Summary
Haemolysis is a major feature of sickle cell disease (SCD) that contributes to organ damage. It is well established that haem, a product of haemolysis, induces expression of the enzyme that degrades it, haem oxygenase-1 (HMOX1). We have also shown that haem induces expression of placental growth factor (PGF), but the organ specificity of these responses has not been well-defined. As expected, we found high level expression of Hmox1 and Pgf transcripts in the reticuloendothelial system organs of transgenic sickle cell mice, but surprisingly strong expression in the heart (P < 0.0001). This pattern was largely replicated in wild type mice by intravenous injection of exogenous haem. In the heart, haem induced unexpectedly strong mRNA responses for Hmox1 (18-fold), Pgf (4-fold), and the haem transporter Slc48a1 (also termed Hrg1; 2.4-fold). This was comparable to the liver, the principal known haem-detoxifying organ. The NFE2L2 (also termed NRF2) transcription factor mediated much of the haem induction of Hmox1 and Hrg1 in all organs, but less so for Pgf. Our results indicate that the heart expresses haem response pathway genes at surprisingly high basal levels and shares with the liver a similar transcriptional response to circulating haem. The role of the heart in haem response should be investigated further.
Keywords: haemolysis, sickle cell disease, haem, Hmox1, Pgf
Introduction
Advancements in medicine have contributed to a longer life span for patients with sickle cell disease (SCD), mainly in developed countries (Crocker et al, 1990), with increased development of age-related progressive end-organ injury, including kidney disease, pulmonary hypertension and diastolic heart failure. Even transplanted organs in patients with SCD have high risks of damage and failure circumstantially related to haemolysis and sickling (Huang et al, 2013; Kerins & Ooi, 2017).
Products of intravascular haemolysis contribute to impaired nitric oxide (NO) bioavailability, endothelial dysfunction and organ damage in SCD (Kato et al, 2007; Kato et al, 2017; Vichinsky, 2017). In addition, excess haem (iron protoporphyrin IX) released from damaged sickle red blood cells also acts as a damage-associated molecular pattern (DAMP), thereby activating inflammatory pathways (Ghosh et al, 2013; Belcher et al, 2014; Gladwin & Ofori-Acquah, 2014). Haem is well-documented to induce haem oxygenase-1 (HMOX1) in diverse cell types, a response that detoxifies haem (Vercellotti et al, 2016; Ingoglia et al, 2017). Hmox1 generates equimolar amounts of carbon monoxide and bilirubin, which exerts antioxidant and anti-inflammatory effects (Wagener et al, 2001; Otterbein et al, 2003; Wagener et al, 2003). SLC48A1 (also termed HRG1), a haem transporter, is important for haem transport from the phagolysosome of macrophages during erythrophagocytosis (Rajagopal et al, 2008; White et al, 2013). Hmox1 and Hrg1 expression is regulated by Nuclear factor (erythroid derived 2)-like 2 (NFE2L2, also termed NRF2), a key cytoprotective transcription factor that also regulates the basal and inducible expression of multiple antioxidant proteins and detoxification enzymes (Alam et al, 2003; Motohashi & Yamamoto, 2004). Recent studies in mice have shown a protective effect of NRF2 in attenuating complications of SCD, such as inflammation, acute lung injury, anaemia (Ghosh et al, 2016; Ghosh et al, 2018) and spleen damage (Zhu et al, 2018).
Our laboratory has shown that haem also induces Placental Growth Factor (PGF) (Wang et al, 2014), which is an angiogenic growth factor secreted by proliferating erythroblasts during normal development (Tordjman et al, 2001.). Pgf promotes the expression of the vasoconstrictor endothelin-1 by endothelial cells, associated with pulmonary hypertension in patients with SCD (Perelman et al, 2003; Sundaram et al, 2010) and thalassaemia intermedia (Kelaidi et al, 2018). In non-haemolytic disease, such as chronic kidney disease, elevated PGF is associated with increased left ventricular mass index (Peiskerova et al, 2013) and other cardiovascular events (Heeschen et al, 2003; Matsui et al, 2015).
Organ damage in SCD is a multifactorial process and different organs may be affected in distinct ways by primary events, such as haemolysis and circulating non-haemoglobin bound haem. Because haemolysis contributes to organ damage in SCD, we investigated organ-specific expression patterns of Hmox1 and Pgf in sickle cell mice and modelled this in wild type mice by increasing circulating extracellular haem. Furthermore, we investigated the role of Nfe2l2 (also termed Nrf2) in the organ-specific transcriptional response pattern of Hmox1, Pgf and Hrg1.
Methods
Mice
Male and female Townes’ knocked-in transgenic sickle mouse (SS) and strain controls expressing normal human haemoglobin (AA mice), C57BL/6J (Nrf2+/+) and Nrf2−/− mice were used. C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA; stock number 000664) while SS, AA and Nrf2−/− mice were obtained from a colony maintained by Dr Solomon Ofori-Acquah’s laboratory in our institution. Mouse genotypes were confirmed by polymerase chain reaction (PCR). Haemin [Fe(III)PPIX, Sigma-Aldrich, St. Louis, MO] was first dissolved in 0.25 mol/l NaOH and then adjusted to pH 7.5 with HCl before filter sterilization. The haemin solution was protected from light and injected into the tail vein of 14- to 16-week-old mice. The haemin dose was 50 μmol/kg body weight for SS and AA mice, and 120 μmol/kg body weight for Nrf2+/+ and Nrf2−/− mice. Some of the mice received sterile vehicle (0.25 mol/l NaOH adjusted to pH 7.5 with HCl used in preparation of haemin) as a control. A range of doses and times were tested; 3 h after injection produced consistent survival with no adverse effects on all strains of mice in this study.
Isolation of haematopoietic progenitors from bone marrow and spleen
Mouse bone marrow cells were isolated from the femur and tibia with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin and 2 mmol/l EDTA from mice untreated and treated with vehicle or haemin for 3 h. The bone marrow suspension was filtered through a 70-μm-pore size cell strainer (Fisherbrand, Pittsburgh, PA, USA; Catalogue number 22363548) centrifuged at 300 g for 5 min. Splenocytes were separated from stromal elements by passing spleen tissue through 70-μm filters. The isolated splenocytes were incubated in 5 ml of red blood cell lysis buffer (155 mmol/l NH4Cl, 14 mmol/l NaHCO3 and 127 mmol/l EDTA) for 5 min at 8°C and washed twice with PBS.
Real-time PCR
Genes of interest (Table SI) were evaluated using the TaqMan® Gene expression assay and the TaqMan® RNA-to-Ct™ 1-Step Kit (both from ThermoFisher Scientific, Pittsburgh, PA, USA) according to the manufacturer’s instructions. Whole organs were harvested from mice immediately after death or 3 h after haemin injection. Freshly isolated organs (300 mg) were snap-frozen and kept at 80°C until use. Organs were homogenized in Qiazol lysis reagent using the Next Advance Bullet Blender (Next Advance, Inc. Troy, NY, USA). Homogenized samples were centrifuged at 18 800 g for 10 min to obtain clear lysates. All tissue processing was carried out at 4°C. Total RNA was extracted from the tissue lysates using the miRNeasy Mini Kit (Catalogue number 217004, Qiagen, Germantown, MD, USA) and quantified using the Nanodrop 8000 micro-volume spectrophotometer (ThermoFisher Scientific). Real-time PCR reactions were set-up with 50 ng of RNA in duplicates. Genes of interest were evaluated using the TaqMan® Gene expression assay and the TaqMan® RNA-to-Ct™ 1-Step Kit (both from ThermoFisher) according to the manufacturer’s instructions. Relative quantification was calculated with the standard ΔΔCt method; amplification signals from target gene transcripts were normalized to those from beta-glucuronidase (Gusb) transcripts. Relative fold induction was calculated by further normalization to gene transcripts from vehicle-treated animals. Gusb gene expressions were similar across all mouse strains used and across all organs within a given mouse strain (Figure S1A–C). Gusb gene expression in organs from control mice was similar to that from the corresponding organs from haemin-injected mice.
