Abstract
Background & aims
Early reports suggest significant difficulty with enteral feeding in critically ill COVID-19 patients. This study aimed to characterize the prevalence, clinical manifestations, and outcomes of feeding intolerance in critically ill patients with COVID-19.
Methods
We examined 323 adult patients with COVID-19 admitted to the intensive care units (ICUs) of Massachusetts General Hospital between March 11 and June 28, 2020 who received enteral nutrition. Systematic chart review determined prevalence, clinical characteristics, and hospital outcomes (ICU complications, length of stay, and mortality) of feeding intolerance.
Results
Feeding intolerance developed in 56% of the patients and most commonly manifested as large gastric residual volumes (83.9%), abdominal distension (67.2%), and vomiting (63.9%). Length of intubation (OR 1.05, 95% CI 1.03–1.08), ≥1 GI symptom on presentation (OR 0.76, 95% CI 0.59–0.97), and severe obesity (OR 0.29, 95% CI 0.13–0.66) were independently associated with development of feeding intolerance. Compared to feed-tolerant patients, patients with incident feeding intolerance were significantly more likely to suffer cardiac, renal, hepatic, and hematologic complications during their hospitalization. Feeding intolerance was similarly associated with poor outcomes including longer ICU stay (median [IQR] 21.5 [14–30] vs. 15 [9–22] days, P < 0.001), overall hospitalization time (median [IQR] 30.5 [19–42] vs. 24 [15–35], P < 0.001) and in-hospital mortality (33.9% vs. 16.1%, P < 0.001). Feeding intolerance was independently associated with an increased risk of death (HR 3.32; 95% CI 1.97–5.6).
Conclusions
Feeding intolerance is a frequently encountered complication in critically ill COVID-19 patients in a large tertiary care experience and is associated with poor outcomes.
Keywords: GI dysmotility, SARS-CoV-2, ICU, Feeding intolerance, Malnutrition
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative pathogen of coronavirus disease 2019 (COVID-19), has led to an ongoing pandemic resulting in a sudden and substantial increase in morbidity and mortality worldwide [1]. Among hospitalized patients with COVID-19, a disproportionate number (approximately 15–40%) are critically ill, requiring intensive care unit (ICU) care and invasive mechanical ventilation [[2], [3], [4], [5], [6]]. Despite primary lung pathology, severe COVID-19 infection is frequently associated with extrapulmonary complications involving multiple organ systems, including dysfunction of the gastrointestinal (GI) tract [7]. GI complications observed in this population include GI bleeding, ischemic bowel, ileus, colonic pseudo-obstruction, pancreatitis, and transaminitis [8]. While the factors driving these observations remain unknown, proposed explanations include intrinsic tropism of SARS-CoV-2 for the GI tract and GI dysmotility in the setting of critical illness with high sedative use and prolonged mechanical ventilation [9,10].
Enteral nutrition is an integral part of care for critically ill patients, particularly for those on mechanical ventilation who cannot eat volitionally. Successful delivery of enteral nutrition is commonly impeded by signs and symptoms of feeding intolerance, including vomiting, abdominal distension, constipation, diarrhea, and increased gastric residual volumes [11,12]. Failure to achieve enteral nutrition goals in critically ill patients is associated with accumulated energy deficits, prolonged ICU stays, greater infectious complications, and increased mortality [13]. Our own experience of caring for COVID-19 patients in the ICUs has been marked by unprecedented difficulty with providing enteral nutrition for this population due to frequent, severe feeding intolerance [14]. We therefore sought to examine the prevalence, clinical manifestations, and outcomes of feeding intolerance in a large cohort of critically ill patients with COVID-19.
2. Methods
2.1. Patient population and data collection
We enrolled adult patients with laboratory-confirmed COVID-19 admitted to the ICUs of Massachusetts General Hospital (MGH) during the first surge of the pandemic between March 11 and June 28, 2020 who received enteral nutrition in the ICU. Patients were identified through the Mass General Brigham electronic health record (EHR) database. Laboratory-confirmed SARS-CoV-2 infection was determined by a positive reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assay of a nasopharyngeal swab specimen. We excluded patients who were never started on enteral nutrition to determine the final ICU cohort. This study was approved by the MGH Institutional Review Board, which waived the need for informed consent from individual patients.
We used systematic EHR review to collect relevant admission data including demographics, body mass index (BMI), medical comorbidities, initial Sequential Organ Failure Assessment (SOFA) scores, laboratory values, and presence and nature of COVID-related GI symptoms. The Glasgow Coma Scale was excluded from SOFA score calculation due to its reported lack of robustness and reliability, and its inconsistent recording in daily clinical practice [15,16]. Similar exclusion of the neurologic component score has been previously reported [17,18]. The remaining five component scores (cardiovascular, respiratory, renal, hepatic, and coagulative) yield a maximum score of 20 points. Identified patients were followed serially to determine COVID-related medical complications, GI-specific complications, ICU interventions, lengths of ICU stay and overall hospitalization, and mortality. Patients were followed until hospital discharge, death, or September 15, 2020, whichever came first.
