Abstract
Objectives:
Despite public health concerns about hookworm infection in pregnancy, little is known about immune profiles associated with hookworm (Necator americanus and Ancylostoma duodenale) infection during pregnancy. Fetal tolerance requirements may constrain maternal immune response to hookworm, thereby increasing susceptibility to new infections or increasing hemoglobin loss. To explore this possibility, we study systemic immune response and hemoglobin levels in a natural fertility population with endemic helminthic infection.
Methods:
We used Bayesian multilevel models to analyze mixed longitudinal data on hemoglobin, hookworm infection, reproductive state, eosinophils, and erythrocyte sedimentation rate (ESR) to examine the effects of pregnancy and hookworm infection on nonspecific inflammation, cellular parasite response, and hemoglobin among 612 Tsimane women aged 15-45 (1016 observations).
Results:
Pregnancy is associated with lower eosinophil counts and lower eosinophil response to hookworm, particularly during the second and third trimesters. Both hookworm and pregnancy are associated with higher ESR, with evidence for an interaction between the two causing further increases in the first trimester. Pregnancy is moderately associated with higher odds of hookworm infection (OR: 1.23, 95% CI: 0.83 to 1.83). Pregnancy and hookworm both decrease hemoglobin and may interact to accentuate this effect in the first-trimester of pregnancy (Interaction: β: −0.30 g/dL; CI: −0.870 to 0.24).
Conclusions:
Our findings are consistent with a possible trade-off between hookworm immunity and successful pregnancy, and with the suggestion that hookworm and pregnancy may have synergistic effects, particularly in the first trimester.
1 |. INTRODUCTION
Hookworm (spp. Necator americanus and Ancylostoma duodenale) presents the second highest global morbidity burden of any parasite, following malaria (Brooker, Bethony, & Hotez, 2004). Hookworm infection is a common cause of anemia, a deficiency in functional red blood cells that leads to poor tissue oxygenation and subsequent symptoms such as fatigue, particularly among reproductive-aged women (Aphijirawat et al., 2011). Over 40% of pregnant women worldwide are anemic and almost 25% of pregnant women are infected with hookworm, with prevalence often higher in equatorial regions (McClure et al., 2014; WHO, 2008). Yet despite public health concerns about poor birth outcomes from hookworm-associated anemia during pregnancy (Bundyl, Chant, & Savioliz, 1995; Dreyfuss et al., 2000; Gyorkos & Gilbert, 2011; Ndyomugyenyi, Kabatereine, Olsen, & Magnussen, 2008), little is known about interactions between pregnancy and hookworm or the implications for maternal and fetal health. Hookworm and pregnancy might interact not only through exacerbated anemia, but also because both pregnancy and hookworm-infection affect systemic immune responses. Most studies of hookworm immunology intentionally exclude pregnant women (eg, Gaze, Bethony, & Periago, 2014), while most research on pregnancy immunology is conducted in populations with low hookworm prevalence (Kaur, Khan, & Nigam, 2014), meaning we know very little about interactions between the two.
In high-fertility populations with endemic parasitism, helminth infections frequently coincide with pregnancy, a cooccurrence that may have been the norm rather than the exception throughout much of human history. An evolutionary framework leads to a number of testable hypotheses about the confluence of pregnancy and helminth infection. Namely, we hypothesize that, due to immunological characteristics shared by hookworm and fetus, changes in maternal immunity aimed at ensuring immune tolerance of a fetus may dampen the immune response to hookworm during pregnancy. As a result, pregnant women may experience both higher susceptibility to new hookworm infections and higher morbidity due to existing infections, manifesting as greater hookworm-driven blood loss. Here, we investigate these predictions using mixed longitudinal data on hemoglobin, hookworm infection, reproductive state, eosinophil counts, and erythrocyte sedimentation rate (ESR) among Tsimane horticulturalists in Bolivia.
1.1 |. Hookworm: old friend, old foe
Hookworm infections are transmitted mainly through skin contact with soil (often near open latrines), as the worms release eggs into their hosts’ feces and then hatch and live in larval form in soil around the latrine. When contacting skin, larvae burrow directly into the skin of their new host. Larvae travel passively through venous circulation to the lungs, and ultimately through the alimentary tract to the small intestine where they mature and repeat the cycle (Hotez et al., 2004). Adult hookworms reside in the small intestine and, unlike other helminths, are often a direct cause of anemia because mature worms attach to their hosts’ intestinal lining, where they feed on epithelial tissue and blood.
Host immune response to hookworm involves both cellular and antibody-mediated strategies, with eosinophils targeting the larval stage of the infection and higher levels of circulating immunoglobulin E (IgE) across all stages of the infection (McSorley & Loukas, 2010).
Helminths, like hookworm, are sometimes considered “old friends” because of tradeoffs between helminth exposure and allergic responses that highlight an apparent evolved dependency on these parasites for calibrating immune function (McSorley & Loukas, 2010). Many of the same immune responses that respond to helminths, such as eosinophils and IgE, are also activated in allergic responses. Helminths also cause systemic shifts toward antibody-mediated type 2 helper T cell (TH2) responses (Blackwell, 2016; Geiger et al., 2002; Jiang et al., 2014; Maizels & Yazdanbakhsh, 2003), which may lead to decreases in inflammatory responses and affect susceptibility to other diseases. There is also evidence that helminth infections may affect fertility, perhaps as a consequence of these immunological changes (Blackwell, Tamayo, Beheim, Trumble, Stieglitz, et al., 2015).
1.2 |. Pregnancy and systemic immunity
Like helminth infections, pregnancy is characterized by dynamic immune modulation. The role of the immune system is to identify and eliminate any nonself antigens it encounters, yet the maternal immune system tolerates a growing fetus for 9 months, despite the fetus’ paternally derived DNA. Many known mechanisms of fetal tolerance are highly localized (La Rocca, Carbone, Longobardi, & Matarese, 2014; Luppi, 2003; Trowsdale & Betz, 2006), but fetal tolerance writ large includes any systemic shifts in maternal immune profiles that facilitate the tolerance of a foreign body and navigation of a successful pregnancy.
During pregnancy total blood volume increases in order to adequately nourish the mother, growing infant, and placenta. This increase is mainly due to increased plasma production, which dilutes biomarkers and cells that measured by volume, and thus alters multiple hematological parameters. Thus, hemoglobin concentration decreases during pregnancy, despite increased hemoglobin production. Consequently, the clinical hemoglobin threshold for diagnosing anemia is lowered from 12 to 11 g/dL to accommodate the hemoglobin nadir near the end of the second trimester. Plasma dilution also affects immunological factors and may be the cause of declines in some immunological factors during the first trimester.
Measures of systemic immune function vary over the course of pregnancy and between populations. In women from high-income countries, the first trimester is marked by localized proinflammatory processes that support tissue repair following implantation and placentation, while the second trimester is characterized by predominantly anti-inflammatory processes (Cardenas & Mor, 2011). Labor is preceded by a cascade of proinflammatory cytokines (Cardenas & Mor, 2011). ESR is an indicator of systemic inflammation. Its half-life is longer than that of C-reactive protein, making it a more suitable biomarker of chronic inflammation (Kaori, Litao, & Kamat, 2014). ESR increases across trimesters due to a sharp rise in blood coagulants that mitigate the risk of postpartum hemorrhage (Brenner, 2004). In Industrialized populations Overall WBC count also rises, due to a marked increase in circulating neutrophils, a major part of the innate inflammatory response (Kaur et al., 2014). Conversely, counts of lymphocytes, which belong to the adaptive arm of the immune system, diminish over the first two trimesters and begin to rise in the third, but do not return to prepregnancy levels until several weeks postpartum (Ramsay, 2010). In industrialized populations, eosinophil levels are generally low (<5% of total WBC count) and remain unchanged or decrease over pregnancy (Ramsay, 2010). In nonindustrialized population eosinophils may be quite high, but still decline during pregnancy (Hové et al., in press).
1.3 |. Theoretical interactions between pregnancy and hookworm
Though known mechanisms of fetal tolerance are primarily localized, fetal tolerance may have effects on systemic immunity, much as local immune mechanisms target hookworm infections at the site of attachment but systemic indicators of an immune response to hookworm can still be detected in peripheral blood. From an immunological viewpoint, a human fetus shares a number of broad similarities with hookworm and other helminths. Both are fairly long-term inhabitants of the maternal body, are genetically distinct from the host/mother, and rely on the resources of the maternal body for sustenance. In light of this, it is plausible that helminths may have evolved to utilize strategies similar to the human fetus for avoiding the immune response of the maternal body (Blackwell, 2016). Both helminth infections and successful pregnancies are associated with an immune shift toward a greater reliance on TH2 responses and suppression of TH1 responses, (Blackwell, 2016; Geiger et al., 2002; Jiang et al., 2014; Maizels & Yazdanbakhsh, 2003), which may facilitate immunological tolerance. Both pregnancy and helminth infections can be associated with reductions in autoimmune and allergic responses, and both show evidence of shifts in susceptibility to other infections (Klion & Nutman, 2004; Luppi, 2003). Chronic hookworm infection was probably common in prehistory (Araújo, Ferreira, Confalonieri, & Chame, 1988), as it is today among subsistence populations in the tropics (Hurtado, Frey, Hurtado, Kill, & Baker, 2008), yet we know very little about how hookworms interact with pregnancy. Such interactions might be detrimental, exacerbating anemia and susceptibility to comorbid infections. They might also be positive, facilitating maternal tolerance of the fetus and decreasing risk for preeclampsia and miscarriage. Indeed, different helminth species influence human fertility in both positive and negative ways, an effect that may be due to immunological interactions (Blackwell, Tamayo, Beheim, Trumble, Stieglitz, et al., 2015).
