Abstract
Objectives
We investigated the impact of anemia based on admission hemoglobin (Hb) level as a prognostic risk factor for severe outcomes in hospitalized patients with coronavirus disease 2019 (COVID-19).
Methods
A single-center, retrospective cohort study was conducted from a random sample of 733 adult patients (age ≥ 18 years) obtained from a total of 4356 laboratory confirmed SARS-CoV-2 cases who presented to the Emergency Department of Montefiore Medical Center between March–June 2020. The primary outcome was a composite endpoint of in-hospital severe outcomes of COVID-19. A secondary outcome was in-hospital all-cause mortality.
Results
Among the 733 patients included in our final analysis, 438 patients (59.8%) presented with anemia. 105 patients (14.3%) had mild, and 333 patients (45.5%) had moderate-severe anemia. Overall, 437 patients (59.6%) had a composite endpoint of severe outcomes. On-admission anemia was an independent risk factor for all-cause mortality, (Odds Ratio 1.52, 95% CI [1.01–2.30], p = 0.046) but not for composite severe outcomes. However, moderate-severe anemia (Hb < 11 g/dL) on admission was independently associated with both severe outcomes (OR1.53, 95% CI [1.05–2.23], p = 0.028) and mortality (OR 1.67, 95% CI [1.09–2.56], p = 0.019) during hospitalization.
Conclusion
Anemia on admission was independently associated with increased odds of all-cause mortality in patients hospitalized with COVID-19. Furthermore, moderate-severe anemia (Hb <11 g/dL) was an independent risk factor for severe COVID-19 outcomes. Moving forward, COVID-19 patient management and risk stratification may benefit from addressing anemia on admission.
Keywords: Anemia, COVID-19, Mortality, Emergency department, Hypoxic respiratory failure, Sepsis
1. Introduction
Since its discovery in December 2019 in Wuhan, China, the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) has rapidly evolved into a global pandemic with over 100 million confirmed cases worldwide and total deaths exceeding 2 million as of March 2021 [1]. Despite much progress that has been made over the past year to combat the pandemic including vaccinations which began in January 2021, efforts to respond to and understand the virus continue as reports of new coronavirus variants have emerged across the globe [2], posing a challenge for healthcare providers to predict the outcomes in COVID-19 patients.
Older age, male sex, and multiple comorbid conditions, including hypertension, diabetes, hypercholesterolemia, cardiovascular disease, and kidney disease have been associated with mortality and critical outcomes of COVID-19 [[3], [4], [5], [6]]. Anemia is a global health concern affecting over 1.6 billion people, approximately 24.8% of the world's population, and is often associated with common medical conditions and critical illness [7,8]. Anemia has been identified as a significant risk factor for mortality and hospitalization in several diseases, such as heart failure, chronic obstructive pulmonary disease, and myocardial infarction [[9], [10], [11]]. While iron deficiency anemia has been reported as a risk factor for lower respiratory tract infection in the pediatric population [12], little information regarding anemia as a risk factor for respiratory infection outcomes was available in the adult literature. However, anemia on admission has been implicated as a possible risk factor for severe COVID-19 outcomes, albeit without consistency [3,5,13,14]. In a recently published study, anemia was associated with higher rates of mortality in COVID-19 patients who displayed signs of immune-mediated alteration of iron homeostasis [15]. Furthermore, reduction in hemoglobin has been observed in critically ill patients [16,17], but the relationship between anemia and severe illness of COVID-19 remains poorly understood, with no studies looking at pre-existing history of anemia or iron deficiency anemia [18].
In this study, we investigated anemia based on admission hemoglobin level as a prognostic risk factor for severe COVID-19 outcomes in hospitalized patients. Additionally, we evaluated the association between increasing severities of anemia, anemia as a comorbidity, and reduction in hemoglobin levels during hospitalization with severe COVID-19 outcomes. Investigation of its association with severe cases of COVID-19 was warranted since anemia is a preventable and modifiable factor.
2. Methods
2.1. Study design and data collection
We conducted a retrospective study of COVID-19 patients, confirmed by positive nasopharyngeal RT-PCR test for SARS-CoV-2, diagnosed at the Montefiore Health System from March 12 – June 15, 2020. From a total of 4356 identified COVID-confirmed patients who presented to the ED, we used arbitrary cutoffs of Hb values (9 g/dL and 13 g/dL) to divide patients and randomly sampled 250 patients from each group for a total of 750 patients. Sample size was determined from a preliminary study to compare inpatient admission rates between groups based on Hb cutoff values. We then used the WHO classification (Hb <12 g/dL for females, <13 g/dL for males) to identify patients who presented with anemia on admission and subclassified those cases of anemia as mild (Hb 11–12.9 g/dL in men and 11–11.9 g/dL in women) or moderate-severe (Hb < 11 g/dL) [19]. The data were obtained using Clinical Looking Glass (CLG), an electronic data support software used at Montefiore, and through manual review of electronic medical records.
We collected data on baseline demographics, clinical comorbidities, laboratory values pertaining to anemia and inflammation, in-hospital complications, clinical outcomes, and interventions given during the hospital course such as the use of vasopressors, invasive mechanical ventilation (IMV), hemodialysis, transfusions, and admission to the intensive care unit (ICU).
2.2. Definitions and outcomes
Our primary outcome was a composite endpoint of severe COVID-19 outcomes among the different anemia cohorts. We defined the composite endpoint as the occurrence of any events such as death, prolonged mechanical ventilation, acute hypoxic respiratory failure, and sepsis based on the WHO COVID-19 management guidelines [20] during the hospitalization. In brief, acute hypoxic respiratory failure was defined as clinical signs of pneumonia plus severe respiratory distress or O2 saturation < 90% on room air. Sepsis was defined as acute life-threatening signs of organ dysfunction caused by a documented infection, and a clinical diagnosis of sepsis or septic shock in the medical notes. Secondary outcome was all-cause mortality. Hospitalization was defined as admission to the hospital for at least 24 consecutive hours or death in the emergency department within 24 h of presentation.