2.2. Determination and characterization of feeding intolerance
Four coders were trained to systematically review inpatient records for evidence of feeding intolerance. Because no systematic definition of feeding intolerance exists, coders examined inpatient documentation to evaluate the onset and nature of signs and symptoms that correlated with administration of feeds, requirement to temporarily hold or terminate tube feeds due to GI intolerance, improvement of signs and symptoms after holding or terminating tube feeds, or requirement to initiate of total parenteral nutrition (TPN) due to persistent intolerance to enteral feeding. Episodes of feeding intolerance were characterized according to presenting signs and symptoms and by duration of symptoms. A radiologist (JR) blinded to the clinical assessment of the patient examined associated abdominal imaging to provide an independent radiological diagnosis of imaging abnormalities associated with feeding intolerance episodes. We reviewed daily progress notes to determine whether identified episodes led to any complications arising from feeding intolerance, specifically: malnutrition, documented vomiting or regurgitation while intubated, bowel perforation, or a new bloodstream infection while on TPN.
2.3. Enteral feeding
Per hospital practice, early enteral nutrition was initiated in critically ill patients when medically feasible within 12–36 hr of ICU admission. Common reasons for delayed initiation including worsening hemodynamics with increasing pressor requirements or non-functional gut such as bowel obstructions or ileus. Where possible, trophic feeds were started at 10 cc/hr on ICU day 1 through day 3 with slow advancement thereafter per clinical judgement. For patients presenting with at least one GI symptom (i.e. nausea, vomiting, diarrhea or abdominal distention), trophic feeds (10 cc/hr) were initiated on ICU day 1 through day 5 with slow advancement thereafter per clinical judgement. Modular liquid protein was initiated on ICU day 4.
Formula selection and goal tube feed rate were recommended by the clinical dietitian. Typical formula choice was a standard polymeric, fiber free formula (i.e. Promote 1.0 or Osmolite 1.0). Fluid restricted formulas (i.e. Osmolite 1.5) were used in the setting of worsening respiratory distress, volume overload, and for patients on renal replacement therapy that experienced frequent interruptions to the circuit. Low electrolyte formulas were used when necessary in the setting of acute kidney injury. For patients with BMI <30, the caloric goals were set at 15–20 calories per kilogram (kg) of actual weight during the first week of critical illness, which would be an equivalent of 70–80% of estimated energy needs. Energy needs were re-assessed during the second week of critical illness to meet the need of usual caloric requirement of 25–30 calories per kg of actual weight. For patients with BMI ≥30, the caloric goals were 11–14 calories per kg of actual weight or 22–25 calories per kg of ideal body weight. Dietitians recommended a goal tube feed rate to meet these targets while also accounting for additional calories received from alternative sources such as propofol and dextrose-containing intravenous fluid.
Route of feeding was typically via nasogastric tube unless patients were dependent on percutaneous endoscopic gastrostomy tube at baseline. Tube feeds were infused continuously while in ICU. After extubation or when stable for floor transfer, the need to stop or adjust tube feeds was reassessed based on the safety and adequacy of oral intake. The initiation of parenteral nutrition was reserved for patients with non-functional gut or inadequate enteral intake <60% of estimated energy needs over 7–10 days in the ICU.
2.4. Assessment of nutritional status
Malnutrition was defined using standardized diagnostic characteristics according to the Consensus Statement released by the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition [19]. The six diagnostic characteristics include insufficient energy intake, weight loss, loss of muscle mass, loss of subcutaneous fat, localized or generalized fluid accumulation and diminished functional status. Each of these characteristics has associated criteria that help determine the severity of malnutrition in the context of patients’ illness. Nutrition assessment by clinical dietitians identified one or more of the above characteristics to support a malnutrition diagnosis and to determine the severity of diagnosis.
2.5. Statistical analysis
Categorical variables are reported as frequencies and continuous variables as means (with SDs) or medians (with interquartile ranges [IQRs]) according to distribution. Groups were compared with Wilcoxon rank sum tests for continuous variables, and with Pearson χ2 tests (Fisher exact tests where appropriate) for categorical variables. Any missing values were indicated in the table footnotes. We used multivariable logistic regression models to determine factors associated with feeding intolerance. Covariates were selected a priori incorporating demographic information (age, sex, and race), SOFA score (a composite reflecting multiple markers of admission disease severity), variables putatively-associated with both GI dysmotility and COVID-19 per clinical judgement and existing COVID-19 literature (BMI, diabetes, chronic kidney disease, neurologic disease), and a variable of experimental interest (presence of GI symptoms on admission). We included length of intubation as a key covariate, as it likely serves as a proxy of severity of illness in addition to a risk factor for development of feeding intolerance (via sedation requirement and presence of critical illness).
