Abstract
Cell lines derived from Trichoplusia ni (Tn) are widely used as hosts in the baculovirus-insect cell system (BICS). One advantage of Tn cell lines is they can produce recombinant proteins at higher levels than cell lines derived from other insects. However, Tn cell lines are persistently infected with an alphanodavirus, Tn5 cell line virus (TnCLV), which reduces their utility as a host for the BICS. Several groups have isolated TnCLV-negative Tn cell lines, but none were thoroughly characterized and shown to be free of other adventitious viruses. Thus, we isolated and extensively characterized a new TnCLV-negative line, Tn-Nodavirus Negative (Tn-NVN). Tn-NVN cells have no detectable TnCLV, no other previously identified viral contaminants of lepidopteran insect cell lines, and no sequences associated with any replicating virus or other viral adventitious agent. Tn-NVN cells tested negative for >60 species of Mycoplasma, Acholeplasma, Spiroplasma, and Ureaplasma. Finally, Tn-NVN cells grow well as a single cell suspension culture in serum-free medium, produce recombinant proteins at levels similar to High Five™ cells, and do not produce recombinant glycoproteins with immunogenic core α1,3-fucosylation. Thus, Tn-NVN is a new, well-characterized TnCLV-negative cell line with several other features enhancing its utility as a host for the BICS.
Keywords: adventitious virus, alphanodavirus, baculovirus-insect cell system, massively parallel sequencing, Trichoplusia ni cell line virus, Tn-NVN
1 |. INTRODUCTION
The baculovirus-insect cell system (BICS) is widely utilized as a recombinant protein production platform for research and biological products manufacturing (Contreras-Gómez, Sánchez-Mirón, García-Camacho, Molina-Grima, & Chisti, 2014; Kost, Condreay, & Jarvis, 2005). The insect cell lines most frequently used as hosts in the BICS are derived from two moth species: the fall armyworm, Spodoptera frugiperda (Sf), and the cabbage looper, Trichoplusia ni (Tn). The most commonly used Tn cell line is BTI-Tn-5B1–4, also known as “High Five™” (Wickham, Davis, Granados, Shuler, & Wood, 1992). It has been reported that High Five™ cells can produce recombinant proteins at higher levels than Sf cell lines, such as Sf9 (Davis et al., 1993; Krammer et al., 2010; Rhiel, Mitchell-Logean, & Murhammer, 1997; Sander & Harrysson, 2007; R. A. Taticek, Choi, Phan, Palomares, & Shuler, 2001; Wickham et al., 1992; Wickham & Nemerow, 1993; Wilde, Klausberger, Palmberger, Ernst, & Grabherr, 2014). Thus, higher productivity is widely considered to be an important advantage of High Five™ cells. However, there is a significant problem with High Five™ and other Tn cell lines, which is these cells are contaminated with a novel alphanodavirus, Tn5 cell line virus (TnCLV; (Li, Scotti, Miyamura, & Takeda, 2007).
The true extent of the problem posed by this viral contaminant is unclear. It certainly complicates the use of Tn cells as a substrate for virus-like particle (VLP) production and purification, as illustrated by the fact that TnCLV was originally discovered as a contaminant of hepatitis E VLPs produced using these cells (Li et al., 2007). Perhaps more importantly, contamination of Tn cell lines with TnCLV generally diminishes their biosafety profile. While alphanodaviruses appear to be restricted to invertebrates (Schneemann, Reddy, & Johnson, 1998), published studies on the TnCLV host range have only been performed using insect cell lines (Geisler & Jarvis, 2018). Thus, it is unknown whether or not TnCLV can infect mammalian cells. On the other hand, several studies have shown Nodamuravirus, another alphanodavirus, can induce flaccid paralysis and death in suckling mice (Garzon, Strykowski, & Charpentier, 1990; Murphy, Scherer, Harrison, Dunne, & Gary, 1970; Scherer, Verna, & Richter, 1968). This finding validates concerns about the potential risks associated with TnCLV, particularly considering Tn cells are used not only as a substrate to produce recombinant proteins for research purposes, but also to produce biological products for human medicine in the biotechnology industry (CDC, 2010; Felberbaum, 2015).
Because the risk to human patients has not yet been clearly assessed, effective TnCLV clearance and/or inactivation and strict quality control are needed to ensure biological products manufactured in TnCLV-contaminated cell lines are safe. However, a more direct and reliable approach would be to use a TnCLV-negative cell line. Several TnCLV-negative Tn cell lines have been isolated and presented as potential alternatives for TnCLV-contaminated lines (Table 1). The best alternative would also have some other features to enhance its utility as a host for the BICS. One would be the ability to produce recombinant proteins at high yields in serum-free medium. Another would be the ability to grow quickly and consistently as a suspension culture in serum-free medium without clumping, which is a notorious problem with High Five™ cells (Dee, Shuler, & Wood, 1997; Dee, Wood, & Shuler, 1997; Donaldson & Shuler, 1998; M. A. Saarinen & Murhammer, 2000; R. A. Taticek et al., 2001; R.A. Taticek, McKenna, Granados, & Shuler, 1997; Wickham & Nemerow, 1993). The species of origin would be confirmed, the cell line would be clonally derived, phenotypically stable, and its history well documented. Finally, the best alternative would be a Tn cell line that not only has no detectable TnCLV, but also has no other adventitious viruses, several of which were recently discovered in other cell lines used as hosts for the BICS (Geisler & Jarvis, 2018). While previously described TnCLV-negative Tn cell lines have been characterized to varying degrees, none has been shown to have the full set of other beneficial features described above (see Table 1).
Table 1:
Key properties of High Five™, previously reported TnCLV-negative Tn, and Tn-NVN cell lines.
| Cell line | References | Growth in SFM | Productivity in SFM | Single cell suspension culture | Peak cell density [106 cells / mL] | Clonal | Species of origin confirmed | Tn-CLV free | Free of other adventitious agents | Genome / transcrtiptome available |
Free of immunogenic Coreα−3 fucosylated N-glycans? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BTI-Tn-5B1–4 (High Five™) | (R. R. Granados, 1991; Robert R. Granados, Guoxun, Derksen, & McKenna, 1994; Wickham et al., 1992) | YES | YES | NO1 | ~ 5 | YES | YES | NO | ? | YES (Fu et al., 2018)/ YES (Yu et al., 2016) | NO |
| MSU-TnT4 (TnT4) | (Thiem, 2011; Zhang, Manzan, Peplinski, & Thiem, 2008; Zhang & Thiem, 2010) | ? | ? | ? | < 1 | NO | NO2 | YES | ? | NO / NO | ? |
| QAU-BTI-Tn9–4s | (Meng, Li, Li, & Li, 2008; Zheng, Zhou, & Li, 2014) | YES | YES | YES | ~ 4.5 | NO | NO3 | YES | ? | NO / NO | ? |
| QB-CL-B | (Shan, Zhang, Jiang, Ma, & Li, 2011) | ? | ? | YES | 1.6 | YES | NO | ?4 | ? | NO / NO | ? |
| BTI-Tnao38 | (Y. Hashimoto et al., 2010; Yoshi Hashimoto et al., 2012) | YES | YES | YES5 | 2.5 | YES | YES | YES | ? | NO / NO | ? |
| Tnms42 | (Chen et al., 2014; Chen et al., 2013) | ? | ? | ? | ? | YES | YES6 | YES | ? | NO / YES (Chen et al., 2013) | ? |
| Tn NVN | This study | YES | YES | YES | 6.5 | YES | YES | YES | YES | YES / YES This study | YES |
Some labs have reportedly adapted High Five™ to single cell suspension cultures (D. Esposito, personal communication) (Rhiel et al., 1997; Mark A. Saarinen, Troutner, Gladden, Mitchell-Logean, & Murhammer, 1999), but without adaptation or polyanionic supplements, High Five cells typically form large aggregates when cultured in suspension.
DNA fingerprinting was used to determine TnT4 was genetically distinct from Sf21.
DNA fingerprinting was used to determine QAU-BTI-Tn9–4s is genetically similar to T. ni eggs and High Five™ cells, but distinct from Sf9.
QB-CL-B has not been assayed directly for TnCLV genetic material, but is likely TnCLV-negative because it is a clonal derivative of the TnCLV-negative QAU-BTI-Tn9–4s cell line.
“The majority of cells in the shaker culture appeared to be single suspended cells …” (Y. Hashimoto et al., 2010).
Cell line species of origin confirmed through transcriptomic sequences.
The purpose of this study was to bridge this gap by isolating and extensively characterizing a new TnCLV-negative Tn cell line, Tn nodavirus negative (Tn-NVN), which can be used for safe biological products manufacturing. Tn-NVN cells produced ~2–5 fold higher levels of three model glycoproteins as compared to Sf cells, which is similar to the increase in yields previously reported for High Five™. Unlike High Five™, Tn-NVN cells grew consistently well as a single cell suspension culture in a serum-free medium. Reverse transcription-polymerase chain reaction (RT-PCR) assays showed Tn-NVN cells had no detectable TnCLV over the course of 200 passages in continuous culture. Tn-NVN cells also tested negative for >60 species of Mycoplasma, Acholeplasma, Spiroplasma, and Ureaplasma and had no detectable Sf-rhabdovirus (Sf-RV) or Bombyx mori macula-like virus (BmMLV), which are two other known insect cell line contaminants (Geisler & Jarvis, 2018). Finally, using a recently described bioinformatics approach (Geisler, 2018), Tn-NVN cells were found to have no detectable bacterial, fungal, or viral sequences, suggesting they are not contaminated with any adventitious agents. Together, these results demonstrate Tn-NVN cells are an excellent alternative to other Tn lines as hosts for the BICS, particularly for manufacturing biological products for clinical applications.
2 |. MATERIALS AND METHODS
2.1 |. Tn-NVN cell line isolation
TN-368 cells (Hink, 1970) were originally obtained from Max Summers (Department of Entomology, Texas A&M University) and routinely maintained as adherent cultures at 28°C in complete TNMFH medium (C-TNMFH), which is defined as Grace’s insect cell medium containing lactalbumin hydrolysate (Invitrogen, Carlsbad, CA), yeastolate (Invitrogen), 10% fetal bovine serum (Atlanta Biologicals, Inc., Flowery Branch, GA), and 1% pluronic F-68 (Corning Cellgro).
Tn-NVN cells were produced by using limiting dilution to isolate single TN-368 cells, then culturing those cells in C-TNMFH medium supplemented with 200 μg/mL ribavirin (Oxchem Corporation, Irwindale, CA). Wells containing single cells were identified by microscopy and progressively expanded through a series of larger culture vessels as they continued to divide. After one month, several clones exhibiting fast growth rates were screened for TnCLV RNA by RT-PCR. TnCLV-negative clones were further amplified as adherent cultures in C-TNMFH medium supplemented with 200 μg/mL ribavirin, then screened for high recombinant protein production levels and to confirm the absence of detectable TnCLV RNA segments 1 and 2 after baculovirus infection. This screen identified two clonally-derived Tn-NVN candidates, which were then amplified as adherent cultures in C-TNMFH medium lacking ribavirin. Each was adapted to suspension culture by growth in spinner flasks for one passage in C-TNMFH and two passages in ESF-921 (Expression Systems LLC, Woodland, CA) supplemented with 10% FCS. Finally, each was adapted to serum-free ESF-921 by cultivation in ESF-921 with incremental reductions in the serum concentration from 10% to 0% and then they were transferred to shake flasks. These shake flask cultures were routinely maintained in serum-free ESF-921 medium with passages every three days and cell growth was monitored. One clone grew faster than the other and its doubling time stabilized around 26.6 hr after ~100 passages (data not shown). This clone was designated Tn-NVN and used for the remainder of the experiments performed in this study.
2.2 |. RT-PCR assays
RT-PCR assays were designed to detect TnCLV RNA segments 1 or 2, BmMLV RNA, or Sf-RV RNA. Samples containing ~106 cells were removed from shake flask cultures and the cells were pelleted by low speed centrifugation (5 minutes at 800 ×g). The cell-free supernatants were then filtered through a 0.22 μm filter (CELLTREAT Scientific, Shirley, MA) and the filtrate was centrifuged at 131,000 × g for 22 h at 4°C. Total RNA was extracted from both the low speed cell and high speed cell-free pellets using RNASolv reagent (Omega Bio-Tek, Inc., Norcross, GA), according to the manufacturer’s protocol. Total RNA was then quantified and used as the template for cDNA synthesis with the ProtoScript II First Strand cDNA synthesis kit (New England Biolabs, Ipswich, MA) and a Tn-nodavirus-specific primer (Noda-7) or the oligo(dT)23-VN primer included in the kit, according to the manufacturer’s protocol. Equivalent amounts of each cDNA preparation were then used for PCRs with Taq DNA polymerase in ThermoPol reaction buffer (New England Biolabs) and either TnCLV- (Noda-1 and Noda-2 for RNA-1; Noda-6 and Noda-7 for RNA-2), BmMLV- (MLVSP and MLVASP), or Sf-RV- (NSP and NASP) specific primers. All PCRs were incubated at 94°C for 3 min, subjected to 35 cycles of 94°C for 30s, 60°C for 1 min, and 72°C for 1 min, and finally incubated at 72°C for 10 min. In some cases, 1 μL of the primary PCR was used as the template for nested PCR, which was performed under the same conditions described above with internal primer pairs specific for Tn-nodavirus RNA segment 1 (Noda-1i and Noda-2i) or 2 (Noda-6i and Noda-7i). All primer sequences are given in Table 2 and all PCR products were analyzed by agarose gel electrophoresis with ethidium bromide staining.