Haem quantification
Mice were perfused with PBS under anaesthesia. Harvested organs (300 mg) were homogenized in radio-immunoprecipitate assay buffer using the Next Advance Bullet Blender (Next Advance, Inc.). Homogenized samples were centrifuged at 18 800 g for 10 min to obtain clear lysates. All tissue processing was carried out at 4°C. Total haem in tissue lysates was quantified using a colourimetric assay kit (QuantiChrom haem assay kit; Bioassay Systems, Hayward, CA, USA) as described in previous studies (Ghosh et al, 2018). The bicinchoninic acid (BCA) assay kit (ThermoFisher Scientific, Catalogue number 23225) was used to quantify total protein in the lysates. Total haem values were first normalized to total protein, and then the haem values from vehicle-injected control organs were subtracted from the corresponding haem-injected organ values to determine the increase in haem in each organ after haemin injection.
HMOX1 and PGF protein quantification
Heart HMOX1 and PGF concentration were measured using the mouse HMOX-1 enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Sciences, Farmingdale, NY, USA) and the PGF ELISA kit (Sigma-Aldrich) following the manufacturer’s instructions. Total protein was quantified using the BCA method.
Statistical analysis
GraphPad Prism 7 software (GraphPad Software Inc., San Diego, CA, USA) was used for all statistical analyses. Results are reported as mean ± standard error of the mean. Group means were compared using parametric tests, such as t-test (for two groups) and one-way analysis of variance for more than two conditions. Statistical significance was set at P values of <0.05.
Study approval
The Institutional Animal Care and Use Committee at the University of Pittsburgh approved all experimental procedures performed on mice (Protocol number 16099101).
Results
Prominent cardiac expression of Hmox1 and Pgf in sickle cell mice
We observed differential expression of Hmox1 transcripts in untreated SS mice with highest expression level in the spleen, liver and kidney (P < 0.0001, Fig 1A). We hypothesized that uptake of haem from haemolysis induces this heterogeneous expression. To investigate this hypothesis, we increased circulating haem in SS mice with intravenous injection of haemin. Haem injection produced a sharp increase in Hmox1 gene expression in all organs analysed after 3 h. Hmox1 transcripts significantly increased, as expected, by about 20-fold in the kidney, 8-fold in the liver, 6-fold in the spleen, 5-fold in the lung and 4-fold in the BM after haemin injection compared to vehicle injected controls (Fig 1). Surprisingly, the heart showed 18-fold induction of Hmox1, implying an unexpected capacity of the heart to take up haem and induce its degradation pathway. The spleen showed little increase in Hmox1 expression following haemin injection, probably because its basal expression was already quite high in the spleens of the untreated SS mice (Fig 1A), presumably due to the high rate of splenic macrophage turnover of sickle erythrocytes. All the organs of AA strain control mice were similarly affected by haemin injections. A second surprise was that the haemin-induced liver Hmox1 transcript level was significantly lower in SS mice than in AA mice (Fig 1C). This result implies that SS mice may have an impaired reserve capacity for induction of Hmox1, a vital enzyme for detoxifying and clearing haem.
Fig 1.
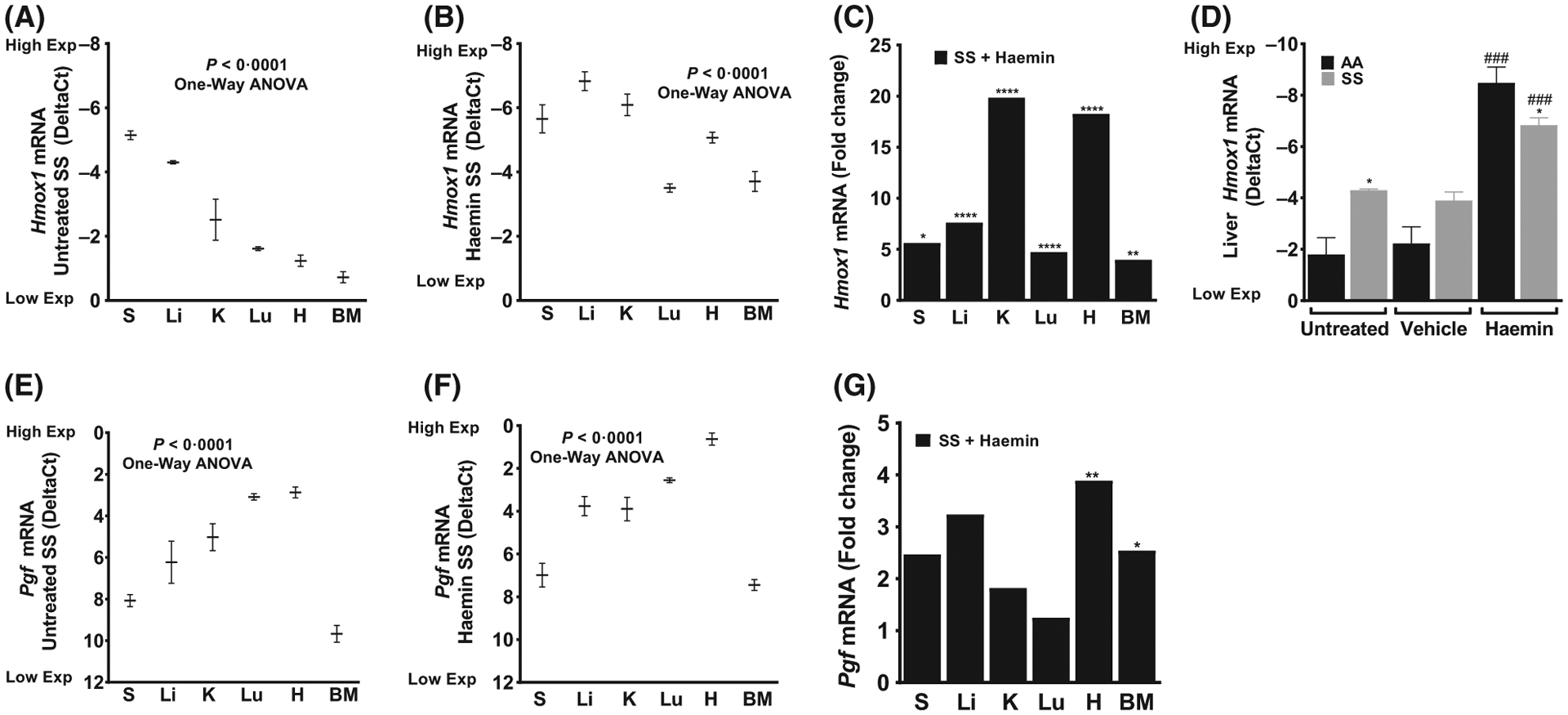
Heterogeneity in basal and haem-induced Hmox1 and Pgf expression in organs from sickle cell mice. (A) Endogenous Hmox1 expression in untreated SS mice (n = 3–5). Haem-induced Hmox1 expression in SS mice presented as (B) DeltaCt and (C) Relative fold change (n = 7–10). (D) Impaired Hmox1 expression in the liver of SS mice compared to AA mice (n = 3–10, *P < 0.05 *AA compared to SS and ###P < 0·001 #vehicle compared to haemin within strain). (E) Endogenous Pgf expression in SS mice (n = 3–5). Haemin induced Pgf expression presented as (F) DeltaCt and (G) Relative fold change (n = 7–10). For DeltaCt, lowest value = highest expression and highest value = lowest expression. Target gene transcripts were normalized to Gusb for all mRNA expression level. Gusb expression was similar in all mice strains used and in all of these organs in animals injected with either vehicle or haemin. For relative fold change, samples were further normalized to vehicle gene transcripts. One-Way ANOVA or Student’s T-test; All values are mean ± SEM, *compared to vehicle. *P < 0·05, **P < 0·01, ****P < 0·0001. ANOVA, analysis of variance; BM, bone marrow; Exp, Expression; H, heart; K, kidney; Li, liver; Lu, lung; S, spleen.
