [Google Scholar]
- [8].Mauri MC, Paletta S, Maffini M, Colasanti A, Dragogna F, Di Pace C, Altamura AC, Clinical pharmacology of atypical antipsychotics: an update, EXCLI J 13 (2014) 1163–91. [PMC free article] [PubMed] [Google Scholar]
- [9].Lieberman JA, Dopamine partial agonists: a new class of antipsychotic, CNS Drugs 18(4) (2004) 251–67. [DOI] [PubMed] [Google Scholar]
- [10].Nasrallah HA, Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles, Mol Psychiatry 13(1) (2008) 27–35. [DOI] [PubMed] [Google Scholar]
- [11].Iversen TSJ, Steen NE, Dieset I, Hope S, Morch R, Gardsjord ES, Jorgensen KN, Melle I, Andreassen OA, Molden E, Jonsson EG, Side effect burden of antipsychotic drugs in real life - Impact of gender and polypharmacy, Prog Neuropsychopharmacol Biol Psychiatry 82 (2018) 263–271. [DOI] [PubMed] [Google Scholar]
- [12].Purdon SE, Woodward N, Lindborg SR, Stip E, Procedural learning in schizophrenia after 6 months of double-blind treatment with olanzapine, risperidone, and haloperidol, Psychopharmacology (Berl) 169(3–4) (2003) 390–7. [DOI] [PubMed] [Google Scholar]
- [13].Lally J, Gaughran F, Timms P, Curran SR, Treatment-resistant schizophrenia: current insights on the pharmacogenomics of antipsychotics, Pharmgenomics Pers Med 9 (2016) 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Psychosis and Schizophrenia in Adults: Treatment and Management: Updated Edition 2014, London, 2014. [PubMed] [Google Scholar]
- [15].Meltzer HY, Treatment of the neuroleptic-nonresponsive schizophrenic patient, Schizophr Bull 18(3) (1992) 515–42. [DOI] [PubMed] [Google Scholar]
- [16].Hagger C, Buckley P, Kenny JT, Friedman L, Ubogy D, Meltzer HY, Improvement in cognitive functions and psychiatric symptoms in treatment-refractory schizophrenic patients receiving clozapine, Biol Psychiatry 34(10) (1993) 702–12. [DOI] [PubMed] [Google Scholar]
- [17].Nielsen RE, Levander S, Kjaersdam Telleus G, Jensen SO, Ostergaard Christensen T, Leucht S, Second-generation antipsychotic effect on cognition in patients with schizophrenia--a meta-analysis of randomized clinical trials, Acta Psychiatr Scand 131(3) (2015) 185–96. [DOI] [PubMed] [Google Scholar]
- [18].Meltzer HY, Matsubara S, Lee JC, Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values, J Pharmacol Exp Ther 251(1) (1989) 238–46. [PubMed] [Google Scholar]
- [19].Seeman P, Dopamine receptor sequences. Therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4, Neuropsychopharmacology 7(4) (1992) 261–84. [PubMed] [Google Scholar]
- [20].Miyamoto S, Duncan GE, Marx CE, Lieberman JA, Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs, Mol Psychiatry 10(1) (2005) 79–104. [DOI] [PubMed] [Google Scholar]
- [21].Olianas MC, Maullu C, Onali P, Mixed agonist-antagonist properties of clozapine at different human cloned muscarinic receptor subtypes expressed in Chinese hamster ovary cells, Neuropsychopharmacology 20(3) (1999) 263–70. [DOI] [PubMed] [Google Scholar]
- [22].Zorn SH, Jones SB, Ward KM, Liston DR, Clozapine is a potent and selective muscarinic M4 receptor agonist, Eur J Pharmacol 269(3) (1994) R1–2. [DOI] [PubMed] [Google Scholar]
- [23].Bolden C, Cusack B, Richelson E, Clozapine is a potent and selective muscarinic antagonist at the five cloned human muscarinic acetylcholine receptors expressed in CHO-K1 cells, Eur J Pharmacol 192(1) (1991) 205–6. [DOI] [PubMed] [Google Scholar]