Table 2:
Primer sequences
| Primer | Primer Sequence (5’ to 3’) | Product Size (bp) |
|---|---|---|
| Noda-1 Noda-2 |
GGG AAC CGA GTT ACA CGC GCA TTG C CCG CCC TAA GTT GTA GTT GTT GGG ACG G |
1342 |
| Noda-li Noda-2i |
GAT GCT GAC TCA CCA TTC ACC CCG ATA AGC CTA GCG TTG ACA GAT TG |
503 |
| Noda-6 Noda-7 |
GCC TTC GCA CCA CCT GAC TTC GCC AGG AAT GTT GCT TGC AAC AGC |
951 |
| Noda-6i Noda-7i |
CAT CCA GAT CCG ATC AAG TGT C CAC GGA TGA CAA TGG TGT CC |
432 |
| Noda-8 | ACC TTA GTC TGT TGA CTT AAA CTG | n/a |
| TnRPL3-SP TnRPL3-ASP |
GTC ATC GTG GTA AGG TCA AG GCT TCT TAG GTC CCA TGC AAC |
957 |
| TnCOX1-SP TnCOX1-ASP |
GGA AAC ATT TGG ATG CTT AGG GAA TGT TCA GCT GGA GGT A |
717 |
| TnGAPDH-SP TnGAPDH-ASP |
GTC GAA AAT CGG AAT CAA CG GTA CTT GAT GAG ATC GAT GA |
979 |
| Sf-RV-NSP Sf-RV-NASP |
GAG TGT TGA TAC ATG TCG GTG ACC AAC CTC TTC CAG |
1020 |
| BmMLV-SP BmMLV-ASP |
GTC TCC TCC ATC ATC AAA GG GGA TCG AAG ACG TAG ACT CG |
755 |
2.3 |. Mycoplasma detection assays.
Samples containing about 105 Tn-NVN cells were assayed using the Universal Mycoplasma Detection kit (American Type Culture Collection, Manassas, VA), according to the manufacturer’s protocol. This kit can be used to detect DNAs from >60 different species of Mycoplasma, Acholeplasma, Spiroplasma, and Ureaplasma, including the eight most frequent contaminants of laboratory-cultured cell lines (ATCC).
2.4 |. Species identity confirmation
Fragments of the Tn RPL-3, COX-1, and GAPDH genes were PCR amplified from reverse-transcribed total RNA isolated from Tn-NVN cells using primer pairs TnRPL3-SP and -ASP, TnCOX1-SP and -ASP, and TnGAPDH-SP and –ASP, respectively (Table 2), as described in section 2.2. The PCR products were gel-purified using HiBind DNA Mini Columns (Omega-Biotek, Norcross, GA) according to the manufacturer’s protocol and directly sequenced (Genewiz, South Plainfield, NJ). The sequences were then assembled and aligned with reference sequences using Vector NTI 10.3.1 (Invitrogen), as described previously (Maghodia, Geisler, & Jarvis, 2016).
2.5 |. Cell growth properties, morphologies, and diameters
Following adaptation to serum-free medium and suspension culture, Tn-NVN and TN-368 cells were seeded into shake flasks at a density of 106 cells/mL in ESF-921. Triplicate samples were harvested at various times after seeding and viable cell counts and diameters were measured using a Countess automated cell counter (Invitrogen), as described previously (Maghodia et al., 2016). We also photographed samples of these cells using an Olympus FSX-100 microscope to document their morphologies.
2.6 |. Recombinant protein production, purification, and quantification
Recombinant baculovirus expression vectors encoding full-length, untagged E. coli β-galactosidase (ß-gal) and 8X HIS-tagged forms of human secreted alkaline phosphatase (hSEAP) and human erythropoietin (hEPO), were isolated and characterized as described previously (Maghodia et al., 2016). Sf-RVN cells were used to isolate and produce working stocks of all baculovirus vectors to ensure the absence of TnCLV and other adventitious viruses in the inocula required for the recombinant protein production experiments. These working baculovirus stocks were used to infect Tn-NVN, TN-368, Sf-RVN, Tn-PRO™ (Expression Systems, Davis, CA), and High Five™ cell cultures. All cells were seeded into six well plates at a density of 1 × 106 cells/mL and infected at a multiplicity of infection of 5 plaque-forming units per cell, as described previously (Maghodia et al., 2016). The infected cell cultures were sampled at the times shown in Figures 4–6, the cell pellets or cell-free media were then analyzed for intracellular ß-gal or extracellular hSEAP and hEPO, respectively, by enzyme activity and/or western blotting assays, and the results were quantified by laser scanning densitometry, as described previously (Maghodia et al., 2016).
FIGURE 4:
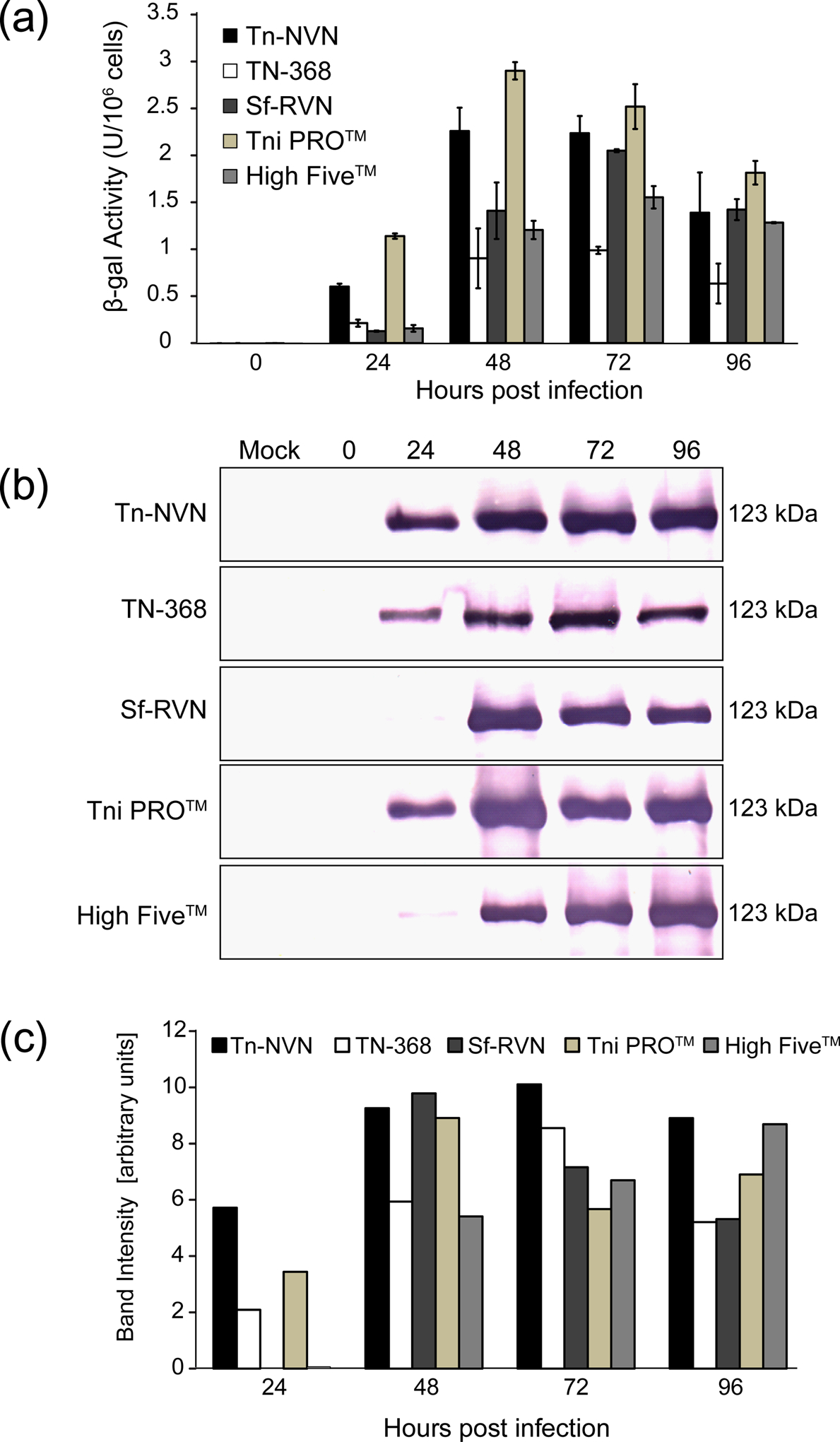
Recombinant β-galactosidase production levels. Tn-NVN and four other insect cell lines were infected with a recombinant baculovirus encoding E. coli β-galactosidase, cell extracts were prepared at various times post-infection, and (a) β-galactosidase activity assays and (b) western blotting analyses were performed on normalized samples. Panel (c) shows quantification of the western blotting results by laser scanning densitometry. The error bars in the graph shown in Panel (a) represent 95% confidence intervals.
FIGURE 6:
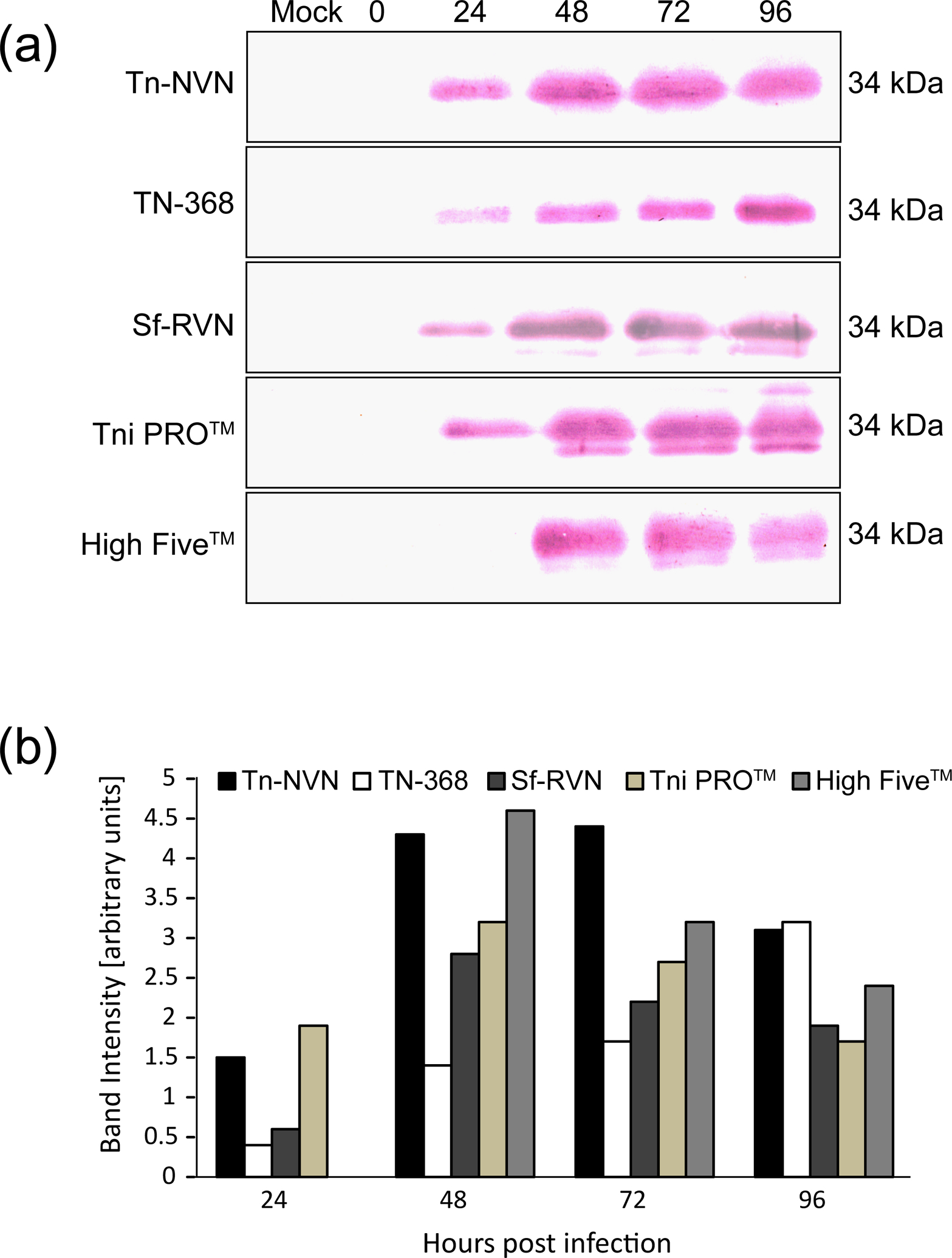
Recombinant hEPO production and secretion levels. Tn-NVN and four other insect cell lines were infected with a recombinant baculovirus encoding human EPO, CFM were prepared at various times post-infection, and (a) western blotting analyses were performed on normalized samples and (b) the western blotting results were quantified by laser scanning densitometry.