Previous work from our laboratory (Sundaram et al, 2010; Wang et al, 2014) and others (Heeschen et al, 2003; Perelman et al, 2003; Peiskerova et al, 2013; Matsui et al, 2015; Gu et al, 2018; Kelaidi et al, 2018) has shown a link between haem, elevated PGF and vasculopathy in diseases with chronic haemolytic anaemia, such as SCD and thalassaemia. Therefore, we evaluated the expression of Pgf in the organs of SS mice, and determined that there was heterogeneity (P < 0.0001, Fig 1E) in basal Pgf expression in these mice, surprisingly with the highest expression in the heart (DeltaCt 2.9 ± 0.3), lung (3.1 ± 0.2) and kidney (5.0 ± 0.6). With haemin injection, cardiac Pgf transcript showed a robust 4-fold further increase compared to vehicle-treated control mice (Fig 1F, G). Cardiac expressions of PGF and HMOX1 in SS mice are surprisingly high, and induced to even higher levels by circulating haem.
Strong cardiac inducibility of Hmox1 and Pgf in haemin-treated wild type mice
We hypothesized that the haemin treatment of mice would be sufficient to replicate the organ-specific variability in Hmox1 and Pgf expression seen in SS mice. Nrf2+/+ mice were injected intravenously with vehicle or a higher dose of haemin (120 μmol/kg) and organs were harvested 3 h later. Hmox1 mRNA levels were significantly increased (P < 0.0001, Fig 2A) in all organs from Nrf2+/+ mice that received haemin compared with vehicle controls. Most importantly, different organs demonstrated variable degrees of Hmox1 inducibility with an increase of 207-fold in the liver, 201-fold in the heart, 101-fold in the kidney, 69-fold in the lung and 7-fold in the spleen (Fig 2B). The strong inducibility of Hmox1 expression by haem in liver and kidney was expected, but comparable level of haem inducibility of Hmox1 in the heart is a novel observation. For Pgf expression, we observed the highest basal expression in the heart, followed by lung and kidney (P < 0.0001, Fig 2C). Following haem exposure, heart tissue had the highest elevation of Pgf expression (Fig 2C). Interestingly, haemin treatment of Nrf2+/+ mice largely recreated the organ-specific expression pattern of Pgf in untreated SS mice (Fig 1E compared to Fig 2C). This suggests that in sickle cell mice, haem exposure from haemolysis is a factor regulating organ-specific Pgf expression (Fig 2C). The high basal and haem-induced expression of Hmox1 and Pgf in the heart is remarkable.
Fig 2.
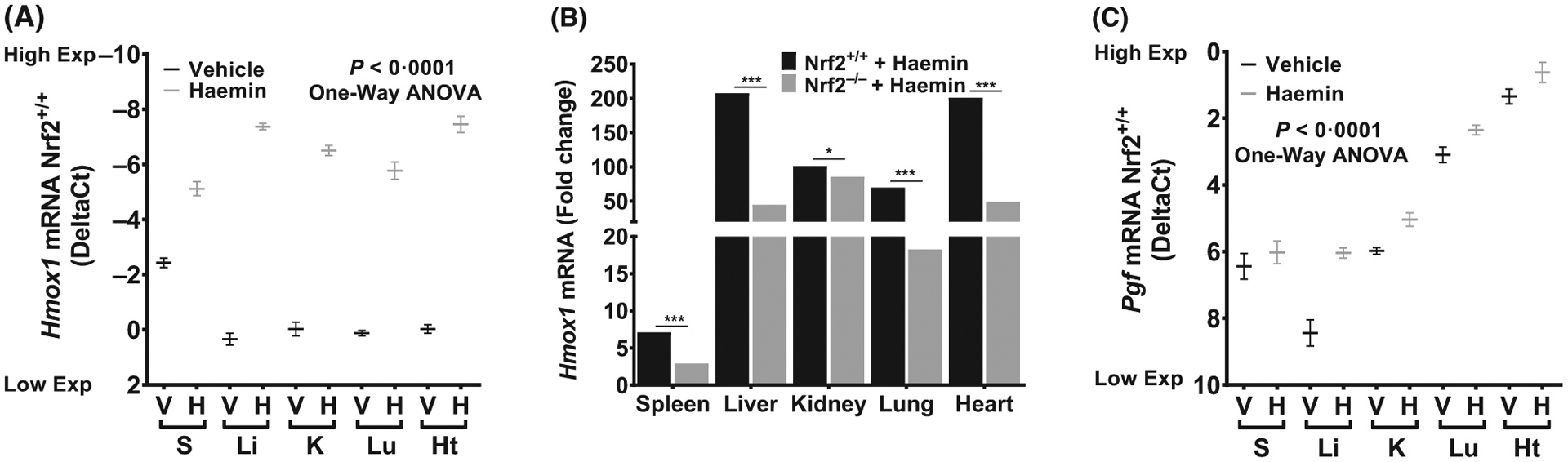
Organ-specific variability in Hmox1 inducibility is Nrf2 dependent. (A) Hmox1 expression in wild type mice injected with vehicle or haemin (DeltaCt, n = 8–11, P < 0.0001 vehicle vs haemin in all organs shown). (B) Hmox1 expression is Nrf2 dependent in wild type mice injected with haemin (n = 6–11). (C) Haemin induced Pgf expression in wild type mice mimics the pattern seen in sickle cell mice (n = 8–11, P < 0.05 vehicle vs haemin in all organs shown except spleen). For DeltaCt, lowest value = highest expression and highest value = lowest expression. Target gene transcripts were normalized to Gusb for all mRNA expression level. Gusb expression was similar in all mice strains used and in all of these organs in animals injected with either vehicle or haemin. For relative fold change, samples were further normalized to vehicle control gene transcripts. One-Way ANOVA or Student’s t-test; All values are mean ±SEM, *compared to vehicle. *P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001. ANOVA, analysis of variance; BM, bone marrow; Exp, Expression; H, haemin; Ht, heart; K, kidney; Li, liver; Lu, lung; S, spleen; V, vehicle.
