Abstract
SARS-CoV-2 has high human-human transmission rate. The aerosols and saliva droplets are the main contamination source. Thus, it is crucial to point out that dental practitioners become a high-risk group of contagion by SARS-CoV-2. Based on this, protocols have been recommended to avoid cross-contamination during dental care; however, appropriate evidence has not yet been established.
Objective
Our study sought to make a screening, by in silico analysis, of the potential of mouth rinses used in dental practices to prevent the dental workers' contamination by SARS-CoV-2.
Methodology
Multiple sequence comparisons and construction of the phylogenetic tree were conducted using the FASTA code. Therefore, molecular docking investigation between SARS-CoV-2 proteins (Main Protease, Spike Glycoprotein, Non-structure Protein, and Papain-like Protease) and molecules used in dental practices (chlorhexidine digluconate, hydrogen peroxide, cetylpyridinium chloride, povidone-iodine, gallic acid, β-cyclodextrin, catechin, and quercetin) was performed using AutoDock Vina. Moreover, 2D interactions of the complex protein-ligand structure were analyzed by Ligplot+.
Results
The obtained results showed a remarkable affinity between SARS-CoV-2 proteins and all tested compounds. The chlorhexidine digluconate, catechin, and quercetin presented a higher affinity with SARS-CoV-2.
Conclusions
The overall results allowed us to suggest that chlorhexidine is the most suitable active compound in reducing the SARS-CoV-2 salivary load due to its better binding energy. However, in vivo studies should be conducted to confirm their clinical use.
Keywords: Molecular docking simulation; Severe acute respiratory syndrome-related coronavirus; Practice management, Dental; Containment of biohazards
Introduction
The coronavirus disease 2019 (COVID-19) outbreak has drawn attention worldwide since its first identified case in Wuhan – China.1 This infectious disease, caused by the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV-2), has spread globally and infected millions of people, leading thousands of individuals to death.2 The SARS-CoV-2 has high human-human transmissibility, and the saliva plays an essential role in it. Through the saliva droplets/aerosols inhalation or ingestion from infected people, health people may fall ill.3,4 Based on this rationale, asymptomatic patients also are considered a transmission vector, since they are freely carrying out their activities and endangering the population’s health.5
Salivary glands and saliva are the main reservoirs to SARS-CoV-2 due to the high-affinity with the host-cell receptor angiotensin-converting enzyme II (ACE2), also found in salivary glands.6,7 Then, dental practitioners can be considered a high-risk group of contagion by SARS-CoV-2 due to their exposure to aerosols-generated procedures during dental care.8,9 Moreover, a lack of evidence regarding what to do as a pre-procedural protocol to avoid SARS-CoV-2 cross-infection is worrying. It should be resolved to offer more protection to dental workers.
Regarding this issue, some protocols have been recommended worldwide to avoid SARS-CoV-2 dissemination during dental care. Based on this, mouth rinses with hydrogen peroxide, povidone-iodine, or chlorhexidine are used before any dental procedure.10 However, once these suggestions have inadequate scientific support,11 appropriate evidence must be produced to understand the possible mechanism of action of these compounds. Additionally, clinical studies involving drug testing require time and involve risks for both research groups and researchers.12
In this context, in silico analyses play a fundamental role in simulating molecular processes to support validation studies between molecular and cellular processes.13 Among in silico analyses, the docking studies can be emphasized, which evaluate protein-ligand complexes through a series of algorithms to generate scoring functions. Thus, these analyses can predict the biological effects of chemical compounds due to their ability to interact with proteins responsible for the virulence present in the surface.14 Therefore, the fastest scenario to test existing drugs on the SARS-CoV-2 proteins (such as Main or Papain-like proteases, Spike glycoprotein, and non-structured proteins) is the in silico analysis, which is a robust approach to provide remarkable results. Thus, it could propose initial therapeutic strategies to prevent SARS-CoV-2 contamination by dental workers.15
Then, our study aimed at providing preliminary data, using computational tools, of the therapeutic potential of mouth rinses, widely used in dentistry practices, to prevent the contamination of dental workers by SARS-CoV-2 during dental procedures. The absence of interaction between the SARS-CoV-2 proteins and mouth rinses’ compounds was considered the null hypothesis.