2.3. Statistical analysis
We report demographic and clinical characteristics as frequencies and percentages for categorical variables and as means and standard deviations for continuous variables. Sampling weights were utilized to take into account the change of classification for the post-stratified random samples on statistical tests. We used Chi-squared tests and t-test or the one-way analysis of variance (ANOVA) for categorical and continuous variables, respectively, to compare between patients with and without severe outcomes and between anemia statuses on admission. Log transformation was applied for non-normally distributed continuous variables. We employed multivariable logistic regression to evaluate the association of anemia on admission with each outcome – a composite endpoint of severe events (primary outcome) and all-cause mortality (secondary outcome) – while adjusting for potential confounding factors. Variables with p-value <0.1 on bivariate analysis or established clinical significance were included: age, sex, diabetes mellitus, hypertension, hyperlipidemia, heart disease, chronic kidney disease, levels of AST and platelet. For anemia on admission, binary anemia status and categorical anemia severity were each used in multivariable models. Odds ratios (ORs) with associated 95% confidence intervals (CIs) were estimated for effects of variables on each outcome. In addition, we performed two subgroup analyses for patients with no anemia on admission and patients with ferritin values prior to admission to investigate the associations of becoming anemic during hospitalization and pre-existing iron deficiency anemia with the composite endpoint of severe outcome. All tests of statistical significance were two-sided, and p-values <0.05 were considered statistically significant. Data were analyzed using Stata (16.1, StataCorp LLC, College Station, TX).
3. Results
From a total of 4356 patients with RT-PCR-confirmed SARS-CoV2 infections who presented to the emergency department, anemia was identified in 1887 (43.3%) patients. Among the randomly sampled 750 patients, 14 patients were excluded because they were discharged from the ED in less than 24 h. One patient remained admitted at the end of the data collection period and was excluded from analysis. Two patients with no prior medical records admitted with altered mental status were also excluded. We report the findings from a final cohort of 733 patients.
3.1. Baseline demographics, clinical characters, and outcomes
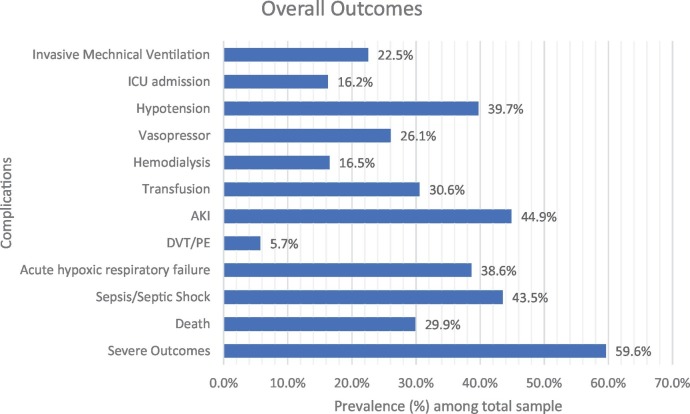
Baseline demographics and clinical characteristics are depicted in Table 1 . The mean age was 65 (±16) years, with 372 (50.8%) men. Hypertension (76.3%) was the most common comorbidity, followed by hyperlipidemia (49.9%), diabetes mellitus (48.7%), and chronic kidney disease (37.0%). Baseline laboratory parameters were elevated for ferritin, D-dimer, and CRP. Overall, 219 (29.9%) patients died during hospitalization, and 437 (59.6%) reached the composite endpoint of severe outcomes (Fig. 1 ).
Table 1.
Demographic and clinical characteristics by severe outcome status.⁎
| All patients N = 733 |
No Severe Outcomes N = 296 (40.4%) |
Severe Outcomes N = 437 (59.6%) |
P-value (n1) |
|
|---|---|---|---|---|
| Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | ||
| Demographics | ||||
| Age (mean) | 65 (±16) | 62 (±17) | 67 (±15) | <0.001 |
| Sex, Male (%) | 372 (50.8) | 146 (39.2) | 226 (60.8) | 0.324 |
| Race or Ethnic Group | 0.77 | |||
| White | 72 (9.8) | 29 (9.8) | 43 (9.8) | |
| Hispanic or Latino | 260 (35.5) | 109 (36.8) | 151 (34.6) | |
| Black or African American | 280 (38.2) | 109 (36.8) | 171 (39.1) | |
| Asian | 22 (3.0) | 7 (2.4) | 15 (3.4) | |
| Other2 | 99 (13.5) | 42 (14.2) | 57 (13.0) | |
| Body Mass Index (kg/m2) | 29.39 (± 7.81) | 28.51 (±7.61) | 29.03 (±7.94) | 0.598 (n=693) |
| Comorbidities and risk factors | ||||
| Comorbidities | ||||
| Anemia3 | 304 (41.5) | 114 (38.5) | 190 (43.5) | 0.125 |
| Diabetes Mellitus | 357 (48.7) | 130 (43.9) | 227 (51.9) | 0.078 |
| Hypertension | 559 (76.3) | 211 (71.3) | 348 (79.6) | 0.008 |
| Hyperlipidemia | 366 (49.9) | 130 (43.9) | 236 (54.0) | 0.009 |
| Coronary Artery Disease | 202 (27.6) | 73 (24.7) | 129 (29.5) | 0.165 |
| Heart Failure | 117 (16.0) | 32 (10.8) | 85 (19.5) | 0.003 |
| Asthma | 112 (15.3) | 42 (14.2) | 70 (16.0) | 0.309 |
| COPD | 88 (12.0) | 31 (10.5) | 57 (13.0) | 0.317 |