2.7 |. N-glycan analysis
Human erythropoietin (hEPO) was produced in various insect cell lines, including Tn-NVN and purified as described previously (Maghodia et al., 2016). The purified product was treated with PNGase F (New England Biolabs), an endoglycosidase that cannot remove N-glycans containing core α1,3-linked fucose residues (Maley, Trimble, Tarentino, & Plummer, 1989). PNGase F-treated hEPO was then resolved by SDS–PAGE and transferred to PVDF membranes as described previously (Mabashi-Asazuma, Kuo, Khoo, & Jarvis, 2014). The membranes were probed with anti-hEPO (U-CyTech, Utrecht, The Netherlands) to detect hEPO, with Aleuria aurantia lectin (AAL; Vector labs, Burlingame, CA) to detect fucose residues, or with anti-HRP (Biogenesis, Poole, UK) to detect N-glycans with core α1,3-linked fucose residues, as described previously (Geisler & Jarvis, 2012; Mabashi-Asazuma et al., 2014).
Total N-glycans were isolated for MALDI-TOF MS analysis using PNGase Ar (New England Biolabs), an endoglycosidase that can remove all N-glycans, whether or not they have core α1,3-linked fucose residues (Ftouhi-Paquin, Hauer, Stack, Tarentino, & Plummer, 1997). The released N-glycans were then purified, permethylated, and analyzed by MALDI-TOF MS, as described previously (Mabashi-Asazuma et al., 2014).
2.8 |. Massively parallel sequencing and adventitious virus search
DNA and RNA were extracted as described previously (Geisler & Jarvis, 2016). DNA and RNA library preparation, sequencing, and initial bioinformatics analysis were conducted by Genewiz, LLC (South Plainfield, NJ, USA) as described previously (Geisler, 2018). De novo assembly was conducted using CLC Genomics Server 8.0.3 and Soapdenovo, the resulting reads were assembled in CLC Genomics Workbench, and the resulting assemblies were uploaded to GenBank (BioProject PRJNA626441). Searchable Tn-NVN nucleotide databases were generated using the NCBI BLAST+ suite and queried through the prfectBLAST Java front end (Santiago-Sotelo & Ramirez-Prado, 2012) (BLAST+ version 2.4.0) with an in-house viral protein database and TBLASTN searches, as described previously (Geisler, 2018). Significant hits (E-value < 0.1) were further investigated by comparison to insect and viral protein sequences in Genbank.
3 |. RESULTS
3.1 |. Tn-NVN cells are not detectably contaminated with TnCLV
The approach used to isolate the Tn-NVN cell line involved treating single cell clones with ribavirin, as described in Materials and methods. Six of these clones were initially tested for TnCLV by RT-PCR and the results showed four were negative and the other two were positive (data not shown). The two fastest-growing TnCLV-negative clones were amplified in C-TNMFH without ribavirin, adapted to grow in suspension in C-TNMFH, and then adapted to grow in ESF-921 by starting with ESF supplemented with 10% serum and incrementally reducing the serum concentration from 10% to 0% over several passages. Ultimately, one clone adapted better than the other and was used to establish a continuous shake flask suspension culture in ESF-921 containing no antiviral drugs. This putative Tn-NVN culture was passaged about every three days for about the next 1.5 years. During this time, routine monitoring of cell growth showed the average doubling time stabilized at 26.6 hr after about 100 passages (data not shown). In addition, this continuous shake flask culture was routinely sampled to determine if the TnCLV-negative phenotype of our putative Tn-NVN cell line was stable. Cells were pelleted from each sample, total RNA was isolated from the cell pellets, and those cellular RNAs were assayed by nested RT-PCR with primers specific for TnCLV RNA segments 1 and 2. The results showed these cells contained no detectable TnCLV-specific RNA over the course of 210 serial passages in this continuous culture (Figure 1a). These results were validated by positive and negative control RT-PCRs using total RNAs from TN-368 cells, which are contaminated with TnCLV, and Sf9 cells, which are not. In addition, a negative control RT-PCR assay with no RNA template (H2O) was performed. The results obtained using these controls showed the RT-PCR assay was specific for TnCLV (Figure 1a). Finally, in control RT-PCRs with primers specific for a cellular housekeeping gene (GAPDH), which were performed to validate the quality of the RNA preparations, a strong amplimer of the expected size was observed with each RNA preparation (Figure 1a).
FIGURE 1:
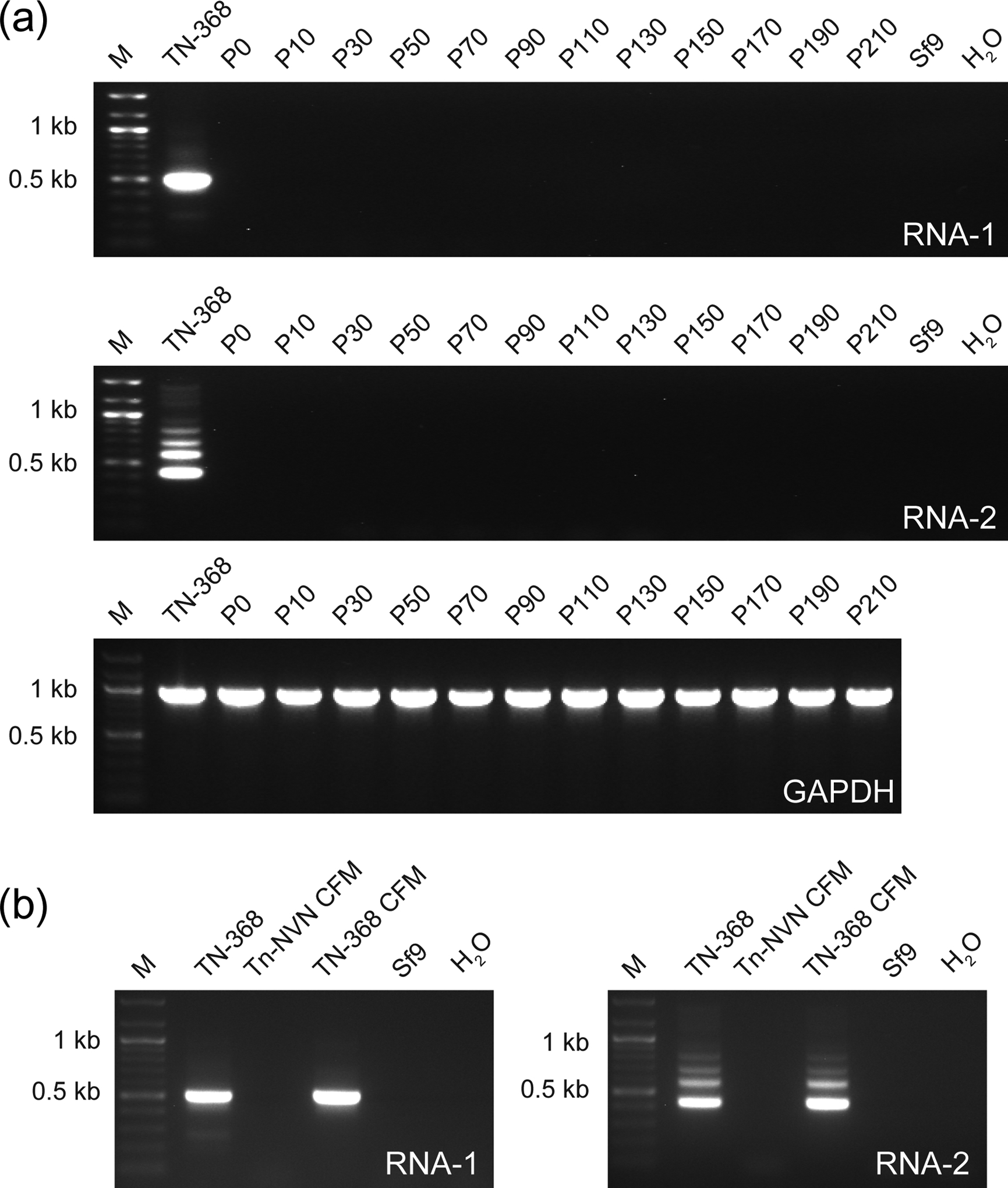
Tn-NVN cells have no detectable TnCLV. (a) A Tn-NVN suspension culture was maintained for 210 serial passages in ESF-921 medium containing no antiviral drugs. Samples were removed periodically and total RNA was isolated from cell pellets and assayed for TnCLV RNA 1, TnCLV RNA 2 by RT-PCR/nested PCR, or Tn GAPDH by RT-PCR. (b) CFM was isolated from a sample taken from the Tn-NVN suspension culture at P55 and ultracentrifuged as described in Materials and methods. Total RNA was then isolated from the resulting high speed pellet and assayed for TnCLV RNA 1 and 2 by RT-PCR/nested PCR. Total RNAs from TN-368 cells and the pellet obtained by ultracentrifuging TN-368 cell-free media were used as positive controls and total RNA from Sf9 cells or PCRs with no RNA template (H2O) were used as negative controls. Each panel in (a) and (b) shows the amplification products obtained using the indicated RNA samples, as determined by agarose gel electrophoresis with ethidium bromide staining. Each lane marked M shows a 100 bp DNA ladder used as a size standard.
To address the remote possibility that the putative Tn-NVN clone was contaminated, but had shed virtually all TnCLV into the extracellular milieu, leaving no detectable intracellular viral RNA, the cell free medium (CFM) from a sample taken from the continuous culture at passage 55 was ultracentrifuged, total RNA was extracted from the high-speed pellet, and RT-PCRs were performed with TnCLV-specific primers, as described in Materials and methods. RT-PCRs on total RNAs from TN-368 cells and a TN-368 CFM high-speed pellet were used as positive controls. Both positive controls yielded strong amplimers of the expected sizes with primers specific for both TnCLV RNA segments, but total RNA isolated from the Tn-NVN CFM high-speed pellet produced no amplification products in either assay (Figure 1b). These results showed the putative Tn-NVN clone, unlike TN-368 cells, does not shed particles containing TnCLV segment 1 or 2 genomic RNAs into the extracellular growth medium. RT-PCRs with total RNA from Sf9 cells and with no RNA template (H2O) were used as the negative controls. These results confirmed this clone was TnCLV-negative and validated its designation as a new Tn-NVN cell line, which was used for the remainder of the experiments performed in this study.
3.2 |. Tn-NVN cells lack other known cell line contaminants.
In addition to TnCLV, Sf-RV and BmMLV are also known to be common contaminants of lepidopteran insect cell lines (Geisler & Jarvis, 2018). Cross contamination is certainly possible, particularly in laboratories like ours, which sometimes cultivate Tn, Sf, and Bm cell lines in essentially the same environment. Accordingly, it was important to assay Tn-NVN cells for contamination with Sf-RV and BmMLV, in addition to TnCLV. Thus, RT-PCRs were performed with Sf-RV- and BmMLV-specific primers on total RNAs isolated from Tn-NVN cells, as described in Materials and methods. Total RNAs from Sf9 cells, which are contaminated with Sf-RV, and from BmN cells, which are contaminated with BmMLV, were used as the positive controls. While the control RT-PCRs produced strong amplimers of the expected sizes, RT-PCRs with Tn-NVN total RNA produced no amplimers, indicating Tn-NVN cells are not detectably contaminated with either Sf-RV (Figure 2a) or BmMLV (Figure 2b).