We used time-to-event techniques to calculate person-time for each patient using days from feed initiation to death (event), discharge (censored), or September 15, 2020. We calculated Kaplan–Meier survival estimates and used the log-rank test to compare groups. We used Cox proportional hazards modeling to calculate multivariable-adjusted hazard ratios (HR) and 95% confidence intervals for mortality according to feeding intolerance. As above, covariates were selected a priori and included demographics, BMI, presence of GI symptoms on admission, length of intubation, and presence of diabetes, and kidney and neurologic diseases (related to both feeding intolerance and COVID-19 mortality) [20,21]. All statistical analyses were performed using Python Version 3.8.5 (Python Software Foundation, https://www.python.org/). Statistical significance was defined by a two-sided p-value less than 0.05.
3. Results
3.1. Description of the cohort
From March 11 through June 28, 2020, we documented a total of 402 adult patients with COVID-19 admitted to the ICU, of which 323 (mean [SD] age 59.6 [14.9] years; 207 [64.1%] male) received enteral nutrition. Amongst the 79 patients who did not receive enteral feeding, the median length of ICU stay was only 2 days. Of this group, 18 (22.8%) died prior to initiation of enteral feeding (median ICU length of stay [LOS] 2 days; IQR 2–3 days), while the remaining surviving patients (n = 61) were quickly transferred to the medical floors primarily for lower acuity (median ICU LOS 2 days; IQR 2–4 days) and 53 (86.9%) did not require mechanical ventilation. Baseline patient characteristics, comorbidities, and outcomes of patients who did versus did not receive enteral nutrition are listed in (Supplementary Table 1).
We documented 180 (56%) incident cases of feeding intolerance (mean [SD] age, 59.9 [18.8] years; 125 (69.4%) male) among the 323 patients receiving ICU enteral nutrition. Compared to patients without feeding intolerance, feeding-intolerant patients were more likely to be male and present with higher SOFA scores on admission. Age, race, medical comorbidities, and initial laboratory data on admission were not significantly different between patients with feeding intolerance versus those without (Table 1 and Supplementary Table 2). However, patients with severe obesity (defined as BMI ≥ 40 kg/m2) were less likely than others to develop feeding intolerance.
Table 1.
Admission patient characteristics and comorbidities.a
| Characteristic or condition | Feeding intoleranceb (N = 180) |
No feeding intolerance (N = 143) |
p-value |
|---|---|---|---|
| Demographics | |||
| Age (years) | |||
| <40 | 18 (10.0) | 15 (10.5) | 1.0 |
| 40–59 | 60 (33.3) | 58 (40.6) | 0.20 |
| 60–79 | 85 (47.2) | 55 (38.5) | 0.14 |
| ≥80 | 17 (9.4) | 15 (10.5) | 0.85 |
| Female | 55 (30.6) | 61 (42.7) | 0.03 |
| Race or ethnic group | |||
| White | 56 (31.1) | 53 (37.1) | 0.29 |
| Black | 26 (14.4) | 13 (9.1) | 0.17 |
| Hispanic | 13 (7.2) | 16 (11.2) | 0.24 |
| Asian | 10 (5.6) | 5 (3.5) | 0.44 |
| Other/unknown | 75 (41.7) | 56 (39.2) | 0.73 |
| BMI on admission | |||
| <18.5 | 2 (1.1) | 0 (0) | 0.51 |
| 18.5–24.9 | 26 (14.4) | 20 (14.0) | 1.0 |
| 25–29.9 | 60 (33.3) | 44 (30.8) | 0.63 |
| 30–39.9 | 77 (42.8) | 55 (38.5) | 0.49 |
| ≥40 | 15 (8.3) | 24 (16.8) | 0.03 |
| Comorbidities | |||
| Diabetes | 82 (45.6) | 61 (42.7) | 0.65 |
| Immunocompromise | 17 (9.4) | 13 (9.1) | 1.0 |
| Respiratory disease | 30 (16.7) | 35 (24.5) | 0.09 |
| Cardiovascular disease | 37 (20.6) | 34 (23.8) | 0.50 |
| Hypertension | 107 (59.4) | 76 (53.1) | 0.26 |
| Cerebrovascular disease | 14 (7.8) | 6 (4.2) | 0.25 |
| Chronic kidney disease | 31 (17.2) | 19 (13.3) | 0.36 |
| Liver cirrhosis | 4 (2.2) | 5 (3.5) | 0.52 |
| Chronic gastrointestinal disease | 38 (21.1) | 33 (23.1) | 0.69 |
| Neurologic condition | 14 (7.8) | 17 (11.9) | 0.25 |
| Sequential organ failure assessment (SOFA) score | |||
| SOFA score on ICU admission, mean (SD)c | 6.8 (2.1) | 6.1 (2.3) | <0.005 |
Abbreviations: BMI, body mass index; GRV, gastric residual volume; ICU, intensive care unit.