1.4 |. Hypotheses and predictions
In the present study, we investigate whether hookworm and pregnancy interact to affect the hematological and immunological values of Tsimane women from the Bolivian Amazon, since these factors are critical for maternal health and fetal outcomes. Given the dynamic changes in maternal immunity that occur across pregnancy, we explore whether immune responses to hookworm are consistent across trimesters. We also examine women experiencing lactational amenorrhea, to examine the postpartum return to cycling levels of systemic immune measures. Additionally, very little is known about the effects of lactation on immunity and infection and we use this as an opportunity to examine immunity in the context of lactation.
We propose that pregnancy and hookworm infection may interact through several avenues (summarized in Table 1), and test the following hypotheses:
TABLE 1.
Summary of predictions
| Biomarker | Function | Change during pregnancy | Change with hookworm infection | Predicted change with both |
|---|---|---|---|---|
| Hemoglobin | Delivers oxygen to somatic tissues | ↓ | ↓ | ↓ |
| ESR | General marker of inflammation | ↑ | ↑ | ↑ |
| Eosinophils | Attack macroparasites | - | ↑ | ↑ |
| Neutrophils | Innate inflammatory response | - | - | |
| Lymphocytes | Adaptive antibody-mediated immune response | ↓ | - | - |
H1) Given some of the immunologically similar characteristics of helminth and fetus, pregnancy may downregulate the immune response to hookworm. In particular, pregnancy may downregulate eosinophils, since these white blood cells tend to target helminths and other macroparasites and may pose a risk to a developing fetus.
P1) Eosinophil association with hookworm will differ between pregnant and nonpregnant women, and across trimesters.
H2) Early pregnancy and hookworm infection are both associated with increases in systemic inflammation, and may synergize to further increase inflammatory processes, potentially with detrimental effects for mother and fetus.
P2) ESR (inflammation) will be more highly correlated with hookworm infection among women in their first trimester.
H3) If effectors of hookworm immunity are downregulated during pregnancy, then the immune response to hookworm infection may be compromised, resulting in higher susceptibility to new infections.
P3) We predict a greater prevalence of hookworm infection in pregnant and lactating women compared to cycling women, reflecting higher cumulative odds of infection over the course of pregnancy.
H4) Given the higher overall demands for hemoglobin production during pregnancy, pregnant women may be less able to compensate for hemoglobin loss than women in other reproductive states, resulting in greater hemoglobin loss from hookworm infection compared to nonpregnant women.
P4) We predict that among pregnant women the difference in hemoglobin concentration between hookworm-infected and uninfected individuals will be greater than for other groups.
2 |. METHODS
2.1 |. Study population
This study focuses on Tsimane women in the Bolivian lowlands. The Tsimane are a population of ~16 000 Amerindian forager-horticulturalists who inhabit over 90 villages in the Maniqui River basin in the Beni department of Amazonian Bolivia (Gurven et al., 2017). They fish, hunt, and engage in subsistence-level horticulture of plantains, rice, sweet manioc, and corn (Kraft et al., 2018). The Tsimane health environment differs from industrialized contexts by its high pathogen burden, including endemic hookworm, energetic limitations, and high fertility (Blackwell, Trumble, Suarez, Stieglitz, et al., 2016; Costa, Trumble, Kaplan, & Gurven, 2018). Most villages have little to no public health infrastructure, no running water, and limited access to modern medicine. In past reports, 57% of Tsimane participants were infected with at least one helminth species, with hookworm (species undetermined, but likely Necator americanus) (45.3%) and Ascaris lumbricoides, a species of roundworm (19.9%), being the most prevalent (Blackwell et al., 2011; Vasunilashorn et al., 2010).
Tsimane white blood cell counts are roughly 1.5 times higher than US National Health and Nutrition Examination Survey (NHANES) values, and 89% of Tsimane have eosinophil counts higher than the NHANES 95th percentile (Blackwell, Trumble, Suarez, Stieglitz, et al., 2016). Levels of immunoglobulin-E (IgE), a class of antibody produced in response to parasitic worm infections, are 150-200 times higher among Tsimane than in age-matched Americans, though most women in our current study sample did not have available IgE measures. Roughly a third of Tsimane adults are anemic by WHO standards (Vasunilashorn et al., 2010). Considering that dietary iron intake among Tsimane is almost twice the recommended daily value (Kraft et al., 2018) and their environment exposes them to chronic pathogen risks (though notably for anemia risk, malaria is not endemic to the Tsimane territory), the most probable cause of anemia in this population is secondary iron deficiency from a combination of chronic infection, gastritis, and hookworm-driven blood loss.
The Tsimane are a natural-fertility population, employing minimal pharmaceutical birth control (<5% prevalence) (Blackwell, Tamayo, Beheim, Trumble, Stieglitz, et al., 2015). The average Tsimane woman will give birth to nine children over her lifetime (Gurven, Costa, et al., 2016; Mcallister, Gurven, Kaplan, & Stieglitz, 2012). Tsimane women breastfeed on demand, with a mean weaning age of 19 months (Martin, Garcia, Kaplan, & Gurven, 2016; Veile, Martin, McAllister, & Gurven, 2014) and an average length of lactation amenorrhea of 13 months (Blackwell, unpublished analysis).
2.2 |. Data collection
Mixed cross-sectional and longitudinal data were collected between 2005 and 2014 by the on-going Tsimane Health and Life History Project (THLHP) (Gurven et al., 2017). Data collection followed the standard THLHP protocol (explained in detail in Blackwell et al., 2011; Blackwell, Tamayo, Beheim, Trumble, Stieglitz, et al., 2015; Martin, Blackwell, Gurven, & Kaplan, 2013; Blackwell, Trumble, Suarez, Beheim, et al., 2016; Gurven, Trumble, et al., 2016). The THLHP team made annual or biannual trips to provide medical assistance and collect data. Following routine medical examinations, blood was collected by venipuncture in a heparin-coated vacutainer and hemoglobin and total blood count were measured on-site using a QBC Autoread Plus Dry Hematology System (QBC Diagnostics). WBC subtypes were counted manually by a certified biochemist using microscopy and a hemocytometer. Erythrocyte sedimentation rate (ESR) as a marker of generalized inflammation (Erikssen et al., 2000) was calculated using the Westergren (1957) method. Fecal samples were collected to analyze parasite prevalence. Each fecal sample was analyzed by direct identification on wet mounts or by a modified Percoll method (see Blackwell et al., 2011; Blackwell, Martin, Kaplan, & Gurven, 2013) for the presence of helminth eggs, larvae, protozoa, and other parasites. Parasite infection was recorded as presence or absence of each species. Following on-site analysis, physicians administered vitamins, medications, and anti-helminthics (mebendazole or albendazole) as needed. The period between a given observation and follow-up measures on an individual was generally long enough that, in the helminth-endemic environment, individuals treated at one time-point were largely as or slightly less likely to be infected at later follow-up (Blackwell et al., 2013).
Female reproductive state (FRS), classified as either pregnant, cycling, or experiencing lactational amenorrhea, was determined based on reported date of last menstrual cycle in combination with date of last birth, and often confirmed by visual evidence of pregnancy. Pregnancy tests were administered at the discretion of the physician when pregnancy was suspected. To capture pregnancies overlooked during medical visits or occurring between medical visits, pregnancy status was cross-validated against later demographic and census data on child births (see Blackwell, Tamayo, Beheim, & Trumble, 2015). Women whose later births indicated they would have been pregnant at the time of a medical visit were classified as pregnant, even if it wasn’t noted by the physician at the time (n = 16 women in the first month of pregnancy). Lactational amenorrhea was defined as actively lactating and not having resumed cycling after a birth.
2.3 |. Sample criteria
Our sample of women comprised individuals aged 15 to 45 years for whom concurrent data on hemoglobin, hookworm infection, ESR, and eosinophil count were collected (1099 obs; n = 652 women). None of the women in our sample had been treated with anti-helminthics within the 6 months prior to data collection. Since fecal analysis was sometimes collected for medical reasons, and not completely at random, we compared this sample to women who had not had parasite data collected, to assess bias in the sample. For clinic visits with associated parasite data, mean levels of hemoglobin, WBC count, and reproductive state did not differ from clinic visits without parasite data (n = 2470). However, individuals in our sample with parasite data were likely to have a slightly higher eosinophil count (1774, SD = 1106) than the broader sample (1742, SD = 1432).