Methodology
Retrieval of proteins sequences
The crystal structures and FASTA code of SARS-CoV-2 proteins were obtained from the Research Collaboratory for Structural Bioinformatics Protein Data Bank – RCSB PDB (RRID: SCR_012820). Therefore, four different SARS-CoV-2 proteins groups [Main Protease – Mpro (6LU7, 6Y2E, 6Y84, 6YB7), Spike glycoprotein (6LVN, 6VSB, 6VXX, 6VYB), Non-structure Protein – NSP (6YHU, 6W4B, 6W4H, 6W37, 6WEY, 6WIQ, 6WIJ), and Papain-like Protease (6W9C)] were selected as molecular targets.
Sequence alignment, multiple sequence comparisons, and construction of the phylogenetic tree
Multiple sequence comparisons of proteins from SARS-CoV-2 was conducted using a Constraint-based Multiple Alignment Tool (COBALT, RRID: SCR_004152) through the FASTA code, which allowed to construct the phylogenetic tree by using the neighbor-joining method based on the alignment sequences.
Ligand selection and structure preparation
Eight compounds were selected for in silico analyses: five substances commercially used as mouth rinses/mouthwashes [chlorhexidine digluconate – CHX (C34H54Cl2N10O14 – PubChem CID: 9552081), hydrogen peroxide – HP (H2O2 – PubChem CID: 784), cetylpyridinium chloride – CCP (C21H38ClN – PubChem CID: 31239), povidone-iodine – PVPI (C6H9I2NO – PubChem CID: 410087)] and three antimicrobial compounds [gallic acid – GA (C7H6O5 – PubChem CID: 370), β-cyclodextrin – BCD (C42H70O35 – PubChem CID: 444041), catechin – CAT (C15H14O6 – PubChem CID: 9064), quercetin – QTN (C15H10O7 – CID: 5280343)].
The 2D structures were retrieved from the National Center for Biotechnology Information (NCBI) chemical structure library (PubChem, RRID: SCR_004284). The files were imported in 2D SDF format and converted to 3D Protein Data Bank format (.pdb) by the Open Babel (RRID: SCR_014920).