| Chronic Kidney Disease | 271 (37.0) | 94 (31.9) | 177 (40.6) | 0.031 |
| Cancer | 145 (19.8) | 56 (18.9) | 89 (20.4) | 0.359 |
| Immunosuppressed | 83 (11.3) | 32 (10.8) | 51 (11.7) | 0.368 |
| Current/Former smoker | 235 (38.9) | 81 (32.8) | 154 (43.1) | 0.029 |
| Laboratory Parameters | ||||
| Hemoglobin (g/dL) | 11.15 (±2.94) | 11.22 (±2.99) | 11.10 (±2.91) | 0.658 |
| Hgb min (g/dL) | 9.53 (±2.81) | 9.98 (±2.91) | 9.23 (±2.70) | <0.001 (n=728) |
| Hgb Max (g/dL) | 11.85 (±2.57) | 11.95 (±2.50 | 11.79 (±2.62) | 0.671 (n=728) |
| Ferritin (ng/mL) | 1716 (±4713) | 1043 (±1339) | 2142 (±5891) | <0.001 (n=573) |
| Transferrin (mg/dL) | 153.87 (±57.50) | 156.60 (±59.98) | 151.93 (±54.23) | 0.301 (n=125) |
| Ferritin/Transferrin | 13.78 (±31.07) | 9.00 (±10.51) | 17.19 (±39.45) | 0.729 (n=125) |
| D-dimer (ug/mL) | 4.22 (±5.33) | 2.75 (±3.61) | 5.21(±6.03) | <0.001 (n=586) |
| MCV (fL) | 89.71 (±8.44) | 88.49 (±8.25) | 90.53 (±8.48) | 0. 003 |
| WBC (k/uL) | 9.32 (±9.13) | 8.91 (±10.96) | 9.77 (±7.66) | 0.002 (n=730) |
| IL-6 (pg/mL) | 149.44 (±242.11) | 40.76 (±33.65) | 195.63 (±276.34) | 0. 027 (n=57) |
| CRP (ug/mL) | 13.24 (±10.77) | 8.26 (±7.79) | 16.68 (±11.19) | <0.001 (n=541) |
| Platelet (k/uL) | 245.84 (±130.61) | 252.64 (±129.08) | 241.25 (±131.57) | 0.676 (n=730) |
| Albumin (g/dL) | 3.58 (±0.61) | 3.71 (±0.58) | 3.48 (±0.61) | <0.001 (n=712) |
| ALT (U/L) | 48.19 (±140.26) | 37.46 (±61.68) | 55.39 (±173.87) | 0.013 (n=712) |
| AST (U/L) | 68.23 (±179.75) | 49.07 (±55.45) | 81.31 (±227.82) | <0.001 (n=700) |
| Creatinine (mg/dL) | 2.51 (±3.06) | 2.12 (±2.50) | 2.77 (±3.37) | <0.001 (n=730) |
| Anemia on Admission | 438 (59.8) | 162 (54.7) | 276 (63.2) | 0.038 |
| Length of Stay** | ||||
| Mean (days) | 11 (±13) | 7 (±7) | 16 (±16) | <0.001 (n=502) |
| Prolonged stay (>11 days) | 129 (17.6) | 40 (13.8) | 89 (42.0) | < 0.001 (n=502) |
*All raw sample values are reported. P-values applied with sampling weights are reported. 1n reports counts of available values and frequencies reported. If no n is written, n = 733, whole sample.2Other category includes all patients who either declined to answer, fit in to none of the categories, or information was unavailable.3Comorbidities and risk factors Anemia is information on any medical history or prior diagnosis of Anemia, regardless of current admission hemoglobin status.
**Length of stay excludes patients who died during hospital stay. Abbreviations: ED: Emergency Department, DM: Diabetes Mellitus, HTN: Hypertension, HLD: Hyperlipidemia, CAD: Coronary Artery Disease, CHF: Chronic Heart failure CKD: Chronic Kidney Disease, COPD: Chronic Obstructive Pulmonary Disease.
Fig. 1.
Prevalence of clinical outcomes in our total sample cohort.
Representation of prevalence of disease outcomes in total sample cohort. ICU admission signifies admission to the traditional Intensive Care Units, and may not reflect the true prevalence for need of intensive cares since patients admitted to make-shift ICUs and higher level care floors could not be accurately counted. Hypotension was defined as systolic blood pressure < 100 and diastolic blood pressure < 60, or a mention of hypotension in the physician notes. AKI was defined as an elevation in creatinine by ≥0.3 mg/dL within 48 h or increase in serum creatinine to ≥1.5 times baseline, or urine volume < 0.5 mL/kg/h for six hours according to the KDIGO guidelines [30]. DVT/PE: Deep Vein Thrombosis/Pulmonary Embolism, ICU: Intensive Care Unit, AKI: Acute Kidney Injury.
Patients with severe outcomes were older (p < 0.001, Table 1) with a higher rate of pre-existing comorbidities such as hyperlipidemia (p = 0.009), heart failure (p = 0.003), and chronic kidney disease (p = 0.031) but did not have a higher rate of history of anemia. Supplementary Table S1 depicts complications and management during hospitalization. More patients with severe outcomes received transfusion (p < 0.001), and the rates of all complications were higher in patients with severe outcomes (p < 0.003). Serum chemistry values showed significantly elevated acute phase reactants such as ferritin, D-dimer, and CRP (p < 0.001, Table 1). Indices of functional impairment of major organs such as AST and creatinine were also significantly elevated in the severe outcome group (p < 0.001). Based on admitting hemoglobin values, more patients in the severe outcome group met the diagnostic criteria for anemia on admission (63.2% vs. 54.7%, p = 0.038).