As the focus of this study was the interaction between hookworm infection and reproductive state, we additionally excluded women who were suspected to have reached menopause (no cycle in 6 months and not lactating) (n = 11 obs; 10 women) and those who had not yet reached menarche at age 15 (n = 21 obs; 21 women). Because the Tsimane are a natural fertility society, we excluded nulliparous women over the age of 25 (n = 14 obs; nine women), and women who had last given birth more than 10 years prior to the observation (n = 10 obs; seven women) in order to control for possible underlying fecundity issues.
For women who had repeat clinical visits but lacked body mass index (BMI) data for at least one visit, we imputed individual average BMI, standardized by trimester/reproductive state. Seven women were excluded because they lacked BMI data for all observations. Thus, the final dataset for analysis includes 1016 observations of 612 women from 69 villages over 11 years.
The mean age of women in the sample was 33.4 years. Of the 596 observations of cycling women in our sample, 17.8% had given birth within the previous year and 20.8% had given birth between 1 and 2 years prior to sampling. Of the 203 cases of women experiencing lactational amenorrhea, 51.2% had given birth within the previous year and 7.0% had given birth between 1 and 2 years prior. All three trimesters were represented fairly evenly by the 217 pregnant women in the sample: 70 women in the first trimester, 78 in the second, and 69 in the third. Table 2 provides complete sample information for the women included in the study.
TABLE 2.
Descriptive statistics of sample
| Reproductive state | Cycling | Pregnant All pregnant | Trimester 1 | Trimester 2 | Trimester 3 | Lactational amenorrhea |
|---|---|---|---|---|---|---|
| Observations (individuals) | 596 (424) | 217 (191) | 70 (69) | 78 (76) | 69 (63) | 203 (127) |
| Mean age, years (SD) | 34.8 (8.8) | 30.8 (8.9) | 30.0 (9.0) | 30.4 (8.9) | 31.9 (8.7) | 32.1 (8.7) |
| Mean hemoglobin, g/dL (SD) | 12.8 (1.3) | 12.0 (1.2) | 12.4 (1.0) | 11.8 (1.3) | 11.9 (1.1) | 12.4 (0.9) |
| Anemic (%) | 18.3 | 13.4 | 7.1 | 17.9 | 14.5 | 31 |
| BMI, kg/m2 (SD) | 28.3 (3.5) | 26.1 (1.3) | 24.5 (3.4) | 25.9 (2.8) | 28.0 (2.5) | 29.1 (3.4) |
| Hookworm (%) | 51 | 52.1 | 52.9 | 52.6 | 50.7 | 57.1 |
| Median eosinophils, cells/μL (95% range) | 1581 (390-3924) | 1320 (286-3190) | 1670 (541-3863) | 1290 (225-2502) | 979 (263-2991) | 1921 (566-4828) |
| Median neutrophils (cells/μL) | 4568 (2499-8433) | 5472 (3239-8330) | 5062 (2859-8451) | 5645 (3713-8322) | 5814 (3467-8100) | 5143 (3052-8676) |
| Median lymphocytes, cells/μL (95% range) | 2705 (1604-4499) | 2673 (1397-4162) | 2912 (1649-5054) | 2548 (1179-3622) | 2368 (1359-3834) | 2997 (1598-4802) |
| Median erythrocyte sedimentation rate, mm/h (95% range) | 28 (10-73.2) | 46 (15.8-89.2) | 35 (9.8-78.9) | 46.5 (19.6-89.2) | 53 (25-93.4) | 32 (10.9-80.2) |
Abbreviation: BMI, body mass index.
2.4 |. Ethics approval
The Gran Consejo Tsimane, the governing body overseeing Tsimane affairs and research projects, and the Institutional Review Boards of the University of California-Santa Barbara and the University of New Mexico reviewed and approved the study from which this secondary analysis is drawn. Informed consent was obtained at both the community and individual participant levels. During a community meeting open to all residents, communities determined collectively whether the study would be conducted. To date, all communities that have been approached have approved the study. Individuals gave informed consent before each medical visit, and biospecimen collection. For minors, both parental consent and child assent were obtained.
2.5 |. Statistical analyses
Due to the mixed longitudinal aspect of the THLHP data, we employed mixed models with random effects for individual. All statistical analyses followed a Bayesian framework using the brms package (Burkner, 2017) in (R Core Team, 2018). The importance of results was determined by a combined assessment of the biological effect size and the certainty of the posterior estimate. Models were run with default, non- or weakly-informative priors. For all parameters, we report the mean and 95% credibility interval as a summary of the posterior parameter distribution.
All models included main effects for presence/absence of hookworm infection, age, and BMI, with separate random effects for individual, community, and year. To eliminate covariance between BMI and pregnancy, we ran a linear model to estimate the effect of reproductive state (trimester, cycling, lactational amenorrhea) and subtracted the resulting coefficients for each state from individual BMIs. All models utilized these trimester-corrected BMI scores.
For assessing differences across reproductive states in immune response to hookworm infection (H2, H3) we ran regressions with log-normal distributions for eosinophil count (model 1, model 2) and ESR (model 3, model 4) as a function of hookworm infection status and reproductive state. Binomial logistic regressions assessed whether pregnancy was associated with increased susceptibility to hookworm infection (H1). Models 5 and 6 tested hookworm infection’s association with different reproductive states. To test whether the effect of hookworm on hemoglobin is greater among pregnant women (H4) we ran regressions with an interaction effect between hookworm infection and reproductive state (models 7 and 8). Models 1, 3, 5, and 7 treated pregnancy as a single reproductive state, while models 3, 4, 6, and 8 assessed trimesters separately. Models 3 and 4 included only the 1008 observations for which ESR values were available. All other models included the full sample of 1016 observations.
We also ran analyses of women with longitudinal observations (n = 235 women, Table S1) to help improve causal inference. Since the results of the longitudinal analysis generally corroborated the findings of the larger mixed sample, we report these results in Table S2. Finally, we also ran models controlling for roundworm (Ascaris lumbricoides) (co-) infection (Table S4). The effects of hookworm reported here were not substantially altered by controlling for roundworm so we report these models in Table S5.
3 |. RESULTS
Hemoglobin varied by reproductive state, with the highest mean hemoglobin in cycling women (12.8 g/dL) and the lowest in second-trimester women (11.8 g/dL). About 52.5% of the women in the sample tested positive for hookworm infection. Following the World Health Organization age- and sex-specific hemoglobin thresholds of 12 g/dL for non-pregnant women, and <11 g/dL for pregnant women (Chan, 2011), 19.8% of women in the sample were anemic. Median eosinophil count for all women was 1584 cells/μL, but across reproductive states the median eosinophil count ranged from 979 cells/μL for women in their third trimester to 1921 cells/μL for women in lactational amenorrhea. ESR increased with successive trimesters, and was 35, 46.3, and 53 mm/h for first, second, and third trimesters, respectively.
3.1 |. Hookworm infection was associated with higher eosinophil counts across reproductive states, but this effect was blunted in the second and third trimesters, and in lactating women
Among the hookworm-negative women in our sample, eosinophil counts varied across reproductive states (Table 3). Overall, hookworm-positive women had higher predicted eosinophil counts than their hookworm-negative counterparts, and pregnant women had lower eosinophil counts than women who were cycling or in lactational amenorrhea (Figure 1). Among cycling women, hookworm infection was associated with an eosinophil count 20% (95% CI: 8%, 32%) higher. Eosinophil counts varied less among pregnant women with and without hookworm (Figure 1). Compared to an uninfected woman in the same trimester, a hookworm-positive first-, second-, and third-trimester woman had mean predicted eosinophil counts 12%, 1%, and 15% higher, respectively (Table 3). Among women in lactational amenorrhea, who had the highest eosinophil counts, hookworm infection was not associated with eosinophil count.
TABLE 3.
Hookworm and eosinophils across reproductive states
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Eosinophils (log cells/μL) | Eosinophils (log cells/μL) | ||||
| β | 95% CI | β | 95% CI | ||
| Intercept | 7.67 | (7.2, 8.12) | Intercept | 7.5 | (7.02, 7.97) |
| Hookworm | 0.2 | (0.08, 0.33) | Hookworm | 0.2 | (0.08, 0.32) |
| Age | 0 | (−0.01, 0.01) | Age | 0 | (−0.01, 0.01) |
| BMIa | −0.02 | (−0.03, 0) | BMIa | −0.01 | (−0.03, 0) |
| Pregnant | −0.21 0.23 −0.15 |
(−0.37, −0.04) (0.05, 0.41) (−0.38, 0.08) |
T1b T2b T3b |
0.08 −0.25 −0.38 |
(−0.19, 0.34) (−0.49, −0.01) (−0.64, −0.14) |
| Lactating | −0.1 | (−0.33, 0.12) | Lactating | 0.23 | (0.06, 0.4) |
| Hwc: Lactating | 7.67 | (7.2, 8.12) | Hwc: Lactating | −0.14 | (−0.37, 0.09) |
| Hwc: Pregnant | 0.2 | (0.08, 0.33) | Hw:T1b,c Hw:T2b,c Hw:T3b,c |
−0.08 −0.19 −0.06 |
(−0.44, 0.29) (−0.52, 0.14) (−0.4, 0.3) |
Body mass index, correct for trimester.