The ligand’ rotatable bonds were defined using AutoDock, and the structures were saved as pdbqt files for the use in the docking studies.
SARS-CoV-2 proteins preparation
The AutoDock (RRID: SCR_012746) was used to delete repeated chains, heteroatoms, and water molecules, add polar hydrogens atoms, and add the charge atoms to the protein structure. Gasteiger charges were computed, and the structure was saved as a pdbqt file for the docking studies.
Molecular docking procedure
The 3D grids were created by the Autogrid algorithm to generate the grid parameter files (Autogrid, RRID: SCR_015982). Each grid map was set to the center of the Chain A. Docking parameters were set according to the protein (Table 1), and all analyses were conducted with “exhaustiveness = 8”.
Table 1. Grid parameters of SARS-CoV-2 proteins.
| SARS-CoV-2 Proteins | Center | Size | ||||
|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | |
| 6LU7 | -26 | 13 | 59 | 126 | 126 | 126 |
| 6LVN | 10 | 34 | 29 | 46 | 68 | 40 |
| 6VSB | 206 | 223 | 227 | 104 | 92 | 126 |
| 6VXX | 198 | 223 | 207 | 90 | 102 | 126 |
| 6VYB | 197 | 223 | 206 | 126 | 102 | 126 |
| 6W4B | 54 | -11 | 23 | 56 | 68 | 54 |
| 6W4H | 92 | 24 | 23 | 102 | 126 | 92 |
| 6W9C | -32 | 34 | 26 | 126 | 126 | 126 |
| 6W37 | -24 | 14 | 15 | 40 | 40 | 40 |
| 6WEY | -2 | 5 | 13 | 126 | 90 | 122 |
| 6WIQ | -4 | -8 | -6 | 96 | 100 | 114 |
| 6WJI | 8 | 1 | -14 | 126 | 100 | 96 |
| 6Y2E | -17 | -26 | 18 | 126 | 126 | 126 |
| 6Y84 | 12 | 1 | 5 | 72 | 84 | 90 |
| 6YB7 | 12 | 1 | 5 | 106 | 126 | 126 |
| 6YHU | -25 | 25 | 51 | 70 | 100 | 114 |
Molecular docking was conducted using AutoDock Vina (RRID: SCR_011958), and the best ligand/protein model was identified based on the binding energy (ΔG – kcal/mol).16
Docking visualization