Organ-specific haem induction of Hmox1 is largely Nrf2 dependent
The Nrf2 gene regulates the basal and inducible expression of Hmox1 and to understand the mechanisms and molecular pathways involved in the haemin-induced organ-specific Hmox1 expression, we utilized Nrf2 null mice. Similar to results from other strains described above, we observed organ-specific heterogeneity in haemin-induced Hmox1 expression in Nrf2−/− mice (Fig 2B), with the kidney (86-fold), heart (49-fold) and liver (44-fold) showing relatively higher induction of Hmox1. Most importantly, haemin induction of Hmox1 was largely Nrf2-dependent, with 59–78% lower response in Nrf2−/− mice in every organ except kidney. The 101-fold haem-induction of Hmox1 in the kidney was essentially Nrf2-independent (Fig 2B). Interestingly, Pgf expression in the organs of Nrf2−/− mice (Figure S2) were similar to those of Nrf2+/+ mice (Fig 2C), with the basal expression of Pgf highest in the heart (2.15 DeltaCt), followed by lung and kidney (Figure S2). As observed in Nrf2+/+ mice, heart and lung had the highest expression of Pgf post-haemin exposure in Nrf2−/− mice. Haemin injections resulted in significant Pgf induction in both Nrf2+/+ and Nrf2−/− mice (Figure S3) with no significant change between the two strains, suggesting that haem-induced Pgf expression in the organs examined was Nrf2 independent.
HMOX1 and PGF proteins are highly inducible by haem and Nrf2-dependent
We confirmed at the protein level the strong cardiac inducibility of HMOX1 and PGF expression in the heart tissue lysates 3 h after haem injection. We further analysed HMOX1 protein as a percentage of total heart protein in both vehicle and haemin treated animals. In agreement with our mRNA measurements above, HMOX1 protein increased by approximately 49% (P < 0.05), 57% (P < 0.001), and 33% (P < 0.05) in SS, Nrf2+/+ and Nrf2−/− mice respectively (Fig 3A). HMOX1 protein expression in the heart lysates was Nrf2-dependent in both vehicle-treated (P < 0.001) and haemin-injected mice (P < 0.05, Fig 3A), also supporting the mRNA measurements described above. In the same heart lysates, PGF protein as a percentage of total heart protein increased by about 22% (P < 0.01), 29% (P < 0.05), and 18% (P < 0.01) in SS, Nrf2+/+ and Nrf2−/− mice respectively (Fig 3B). However, the percentage of cardiac PGF was not significantly different between Nrf2+/+ and Nrf2−/− mice (Fig 3B).
Fig 3.
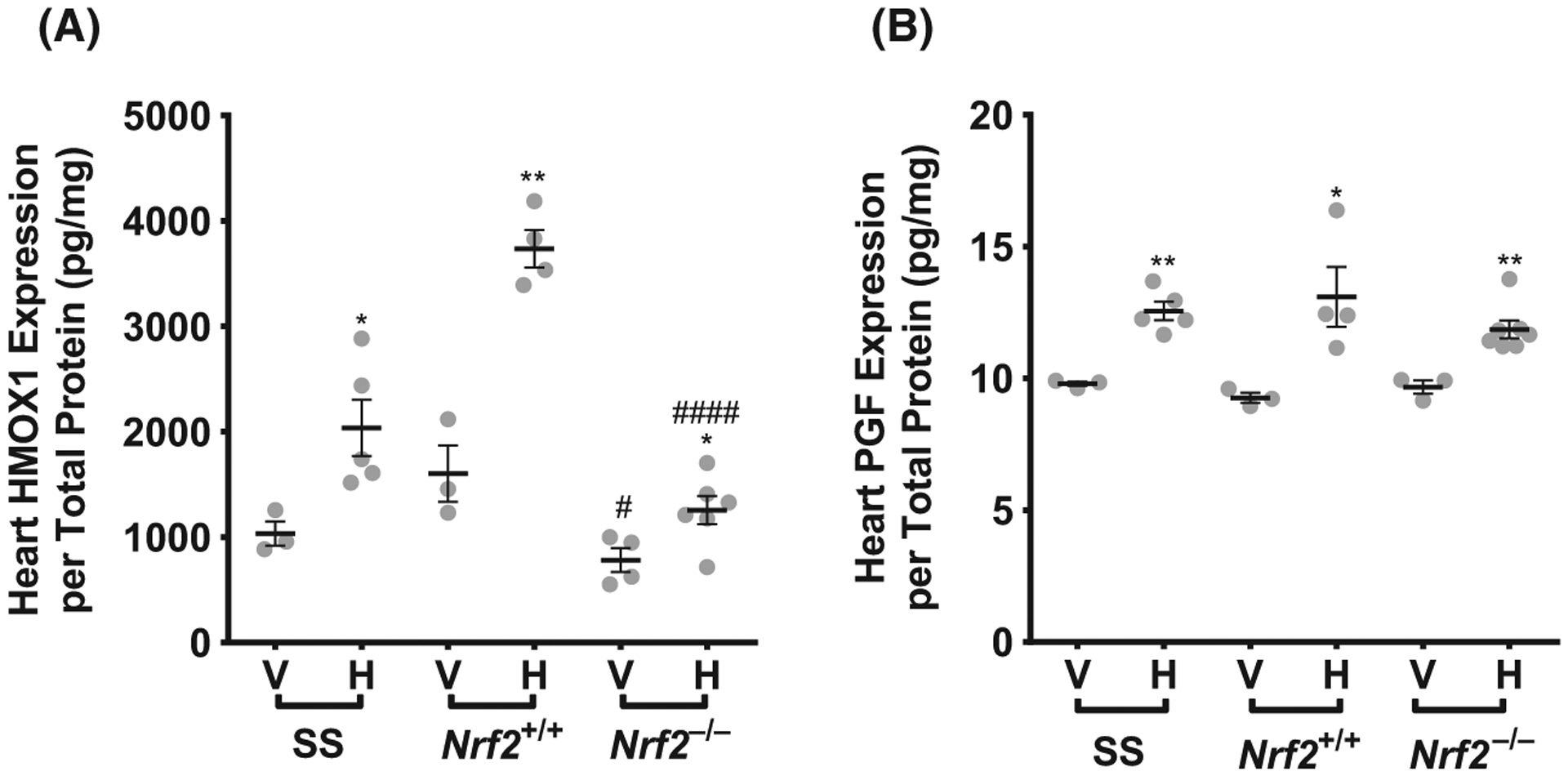
HMOX1 and PGF proteins are highly inducible by haem and Nrf2-dependent. (A) Vehicle-treated and haemin induced HMOX1 protein in the heart as a percentage of total protein concentration (n = 3–7). (B) Vehicle-treated and haemin induced PGF and protein in the heart as a percentage of total protein concentration (n = 3–7). Student’s t test, *Vehicle-treated compared to haemin-treated within strain, # Vehicle/haemin-treated Nrf2+/+ compared to Nrf2−/−. All values are mean ± SEM. *P < 0·05, **P < 0·01, ***P < 0·001, #P < 0·05, ####P < 0001. H, haemin; V, vehicle.
Haem levels in different organs after haem injection correlates with Hmox1 induction
Our previous results consistently demonstrated a heterogeneous Hmox1 response to haemin among different organs that were examined, suggesting variability in haem degradation or haem distribution. To investigate this further, we measured intracellular haem in Nrf2+/+ and Nrf2−/− mice that received vehicle or haemin. The organs were perfused to remove circulating red blood cells before processing for haem quantification. There was variability in haem biodistribution 3 h after intravenous injection of haemin (Fig 4A). Our results indicate that haem levels at 3 h remained significantly increased by about 67% (P < 0.05) in the spleen and 53% (P < 0.05) in the liver of Nrf2+/+ animals while the other organs were largely unaffected. Showing a remarkable degree of correlation, the organs with the highest haemin induction of Hmox1 mRNA showed the lowest levels of intracellular haem, suggesting a more rapid clearance of haem by enhanced Hmox1 expression in the heart, liver, kidney and lung (Pearson R2 = 0.82, P = 0.03, Fig 4B).
Fig 4.
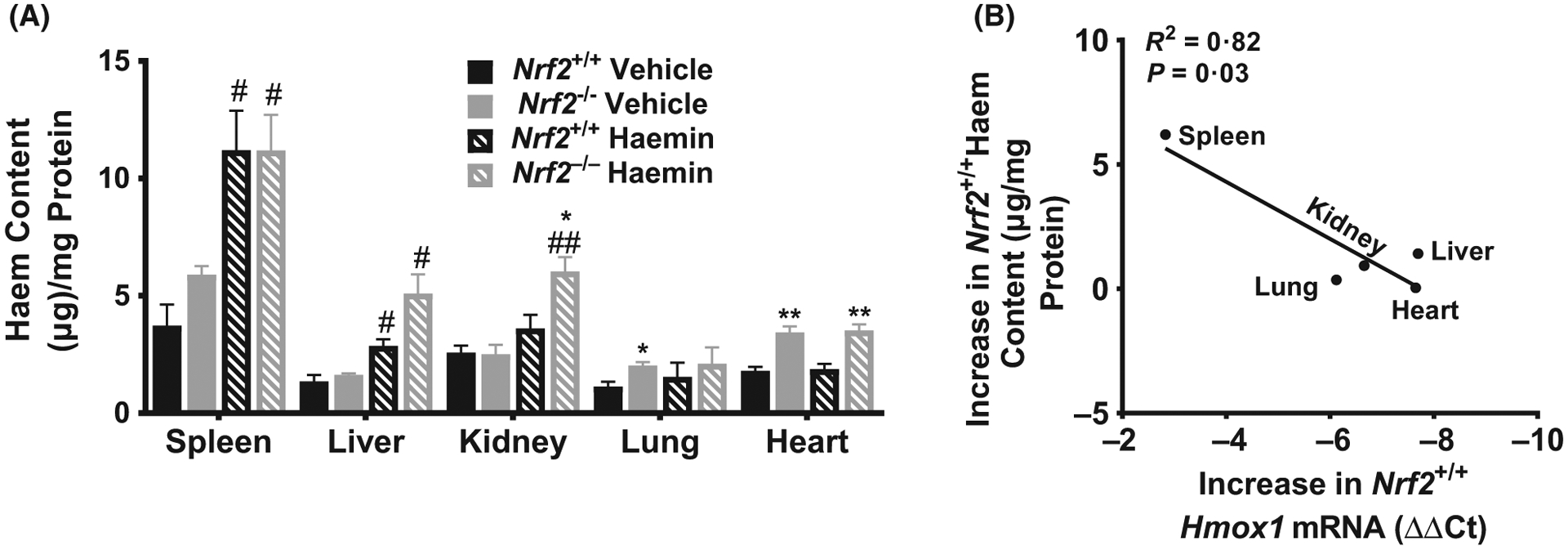
Biodistribution of intracellular haem content in mice. (A) Haem content was quantified as a ratio of total protein from tissue lysate in organs from Nrf2+/+ and Nrf2−/− mice 3 h after haemin injection (n = 3–6). (B) Change in haem content is correlated with haemin-induced Hmox1 expression in wild type mice (n = 3–11). For DeltaCt, lowest value = highest expression and highest value = lowest expression. Target gene transcripts were normalized to Gusb for all mRNA expression level. Gusb expression was similar in all mice strains used and in all of these organs in animals injected with either vehicle or haemin. All values are mean ± SEM. Student’s T-test, *Nrf2+/+ compared to Nrf2−/− within same treatment, # Haemin compared to Vehicle within strain. #P < 0·05, ##P < 0·01, *P < 0·05, **P < 0·01.
Nrf2 deficiency altered the haem distribution in haemin-injected mice. In haemin-injected Nrf2−/− animals, organ-specific haem levels were significantly higher by about 47% (P < 0.05) in the spleen, 58% (P < 0.01) in the kidney and 68% (P < 0.05) in the liver compared to haemin-injected Nrf2+/+ mice. Interestingly, vehicle-treated Nrf2−/− mice showed mild but statistically significant haem accumulation in the heart (P < 0.01) and lung (P < 0.05) compared to Nrf2+/+ mice, presumably due to basal state effects of Nrf2 deficiency on chronic metabolism of endogenous haem. After haemin injection, the Nrf2−/− mice accumulated significantly higher levels of haem in the heart (P < 0.01) and kidney (P < 0.05) compared to haemin-injected Nrf2+/+ mice. This may be due to impaired inducible haem clearance in the heart and kidney of the Nrf2-deficient mice compared to Nrf2+/+ mice (Fig 2B). The liver and lung haem concentrations were not significantly different between the Nrf2−/− and Nrf2+/+ mice.
Haem clearance is associated with haem transporter expression
Our results above suggested that extracellular haem induced the expression of Hmox1 and Pgf genes in an organ-specific manner. Therefore, we hypothesized that differences in haem trafficking might contribute to this phenotype. Because Hrg1 functions as a haem transporter (Rajagopal et al, 2008; White et al, 2013), we evaluated Hrg1 mRNA expression in these organs. Haemin injection significantly induces the expression of Hrg1 gene in most organs (P < 0.05), which was strongly dependent on Nrf2 in most organs (Fig 5A). Depending on the organ, Hrg1 induction by haemin was between 54% and 75% lower in Nrf2−/− mice. Compared to AA controls, SS mice showed significantly higher (P < 0.05) haemin induction of Hrg1 in the spleen (~3-fold), liver (~3-fold) and kidney (~2-fold) (Fig 5B). Hrg1 induction closely paralleled the same organ-specific induction pattern as Hmox1 in relation to haem content, probably reflecting similar regulatory mechanisms (R2 = 0.82, P = 0.004, Fig 5C).
Fig 5.
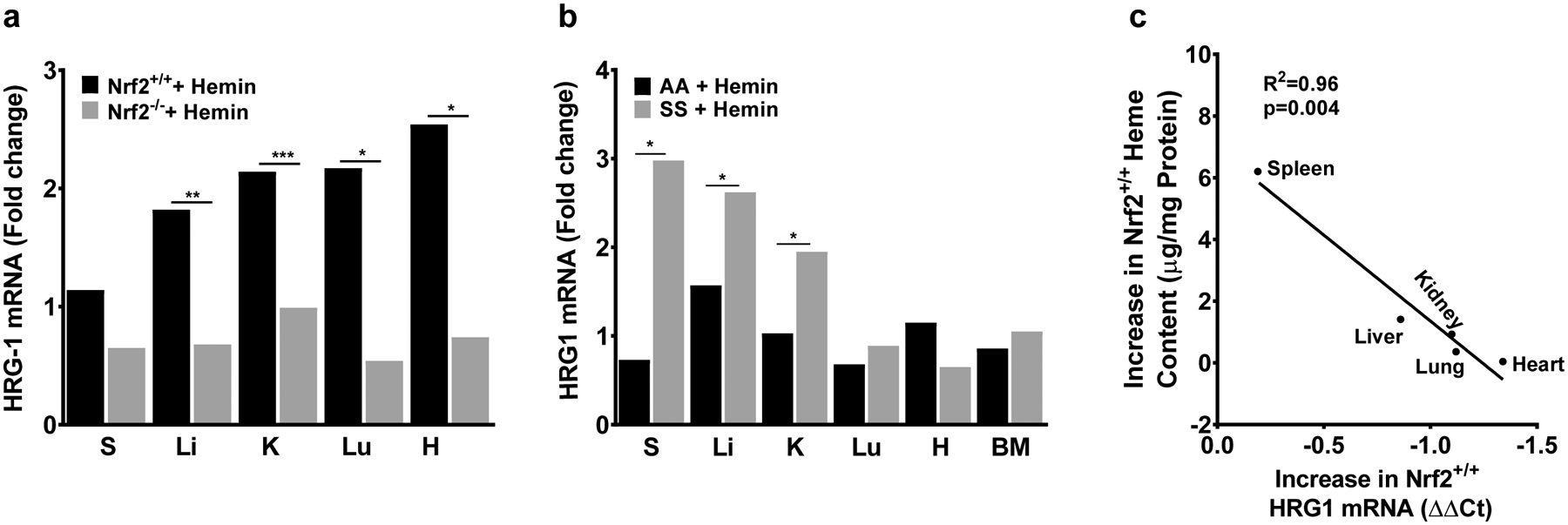
Haem clearance is positively associated with Hmox1 induction and haem trafficking. (A) Nrf2-dependent, organ specific induction of haem importer Hrg1 (n = 4). (B) Haem importer Hrg1 expression is more highly induced by haem in liver, kidney and spleen of sickle cell mice (n = 3–10). (C) Change in haem content is correlated with haemin-induced Hrg1 expression in wild type mice (n = 3–4). For DeltaCt, lowest value = highest expression and highest value = lowest expression. Target gene transcripts were normalized to Gusb for all mRNA expression level. Gusb expression was similar in all mice strains used and in all of these organs in animals injected with either vehicle or haemin. For relative fold change, samples were further normalized to vehicle control gene transcripts. R2 was calculated with Pearson correlation. All values are mean ±SEM. *P < 0·05, **P < 0·01, ***P < 0.001. BM, bone marrow; H, heart; K, kidney; Li, liver; Lu, lung; S, spleen.
Discussion
Sickle cell disease is no longer the ‘disease of childhood’ as described in the 1960’s (Maglione et al, 1993). Recent studies have shown that over 90% of the patients live to adulthood, especially in developed countries (Maglione et al, 1993; Platt et al, 1994). The improvement in survival is due to several interventions, such as new-born screening, use of penicillin prophylaxis and pneumococcal vaccination, hydroxycarbamide therapy and advancement in research and translational studies (Platt et al, 1994; Nouraie et al, 2013). These advances in medicine have contributed to better prognosis and quality of care for patients with SCD compared to five decades ago. Despite this progress in medicine and treatment of SCD patients, mortality in adult patients remains high (Platt et al, 1994). Organ failure due to accumulated injury from persistent haemolysis (Gardner et al, 2016) and sickling remains one of the major causes of death in patients with SCD (Vichinsky, 2017). The current study assessed organ-specific haem induction of Pgf and Hmox1, as a way to identify the most at-risk organs in SCD during elevation of circulating extracellular haem. The SCD pattern of Hmox1 and Pgf expression is largely replicated by haem injection, mimicking acute haemolysis.
HMOX1 is a cytoprotective enzyme that metabolizes haem. Its deletion in mice amplified the severity of cerebral (Paulukonis et al, 2016) and noncerebral malaria (Seixas et al, 2009), ischaemia-reperfusion injury (Maitra et al, 2017), inflammation and vascular injury in different organs in SCD (Belcher et al, 2006; Belcher et al, 2010). Our data support and extend the prior work of Belcher et al. (2006, 2010), which showed Hmox1 expression and haem-inducibility in some organs of transgenic sickle mice. We have characterized the expression of Hmox1 in organs from naïve and haemin-treated Townes sickle mice. We observed wide heterogeneity in the induction of transcript levels of Hmox1 in different organs, but, more importantly, we noticed a significant but very distinct haemin induction pattern depending on the organ. Spleen and liver showed the highest expression of Hmox1 as expected, as these are key organs in the reticuloendothelial system, involved in turnover of old and senescent red blood cells (RBCs). Exposure to extracellular haem further increased the transcript level of Hmox1 in these organs, with an unexpected strongest induction in the kidney and the heart of SS mice, which had lower baseline expression. The role of Hmox1 in the heart is controversial. Cardioprotective roles of Hmox1 in the heart includes reduction in oxidative stress (van Berlo et al, 2013), improved mitochondrial function (Hull et al, 2016), prevention of vascular remodelling (Mito et al, 2008), inhibition of apoptosis (Wang et al, 2010; Vichinsky, 2017) and prevention of atherosclerosis. Increased Hmox1 expression and activity is also linked to diabetes-induced oxidative stress in the heart via accumulation of iron (Farhangkhoee et al, 2003). Hmox1 expression in the heart of SS mice comprises part of the previously reported atheroprotective effect of SCD in SS/apolipoprotein E-deficient mice (Wang et al, 2013).
Interestingly, our result also suggests a previously unreported impaired inducible reserve capacity of Hmox1 induction in the liver (Fig 1C). Our finding is consistent with the published effectiveness of augmented Hmox1 expression as a protective therapy through gene transfer (Belcher et al, 2010) or through Nrf2 activators (Ghosh et al, 2016; Ghosh et al, 2018) in sickle cell mice. In agreement with the current literature (Boyle et al, 2011; Loboda et al, 2016), our data offers novel insights regarding the organ-specific Hmox1 expression in vivo. Although Nrf2 depletion ubiquitously blunted haemin-induced Hmox1 levels, the magnitude of the Hmox1 reduction varied in the different organs, suggesting the presence of additional unknown mechanisms that control Hmox1 expression. These mechanisms deserve future investigation.
Similar to Hmox1, we have investigated Pgf haemin-induction in organs of SS, Nrf2+/+ and Nrf2−/− mice. For the first time, we describe high-level expression of Pgf in the heart of sickle cell mice. Pgf is expressed in several organs including placenta, erythroid cells, heart and skeletal muscle (Pamplona et al, 2007). We observed the highest Pgf expression in the heart, followed by the lung and kidney in organs isolated from na€ıve adult SS mice. On exposure to extracellular haem in the circulation, we found a surprisingly high increase in Pgf transcript and total protein levels in the heart. Similarly, Pgf heart levels in haemin injected Nrf2+/+ animals rose higher than all other organs. Elevated circulating level of PGF protein is associated with worsening cardiac outcomes in patients with chronic kidney disease (Heeschen et al, 2003; Matsui et al, 2015), atherosclerosis (Cao et al, 1997) and asthma (Maglione et al, 1993). We have previously reported the association between Pgf, inflammation, pulmonary hypertension (PH) and high mortality in patients with SCD (Perelman et al, 2003; Sundaram et al, 2010). This present result, showing the highest basal expression of Pgf in the heart followed by the lung in SS and Nrf2+/+ mice, is unexpected and in contrast to previous reports of low expression levels in these organs (Alam et al, 2003; Liu et al, 2005; Pamplona et al, 2007). This apparent inconsistency might be due to differences in mouse strains, although our own results were consistent across four different strains of mice. Additionally, our group and others have shown that erythroid hyperplasia, hypoxia and erythropoietin in SCD contribute to elevated circulating PGF levels in both humans and mice with SCD (Tordjman et al, 2001; Perelman et al, 2003; Sundaram et al, 2010; Wang et al, 2014). High expression of Pgf in the heart is associated with angiogenesis and ischaemia (Maglione et al, 1993), though its role in cardiovascular disorders is somewhat controversial. Kolakowski et al., (2006) reported that direct intramyocardial injection of PGF following a large myocardial infarction attenuated adverse ventricular remodelling and improved myocardial function. On the other hand, elevated serum PGF is an independent risk factor for mortality (Matsui et al, 2015) and left ventricular diastolic dysfunction in patients with chronic kidney disease (Peiskerova et al, 2013). PGF contributes to cardiac hypertrophy (Dewerchin & Carmeliet, 2012, Eiymo Mwa Mpollo et al, 2016, Roncal et al, 2010), through intermediate paracrine growth factors such as interleukin 6 (Accornero et al, 2011; Dewerchin & Carmeliet, 2012), which is known to be elevated in the serum of SCD patients (Taylor et al, 1995; Qari et al, 2012; Sarray et al, 2015).
The third gene that we evaluated was the haem transporter Hrg1, which is known to facilitate haem export from the phagolysosome into the cytosol during RBC recycling in macrophages (Rajagopal et al, 2008; White et al, 2013). Our results describe, for the first time, how Hrg1 is regulated in vivo by the addition of an extracellular haem analogue with or without Nrf2. Similar to Hmox1 and Pgf, HRG1 expression in Nrf2+/+ mice was stimulated by haemin injections in an organ specific manner. Furthermore, the absence of Nrf2 in the Nrf2 null animals eradicated the haemin effect in all organs in agreement with a previous publication reporting the role of NRF2 in HRG1 regulation (Alam et al, 2003). Interestingly, Hrg1 induction by haemin strongly correlates with intracellular haem content in haemin-injected mice. Moreover, intracellular haem content strongly correlates with Hmox1 expression in the same mice. The relationship of haem transport to haem clearance requires further investigation.
It is interesting that haemin injection in wild-type mice largely recreates the profile of Hmox1 and Pgf expression by organ seen in untreated SS mice. This suggests that sickling, vaso-occlusion or anaemia is not specifically required for this Hmox1 and Pgf pattern. Further stimulation of the SS mice with additional haem simply amplifies this pattern, but retains the dominance of liver and heart expression of Hmox1 and Pgf. Although the change in expression of haem-regulated genes is not surprising in liver and spleen, organs involved in haem clearance, we report previously underappreciated Hmox1 basal and inducible expression in the heart.
We found prominent organ-specific Hmox1 and Pgf expression patterns, which could promote variable protective and maladaptive responses among these organs. A recent investigation of blockade of PGF suggested beneficial effects in SS mice (Gu et al, 2018). The remarkable regulation of Hmox1, Pgf and Hrg1 in the heart by haem offers a particular new avenue for investigation.
Supplementary Material
Fig S1. Similarity in GUSB expression in vehicle and haem-injected mice.
Fig S2. Basal PlGF expression is highly variable in Nrf2−/− mice.
Fig S3. Haem-induced PlGF expression in solid organs is not Nrf2 dependent.
Table SI. Rt-qPCR Taqman probes.
Acknowledgements
We thank Diane Lenhart, Bethany Flage and Danielle Crosby for technical assistance.
Funding
Dr. Kato received support from NIH grants HL133864, MD009162 and from the Institute for Transfusion Medicine Hemostasis and Vascular Biology Research Institute at the University of Pittsburgh School of Medicine. Dr. Ofori-Acquah is supported by NIH grants R01HL106192, U01HL117721 and U54HL141011. Dr. Bullock is supported by the University of Pittsburgh Department of Pathology.
Footnotes
Conflicts of interest
The authors declare no competing financial interest in relation to the study.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- Accornero F, van Berlo JH, Benard MJ, Lorenz JN, Carmeliet P & Molkentin JD (2011) Placental growth factor regulates cardiac adaptation and hypertrophy through a paracrine mechanism. Circulation Research, 109, 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam J, Killeen E, Gong P, Naquin R, Hu B, Stewart D, Ingelfinger JR & Nath KA (2003) Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2. American Journal of Physiology. Renal Physiology, 284, F743–752. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP & Vercellotti GM (2006) Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. Journal of Clinical Investigation, 116, 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher JD, Vineyard JV, Bruzzone CM, Chen C, Beckman JD, Nguyen J, Steer CJ & Vercellotti GM (2010) Heme oxygenase-1 gene delivery by Sleeping Beauty inhibits vascular stasis in a murine model of sickle cell disease. Journal of Molecular Medicine (Berlin), 88, 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP & Vercellotti GM (2014) Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood, 123, 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Maillet M & Molkentin JD (2013) Signaling effectors underlying pathologic growth and remodeling of the heart. Journal of Clinical Investigation, 123, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JJ, Johns M, Lo J, Chiodini A, Ambrose N, Evans PC, Mason JC & Haskard DO (2011) Heme induces heme oxygenase 1 via Nrf2: role in the homeostatic macrophage response to intraplaque hemorrhage. Arteriosclerosis, Thrombosis, and Vascular Biology, 31, 2685–2691. [DOI] [PubMed] [Google Scholar]
- Cao Y, Ji WR, Qi P, Rosin A & Cao Y (1997) Placenta growth factor: identification and characterization of a novel isoform generated by RNA alternative splicing. Biochemical and Biophysical Research Communications, 235, 493–498. [DOI] [PubMed] [Google Scholar]
- Crocker P, Werb Z, Gordon S & Bainton D (1990) Ultrastructural localization of a macrophage-restricted sialic acid binding hemagglutinin, SER, in macrophage-hematopoietic cell clusters. Blood, 76, 1131–1138. [PubMed] [Google Scholar]
- Dewerchin M & Carmeliet P (2012) Pgf: a multitasking cytokine with disease-restricted activity. Cold Spring Harbor Perspectives in Medicine, 2, abstract a011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhangkhoee H, Khan ZA, Mukherjee S, Cukiernik M, Barbin YP, Karmazyn M & Chakrabarti S (2003) Heme oxygenase in diabetes-induced oxidative stress in the heart. Journal of Molecular and Cellular Cardiology, 35, 1439–1448. [DOI] [PubMed] [Google Scholar]
- Gardner K, Douiri A, Drasar E, Allman M, Mwirigi A, Awogbade M & Thein SL (2016) Survival in adults with sickle cell disease in a high-income setting. Blood, 128, 1436–1438. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Adisa OA, Chappa P, Tan F, Jackson KA, Archer DR & Ofori-Acquah SF (2013) Extracellular haemin crisis triggers acute chest syndrome in sickle mice. Journal of Clinical Investigation, 123, 4809–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Ihunnah CA, Hazra R, Walker AL, Hansen JM, Archer DR, Owusu-Ansah AT & Ofori-Acquah SF (2016) Nonhematopoietic Nrf2 dominantly impedes adult progression of sickle cell anemia in mice. JCI. Insight, 1. 10.1172/jci.insight.81090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hazra R, Ihunnah CA, Weidert F, Flage B & Ofori-Acquah SF (2018) Augmented NRF2 activation protects adult sickle mice from lethal acute chest syndrome. British Journal of Haematology, 182, 271–275. [DOI] [PubMed] [Google Scholar]
- Gladwin MT & Ofori-Acquah SF (2014) Erythroid DAMPs drive inflammation in SCD. Blood, 123, 3689–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JM, Yuan S, Sim D, Abe K, Liu P, Rosenbruch M, Bringmann P & Kauser K (2018) Blockade of placental growth factor reduces vaso-occlusive complications in murine models of sickle cell disease. Experimental Hematology, 60, 73–82.e73. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Dimmeler S, Hamm CW, Boersma E, Zeiher AM & Simoons ML (2003) Prognostic significance of angiogenic growth factor serum levels in patients with acute coronary syndromes. Circulation, 107, 524–530. [DOI] [PubMed] [Google Scholar]