Trimester 1, Trimester 2, Trimester 3.
Hookworm.
FIGURE 1.
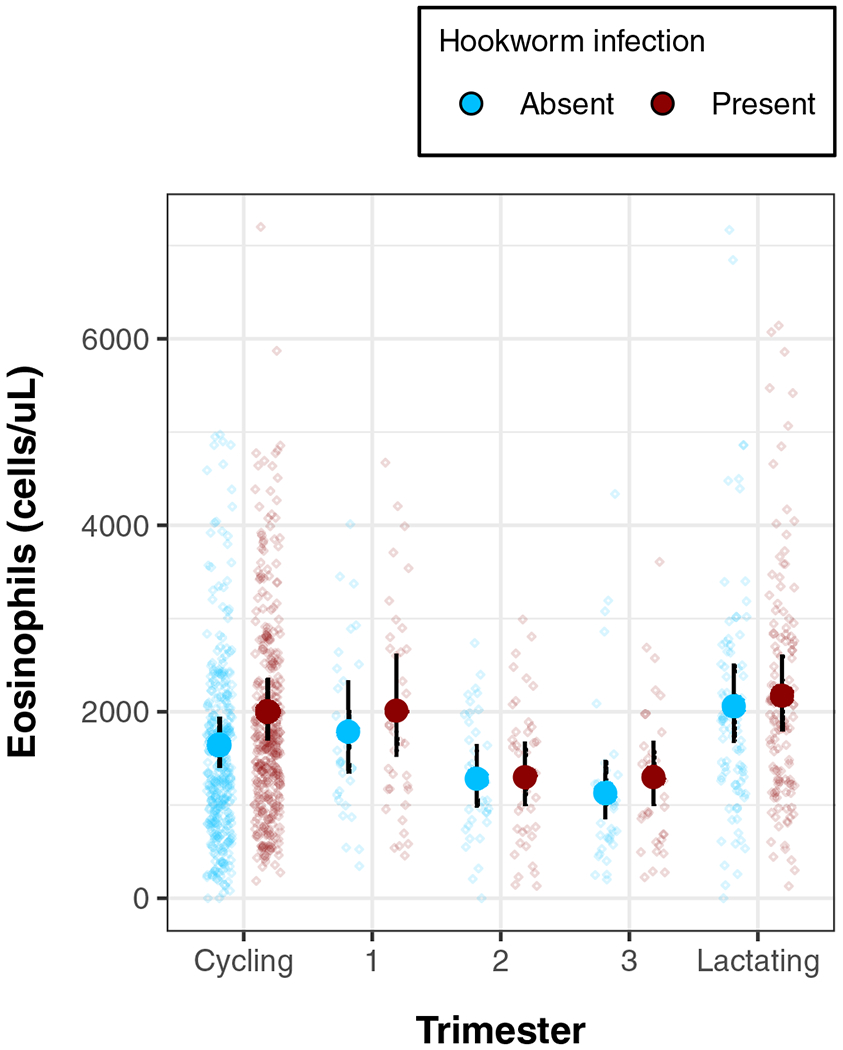
Hookworm-eosinophil association by reproductive state (model 2). Scatter values show individual eosinophil values controlling for age and BMI, (corrected for effect of trimester) while overlaid forest plot gives predicted means and 95% credibility intervals. The dashed line shows the minimum clinical threshold for diagnosing eosinophilia
To verify results, we used a subset of observations allowing for longitudinal analysis of the same women across medical visits to examine changes in eosinophils associated with changes in reproductive status (Table S1), though the smaller sample size precluded trimester-level analysis. These models produced similar results, with new hookworm infections leading to an increase in eosinophils, new pregnancies in a decrease, and the transition from pregnant to lactating being associated with an overall increase in eosinophils.
As a further check on the results from the eosinophil models, we ran identical models for neutrophils and lymphocytes, cell types which play a less prominent role in the response to hookworm infection (Table S6). Counts for these leukocyte subtypes showed no association with hookworm infection, though both were higher among pregnant women.
3.2 |. Hookworm infection was associated with higher inflammation among first-trimester women
Among hookworm-negative women, pregnant individuals had higher inflammation than cycling women: predicted ESR for a pregnant woman was 42.75 mm/h, compared to 29.88 mm/h for a cycling woman (Table 4). The higher mean value for pregnancy was driven by values in the second and third trimesters (model 4). Additionally, hookworm infection was positively associated with ESR for all women combined (β: 1.10; 95% CI: 1.00, 1.22). This association was further strengthened by pregnancy (Interaction β: 1.12; 95% CI: 0.95, 1.34), but this interaction was largely due to first-trimester women (Interaction: β: 1.26; CI: 0.94, 1.66); the overall predicted mean ESR for first-trimester women with hookworm was 39% (CI: 11%, 72%) higher than for a first-trimester woman without hookworm, compared to 10% (CI: −4%, 27%) higher for cycling women with vs without hookworm (Figure 2).
TABLE 4.
Hookworm and ESR across reproductive states
| Model 3 | Model 4 | ||||
|---|---|---|---|---|---|
| ESR (log-mm/h) | ESR (log-mm/h) | ||||
| β | 95% CI | β | 95% CI | ||
| Intercept | 2.85 | (2.48, 3.20) | Intercept | 2.85 | (2.49, 3.22) |
| Hookworm | 0.09 | (0.00, 0.19) | Hookworm | 0.10 | (0.00, 0.19) |
| Age | 0.00 | (0.00, 0.01) | Age | 0.00 | (0.00, 0.01) |
| BMI† | 0.01 | (0.00, 0.03) | BMI† | 0.01 | (0.00, 0.03) |
| Pregnant | 0.33 | (0.20, 0.46) | T1 T2 T3 |
0.01 0.40 0.53 |
(−0.20, 0.22) (0.21, 0.60) (0.33, 0.74) |
| Lactating | 0.08 | (−0.07, 0.22) | Lactating | 0.08 | (−0.06, 0.22) |
| Hw§: Lactating | −0.02 | (−0.21, 0.17) | Hw§: Lactating | −0.02 | (−0.20, 0.17) |
| Hw§: Pregnant | 0.12 | (−0.06, 0.29) | Hw:T1§‡ Hw:T2§‡ Hw:T3§‡ |
0.23 0.07 0.09 |
(−0.06, 0.51) (−0.19, 0.33) (−0.19, 0.37) |
= body mass index.
= hookworm.
= trimester.
FIGURE 2.
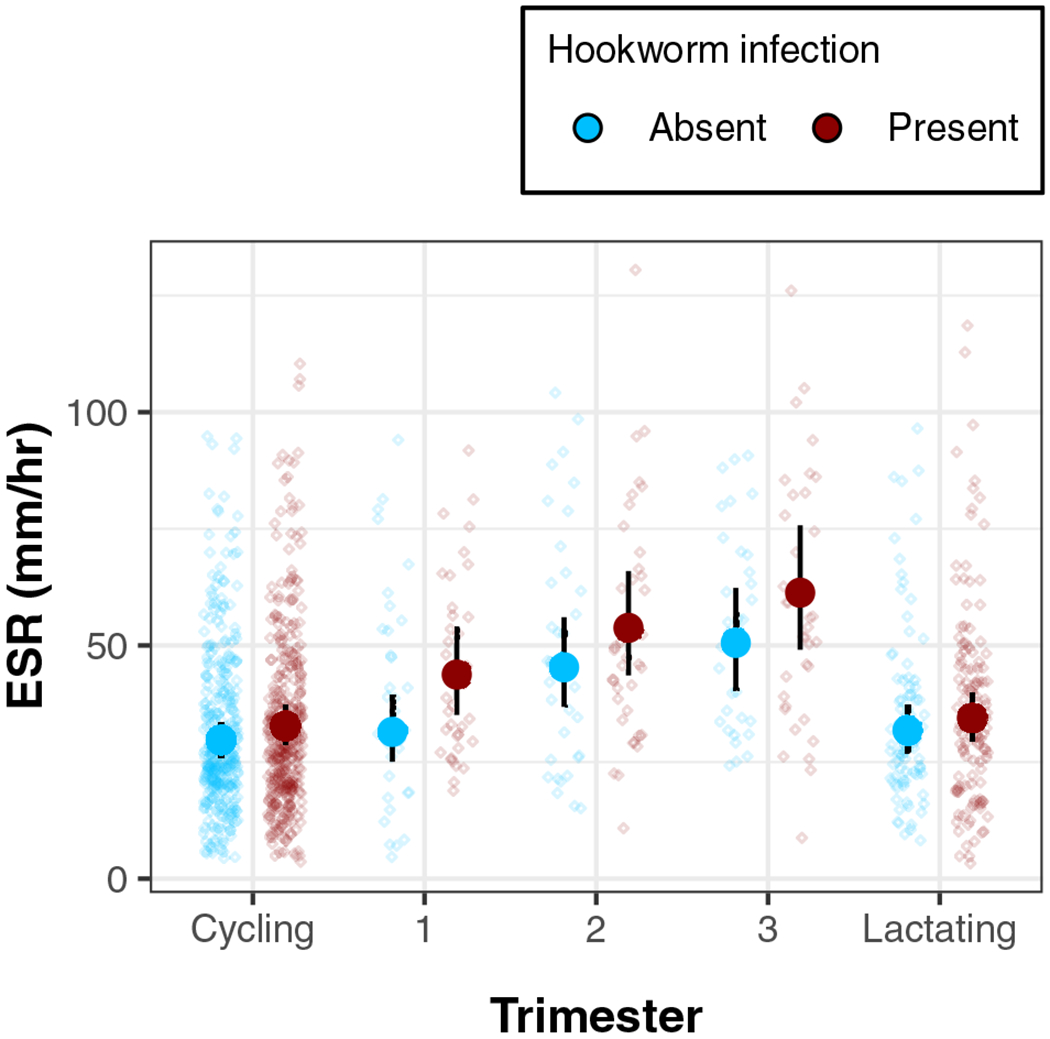
Association between hookworm infection and ESR by reproductive state (model 4). Points show individual ESR controlling for age and trimester-corrected BMI, with predicted means and credibility intervals
In longitudinal analyses (Table S2), becoming pregnant was associated with an increase in ESR. Interestingly, new hookworm infections were not associated with an increase in ESR except during pregnancy, during which ESR increased even more than from pregnancy alone.