- [24].Weiner DM, Meltzer HY, Veinbergs I, Donohue EM, Spalding TA, Smith TT, Mohell N, Harvey SC, Lameh J, Nash N, Vanover KE, Olsson R, Jayathilake K, Lee M, Levey AI, Hacksell U, Burstein ES, Davis RE, Brann MR, The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical effects of clozapine, Psychopharmacology (Berl) 177(1–2) (2004) 207–16. [DOI] [PubMed] [Google Scholar]
- [25].Olianas MC, Maullu C, Onali P, Effects of clozapine on rat striatal muscarinic receptors coupled to inhibition of adenylyl cyclase activity and on the human cloned m4 receptor, Br J Pharmacol 122(3) (1997) 401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dragovic S, Gunness P, Ingelman-Sundberg M, Vermeulen NP, Commandeur JN, Characterization of human cytochrome P450s involved in the bioactivation of clozapine, Drug Metab Dispos 41(3) (2013) 651–8. [DOI] [PubMed] [Google Scholar]
- [27].Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, Brandish PE, Pettibone DJ, Scolnick EM, Conn PJ, N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-D-aspartate receptor activity, Proc Natl Acad Sci U S A 100(23) (2003) 13674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miyauchi M, Neugebauer NM, Sato T, Ardehali H, Meltzer HY, Muscarinic receptor signaling contributes to atypical antipsychotic drug reversal of the phencyclidine-induced deficit in novel object recognition in rats, J Psychopharmacol 31(12) (2017) 1588–1604. [DOI] [PubMed] [Google Scholar]
- [29].Choy KH, Shackleford DM, Malone DT, Mistry SN, Patil RT, Scammells PJ, Langmead CJ, Pantelis C, Sexton PM, Lane JR, Christopoulos A, Positive Allosteric Modulation of the Muscarinic M1 Receptor Improves Efficacy of Antipsychotics in Mouse Glutamatergic Deficit Models of Behavior, J Pharmacol Exp Ther 359(2) (2016) 354–365. [DOI] [PubMed] [Google Scholar]
- [30].Li Z, Huang M, Ichikawa J, Dai J, Meltzer HY, N-desmethylclozapine, a major metabolite of clozapine, increases cortical acetylcholine and dopamine release in vivo via stimulation of M1 muscarinic receptors, Neuropsychopharmacology 30(11) (2005) 1986–95. [DOI] [PubMed] [Google Scholar]
- [31].Molins C, Carceller-Sindreu M, Navarro H, Carmona C, Pineiro M, Martinez E, Alvarez E, Portella MJ, Plasma ratio of clozapine to N-desmethylclozapine can predict cognitive performance in treatment-resistant psychotic patients, Psychiatry Res 258 (2017) 153–157. [DOI] [PubMed] [Google Scholar]
- [32].Rajji TK, Mulsant BH, Davies S, Kalache SM, Tsoutsoulas C, Pollock BG, Remington G, Prediction of working memory performance in schizophrenia by plasma ratio of clozapine to N-desmethylclozapine, Am J Psychiatry 172(6) (2015) 579–85. [DOI] [PubMed] [Google Scholar]
- [33].Safety Study of ACP-104: To Demonstrate the Safety, Tolerability, and Pharmacokinetics. https://ClinicalTrials.gov/show/NCT00628420.
- [34].Acadia Pharmaceuticals, 2008. https://ir.acadia-pharm.com/news-releases/news-release-details/acadia-pharmaceuticals-announces-results-acp-104-phase-iib?field_nir_news_date_value%5Bmin%5D=.
- [35].A Multiple Ascending Dose Trial of CVL-231 in Subjects With Schizophrenia. https://ClinicalTrials.gov/show/NCT04136873.