Data are shown as number (percentage) unless otherwise noted. Percentages are rounded.
Defined as receiving tube feeds and diagnosed with feeding intolerance during the ICU admission, per chart review of signs and symptoms.
There were 5 patients in the feeding intolerance group and 5 patients in the no feeding intolerance group with missing admission SOFA scores who were not included in this analysis.
We also assessed COVID-19-related GI symptoms on presentation. A significantly higher proportion of patients who tolerated enteral feeding presented with diarrheal symptoms on admission than those who were feeding intolerant (27.3% vs 14.4%, p = 0.005; Supplementary Table 2). While a higher proportion of feed-tolerant patients presented with at least one GI symptom on admission compared to the feed-intolerant group (48.3% vs 37.8%), this difference did not reach statistical significance (p = 0.070).
3.2. Characteristics and predictors of feeding intolerance
The median time to tube feed initiation from ICU admission was 1 day (IQR = 1) for both the feeding tolerant and feeding intolerant groups (p = 0.56). The most common signs or symptoms of feeding intolerance was large gastric residual volumes, though the majority of these patients (76.1%) had other manifestations of feeding intolerance as well (Table 2 ). Abdominal distension and vomiting were also highly prevalent. The median time to development of feeding intolerance was 4 days (IQR 2–8) with a median duration of 7 days (IQR 4–12). A further 42 (23.3%) patients developed additional episodes of feeding intolerance after resolution of the initial episode. Ileus was the most common abnormality noted on systematic review of abdominal imaging obtained during feeding intolerance episodes. Thirty-three (18.3%) patients required TPN. The median duration of TPN was 8 days with five (15.2%) TPN patients developing a new bloodstream infection while on TPN. Common complications related to feeding intolerance included documented vomiting or regurgitation while intubated and malnourishment. Bowel perforation occurred in three patients. Metoclopramide and erythromycin were the most commonly used prokinetic agents for feeding intolerance (Supplementary Table 3).
Table 2.
Characterization of feeding intolerance in critically ill patients with COVID-19.a
| Feeding intolerance (N = 180) |
|
|---|---|
| Signs & symptoms of feeding intolerance | |
| Nausea | 44 (24.4) |
| Retching | 13 (7.2) |
| Vomiting | 115 (63.9) |
| Abdominal pain | 30 (16.7) |
| Abdominal distension | 121 (67.2) |
| Diarrhea | 50 (27.8) |
| Constipation | 67 (37.2) |
| Large GRV that required holding tube feed with one or more s/s above | 137 (76.1) |
| Large GRV that required holding tube feed without s/s | 14 (7.8) |
| Largest documented GRV in ICU, mean (IQR), mLb | 473 (300–600) |
| Time course of feeding intolerance | |
| Time to development of feeding intolerance since initiation of tube feed, median (IQR), days | 4 (2–8) |
| Duration of feeding intolerance, median (IQR), days | 7 (4–12) |
| Development of subsequent feeding intolerance episode(s) | 42 (23.3) |
| Route of feeding during ICU admission | |
| OG/NG | 176 (97.8) |
| PEG tube | 52 (28.9) |
| J tube | 2 (1.1) |
| Type of GI dysmotility by imaging assessmentc | |
| Gastroparesis | 2 (1.3) |
| Ileus | 30 (19.4) |
| Colonic pseudo-obstruction (Ogilvie) | 3 (1.9) |
| Unspecified/cannot be determined | 123 (79.4) |
| Total parenteral nutrition (TPN) | |
| Initiation of TPN | 33 (18.3) |
| Duration of TPN, median (IQR), days | 8 (4–12) |
| Complications from feeding intolerance | |
| Malnutrition | 90 (50.0) |
| Documented vomiting or regurgitation while intubated | 116 (64.4) |
| Bowel perforation | 3 (1.7) |
| New bloodstream infection while on TPN | 5 (15.2) |
Abbreviations: GRV, gastric residual volume; s/s, signs and symptoms; ICU, intensive care unit; IQR, interquartile range; OG/NG, orogastric/nasogastric tube; PEG tube, percutaneous endoscopic gastrostomy tube; J tube, jejunostomy tube.
Data are shown as number (percentage) unless otherwise noted. Percentages are rounded.
There were 4 patients with missing GRV values during the ICU admission who were not included in this analysis.
Imaging assessment was determined by radiologist review of gastrointestinal imaging (abdominal x-ray and/or CT abdomen/pelvis) during feeding intolerance episodes. 25 patients did not have available abdominal imaging during feeding intolerance episodes and were not included in this analysis.