3.3 |. Pregnant women had relatively higher hookworm prevalence than cycling women, but the absolute difference was small
Pregnant women were more likely to be infected with hookworm than cycling women (OR: 1.23; 95% CI: 0.83, 1.82; Table 5: model 5). Women with lactational amenorrhea also had higher odds of infection compared to cycling women (OR: 1.40; 95% CI: 0.92-2.13; Table 5). However, there was some uncertainty in the direction of these posterior predictions as indicated by the credibility intervals, and on an absolute scale, reproductive state had a relatively modest effect on the predicted probability of hookworm infection: predicted probability of hookworm infection: cycling 49%, pregnant 53%, amenorrhea 57%.
TABLE 5.
Hookworm prevalence and reproductive state
| Model 5 | Model 6 | ||||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Intercept | 6.85 | (1.48, 32.85) | Intercept | 6.820 | (1.39, 33.41) |
| Age | 1.00 | (0.98, 1.02) | Age | 1.005 | (0.99, 1.03) |
| BMIa | 0.93 | (0.89, 0.97) | BMIa | 0.926 | (0.88, 0.97) |
| Pregnant | 1.23 | (0.83, 1.82) | T1b | 1.755 | (0.96, 3.26) |
| T2b | 1.240 | (0.68, 2.22) | |||
| T3b | 1.209 | (0.66, 2.20) | |||
| Lactating | 1.40 | (0.92, 2.13) | Lactating | 1.449 | (0.95, 2.24) |
Body mass index, correct for trimester.
Trimester 1, Trimester 2, Trimester 3.
3.4 |. The negative association between hookworm and hemoglobin was greater for women in their first trimester
Pregnant women of all trimesters had lower hemoglobin than cycling women (see Table 6, model 7). First-trimester women had 0.16 g/dL lower (95% CI: 0.25, −0.56) predicted hemoglobin than cycling women, while second-trimester women had predicted hemoglobin values 0.91 g/dL lower (95% CI: −1.29, −0.54) than cycling women, and third-trimester women had hemoglobin 0.73 g/dL lower (95% CI: −1.12, −0.34). Lactating women have 0.39 g/dL lower (−0.67, −0.12) hemoglobin than cycling women. Hookworm infection was associated with 0.23 g/dL lower hemoglobin (95% CI: −0.42, −0.04, model 7, Table 6; Figure 3).
TABLE 6.
Hookworm and hemoglobin across reproductive states
| Response variable | Model 7 | Model 8 | 95% CI | ||
|---|---|---|---|---|---|
| Hemoglobin | Hemoglobin | ||||
| β | 95% CI | β | |||
| Intercept | 11.73 | (11.07, 12.42) | Intercept | 11.72 | (11.07, 12.38) |
| Hookworm | −0.23 | (−0.42, −0.03) | Hookworm | −0.23 | (−0.42, −0.04) |
| Age | 0.01 | (0.00, 0.02) | Age | 0.01 | (−0.00, 0.02) |
| BMIa | 0.04 | (0.01, 0.06) | BMIa | 0.04 | (0.01, 0.06) |
| Pregnant | −0.63 | (−0.90, −0.37) | T1b T2b T3b |
−0.16 −0.91 −0.73 |
(−0.56, 0.25) (−1.29, −0.53) (−1.12, −0.34) |
| Lactating | −0.40 | (−0.68, −0.13) | Lactating | −0.39 | (−0.67, −0.11) |
| Hwc: Lactating | 0.21 | (−0.16, 0.58) | Hwc: Lactating | 0.20 | (−0.17, 0.57) |
| Hwc: Pregnant | −0.14 | (−0.48, 0.21) | Hw:T1b,c Hw:T2b,c Hw:T3b,c |
−0.30 0.04 −0.22 |
(−0.87, 0.24) (−0.48, 0.24) (−0.78, 0.34) |
Body mass index, correct for trimester.
Trimester 1, Trimester 2, Trimester 3.
Hookworm.
FIGURE 3.
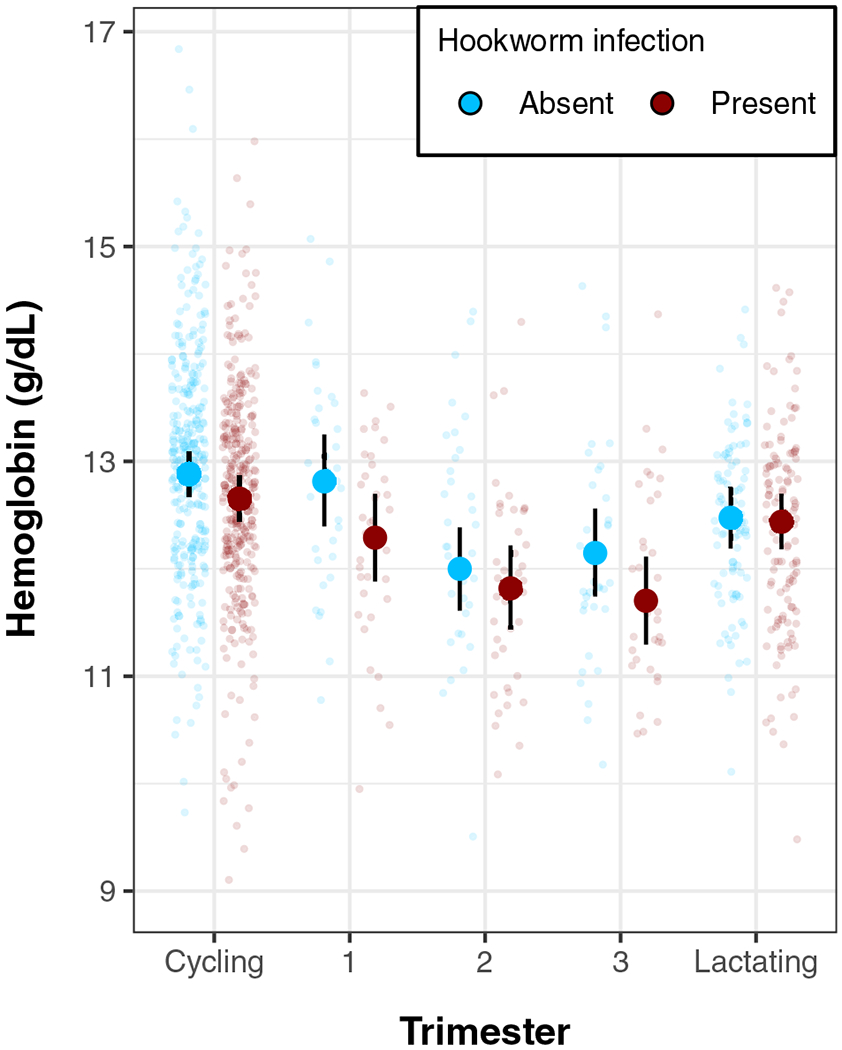
Hemoglobin levels (g/dL) by hookworm infection and reproductive state (model 8). Points show individual hemoglobin values controlling for age and trimester-corrected BMI, with overlaid model-predicted means and 95% credibility intervals. Women in their first trimester show the largest difference in hemoglobin level by hookworm infection status, while women in lactational amenorrhea show minimal differences in hemoglobin by hookworm infection status
The association between hookworm and hemoglobin varied slightly across trimesters and reproductive states (Figure 3). Compared to cycling women, the extent to which hemoglobin was lower among hookworm-infected women was somewhat moderated for women in lactational amenorrhea, though with wide uncertainty on this moderation (Interaction: β: 0.20 g/dL; 95% CI: −0.17, 0.57). Overall, there was only weak evidence for a modified negative effect of hookworm on hemoglobin in all pregnant women combined (model 7; Interaction: β: −0.14 g/dL, 95% CI: −0.48, 0.21). However, the interaction between hookworm and pregnancy was somewhat stronger, though still fairly uncertain for first-trimester women (interaction: β: −0.30 g/dL; 95% CI: −0.87, 0.24) and third-trimester women (interaction: β: −0.22 g/dL, 95% CI: −0.79, 0.34). Second-trimester women showed little evidence for an interaction (Trimester 2 interaction: β: 0.04 g/dL, 95% CI: −0.48, 0.56).