- [36].Natesan S, Reckless GE, Barlow KB, Nobrega JN, Kapur S, Evaluation of N-desmethylclozapine as a potential antipsychotic--preclinical studies, Neuropsychopharmacology 32(7) (2007) 1540–9. [DOI] [PubMed] [Google Scholar]
- [37].Baldessarini RJ, Frankenburg FR, Clozapine. A novel antipsychotic agent, N Engl J Med 324(11) (1991) 746–54. [DOI] [PubMed] [Google Scholar]
- [38].McArdle PA, De Mel V, DeMonte V, Winckel K, Gore-Jones V, Foley S, Korman N, Parker S, Dark F, Siskind D, An investigation into the relationship between clozapine treatment and cognitive performance in patients with treatment resistant schizophrenia, Schizophr Res 206 (2019) 450–451. [DOI] [PubMed] [Google Scholar]
- [39].Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G, Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications, Handb Exp Pharmacol (213) (2012) 267–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Moghaddam B, Javitt D, From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment, Neuropsychopharmacology 37(1) (2012) 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Egerton A, Modinos G, Ferrera D, McGuire P, Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis, Transl Psychiatry 7(6) (2017) e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jones CK, Byun N, Bubser M, Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia, Neuropsychopharmacology 37(1) (2012) 16–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Foster DJ, Conn PJ, Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders, Neuron 94(3) (2017) 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Osterholm RK, Camoriano JK, Transdermal scopolamine psychosis, JAMA 247(22) (1982) 3081. [PubMed] [Google Scholar]
- [45].Tandon R, Shipley JE, Greden JF, Mann NA, Eisner WH, Goodson JA, Muscarinic cholinergic hyperactivity in schizophrenia. Relationship to positive and negative symptoms, Schizophr Res 4(1) (1991) 23–30. [DOI] [PubMed] [Google Scholar]
- [46].Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC, Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity, Eur J Neurosci 17(7) (2003) 1403–10. [DOI] [PubMed] [Google Scholar]
- [47].Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM, Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease, Arch Neurol 54(4) (1997) 465–73. [DOI] [PubMed] [Google Scholar]
- [48].Bodick NC, Offen WW, Shannon HE, Satterwhite J, Lucas R, van Lier R, Paul SM, The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease, Alzheimer Dis Assoc Disord 11 Suppl 4 (1997) S16–22. [PubMed] [Google Scholar]
- [49].Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mallinckrodt C, Bymaster FP, McKinzie DL, Felder CC, Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia, Am J Psychiatry 165(8) (2008) 1033–9. [DOI] [PubMed] [Google Scholar]
- [50].Halaska M, Ralph G, Wiedemann A, Primus G, Ballering-Bruhl B, Hofner K, Jonas U, Controlled, double-blind, multicentre clinical trial to investigate long-term tolerability and efficacy of trospium chloride in patients with detrusor instability, World J Urol 20(6) (2003) 392–9. [DOI] [PubMed] [Google Scholar]
- [51].Todorova A, Vonderheid-Guth B, Dimpfel W, Effects of tolterodine, trospium chloride, and oxybutynin on the central nervous system, J Clin Pharmacol 41(6) (2001) 636–44. [DOI] [PubMed] [Google Scholar]
- [52].Karuna Therapeutics, 2019. https://investors.karunatx.com/news-releases/news-release-details/karuna-therapeutics-announces-karxt-met-primary-endpoint-phase-2.
- [53].Shannon HE, Bymaster FP, Calligaro DO, Greenwood B, Mitch CH, Sawyer BD, Ward JS, Wong DT, Olesen PH, Sheardown MJ, Swedberg MD, Suzdak PD, Sauerberg P, Xanomeline: a novel muscarinic receptor agonist with functional selectivity for M1 receptors, J Pharmacol Exp Ther 269(1) (1994) 271–81. [PubMed] [Google Scholar]
- [54].Bymaster FPW, C.A.; Shannon HE; DeLapp N; Ward JS; Calligaro DO; Shipley LA; Buelke-Sam JL; Bodick NC; Farde L; Sheardown MJ; Olesen PH; Hansen KT; Suzdak PD; Swedberg MDB; Sauerberg P; Mitch CH, Xanomeline: A Selective Muscarinic Agonist for the Treatment of Alzheimer’s Disease, DRUG DEVELOPMENT RESEARCH 40(2) (1997) 158–170. [Google Scholar]