Multivariable logistic regression analysis showed that length of intubation was an independent predictor of feeding intolerance (OR 1.05, 95% CI 1.03–1.08) (Fig. 1 ). Age, sex initial SOFA score, diabetes, chronic kidney disease, and comorbid neurologic conditions were not associated with increased risk of feeding intolerance. Of note, one or more GI symptoms on presentation (OR 0.76, 95% CI 0.59–0.97) and severe obesity (OR 0.29, 95% CI 0.13–0.66) were associated with decreased risk of feeding intolerance.
Fig. 1.
Predictors of feeding intolerance in critically ill patients with COVID-19. Odds ratios with 95% confidence intervals of risk factors for developing feeding intolerance in critically ill patients with COVID-19. Risk factors shown were chosen using both clinical and univariate predictor analysis. SOFA, Sequential Organ Failure Assessment; GI, gastrointestinal; BMI, body mass index, with categories defined as: severe obesity with a BMI of ≥40 kg/m2, obesity 30–39.9 kg/m2, overweight 25–29.9 kg/m2, normal 18.5–24.9 kg/m2, and underweight <18.5 kg/m2; Racial categories are expressed relative to white race; OR (95% CI), odds ratio and 95% confidence interval.
3.3. ICU interventions, complications, and mortality
Compared to patients without feeding intolerance, patients with incident feeding intolerance were significantly more likely to suffer cardiac, renal, hepatic, and hematologic complications during their hospitalization (Table 3 ). Patients with feeding intolerance were also more likely to develop ischemic colitis. The median length of intubation was 6 days longer in feeding intolerant patients and these patients were more likely to require proning for respiratory optimization. Overall hospitalization was also 6 days longer in patients with feeding intolerance.
Table 3.
ICU outcomes, complications, interventions of patients by presence of feeding intolerance.a
| Feeding intolerance (N = 180) |
No feeding intolerance (N = 143) |
p-value | |
|---|---|---|---|
| Outcomes | |||
| Length of ICU admission, median (IQR), days | 21.5 (14–30) | 15 (9–22) | <0.001 |
| ICU readmissionb | 13 (7.2) | 8 (5.6) | 0.65 |
| Length of overall hospitalization, median (IQR), days | 30.5 (19–42) | 24 (15–35) | <0.001 |
| Died | 61 (33.9) | 23 (16.1) | <0.001 |
| COVID-19 associated complications | |||
| Myocardial injury | 86 (47.8) | 19 (13.3) | <0.001 |
| VF/VT | 13 (7.2) | 25 (17.5) | <0.01 |
| AKI | 143 (79.4) | 83 (58.0) | <0.001 |
| Kidney failure requiring RRT | 63 (35.0) | 21 (14.7) | <0.001 |
| Acute liver injury | 96 (53.3) | 31 (21.7) | <0.001 |
| Septic shock | 120 (66.7) | 92 (64.3) | 0.72 |
| Coagulopathy or hypercoagulable state | 91 (50.6) | 37 (25.9) | <0.001 |
| GI complications | |||
| Mesenteric ischemia | 4 (2.2) | 1 (0.7) | 0.39 |
| Ischemic colitis | 9 (5.0) | 0 (0) | <0.01 |
| C. diff infection | 6 (3.3) | 2 (1.4) | 0.31 |
| Need for operative exploration/surgery | 10 (5.6) | 6 (4.2) | 0.62 |
| Need for GI tract resection | 9 (5.0) | 4 (2.8) | 0.40 |
| ICU interventions | |||
| Mechanical ventilation | 179 (99.4) | 142 (99.3) | 1 |
| Length of intubation, median (IQR), daysc | 19 (13.5–28.0) | 13 (7.5–18.8) | <0.001 |
| Tracheostomy | 71 (39.4) | 41 (28.7) | 0.05 |
| ECMO | 12 (6.7) | 6 (4.2) | 0.46 |
| Proning | 150 (83.3) | 91 (63.6) | <0.001 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; VF/VT, ventricular fibrillation/ventricular tachycardia; AKI, acute kidney injury; RRT, renal replacement therapy; ECMO, extracorporeal membrane oxygenation.
Data are shown as number (percentage) unless otherwise noted. Percentages are rounded.
Defined as the number of patients who were re-admitted to the ICU during the same or a subsequent hospitalization within the study period.
There was 1 patient from the feeding intolerance group and 1 patient from the no feeding intolerance group who were not intubated and who were not included in this analysis.
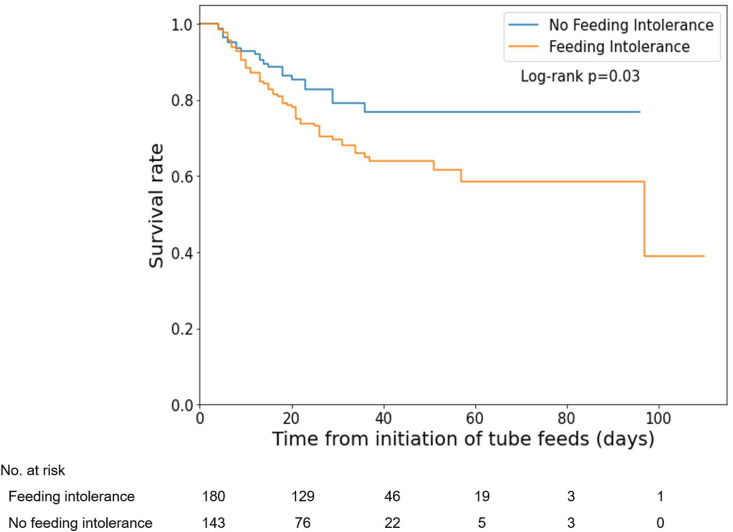
Finally, in-hospital mortality rates for patients with and without feeding intolerance were 33.9% and 16.1%, respectively (p < 0.001). Kaplan–Meier estimates of time to death from any cause showed that patients with feeding intolerance had a significantly increased risk of early death (log-rank test, p = 0.03, Fig. 2 ). Feeding intolerance was independently associated with an increased risk of death (HR 3.32; 95% CI 1.97–5.6) even after adjusting for multiple demographic and medical covariates. On the other hand, presentation with at least one COVID-related GI symptom was associated with a lower mortality rate (HR 0.69; 95% CI 0.52–0.91).
Fig. 2.
Survival of critically ill COVID-19 patients by presence of feeding intolerance.
4. Discussion
In this large cohort study of critically ill patients with COVID-19, feeding intolerance occurred in more than half of patients and was associated with substantial morbidity and mortality. We found COVID-19 patients with feeding intolerance to have a nearly universal increase in length of stay and rates of COVID-19-related complications and death. Our findings provide novel evidence for the potential roles of feeding intolerance as a marker of illness severity or harbinger of poor outcomes.
To date, there is very little evidence characterizing feeding intolerance in critically ill COVID-19 patients, with most accounts limited to case series or anecdotal reports. Nevertheless, providing enteral nutrition in this population has been shown to be unprecedentedly difficult according to observations from multiple frontline series [14,22,23]. In an earlier systemic review and meta-analysis of feeding intolerance in intensive care settings, the pooled prevalence was 38% (compared to 56% in our cohort), though the reported prevalence varied widely between individual studies likely due to patient heterogeneity across different ICUs and the lack of a standardized definition of feeding intolerance [11]. Men infected with COVID-19 are more likely to experience more severe disease and higher mortality compared to women [24,25]. Our finding that feeding intolerance was similarly more common in men suggests a potential link between the degree of GI dysfunction and severity of illness in critically ill patients with COVID-19. Other parameters that have been shown to predict COVID-19 disease severity, such as older age, race, increased BMI, and certain co-morbidities, however, did not appear to be risk factors for feeding intolerance. In fact, we found that severe obesity may be protective against development of feeding intolerance. Obesity has been associated with a myriad of GI comorbidities, including gastroesophageal reflux, dysphagia, and altered bowel habits, but majority of these studies were not done in intensive care settings [26,27]. While challenges of providing nutritional support for individuals with obesity in the ICU are well-recognized, there is currently little data systematically examining feeding intolerance in critically ill patients with versus without obesity. The relationship between severe obesity and feeding intolerance in severe COVID-19, or in critical illness generally, should be further evaluated in a larger cohort. We also found that COVID-19 patients presenting with diarrheal symptoms on admission were less likely to suffer feeding intolerance. Multivariable analyses revealed that presenting with at least one COVID-related GI symptom on admission was associated with a decreased odds of feeding intolerance. One may theorize that diarrhea effectively clears enteric pathogens, thereby diminishing viral burden within the GI tract and reducing rates of subsequent GI dysfunction. Data supporting this concept includes early observations that antimotility agents may lead to delayed bacterial pathogen clearance [28].
Nutritional status is strongly associated with clinical outcomes in critical illness [29], with poor nutrition linked to impaired immunity, compromised respiratory function, increased infectious complications, and death [13,30]. Patients with severe COVID-19 are likely at particular risk for adverse consequences of inadequate nutrition due to increased energy demands from ventilatory efforts and impaired energy utilization in the setting of associated systemic inflammation and a hypercatabolic state [31]. Our data suggest that COVID-19 patients with feeding intolerance are at increased risk for the development of cardiac, renal, hepatic and hematologic complications during their hospitalizations and have longer ICU stays. Most importantly, feeding intolerance appeared to be independently associated with an increased likelihood of in-hospital mortality despite adjustment for putative factors associated with increased mortality in this population.