FIGURE 2:
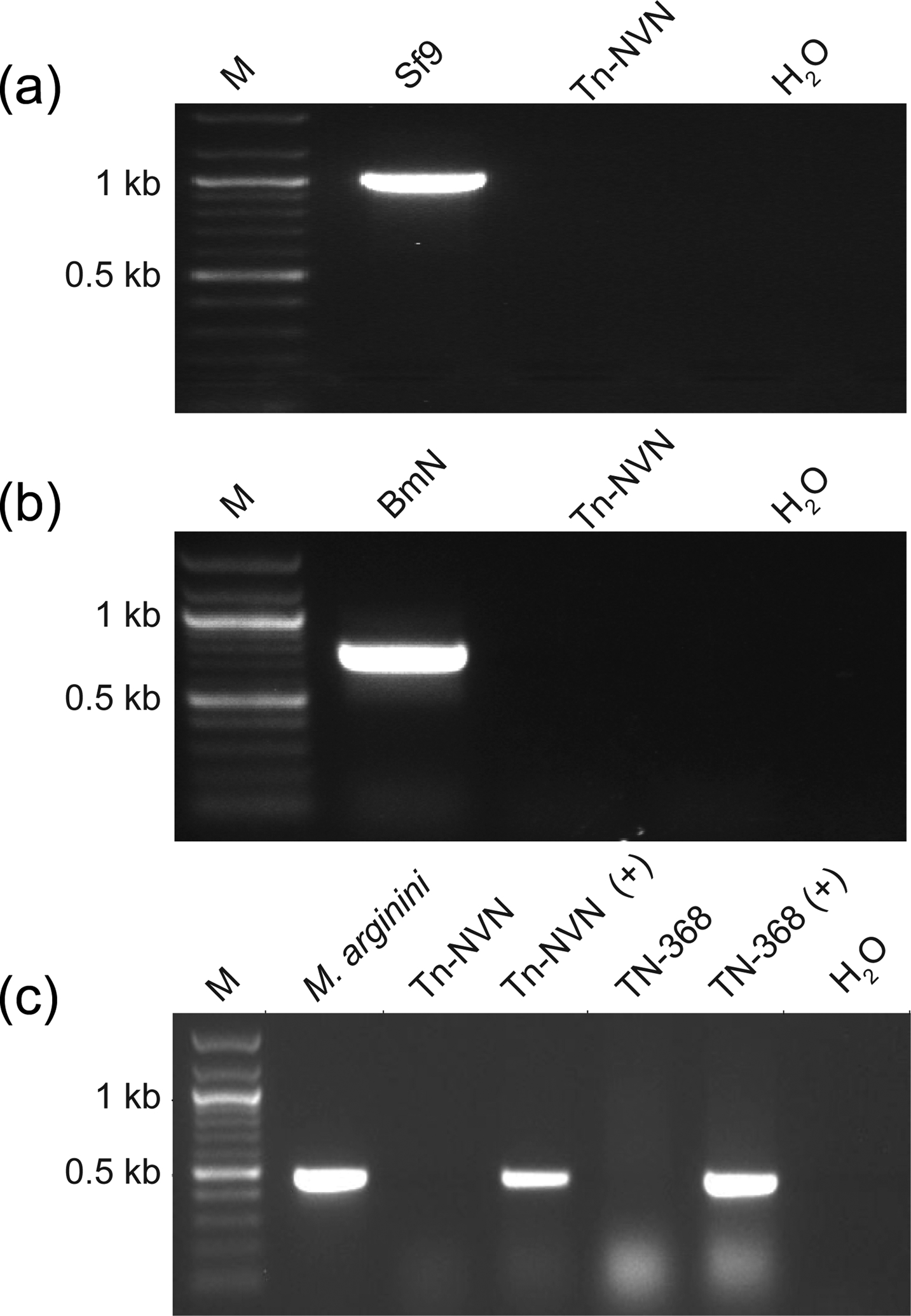
Tn-NVN cells lack other known cell line contaminants. Total RNA was isolated from Tn-NVN cells and assayed for (a) Sf-RV or (b) BmMLV by RT-PCR, as described in Materials and methods. Total RNAs from Sf9 cells, which are contaminated with Sf-RV, and BmN cells, which are contaminated with BmMLV, were used as the positive controls. (c) Tn-NVN and TN-368 cell lysates were assayed for Mycoplasma, Acholeplasma, Spiroplasma, and Ureaplasma using a PCR-based Universal Mycoplasma Detection Kit (ATCC). A plasmid encoding an M. arginini rRNA sequence (M. arginini) was used as the positive control. Additional controls were Tn-NVN and TN-368 cell lysates spiked with the positive control plasmid (Tn-NVN + and TN-368 +), which were included to determine if the lysate interfered with the assay. A PCR with no template (H2O) was used as the negative control. Each panel in (a), (b) and (c) shows the amplification products obtained using the indicated RNA samples, as determined by agarose gel electrophoresis with ethidium bromide staining and the lanes marked M show a 100 bp DNA ladder used as a size standard.
In addition, ATCC’s Universal Mycoplasma Testing Kit (ATCC) was used to assay extracts from Tn-NVN and the parental TN-368 cell line for the presence of DNA sequences derived from >60 different species of Mycoplasma, Acholeplasma, Spiroplasma, and Ureaplasma. A plasmid encoding an M. arginini rRNA sequence was used not only as a direct positive control, but also as a spiking control to determine if Tn-NVN and/or TN-368 cell lysate interfered with the PCR assay. A negative control reaction was performed with no template DNA (H2O). The results showed all three positive controls, but neither the Tn-NVN nor the TN-368 lysates produced amplimers of the expected size (Figure 2c). These results indicated Tn-NVN cells are not detectably contaminated with any of the Mycoplasma, Acholeplasma, Spiroplasma, and Ureaplasma species covered by the primers included in this kit.
3.3 |. General properties of Tn-NVN
The next goal was to document some of the biotechnologically relevant general properties of Tn-NVN cells, including their peak culture densities, diameters, and morphologies, in comparison to the parental TN-368 cell line. The results showed Tn-NVN and TN-368 cells achieved similar densities, reaching ~7 × 106 cells/mL after 5 days of shake flask culture in ESF-921 (Figure 3a). However, Tn-NVN cells had significantly and consistently larger average diameters than TN-368 cells throughout this 5-day culture period (Figure 3b). Despite being clonally derived, Tn-NVN cells also had surprisingly diverse morphologies, as a typical culture included cells of various sizes and shapes (Figure 3c). TN-368 cells were also morphologically diverse (Figure 3d), which was consistent with the morphological diversity of TN-368 cells noted in the original description of this cell line in 1970 (Hink, 1970).
FIGURE 3:
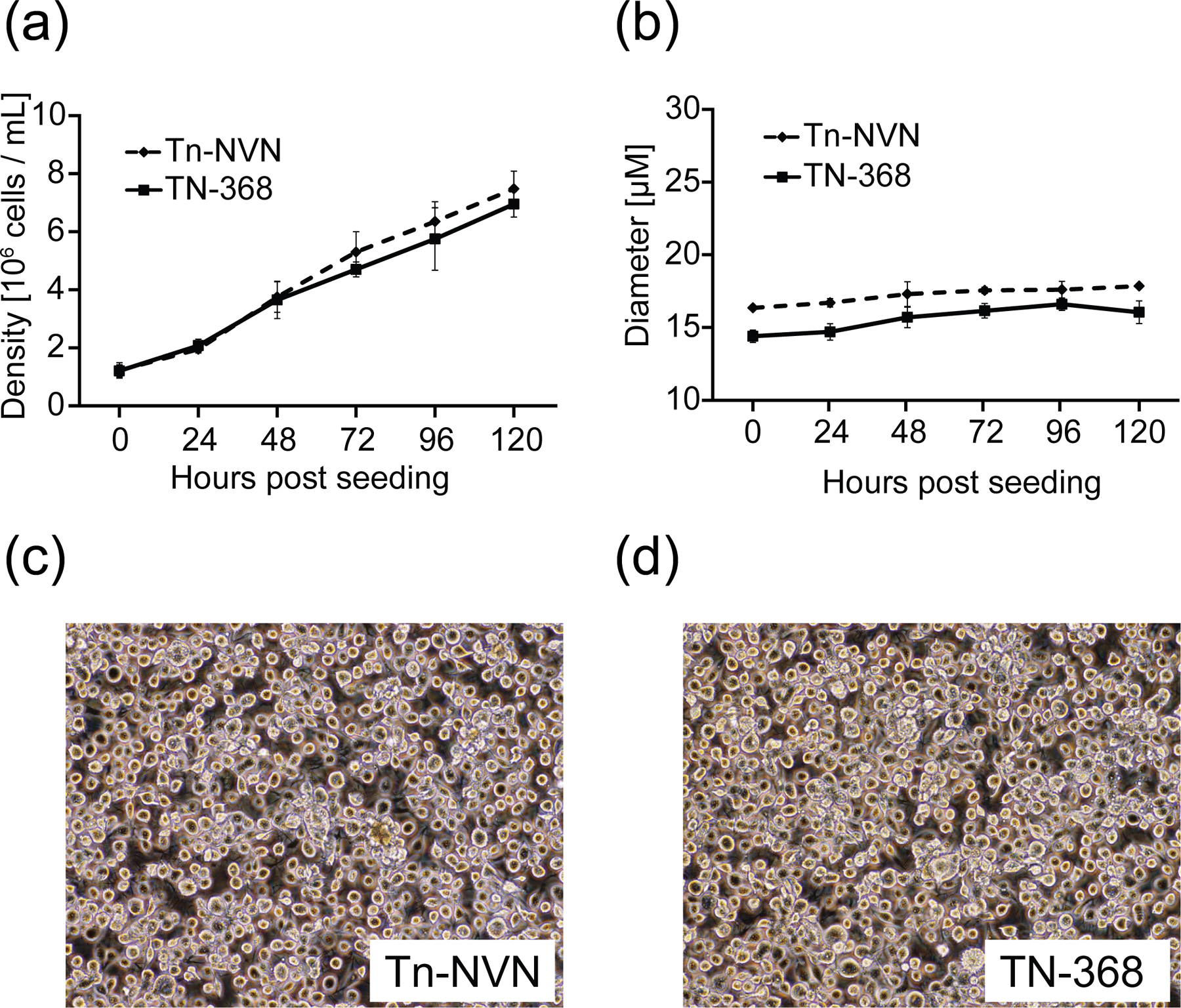
Tn-NVN growth, size, and morphology. Tn-NVN and TN-368 cells were seeded into shake flasks at a density of 106 cells/mL in ESF 921 medium, then triplicate samples were taken at various times and (a) viable cell counts and (b) diameters were measured using a Countess automated cell counter and plotted against hours post seeding. The error bars represent 95% confidence intervals. Representative samples of (c) Tn-NVN and (d) TN-368 cells also were photographed a magnification of 10X using an Olympus FSX-100 microscope to document cell morphologies.
3.4 |. Tn-NVN supports high levels of recombinant protein production
The next set of experiments was designed to assess the levels of baculovirus-mediated recombinant protein production supported by Tn-NVN cells, as compared to other insect cell lines used as hosts for the BICS. This involved measuring the levels of three different recombinant proteins produced by Tn-NVN, TN-368, Tni PRO™, High Five™, and Sf-RVN cells. The four Tn cell lines were included to compare the recombinant protein production levels provided by the new, TnCLV-free line to those provided by other Tn lines available for use in the BICS, all of which are contaminated with TnCLV (Maghodia, Geisler, and Jarvis, unpublished). Sf-RVN cells were included to compare the recombinant protein production levels obtained using virus-free Tn or Sf cells as the hosts. Untagged E. coli ß-galactosidase (ß-gal) and 8X HIS-tagged forms of human secreted alkaline phosphatase (hSEAP) and human erythropoietin (hEPO) were used as the model recombinant proteins for these experiments. These models were chosen because they represent a diverse range of proteins, including both intracellular and secreted proteins, which are often used to assess recombinant protein production levels in the BICS. Moreover, correct folding of ß-gal and hSEAP is required for their enzymatic activities.
In general, the results of these recombinant protein production experiments revealed Tn-NVN and the other four insect cell lines produced comparable levels of all three recombinant proteins. The production levels observed with Tn-NVN cells were already high at 24 hours post-infection (hpi) and reached a plateau by 48 hpi. As compared to the parental TN-368 cell line, Tn-NVN consistently produced higher levels of recombinant protein.
3.4.1 |. β-gal production levels
The results of the recombinant ß-gal production experiments showed Tni PRO™ cells produced the highest levels of enzymatically active ß-gal, but Tn-NVN and each of the other three cell lines tested produced comparable levels over the 4-day time course of the experiment (Figure 4a). In addition, Tn-NVN and the other four cell lines tested produced comparable amounts of immunoreactive ß-gal protein (Figure 4b–4c) over the 4-day time course of the experiment.
3.4.2 |. hSEAP production levels
The results of the recombinant human SEAP production experiments showed Tn-NVN cells produced and secreted the highest levels of SEAP activity (Figure 5a) and total immunoreactive protein (Figure 5b–5c) over the 4-day time course of baculovirus infection. Interestingly, both Tn-NVN and the parental TN-368 cell line produced and secreted higher levels of recombinant SEAP at 24 hpi than all other cell lines, suggesting the baculovirus infections may have progressed faster in these cell lines than in the others.
FIGURE 5:
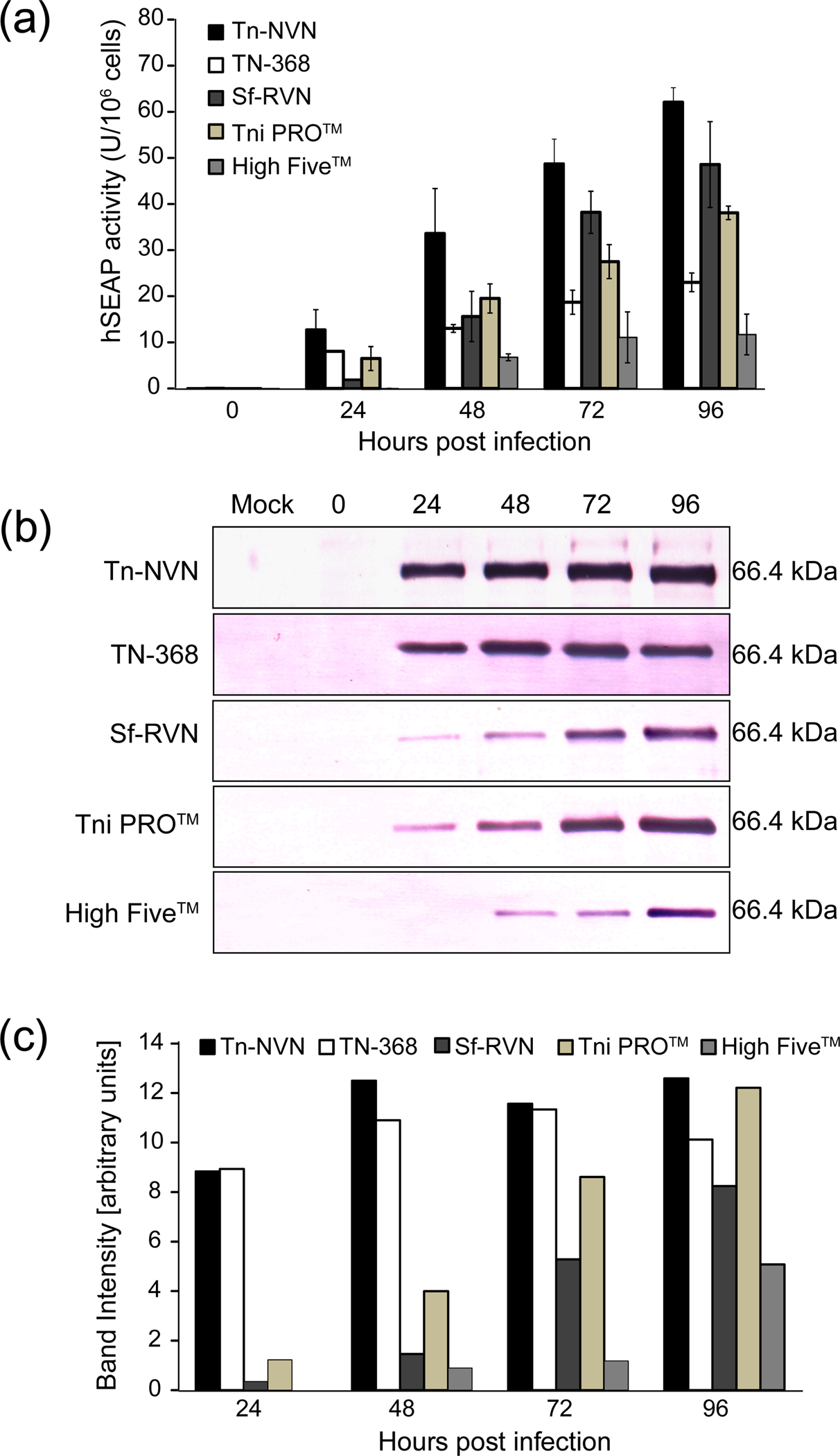
Recombinant hSEAP production and secretion levels. Tn-NVN and four other insect cell lines were infected with a recombinant baculovirus encoding human SEAP, CFM were prepared at various times post-infection, and (a) SEAP activity assays and (b) western blotting analyses were performed on normalized samples. Panel (c) shows quantification of the western blotting results by laser scanning densitometry. The error bars in the graph shown in Panel (a) represent 95% confidence intervals.