In longitudinal analyses (Table S2), hemoglobin declined during pregnancy. Interestingly, new hookworm infections are not associated with a reduction in hemoglobin, except in pregnant women.
4 |. DISCUSSION
In this study, we sought to examine whether hookworm infection interacts with women’s reproductive state to produce alterations in hemoglobin levels or changes in immune responses, and whether prevalence of hookworm itself varies by reproductive state. We found that both hookworm and pregnancy exert independent and additive effects on the biological markers hypothesized to be affected by both factors: hemoglobin, eosinophil counts, and ESR. Both hookworm and pregnancy lower hemoglobin counts and elevate ESR, while hookworm and pregnancy had inverse effects on eosinophils. We also find some evidence for interactions: pregnancy was associated not only with lower eosinophil counts, but lower hookworm-associated elevation of eosinophils, particularly in the second trimester. Interestingly, lactating women had overall elevated eosinophils, but little dependence on hookworm for these elevations. Additionally, women in their first trimester showed the greatest evidence for an interaction between pregnancy and hookworm infection, for whom hookworm was associated with further reduced hemoglobin and further elevated ESR, beyond the additive effects of hookworm and pregnancy.
The clinical implications of these effects are unknown. Overall effects on hemoglobin levels were relatively small, with little effect on clinical anemia diagnoses (Figure S1, Table S3). However, first trimester effects may be important because of the possibility of early fetal loss, which is consistent with previously reported effects of hookworm on reduced fertility in Tsimane women (Blackwell, Tamayo, Beheim, & Trumble, 2015). Second, lowered eosinophil response to infection was most apparent in the second trimester, where infected women had marginally lower eosinophil counts than uninfected women, though with a wide credible interval.
4.1 |. Infection risk and the eosinophil response
We found that women experiencing pregnancy or lactational amenorrhea had higher odds of being infected with hookworm, which suggests that susceptibility to new hookworm infections may be higher during pregnancy. These results are in agreement with previous analyses of hookworm infection in the Tsimane, which found that odds of infection are higher during pregnancy than prepregnancy, and that prevalence of infection increased over the course of pregnancy (Blackwell, Tamayo, Beheim, & Trumble, 2015). If increases in hookworm-positive fecal smears are observed among pregnant women, these increases are likely due to new infection events during pregnancy, since time from initial infection to onset of egg production is 5 to 9 weeks (Cline et al., 1984). Animal studies of experimental helminth infection have found that helminth egg production increases late in host pregnancy, which likewise suggests an increased susceptibility to infections during pregnancy (Mpairwe, Tweyongyere, & Elliott, 2014). However, studies of human infection risk during pregnancy report mixed results, with some finding higher egg counts among pregnant women (Herter et al., 2007) and some finding similar egg counts (Adegnika et al., 2010).
Based on our results, we posit a mechanism for this potentially increased hookworm susceptibility in pregnant women. Eosinophils attack hookworm larvae as they invade host tissues, preventing them from successfully attaching to the host’s intestinal wall, where they mature into egg-laying adults (McSorley & Loukas, 2010). We found that while eosinophil counts were higher among infected women compared to uninfected women across reproductive states, the difference in eosinophils was smaller among pregnant women. Moreover, absolute values for eosinophils were lower overall in the second and third trimesters of pregnancy, regardless of infection status. Suppression of the eosinophil response during pregnancy may lower the odds that migrating larvae are cleared by pregnant hosts. However, since mature (egg-producing) hookworms are not the target of eosinophils, we should not necessarily expect eosinophil counts and fecal egg counts to be correlated.
Among women in lactational amenorrhea, we found that predicted eosinophil counts for both hookworm-infected and uninfected individuals were similar to those observed among infected cycling women. High eosinophil levels are a normal part of postpartum physiology (Dawson, 1951), and it could be that there is no benefit to increasing an already high eosinophil count in the face of mild-to-moderate hookworm infection.
4.2 |. Fetal tolerance and the maternal immune response to hookworm
Changes in eosinophil count, or reactivity, to helminth infection during pregnancy could present a mechanism of fetal tolerance with measurable systemic changes. It follows that systemic immunological changes during pregnancy may render pregnant women more susceptible to new hookworm infections or to greater morbidity from existing infections.
Eosinophils may be dampened during pregnancy because the cost of lowering protection against hookworm infection is outweighed by the danger of harming the fetus with “friendly fire” from the maternal immune system. Eosinophilia (eosinophil counts greater than 500 cells/uL) is not a feature of healthy pregnancy and appears to be associated with a higher risk of miscarriage and preterm birth (Lorraine, 1996; Ogasawara et al., 1995; Romero et al., 2010). Despite their high eosinophil counts relative to industrialized populations, Tsimane women experience rates of second- or third-term miscarriage similar to other documented populations (Gurven, 2012) and high total fertility, indicating that Tsimane women successfully navigate the immune trade-offs of a parasite-endemic environment during pregnancy.
There are likely to be compensatory mechanisms of helminth immunity during pregnancy, since pregnant women in their second and third trimesters showed similar levels of hookworm-related hemoglobin loss. Since pregnancy induces a shift toward antibody-mediated immunity, the down-regulation of eosinophils may be partly countered by increased Immunoglobulin G (IgG) and Immunoglobulin E (IgE) production in the intestinal mucosae. This is something to be explored in future studies. However, based on our findings with the Tsimane, we suspect that hookworm infection may result in greater morbidity for first-trimester women, among whom hookworm had the greatest effect on both ESR and hemoglobin.
4.3 |. Anemia and reproductive state
It appears that the relationship between hookworm infection and hemoglobin also varies across pregnancy, with the strongest effects observed during the first trimester. Measuring first-trimester women against thresholds designed with later stages of pregnancy in mind could result in underestimating the cost of hookworm in early pregnancy; our analysis estimates that for first-trimester women hookworm infection is associated with an additional hemoglobin reduction of 0.30 g/dL (CI = −0.87, 0.24) more than the 0.23 g/dL (CI = −0.42, −0.04) cost of hookworm for cycling women. This additional cost to maternal resources in the first trimester may have long-term developmental costs for offspring, as well as immediate costs to maternal well-being. Individual costs will of course vary based on infection intensity; hookworm-mediated hemoglobin loss is negatively correlated with worm burden (Hotez et al., 2004) and an infection with a low worm count may not have a biologically meaningful effect on host hemoglobin.
The postpartum period, like pregnancy, is characterized by dynamic physiological shifts, including decreasing plasma levels and concomitant increase in hemoglobin concentration. We found that among women with lactational amenorrhea, hookworm infection bore no association with hemoglobin levels. This may be due to compensatory effects of decreasing blood plasma levels in more recently postpartum individuals, and possibly also the hemoglobin-buffering effect of amenorrhea compared to menstruation (although see Clancy, Nenko, & Jasienska, 2006 for the case against this pathway).
Barring severe infections, hookworm is rarely the sole cause of anemia in a healthy human (Brooker et al., 2004). In a context of chronic pathogen infection, inflammation likely plays a role in lowering hemoglobin levels. Tsimane ESR is roughly four times that of US references, average eosinophil count for a Tsimane individual is three times higher than the clinical US threshold for eosinophilia, and other inflammatory biomarkers are also high relative to US references (Blackwell, Trumble, Suarez, Beheim, et al., 2016). Among the Tsimane, many cases of anemia are probably combined cases of iron-deficiency anemia due to blood loss and anemia of inflammation due to long-term iron sequestration induced by a state of chronic inflammation.
4.4 |. Old friends?
