Abstract
The goal of this narrative review of pharyngitis is to summarize the practical aspects of the management of sore throat in children in high- and middle-income countries. A traditional review of the literature was performed. Most cases of pharyngitis are viral and self-limited, although rarely viral pharyngitis due to Epstein–Barr leads to airway obstruction. Bacterial pharyngitis is usually due to group A streptococcus (GAS), occurs primarily in children aged 5–15 years, and presents as sore throat in the absence of rhinitis, laryngitis or cough. Again, most cases are self-limited; antibiotics hasten recovery by only 1–2 days. Guidelines vary by country, but antibiotics are commonly recommended for proven GAS pharyngitis as they may prevent rare but severe complications, in particular rheumatic fever (RF). In this era of antimicrobial stewardship, it should be extremely rare that antibiotics are prescribed for presumed GAS pharyngitis until GAS has been detected. Even with proven GAS pharyngitis, it is controversial whether children at low risk for RF should routinely be prescribed antibiotics as the number needed to treat to prevent one case of RF is undoubtedly very large. When treatment is offered, the antibiotics of choice are penicillin or amoxicillin as they are narrow spectrum and resistance resulting in clinical failure is yet to be documented. A 10-day oral course is recommended as shorter courses appear to be less likely to clear carriage of GAS. However, the evidence that one needs to clear carriage to prevent RF is low quality and indirect.
Keywords: group A streptococcus, pharyngitis
Introduction
Pharyngitis is inflammation of the oropharynx (the cavity behind the mouth) and manifests as intermittent or constant sore throat. It is commonly referred to as ‘tonsillitis’ by clinicians and patients. Most acute pharyngitis is due to viral or bacterial infection but can also result from non-infectious aetiologies such as gastroesophageal reflux or foreign body ingestion. This review will focus on acute infectious pharyngitis in the immunocompetent child in high- and middle-income countries. The primary goal is to explain the rationale behind current pharyngitis guidelines as studies have shown that clinicians commonly do not adhere to them.1,2 A secondary goal is to provide clinicians with an expanded view of the infectious aetiologies of pharyngitis and the sequelae of group A streptococcal (GAS) pharyngitis.
Methods
A traditional review of the literature was performed, including a PubMed search of articles with “pharyngitis” or “sore throat” in the title for 2016 through to 2020.
Review
Viruses and bacteria in the oropharynx of the asymptomatic child
The oropharynx can harbour respiratory viruses, such as rhinovirus, coronaviruses (including severe acute respiratory syndrome coronavirus 2), influenza, respiratory syncytial virus, human metapneumovirus, parainfluenza virus and adenovirus, with or without symptoms. The concentration of viruses appears to be higher in the nasopharynx than in the oropharynx but, using molecular techniques, respiratory viruses can often be detected from oropharyngeal specimens (throat swabs or saliva).3 The ratio of symptomatic-to-asymptomatic infection and the duration of shedding vary by virus and by age.
Normal mouth flora consists predominately of anaerobes and viridians group streptococci. However, it is common for children or adults to also have GAS in their oropharynx. A small percentage have GAS pharyngitis but the vast majority are simply GAS carriers. GAS carriage is asymptomatic, can persist for weeks to years, and is thought to be benign and without sequelae. A meta-analysis of studies up to 2017 reported that 8% of asymptomatic children had GAS detected by throat swabs.4 To estimate the incidence of carriage over time during colder months, children aged 5–15 years in the United States had throat swabs performed every 2 weeks from October through to May with some children enrolled in consecutive years. Approximately 15% of children were carriers in any given month (defined as detection of GAS on two or more sequential swabs in the absence of symptoms) while 30% were carriers at least once over an 8-month period.5 Carriage persisted for a mean of 11 and a maximum of 127 weeks.6 Carriers appear to be far less infectious than are symptomatic patients.6
Viruses and bacteria implicated in pharyngitis
Viral pharyngitis
Symptomatic infection with rhinovirus, coronaviruses, influenza, respiratory syncytial virus, human metapneumovirus, parainfluenza virus and adenovirus accounts for the majority of pharyngitis cases in all age groups. The initial phase in infection is often invasion of the oropharynx by the virus, resulting in inflammation and pain. It is therefore common for pharyngitis to precede all other respiratory tract symptoms (such as rhinitis, laryngitis and cough) by 1–2 days. The sore throat is typically mild to moderate in severity and resolves within a few days.
Infection with non-respiratory viruses can also present as pharyngitis. Primarily in young children, enteroviruses can cause herpangina (shallow ulcers on the posterior tongue and oropharynx).7 This may be accompanied by vesicles on the hands, feet or buttocks (hand, foot and mouth disease). The patient or parent is typically not aware of the mouth ulcers as the ulcers are posterior and not readily visible. Herpes simplex virus classically causes vesicles on the lips or anterior mouth but there is evidence that, particularly in older adolescents, pharyngitis can occur due to lesions limited to the posterior mouth that may extend into the oesophagus.8,9
Epstein–Barr virus (EBV) is another non-respiratory virus that commonly presents with pharyngitis as one component of infectious mononucleosis. The sore throat can be relatively severe and persists for days to weeks. Accompanying fatigue is often the dominant symptom. There is significant overlap in symptoms and signs between EBV infection and acute HIV infection so one should always consider testing for HIV in a sexually active person being tested for EBV.10
Bacterial pharyngitis
A meta-analysis of studies up to 2017 estimated that 16.6% of children younger than 5 years and 24.3% aged 5–19 years with a sore throat had GAS detected in their oropharynx.4 GAS pharyngitis almost always occurs in the absence of new-onset rhinitis, laryngitis or cough.11 Complicated cases can present as peritonsillar, parapharyngeal or retropharyngeal abscesses. The treatment of GAS pharyngitis with antibiotics may decrease the risk of rheumatic fever (RF).11
Like GAS, Streptococcus dysgalactiae subsp equisimilis, which express group C or G Lancefield antigens (Group C or G streptococci), can be part of normal flora or cause pharyngitis.12 They sometimes originate from food-borne sources. Pharyngitis with these serotypes rarely, if ever, leads to abscesses or sequelae such as RF.11
Clinicians should be familiar with the non-streptococcal bacterial aetiologies of pharyngitis, which are rare but can result in serious sequelae. Corynebacterium diphtheria remains a cause of pharyngitis in under immunized adults and children. Despite the vaccine preventing only disease and not infection, immunization programmes have been remarkably successful; there are now less than 10,000 reported cases worldwide annually, primarily in southeast Asia and Africa.13
Arcanobacterium haemolyticum (formerly classified in the genus Corynebacterium) was first linked to pharyngitis in 1946;14 it is not known whether it occurs worldwide. In a study performed in Ottawa in 1995, A. haemolyticum was detected in 26 of 1019 adolescents aged 15–18 years (2.6%), in 9 of 8180 younger children (0.1%), and in 7 of 1960 adults (0.4%) with pharyngitis versus in 0 of 2241 controls, suggesting that it is a rare but true cause of pharyngitis. Additionally, 18 of 42 (44%) cases had a non-distinctive rash and 5 of 13 (38%) tested had a positive monospot test, suggesting a potential overlap with EBV infection.15 In a subsequent study from Israel in patients aged 1–90 years, A. haemolyticum was detected in only 1 of 518 (0.2%) throat cultures.16
As suggested by their names, Mycoplasma pneumoniae and Chlamydia pneumoniae cause atypical pneumonia. However, they can also cause upper respiratory tract infections, (RTIs) which are indistinguishable from viral upper RTIs. In a study of 2433 children hospitalized in China with upper or lower RTIs and evidence for recent M. pneumoniae infection (positive IgM or molecular detection), approximately 20% had sore throat and 20% had pharyngitis on examination.17 In a study from Italy, molecular detection and serology for M. pneumoniae and C. pneumoniae were performed on 133 children aged 6 months to 14 years with pharyngitis without evidence for lower RTI;18 M. pneumoniae was detected in seven (5%) children and C. pneumoniae in six (5%). IgM without molecular detection was reported for M. pneumoniae in 36 (27%) children and C. pneumoniae in 12 (9%). However, the significance of detection of IgM is not clear as it can be falsely positive or persist from symptomatic or asymptomatic infections that occurred months prior to the pharyngitis.19
Sexually active persons can develop gonococcal pharyngitis. Approximately 10% of cases are estimated to be symptomatic.20 There are rare case reports of syphilis presenting as pharyngitis with molecular detection of Treponema pallidum in the oropharynx.21
Plague, caused by Yersinia pestis, is a rare but very serious cause of pharyngitis. It occurs primarily in rural areas in Africa, although epidemics have occurred in Asia and South America. Scattered cases continue to occur in the rural United States.22 The life cycle involves rodents, lagomorphs and fleas. Humans acquire plague after a bite by an infected flea, handling an infected animal or being in contact with a person with pneumonic plague. When the portal of entry is through the mouth, plague can present with pharyngitis, sometimes with cervical buboes (very swollen tender lymph nodes). There are rare reports of Yersinia enterocolitica infection presenting with pharyngitis rather than diarrhoea.23,24
A wide variety of animals (including domestic cats) can be infected with Francisella tularensis, another rare cause of pharyngitis that remains endemic in North America and in Nordic countries.25 Humans become infected after a bite by an infected tick or deer fly, handling an infected animal, inhaling infected aerosols, or swallowing contaminated food or water. Person-to-person transmission does not occur. Again, pharyngitis can be the initial presentation if entry is through the mouth.
It is controversial whether the anaerobe Fusobacterium necrophorum (part of normal mouth flora) ever causes pharyngitis. This is of particular interest as it is the primary cause of Lemierre’s syndrome (suppurative thrombophlebitis of the internal jugular vein leading to systemic venous emboli), which is sometimes preceded by sore throat. A 2016 meta-analysis of six primarily low-quality studies reported detection from 213/1065 (21%) patients with pharyngitis versus 59/776 (8%) controls (p<0.05).26 A subsequent small study demonstrated no difference in detection in patients with or without pharyngitis but a higher bacterial load in those with pharyngitis.27
Linking the clinical presentation to the most likely aetiology of pharyngitis
Children who have respiratory distress (difficulty breathing) at presentation or rapidly progress to severe disease
A minor percentage (<1%) of children who present initially with pharyngitis have a potentially life-threatening infection and require emergent care. This can result from the following situations:
Peritonsillar abscess (quinsy)
A pocket of pus forms adjacent to the tonsil, resulting in fever, severe pain on swallowing and a muffled voice. This occurs more commonly in adults than in children and can lead to airway obstruction. On examination, there is usually trismus. The abscess itself may or may not be readily apparent but will push the adjacent tonsil and the uvula beyond the mid-line. Many cases are due to GAS but Staphylococcus aureus (which is commonly found in the nasopharynx) and almost all components of mouth flora alone or in combination have been detected in peritonsillar abscesses.28 Drainage of an early abscess can sometimes be accomplished with a needle in an awake, co-operative older child but often requires surgical drainage with a general anaesthetic. In select cases with early diagnosis, antibiotics alone are curative.29
Retropharyngeal/parapharyngeal abscess
Retropharyngeal/parapharyngeal abscess presents as a pocket of pus in a deep neck space. The child presents with fever, pain on swallowing and torticollis. This occurs primarily in children younger than 6 years of age and can lead to airway obstruction or spread of pus to other spaces, including the mediastinum. Imaging is usually required to confirm the diagnosis as there are no distinctive findings on examination. As with peritonsillar abscesses, any component of mouth flora can be found in the abscess with viridans streptococci groups perhaps being most common.30 The incidence of retropharyngeal abscess with methicillin-resistant S. aureus appears to be increasing.31 Again, antibiotics alone, needle drainage or surgical drainage may be appropriate depending on the age of the child and the size of the abscess.32
Diphtheria
The initial presentation is indistinguishable from viral or GAS pharyngitis but 2–3 days later, about one-third of patients develop a white or blue-grey leathery membrane on the tonsils with extension to the uvula and soft palate. The membrane can spread down the trachea to the bronchi. Airway obstruction occurs from the swelling or from the membrane being aspirated. Clues on examination are the presence of the membrane (with bleeding if it is scraped off) and significant neck swelling (‘bull neck’) from the combination of cervical adenitis and mucosal oedema.33 The toxin can lead to myocarditis or neuritis (including paralysis of the soft palate).
Other situations
Plague or tularaemia can present initially as pharyngitis and then progress to pneumonia or systemic disease. Bacterial tracheitis or epiglottitis can present with sore throat but will rapidly progress to respiratory distress so will not be confused with other causes of pharyngitis. Very rarely, inflammation from EBV pharyngitis is sufficient to lead to upper airway obstruction requiring intubation.34
Non-emergent cases
This applies to children with sore throat who are systemically well and do not have respiratory distress.
History
The history is the most important tool in determining the aetiology of pharyngitis. The presence of fever is not a useful clue as it can occur with viral or bacterial pharyngitis. However, if a child of any age has any new-onset respiratory symptoms, including rhinitis, laryngitis or cough, their pharyngitis is highly likely to be viral. Conjunctivitis and diarrhoea are also suggestive of viral illness.35
If the child presents with pharyngitis without rhinitis, laryngitis, cough, conjunctivitis or diarrhoea, one should consider EBV or GAS as possible aetiologies. However, many such cases are still due to respiratory viruses with other respiratory symptoms lagging behind the pharyngitis.
EBV pharyngitis presents primarily in adolescents and young adults but can occur in younger children. Clues on history to the diagnosis of EBV are fatigue, facial swelling (from poor drainage of the lymphatics due to cervical lymphadenopathy) and/or development of a generalized maculopapular rash, especially if given amoxicillin.36
GAS pharyngitis is most common in children aged 5–15 years. When compared to viral pharyngitis, it typically starts more abruptly and is more severe. It is accompanied by abdominal pain of unknown pathogenesis in approximately 7% of febrile cases.37 A small percentage of patients with GAS pharyngitis develop scarlet fever, which is a hypersensitivity rash to a GAS exotoxin. The incidence fluctuates over time and increased from 8.2 to 33.2 per 100,000 in England from 2013 to 2016.38 It presents as an erythematous ‘sandpaper’ rash on the trunk and limbs (accentuated in the flexor creases) with sparing of the palms, soles and often the face (although there may be perioral pallor).39
The other previously mentioned bacterial aetiologies typically present in non-specific ways that overlap with viral or GAS pharyngitis.
Physical examination
With pharyngitis due to respiratory viruses, one expects to find a red oropharynx and small, anterior cervical lymph nodes that are not very tender. The presence of exudate in the oropharynx, tender anterior cervical nodes or palatal petechiae is suggestive of infection with GAS or EBV, although adenovirus can also produce exudates. Generalized lymphadenopathy, splenomegaly or facial swelling make EBV the likely diagnosis.
Clinical scoring systems for GAS
Common scoring systems that use criteria from history and physical examination to estimate the risk of GAS pharyngitis are the Centor/McIsaac40 or FeverPAIN.41 In a recent review using GAS culture as the reference standard, the sensitivity of the scoring systems ranged from 74% to 97% and the specificity from 17% to 65%.42 Because even patients with the highest scores still commonly have viral pharyngitis, these systems should only be considered to determine which children to test for GAS and not which children to empirically treat with antibiotics.
Laboratory confirmation of the aetiology of pharyngitis
Suspected viral pharyngitis
When respiratory viruses are suspected, neither a throat swab (to rule out GAS) nor any other investigations are indicated unless the child is immunocompromised or more ill than expected or there is a need to know their severe acute respiratory syndrome coronavirus 2 or influenza status, in which case viral detection can be performed (typically on a nasopharyngeal specimen).
If EBV seems likely, a test for heterophile antibodies (such as a monospot) should be ordered. Results can often be obtained on the same day. False-positive results are rare but false negative results are common in pre-school-aged children.43 EBV serology is the definitive test in all age groups,43 but there is often a delay of several days before results are available. With acute infection, EBV IgM is expected to be positive (although it may not be in the first few days of illness) and to stay positive for 4–8 weeks.44 EBV IgG takes a few days longer than IgM to become positive, while EBV nuclear antigen becomes positive 3–4 weeks into illness.44 Unfortunately, a false-positive EBV IgM is relatively common with other viral infections.45 Atypical lymphocytes, mild thrombocytopenia and subclinical hepatitis are suggestive of EBV infection, but blood work other than serology should only be performed if the child is sufficiently ill to require admission or if the clinical picture is puzzling.
Suspected bacterial pharyngitis
A throat swab is typically obtained to confirm GAS pharyngitis. The tonsils or tonsillar bed and the posterior pharynx should be swabbed bilaterally. The swab is most commonly processed by culture (results take 18–48 hours)44 or by rapid antigen detection test (RADT; a point-of-care test). Groups A, C and G streptococci are the only bacteria detected by routine throat cultures while RADT detects only GAS. Cultures or RADT should be performed only if the history and examination fit best with GAS pharyngitis and the clinician intends to treat with antibiotics if GAS is confirmed. Culture appears to be over 90% sensitive for GAS in adults,11 but it seems likely that sensitivity is lower in uncooperative children, where it is difficult to perform an optimal throat swab. RADT is less sensitive than culture. Because of this, some experts recommend confirming negative RADTs in children with throat culture,11 while others disagree that this is indicated.46
Molecular tests for the detection of GAS from throat swabs have recently become available in the United States and appear to have superior sensitivity and specificity to culture or RADT;47 they can be used as a point-of-care test but this requires training of staff prior to implementation and is not widely available.47 One concern is that the increased sensitivity may lead to the diagnosis and treatment of cases that have very low bacterial loads such that the risk of RF is negligible.48
A recent study showed that GAS could be cultured in 19 or 20 cases from having a child suck on a swab,49 in keeping with results of a study that reported a sensitivity of 79% and a specificity of 91% for saliva.50 Eventually, saliva may be used in place of throat swabs for the detection of GAS.
Because of the overlap in symptoms and signs for bacterial and viral pharyngitis, it is the author’s opinion that antibiotics should not be started prior to the detection of GAS. The exception would be patients with no fixed address, where it may be impossible to find them once GAS is detected. However, guidelines from New Zealand51 and Australia52 recommend empirical therapy of those at high risk of RF, which may be appropriate in settings with a high incidence of RF. Many clinics and emergency departments have protocols that allow an administrative person to efficiently contact the patient and their preferred pharmacy if cultures grow GAS. A delay in starting penicillin of up to 9 days did not increase the incidence of RF in a study of 1199 patients.53 Three children developed acute RF less than 9 days after being swabbed for pharyngitis in a study of school-based clinics despite one having already been started on penicillin.54
Streptococcal serology measures titres to the GAS extracellular antigens antistreptolysin O (ASO) or antideoxyribonuclease B (ADB). ASO increases 1 week following infection, peaks at 3–5 weeks following infection,55 and sometimes persists for over 1 year. ADB peaks at 6–8 weeks following infection.55 ASO titres are higher following a throat infection than after skin infection.55 Increases in ASO but not in ADB can occur following group C or G streptococcal infection56 or in non-infectious conditions such as liver or autoimmune disease.55 Approximately 20% of patients with GAS pharyngitis do not have an increase in ASO.55 Although it was once dogma that GAS carriers do not have an increase in titres,6 this is not true.56 Serologies are of no value for the diagnosis of pharyngitis. A four-fold increase in titres is specific but not sensitive for infection in recent weeks55,56 and may be useful in linking sequelae to GAS.
To diagnose diphtheria, a throat swab should be cultured for C. diphtheria on selective media and toxin production confirmed.
Given the low incidence of infection, the lack of evidence for sequelae and the lack of evidence that antibiotics shorten the clinical course, the detection of or serology for A. haemolyticum, M. pneumoniae or C. pneumoniae is rarely performed.
Molecular detection or culture of gonococcus in the oropharynx should be performed in patients at risk as treatment can prevent disseminated infection or transmission to others. Serology is highly likely to be reactive if pharyngitis is due to syphilis.
Plague is usually diagnosed by detection of the organism in buboes or blood, Y. enterocolitica by detection in stool, and tularaemia by detection in infected tissues or by serology.
Some experts advocate that throat swabs from adolescents be plated on media for the detection of F. necrophorum and antibiotics offered if the bacteria is detected.57 An argument against this is that, although the incidence of Lemierre’s syndrome may be comparable to that of RF, both entities are rare and it is not known how commonly symptomatic pharyngitis precedes Lemierre’s syndrome and whether antibiotics prevent this serious complication.26,58
Potential sequelae of GAS pharyngitis
Suppurative complications
As mentioned previously, suppurative complications include peritonsillar, parapharyngeal or retropharyngeal abscesses. Typically the child was reported to be well until the day they presented with the abscess,59 suggesting that these complications arise quickly and are not necessarily due to failure to recognize or treat GAS pharyngitis. However, a 2013 Cochrane review of 8 studies including 2433 patients reported that antibiotics reduced the incidence of peritonsillar abscess in the subsequent 2 months (RR 0.15, 95% CI 0.05–0.47).60
Non-suppurative complications
Post-streptococcal glomerulonephritis
This primarily follows skin infections but can also be preceded by pharyngitis. Treatment of the preceding GAS infection is not thought to decrease the risk of this complication.
Rheumatic fever
RF is postulated to be due to cross-reactivity between cardiac myosin and laminin and either GAS M protein or collagen bound to the M protein.61 RF typically occurs 14–21 days after symptomatic or asymptomatic GAS pharyngitis. Following decades of study,62 there is still no convincing evidence that specific strains of GAS increase the risk of RF.61 Many patients remain asymptomatic carriers of GAS following treatment but it is thought to be exceedingly rare (or perhaps not possible) for carriers to develop RF.
Post-streptococcal arthritis
Post-streptococcal arthritis differs from arthritis due to RF in that it starts earlier (10 days versus 14–21 days following the pharyngitis), does not respond to aspirin, and is more likely to be persistent and to involve small joints and the axial skeleton in addition to large joints.63
Psoriasis
Elevated titres of streptococcal antibodies in patients with recent-onset psoriasis were first described in 1955.64 It is commonly observed that GAS pharyngitis (or less commonly vulvar–vaginal GAS infection65) precedes the initial manifestations of guttate psoriasis or leads to worsening of plaque psoriasis.66 HLA-C*06:02 increases the risk of both psoriasis and recurrent tonsillitis,67 so the relationship is not necessarily causal. A Cochrane review of five studies of antibiotics and one of tonsillectomy found inconclusive evidence that treatment or prevention of streptococcal infection improves the prognosis of psoriasis.66
Paediatric acute-onset neuropsychiatric syndrome
Paediatric acute-onset neuropsychiatric syndrome (formerly referred to as paediatric autoimmune neuropsychiatric disorder associated with Streptococcus pyogenes infection) is a syndrome with new onset or worsening of tics or obsessive-compulsive disorder or both following an infection. GAS pharyngitis was the first infection to be implicated. As with psoriasis, it seems likely that the neuropsychiatric sequelae would have eventually occurred even if the child had not developed GAS pharyngitis at that time. Evidence for the use of antibiotics as treatment or prophylaxis is limited and inconclusive.68
Treatment of pharyngitis
In a randomized trial of children with pharyngitis (throat swabs were not part of the study protocol and all were given penicillin), pain resolved by 48 hours in 80% of children treated with ibuprofen, in 71% of those treated with acetaminophen and in 55% of those treated with placebo (p<0.05 for superiority of either analgesic over placebo).69 A 2020 Cochrane review of nine studies using primarily one dose of intramuscular or oral corticosteroids in adults or children (all of whom received antibiotics) reported improvement of symptoms 6 hours earlier and a greater chance of complete resolution of symptoms at 24 hours and 48 hours in the corticosteroid recipients as compared to the placebo group but no decrease in time missed from school or work.70 This degree of improvement in outcomes would not seem to justify an increase in the number of outpatient visits for pharyngitis and the potential adverse events were corticosteroids to be recommended for every sore throat. Lozenges have unclear efficacy for pain relief in adults with pharyngitis71 and do not appear to have been studied in children.
Viral pharyngitis
Treatment of viral pharyngitis is primarily symptomatic. Corticosteroids are recommended for airway obstruction from EBV pharyngitis.34 Indications for oseltamivir vary by country but generally would be used for influenza pharyngitis only if the child was at high risk for a lower RTI.
Bacterial pharyngitis
Even without antibiotics, symptoms of GAS pharyngitis are typically markedly improved after 3–4 days.44 Symptoms resolve only 1 or 2 days sooner with antibiotics than with placebo.60 Therefore, antibiotics are used primarily to prevent complications, especially RF. Until the 1950s, antibiotics were not recommended for GAS pharyngitis and throat cultures were performed primarily to rule out diphtheria.72 A pivotal trial in 1950 demonstrated that intramuscular penicillin decreased the incidence of RF in adult military recruits from approximately 3% to 1%.72 A 2005 meta-analysis of 10 poor-quality studies from the subsequent decade confirmed a reduction in the incidence of RF in those with GAS pharyngitis treated with intramuscular penicillin using various regimens, with a number needed to treat of 53.73 The two studies that included children were inconclusive as one study had no cases of RF and the other had only two cases.
The intramuscular route for the administration of penicillin has mainly been abandoned in resource-rich countries because of painful local reactions. Studies of oral antibiotics for the prevention of RF have all employed the surrogate marker of ‘clearance of carriage’. The conclusions from these studies were that patients should receive approximately 10 days of penicillin,72 although there were minimal comparisons to shorter courses. A recent study reported that carriage cleared in 156 of 194 children and adults aged 6 years or older randomized to penicillin 800 mg qid for 5 days (80%) versus in 165 of 182 randomized to 1000 mg tid for 10 days (91%; difference −10.2%, 95% CI −17.8 to −2.7%).74 Symptoms resolved earlier with the higher daily dose but rates of clinical cure at 5–7 days, relapse and complications were equivalent with the two regimens. To sum up, the optimal duration of treatment with oral penicillin to prevent RF is not known but 10 days is slightly more likely than 5 days to clear carriage.
RF is a disease of poverty.75 The current incidence is not known but appears to be markedly increased in some identifiable populations such as indigenous people in Australia76 and in Canada.77 The role that treatment of GAS pharyngitis has played over time and still plays in 2021 in preventing RF is not clear. It therefore remains controversial whether GAS should routinely be treated to prevent RF. Figure 1 outlines the arguments for and against this practice in populations at low risk for RF. The Infectious Diseases Society of America recommends a 10-day course of penicillin for all proven cases of GAS pharyngitis in children over 3 years of age (RF was always rare in younger children and they tend to present with coryza rather than pharyngitis when they have GAS). In contrast, in Europe, testing for GAS is recommended only if the patient has three of the following four Centor criteria: tonsillar exudate, swollen tender anterior cervical nodes, fever and lack of cough.46 Perhaps given concerns about the overuse of antibiotics, worldwide, the focus should shift to treatment of GAS pharyngitis only in children at high risk of RF because of poverty or ethnicity.
Figure 1.
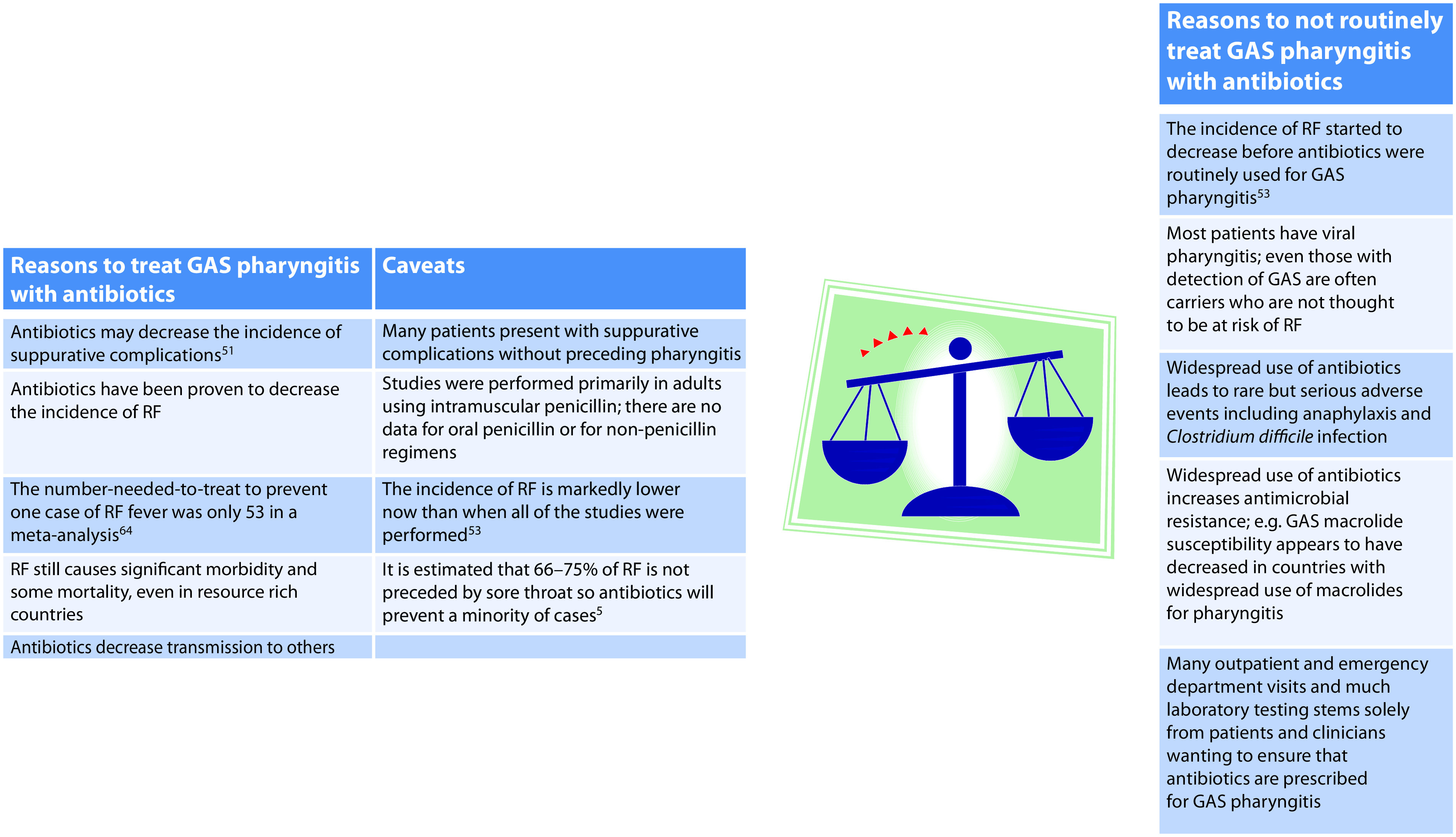
Considerations in deriving guidelines for treatment of GAS pharyngitis in children at low risk for rheumatic fever.
GAS, group A streptococcus; RF, rheumatic fever.
If treatment for GAS pharyngitis is indicated, penicillin is the narrowest spectrum antibiotic. There are just starting to be reports of isolates with elevated minimal inhibitory concentrations for penicillin78 but, for now, it is highly likely to be effective. Although intramuscular administration is rarely used, warming benzathine penicillin to room temperature63 and adding procaine to benzathine penicillin79 may lessen local reactions. A common oral regimen is 40 mg/kg/day (maximum 1000 mg) divided into two doses. Once-daily dosing of amoxicillin was non-inferior to twice-daily dosing with the outcomes being bacteriologic failure and clinical recurrence risk.80 Given that amoxicillin suspension is more palatable than penicillin, amoxicillin daily (50 mg/kg; maximum 1000 mg) is preferable to penicillin for patients who cannot swallow pills and for cases where compliance with twice daily dosing is likely to be poor. For the reasons explained above, all guidelines still recommend a 10-day course with penicillin or amoxicillin give the indirect evidence that this will decrease the risk of RF. However, clinical cure is usually achieved within the first few days. A 2012 Cochrane review reported that, when compared to penicillin, cephalosporins shortened the duration of fever and sore throat by a statistically significant (but perhaps not clinically significant) duration of 0.3 and 0.5 days, respectively.81 This more rapid clinical cure with cephalosporins was confirmed by a 2020 meta-analysis.82 A 2016 Cochrane review reported that the more rapid clinical cure with cephalosporins was not apparent in intention-to-treat analyses but did report a lower risk of relapse in adults (but not children) treated with cephalosporins.83 Despite the potential benefits of cephalosporins, a 10-day course of oral penicillin (or amoxicillin if the child requires suspension) is still generally considered the treatment of choice given the risk of potentiating antibiotic resistance through using broader spectrum agents such as cephalosporins. Commonly recommended second-line options (or alternatives for the penicillin-allergic patient) include first-generation cephalosporins, macrolides or clindamycin.11 However, one should be aware of local resistance patterns for the latter two options before prescribing them84 and consider the risk of Clostridium difficile infection with the use of clindamycin.85
An end-of-therapy test-of-cure swab is not indicated as it is not expected that antibiotics will reliably clear GAS carriage. There are no well-established indications for prescribing antibiotics to clear persistent GAS carriage and this is generally not recommended. However, it is sometimes attempted when a child is at risk for recurrent RF or has bothersome recurrent pharyngitis suspected to be due to GAS (although many of these children are GAS carriers with recurrent viral pharyngitis). Regimens that have been studied for clearing carriage include a 10-day course of clindamycin or amoxicillin-clavulanate, rifampin for the last 4 days of a 10-day course of penicillin, or intramuscular benzathine penicillin followed by a 2-day course of rifampin.11
Antibiotics probably shorten the duration of pharyngitis due to group C or G streptococci but, typically, symptoms are improved by the time culture results are evident, in which case antibiotics are not indicated. The value of treatment for pharyngitis due to A. haemolyticum, M. pneumoniae, C. pneumoniae, or F. necrophorum is not clear. The presence or absence of pharyngitis does not alter treatment recommendations for diphtheria, gonococcus, syphilis, Yersinia species or tularaemia, with the exception that pharyngeal gonococcus always warrants ceftriaxone.
Prevention of pharyngitis
The incidence of RTIs due to other respiratory viruses has plummeted during the coronavirus disease 2019 pandemic.86 It is not clear whether this is due to social distancing, improved hand hygiene, viral interference or a combination of mechanisms, but perhaps the lessons learnt from this pandemic can be applied in the future to decrease the incidence of viral pharyngitis. The prevention of poverty and overcrowding is key to decreasing the incidence of GAS infections and ultimately of RF.52
As mentioned previously, children with recurrent pharyngitis and repeated detection of GAS are commonly carriers with recurrent viral pharyngitis but this is difficult to establish; an attempt to clear GAS can be made but this is generally not advised as it will not reliably prevent further bouts of pharyngitis. Tonsillectomy with or without adenoidectomy is often contemplated. However, in randomized trials, the number of episodes of pharyngitis decreased sufficiently over time in the control group that most would consider the benefits to be minimal; 8–14% of patients had surgical complications87,88 and therefore surgery should generally be avoided for recurrent pharyngitis.
Conclusion
Most children have at least one episode of pharyngitis annually. Given the rising rates of antimicrobial resistance, antibiotics should generally be provided only to children with proven GAS. It remains controversial whether testing for GAS (and antibiotics administration) can routinely be avoided for children with mild symptoms who lack risk factors for RF but the author predicts that, eventually, all guidelines will consider this practice to be acceptable.
Key practice points
Most cases of pharyngitis are viral and self-limited.
Bacterial pharyngitis is usually due to group A streptococcus (GAS); antibiotics hasten recovery by only 1–2 days but are prescribed to potentially prevent rare but severe complications in proven GAS, in particular RF.
For presumed GAS pharyngitis, in this era of antimicrobial stewardship, an antibiotic prescription should be withheld until GAS has been detected.
When treatment is offered, the antibiotics of choice are penicillin or amoxicillin as they are narrow spectrum and GAS is yet to develop resistance leading to clinical failure.
A 10-day oral course is recommended as shorter courses appear to be less likely to clear carriage of GAS.
The evidence that one needs to clear carriage to prevent RF is low quality and indirect.
Acknowledgements
The author would like to thank Drs Dolores Freire, Alexandra Seal-Grant and Catherine Burton for pre-submission review of this manuscript.
Footnotes
Contributions: The named author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity of the work as a whole, and has given her approval for this version to be published.
Disclosure and potential conflicts of interest: The author declares that she has no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/03/dic.2020-11-6-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2021 Robinson JL. https://doi.org/10.7573/dic.2020-11-6. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Reinholdt KB, Rusan M, Hansen PR, Klug TE. Management of sore throat in Danish general practices. BMC Family Pract. 2019;20(1):75. doi: 10.1186/s12875-019-0970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan-Krohn T, Ozonoff A, Sandora TJ. Adherence to guidelines for testing and treatment of children with pharyngitis: a retrospective study. BMC Pediatr. 2018;18(1):43. doi: 10.1186/s12887-018-0988-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson JL, Lee BE, Kothapalli S, Craig WR, Fox JD. Use of throat swab or saliva specimens for detection of respiratory viruses in children. Clinical Infect Dis. 2008;46(7):e61–64. doi: 10.1086/529386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver J, Malliya Wadu E, Pierse N, Moreland NJ, Williamson DA, Baker MG. Group A streptococcus pharyngitis and pharyngeal carriage: a meta-analysis. PLoS Negl Trop Dis. 2018;12(3):e0006335. doi: 10.1371/journal.pntd.0006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin JM, Green M, Barbadora KA, Wald ER. Group A streptococci among school-aged children: clinical characteristics and the carrier state. Pediatrics. 2004;114(5):1212–1219. doi: 10.1542/peds.2004-0133. [DOI] [PubMed] [Google Scholar]
- 6.DeMuri GP, Wald ER. The group A streptococcal carrier state reviewed: still an enigma. J Ped Infect Dis Soc. 2014;3(4):336–342. doi: 10.1093/jpids/piu030. [DOI] [PubMed] [Google Scholar]
- 7.Lee CJ, Huang YC, Yang S, et al. Clinical features of coxsackievirus A4, B3 and B4 infections in children. PloS ONE. 2014;9(2):e87391. doi: 10.1371/journal.pone.0087391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMillan JA, Weiner LB, Higgins AM, Lamparella VJ. Pharyngitis associated with herpes simplex virus in college students. Pediatr Infect Dis J. 1993;12(4):280–284. doi: 10.1097/00006454-199304000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Haddad JD, Ng P, James T. Empiric treatment for acute pharyngitis. Am Fam physician. 2019;100(11):713–714. [PubMed] [Google Scholar]
- 10.Rosenberg ES, Caliendo AM, Walker BD. Acute HIV infection among patients tested for mononucleosis. N Engl J Med. 1999;340(12):969. doi: 10.1056/nejm199903253401217. [DOI] [PubMed] [Google Scholar]
- 11.Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clinical Infect Dis. 2012;55(10):e86–102. doi: 10.1093/cid/cis629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baracco GJ. Infections caused by group C and G streptococcus (streptococcus dysgalactiae subsp. equisimilis and others): epidemiological and clinical aspects. Microbiol Spectr. 2019;7(2) doi: 10.1128/microbiolspec.GPP3-0016-2018. [DOI] [PubMed] [Google Scholar]
- 13.Clarke KEN, MacNeil A, Hadler S, Scott C, Tiwari TSP, Cherian T. Global epidemiology of diphtheria, 2000–2017. Emerg Infect Dis. 2019;25(10):1834–1842. doi: 10.3201/eid2510.190271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maclean PD, Liebow AA, Rosenberg AA. A hemolytic corynebacterium resembling Corynebacterium ovis and Corynebacterium pyogenes in man. J Infect Dis. 1946;79:69–90. doi: 10.1093/infdis/79.1.69. [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie A, Fuite LA, Chan FT, et al. Incidence and pathogenicity of Arcanobacterium haemolyticum during a 2-year study in Ottawa. Clinical Infect Dis. 1995;21(1):177–181. doi: 10.1093/clinids/21.1.177. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Colodner R, Chazan B, Raz R. Pharyngotonsillitis due to arcanobacterium haemolyticum in northern Israel. Israel Med Assoc J. 2005;7(4):241–242. [PubMed] [Google Scholar]
- 17.He XY, Wang XB, Zhang R, et al. Investigation of Mycoplasma pneumoniae infection in pediatric population from 12,025 cases with respiratory infection. Diagn Microbiol Infect Dis. 2013;75(1):22–27. doi: 10.1016/j.diagmicrobio.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Esposito S, Bosis S, Begliatti E, et al. Acute tonsillopharyngitis associated with atypical bacterial infection in children: natural history and impact of macrolide therapy. Clinical Infect Dis. 2006;43(2):206–209. doi: 10.1086/505120. [DOI] [PubMed] [Google Scholar]
- 19.Esposito S, Mencacci A, Cenci E, Camilloni B, Silvestri E, Principi N. Multiplex platforms for the identification of respiratory pathogens: are they useful in pediatric clinical practice? Front Cell Infec Microbiol. 2019;9:196. doi: 10.3389/fcimb.2019.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balmelli C, Günthard HF. Gonococcal tonsillar infection--a case report and literature review. Infection. 2003;31(5):362–365. doi: 10.1007/s15010-003-4003-7. [DOI] [PubMed] [Google Scholar]
- 21.Smith JR, Tsang RS, Kadkhoda K. Tonsillar syphilis: an unusual site of infection detected by treponema pallidum PCR. J Clin Microbiol. 2015;53(9):3089–3091. doi: 10.1128/jcm.01634-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC. Maps and statistics. Plague in the United States. [Accessed March 17, 2021]. https://www.cdc.gov/plague/maps/index.html.
- 23.Fenwick SG. Pharyngitis and infections with Yersinia enterocolitica. N Z Med J. 1992;105(930):112. [PubMed] [Google Scholar]
- 24.Tacket CO, Davis BR, Carter GP, Randolph JF, Cohen ML. Yersinia enterocolitica pharyngitis. Ann Intern Med. 1983;99(1):40–42. doi: 10.7326/0003-4819-99-1-40. [DOI] [PubMed] [Google Scholar]
- 25.Gürcan S. Epidemiology of tularemia. Balkan Med J. 2014;31(1):3–10. doi: 10.5152/balkanmedj.2014.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klug TE, Rusan M, Fuursted K, Ovesen T, Jorgensen AW. A systematic review of Fusobacterium necrophorum-positive acute tonsillitis: prevalence, methods of detection, patient characteristics, and the usefulness of the Centor score. Eur J Clin Microbiol Infect Dis. 2016;35(12):1903–1912. doi: 10.1007/s10096-016-2757-y. [DOI] [PubMed] [Google Scholar]
- 27.Hayakawa K, Nagashima M, Kanehisa E, et al. Real-time PCR investigation of the prevalence of Fusobacterium necrophorum in patients with pharyngitis in Japan. J Infect Chemother. 2018;24(12):969–974. doi: 10.1016/j.jiac.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Slouka D, Hanakova J, Kostlivy T, et al. Epidemiological and microbiological aspects of the peritonsillar abscess. Int J Environ Res Public Health. 2020;17(11):4020. doi: 10.3390/ijerph17114020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forner D, Curry DE, Hancock K, et al. Medical intervention alone vs surgical drainage for treatment of peritonsillar abscess: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020;163(5):915–922. doi: 10.1177/0194599820927328. [DOI] [PubMed] [Google Scholar]
- 30.Philpott CM, Selvadurai D, Banerjee AR. Paediatric retropharyngeal abscess. J laryngol Otol. 2004;118(12):919–926. doi: 10.1258/0022215042790538. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Haq N, Quezada M, Asmar BI. Retropharyngeal abscess in children: the rising incidence of methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2012;31(7):696–699. doi: 10.1097/INF.0b013e318256fff0. [DOI] [PubMed] [Google Scholar]
- 32.Kosko J, Casey J. Retropharyngeal and parapharyngeal abscesses: factors in medical management failure. Ear Nose Throat J. 2017;96(1):e12–e15. [PubMed] [Google Scholar]
- 33.Lo BM. Diphtheria clinical presentation. Emedicine. [Accessed March 3, 2021]. https://emedicine.medscape.com/article/782051-clinical.
- 34.Wohl DL, Isaacson JE. Airway obstruction in children with infectious mononucleosis. Ear Nose Throat J. 1995;74(9):630–638. [PubMed] [Google Scholar]
- 35.Wessels MR. Clinical practice. Streptococcal pharyngitis. N Engl J Med. 2011;364(7):648–655. doi: 10.1056/NEJMcp1009126. [DOI] [PubMed] [Google Scholar]
- 36.Ciccarese G, Trave I, Herzum A, Parodi A, Drago F. Dermatological manifestations of Epstein-Barr virus systemic infection: a case report and literature review. Int J Dermatol. 2020;59(10):1202–1209. doi: 10.1111/ijd.14887. [DOI] [PubMed] [Google Scholar]
- 37.Igarashi H, Nago N, Kiyokawa H, Fukushi M. Abdominal pain and nausea in the diagnosis of streptococcal pharyngitis in boys. Int J Gen Med. 2017;10:311–318. doi: 10.2147/ijgm.S144310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamagni T, Guy R, Chand M, et al. Resurgence of scarlet fever in England, 2014–16: a population-based surveillance study. Lancet Infect Dis. 2018;18(2):180–187. doi: 10.1016/s1473-3099(17)30693-x. [DOI] [PubMed] [Google Scholar]
- 39.Brinker A. Scarlet Fever. N Engl J Med. 2017;376(20):1972. doi: 10.1056/NEJMicm1612308. [DOI] [PubMed] [Google Scholar]
- 40.Fine AM, Nizet V, Mandl KD. Large-scale validation of the Centor and McIsaac scores to predict group A streptococcal pharyngitis. Arch Intern Med. 2012;172(11):847–852. doi: 10.1001/archinternmed.2012.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Little P. FeverPAIN score for strep pharyngitis. [Accessed March 17, 2021]. https://www.mdcalc.com/feverpain-score-strep-pharyngitis.
- 42.Fraser H, Gallacher D, Achana F, et al. Rapid antigen detection and molecular tests for group A streptococcal infections for acute sore throat: systematic reviews and economic evaluation. Health Technol Assess. 2020;24(31):1–232. doi: 10.3310/hta24310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall-Andon T, Heinz P. How to use … the Monospot and other heterophile antibody tests. Arch Dis Child Educ Pract Ed. 2017;102(4):188–193. doi: 10.1136/archdischild-2016-311526. [DOI] [PubMed] [Google Scholar]
- 44.Alcaide ML, Bisno AL. Pharyngitis and epiglottitis. Infect Dis Clin North Am. 2007;21(2):449–469. vii. doi: 10.1016/j.idc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valota M, Thienemann F, Misselwitz B. False-positive serologies for acute hepatitis A and autoimmune hepatitis in a patient with acute Epstein-Barr virus infection. BMJ Case Rep. 2019;12:e228356. doi: 10.1136/bcr-2018-228356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelucchi C, Grigoryan L, Galeone C, et al. Guideline for the management of acute sore throat. Clin Microbiol Infect. 2012;18(Suppl 1):1–28. doi: 10.1111/j.1469-0691.2012.03766.x. [DOI] [PubMed] [Google Scholar]
- 47.Rao A, Berg B, Quezada T, et al. Diagnosis and antibiotic treatment of group a streptococcal pharyngitis in children in a primary care setting: impact of point-of-care polymerase chain reaction. BMC Pediatr. 2019;19(1):24. doi: 10.1186/s12887-019-1393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaggi P, Leber A. Molecular testing for group A streptococcal pharyngitis: to test or not to test, that is the question. J Ped Infect Dis Soc. 2018 doi: 10.1093/jpids/pix106. [DOI] [PubMed] [Google Scholar]
- 49.DeMuri G, Wald ER. Detection of group A streptococcus in the saliva of children presenting with pharyngitis using the Cobas liat PCR system. Clin Ped. 2020;59(9–10):856–858. doi: 10.1177/0009922820920936. [DOI] [PubMed] [Google Scholar]
- 50.Hashavya S, Pines N, Gayego A, Schechter A, Gross I, Moses A. The use of bacterial DNA from saliva for the detection of GAS pharyngitis. J Oral Microbiol. 2020;12(1):1771065. doi: 10.1080/20002297.2020.1771065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The National Heart Foundation of New Zealand. Group A streptococcal sore throat management – guideline. 2019. https://www.heartfoundation.org.nz/resources/group-a-streptococcal-sore-throat-management.
- 52.Ralph AP, Noonan S, Wade V, Currie BJ. The 2020 Australian guideline for prevention, diagnosis and management of acute rheumatic fever and rheumatic heart disease. Med J Aust. 2020 doi: 10.5694/mja2.50851. [DOI] [PubMed] [Google Scholar]
- 53.Catanzaro FJ, Stetson CA, Morris AJ, et al. The role of the streptococcus in the pathogenesis of rheumatic fever. Am J Med. 1954;17(6):749–756. doi: 10.1016/0002-9343(54)90219-3. [DOI] [PubMed] [Google Scholar]
- 54.Lennon D, Stewart J, Farrell E, Palmer A, Mason H. School-based prevention of acute rheumatic fever: a group randomized trial in New Zealand. Pediatr Infect Dis J. 2009;28(9):787–794. doi: 10.1097/INF.0b013e3181a282be. [DOI] [PubMed] [Google Scholar]
- 55.Steer AC, Smeesters PR, Curtis N. Streptococcal serology: secrets for the specialist. Pediatr Infect Dis J. 2015;34(11):1250–1252. doi: 10.1097/inf.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 56.Johnson DR, Kurlan R, Leckman J, Kaplan EL. The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications. Clinical Infect Dis. 2010;50(4):481–490. doi: 10.1086/650167. [DOI] [PubMed] [Google Scholar]
- 57.Centor RM, Linder JA. Web exclusive. Annals on call – Fusobacterium pharyngitis debate. Ann Intern Med. 2019;171(4):Oc1. doi: 10.7326/a19-0010. [DOI] [PubMed] [Google Scholar]
- 58.Linder JA. Sore throat: avoid overcomplicating the uncomplicated. Ann Intern Med. 2015;162(4):311–312. doi: 10.7326/m14-2899. [DOI] [PubMed] [Google Scholar]
- 59.Bisno AL. Are cephalosporins superior to penicillin for treatment of acute streptococcal pharyngitis? Clinical Infect Dis. 2004;38(11):1535–1537. doi: 10.1086/392520. [DOI] [PubMed] [Google Scholar]
- 60.Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane database Syst Rev. 2013;2013(11):CD000023. doi: 10.1002/14651858.CD000023.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Crombrugghe G, Baroux N, Botteaux A, et al. The limitations of the rheumatogenic concept for group a streptococcus: systematic review and genetic analysis. Clinical Infect Dis. 2020;70(7):1453–1460. doi: 10.1093/cid/ciz425. [DOI] [PubMed] [Google Scholar]
- 62.Bisno AL. Group A streptococcal infections and acute rheumatic fever. N Engl J Med. 1991;325(11):783–793. doi: 10.1056/nejm199109123251106. [DOI] [PubMed] [Google Scholar]
- 63.Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119(11):1541–1551. doi: 10.1161/circulationaha.109.191959. [DOI] [PubMed] [Google Scholar]
- 64.Norrlind R. The significance of infections in the origination of psoriasis. Acta Rheumatol Scand. 1955;1(2):135–144. [PubMed] [Google Scholar]
- 65.Hernandez M, Simms-Cendan J, Zendell K. Guttate psoriasis following streptococcal vulvovaginitis in a five-year-old girl. J Pediatr Adolesc Gynecol. 2015;28(5):e127–129. doi: 10.1016/j.jpag.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Dupire G, Droitcourt C, Hughes C, Le Cleach L. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haapasalo K, Koskinen LLE, Suvilehto J, et al. The psoriasis risk allele HLA-C*06:02 shows evidence of association with chronic or recurrent streptococcal tonsillitis. Infect immun. 2018;86(10):e00304–18. doi: 10.1128/iai.00304-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sigra S, Hesselmark E, Bejerot S. Treatment of PANDAS and PANS: a systematic review. Neurosci Biobehav Rev. 2018;86:51–65. doi: 10.1016/j.neubiorev.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Bertin L, Pons G, d’Athis P, et al. Randomized, double-blind, multicenter, controlled trial of ibuprofen versus acetaminophen (paracetamol) and placebo for treatment of symptoms of tonsillitis and pharyngitis in children. J Pediatr. 1991;119(5):811–814. doi: 10.1016/s0022-3476(05)80308-7. [DOI] [PubMed] [Google Scholar]
- 70.Cassan S, Thompson MJ, Perera R, et al. Corticosteroids as standalone or add-on treatment for sore throat. Cochrane database Syst Rev. 2020;5(5):CD008268. doi: 10.1002/14651858.CD008268.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sousa R, Lakha DR, Brette S, Hitier S. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of ambroxol hard-boiled lozenges in patients with acute pharyngitis. Pulm Ther. 2019;5(2):201–211. doi: 10.1007/s41030-019-00100-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radetsky M. Hostage to History: the duration of antimicrobial treatment for acute streptococcal pharyngitis. Pediatr Infect Dis J. 2017;36(5):507–512. doi: 10.1097/inf.0000000000001480. [DOI] [PubMed] [Google Scholar]
- 73.Robertson KA, Volmink JA, Mayosi BM. Antibiotics for the primary prevention of acute rheumatic fever: a meta-analysis. BMC Cardiovasc Disord. 2005;5(1):11. doi: 10.1186/1471-2261-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skoog Ståhlgren G, Tyrstrup M, Edlund C, et al. Penicillin V four times daily for five days versus three times daily for 10 days in patients with pharyngotonsillitis caused by group A streptococci: randomised controlled, open label, non-inferiority study. BMJ. 2019;367:l5337. doi: 10.1136/bmj.l5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yacoub M, Mayosi B, ElGuindy A, Carpentier A, Yusuf S. Eliminating acute rheumatic fever and rheumatic heart disease. Lancet. 2017;390(10091):212–213. doi: 10.1016/s0140-6736(17)31608-2. [DOI] [PubMed] [Google Scholar]
- 76.Katzenellenbogen JM, Bond-Smith D, Seth RJ, et al. Contemporary incidence and prevalence of rheumatic fever and rheumatic heart disease in Australia using linked data: the case for policy change. J Am Heart Assoc. 2020;9(19):e016851. doi: 10.1161/jaha.120.016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madden S, Kelly L. Update on acute rheumatic fever: it still exists in remote communities. Can Fam Physician. 2009;55(5):475–478. [PMC free article] [PubMed] [Google Scholar]
- 78.Musser JM, Beres SB, Zhu L, et al. Reduced in vitro susceptibility of streptococcus pyogenes to β-lactam antibiotics associated with mutations in the pbp2x gene is geographically widespread. J Clin Microbiol. 2020;58:e01993–19. doi: 10.1128/jcm.01993-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bass JW. A review of the rationale and advantages of various mixtures of benzathine penicillin G. Pediatrics. 1996;97(6 Pt 2):960–963. [PubMed] [Google Scholar]
- 80.Clegg HW, Ryan AG, Dallas SD, et al. Treatment of streptococcal pharyngitis with once-daily compared with twice-daily amoxicillin: a noninferiority trial. Pediatr Infect Dis J. 2006;25(9):761–767. doi: 10.1097/01.inf.0000235678.46805.92. [DOI] [PubMed] [Google Scholar]
- 81.Altamimi S, Khalil A, Khalaiwi KA, Milner RA, Pusic MV, Al Othman MA. Short-term late-generation antibiotics versus longer term penicillin for acute streptococcal pharyngitis in children. Cochrane database Syst Rev. 2012;8:CD004872. doi: 10.1002/14651858.CD004872.pub3. [DOI] [PubMed] [Google Scholar]
- 82.Holm AE, Llor C, Bjerrum L, Cordoba G. Short-vs. long-course antibiotic treatment for acute streptococcal pharyngitis: systematic review and meta-analysis of randomized controlled trials. Antibiotics. 2020;9(11):733. doi: 10.3390/antibiotics9110733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Driel ML, De Sutter AI, Habraken H, Thorning S, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane database Syst Rev. 2016;9(9):CD004406. doi: 10.1002/14651858.CD004406.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oppegaard O, Skrede S, Mylvaganam H, Kittang BR. Emerging threat of antimicrobial resistance in β-hemolytic streptococci. Front Microbiol. 2020;11:797. doi: 10.3389/fmicb.2020.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013;68(9):1951–1961. doi: 10.1093/jac/dkt129. [DOI] [PubMed] [Google Scholar]
- 86.Leuzinger K, Roloff T, Gosert R, et al. Epidemiology of severe acute respiratory syndrome Coronavirus 2 emergence amidst community-acquired respiratory viruses. J Infect Dis. 2020;222(8):1270–1279. doi: 10.1093/infdis/jiaa464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paradise JL, Bluestone CD, Colborn DK, Bernard BS, Rockette HE, Kurs-Lasky M. Tonsillectomy and adenotonsillectomy for recurrent throat infection in moderately affected children. Pediatrics. 2002;110(1 Pt 1):7–15. doi: 10.1542/peds.110.1.7. [DOI] [PubMed] [Google Scholar]
- 88.Paradise JL, Bluestone CD, Bachman RZ, et al. Efficacy of tonsillectomy for recurrent throat infection in severely affected children. Results of parallel randomized and nonrandomized clinical trials. N Engl J Med. 1984;310(11):674–683. doi: 10.1056/nejm198403153101102. [DOI] [PubMed] [Google Scholar]