3.4.3 |. hEPO production levels
The recombinant hEPO production experiments showed Tn-NVN cells produced and secreted slightly less immunoreactive hEPO protein than Tni PRO™ cells at 24 hpi and slightly less than High Five™ cells at 48 hpi (Figure 6). However, the levels produced by Tn-NVN and Tni PRO™ at 24 and 48 hpi were similar and Tn-NVN cells produced higher levels at 72 hpi. Overall, the results of these experiments showed Tn-NVN and all other cell lines tested produced and secreted hEPO at comparable levels over the 4-day course of baculovirus infection (Figure 6).
3.5 |. Tn-NVN produces glycoproteins with no detectable core α1–3 fucose
Mammalian and insect cells commonly produce glycoproteins with N-glycans that include an α1,6-linked fucose residue on the asparagine-bound N-acetylglucosamine residue. However, some insects can produce glycoproteins that also have an α1,3-linked fucose residue in the same position (Fabini, Freilinger, Altmann, & Wilson, 2001; Geisler et al., 2012). The glycobiological processes resulting in the addition of these sugars are known as core α1,6- and core α1,3-fucosylation, respectively. While the only difference in these modifications is in the way the fucose residues are linked to the core N-acetylglucosamine residue, this is a significant difference because glycoproteins containing core α1,3-fucosylated N-glycans can be immunogenic in mammals (Bardor et al., 2003; Prenner, Mach, Glössl, & März, 1992). Moreover, core α1,3-fucosylated N-glycans can be recognized not only by sera from individuals allergic to honey bee venom (Tretter, Altmann, Kubelka, März, & Becker, 1993), but also by sera from up to 25% of the general population (Bardor et al., 2003). Thus, if biological products manufactured in insect cells have this foreign N-glycan epitope, their administration to humans or other mammals could have potentially undesirable side effects. Interestingly, High Five™, which is the most commonly used Tn cell line, produces glycoproteins with high levels of core α1,3-fucosylated N-glycans (Seismann et al., 2010) that have been shown to react with human sera (Hancock et al., 2008).
To determine if Tn-NVN cells also produce core α1,3-fucosylated N-glycans, these cells were infected with a baculovirus vector encoding hEPO, and then the secreted product was purified and its N-glycans were isolated and analyzed in various ways, with High Five™, Sf-RVN, and TN-368 cells as alternative hosts for comparison. Our first level of glycoanalysis involved probing the purified hEPO preparations with Aleuria aurantia lectin (AAL), which is a lectin that specifically recognizes fucose (Kochibe & Furukawa, 1980). AAL bound to the purified hEPOs from all four cell lines, indicating each can produce fucosylated N-glycans, which was no surprise (Figure 7a). The fucose linkages were then analyzed by pre-treating the hEPOs with PNGase F, which can remove core α1,6-, but not core α1,3-fucosylated N-glycans. Pre-treatment with PNGase F eliminated AAL binding to the hEPOs produced by Sf-RVN, TN-368, and Tn-NVN cells, but not to the hEPO produced by High Five™ cells (Figure 7a). These results indicated, in contrast to High Five™ cells, neither TN-368 nor Tn-NVN cells produced hEPO with any detectable core α1,3-fucosylated N-glycans.
FIGURE 7:
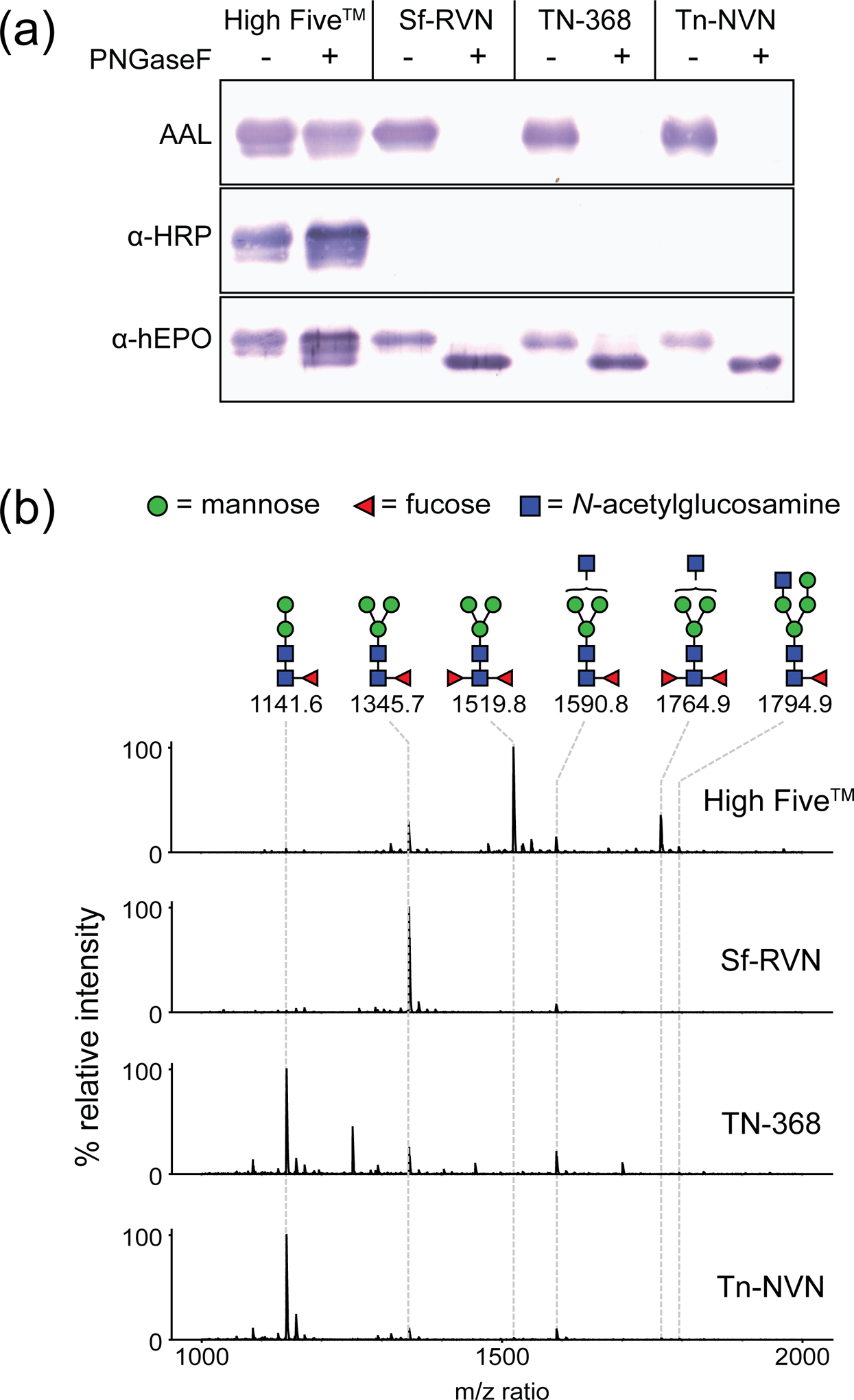
Analysis of N-glycan fucosylation by various cell lines. Tn-NVN and three other insect cell lines were infected with a recombinant baculovirus encoding human EPO, the recombinant protein product was purified from the CFM, and samples of the purified product were (a) either mock-digested or digested with PNGase F, resolved by SDS–PAGE and transferred to a PVDF membrane. The membranes were then probed with AAL to detect fucose residues, with anti-HRP to detect N-glycans with core α1,3-linked fucose residues, or with anti-hEPO to detect hEPO. Other samples of the purified hEPO product were (b) treated with PNGase Ar, and the released N-glycans were then purified, permethylated, and analyzed by MALDI-TOF MS, as described in Materials and methods. Only the fucosylated N-glycan peaks in the MALDI spectrum are annotated.
This conclusion was supported by the results of a second level of glycoanalysis, which involved probing the purified hEPO preparations with anti-horseradish peroxidase (anti-HRP), an antibody specific for core α1,3-fucosylated N-glycans. While anti-HRP clearly recognized N-glycans on hEPO from High Five™ cells, it failed to recognize N-glycans on hEPO from Sf-RVN, TN-368, or Tn-NVN cells, indicating only the former had detectable core α1,3-fucosylated N-glycans (Figure 7a). The fact that PNGase-F pretreatment did not eliminate binding of anti-HRP to N-glycans on hEPO from High Five™ cells is consistent with the interpretation that this hEPO preparation includes core α1,3-fucosylated N-glycans that cannot be removed using this endoglycosidase.
Finally, MALDI-TOF MS was used as a third level of analysis to directly analyze the N-glycans from the hEPOs produced by the four different cell lines. In this case, PNGase Ar, which is an endoglycosidase that can remove all N-glycans, including those with and without core α1,3-fucose residues (Mabashi-Asazuma et al., 2014; Yan et al., 2018), was used to enzymatically release the N-glycans from each purified hEPO preparation. The released N-glycans were then purified, permethylated, re-purified, and analyzed by MALDI-TOF MS. The resulting spectra confirmed the hEPOs from TN-368, Tn-NVN, and Sf-RVN cells had only monofucosylated N-glycans, whereas the hEPO from High Five™ cells had predominantly difucosylated N-glycans that included both α1,3- and α1,6-linked core fucose residues (Figure 7b).
3.6 |. Elucidation of Tn-NVN genomic and transcriptomic data bases
Illumina sequencing yielded 63,763 Mb of reads from Tn-NVN genomic DNA, and 48,699 Mb of reads from Tn-NVN cDNA. Following assembly, the Tn-NVN cell genome comprised 357.5 Mb in 150,340 contigs (178-fold coverage), and the Tn-NVN cell transcriptome comprised 68.7 Mb in 97,994 contigs (708-fold coverage). The previously released, higher quality chromosomal draft genome and transcriptome of High Five™ cells comprised 368.2 Mb (Fu et al., 2018), and 39.4 Mb (Yu et al., 2016), respectively. The close match in genome assembly size suggests the current assembly is essentially complete, but more fragmented, as it had a lower contig N50 (4,542 bps vs. 621.9 kb by Fu et al.) from the use of Illumina HiSeq short reads. The difference in transcriptome assembly size suggests the current assembly is not only more fragmented, but also more redundant, as compared to the previously published assembly. The current assembly included 97,994 contigs with an N50 of 943 bps, whereas the Yu et al. assembly included 31,068 contigs with an N50 of 2,276 bp.
3.7 |. TBLASTN searches for virus-like sequences
RT-PCR assays showed directly that Tn-NVN cells are not detectably contaminated with TnCLV, Sf-RV, and BmMLV, which are known adventitious viruses of insect cells, as described above. However, these results did not exclude the possibility that Tn-NVN cells are contaminated with one or more other viruses, including potentially unknown viruses, which could not be detected in those assays.
To address this possibility, Tn-NVN cells were probed for potential adventitious viral sequences using a recently described bioinformatics approach (Geisler, 2018). Briefly, TBLASTN was used to search the assembled Tn-NVN cell genome and transcriptome using an in-house viral protein database as the query. In summary, no bona fide viral RNA or DNA sequences were detected, indicating Tn-NVN cells are most likely not contaminated with any adventitious viruses. However, various virus-like sequences were detected and found to be novel endogenous viral elements (EVEs), R1 LINEs with virus-like C-terminal superfamily 1 helicase (S1H) domains, and Maverick/Polinton elements. Each of these sequences is briefly described below.