3.2. Comparisons between anemia status on admission
In our cohort, 438 (59.8%) of patients had hemoglobin levels corresponding to anemia diagnosis on admission. As shown in Table 2 , patients with anemia on admission were older, more likely female, and had significantly higher rates of comorbidities, including chronic kidney disease (49.1% vs. 19.3%, p < 0.001), heart failure (21.0% vs. 8.5%, p < 0.001), and cancer (25.3% vs. 11.5%, p < 0.001). Not all patients with the comorbidity of anemia in the past presented with anemia on admission (248/304, 81.6%).
Table 2.
Demographics and patient characteristics by anemia status on admission (no anemia, mild, and moderate-severe anemia)
| No Anemia N = 295 (40.2) |
Anemia N = 438 (59.8) |
P-value† | Mild N = 105 (14.3) |
Mod-Severe N = 333 (45.5) |
P-value‡ (n1) |
|
|---|---|---|---|---|---|---|
| Mean (SD) Or n (%) |
Mean (SD) Or n (%) |
Mean (SD) Or n (%) |
Mean (SD) Or n (%) |
|||
| Demographics | ||||||
| Age (years) | 63 (±16) | 66 (±16) | 0.009 | 67 (±15) | 66 (±16) | 0.032 |
| Sex, Male | 163 (55.3) | 209 (47.7) | 0.045 | 54 (51.4) | 155 (46.5) | 0.018 |
| BMI (kg/m2) | 30.59 (±7.80) | 27.69 (±7.61) | <0.001 | 28.65 (±7.02) | 27.39 (±7.78) | <0.001(n = 693) |
| Comorbidities | ||||||
| Anemia | 56 (19.0) | 248 (56.5) | <0.001 | 36 (34.3) | 212 (63.7) | <0.001 |
| Iron supplementation | 27 (9.2) | 160 (36.5) | <0.001 | 20 (19.0) | 140 (42.0) | <0.001 |
| DM | 114 (38.6) | 243 (55.5) | <0.001 | 57 (54.3) | 186 (55.9) | <0.001 |
| HTN | 199 (67.5) | 360 (82.2) | <0.001 | 86 (81.9) | 274 (82.3) | <0.001 |
| HLD | 138 (46.8) | 228 (52.1) | 0.317 | 51 (48.6) | 177 (53.2) | 0.410 |
| CAD | 55 (18.6) | 147 (33.6) | <0.001 | 36 (34.3) | 111 (33.4) | <0.001 |
| HF | 25 (8.5) | 92 (21.0) | <0.001 | 25 (8.5) | 20 (19.0) | <0.001 |
| Asthma | 34 (11.5) | 78 (17.8) | 0.058 | 18 (17.1) | 60 (18.1) | 0.115 |
| COPD | 24 (8.1) | 64 (14.6) | 0.085 | 7 (6.7) | 57 (17.2) | 0.011 |
| CKD | 57 (19.3) | 214 (49.1) | <0.001 | 38 (36.2) | 176 (53.2) | <0.001 |
| Cancer | 34 (11.5) | 111 (25.3) | <0.001 | 18 (17.1) | 93 (27.9) | <0.001 |
| Immunosuppressed | 9 (3.1) | 74 (17.0) | <0.001 | 12 (11.4) | 62 (18.7) | <0.001 |
| Clinical Outcomes | ||||||
| Length of Stay* | 9 (±12) | 12 (±13) | 0.012 | 10 (±10) | 12 (±13) | 0.001 (n = 502) |
| Prolonged Stay* | 40 (18.2) | 89 (31.6) | 0.022 | 14 (20.6) | 75 (35.0) | 0.005 (n = 502) |
| Transfusion | 19 (6.4) | 205 (46.8) | <0.001 | 16 (15.2) | 189 (56.8) | <0.001 |
1n reports counts of available values and frequencies reported. If no n is written, n = 733, whole sample. Abbreviations: ED: Emergency Department, DM: Diabetes Mellitus, HTN: Hypertension, HLD: Hyperlipidemia, CAD: Coronary Artery Disease, HF: Heart failure CKD: Chronic Kidney Disease, COPD: Chronic Obstructive Pulmonary Disease, PE/DVT: Pulmonary Embolism/Deep Vein Thrombosis. †: P- value for the test comparing anemia vs. no anemia. ‡: P-value for the test comparing three categories of no anemia, mild, and moderate-severe anemia.
Anemia was mild in 105 (14.3%) patients and moderate-severe in 333 (45.5%) patients. Overall, rates of comorbidities were lowest in the no anemia group compared to each cohort of anemia severity, except for COPD (8.1% in no anemia, 6.7% in mild anemia, and 17.2% in moderate-severe anemia). The rate of prior history of anemia increased with anemia severity (19% vs. 34.3% vs. 63.7%, p < 0.001). With increasing severities of anemia on admission, patients showed elevated inflammatory markers such as D-dimer (p < 0.001) and presented with lower albumin and higher creatinine on admission (p < 0.001, Supplementary Table S2).
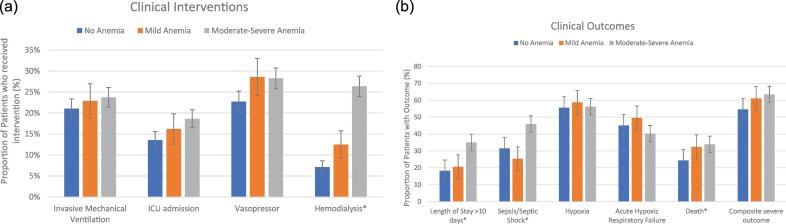
Patients who presented with anemia on admission had a longer length of stay (p = 0.001, Supplementary Table S2) and a significantly higher rate of complications such as sepsis (43.4% vs. 31.5%, p = 0.015) and hemodialysis (23.0% vs. 7.1%, p < 0.001). Accordingly, anemic patients had significantly higher rates of mortality (33.6% vs. 24.4%, p = 0.017) and severe outcomes (63.0% vs. 54.6%, p = 0.038). When comparing different anemia severities, rates of adverse events such as prolonged stay, hypotension, and hemodialysis increased significantly with anemia severity (Fig. 2a, b). However, the mild anemia group had highest rate of hypoxia (55.6% in no anemia, 58.7% in mild anemia, and 56.2% in moderate-severe anemia) and acute respiratory failure (45.1% vs. 49.5% vs. 40.2%) than no anemia and moderate-severe anemia groups. Overall, although rates of mortality and severe composite outcomes increased with increasing anemia severity, the differences were not significant.