This paper has focused on the cost of hookworm infection. However, hookworm tolerance may be the least costly option in an environment where parasitism is practically inevitable. For example, in a recent study hookworm infection was protective against malaria (Plasmodium vivax) (Budischak et al. 2018). Though malaria is not endemic in the Tsimane territory, previous work with the Tsimane found evidence that individuals infected with hookworm are less likely to become infected with Giardia lamblia (Blackwell et al., 2013). Hookworm infection among the Tsimane is associated with longer interbirth intervals (Blackwell, Tamayo, Beheim, & Trumble, 2015). However, since the symptoms of G. lamblia infection can be more acute than the symptoms of hookworm infection, hookworm-positive pregnant women may have some advantages over uninfected women in an environment where G. lamblia is endemic. Moreover, if eosinophilia can also result in fetal rejection, then the costs of hookworm infection may be lower than the potential costs of mounting or maintaining a full immune response against hookworm. Future studies will be needed to parse these complex interactions more fully.
4.5 |. Limitations of current study
The use of secondary data for these analyses creates several limitations. On-demand breastfeeding is ubiquitous among the Tsimane, with a mean weaning age of 19 months, and women often begin weaning their current infant when they discover that they are pregnant again (Martin et al., 2016). However, explicit data on lactation were not collected in this study. As a result, many of the women in our cycling sample were likely still lactating, though not experiencing amenorrhea. A complete study of the effects of lactation on infection would need more nuanced measures of lactation and energy throughput.
Parasite load is a major determinant of morbidity in infected individuals. Though inexact, parasite load is often indirectly assessed through fecal egg counts, which were not reliably available for this study; we instead relied on a binary presence/absence of eggs or larvae. Additionally, as we were limited to only a single fecal sample per clinic visit from study participants, our analysis likely includes some false negative diagnoses. The possibility of false negatives and the likelihood of other concurrent or recent infections affecting the measured immune markers renders this study a more conservative analysis of the effects of hookworm infection. Finally, we measured eosinophil prevalence in peripheral blood, but cell reactivity can also be modulated. Considering that fetal cells in the placenta are known to produce major basic protein (MBP), the main cytotoxic product of eosinophils (Wagner et al., 1993), there may be changes in reactivity of circulating eosinophils that could be further related to fetal tolerance. The possible trade-off between MBP from placental cells and MBP from eosinophils in a context of helminth infection is an intriguing area for future study.
5 |. CONCLUSIONS
Our findings suggest that women may be slightly more susceptible to the morbidity effects of hookworm infection in the first trimester of pregnancy, and that the higher eosinophil counts typically associated with hookworm infection are less marked during pregnancy, though they rebound to even higher levels during postpartum amenorrhea. From an evolutionary perspective, these immunological changes may be due to a trade-off between hookworm immunity and successful pregnancy; mounting an eosinophil response to hookworm may not be worth the risk of subjecting the fetus to friendly fire. Other mechanisms, such as Major basic protein, Immunoglobulin G, and Immunoglobulin E, likely compensate for down-regulation of eosinophil reactivity or prevalence, but may not be as effective as eosinophils in responding to the larval stage of new infections, resulting in higher susceptibility to hookworm infection during pregnancy. However, existing chronic infections do not appear to have greater physiological costs beyond the first trimester, though the long-term developmental implications of lower maternal hemoglobin in the first trimester are unknown. Overall, it appears that while hookworm may incur different costs and immune responses for women in different reproductive states, the magnitude of these differences across reproductive states is not large. Overall, our results merit further investigation, particularly to explore whether IgE levels might be a compensatory mechanism of immune response to hookworm that offsets lower eosinophil counts during pregnancy. Further study of the dynamics of immune response during pregnancy may increase our understanding of fetal tolerance and the evolutionary relationship between humans and helminths and inform our ability to alleviate the risks of pregnancy in different ecological contexts.
Supplementary Material
ACKNOWLEDGMENTS
Our deep gratitude is owed to the members of the Tsimane population who have allowed us to work with them for the last 17 years, and to the THLHP medical team and personnel, who made this and many other projects possible. Many thanks are also due to the agencies whose funding supported data collection: NIH/NIA (R01AG024119-01, R21AG031988, R01AG024119-02, R56AG024119, P01AG022500), NSF BCS-0422690.
Funding information
Division of Behavioral and Cognitive Sciences, Grant/Award Number: BCS-0422690; National Institute on Aging, Grant/Award Numbers: P01AG022500, R01AG024119-01, R01AG024119-02, R21AG031988, R56AG024119
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- Adegnika A, Ramharter M, Agnandji ST, Ngoa UA, Issifou S, Yazdanbahksh M, & Kremsner PG (2010). Epidemiology of parasitic co-infections during pregnancy in Lambarene, Gabon. Tropical Medicine and International Health, 15(10), 1204–1209. [DOI] [PubMed] [Google Scholar]
- Aphijirawat W, Piyaraj P, Leelayoova S, Khositnithikul R, Jiraanankul V, Taamasri P, … Rangsin R (2011). Incidence and risk factors of hookworm infection in a rural Community of Central Thailand. The American Journal of Tropical Medicine and Hygiene, 84(4), 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo A, Ferreira LF, Confalonieri U, & Chame M. (1988). Hookworms and the peopling of America. Cadernos de Saúde Pública, 4(2), 226–233. [Google Scholar]
- Blackwell AD (2016). Helminth infection during pregnancy: Insights from evolutionary ecology. International Journal of Women’s Health, 8, 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Gurven MD, Sugiyama LS, Madimenos FC, Liebert MA, Martin MA, … Snodgrass JJ (2011). Evidence for a peak shift in a humoral response to helminths: Age profiles of ige in the shuar of Ecuador, the Tsimane of Bolivia, and the U.S. NHANES. PLoS Neglected Tropical Diseases, 5(6), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Martin M, Kaplan H, & Gurven M. (2013). Antagonism between two intestinal parasites in humans: The importance of co-infection for infection risk and recovery dynamics. Proceedings of the Royal Society B: Biological Sciences, 280 (1769), 20131671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Tamayo M, Beheim B, & Trumble BC (2015). Helminth infection, fecundity, and age of first pregnancy in human females. Science, 350(6263), 970–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Tamayo MA, Beheim B, Trumble BC, Stieglitz J, Hooper PL, … Gurven M. (2015). Helminth infection, fecundity, and age of first pregnancy in women. Science, 350 (6263), 970–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Trumble BC, Suarez IM, Beheim B, Snodgrass JJ, Kaplan H, & Gurven M. (2016). Annals of human biology, 4460 (May). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Trumble BC, Suarez IM, Stieglitz J, Beheim B, Snodgrass JJ, … Gurven M. (2016). Immune function in Amazonian horticulturalists. Annals of Human Biology, 43 (4), 382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. (2004). Haemostatic changes in pregnancy. Thrombosis Research, 114(5–6 SPEC. ISS), 409–414. [DOI] [PubMed] [Google Scholar]
- Brooker S, Bethony J, & Hotez PJ (2004). Human hookworm infection in the 21 st century. Advances in Parasitology, 58, 197–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budischak SA, Wiria AE, Hamid F, Wammes LJ, Kaisar MM, van Lieshout L, Sartono E, Supali T, Yazdanbakhsh M, Graham AL (2018). Competing for blood: the ecology of parasite resource competition in human malaria – helminth co-infections. Ecology Letters, 21, 536–545. 10.1111/ele.12919 [DOI] [PubMed] [Google Scholar]
- Bundyl DAP, Chant MS, & Savioliz L. (1995). Hookworm infection in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene, 89, 521–522. [DOI] [PubMed] [Google Scholar]
- Burkner PC (2017). Brms : An R package for Bayesian generalized linear mixed models using Stan, (Plummer 2013). [Google Scholar]
- Cardenas I, & Mor G. (2011). The immune system in pregnancy: A unique complexity. American Journal of Reproductive Immunology, 63(6), 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M. (2011). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity (pp. 1–6). Geneva, Switzerland: World Health Organization. [Google Scholar]
- Clancy KBH, Nenko I, & Jasienska G. (2006). Menstruation does not cause anemia: Endometrial thickness correlates positively with erythrocyte count and hemoglobin concentration in premenopausal women. American Journal of Human Biology, 19(2), 165–180. [DOI] [PubMed] [Google Scholar]