The results were viewed on UCSF Chimera 1.14 (RRID: SCR_004097). Only one protein from each group was selected for the visualization. The 2D interactions of the complex protein-ligand structure, including hydrogen bonds and the bond lengths, were analyzed by Ligplot+ (RRID: SCR_018249) for the high-affinity bindings.17
Results
Multiple sequence comparisons and construction of the phylogenetic tree
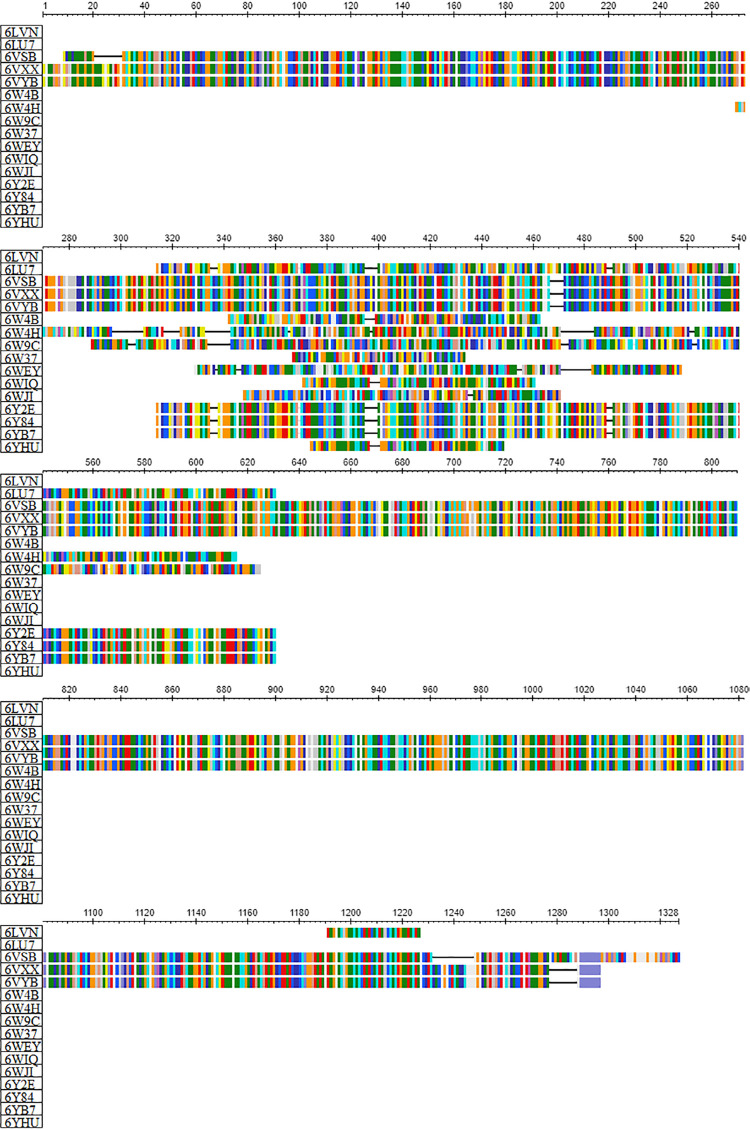
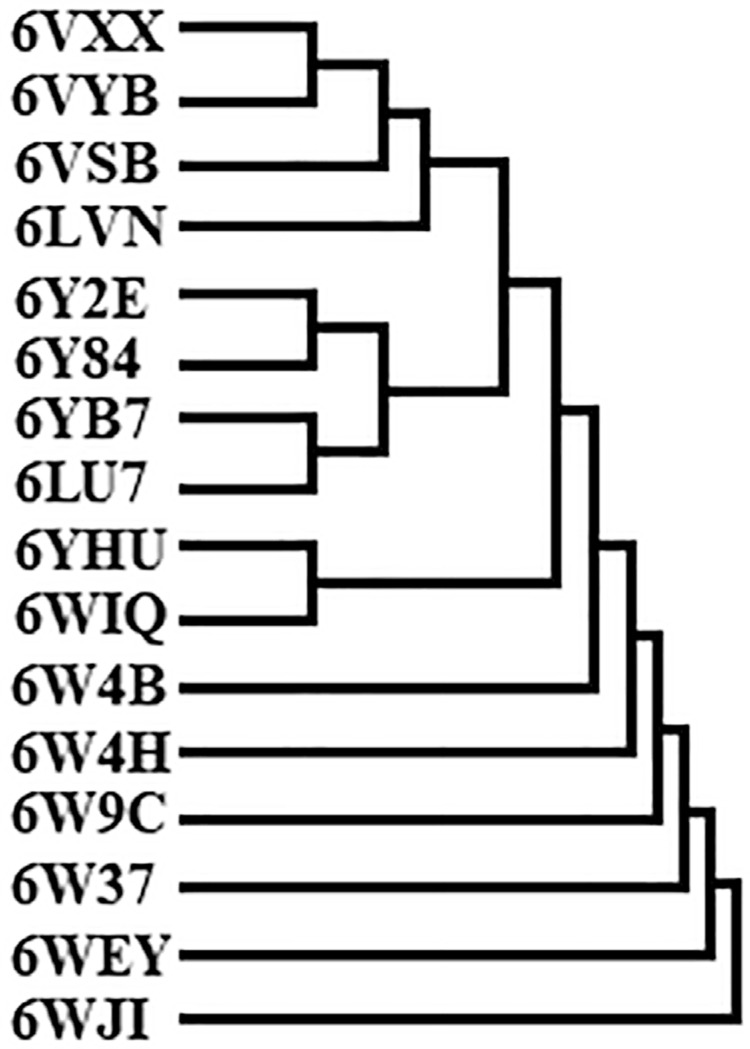
Sequence alignment and multiple sequence comparisons of the studied proteins from SARS-CoV-2 (Figure 1) allowed observing their similarity (Figure 2). Moreover, not only the studied Mpro presented similarity to the others, but also the Glycoproteins Spike. Besides, two Non-structure Proteins (PDB: 6YHU and 6WIQ) showed similarity, since they presented the same NSP7-NSP8 complex, whereas the other NSP presented several different complexes. In general, the similarity observed in the phylogenetic tree of the studied proteins classes is reflected in the binding energies.
Figure 1. Sequence alignment and multiple sequence comparisons of studied proteins from SARS-CoV-2.
Figure 2. Phylogenetic tree of SARS-CoV-2 studied proteins.
Molecular docking
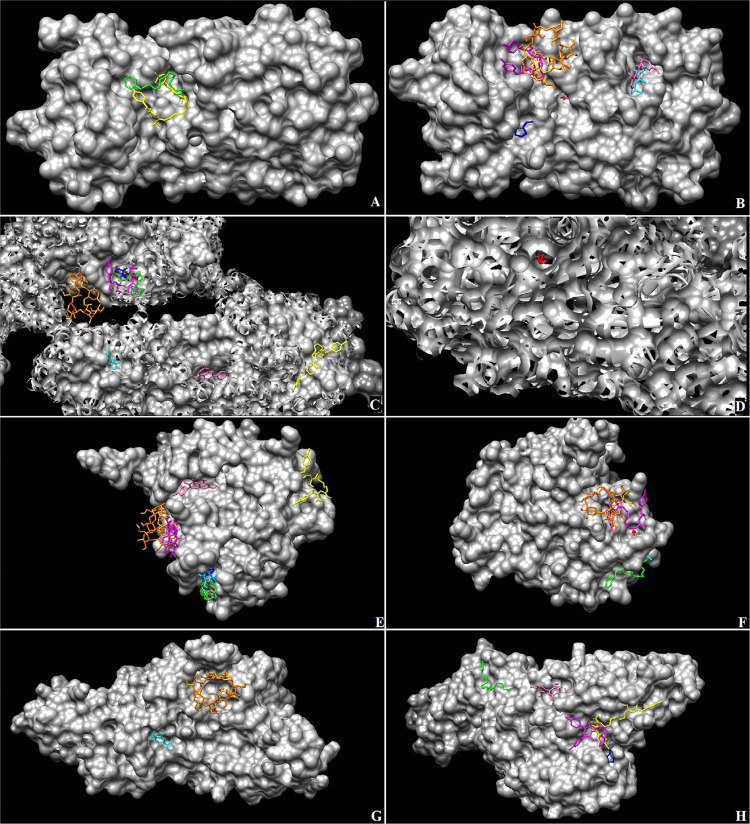
The affinity between selected compounds and SARS-CoV-2 proteins was observed in our study (Table 2). Nonetheless, chlorhexidine digluconate, catechin, and quercetin showed higher binding energy than others. A remarkable affinity between chlorhexidine and Mpro (PDB: 6Y84, -10.4 kcal/mol) was observed. Hydrogen peroxide showed to be the least recommended compound, since they presented the lowest affinity (-2.1 kcal/mol) with Spike glycoprotein (PBD: 6LVN) and Non-structure Protein (PDB: 6WIQ). The specific binding sites to each compound are shown in Figure 3, in which we could observe that some compounds have the same binding site.