Fig. 2.
a) Prevalence of clinical interventions among patients without anemia and different severities of anemia. *Chi-square p < 0.05. Error bars are of standard errors calculated based on sample statistics. Moderate-severe anemia patient group was significantly more likely to receive hemodialysis than the other two groups. No statistically significant differences were observed in the rates of mechanical ventilation, ICU admission, or vasopressor use. However, ICU admission rate may not reflect the true prevalence for need of intensive cares since patients admitted to make-shift ICUs and higher-level care floors could not be accurately counted.
b) Prevalence of clinical outcomes and diagnoses among patients without anemia and with different severities of anemia. *Chi square p < 0.05. Error bars are of standard errors calculated based on sample statistics. Moderate-Severe anemia group had significantly higher rates of prolonged stay, sepsis/septic shock, and death compared to the other two groups. The rate of acute hypoxic respiratory failure was highest in the mild anemia group, and lowest in the moderate-severe anemia group. Although lacking statistical significance, the rate of composite severe outcomes increases with anemia severity.
3.3. Independent risk factors for death and severe outcomes
Tables 3 depicts the results of the multivariable logistic regression analysis for severe outcomes. After controlling for confounders, anemia on admission was not predictive of severe outcomes (Model I), but moderate-severe anemia was significantly associated with increased odds of severe outcomes (OR 1.532, CI [1.048–2.230], p = 0.028, Model II). In the multivariable analysis for morality (Table 4 ), anemia on admission remained an independent risk factor for mortality (OR 1.523, CI [1.008–2.303], p = 0.046, Model I). Further analysis showed that moderate-severe anemia was significantly associated with increased risk of death in COVID-19 patients (OR 1.671, CI [1.090–2.563], p = 0.019, Model II).
Table 3.
Logistic regression models of the composite endpoint of severe outcomes
| Unadjusted Model |
Adjusted Model I |
Adjusted Model II |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | aOR1 | 95% CI | P-value | aOR1 | 95% CI | P-value | |
| Demographics | |||||||||
| Age > 65 | 1.989 | 1.427–2.772 | <0.001 | 1.715 | 1.171–2.510 | 0.006 | 1.727 | 1.180–2.528 | 0.005 |
| BMI | 1.006 | 0.984–1.028 | 0.601 | ||||||
| Male Sex | 1.179 | 0.850–1.635 | 0.325 | 1.271 | 0.897–1.801 | 0.176 | 1.287 | 0.908–1.824 | 0.156 |
| Comorbidities | |||||||||
| DM | 1.346 | 0.967–1.873 | 0.078 | 1.050 | 0.709–1.555 | 0.807 | 1.053 | 0.711–1.559 | 0.796 |
| HTN | 1.654 | 1.137–2.408 | 0.009 | 1.129 | 0.693–1.840 | 0.627 | 1.121 | 0.694–1.842 | 0.622 |
| HLD | 1.556 | 1.119–2.165 | 0.009 | 1.373 | 0.935–2.015 | 0.105 | 1.370 | 0.933–2.011 | 0.109 |
| Heart Disease | 1.443 | 1.009–2.063 | 0.044 | 0.952 | 0.626–1.448 | 0.818 | 0.953 | 0.626–1.451 | 0.822 |
| Asthma | 1.274 | 0.798–2.032 | 0.310 | ||||||
| COPD | 1.314 | 0.768–2.249 | 0.318 | ||||||
| Kidney Disease | 1.472 | 1.035–2.093 | 0.031 | 1.122 | 0.740–1.700 | 0.588 | 1.101 | 0.724–1.674 | 0.652 |
| Cancer | 1.221 | 0.797–1.870 | 0.360 | ||||||
| Lab Findings | |||||||||
| Hemoglobin | 0.988 | 0.937–1.041 | 0.645 | ||||||
| Ferritin* | 1.000 | 1.000–1.000 | 0.001 | ||||||
| D-dimer* | 1.134 | 1.077–1.194 | <0.001 | ||||||
| WBC | 1.047 | 0.990–1.107 | 0.107 | ||||||
| CRP* | 1.096 | 1.066–1.128 | <0.001 | ||||||
| Platelet | 0.999 | 0.998–1.001 | 0.392 | ||||||
| ALT** | 1.003 | 0.999–1.007 | 0.096 | ||||||
| AST | 1.006 | 1.003–1.010 | 0.001 | 1.007 | 1.002–1.011 | 0.002 | 1.007 | 1.002–1.011 | 0.002 |
| Creatinine*** | 1.087 | 1.011–1.170 | 0.025 | ||||||
| Classifications | |||||||||
| Anemia (on admission) vs. none | 1.411 | 1.019–1.954 | 0.038 | 1.403 | 0.969–2.032 | 0.073 | |||
| Anemia Severity | |||||||||
| Mild vs. none | 1.383 | 0.872–2.200 | 0.168 | 1.297 | 0.792–2.125 | 0.301 | |||
| Mod-Sev vs. none | 1.441 | 1.045–1.985 | 0.026 | 1.532 | 1.048–2.230 | 0.028 | |||
Model I: Adjusted model for the association between anemia and composite endpoint of severe outcomes. Model II: Adjusted model for the association between different severities of anemia and composite endpoint of severe outcomes.1Adjusted Odds Ratio. *Variables with 20% or missing values were not included in the multivariable model due to too many missing values. **ALT was not included in multivariable models because of the high correlation with AST which appeared to be the highest predictive variable in bivariate analysis. ***Creatinine was not included in multivariable models because of the high correlation with CKD which appeared to be the highest predictive variable in bivariate analysis.