3.7.1 |. Novel endogenous viral elements (EVEs)
Six contigs encoding predicted proteins that are highly similar to various viral proteins (Table 3) were identified. Five were derived from negative stranded, ssRNA viruses, which are often the source of EVEs (ter Horst, Nigg, Dekker, & Falk, 2019; Supplementary Sequence Files 1–5), but one was derived from a dsDNA virus related to granuloviruses or ascoviruses (Supplementary Sequence File 6).
Table 3:
Newly identified EVEs in Tn-NVN cells
| EVE | Highest BLASTN E value |
Highest TBLASTX E value |
Length (bps) |
Nonsense / frameshift mutations |
|---|---|---|---|---|
| Orthomyxovirus N-like | 0.25* | 5e-54 (1) | 1638 | 1 / 0 |
| Orthomyxovirus PA-like 1 | 0.045* | 1e-14 (2) | 642 | 0 / 2 |
| Orthomyxovirus PA-like 2 | 3.2* | 2e-30 (3) | 1474 | 4 / 2 |
| Sf-Rhabdovirus P-like EVE | 0.30* | 1e-41 (5) | 650 | 2 / 1 |
| Taï virus N-like EVE | 0.15* | 3e-18 (6) | 687 | 0 / 0 |
| Granulovirus 65.5 kDA-like EVE | 6e-11 (4) | 3e-29 (4) | 512 | 2 / 2 |
Against Hubei Earwig Virus Nucleocapsid APG77883.1.
Against Jingshan Fly Virus 1 polymerase PA APG77878.1.
Against Hubei earwig virus 1 strain GCM19162 polymerase PA APG77882.1.
Against Sf-Rhabdovirus P YP_009094308.1.
Against Taï virus N.
Against Pseudaletia unipuncta granulovirus gp039 YP_003422378.1 / Helicoverpa armigera granulovirus EU255577.1.
no significant hits
3.7.1.1 |. Orthomyxovirus-derived EVEs
Three of the five newly discovered EVEs cited above encoded predicted translation products similar to orthomyxovirus proteins (Table 3). Two of these encoded predicted translation products similar to orthomyxovirus PA (polymerase acidic), and the third encoded a product similar to orthomyxovirus N (nucleocapsid).
Surprisingly, the predicted translation products of both PA-like EVEs were more closely related to each other than to other orthomyxovirus PA protein sequences in Genbank, suggesting they are derived from the same virus or two very closely related viruses. Among all the protein sequences in Genbank, the PA-like proteins encoded by both of these EVEs were most similar to sequences from invertebrate orthomyxovirus virus proteins (L’Vov D et al., 2014; Shi et al., 2016). In addition, both PA-like EVE’s had accumulated missense mutations, frameshift mutations, and other insertions, suggesting they have decayed substantially since their acquisition. The EVE designated Orthomyxovirus PA-like 2 was embedded in the antisense orientation in the 3’UTR of a gene putatively encoding the PHD finger protein rhinoceros. The predicted translation product of the orthomyxovirus N-like EVE was most similar to the N protein from Hubei earwig virus 1 (Shi et al., 2016), and was also similar to N proteins from a range of other orthomyxoviruses (Table 3). This EVE was embedded in the antisense orientation in the 3’UTR of a gene putatively encoding negative elongation factor A.
3.7.1.2 |. Rhabdovirus P-like EVE
One of the Tn-NVN cell line EVEs encoded a predicted translation product similar to the Sf-RV P protein (Ma, Galvin, Glasner, Shaheduzzaman, & Khan, 2014) and to a previously discovered, Sf-RV-related EVE encoding a predicted translation product similar to the Sf-RV P protein (Geisler & Jarvis, 2016). Rhabdoviral P proteins are not conserved; therefore, the putative protein encoded by the Tn-NVN P-like EVE was not similar to any other viral proteins in Genbank.
3.7.1.3 |. Taï virus N-like EVE
Tn-NVN cells also had a previously identified S. frugiperda gene containing a bunyavirus N-like region (Genbank accession number MF327144). Approximately 576 bp of the 687 bp ORF in this EVE encoded a protein similar to bunyaviral N proteins, with highest similarity to the N protein of a mosquito bunyavirus known as Taï virus. The presence of homologs of this EVE in at least two insect species confirms the notion that it includes an exapted viral gene.
3.7.1.4 |. Ascovirus 65.5 kDa-like EVE
Finally, a novel EVE identified in Tn-NVN cells appeared to be derived from a large dsDNA virus. This EVE encodes a product very similar to the N-terminal one-third of a set of viral gene products that is highly conserved between several granuloviruses, ascoviruses, and nucleopolyhedroviruses. This gene product has not yet been characterized and is not similar to any non-viral proteins in Genbank. Other than a C-terminal DNA-binding zinc ribbon domain, this viral gene product does not have any other putative conserved domains. This EVE was originally identified using the Spodoptera frugiperda ascovirus 1a 65.6 kDa protein as the query, but was later found to be more similar to the 65.5 kDa protein of various granuloviruses, which are dsDNA viruses that infect arthropods. Surprisingly, the granulovirus 65.5 kDA-like EVE was similar to several granuloviruses at the nucleotide level, indicating a relatively close relation to extant viruses (Table 3). Apart from reports of genomically integrated intact herpesviral genomes (Aswad & Katzourakis, 2014; Katzourakis & Gifford, 2010), this is to our knowledge the first report of a dsDNA-virus-derived EVE.
3.7.2 |. Transposons encoding virus-like proteins
3.7.2.1 |. Maverick / Polinton elements
Mavericks or Polintons (Kapitonov & Jurka, 2006) are a class of very large DNA transposons that are present in the genomes of many eukaryotes, including Lepidoptera (Dupuy et al., 2011; Geisler, 2018). Lepidopteran Mavericks are distantly related to various viruses and encode putative proteins similar to the poxvirus A32 ATPase, retroviral integrase, adenoviral cysteine protease, densoviral DNA polymerase B2, and parvovirus VP1 (Geisler, 2018).
Using TBLASTN to search the assembled Tn-NVN cell transcriptome and genome with the VPD, two distinct lineages of Maverick elements were identified through their similarity with these viral proteins (Haapa-Paananen, Wahlberg, & Savilahti, 2014; Kapitonov & Jurka, 2006). The Tn Maverick elements had lengths, gene contents, and organizations that were very similar to previously reported lepidopteran Mavericks and distantly related to viruses (Geisler, 2018). Thus, Tn-NVN cells contain Maverick transposons that encode virus-like proteins, but this does not indicate these cells are contaminated with any adventitious viruses.
3.7.2.2 |. R1 LINEs with virus-like C-terminal superfamily 1 RNA helicase domain (S1H)
Long interspersed nuclear elements (LINEs) are RNA transposons that can be found in the genomes of many eukaryotes, including Lepidoptera (Kumar, Nei, Dudley, & Tamura, 2008; Lazareva, Lezzhov, Vassetzky, Solovyev, & Morozov, 2015). R1 LINEs of some lepidopteran species are unusual in that the proteins encoded by their second large ORFs contain a C-terminal S1H domain with extensive similarity to the RNA helicases of positive sense single stranded RNA viruses (Lazareva et al., 2015).
TBLASTN searching of the assembled Tn-NVN cell transcriptome and genome with our VPD identified R1 LINEs through their similarity with viral S1H domains. The Tn R1 LINEs had lengths, gene contents, and organizations that were very similar to previously reported lepidopteran R1 LINEs, and similarly distantly related to viruses (Geisler, 2018). Thus, we conclude that Tn-NVN cell S1H sequences, though related to positive sense single stranded RNA viruses, are components of R1 LINE transposons whose presence are not indicators of viral contamination.
3.7.2.3 |. TED transposons (“errantiviruses”)
Errantiviruses are a type of insect-specific long terminal repeat (LTR) retrotransposons derived from typical LTR retrotransposons that also contain gag and pol genes. Errantiviruses are similar to endogenous retroviruses in genome structure and organization and they have a third ORF (ORF3) putatively encoding an env-like membrane fusion protein (Malik, Henikoff, & Eickbush, 2000; Misseri, Cerutti, Devauchelle, Bucheton, & Terzian, 2004; Ozers & Friesen, 1996; Pearson & Rohrmann, 2004; Pélisson et al., 1994; G. Rohrmann & Karplus, 2001; G. F. Rohrmann, 2019; Teysset et al., 1998); however, the name “errantivirus” is misleading, as these genetic elements are not viruses.
TED (Transposable Element D) is an errantiviral retrotransposon originally identified in TN-368 cells, from which Tn-NVN cells were derived. Thus, as expected, intact, transcribed TED sequences were found in the Tn-NVN genome and transcriptome, indicating this retrotransposon is intact and active in this cell line. The TED sequence in Tn-NVN cells was essentially the same as the previously published sequence, with 3 silent and 1 non-silent mutation in ORF2 and 1 silent mutation in ORF3, as well as 6 mutations in the terminal repeats as compared to the Genbank sequence. Tn-NVN cells also encoded a variety of other errantiviral retrotransposons, which was previously shown for another Tn cell line (Menzel & Rohrmann, 2008).
3.8 |. Tn-NVN transcriptome and genome include no detectable bacterial and fungal sequences
In addition to enabling an effective, non-biased search for virus-specific proteins, the Tn-NVN genome and transcriptome obtained in this study presented the opportunity to search these databases for evidence of Bacterial, Archaeal, and Fungal contamination. Given no such contamination became apparent over hundreds of continuous passages in growth medium lacking antibiotics or antimycotics, and given the negative results of our search for mycoplasma, it would have been surprising to find bacterial or fungal sequences. Nonetheless, the Tn-NVN genome and transcriptome were searched using non-redundant databases containing bacterial and archaeal 5S, 16S and 23S sequences, and fungal ITS sequences from the curated NCBI RefSeq Targeted Loci Project. As expected, the results revealed no evidence of bacterial, archeal, or fungal contamination.
3.9 |. Tn-NVN is a Tn cell line
The initial confusion surrounding the species of origin of AO38, now known as Tnao38, which is a previously described TnCLV-negative High Five subclone (Y. Hashimoto, Zhang, & Blissard, 2010; Yoshi Hashimoto, Zhang, Zhang, Chen, & Blissard, 2012), prompted us to unambiguously confirm the species identity of Tn-NVN cells. This goal was achieved by using BLAST searches to compare the sequences of housekeeping genes encoding ribosomal protein L3 (RPL-3), cytochrome c oxidase 1 (COX-1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) assembled from the Tn-NVN transcriptome to their genbank reference sequences (XM_026889803.1, XM_026884566.1, and XM_026892236.1, respectively). The results showed all three Tn-NVN sequences matched their genbank reference sequences, but not the previously reported S. frugiperda or B. mori reference sequences (Maghodia et al., 2016). These results indicate Tn-NVN is, indeed, a Tn cell line.
4 |. DISCUSSION
Tn cell lines are commonly used as hosts for recombinant protein production in the BICS and some studies have shown at least some Tn cell lines can produce recombinant proteins at higher levels than Sf lines (Davis et al., 1993; Krammer et al., 2010; R. A. Taticek et al., 2001; Wickham et al., 1992; Wickham & Nemerow, 1993; Wilde et al., 2014). Recombinant proteins produced by Tn cells can be used for diverse applications in basic research. In addition, some recombinant proteins produced by these cells have been and are being used to develop biological products for human medicine (CDC, 2010; Felberbaum, 2015; Kamili, 2011; Krammer et al., 2010). In some cases, production of adequate amounts of recombinant proteins for these applications requires scaled-up Tn cell cultures. However, scaling-up High Five™, the most commonly used Tn cell line, is hampered by its poor adaptability to single cell suspension culture in serum-free media, which is a preferred format for recombinant protein production (Dee, Shuler, et al., 1997; Dee, Wood, et al., 1997; Donaldson & Shuler, 1998; M. A. Saarinen & Murhammer, 2000; R. A. Taticek et al., 2001; R.A. Taticek et al., 1997; Wickham & Nemerow, 1993). The use of Tn cell lines to produce recombinant proteins for direct clinical applications in human medicine is also complicated by Tn cell line contamination with an adventitious virus, TnCLV (Li et al., 2007). In fact, using a TnCLV-contaminated Tn cell line as the host to manufacture biological products in the BICS would demand highly effective viral clearance and/or inactivation and product quality control measures to avoid the potential hazard(s) associated with the viral contaminant. However, an obvious alternative would be to circumvent this potential biohazard by using a TnCLV-negative Tn cell line to manufacture biological products for human clinical applications.