Table 2. Binding energy between the tested compounds and the SARS-CoV-2 proteins.
| SARS-CoV-2 Proteins | Binding Energy (ΔG – kcal/mol) | |||||||
|---|---|---|---|---|---|---|---|---|
| CHX | CCP | PVPI | HP | GA | BCD | CAT | QTN | |
| 6LU7 | -6.9 | -4.2 | -3.9 | -2.8 | -5.5 | -5.9 | -7.4 | -7.5 |
| 6LVN | -6.0 | -3.6 | -2.7 | -2.1 | -3.8 | -4.0 | -5.6 | -4.8 |
| 6VSB | -7.1 | -4.6 | -4.4 | -3.4 | -5.0 | -5.5 | -6.8 | -6.4 |
| 6VXX | -7.1 | -4.5 | -3.9 | -3.5 | -5.5 | -6.0 | -7.6 | -7.2 |
| 6VYB | -6.3 | -4.3 | -3.9 | -3.1 | -5.3 | -5.9 | -7.0 | -8.5 |
| 6W4B | -7.2 | -4.3 | -3.5 | -2.5 | -4.7 | -4.8 | -6.9 | -6.7 |
| 6W4H | -8.0 | -4.9 | -4.4 | -3.5 | -5.9 | -7.2 | -7.6 | -8.6 |
| 6W9C | -6.3 | -4.7 | -4.0 | -2.9 | -5.2 | -5.7 | -7.5 | -6.6 |
| 6W37 | -6.6 | -3.8 | -3.2 | -2.5 | -4.7 | -4.6 | -6.1 | -6.5 |
| 6WEY | -9.6 | -4.3 | -4.4 | -3.4 | -5.4 | -6.1 | -8.0 | -8.4 |
| 6WIQ | -5.1 | -3.6 | -3.1 | -2.1 | -3.6 | -4.5 | -4.6 | -5.8 |
| 6WJI | -7.4 | -3.5 | -3.9 | -3.0 | -4.8 | -6.2 | -7.2 | -7.4 |
| 6Y2E | -5.9 | -3.6 | -3.8 | -2.9 | -4.5 | -6.1 | -6.0 | -7.2 |
| 6Y84 | -10.4 | -6.0 | -4.5 | -3.2 | -6.9 | -7.9 | -9.1 | -9.2 |
| 6YB7 | -9.1 | -5.1 | -4.2 | -3.4 | -6.0 | -7.1 | -8.3 | -8.2 |
| 6YHU | -5.3 | -3.4 | -3.4 | -2.5 | -4.5 | -5.0 | -4.9 | -5.4 |
CHX: chlorhexidine digluconate; CCP: cetylpyridinium chloride; PVPI: povidone-iodine; HP: hydrogen peroxide; GA: gallic acid; BCD: β-cyclodextrin; CAT: catechin; QTN:
Figure 3. Binding complex and interaction visualization of tested compounds with SARS-CoV-2 proteins. Magenta: chlorhexidine digluconate; Green: cetylpyridinium chloride; Blue: povidone-iodine; Red: hydrogen peroxide; Cyan: gallic acid; Orange: β-cyclodextrin; Yellow: catechin; Pink: quercetin. Main protease - PDB: 6LU7 complex (a, b); Spike Glycoprotein – PDB: 6VYB complex (c, d); Non-structure Protein – PDB: 6W4H complex (e, f); Papain-like Protease – PDB: 6W9C complex (g, h).
Interactions of the complex protein-ligand structure
The results of LigPlot+ analyses showed the interaction of chlorhexidine, catechin, and quercetin with Mpro or Non-structure Protein (Figure 4, Table 3). Importantly, these compounds shared the same binding pocket in the Mpro (PDB: 6Y84).
Figure 4. Visualization of residue interactions. Chlorhexidine digluconate-Non structure protein (PDB: 6WEY) binding complex (a); Chlorhexidine digluconate-Mpro (PDB: 6YB7) binding complex (b); Cathechin- Mpro (PDB: 6Y84) binding complex (c); Quercetin- Mpro (PDB: 6Y84) binding complex (d), Chlorhexidine digluconate- Mpro (PDB: 6Y84) binding complex (e).