- [55].Thorn CA, Moon J, Bourbonais CA, Harms J, Edgerton JR, Stark E, Steyn SJ, Butter CR, Lazzaro JT, O’Connor RE, Popiolek M, Striatal, Hippocampal, and Cortical Networks Are Differentially Responsive to the M4- and M1-Muscarinic Acetylcholine Receptor Mediated Effects of Xanomeline, ACS Chem Neurosci 10(3) (2019) 1753–1764. [DOI] [PubMed] [Google Scholar]
- [56].Heinrich JN, Butera JA, Carrick T, Kramer A, Kowal D, Lock T, Marquis KL, Pausch MH, Popiolek M, Sun SC, Tseng E, Uveges AJ, Mayer SC, Pharmacological comparison of muscarinic ligands: historical versus more recent muscarinic M1-preferring receptor agonists, Eur J Pharmacol 605(1–3) (2009) 53–6. [DOI] [PubMed] [Google Scholar]
- [57].Woolley ML, Carter HJ, Gartlon JE, Watson JM, Dawson LA, Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice, Eur J Pharmacol 603(1–3) (2009) 147–9. [DOI] [PubMed] [Google Scholar]
- [58].Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, Lamsal A, Niswender CM, Daniels JS, Poslusney MS, Melancon BJ, Tarr JC, Byers FW, Wess J, Duggan ME, Dunlop J, Wood MW, Brandon NJ, Wood MR, Lindsley CW, Conn PJ, Jones CK, Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents, ACS Chem Neurosci 5(10) (2014) 920–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, Christopoulos A, Lazareno S, Birdsall NJ, Bymaster FP, Felder CC, Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia, Proc Natl Acad Sci U S A 105(31) (2008) 10978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM, Conn PJ, Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release, Neuron 91(6) (2016) 1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Leach K, Loiacono RE, Felder CC, McKinzie DL, Mogg A, Shaw DB, Sexton PM, Christopoulos A, Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties, Neuropsychopharmacology 35(4) (2010) 855–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Suratman S, Leach K, Sexton P, Felder C, Loiacono R, Christopoulos A, Impact of species variability and ‘probe-dependence’ on the detection and in vivo validation of allosteric modulation at the M4 muscarinic acetylcholine receptor, Br J Pharmacol 162(7) (2011) 1659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rice ME, Patel JC, Cragg SJ, Dopamine release in the basal ganglia, Neuroscience 198 (2011) 112–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA, Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain, Neuroscience 63(1) (1994) 207–21. [DOI] [PubMed] [Google Scholar]
- [65].Ince E, Ciliax BJ, Levey AI, Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons, Synapse 27(4) (1997) 357–66. [DOI] [PubMed] [Google Scholar]
- [66].Yan Z, Surmeier DJ, Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway, J Neurosci 16(8) (1996) 2592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Weiner DM, Levey AI, Brann MR, Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia, Proc Natl Acad Sci U S A 87(18) (1990) 7050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Foster DJ, Gentry PR, Lizardi-Ortiz JE, Bridges TM, Wood MR, Niswender CM, Sulzer D, Lindsley CW, Xiang Z, Conn PJ, M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location, J Neurosci 34(9) (2014) 3253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shin JH, Adrover MF, Wess J, Alvarez VA, Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens, Proc Natl Acad Sci U S A 112(26) (2015) 8124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shin JH, Adrover MF, Alvarez VA, Distinctive Modulation of Dopamine Release in the Nucleus Accumbens Shell Mediated by Dopamine and Acetylcholine Receptors, J Neurosci 37(46) (2017) 11166–11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ, Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum, J Neurosci 30(9) (2010) 3398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pancani T, Bolarinwa C, Smith Y, Lindsley CW, Conn PJ, Xiang Z, M4 mAChR-mediated modulation of glutamatergic transmission at corticostriatal synapses, ACS Chem