In addition to having no detectable TnCLV, an improved Tn cell line for the BICS would have some other desirable properties, such as the ability to support high-level recombinant protein production in scalable, single cell suspension cultures in serum-free media. In addition, this new cell line would not produce recombinant glycoproteins with immunogenic N-glycans. To date, several TnCLV-negative Tn cell lines have been described in the peer-reviewed literature. However, none have been characterized well enough to document the full extent of their utility as hosts for the BICS. For this reason, we undertook an effort to isolate and extensively characterize Tn-NVN, a new TnCLV-negative Tn cell line. The results obtained in this study showed Tn-NVN is not detectably contaminated with TnCLV or any other adventitious agents, it grows well as a single cell suspension culture in serum-free growth medium, it supports high-level recombinant protein production, and it does not produce recombinant glycoproteins with immunogenic N-glycans
Our conclusion that Tn-NVN has no detectable TnCLV was based on the results of a sensitive nested RT-PCR assay, which showed Tn-NVN cells and cell-free media had no TnCLV Segment 1 or 2 RNAs over the course of over 200 continuous passages in culture. A similar RT-PCR assay was used to demonstrate Tn-NVN is also free of Sf-RV, which is a common contaminant of Sf cell lines, and BmMLV, which is a common contaminant of Bm cell lines (Geisler & Jarvis, 2018). The search for potential adventitious viruses in Tn-NVN cells was extended by assembling their genome and transcriptome and then using TBLASTN to search those sequences for adventitious contaminants with a novel viral protein database (Geisler, 2018). Using this sensitive and unbiased approach, diverse sequences encoding virus-like proteins including Maverick, R1 LINE, and errantivirus transposons, as well as endogenous viral elements, were identified. However, there was no evidence of contamination with any bona fide viral agents. Similarly, searching the Tn-NVN genome and transcriptome using Bacterial or Archaeal ribosomal 5S, 16S, and 23S databases or Fungal nuclear ribosomal internal transcribed spacer (ITS) sequences revealed no evidence of Bacterial, Archaeal, or fungal contamination. Finally, the results of direct assays of total Tn-NVN cell RNA using a commercial PCR kit designed to detect DNA from >60 species of Mycoplasma, Acholeplasma, Spiroplasma, and Ureaplasma revealed no positive results. Together, these data indicated Tn-NVN cells have no Tn-CLV and no other viral, Bacterial, Archaeal, or Fungal adventitious agents, demonstrating they can be used as a highly suitable host for manufacturing safe biological products in the BICS for human or veterinary clinical applications.
Tn-NVN cells also have several biotechnologically relevant properties that enhance their utility for recombinant protein production. In contrast to High Five™ cells, which adapt poorly to non-adherent growth (Dee, Shuler, et al., 1997; Dee, Wood, et al., 1997; Donaldson & Shuler, 1998; M. A. Saarinen & Murhammer, 2000; R. A. Taticek et al., 2001; R.A. Taticek et al., 1997; Wickham & Nemerow, 1993), Tn-NVN cells grow consistently well as suspension cultures in serum-free medium without clumping. In addition, Tn-NVN cells produced three distinct model recombinant proteins at higher or comparable levels after infection with baculovirus vectors, as compared to four other commonly used insect cell lines, including High Five™. Interestingly, Tn-NVN cells produced an average of about 2-fold higher levels of our model recombinant proteins than the TN-368 cell line from which they were derived, suggesting removal of TnCLV from Tn cells might have contributed to improved recombinant protein productivity. This might seem like a predictable result, as removing a persistent viral contamination would be expected to eliminate a metabolic burden and provide additional resources for recombinant protein production. However, this result was not obtained in a previous study, which showed there was no significant difference in the recombinant protein production levels supported by Sf-RV-contaminated and Sf-RV-negative Sf cell lines after baculovirus infection (Maghodia et al., 2016).
5 |. CONCLUSION
The major outcome of this study was the isolation and characterization of Tn-NVN, which we introduced here as a new TnCLV-negative Tn cell line. This new cell line can be cultured in suspension in a serum free growth medium and can support high level recombinant protein production after baculovirus infection, as assessed using three distinct model proteins in comparison to four other commonly used insect cell lines. Tn-NVN cells also exhibited consistent growth properties, with doubling times of about 26 hours under the culture conditions used in this study, and was not detectably contaminated with any other viral, Bacterial, Archaeal, or Fungal adventitious agents. As a new, extensively characterized Tn-CLV-negative Tn cell line, Tn-NVN should be an excellent alternative host for the BICS with significant advantages for the production of recombinant proteins for both research and clinical applications.
Supplementary Material
6 |. ACKNOWLEDGEMENTS
This work was supported by Award R43 GM125397 from the National Institutes of Health, Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Institute of General Medical Sciences.
Abbreviations
- BICS
Baculovirus insect cell system
- CDD
Conserved domain database
- EVE
Endogenous viral element
- LINE
Long interspersed nuclear element
- LTR
Long terminal repeat
- MPS
Massively parallel sequencing
- ORF
Open reading frame
- RT
Reverse transcriptase
- RPKM
Reads per 1000 bps of transcript per million mapped reads
- Sf
Spodoptera frugiperda
- Tn
Trichoplusia ni
- TSA
Transcriptome sequence assembly
- VLP
Virus-like particles
- VPD
Viral protein database
- WGS
Whole genome shotgun
Footnotes
CONFLICT OF INTEREST
As the President of GlycoBac, LLC, DLJ declares a potential conflict of interest as GlycoBac has provided and expects to provide Tn-NVN cells as a commercial product, which will be of financial benefit to the company. As past employees of GlycoBac who have moved on to other positions and have no financial interest in GlycoBac, A.B.M. and C.G. have no conflicts of interest. Tn-NVN cells are deposited with the ATCC under patent deposit number PTA-125827.
8 | REFERENCES
- Aswad A, & Katzourakis A (2014). The first endogenous herpesvirus, identified in the Tarsier genome, and novel sequences from primate Rhadinoviruses and Lymphocryptoviruses. PLoS Genetics, 10(6), e1004332. doi:https://dx.doi.org/10.1371%2Fjournal.pgen.1004332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATCC. Universal Mycoplasma Detection Kit. Retrieved from https://www.atcc.org/MDK
- Bardor M, Faveeuw C, Fitchette A-C, Gilbert D, Galas L, Trottein F, … Lerouge P (2003). Immunoreactivity in mammals of two typical plant glyco-epitopes, core α(1,3)-fucose and core xylose. Glycobiology, 13(6), 427–434. doi: 10.1093/glycob/cwg024 [DOI] [PubMed] [Google Scholar]
- CDC. (2010). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly Rep, 59(20), 626–629. doi:mm5920a4 [pii] [PubMed] [Google Scholar]
- Chen YR, Zhong S, Fei Z, Gao S, Zhang S, Li Z, … Blissard GW (2014). Transcriptome responses of the host Trichoplusia ni to infection by the baculovirus Autographa californica multiple nucleopolyhedrovirus. J. Virol.ogy, 88(23), 13781–13797. doi:https://dx.doi.org/10.1128%2FJVI.02243-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YR, Zhong S, Fei Z, Hashimoto Y, Xiang JZ, Zhang S, & Blissard GW (2013). The transcriptome of the baculovirus Autographa californica multiple nucleopolyhedrovirus in Trichoplusia ni cells. J. Virol, 87(11), 6391–6405. doi: 10.1128/JVI.00194-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Gómez A, Sánchez-Mirón A, García-Camacho F, Molina-Grima E, & Chisti Y (2014). Protein production using the baculovirus-insect cell expression system. Biotechnol. Progr, 30(1), 1–18. doi: 10.1002/btpr.1842 [DOI] [PubMed] [Google Scholar]
- Davis TR, Wickham TJ, McKenna KA, Granados RR, Shuler ML, & Wood HA (1993). Comparative recombinant protein production of eight insect cell lines. In Vitro Cell. Dev. Biol. Anim, 29A(5), 388–390. doi: 10.1007/bf02633986 [DOI] [PubMed] [Google Scholar]
- Dee KU, Shuler ML, & Wood HA (1997). Inducing single-cell suspension of BTI-TN5B1–4 insect cells: I. The use of sulfated polyanions to prevent cell aggregation and enhance recombinant protein production. Biotechnol. Bioengr, 54(3), 191–205. doi: [DOI] [PubMed] [Google Scholar]
- Dee KU, Wood HA, & Shuler ML (1997). Inducing single-cell suspension of BTI-TN5B1–4 insect cells: II. The effect of sulfated polyanions on baculovirus infection. Biotechnol. Bioengr, 54(3), 206–220. doi: [DOI] [PubMed] [Google Scholar]
- Donaldson MS, & Shuler ML (1998). Effects of long-term passaging of BTI-Tn5B1–4 insect cells on growth and recombinant protein production. Biotechnol. Progr, 14(4), 543–547. doi: 10.1021/bp9800485 [DOI] [PubMed] [Google Scholar]
- Dupuy C, Periquet G, Serbielle C, Bézier A, Louis F, & Drezen J-M (2011). Transfer of a chromosomal Maverick to endogenous bracovirus in a parasitoid wasp. Genetica, 139(4), 489–496. doi: 10.1007/s10709-011-9569-x [DOI] [PubMed] [Google Scholar]
- Fabini G, Freilinger A, Altmann F, & Wilson IB (2001). Identification of core α1,3-fucosylated glycans and cloning of the requisite fucosyltransferase cDNA from Drosophila melanogaster. Potential basis of the neural anti-horseradish peroxidase epitope. J. Biol. Chem, 276(30), 28058–28067. doi: 10.1074/jbc.M100573200 [DOI] [PubMed] [Google Scholar]
- Felberbaum RS (2015). The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J, 10(5), 702–714. doi: 10.1002/biot.201400438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ftouhi-Paquin N, Hauer CR, Stack RF, Tarentino AL, & Plummer TH Jr. (1997). Molecular cloning, primary structure, and properties of a new glycoamidase from the fungus Aspergillus tubigensis. J. Biol. Chem, 272(36), 22960–22965. doi: 10.1074/jbc.272.36.22960 [DOI] [PubMed] [Google Scholar]
- Fu Y, Yang Y, Zhang H, Farley G, Wang J, Quarles KA, … Zamore PD (2018). The genome of the Hi5 germ cell line from Trichoplusia ni, an agricultural pest and novel model for small RNA biology. eLife, 7, e31628. doi: 10.7554/eLife.31628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon S, Strykowski H, & Charpentier G (1990). Implication of mitochondria in the replication of Nodamura virus in larvae of the Lepidoptera, Galleria mellonella (L.) and in suckling mice. Arch. Virol, 113. doi: 10.1007/bf01316670 [DOI] [PubMed] [Google Scholar]
- Geisler C (2018). A new approach for detecting adventitious viruses shows Sf-rhabdovirus-negative Sf-RVN cells are suitable for safe biologicals production. BMC Biotechnol, 18(1), 8. doi: 10.1186/s12896-017-0412-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, & Jarvis DL (2012). Innovative use of a bacterial enzyme involved in sialic acid degradation to initiate sialic acid biosynthesis in glycoengineered insect cells. Met. Engr, 14(6), 642–652. doi: 10.1016/j.ymben.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, & Jarvis DL (2016). Rhabdovirus-like endogenous viral elements in the genome of Spodoptera frugiperda insect cells are actively transcribed: Implications for adventitious virus detection. Biologicals, 44(4), 219–225. doi: 10.1016/j.biologicals.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, & Jarvis DL (2018). Adventitious viruses in insect cell lines used for recombinant protein expression. Prot. Expr. Purif, 144, 25–32. doi: 10.1016/j.pep.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, Kotu V, Sharrow M, Rendic D, Poltl G, Tiemeyer M, … Jarvis DL (2012). The Drosophila neurally altered carbohydrate mutant has a defective Golgi GDP-fucose transporter. J. Biol. Chem, 287(35), 29599–29609. doi: 10.1074/jbc.M112.379313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados RR (1991). United States of America Patent No. 5,300,435. USPTO.