Table 3. Interaction between the compounds and the protein targets.
| Protein | Ligand | Interactions |
|---|---|---|
| 6WEY | CHX | Hydrophobic interaction: Ser370(A), Phe372(A), Leu373(A), Glu374(A), Met375(A), Lys376(A), Ser377(A) |
| 6YB7 | CHX | Hydrogen bond: Arg131(A), Asp197(A), Met276(A), Ala285(A), Asp289(A) Hydrophobic interaction: Thr199(A), Tyr237(A), Asn238(A), Tyr239(A), Leu272(A), Gly275(A), Leu286(A), Leu 287(A) |
| 6Y84 | CAT | Hydrogen bond: Lys5(A), Asp289(A) Hydrophobic interaction: Arg131(A), Lys137(A), Gly138(A), Glu290(A) |
| QTN | Hydrogen bond: Lys5(A), Gln127(A), Asp289(A) Hydrophobic interaction: Arg131(A), Lys137(A), Gly138(A), Glu290(A) | |
| CHX | Hydrogen bond: Lys5(A), Gln127(A), Thr199(A), Leu287(A) Hydrophobic interaction: Arg131(A), Lys137(A), Gly138(A), Tyr239(A), Leu286(A), Glu288(A), Asp289(A), Glu290(A) |
CHX: chlorhexidine digluconate; CAT: catechin; QTN: quercetin.
Discussion
The null hypothesis was rejected, once the binding affinity among some compounds (chlorhexidine, catechin, and quercetin) and all proteins tested (Mpro, Spike glycoprotein, Papain-like protease, and Non-structure Protein) was observed.
We constructed the phylogenetic tree of SARS-CoV-2 proteins. We observe that the major proteins in the same group are similar. However, differences in the amino acid residues may affect the interaction between them and “external” molecules. Different affinity degrees were observed besides the similarity among the proteins in the same group. On the other hand, the non-structure protein (NSP) group shows a diversity in the presented complexes, explaining the different sequences and the binding energies among NSP.
The molecular docking showed the affinity of the tested compounds with SARS-CoV-2 proteins in different degrees. In our study, HP and PVPI presented the lowest affinities with SARS-CoV-2 proteins. However, HP and PVPI are reported in the literature as possible products to decrease the SARS-CoV-2 salivary load in dental practice, due to their oxidative propriety.8,18 Thus, the obtained data allowed us to hypothesize that their mechanism of action against SARS-CoV-2 would not be binding-dependent. Other studies can also support this hypothesis, demonstrating the anti-SARS-CoV-2 effect of HP and PVPI solutions by in vitro studies19,20and case report.21
On the other hand, CHX was tested against SARS-CoV-2, and the results evidenced better binding energies with all studied Mpro and papain-like protease. These SARS-CoV-2 proteases are the main targets of antiviral agents, since they play an essential role in viral RNA replication and controlling host cells.22 Thus, our experiments corroborate the data observed by Yoon, et al.23 (2020), allowing us to suggest that CHX may avoid the COVID-19 dissemination in the dental office. Additionally, QTN and CAT showed a remarkable affinity to Mpro, corroborating the previously published studies.24,25 All these compounds share the same binding pocket in the Mpro (PDB: 6Y84 – Lys5, Arg131, Lys137, Gly138, Asp289, Glu290). Thus, it allows us to suggest their activity against SARS-CoV-2 and possibly develop an effective treatment for COVID-19.
The spike glycoprotein was also studied in our work. The ability to bind to the ACE 2 makes the spike glycoprotein a crucial factor of pathogenicity.26,27 Thus, according to Walls, et al.28 (2020), this group is an essential target to neutralize SARS-CoV-2. Based on this rationale, the scientific reports describe that the spike glycoprotein in an opened state (PDB: 6VYB) would be associated with the most pathogenic coronaviruses. In contrast, the closed state is associated with a common cold.28 In our study, both opened and closed spike glycoprotein had a binding affinity with CHX, CAT, and QTN, showing a possible transmissibility inhibition. However, CHX showed a more expressive binding energy, probably due to the four hydrogen bonds and eight hydrophobic interactions present in its molecule.
Based on our results, we hypothesized that chlorhexidine has two different action mechanisms against SARS-CoV-2: (i) acting on viral RNA replication and controlling host cells; and (ii) neutralizing spike glycoprotein, preventing the binding to the ACE-II. All these mechanisms may decrease pathogenicity in coronaviruses.
Our study revealed the tested compounds’ affinity with SARS-CoV-2 proteins and suggested their effectiveness in preventing virus replication or entering the human cells. Thus, the evidence obtained from molecular docking analysis may guide the development of temporary protocols that can be used to prevent the contamination of dental workers by SARS-CoV-2 during dental procedures in COVID-19 asymptomatic patients.