Table 4.
Logistic regression models of mortality outcome
| Unadjusted Model | Adjusted Model I | Adjusted Model II | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Demographics | OR | 95% CI | P-value | aOR1 | 95% CI | P-value | aOR1 | 95% CI | P-value |
| Age > 65 | 2.790 | 1.914–4.065 | <0.001 | 2.277 | 1.481–3.501 | <0.001 | 2.295 | 1.492–3.529 | <0.001 |
| BMI | 0.986 | 0.962–1.012 | 0.293 | ||||||
| Male Sex | 1.311 | 0.920–1.870 | 0.134 | 1.535 | 1.029–2.288 | 0.036 | 1.557 | 1.044–2.323 | 0.030 |
| Comorbidities | |||||||||
| DM | 1.266 | 0.887–1.808 | 0.194 | 0.856 | 0.565–1.299 | 0.465 | 0.860 | 0.567–1.303 | 0.476 |
| HTN | 2.738 | 1.720–4.358 | <0.001 | 2.157 | 1.181–3.939 | 0.012 | 2.163 | 1.187–3.942 | 0.012 |
| HLD | 1.643 | 1.148–2.351 | 0.007 | 1.392 | 0.924–2.097 | 0.113 | 1.390 | 0.922–2.095 | 0.116 |
| Heart disease | 1.458 | 1.003–2.120 | 0.048 | 0.677 | 0.429–1.069 | 0.094 | 0.676 | 0.428–1.069 | 0.094 |
| Asthma | 1.156 | 0.715–1.868 | 0.554 | ||||||
| COPD | 1.338 | 0.778–2.302 | 0.292 | ||||||
| CKD | 1.889 | 1.309–2.725 | 0.001 | 1.257 | 0.801–1.974 | 0.320 | 1.234 | 0.784–1.942 | 0.364 |
| Cancer | 1.298 | 0.833–2.024 | 0.250 | ||||||
| Lab Findings | |||||||||
| Hemoglobin | 0.972 | 0.914–1.034 | 0.365 | ||||||
| Ferritin* | 1.000 | 1.000–1.000 | <0.001 | ||||||
| D-dimer* | 1.142 | 1.101–1.185 | <0.001 | ||||||
| WBC | 1.027 | 0.986–1.070 | 0.199 | ||||||
| CRP* | 1.080 | 1.058–1.103 | <0.001 | ||||||
| Platelet | 0.998 | 0.006–0.999 | 0.006 | 0.997 | 0.995–0.999 | 0.005 | 0.997 | 0.995–0.999 | 0.005 |
| ALT** | 1.001 | 1.000–1.003 | 0.095 | ||||||
| AST | 1.004 | 1.000–1.008 | 0.048 | 1.004 | 1.000–1.008 | 0.033 | 1.004 | 1.000–1.008 | 0.033 |
| Creatinine*** | 1.091 | 1.025–1.161 | 0.006 | ||||||
| Classifications | |||||||||
| Anemia (on admission) vs. none | 1.541 | 1.079–2.200 | 0.017 | 1.523 | 1.008–2.303 | 0.046 | |||
| Anemia Severity | |||||||||
| Mild vs. none | 1.496 | 0.913–2.451 | 0.011 | 1.406 | 0.823–2.404 | 0.212 | |||
| Mod-Sev vs. none | 1.587 | 1.118–2.253 | 0.010 | 1.671 | 1.090–2.563 | 0.019 | |||
Model I: Adjusted model for the association between anemia and all-cause mortality, Model II: Adjusted model for the association between different severities of anemia and all-cause mortality.1Adjusted Odds Ratio. *Variables with 20% or more missing values were not included in the multivariable model due to too many missing values. **ALT was not included in multivariable models because of the high correlation with AST which appeared to be the highest predictive variable in bivariate analysis. ***Creatinine was not included in multivariable models because of the high correlation with CKD which appeared to be the highest predictive variable in bivariate analysis.
In our subgroup analysis of patients who were not anemic on admission, becoming anemic during the hospitalization was significantly associated with an increase in the risk of having severe COVID-19 outcomes (OR 1.737, CI [1.033–2.922] p = 0.037, Table 5 ) after adjusting for confounders. Another subgroup analysis of patients with ferritin values prior to admission demonstrated that pre-existing iron deficiency anemia [21] was not significantly associated with severe outcomes of COVID-19 in an unadjusted regression analysis (Supplementary Table S3).
Table 5.