- Granados RR, Guoxun L, Derksen ACG, & McKenna KA (1994). A new insect cell line from Trichoplusia ni (BTI-Tn-5B1–4) susceptible to Trichoplusia ni single enveloped nuclear polyhedrosis virus. J. Invert. Patho.l, 64(3), 260–266. doi: 10.1016/S0022-2011(94)90400-6 [DOI] [Google Scholar]
- Haapa-Paananen S, Wahlberg N, & Savilahti H (2014). Phylogenetic analysis of Maverick/Polinton giant transposons across organisms. Mol. Phylogen. and Evolution, 78, 271–274. doi: 10.1016/j.ympev.2014.05.024 [DOI] [PubMed] [Google Scholar]
- Hancock K, Narang S, Pattabhi S, Yushak ML, Khan A, Lin S. c., … Tsang VCW (2008). False positive reactivity of recombinant, diagnostic, glycoproteins produced in High Five™ insect cells: Effect of glycosylation. J. Immunol. Meth, 330(1), 130–136. doi: 10.1016/j.jim.2007.08.002 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Zhang S, & Blissard GW (2010). Ao38, a new cell line from eggs of the black witch moth, Ascalapha odorata (Lepidoptera: Noctuidae), is permissive for AcMNPV infection and produces high levels of recombinant proteins. BMC Biotechnol., 10. doi: 10.1186/1472-6750-10-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Zhang S, Zhang S, Chen Y-R, & Blissard GW (2012). Erratum to: BTI-Tnao38, a new cell line derived from Trichoplusia ni, is permissive for AcMNPV infection and produces high levels of recombinant proteins. BMC Biotechnol., 12(1), 1–4. doi: 10.1186/1472-6750-12-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hink WF (1970). Established insect cell line from the cabbage looper, Trichoplusia ni. Nature, 226(5244), 466–467. doi: 10.1038/226466b0 [DOI] [PubMed] [Google Scholar]
- Kamili S (2011). Toward the development of a hepatitis E vaccine. Virus Res, 161(1), 93–100. doi: 10.1016/j.virusres.2011.05.008 [DOI] [PubMed] [Google Scholar]
- Kapitonov VV, & Jurka J (2006). Self-synthesizing DNA transposons in eukaryotes. Proc. Natl. Acad. Sci. U.S.A, 103(12), 4540–4545. doi: 10.1073/pnas.0600833103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzourakis A, & Gifford RJ (2010). Endogenous viral elements in animal genomes. PLoS Genetics, 6(11). doi: 10.1371/journal.pgen.1001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochibe N, & Furukawa K (1980). Purification and properties of a novel fucose-specific hemagglutinin of Aleuria aurantia. Biochemistry, 19(13), 2841–2846. doi: 10.1021/bi00554a004 [DOI] [PubMed] [Google Scholar]
- Kost TA, Condreay JP, & Jarvis DL (2005). Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol, 23(5), 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Schinko T, Palmberger D, Tauer C, Messner P, & Grabherr R (2010). Trichoplusia ni cells (High Five™) are highly efficient for the production of influenza A virus-like particles: a comparison of two insect cell lines as production platforms for influenza vaccines. Mol. Biotechnol, 45(3), 226–234. doi: 10.1007/s12033-010-9268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Nei M, Dudley J, & Tamura K (2008). MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform, 9. doi: 10.1093/bib/bbn017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Vov DK, Al’khovskii SV, Shchelkanov M, Shchetinin AM, Deriabin PG, Aristova VA, … Botikov AG (2014). Taxonomic status of the Tyulek virus (TLKV) (Orthomyxoviridae, Quaranjavirus, Quaranfil group) isolated from the ticks Argas vulgaris Filippova, 1961 (Argasidae) from the birds burrow nest biotopes in the Kyrgyzstan. Vopr. Virusol, 59(2), 28–32. [PubMed] [Google Scholar]
- Lazareva E, Lezzhov A, Vassetzky N, Solovyev A, & Morozov S (2015). Acquisition of full-length viral helicase domains by insect retrotransposon-encoded polypeptides. Front. Microbiol, 6, 1447. doi: 10.3389/fmicb.2015.01447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T-C, Scotti PD, Miyamura T, & Takeda N (2007). Latent infection of a new alphanodavirus in an insect cell line. J. Virol, 81(20), 10890–10896. doi: 10.1128/JVI.00807-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Galvin TA, Glasner DR, Shaheduzzaman S, & Khan AS (2014). Identification of a novel rhabdovirus in Spodoptera frugiperda cell lines. J. Virol, 88(12), 6576–6585. doi: 10.1128/JVI.00780-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabashi-Asazuma H, Kuo CW, Khoo KH, & Jarvis DL (2014). A novel baculovirus vector for the production of nonfucosylated recombinant glycoproteins in insect cells. Glycobiology, 24(3), 325–340. doi: 10.1093/glycob/cwt161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghodia AB, Geisler C, & Jarvis DL (2016). Characterization of an Sf-rhabdovirus-negative Spodoptera frugiperda cell line as an alternative host for recombinant protein production in the baculovirus-insect cell system. Prot. Expr. Purif, 122(June), 45–55. doi: 10.1016/j.pep.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley F, Trimble RB, Tarentino AL, & Plummer TH Jr. (1989). Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Analyt. Biochem, 180(2), 195–204. doi: 10.1016/0003-2697(89)90115-2 [DOI] [PubMed] [Google Scholar]
- Malik HS, Henikoff S, & Eickbush TH (2000). Poised for contagion: evolutionary origins of the infectious abilities of invertebrate retroviruses. Genome Res, 10(9), 1307–1318. doi: 10.1101/gr.145000 [DOI] [PubMed] [Google Scholar]
- Meng M-J, Li T-L, Li C-Y, & Li G-X (2008). A suspended cell line from Trichoplusia ni (Lepidoptera): Characterization and expression of recombinant proteins. Insect Sci., 15(5), 423–428. doi: 10.1111/j.1744-7917.2008.00229.x [DOI] [Google Scholar]
- Menzel T, & Rohrmann GF (2008). Diversity of errantivirus (retrovirus) sequences in two cell lines used for baculovirus expression, Spodoptera frugiperda and Trichoplusia ni. Virus Genes, 36(3), 583–586. doi: 10.1007/s11262-008-0221-5 [DOI] [PubMed] [Google Scholar]
- Misseri Y, Cerutti M, Devauchelle G, Bucheton A, & Terzian C (2004). Analysis of the Drosophila gypsy endogenous retrovirus envelope glycoprotein. J. Gen. Virol, 85(11), 3325–3331. doi: 10.1099/vir.0.79911-0 [DOI] [PubMed] [Google Scholar]
- Murphy FA, Scherer WF, Harrison AK, Dunne HW, & Gary GW (1970). Characterization of Nodamura virus, an arthropod transmissible picornavirus. Virology, 40(4), 1008–1021. doi: 10.1016/0042-6822(70)90147-9 [DOI] [PubMed] [Google Scholar]
- Ozers MS, & Friesen PD (1996). The env-like open reading frame of the baculovirus-integrated retrotransposon TED encodes a retrovirus-like envelope protein. Virology, 226(2), 252–259. doi: 10.1006/viro.1996.0653 [DOI] [PubMed] [Google Scholar]
- Pearson MN, & Rohrmann GF (2004). Conservation of a proteinase cleavage site between an insect retrovirus (gypsy) Env protein and a baculovirus envelope fusion protein. Virology, 322(1), 61–68. doi: 10.1016/j.virol.2004.01.012 [DOI] [PubMed] [Google Scholar]
- Pélisson A, Song SU, Prud’homme N, Smith PA, Bucheton A, & Corces VG (1994). Gypsy transposition correlates with the production of a retroviral envelope-like protein under the tissue-specific control of the Drosophila flamenco gene. EMBO J, 13(18), 4401–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenner C, Mach L, Glössl J, & März L (1992). The antigenicity of the carbohydrate moiety of an insect glycoprotein, honey-bee (Apis mellifera) venom phospholipase A2. The role of α1,3-fucosylation of the asparagine-bound N-acetylglucosamine. Biochem. J, 284(2), 377–380. doi: 10.1042/bj2840377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhiel M, Mitchell-Logean CM, & Murhammer DW (1997). Comparison of Trichoplusia ni BTI-Tn-5B1–4 (high five™) and Spodoptera frugiperda Sf-9 insect cell line metabolism in suspension cultures. Biotechnol. Bioengr, 55(6), 909–920. doi: [DOI] [PubMed] [Google Scholar]
- Rohrmann G, & Karplus PA (2001). Relatedness of baculovirus and gypsy retrotransposon envelope proteins. BMC Evol. Biol, 1(1), 1. doi:https://dx.doi.org/10.1186%2F1471-2148-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann GF (2019). Baculoviruses, retroviruses, DNA transposons (piggyBac), and insect cells. In Baculovirus Molecular Biology (4th ed.). Bethesda, MD: National Center for Biotechnology Information. [PubMed] [Google Scholar]
- Saarinen MA, & Murhammer DW (2000). Culture in the rotating-wall vessel affects recombinant protein production capability of two insect cell lines in different manners. In Vitro Cell. Dev. Biol.. An, 36(6), 362–366. doi: [DOI] [PubMed] [Google Scholar]
- Saarinen MA, Troutner KA, Gladden SG, Mitchell-Logean CM, & Murhammer DW (1999). Recombinant protein synthesis in Trichoplusia ni BTI-Tn-5B1–4 insect cell aggregates. Biotechnol. Bioengr, 63(5), 612–617. doi: [DOI] [PubMed] [Google Scholar]
- Sander L, & Harrysson A (2007). Using cell size kinetics to determine optimal harvest time for Spodoptera frugiperda and Trichoplusia ni BTI-TN-5B1–4 cells infected with a baculovirus expression vector system expressing enhanced green fluorescent protein. Cytotechnology, 54(1), 35–48. doi: 10.1007/s10616-007-9064-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Sotelo P, & Ramirez-Prado JH (2012). prfectBLAST: a platform-independent portable front end for the command terminal BLAST+ stand-alone suite. Biotechniques, 53(5), 299–300. doi: 10.2144/000113953 [DOI] [PubMed] [Google Scholar]
- Scherer WF, Verna JE, & Richter W (1968). Nodamura virus, an ether- and chloroform-resistant arbovirus from Japan: physical and biological properties, with ecologic observations. Am. J. Trop. Med. Hyg, 17(1), 120–128. doi: 10.4269/ajtmh.1968.17.120 [DOI] [PubMed] [Google Scholar]
- Schneemann A, Reddy V, & Johnson JE (1998). The structure and function of nodavirus particles: a paradigm for understanding chemical biology. Adv. Virus Res, 50. doi: 10.1016/s0065-3527(08)60812-x [DOI] [PubMed] [Google Scholar]
- Seismann H, Blank S, Braren I, Greunke K, Cifuentes L, Grunwald T, … Spillner E (2010). Dissecting cross-reactivity in hymenoptera venom allergy by circumvention of α−1,3-core fucosylation. Mol. Immunol, 47(4), 799–808. doi: 10.1016/j.molimm.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Shan M, Zhang SY, Jiang L, Ma M, & Li GX (2011). Susceptibility to AcMNPV and expression of recombinant proteins by a novel cell clone derived from a Trichoplusia ni QAU-BTI-Tn9–4s cell line. Virol. Sin, 26(5), 297–305. doi: 10.1007/s12250-011-3201-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Lin X-D, Tian J-H, Chen L-J, Chen X, Li C-X, … Zhang Y-Z (2016). Redefining the invertebrate RNA virosphere. Nature, 540(7634), 539–543. doi: 10.1038/nature20167 [DOI] [PubMed] [Google Scholar]
- Taticek RA, Choi C, Phan SE, Palomares LA, & Shuler ML (2001). Comparison of growth and recombinant protein expression in two different insect cell lines in attached and suspension culture. Biotechnol. Progr, 17(4), 676–684. doi: 10.1021/bp010061g [DOI] [PubMed] [Google Scholar]
- Taticek RA, McKenna KA, Granados RR, & Shuler ML (1997). Rapid initiation of suspension cultures of Trichoplusia ni insect cells (TN 5B-1–4) using heparin. Biotechnol. Tech, 11(4), 237–240. doi: 10.1023/A:1018482304052 [DOI] [Google Scholar]
- ter Horst AM, Nigg JC, Dekker FM, & Falk BW (2019). Endogenous Viral Elements Are Widespread in Arthropod Genomes and Commonly Give Rise to PIWI-Interacting RNAs. J. Virol, 93(6), e02124–02118. doi: 10.1128/JVI.02124-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teysset L, Burns JC, Shike H, Sullivan BL, Bucheton A, & Terzian C (1998). A Moloney murine leukemia virus-based retroviral vector pseudotyped by the insect retroviral gypsy envelope can infect Drosophila cells. J. Virol, 72(1), 853–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiem SM (2011). USA Patent No. US8383402B2. USPTO.
- Tretter V, Altmann F, Kubelka V, März L, & Becker WM (1993). Fucose α1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int. Arch. Allergy Imm, 102(3), 259–266. doi: 10.1159/000236534 [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Davis T, Granados RR, Shuler ML, & Wood HA (1992). Screening of insect cell lines for the production of recombinant proteins and infectious virus in the baculovirus expression system. Biotechnol. Progr, 8(5), 391–396. doi: 10.1021/bp00017a003 [DOI] [PubMed] [Google Scholar]
- Wickham TJ, & Nemerow GR (1993). Optimization of growth methods and recombinant protein production in BTI-Tn-5B1–4 insect cells using the baculovirus expression system. Biotechnol. Progr, 9(1), 25–30. doi: 10.1021/bp00019a004 [DOI] [PubMed] [Google Scholar]
- Wilde M, Klausberger M, Palmberger D, Ernst W, & Grabherr R (2014). Tnao38, high five and Sf9 - evaluation of host–virus interactions in three different insect cell lines: baculovirus production and recombinant protein expression. Biotechnol. Lett, 36(4), 743–749. doi:https://dx.doi.org/10.1007%2Fs10529-013-1429-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Vanbeselaere J, Jin C, Blaukopf M, Wöls F, Wilson IBH, & Paschinger K (2018). Core richness of N-glycans of Caenorhabditis elegans: A case study on chemical and enzymatic release. Analyt. Chem, 90(1), 928–935. doi:https://dx.doi.org/10.1021%2Facs.analchem.7b03898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Yu Y, Tang X, Chen H, Xiao J, & Su X-D (2016). Transcriptome analyses of insect cells to facilitate baculovirus-insect expression. Protein Cell, 7(5), 373–382. doi: 10.1007/s13238-016-0260-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Manzan MA, Peplinski HM, & Thiem SM (2008). A new Trichoplusia ni cell line for membrane protein expression using a baculovirus expression vector system. In Vitro Cell. Dev. Biol. An, 44(7), 214–223. doi: 10.1007/s11626-008-9095-z [DOI] [PubMed] [Google Scholar]
- Zhang F, & Thiem S (2010). The Trichoplusia ni cell line MSU-TnT4 does not harbor a latent nodavirus. In Vitro Cell. Dev. Biol. An, 46(1), 1–6. doi: 10.1007/s11626-009-9241-2 [DOI] [PubMed] [Google Scholar]
- Zheng G-L, Zhou H-X, & Li C-Y (2014). Serum-free culture of the suspension cell line QB-Tn9–4s of the cabbage looper, Trichoplusia ni, is highly productive for virus replication and recombinant protein expression. J. Insect Sci, 14(24), 1–14. doi: 10.1673/031.014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