Neurosci 5(4) (2014) 318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Jeon J, Dencker D, Wortwein G, Woldbye DP, Cui Y, Davis AA, Levey AI, Schutz G, Sager TN, Mork A, Li C, Deng CX, Fink-Jensen A, Wess J, A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors, J Neurosci 30(6) (2010) 2396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dencker D, Wortwein G, Weikop P, Jeon J, Thomsen M, Sager TN, Mork A, Woldbye DP, Wess J, Fink-Jensen A, Involvement of a subpopulation of neuronal M4 muscarinic acetylcholine receptors in the antipsychotic-like effects of the M1/M4 preferring muscarinic receptor agonist xanomeline, J Neurosci 31(16) (2011) 5905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Thorn CA, Popiolek M, Stark E, Edgerton JR, Effects of M1 and M4 activation on excitatory synaptic transmission in CA1, Hippocampus 27(7) (2017) 794–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Shirey JK, Xiang Z, Orton D, Brady AE, Johnson KA, Williams R, Ayala JE, Rodriguez AL, Wess J, Weaver D, Niswender CM, Conn PJ, An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission, Nat Chem Biol 4(1) (2008) 42–50. [DOI] [PubMed] [Google Scholar]
- [77].Popiolek M, Mandelblat-Cerf Y, Young D, Garst-Orozco J, Lotarski SM, Stark E, Kramer M, Butler CR, Kozak R, In Vivo Modulation of Hippocampal Excitability by M4 Muscarinic Acetylcholine Receptor Activator: Implications for Treatment of Alzheimer’s Disease and Schizophrenic Patients, ACS Chem Neurosci 10(3) (2019) 1091–1098. [DOI] [PubMed] [Google Scholar]
- [78].Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishankar R, Kelm ND, Damon S, Bridges TM, Melancon BJ, Tarr JC, Brogan JT, Avison MJ, Deutch AY, Wess J, Wood MR, Lindsley CW, Gore JC, Conn PJ, Jones CK, Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100, Neuropsychopharmacology 39(7) (2014) 1578–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gould RW, Grannan MD, Gunter BW, Ball J, Bubser M, Bridges TM, Wess J, Wood MW, Brandon NJ, Duggan ME, Niswender CM, Lindsley CW, Conn PJ, Jones CK, Cognitive enhancement and antipsychotic-like activity following repeated dosing with the selective M4 PAM VU0467154, Neuropharmacology 128 (2018) 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tong L, Li W, Lo MM, Gao X, Wai JM, Rudd M, Tellers D, Joshi A, Zeng Z, Miller P, Salinas C, Riffel K, Haley H, Purcell M, Holahan M, Gantert L, Schubert JW, Jones K, Mulhearn J, Egbertson M, Meng Z, Hanney B, Gomez R, Harrison ST, McQuade P, Bueters T, Uslaner J, Morrow J, Thomson F, Kong J, Liao J, Selyutin O, Bao J, Hastings NB, Agrawal S, Magliaro BC, Monsma FJ Jr., Smith MD, Risso S, Hesk D, Hostetler E, Mazzola R, Discovery of [(11)C]MK-6884: A Positron Emission Tomography (PET) Imaging Agent for the Study of M4Muscarinic Receptor Positive Allosteric Modulators (PAMs) in Neurodegenerative Diseases, J Med Chem 63(5) (2020) 2411–2425. [DOI] [PubMed] [Google Scholar]
- [81].Gould RW, Dencker D, Grannan M, Bubser M, Zhan X, Wess J, Xiang Z, Locuson C, Lindsley CW, Conn PJ, Jones CK, Role for the M1 Muscarinic Acetylcholine Receptor in Top-Down Cognitive Processing Using a Touchscreen Visual Discrimination Task in Mice, ACS Chem Neurosci 6(10) (2015) 1683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, Jones K, Graufelds VK, Xu G, Pearson M, McCampbell A, Gaspar R, Shughrue P, Danziger A, Regan C, Flick R, Pascarella D, Garson S, Doran S, Kreatsoulas C, Veng L, Lindsley CW, Shipe W, Kuduk S, Sur C, Kinney G, Seabrook GR, Ray WJ, Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation, Proc Natl Acad Sci U S A 106(37) (2009) 15950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Moran SP, Dickerson JW, Cho HP, Xiang Z, Maksymetz J, Remke DH, Lv X, Doyle CA, Rajan DH, Niswender CM, Engers DW, Lindsley CW, Rook JM, Conn PJ, M1-positive allosteric modulators lacking agonist activity provide the optimal profile for enhancing cognition, Neuropsychopharmacology 43(8) (2018) 1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rook JM, Bertron JL, Cho HP, Garcia-Barrantes PM, Moran