Subgroup Analysis: Logistic Regression analysis of the composite endpoint of severe outcomes in patients with no anemia on admission who later become anemic
| Unadjusted Model |
Adjusted Model I |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | aOR1 | 95% CI | P-value | |
| Demographics | ||||||
| Age > 65 | 2.361 | 1.470–3.791 | <0.001 | 2.792 | 1.527–5.103 | 0.001 |
| BMI | 1.000 | 0.970–1.031 | 0.993 | |||
| Male Sex | 1.349 | 0.847–2.147 | 0.207 | 1.479 | 0.847–2.585 | 0.168 |
| Comorbidities | ||||||
| DM | 1.236 | 0.768–1.991 | 0.382 | 1.120 | 0.624–2.010 | 0.704 |
| HTN | 1.397 | 0.854–2.286 | 0.182 | 0.876 | 0.450–1.704 | 0.695 |
| HLD | 1.497 | 0.939–2.387 | 0.089 | 1.243 | 0.688–2.246 | 0.469 |
| Heart Disease | 1.328 | 0.764–2.309 | 0.313 | 0.888 | 0.441–1.786 | 0.738 |
| Asthma | 1.160 | 0.559–2.406 | 0.689 | |||
| COPD | 1.661 | 0.684–4.031 | 0.261 | 1.272 | 0.414–3.910 | 0.673 |
| Kidney Disease** | 1.616 | 0.883–2.956 | 0.119 | |||
| Cancer | 1.330 | 0.636–2.781 | 0.448 | |||
| Lab Findings | ||||||
| Ferritin* | 1.000 | 1.000–1.000 | 0.017 | |||
| D-dimer* | 1.135 | 1.062–1.212 | <0.001 | |||
| WBC | 1.152 | 1.080–1.229 | <0.001 | 1.139 | 1.061–1.222 | <0.001 |
| CRP* | 1.108 | 1.062–1.156 | <0.001 | |||
| Platelet | 1.000 | 0.998–1.002 | 0.913 | |||
| ALT*** | 1.004 | 0.999–1.009 | 0.091 | |||
| AST | 1.008 | 1.002–1.014 | 0.005 | 1.007 | 1.001–1.013 | 0.015 |
| Creatinine | 1.325 | 0.995–1.764 | 0.054 | 1.132 | 0.934–1.372 | 0.204 |
| Classifications | ||||||
| Becoming anemic vs. none | 1.693 | 1.062–2.701 | 0.027 | 1.737 | 1.033–2.922 | 0.037 |
Total subgroup n = 295, a subset of sample who did not have anemic hemoglobin levels on admission.1Adjusted Odds Ratio. *Variables with 20% or missing values were not included in the multivariable model: ferritin, D-dimer, CRP due to too many missing values. **Chronic Kidney Disease was not included in multivariable models because of the high correlation with creatinine which appeared to be the highest predictive variable in bivariate analysis. *** ALT was not included in multivariable models because of the high correlation with AST which appeared to be the highest predictive variable in bivariate analysis.
4. Discussion
Our single-center, retrospective study of 733 patients admitted with SARS-CoV2 in the greater New York City area showed that anemia on admission is predictive of higher rate of mortality in hospitalized patients with COVID-19, but was not significant for having severe outcomes during hospitalization. However, moderate-severe anemia on admission was independently associated with both mortality and severe outcomes in COVID-19 patients. We additionally found that anemia acquired during the course of hospitalization was independently associated with severe COVID-19 outcomes.
Our findings on the associations between anemia and death were similar to the findings from a recent study by Bellmann et al., who also reported anemia on admission as an independent predictor of mortality in 259 patients with COVID-19 (OR 3.729, CI[1.02–11.75], p = 0.001) but no association with functional iron deficiency [15]. Another recent study by Tao et al. reported anemia as an independent risk factor for severe COVID-19 in 222 patients (OR 3.47, [1.02–11.75], p = 0.046) [18], which differs from our findings, but their definition of severe cases had a higher cutoff of O2 saturation. Whereas both studies included all patients admitted with COVID-19, we report results from a cohort of patient population with higher prevalence of on-admission anemia (43.3% vs. 24.7%, 35.6%). Our sample size is larger than the total patients included in either study, and our patient population was more heterogenous, with a higher comorbidity burden than either study.
Hemoglobin concentration is one of the most important markers of oxygen-carrying capacity in the blood. In the setting of respiratory compromise and increased oxygen demand in a hyper-metabolic state such as COVID-19, anemia can further reduce oxygen delivery to peripheral tissues. That SARS-CoV-2 directly infects cells expressing the ACE-2 enzyme has been observed in organs throughout the body [22], leading to significant complications such as septic shock and multiple organ dysfunction [5,23] as they may reduce the availability of ACE-2 receptors and thus prevent vasodilation, further compounding peripheral tissue ischemia. Indeed, a meta-analysis by Taneri et al. showed that severity of disease and prognosis of patients with COVID-19 may depend on lower Hb levels as severe cases had significantly lower hemoglobin levels [13], a possible explanation for why anemic patients have higher rate of mortality and severe adverse events.
In addition, our subgroup analysis demonstrated that anemia acquired during hospitalization was independently associated with severe COVID-19 outcomes. Patients infected with COVID-19 may develop anemia through several possible mechanisms. Cavezzi et al. hypothesized that viral entry through receptors located on erythrocytes may induce hemolysis, resulting in hemolytic anemia [17]. Anemia may be a result of severe infection due to alteration of iron homeostasis by the innate immune system, implicated by the pathological value of ferritin observed in severe COVID-19 cases. The innate immune system reduces iron's bioavailability by pro-inflammatory cytokine pathways that upregulate hepcidin, an iron regulatory protein that blocks iron release from macrophages. This leads to decreased intestinal iron absorption and cellular sequestration of iron in macrophages, [24] and an upregulation of cytosolic ferritin, which stores iron to prevent iron-mediated free radical damage [25]. The net result of decreased iron availability, elevated ferritin and hindered erythropoiesis may explain the association between severe COVID-19 and moderate-severe anemia. In COVID-19, marked hyperferritinemia has been reported with the development of cytokine storm characterized by significantly elevated IL-6, CRP, and other inflammatory markers in critically ill patients [26], [27], [28]. Our data also suggest that patients with severe outcomes had significantly elevated levels of acute-phase reactants, suggesting a state of inflammation. While our data suggest that being anemic in the ED alone is inconclusive to predicting severe outcomes of COVID-19, it seems warranted to assess whether moderate-severe anemia could be used as a reliable measure to help guide clinical management of COVID-19.