- Cline BL, Little MD, Bartholomew RK, Halsey NA Larvicidal activity of albendazole against Necator americanus in human volunteers. American Journal of Tropical Medicine and Hygiene, 33(3), 387–394. 10.4269/ajtmh.1984.33.387 [DOI] [PubMed] [Google Scholar]
- Costa ME, Trumble B, Kaplan H, & Gurven MD (2018). Child nutritional status among births exceeding ideal family size in a high fertility population. Maternal and Child Nutrition, 14(4), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DW (1951). Eosinophils and pregnancy. British Journal of Gynecology, 60(5), 727–731. [DOI] [PubMed] [Google Scholar]
- Dreyfuss ML, Stoltzfus RJ, Shrestha JB, Pradhan EK, Leclerq SC, Khatry SK, … Sangh J. (2000). Community and international nutrition hookworms, malaria and vitamin a deficiency contribute to Anemia and Iron deficiency among pregnant women in the plains of Nepal. Journal of Nutrition, 1 (February), 2527–2536. [DOI] [PubMed] [Google Scholar]
- Erikssen G, Liestøl K, Bjørnholt JV, Stormorken H, Thaulow E, & Erikssen J. (2000). Erythrocyte sedimentation rate: A possible marker of atherosclerosis and a strong predictor of coronary heart disease mortality. European Heart Journal, 21(19), 1614–1620. [DOI] [PubMed] [Google Scholar]
- Gaze S, Bethony JM, & Periago MV (2014). Immunology of experimental and natural human hookworm infection. Parasite Immunology, 36(8), 358–366. [DOI] [PubMed] [Google Scholar]
- Geiger SM, Massara CL, Bethony J, Soboslay PT, Carvalho OS, & Corrêa-Oliveira R. (2002). Cellular responses and cytokine profiles in Ascaris lumbricoides and Trichuris trichiura infected patients. Parasite Immunology, 24(11–12), 499–509. [DOI] [PubMed] [Google Scholar]
- Gurven M. (2012). Infant and fetal mortality among a high fertility and mortality population in the Bolivian Amazon. Social Science and Medicine, 75, 2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Costa M, Trumble B, Stieglitz J, Beheim B, Rodriguez DE, … Kaplan H. (2016). Health costs of reproduction are minimal despite high fertility, mortality and subsistence lifestyle. Nature Publishing Group, (June), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Stieglitz J, Trumble B, Blackwell AD, Beheim B, Davis H, … Kaplan H. (2017). The Tsimane health and life history project: Integrating anthropology and biomedicine. Evolutionary Anthropology, 26(2), 54–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven MD, Trumble BC, Stieglitz J, Blackwell AD, Michalik DE, Finch CE, & Kaplan HS (2016). Cardiovascular disease and type 2 diabetes in evolutionary perspective: A critical role for helminths? Evolution, Medicine, and Public Health, 2016(1), 338–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorkos TW, & Gilbert NL (2011). Trichuris and hookworm infections associated with anaemia during pregnancy. Tropical Medicine & International Health, 16(4), 531–537. [DOI] [PubMed] [Google Scholar]
- Herter U, Petney T, Pipitgool V, Sithithaworn P, Vivatpatanakul K, Hinz E, & Andrews R. (2007). The influence of pregnancy on intestinal parasite infection in Thai women. Acta Tropica, 101, 200–206. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Brooker S, Phil D, Bethony JM, Bottazzi ME, Loukas A, & Xiao S. (2004). Hookworm infection. New England Journal of Medicine, 351, 799–807. [DOI] [PubMed] [Google Scholar]
- Hové CM, Trumble BC, Anderson AS, Stieglitz J, Gurven M, Kaplan H, & Blackwell AD (in press) The flexibility of Fetal tolerance: Immune function during pregnancy varies between ecologically distinct populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado M, Frey A, Hurtado I, Kill A, & Baker J. (2008). The role of Helminthes in human evolution implications for Global Health in the 21st century. In Elton S and O’Higgins P (Eds.) Medicine and evolution: Current applications future prospects (Vol. 160), Boca Raton: CRC Press. [Google Scholar]
- Jiang TT, Chaturvedi V, Ertelt JM, Kinder JM, Clark DR, Valent AM, … Way SS (2014). Regulatory T cells: New keys for further unlocking the enigma of fetal tolerance and pregnancy complications. The Journal of Immunology, 192(11), 4949–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaori M, Litao S, & Kamat D. (2014). Erythrocyte sedimentation rate and C-reactive protein: How best to use them in clinical practice. Pediatric Annals, 43(10), 417–420. 10.3928/00904481-20140924-10 [DOI] [PubMed] [Google Scholar]
- Kaur S, Khan S, & Nigam A. (2014). Hematological profile and pregnancy: A review. International Journal of Advances in Medicine, 1(2), 68–70. [Google Scholar]
- Klion AD, & Nutman TB (2004). The role of eosinophils in host defense against helminth parasites. Journal of Allergy and Clinical Immunology, 113(1), 30–37. [DOI] [PubMed] [Google Scholar]
- Kraft TS, Stieglitz J, Trumble BC, Martin M, Kaplan H, & Gurven M. (2018). Nutrition transition in 2 lowland Bolivian subsistence populations. The American Journal of Clinical Nutrition, 108, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rocca C, Carbone F, Longobardi S, & Matarese G. (2014). The immunology of pregnancy: Regulatory T cells control maternal immune tolerance toward the fetus. Immunology Letters, 162, 41–48. [DOI] [PubMed] [Google Scholar]
- Lorraine JK (1996). Successful pregnancy in a woman with cyclic angioedema and eosinophilia. Annals of Allergy, Asthma & Immunology, 77(6), 497–499. [DOI] [PubMed] [Google Scholar]
- Luppi P. (2003). How immune mechanisms are affected by pregnancy. Vaccine, 21(24), 3352–3357. [DOI] [PubMed] [Google Scholar]
- Maizels RM, & Yazdanbakhsh M. (2003). Immune regulation by helminth parasites: Cellular and molecular mechanisms. Nature Reviews Immunology, 3(9), 733–744. [DOI] [PubMed] [Google Scholar]
- Martin M, Blackwell AD, Gurven M, & Kaplan H. (2013). Make new friends and keep the old? Parasite coinfection and comorbidity in Homo sapiens In Brinksworth JF & Pechenkina K (Eds.). Primates, Pathogens, and Evolution: Springer; New York. 10.1007/978-1-4614-7181-3 [DOI] [Google Scholar]
- Martin MA, Garcia G, Kaplan HS, & Gurven MD (2016). Conflict or congruence? Maternal and infant-centric factors associated with shorter exclusive breastfeeding durations among the Tsimane. Social Science and Medicine, 170, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcallister L, Gurven M, Kaplan H, & Stieglitz J. (2012). Original research article why do women have more children than they want ? Understanding differences in women’s ideal and actual family size in a natural fertility. Population, 799(February), 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EM, Meshnick SR, Mungai P, Malhotra I, King CL, Goldenberg RL, … Dent AE (2014). The association of parasitic infections in pregnancy and maternal and fetal anemia: A cohort study in coastal Kenya. PLoS Neglected Tropical Diseases, 8(2), 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley HJ, & Loukas A. (2010). The immunology of human hookworm infections. Parasite Immunology, 32, 549–559. [DOI] [PubMed] [Google Scholar]
- Mpairwe H, Tweyongyere R, & Elliott A. (2014). Pregnancy and helminth infections. Parasite Immunology, 36, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndyomugyenyi R, Kabatereine N, Olsen A, & Magnussen P. (2008). Malaria and hookworm infections in relation to haemoglobin and serum ferritin levels in pregnancy in Masindi district, western Uganda. Transactions of the Royal Society of Hygiene and Tropical Medicine, 102, 130–136. [DOI] [PubMed] [Google Scholar]
- Ogasawara M, Kajiura S, Inagaki H, Sasa H, Aoki K, & Yagami Y. (1995). Successful pregnancy in a Churg-Strausssyndrome patient with a history of intrauterine fetal death. International Archives of Allergy and Immunology, 108(2), 200–202. [DOI] [PubMed] [Google Scholar]
- Ramsay M. (2010). Normal hematological changes during pregnancy and the puerperium. In Hunt B and Pavord S (Eds.), The Obstetric hematology manual (pp. 3–11). Cambridge: Cambridge University Press. [Google Scholar]
- Romero R, Kusanovic JP, Gomez R, Lamont R, Bytautiene E, Garfield RE, … Yeo L. (2010). The clinical significance of eosinophils in the amniotic fluid in preterm labor. The Journal of Maternal-Fetal & Neonatal Medicine, 23(4), 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. [Google Scholar]
- Trowsdale J, & Betz AG (2006). Mother’s little helpers: Mechanisms of maternal-fetal tolerance. Nature Immunology, 7(3), 241–246. [DOI] [PubMed] [Google Scholar]
- Vasunilashorn S, Crimmins EM, Kim JK, Winking J, Gurven M, Kaplan H, & Finch CE (2010). Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. American Journal of Human Biology, 22(6), 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veile A, Martin M, McAllister L, & Gurven M (2014). Modernization is associated with intensive breastfeeding patterns in the Bolivian Amazon. Social Science and Medicine, 100, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JM, Bartemes K, Vernof KK, Dunnette S, Offord KP, Checkel JL, & Gleich GJ (1993). Analysis of pregnancy-associated major basic protein levels throughout gestation. Placenta, 14(6), 671–681. 10.1016/S0143-4004(05)80384-4 [DOI] [PubMed] [Google Scholar]
- Westergren A. (1957). Diagnostic tests: The erythrocyte sedimentation rate range and limitations of the technique. Triangle, 3, 20–25. [PubMed] [Google Scholar]
- World Health Organization. (2008). Worldwide prevalence of anemia 1993–2005: WHO global database on anemia. Geneva: WHO Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