These findings suggest the possible mechanisms of action of the tested compounds that lead to the susceptibility of SARS-CoV-2. Nonetheless, in silico analysis provides preliminary data, which need to be addressed by in vitro and/or in vivo studies once in silico analysis present limitations such as: (i) the evidenced interactions by in silico analysis may not mimic the in vivo interactions; (ii) the compound-protein interaction may be purely physical, with no clinical significance; (iii) the lack of studies about the anti-SARS-CoV-2 activity of the studied compounds to discuss our findings better. On the other hand, some strengths should be emphasized: (i) it is the first report to suggest the mechanisms for CHX against SARS-CoV-2; and (ii) we conducted a range of analyses to better understand the relationship between SARS-CoV-2 proteins and some compounds used as mouth rinses.
Additionally, it is essential to emphasize that there are no scientific reports regarding effective drugs against SARS-CoV-2. However, our results may provide several usual data in screening useful therapeutic compounds, if further studied by in vitro and in vivo assays.
Conclusions
Finally, our findings suggest that chlorhexidine is the most active compound in reducing the SARS-CoV-2 salivary load due to its better binding energy. It can be considered to be used as a mouthwash before dentistry procedures, reducing the SARS-CoV-2 contamination risk of dental workers. However, in vivo studies should be conducted to confirm their clinical use.
Acknowledgement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001
References
- 1.1 - Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-33. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed]
- 2.2 - Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409-12. doi: 10.1126/science.abb3405 [DOI] [PMC free article] [PubMed]
- 3.3 - Tolksdorf K, Buda S, Schuler E, Wieler LH, Haas W. Influenza-associated pneumonia as 350 reference to assess seriousness of coronavirus disease (COVID-19). Euro Surveill. 2020:25(11);2000258. doi: 10.2807/1560-7917.ES.2020.25.11.2000258 [DOI] [PMC free article] [PubMed]
- 4.4 - Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. doi: 10.1186/s40249-020-00646-x [DOI] [PMC free article] [PubMed]
- 5.5 - Meng L, Hua F, Bian Z. Coronavirus Disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. 2020;99(5):481-7. doi: 10.1177/0022034520914246 [DOI] [PMC free article] [PubMed]
- 6.6 - Chen L, Zhao J, Peng J, Li X, Deng X, Geng Z, et al. Detection of 2019-nCoV in Saliva and Characterization of Oral Symptoms in COVID-19 Patients. SSRN [Preprint]. 2020 [cited 2020 Dec 3]. Available from: 10.2139/ssrn.3557140. doi: 10.2139/ssrn.3557140 [DOI] [PMC free article] [PubMed]
- 7.7 - Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12(1):11. doi: 10.1038/s41368-020-0080-z [DOI] [PMC free article] [PubMed]
- 8.8 - Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12(1):9. doi: 10.1038/s41368-020-0075-9 [DOI] [PMC free article] [PubMed]
- 9.9 - Izzetti R, Nisi M, Gabriele M, Graziani F. COVID-19 transmission in dental practice: brief review of preventive measures in italy. J Dent Res. 2020;99(9):1030-8. doi: 10.1177/0022034520920580 [DOI] [PubMed]
- 10.10 - Clarkson J, Ramsay C, Richards D, Robertson C, Aceves-Martins M. Aerosol generating procedures and their mitigation in international dental guidance documents: a rapid review. London: Cochrane; 2020 [cited 2020 Dec 3]. Available from: https://oralhealth.cochrane.org/sites/oralhealth.cochrane.org/files/public/uploads/rapid_review_of_agps_in_international_dental_guidance_documents.pdf
- 11.11 - Sette-de-Souza PH, Martins JC, Martins-de-Barros AV, Vieira BR, Costa MJ, Araújo FA. A critical appraisal of evidence in the use of preprocedural mouthwash to avoid SARS-CoV-2 transmission during oral interventions. Eur Rev Med Pharmacol Sci. 2020;24(19):10238-240. doi: 10.26355/eurrev_202010_23245 [DOI] [PubMed]