SP, Maksymetz JT, Nance KD, Dickerson JW, Remke DH, Chang S, Harp JM, Blobaum AL, Niswender CM, Jones CK, Stauffer SR, Conn PJ, Lindsley CW, A Novel M1 PAM VU0486846 Exerts Efficacy in Cognition Models without Displaying Agonist Activity or Cholinergic Toxicity, ACS Chem Neurosci 9(9) (2018) 2274–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sako Y, Kurimoto E, Mandai T, Suzuki A, Tanaka M, Suzuki M, Shimizu Y, Yamada M, Kimura H, TAK-071, a novel M1 positive allosteric modulator with low cooperativity, improves cognitive function in rodents with few cholinergic side effects, Neuropsychopharmacology 44(5) (2019) 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kucinski A, Phillips KB, Koshy Cherian A, Sarter M, Rescuing the attentional performance of rats with cholinergic losses by the M1 positive allosteric modulator TAK-071, Psychopharmacology (Berl) 237(1) (2020) 137–153. [DOI] [PubMed] [Google Scholar]
- [87].Galvin VC, Yang ST, Paspalas CD, Yang Y, Jin LE, Datta D, Morozov YM, Lightbourne TC, Lowet AS, Rakic P, Arnsten AFT, Wang M, Muscarinic M1 Receptors Modulate Working Memory Performance and Activity via KCNQ Potassium Channels in the Primate Prefrontal Cortex, Neuron 106(4) (2020) 649–661 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lange HS, Cannon CE, Drott JT, Kuduk SD, Uslaner JM, The M1 Muscarinic Positive Allosteric Modulator PQCA Improves Performance on Translatable Tests of Memory and Attention in Rhesus Monkeys, J Pharmacol Exp Ther 355(3) (2015) 442–50. [DOI] [PubMed] [Google Scholar]
- [89].Ghoshal A, Rook JM, Dickerson JW, Roop GN, Morrison RD, Jalan-Sakrikar N, Lamsal A, Noetzel MJ, Poslusney MS, Wood MR, Melancon BJ, Stauffer SR, Xiang Z, Daniels JS, Niswender CM, Jones CK, Lindsley CW, Conn PJ, Potentiation of M1 Muscarinic Receptor Reverses Plasticity Deficits and Negative and Cognitive Symptoms in a Schizophrenia Mouse Model, Neuropsychopharmacology 41(2) (2016) 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, Jadhav SB, Menon UN, Xiang Z, Watson ML, Christian EP, Doherty JJ, Quirk MC, Snyder DH, Lah JJ, Levey AI, Nicolle MM, Lindsley CW, Conn PJ, A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning, J Neurosci 29(45) (2009) 14271–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dasari S, Gulledge AT, M1 and M4 receptors modulate hippocampal pyramidal neurons, J Neurophysiol 105(2) (2011) 779–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Dennis SH, Pasqui F, Colvin EM, Sanger H, Mogg AJ, Felder CC, Broad LM, Fitzjohn SM, Isaac JT, Mellor JR, Activation of Muscarinic M1 Acetylcholine Receptors Induces Long-Term Potentiation in the Hippocampus, Cereb Cortex 26(1) (2016) 414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tigaret CM, Chamberlain SEL, Sadowski J, Hall J, Ashby MC, Mellor JR, Convergent Metabotropic Signaling Pathways Inhibit SK Channels to Promote Synaptic Plasticity in the Hippocampus, J Neurosci 38(43) (2018) 9252–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Martin HG, Bernabeu A, Lassalle O, Bouille C, Beurrier C, Pelissier-Alicot AL, Manzoni OJ, Endocannabinoids Mediate Muscarinic Acetylcholine Receptor-Dependent Long-Term Depression in the Adult Medial Prefrontal Cortex, Front Cell Neurosci 9 (2015) 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Moran SP, Xiang Z, Doyle CA, Maksymetz J, Lv X, Faltin S, Fisher NM, Niswender CM, Rook JM, Lindsley CW, Conn PJ, Biased M1 receptor-positive allosteric modulators reveal role of phospholipase D in M1-dependent rodent cortical plasticity, Sci Signal 12(610) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Maksymetz J, Joffe ME, Moran SP, Stansley BJ, Li B, Temple K, Engers DW, Lawrence JJ, Lindsley CW, Conn PJ, M1 Muscarinic Receptors Modulate Fear-Related Inputs to the Prefrontal Cortex: Implications for Novel Treatments of Posttraumatic Stress Disorder, Biol Psychiatry 85(12) (2019) 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Grannan MD, Mielnik CA, Moran SP, Gould RW, Ball J, Lu Z, Bubser M, Ramsey AJ, Abe M, Cho HP, Nance KD, Blobaum AL, Niswender CM, Conn PJ, Lindsley CW, Jones CK, Prefrontal Cortex-Mediated Impairments in a Genetic Model of NMDA Receptor Hypofunction Are Reversed by the Novel M1 PAM VU6004256, ACS Chem Neurosci (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E, Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia, Mol Psychiatry 7(10) (2002) 1083–91. [DOI] [PubMed] [Google Scholar]