The reasons underlying the high incidence of feeding intolerance in critically ill COVID-19 patients are likely multifactorial. Possible explanations include iatrogenic causes (particularly multiple sedative drugs), GI dysfunction in acute critical illness, and potentially intrinsic effects specific to SARS-CoV-2 on the GI tract. A single-center retrospective study found the duration of mechanical ventilation to be significantly longer for patients with COVID-19 relative to influenza (14 vs. 3 days), suggesting that COVID-19 patients may require higher doses of sedatives and/or paralytics than patients with other respiratory illnesses [32]. Our multivariate analyses revealed that length of intubation was an independent predictor of feeding intolerance, supporting the concept that sedative drugs likely play a role in development of feeding intolerance. Because length of intubation correlates with severity of critical illness, GI dysfunction that occurs in the setting of acute critical illness may similarly be a surrogate for disease severity. Emerging evidence suggests that SARS-CoV-2 directly infects gut enterocytes while also acting on neurons of the central nervous system, though the specific effect of the virus on the enteric nervous system has not been explored [9,33,34]. Whether SARS-CoV-2 directly affects gut neuromuscular function with resultant dysmotility or merely leads to dysmotility through the burden of critical illness alone remains to be seen. Indeed, recent work from Moheb et al. compared GI complications between patients with COVID-19 versus non-COVID-19 acute respiratory distress syndrome (ARDS), and found that the COVID-19 cohort suffered higher rates of GI complications than matched non-COVID ARDS patients [35].
5. Conclusions
We show that feeding intolerance is a frequently encountered complication in critically ill COVID-19 patients in a large, comprehensive tertiary care experience. Our data provide novel evidence that feeding intolerance in this population is linked with poor outcomes, including higher rates of multiorgan dysfunction, longer ICU stays, and increased mortality. Further studies are needed to elucidate the interplay between COVID-19, GI dysfunction, and critical illness.
Funding statement
KS has received research support from the NIH (NIDDK K23DK12094501A1); LS and TL (NIGMS T32GM007753).
Author contributions
Rebecca Liu: Conceptualization, Methodology, Data Curation, Writing – Original Draft; Mary Paz: Conceptualization, Methodology, Data Curation, Writing – Review & Editing, Project Administration; Layla Siraj: Formal Analysis, Visualization, Writing – Review & Editing; Taylor Boyd: Data Curation, Writing – Review & Editing; Silvia Salamone: Data Curation, Writing – Review & Editing; Thúy-Lan Võ Lite: Formal Analysis, Visualization, Writing – Review & Editing; Krystle M. Leung: Data Curation, Writing – Review & Editing; Josue D. Chirinos: Data Curation, Writing – Review & Editing; Helen H. Shang: Data Curation, Writing – Review & Editing; Matthew J. Townsend: Data Curation, Writing – Review & Editing; Junsung Rho: Data Curation, Writing – Review & Editing; Peiyun Ni: Data Curation, Writing – Review & Editing; Kushi Ranganath: Data Curation, Writing – Review & Editing; April D. Violante: Writing – Original Draft, Review & Editing; Zezhou Zhao: Data Curation, Writing – Review & Editing; Casey Silvernale: Data Curation, Writing – Review & Editing; Imama Ahmad: Data Curation, Writing – Review & Editing; Nira A. Krasnow: Data Curation, Writing – Review & Editing; Erica S. Barnett: Data Curation, Writing – Review & Editing; Mukesh Harisinghani: Supervision, Writing – Review & Editing; Braden Kuo: Methodology, Supervision, Writing – Review & Editing; Katharine E. Black: Methodology, Supervision, Writing – Review & Editing; Kyle Staller: Conceptualization, Methodology, Writing – Original Draft, Supervision.
Conflict of interest
KS has received research support from AstraZeneca, Takeda, and Gelesis, has served as a speaker for Shire, and has served as a consultant to Gelesis, Synergy, and Shire.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2021.03.033.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/
- 2.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suleyman G., Fadel R.A., Malette K.M., Hammond C., Abdulla H., Entz A., et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vahidy F.S., Drews A.L., Masud F.N., Schwartz R.L., Askary B.B., Boom M.L., et al. Characteristics and outcomes of COVID-19 patients during initial peak and resurgence in the Houston metropolitan area. J Am Med Assoc. 2020;324:998–1000. doi: 10.1001/jama.2020.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 8.Kaafarani H.M.A., El Moheb M., Hwabejire J.O., Naar L., Christensen M.A., Breen K., et al. Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg. 2020;272:e61–e62. doi: 10.1097/SLA.0000000000004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reintam Blaser A., Preiser J.C., Fruhwald S., Wilmer A., Wernerman J., Benstoem C., et al. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the Section of Metabolism, Endocrinology and Nutrition of the European Society of Intensive Care Medicine. Crit Care. 2020;24:224. doi: 10.1186/s13054-020-02889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaser A.R., Starkopf J., Kirsimagi U., Deane A.M. Definition, prevalence, and outcome of feeding intolerance in intensive care: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2014;58:914–922. doi: 10.1111/aas.12302. [DOI] [PubMed] [Google Scholar]
- 12.Ladopoulos T., Giannaki M., Alexopoulou C., Proklou A., Pediaditis E., Kondili E. Gastrointestinal dysmotility in critically ill patients. Ann Gastroenterol. 2018;31:273–281. doi: 10.20524/aog.2018.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casaer M.P., Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med. 2014;370:1227–1236. doi: 10.1056/NEJMra1304623. [DOI] [PubMed] [Google Scholar]
- 14.Arkin N., Krishnan K., Chang M.G., Bittner E.A. Nutrition in critically ill patients with COVID-19: challenges and special considerations. Clin Nutr. 2020;39:2327–2328. doi: 10.1016/j.clnu.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arts D.G., de Keizer N.F., Vroom M.B., de Jonge E. Reliability and accuracy of sequential organ failure assessment (SOFA) scoring. Crit Care Med. 2005;33:1988–1993. doi: 10.1097/01.ccm.0000178178.02574.ab. [DOI] [PubMed] [Google Scholar]
- 16.Tallgren M., Backlund M., Hynninen M. Accuracy of sequential organ failure assessment (SOFA) scoring in clinical practice. Acta Anaesthesiol Scand. 2009;53:39–45. doi: 10.1111/j.1399-6576.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 17.Chase J.G., Pretty C.G., Pfeifer L., Shaw G.M., Preiser J.C., Le Compte A.J., et al. Organ failure and tight glycemic control in the SPRINT study. Crit Care. 2010;14:R154. doi: 10.1186/cc9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zygun D., Berthiaume L., Laupland K., Kortbeek J., Doig C. SOFA is superior to MOD score for the determination of non-neurologic organ dysfunction in patients with severe traumatic brain injury: a cohort study. Crit Care. 2006;10:R115. doi: 10.1186/cc5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White J.V., Guenter P., Jensen G., Malone A., Schofield M., Academy of N., et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) J Acad Nutr Diet. 2012;112:730–738. doi: 10.1016/j.jand.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deane A.M., Chapman M.J., Reintam Blaser A., McClave S.A., Emmanuel A. Pathophysiology and treatment of gastrointestinal motility disorders in the acutely ill. Nutr Clin Pract. 2019;34:23–36. doi: 10.1002/ncp.10199. [DOI] [PubMed] [Google Scholar]
- 22.Aguila E.J.T., Lontok M.A.D., Francisco C.P.D. Follow your gut: challenges in nutritional therapy during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2020;18:2638–2639. doi: 10.1016/j.cgh.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapple L.S., Fetterplace K., Asrani V., Burrell A., Cheng A.C., Collins P., et al. Nutrition management for critically and acutely unwell hospitalised patients with coronavirus disease 2019 (COVID-19) in Australia and New Zealand. Aust Crit Care. 2020;33:399–406. doi: 10.1016/j.aucc.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie J., Tong Z., Guan X., Du B., Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acosta A., Camilleri M. Gastrointestinal morbidity in obesity. Ann N Y Acad Sci. 2014;1311:42–56. doi: 10.1111/nyas.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huseini M., Wood G.C., Seiler J., Argyropoulos G., Irving B.A., Gerhard G.S., et al. Gastrointestinal symptoms in morbid obesity. Front Med. 2014;1:49. doi: 10.3389/fmed.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DuPont H.L., Hornick R.B. Adverse effect of lomotil therapy in shigellosis. J Am Med Assoc. 1973;226:1525–1528. [PubMed] [Google Scholar]
- 29.Alberda C., Gramlich L., Jones N., Jeejeebhoy K., Day A.G., Dhaliwal R., et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35:1728–1737. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 30.Pingleton S.K., Harmon G.S. Nutritional management in acute respiratory failure. J Am Med Assoc. 1987;257:3094–3099. [PubMed] [Google Scholar]
- 31.Thibault R., Coeffier M., Joly F., Bohe J., Schneider S.M., Dechelotte P. How the Covid-19 epidemic is challenging our practice in clinical nutrition-feedback from the field. Eur J Clin Nutr. 2021;75:407–416. doi: 10.1038/s41430-020-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donnino M.W., Moskowitz A., Thompson G.S., Heydrick S.J., Pawar R.D., Berg K.M., et al. medRxiv; 2020. Comparison between influenza and COVID-19 at a tertiary care center. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27 e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., et al. bioRxiv; 2020. Neuroinvasion of SARS-CoV-2 in human and mouse brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Moheb M., Naar L., Christensen M.A., Kapoen C., Maurer L.R., Farhat M., et al. Gastrointestinal complications in critically ill patients with and without COVID-19. J Am Med Assoc. 2020;324(18):1899–1901. doi: 10.1001/jama.2020.19400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.