- 12.12 - Gawlik M, Trawiński J, Skibiński R. Identification and characterization of citalopram new metabolites with the use of UHPLC-Q-TOF technique: in silico toxicity assessment of the identified transformation products. J Pharm Biomed Anal. 2020;186:113299. doi: 10.1016/j.jpba.2020.113299 [DOI] [PubMed]
- 13.13 - John JP, Thirunavukkarasu P, Ishizuka K, Parekh P, Sawa A. An in-silico approach for discovery of microRNA-TF regulation of DISC1 interactome mediating neuronal migration. NPJ Syst Biol Appl. 2019;5:17. doi: 10.1038/s41540-019-0094-3 [DOI] [PMC free article] [PubMed]
- 14.14 - Luis JA, Barros RP, Sousa NF, Muratov E, Scotti L, Scotti MT. Virtual screening of natural products database. Mini Rev Med Chem. Forthcoming 2020. doi: 10.2174/1389557520666200730161549 [DOI] [PubMed]
- 15.15 - Mothay D, Ramesh KV. Binding site analysis of potential protease inhibitors of COVID-19 using AutoDock. Virusdisease. 2020;31(2):1-6. doi: 10.1007/s13337-020-00585-z [DOI] [PMC free article] [PubMed]
- 16.16 - Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455-61. doi: 10.1002/jcc.21334 [DOI] [PMC free article] [PubMed]
- 17.17 - Abdel Bar FM, Elsbaey M, Taha N, Elgaml A, Abdel-Fattah GM. Phytochemical, antimicrobial and antiquorum-sensing studies of Pulicaria undulata L.: a revision on the structure of 1β,2α,3β,19α,23-pentahydroxy-urs-12-en-28-oic acid. Nat Prod Res. 2020;34(6):804-9. doi: 10.1080/14786419.2018.1503658 [DOI] [PubMed]
- 18.18 - Carrouel F, Conte MP, Fisher J, Gonçalves LS, Dussart C, Llodra JC, et al. COVID-19: a recommendation to examine the effect of mouthrinses with β-Cyclodextrin combined with citrox in preventing infection and progression. J Clin Med. 2020;9(4):1126. doi: 10.3390/jcm9041126 [DOI] [PMC free article] [PubMed]
- 19.19 - Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses. J Prosthodont. Forthcoming 2020 [cited 2020 Dec 3]. Available from: 10.1111/jopr.13220. doi: 10.1111/jopr.13220 [DOI] [PMC free article] [PubMed]
- 20.20 - Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Rapid in-vitro inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse. J Prosthodont. 2020;29(6):529-533. doi: 10.1111/jopr.13209 [DOI] [PMC free article] [PubMed]
- 21.21 - Martínez Lamas L, Diz Dios P, Pérez Rodríguez M, Del Campo P, Cabrera Alvaronzalez JJ, López Domínguez A, et al. Is povidone-iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. Forthcoming 2020 [cited 2020 Dec 3]. Available from: 10.1111/odi.13526. doi: doi.org/10.1111/odi.13526 [DOI] [PMC free article] [PubMed]
- 22.22 - Morse JS, Lalonde T, Xu S, Liu WR. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21(5):730-8. doi: 10.1002/cbic.202000047 [DOI] [PMC free article] [PubMed]
- 23.23 - Yoon JG, Yoon J, Song JY, Yoon SY, Lim CS, Seong H, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020;35(20):e195. doi: 10.3346/jkms.2020.35.e195 [DOI] [PMC free article] [PubMed]
- 24.24 - Omotuyi IO, Nash O, Ajiboye BO, Olumekun VO, Oyinloye BE, Osuntokun OT, et al. Aframomum melegueta secondary metabolites exhibit polypharmacology against SARS-CoV-2 drug targets: in vitro validation of furin inhibition. Phytother Res. Forthcoming 2020 [cited 2020 Dec 3]. Available from: 10.1002/ptr.6843. doi: 10.1002/ptr.6843 [DOI] [PubMed]
- 25.25 - Silva FM, Silva KP, Oliveira LP, Costa EV, Koolen HH, Pinheiro ML, et al. Flavonoid glycosides and their putative human metabolites as potential inhibitors of the SARS-CoV-2 main protease (Mpro) and RNA-dependent RNA polymerase (RdRp). Mem Inst Oswaldo Cruz. 2020;115:e200207. doi: 10.1590/0074-02760200207. [DOI] [PMC free article] [PubMed]
- 26.26 - Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120-34. doi: 10.1016/j.virusres.2014.11.021 [DOI] [PMC free article] [PubMed]
- 27.27 - Walls AC, Xiong X, Park YJ, Tortorici MA, Snijder J, Quispe J, et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176(5):1026-39. doi: 10.1016/j.cell.2018.12.028 [DOI] [PMC free article] [PubMed]
- 28.28 - Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281-92. doi: 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed]