Abstract
Severe coronavirus disease 2019 (COVID-19) infection leads to multifactorial acute respiratory distress syndrome (ARDS), with little therapeutic success. The pathophysiology associated with ARDS or post-ARDS is not yet well understood. We hypothesize that amyloid formation occurring due to protein homeostasis disruption can be one of the complications associated with COVID-19-induced-ARDS.
Keywords: acute respiratory distress syndrome, amyloids, amyloidosis, COVID-19, SARS-CoV-2
Multifactorial ARDS in COVID-19 and possible associated complexities
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)/COVID-19 infection leads to acute respiratory distress syndrome (ARDS) in 20–67% of hospital-admitted patients [1]. In COVID-19, a mild or asymptomatic phase is followed by a pulmonary infection and inflammatory phase (Figure 1A). In severe cases, this might lead to ARDS, and the patients suffer from dyspnea and hypoxemia. The possibilities of multiple organ dysfunction, hypotension, and acidosis increase with the severity of ARDS [1]. Although the mortality rate in ARDS patients is decreasing with efficient healthcare management systems, the rate is still significant (~16–30%) in intensive care unit (ICU) patients. This syndrome is multifactorial and heterogeneous, with many unknown pathological mechanisms.
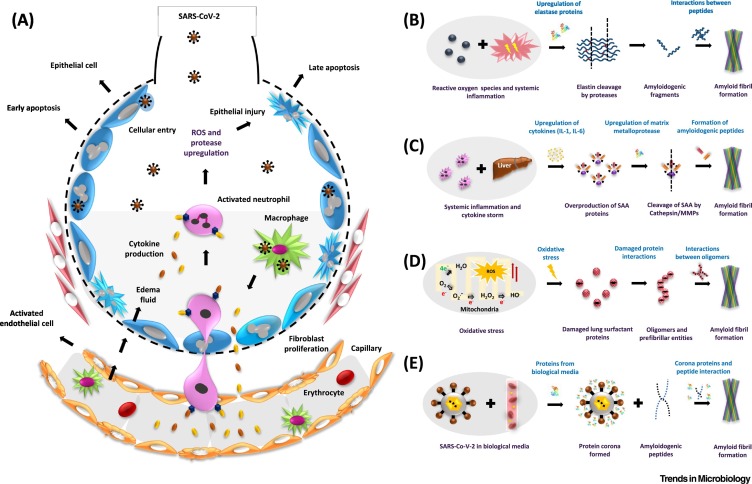
Figure 1.
The possible pathways of amyloid formation in severe acute respiratory syndrome coronavirus-2/coronavirus disease-2019 (SARS-CoV-2/COVID-19)-induced acute respiratory distress syndrome (ARDS).
(A) The pathophysiology of ARDS. The entry of SARS-CoV-2 inside the alveoli and engulfment of the viral particles by macrophages leads to the release of chemokines. These act as signaling molecules for circulating macrophages and neutrophils which then become activated. As a result, neutrophils upregulate the expression of cytokines and proteases that are responsible for inducing systemic inflammation and injury to epithelial and endothelial cells. The loss in permeability of endothelial cells allows entry of fluid into the alveoli, resulting in hypoxia and dyspnea. (B) The inflammatory response upregulates the enzyme elastase which might cleave elastin fibers to form amyloidogenic peptides. (C) Inflammation and subsequent upregulation of cytokines [interleukin (IL)-1, IL-6] also affects distant organs such as the liver and induces overproduction of serum amyloid A (SAA) proteins. SAA is cleaved by upregulated matrix metalloproteases (MMPs) and forms amyloid-forming peptides. (D) Oxidative stress, induced due to SARS-CoV-2 infection and ARDS, could lead to the production of reactive oxygen species (ROS); this perturbs redox homeostasis, resulting in misfolding and aggregation of metastable proteins such as pulmonary surfactant protein SP-C. (E) SARS-CoV-2 in the blood, or in bronchoalveolar lavage fluid, might form an extra protein corona layer around itself – which might induce amyloid formation.
The disease progression in COVID-19 and other related viral infections follows a similar trend. However, COVID-19 infection results in a hyperinflammatory state due to the induction of severe cytokine, chemokine, and interferon upregulation as compared with influenza and related viral diseases. Although recent cohort studies have suggested that COVID-19 is a non-hyperinflammatory syndrome [2], this continues to be a topic of ongoing debate. ARDS and the associated inflammatory conditions in COVID-19 might lead to the added physiological aberrations, thereby complicating the patients' treatment. We propose that one such outcome could be protein aggregation and amyloid formation. Amyloids are involved in more than 35 human diseases, some of which are debilitating and fatal. Disruption of the proteostasis machinery leads to abnormal folding and aggregation of proteins, resulting in amyloid formation and associated pathology. Based on the literature of pulmonary and non-pulmonary amyloidosis occurring due to lung dysfunction and inflammation, we analyzed the pathological mechanisms of COVID-19-induced ARDS and assessed some of the implicating factors linking ARDS to amyloidosis.
Pulmonary and systemic amyloidosis due to infectious and inflammatory diseases
Instances of pulmonary amyloidosis associated with respiratory infections are reported throughout the literature. Systemic amyloidosis in pulmonary tuberculosis patients is well known. Examination of bronchoalveolar lavage fluid in a case report of pulmonary alveoli proteinosis revealed amyloid fibrils of SP-C, a pulmonary surfactant protein [3]. In addition to tuberculosis, bronchitis, chronic pulmonary infections, leprosy, osteomyelitis, aspergillosis, and hepatitis B/C infections have also been found to have a strong association with the occurrence of systemic amyloidosis. Inflammatory conditions, such as cystic fibrosis, pulmonary sarcoidosis, rheumatic diseases, vasculitis, obesity, Crohn’s disease and celiac disease, are also found to have a significant association with systemic amyloid A (AA) amyloidosis [4]. These examples of acute or chronic inflammatory diseases suggest that amyloidosis may occur in COVID-19-induced-ARDS.
ARDS and amyloidosis: can a connection exist?
Direct evidence of amyloid formation in ARDS was reported in 2002. In this study, 10% of the postmortem lung samples of patients suffering from ARDS/acute lung injury exhibited amyloids of the elastin-staining laminar protein [5]. The amyloid formation could be possible because ARDS leads to overexpression of the elastase enzyme in plasma, resulting in the cleavage of elastin proteins to form amyloidogenic peptides [6] (Figure 1B). Serum amyloid A (SAA), an acute-phase protein, and matrix metalloproteases are overexpressed in COVID-19, as observed in multiple cohort studies. SAA cleavage by matrix metalloprotease 3 leads to the formation of amyloidogenic peptides (Figure 1C). These peptides result in amyloid formation and deposition in multiple organs, leading to organ failure and death. The two most common AA amyloidosis outcomes, namely proteinuria and renal failure, are significantly evident in COVID-19 patients. The development of acute kidney injury in ~27.3% of patients was recently reported in a study [7]. Although the kidney injury is of an acute stage 3 type, after recovery, these patients may develop amyloidosis due to persistent subclinical inflammation as observed in familial Mediterranean fever [8]. Therefore, an evaluation of amyloidosis development in COVID-19 and ARDS patients might prove valuable for improving prognosis.
Among COVID-19-patients, the possibility of secondary bacterial and fungal infections seems to be significant due to a compromised immune system. A consequence of secondary infection could be the release of lipopolysaccharide (LPS)-like factors by pathogens such as Klebsiella pneumoniae and Escherichia coli. These molecules are adept at inducing ARDS and AA amyloidosis in animal models by initiating an inflammatory state [9], and might support amyloid formation in secondary infections.
Besides SAA, D-dimer, a fibrin fragment, was found to be significantly upregulated in COVID-19 patients in multiple cohort studies. SAA proteins are also known to bind fibrins and induce amyloid formation of fibrin molecules. Similarly, binding of SAA to D-dimer may induce amyloid formation of this fibrin fragment.
Severe COVID-19 might lead to oxidative stress in the pulmonary system which adversely affects the redox homeostasis of lungs. These conditions might affect the lung surfactant proteins that are crucial for the functioning of lungs. Oxidative stress is known to facilitate protein aggregation and amyloid formation. Among the lung surfactant proteins, SP-C is a metastable protein and is known to form amyloid fibrils due to perturbations in the local environment [10], signifying possibilities of pulmonary amyloidosis in ARDS patients (Figure 1D).
Further, several viruses are capable of inducing protein aggregation. For example, respiratory syncytial virus forms protein corona in human plasma, human bronchoalveolar lavage fluid, and fetal bovine serum. This protein corona causes protein aggregation and amyloid deposition. Another study exhibited that the H1N1 influenza virus induced the aggregation of α-synuclein protein by blocking proteostasis pathways [11]. Since SARS-CoV-2 is closely related to these respiratory viruses, it might also exhibit properties of protein corona formation and subsequently amyloid deposition (Figure 1E).
It is important to note that, according to a recent study, the platelet-poor plasma of 20 COVID-19 patients exhibited a significant amyloid load compared with the control group, and the amyloid formation was implicated in endotheliopathy associated with COVID-19 [12].
Clinical implications of ARDS–amyloidosis association and treatment
In mild ARDS conditions, autophagosomes and proteasomes might degrade the misfolded or damaged proteins. However, truncated fragments of these proteins may initiate the amyloid formation and these, in turn, can act as amyloid-enhancing factors for accelerating amyloidogenicity later in life. The severity of ARDS, old age, chronic infections, and chronic inflammation may further increase the possibility of amyloid formation. Despite the restoration of pulmonary function in ARDS-recovered patients, their quality of life and life expectancy may be reduced. One of the reasons for this could be the development of amyloidosis in the future. The concept of amyloidosis development later in life holds true in other inflammatory conditions such as tuberculosis. Amyloidosis may take several years to develop after the initial diagnosis and even after the complete eradication of the tubercular bacilli [13].
On similar lines, clinicians and scientists are concerned about the long-term consequences of COVID-19. At present, a few follow-up studies on COVID-19 patients have already shown persistent inflammation, proximal muscle weakness, and multiorgan failure involving the cardiovascular and renal systems. To prove the presence or absence of such complications, a continuous monitoring of COVID-19 and especially ARDS patients is required. Moreover, the need for amyloid diagnosis is emphasized by the fact that amyloidosis, including lung amyloids, is underdiagnosed. This was reported in studies where, in postmortem lungs, light-chain (AL) (~92%) and transthyretin (TTR) (>50%) protein amyloid deposits were found in the alveolar septal walls of the lungs [14].
An early diagnosis of amyloidosis plays a pivotal role in its prognosis and might lower the severity of the pathogenesis. Research and development of therapeutics for amyloidosis is ongoing; this has led to the two recent FDA-approved drugs for AL and TTR amyloidosis. Other therapeutic management strategies involve the application of liver transplant, diuretics, anti-inflammatory drugs, stem cell transplantation, immunomodulatory therapies, and other adjunctive approaches [15].
Concluding remarks
ARDS and amyloidosis both seem to be interconnected in the array of an inflammatory cascade. The apparent overlaps in the associated mechanisms suggest that there is a likelihood of amyloidosis induction due to lung damage and overall physiological imbalance. As COVID-19 pathogenesis reveals newer facets every other day, in this forum article we have assessed the risk of amyloid formation due to disruption in physiological homeostasis in COVID-19-induced ARDS as one such complexity.
Experimental evaluation is required to understand the heterogeneous nature of ARDS and its long-term complexities. Postrecovery, COVID-19 and ARDS patients should be evaluated for persistent inflammatory markers and amyloidosis. Conversely, patients diagnosed with amyloidosis should be assessed for a previous history of COVID-19 or ARDS incidence. For the diagnosis and treatment of amyloidosis, the International Society of Amyloidosis guidelines should be considered. Initiation and incorporation of these strategies into COVID-19 management will improve the quality of the patient's life.
Declaration of interests
There are no interests to declare.
References
- 1.Grasselli G., et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir. Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha P., et al. Is a 'cytokine storm' relevant to COVID-19? JAMA Intern. Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson M., et al. Amyloid fibril formation by pulmonary surfactant protein C. FEBS Lett. 1999;464:138–142. doi: 10.1016/s0014-5793(99)01692-0. [DOI] [PubMed] [Google Scholar]
- 4.Brunger A.F., et al. Causes of AA amyloidosis: a systematic review. Amyloid. 2020;27:1–12. doi: 10.1080/13506129.2019.1693359. [DOI] [PubMed] [Google Scholar]
- 5.Fan K., Nagle W.A. Amyloid associated with elastin-staining laminar aggregates in the lungs of patients diagnosed with acute respiratory distress syndrome. BMC Pulm. Med. 2002;2:5. doi: 10.1186/1471-2466-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochicchio B., et al. Amyloidogenesis of proteolytic fragments of human elastin. RSC Adv. 2013;3:13273–13285. [Google Scholar]
- 7.Zahid U., et al. Acute kidney injury in COVID-19 patients: an inner city hospital experience and policy implications. Am. J. Nephrol. 2020;51:786–796. doi: 10.1159/000511160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romano M., et al. Cardiovascular disease risk assessment in patients with familial Mediterranean fever related renal amyloidosis. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-75433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundmark K., et al. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6979–6984. doi: 10.1073/pnas.092205999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dluhy R.A., et al. Deacylated pulmonary surfactant protein SP-C transforms from alpha-helical to amyloid fibril structure via a pH-dependent mechanism: an infrared structural investigation. Biophys. J. 2003;85:2417–2429. doi: 10.1016/s0006-3495(03)74665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marreiros R., et al. Disruption of cellular proteostasis by H1N1 influenza A virus causes α-synuclein aggregation. Proc. Natl. Acad. Sci. U. S. A. 2020;117:6741–6751. doi: 10.1073/pnas.1906466117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pretorius E., et al. Prevalence of readily detected amyloid blood clots in ‘unclotted’ Type 2 diabetes mellitus and COVID-19 plasma: a preliminary report. Cardiovasc. Diabetol. 2020;19:193. doi: 10.1186/s12933-020-01165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kute V.B., et al. Renal transplantation in secondary amyloidosis associated with tuberculosis. Case Rep. Transplant. 2013;2013 doi: 10.1155/2013/353529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govender P., et al. Transbronchial biopsies safely diagnose amyloid lung disease. Amyloid. 2017;24:37–41. doi: 10.1080/13506129.2017.1301917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westermark G.T., et al. AA amyloidosis: pathogenesis and targeted therapy. Annu. Rev. Pathol. Mechan. Dis. 2015;10:321–344. doi: 10.1146/annurev-pathol-020712-163913. [DOI] [PubMed] [Google Scholar]