Our findings may have implications in guiding the management of COVID-19 patients hospitalized with or without anemia. Based on our study results, the timing of anemia and the severity of anemia could offer additional insights into COVID-19 patients' treatment. In the case of COVID-19 infection with mild anemia on presentation, O2 supplementation, in addition to general practice guidelines, may suffice. However, if the patients are moderate-severely anemic in the ED or become newly anemic during hospitalization, the use of steroids may prevent further deterioration, in addition to standard care using iron or blood transfusions. However, the current WHO and NIH guidelines based on the RECOVERY trial have a conditional recommendation against corticosteroids in patients with a mild course of COVID-19 [29]. Given our findings, early detection of anemic hemoglobin levels and their severity could be beneficial in considering the use of systemic steroids before disease progression.
Our study has several limitations. First, this study was conducted using the data from one integrated- delivery health system in New York; hence our results may have limited generalizability. However, as anemia is a global condition with higher prevalence in developing countries, our findings may have potential implications. Secondly, the study was performed during the peak of the pandemic, in which contactless interviews and shortened notes were the norm and limited the amount of information from chart review. Similarly, not all laboratory studies were performed on all patients, and values were missing in laboratory parameters. Lastly, our main limitation was that we could not separate acute from chronic anemia, and did not account for the etiology of anemia including hemoglobinopathies and sickle cell disease, which limits our interpretation. Nonetheless, our study suggests that moderate-severe anemia on admission regardless of its etiology poses as a risk for severe outcomes in COVID-19.
Future research would benefit from an inclusion/exclusion criteria of laboratory values to help distinguish anemia by etiology and strengthen the multivariable regression analysis. Furthermore, a thorough look at all treatments including medications could further control for the differences in outcomes.
5. Conclusion
Anemia is a global disease associated with the prognosis of many clinical diseases, including diseases with respiratory compromises, such as COVID-19. We report that anemia on admission and particularly moderate-severe anemia was independently associated with all-cause mortality in patients hospitalized with COVID-19. Moderate-severe anemia was also predictive of severe outcomes in COVID-19 patients. Anemia on admission and changing hemoglobin levels throughout hospitalization may help further guide risk stratification and management of patients hospitalized with COVID-19. Additional studies addressing more inclusion of laboratory values and treatments received by patients should be the focus of future studies.
Sources of support
None.
Declaration of Interest
None.
Acknowledgments
Acknowledgement
We thank our colleagues Giuseppe Fiorcia and Jasmine Kim for their contribution to data extraction.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajem.2021.03.083.
Appendix A. Supplementary data
Patient outcomes and laboratory findings.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html
- 3.Mendy A., Apewokin S., Wells A.A., Morrow A.L. Factors associated with hospitalization and disease severity in a racially and ethnically diverse population of COVID-19 patients. medRxiv. 2020 [Google Scholar]
- 4.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. medRxiv. 2020 doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S., Hayek S.S., Wang W., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://www.who.int/vmnis/database/anaemia/anaemia_status_summary/en/
- 8.Hayden S.J., Albert T.J., Watkins T.R., Swenson E.R. Anemia in critical illness: insights into etiology, consequences, and management. Am J Respir Crit Care Med. 2012;185(10):1049–1057. doi: 10.1164/rccm.201110-1915CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambellan A., Chailleux E., Similowski T., Group AO Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest. 2005;128(3):1201–1208. doi: 10.1378/chest.128.3.1201. [DOI] [PubMed] [Google Scholar]
- 10.Groenveld H.F., Januzzi J.L., Damman K., et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52(10):818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 11.Salisbury A.C., Alexander K.P., Reid K.J., et al. Incidence, correlates, and outcomes of acute, hospital-acquired anemia in patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3(4):337–346. doi: 10.1161/CIRCOUTCOMES.110.957050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mourad S., Rajab M., Alameddine A., Fares M., Ziade F., Merhi B.A. Hemoglobin level as a risk factor for lower respiratory tract infections in Lebanese children. N Am J Med Sci. 2010;2(10):461–466. doi: 10.4297/najms.2010.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taneri P.E., Gomez-Ochoa S.A., Llanaj E., et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020 doi: 10.1007/s10654-020-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan B.E., Chong V.C.L., Chan S.S.W., et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95(6):E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 15.Bellmann-Weiler R., Lanser L., Barket R., et al. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J Clin Med. 2020;9(8) doi: 10.3390/jcm9082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X., Zhang R., He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol. 2020;99(7):1421–1428. doi: 10.1007/s00277-020-04103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavezzi A., Troiani E., Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10(2):1271. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao Z., Xu J., Chen W., et al. Anaemia is associated with severe illness in COVID-19: a retrospective cohort study. J Med Virol. 2020 doi: 10.1002/jmv.26444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.https://www.who.int/vmnis/indicators/haemoglobin.pdf
- 20.https://www.who.int/publications/i/item/clinical-management-of-covid-19
- 21.Cappellini M.D., Musallam K.M., Taher A.T. Iron deficiency anaemia revisited. J Intern Med. 2020;287(2):153–170. doi: 10.1111/joim.13004. [DOI] [PubMed] [Google Scholar]
- 22.Chu H., Chan J.F.-W., Yuen T.T.-T., et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1(1):e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D., Wang Q., Zhang H., et al. Viral sepsis is a complication in patients with novel corona virus disease (COVID-19) Med Drug Discov. 2020;8:100057. doi: 10.1016/j.medidd.2020.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camaschella C., Nai A., Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105(2):260–272. doi: 10.3324/haematol.2019.232124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drakesmith H., Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6(7):541–552. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- 26.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan X., Huang W., Ye B., et al. Changes of hematological and immunological parameters in COVID-19 patients. Int J Hematol. 2020:1–7. doi: 10.1007/s12185-020-02930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1
- 30.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient outcomes and laboratory findings.