- [99].Scarr E, Keriakous D, Crossland N, Dean B, No change in cortical muscarinic M2, M3 receptors or [35S]GTPgammaS binding in schizophrenia, Life Sci 78(11) (2006) 1231–7. [DOI] [PubMed] [Google Scholar]
- [100].Dean B, Scarr E, Muscarinic M1 and M4 receptors: Hypothesis driven drug development for schizophrenia, Psychiatry Res 288 (2020) 112989. [DOI] [PubMed] [Google Scholar]
- [101].Scarr E, Hopper S, Vos V, Seo MS, Everall IP, Aumann TD, Chana G, Dean B, Low levels of muscarinic M1 receptor-positive neurons in cortical layers III and V in Brodmann areas 9 and 17 from individuals with schizophrenia, J Psychiatry Neurosci 43(5) (2018) 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Nabulsi NB, Holden D, Zheng MQ, Bois F, Lin SF, Najafzadeh S, Gao H, Ropchan J, Lara-Jaime T, Labaree D, Shirali A, Slieker L, Jesudason C, Barth V, Navarro A, Kant N, Carson RE, Huang Y, Evaluation of (11)C-LSN3172176 as a Novel PET Tracer for Imaging M1 Muscarinic Acetylcholine Receptors in Nonhuman Primates, J Nucl Med 60(8) (2019) 1147–1153. [DOI] [PubMed] [Google Scholar]
- [103].Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M, Cortical cholinergic signaling controls the detection of cues, Proc Natl Acad Sci U S A 113(8) (2016) E1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Parikh V, Kozak R, Martinez V, Sarter M, Prefrontal acetylcholine release controls cue detection on multiple timescales, Neuron 56(1) (2007) 141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Voss T, Li J, Cummings J, Farlow M, Assaid C, Froman S, Leibensperger H, Snow-Adami L, McMahon KB, Egan M, Michelson D, Randomized, controlled, proof-of-concept trial of MK-7622 in Alzheimer’s disease, Alzheimers Dement (N Y) 4 (2018) 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mandai T, Kasahara M, Kurimoto E, Tanaka M, Suzuki M, Nakatani A, Kimura H, In Vivo Pharmacological Comparison of TAK-071, a Positive Allosteric Modulator of Muscarinic M1 Receptor, and Xanomeline, an Agonist of Muscarinic M1/M4 Receptor, in Rodents, Neuroscience 414 (2019) 60–76. [DOI] [PubMed] [Google Scholar]
- [107].Davoren JE, Lee CW, Garnsey M, Brodney MA, Cordes J, Dlugolenski K, Edgerton JR, Harris AR, Helal CJ, Jenkinson S, Kauffman GW, Kenakin TP, Lazzaro JT, Lotarski SM, Mao Y, Nason DM, Northcott C, Nottebaum L, O’Neil SV, Pettersen B, Popiolek M, Reinhart V, Salomon-Ferrer R, Steyn SJ, Webb D, Zhang L, Grimwood S, Discovery of the Potent and Selective M1 PAM-Agonist N-[(3R,4S)-3-Hydroxytetrahydro-2H-pyran-4-yl]-5-methyl-4-[4-(1,3-thiazol-4-yl)ben zyl]pyridine-2-carboxamide (PF-06767832): Evaluation of Efficacy and Cholinergic Side Effects, J Med Chem 59(13) (2016) 6313–28. [DOI] [PubMed] [Google Scholar]
- [108].Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, Weisstein JS, Spagnola BV, Wolfe BB, Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain, Mol Pharmacol 43(2) (1993) 149–57. [PubMed] [Google Scholar]
- [109].Gould RW, Gunter BW, Bubser M, Matthews RT, Teal LB, Ragland MG, Bridges TM, Garrison AT, Winder DG, Lindsley CW, Jones CK, Acute Negative Allosteric Modulation of M5 Muscarinic Acetylcholine Receptors Inhibits Oxycodone Self-Administration and Cue-Induced Reactivity with No Effect on Antinociception, ACS Chem Neurosci 10(8) (2019) 3740–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Berizzi AE, Perry CJ, Shackleford DM, Lindsley CW, Jones CK, Chen NA, Sexton PM, Christopoulos A, Langmead CJ, Lawrence AJ, Muscarinic M5 receptors modulate ethanol seeking in rats, Neuropsychopharmacology 43(7) (2018) 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kohlmeier KA, Ishibashi M, Wess J, Bickford ME, Leonard CS, Knockouts reveal overlapping functions of M(2) and M(4) muscarinic receptors and evidence for a local glutamatergic circuit within the laterodorsal tegmental nucleus, J Nesurophysiol 108(10) (2012) 2751–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Moehle MS, Pancani T, Byun N, Yohn SE, Wilson GH 3rd, Dickerson JW, Remke DH, Xiang Z, Niswender CM, Wess J, Jones CK, Lindsley CW, Rook JM, Conn PJ, Cholinergic Projections to the Substantia Nigra Pars Reticulata Inhibit Dopamine Modulation of Basal Ganglia through the M4 Muscarinic Receptor, Neuron 96(6) (2017) 1358–1372 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Tandon R, DeQuardo JR, Goodson J, Mann NA, Greden JF, Effect of anticholinergics on positive and negative symptoms in schizophrenia, Psychopharmacol Bull 28(3) (1992) 297–302. [PubMed] [Google Scholar]
- [114].Kofman O, McGlynn SM, Olmstead MC, Yeomans JS, Differential effects of atropine, procaine and dopamine in the rat ventral tegmentum on lateral hypothalamic rewarding brain stimulation, Behav Brain Res 38(1) (1990) 55–68. [DOI] [PubMed] [Google Scholar]
- [115].Mathur A, Shandarin A, LaViolette SR, Parker J, Yeomans JS, Locomotion and stereotypy induced by scopolamine: contributions of muscarinic receptors near the pedunculopontine tegmental nucleus, Brain Res 775(1–2) (1997) 144–55. [DOI] [PubMed] [Google Scholar]
- [116].Addy NA, Nunes EJ, Wickham RJ, Ventral tegmental area cholinergic mechanisms mediate behavioral responses in the forced swim test, Behav Brain Res 288 (2015) 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Nunes EJ, Rupprecht LE, Foster DJ, Lindsley CW, Conn PJ, Addy NA, Examining the role of muscarinic M5 receptors in VTA cholinergic modulation of depressive-like and anxiety-related behaviors in rats, Neuropharmacology 171 (2020) 108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gunter BW, Gould RW, Bubser M, McGowan KM, Lindsley CW, Jones CK, Selective inhibition of M5 muscarinic acetylcholine receptors attenuates cocaine self-administration in rats, Addict Biol 23(5) (2018) 1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Bender AM, Cho HP, Nance KD, Lingenfelter KS, Luscombe VB, Gentry PR, Voigtritter K, Berizzi AE, Sexton PM, Langmead CJ, Christopoulos A, Locuson CW, Bridges TM, Chang S, O’Neill JC, Zhan X, Niswender CM, Jones CK, Conn PJ, Lindsley CW, Discovery and Optimization of Potent and CNS Penetrant M5-Preferring Positive Allosteric Modulators Derived from a Novel, Chiral N-(Indanyl)piperidine Amide Scaffold, ACS Chem Neurosci 9(7) (2018) 1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].McGowan KM, Nance KD, Cho HP, Bridges TM, Conn PJ, Jones CK, Lindsley CW, Continued optimization of the M5 NAM ML375: Discovery of VU6008667, an M5 NAM with high CNS penetration and a desired short half-life in rat for addiction studies, Bioorg Med Chem Lett 27(6) (2017) 1356–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zachry JE, Nolan SO, Brady LJ, Kelly SJ, Siciliano CA, Calipari ES, Sex differences in dopamine release regulation in the striatum, Neuropsychopharmacology 46(3) (2021) 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Gogos A, Sbisa AM, Sun J, Gibbons A, Udawela M, Dean B, A Role for Estrogen in Schizophrenia: Clinical and Preclinical Findings, Int J Endocrinol 2015 (2015) 615356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Alberich S, Fernandez-Sevillano J, Gonzalez-Ortega I, Usall J, Saenz M, Gonzalez-Fraile E, Gonzalez-Pinto A, A systematic review of sex-based differences in effectiveness and adverse effects of clozapine, Psychiatry Res 280 (2019) 112506. [DOI] [PubMed] [Google Scholar]
- [124].Anderson SG, Livingston M, Couchman L, Smith DJ, Connolly M, Miller J, Flanagan RJ, Heald AH, Sex differences in plasma clozapine and norclozapine concentrations in clinical practice and in relation to body mass index and plasma glucose concentrations: a retrospective survey, Ann Gen Psychiatry 14 (2015) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Putative Cognitive Enhancer VU319. https://ClinicalTrials.gov/show/NCT03220295.