- Huang E, Parke C, Mehrnia A, Kamgar M, Pham PT, Danovitch G & Bunnapradist S (2013) Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrology, Dialysis, Transplantation, 28, 1039–1046. [DOI] [PubMed] [Google Scholar]
- Hull TD, Boddu R, Guo L, Tisher CC, Tray-lor AM, Patel B, Joseph R, Prabhu SD, Suliman HB, Piantadosi CA, Agarwal A & George JF (2016) Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI Insight, 1, e85817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingoglia G, Sag CM, Rex N, De Franceschi L, Vinchi F, Cimino J, Petrillo S, Wagner S, Kreitmeier K, Silengo L, Altruda F, Maier LS, Hirsch E, Ghigo A & Tolosano E (2017) Hemopexin counteracts systolic dysfunction induced by heme-driven oxidative stress. Free Radical Biology and Medicine, 108, 452–464. [DOI] [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT & Steinberg MH (2007) Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Reviews, 21, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Steinberg MH & Gladwin MT (2017) Intravascular hemolysis and the pathophysiology of sickle cell disease. Journal of Clinical Investigation, 127, 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelaidi C, Kattamis A, Apostolakou F, Poziopoulos C, Lazaropoulou C, Delaporta P, Kanavaki I & Papassotiriou I (2018) Pgf and sFlt-1 levels in patients with non-transfusion-dependent thalassemia: correlations with markers of iron burden and endothelial dysfunction. European Journal of Haematology, 100, 630–635. [DOI] [PubMed] [Google Scholar]
- Kerins MJ & Ooi A (2017) The roles of NRF2 in modulating cellular iron homeostasis. Antioxidants & Redox Signaling, 29, 1756–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakowski S Jr, Berry MF, Atluri P, Grand T, Fisher O, Moise MA, Cohen J, Hsu V & Woo YJ (2006) Placental growth factor provides a novel local angiogenic therapy for ischemic cardiomyopathy. Journal of Cardiac Surgery, 21, 559–564. [DOI] [PubMed] [Google Scholar]
- Liu X, Wei J, Peng DH, Layne MD & Yet SF (2005) Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes, 54, 778–784. [DOI] [PubMed] [Google Scholar]
- Loboda A, Damulewicz M, Pyza E, Jozkowicz A & Dulak J (2016) Role of Nrf2/HMOX-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellular and Molecular Life Sciences, 73, 3221–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione D, Guerriero V, Viglietto G, Ferraro MG, Aprelikova O, Alitalo K, Del Vecchio S, Lei KJ, Chou JY & Persico MG (1993) Two alternative mRNAs coding for the angiogenic factor, placenta growth factor (Pgf), are transcribed from a single gene of chromosome 14. Oncogene, 8, 925–931. [PubMed] [Google Scholar]
- Maitra P, Caughey M, Robinson L, Desai PC, Jones S, Nouraie M, Gladwin MT, Hinder-liter A, Cai J & Ataga KI (2017) Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica, 102, 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Uemura S, Takeda Y, Samejima K, Matsumoto T, Hasegawa A, Tsushima H, Hoshino E, Ueda T, Morimoto K, Okamoto K, Okada S, Onoue K, Okayama S, Kawata H, Kawakami R, Maruyama N, Akai Y, Iwano M, Shiiki H & Saito Y (2015) Placental growth factor as a predictor of cardiovascular events in patients with CKD from the NARACKD study. Journal of the American Society of Nephrology, 26, 2871–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito S, Ozono R, Oshima T, Yano Y, Watari Y, Yamamoto Y, Brydun A, Igarashi K & Yoshizumi M (2008) Myocardial protection against pressure overload in mice lacking Bach1, a transcriptional repressor of heme oxygenase-1. Hypertension, 51, 1570–1577. [DOI] [PubMed] [Google Scholar]
- Motohashi H & Yamamoto M (2004) Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends in Molecular Medicine, 10, 549–557. [DOI] [PubMed] [Google Scholar]
- Eiymo Mwa Mpollo MS, Brandt EB, Shanmukhappa SK, Arumugam PI, Tiwari S, Loberg A, Pillis D, Rizvi T, Lindsey M, Jonck B, Carmeliet P, Kalra VK, LeCras TD, Ratner N, Wills-Karp M, Hershey GK & Malik P (2016) Placenta growth factor augments airway hyperresponsiveness via leukotrienes and IL-13. Journal of Clinical Investigation, 126, 571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouraie M, Lee JS, Zhang Y, Kanias T, Zhao X, Xiong Z, Oriss TB, Zeng Q, Kato GJ, Gibbs JS, Hildesheim ME, Sachdev V, Barst RJ, Machado RF, Hassell KL, Little JA, Schraufnagel DE, Krishnamurti L, Novelli E, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Castro OL, Goldsmith JC, Gordeuk VR & Gladwin MT (2013) The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica, 98, 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K & Bach FH (2003) Heme oxygenase-1: unleashing the protective properties of heme. Trends in Immunology, 24, 449–455. [DOI] [PubMed] [Google Scholar]
- Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha-Rodrigues M, Portugal S, Soares MP & Mota MM (2007) Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nature Medicine, 13, 703–710. [DOI] [PubMed] [Google Scholar]
- Paulukonis ST, Eckman JR, Snyder AB, Hagar W, Feuchtbaum LB, Zhou M, Grant AM & Hulihan MM (2016) Defining sickle cell disease mortality using a population-based surveillance system, 2004 through 2008. Public Health Reports, 131, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiskerova M, Kalousova M, Danzig V, Mikova B, Hodkova M, Nemecek E, Bani-Hani A, Ambroz D, Benakova H, Linhart A, Zima T & Tesar V (2013) Placental growth factor may predict increased left ventricular mass index in patients with mild to moderate chronic kidney disease–a prospective observational study. BMC Nephrology, 14, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelman N, Selvaraj SK, Batra S, Luck LR, Erdreich-Epstein A, Coates TD, Kalra VK & Malik P (2003) Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood, 102, 1506–1514. [DOI] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH & Klug PP (1994) Mortality in sickle cell disease. Life expectancy and risk factors for early death. New England Journal of Medicine, 330, 1639–1644. [DOI] [PubMed] [Google Scholar]
- Qari MH, Dier U & Mousa SA (2012) Biomarkers of inflammation, growth factor, and coagulation activation in patients with sickle cell disease. Clinical and Applied Thrombosis/Hemostasis, 18, 195–200. [DOI] [PubMed] [Google Scholar]
- Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, Krause M & Hamza I (2008) Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature, 453, 1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncal C, Buysschaert I, Gerdes N, Georgiadou M, Ovchinnikova O, Fischer C, Stassen JM, Moons L, Collen D, De Bock K, Hansson GK & Carmeliet P (2010) Short-term delivery of anti-Pgf antibody delays progression of atherosclerotic plaques to vulnerable lesions. Cardiovascular Research, 86, 29–36. [DOI] [PubMed] [Google Scholar]
- Sarray S, Saleh LR, Lisa Saldanha F, Al-Habboubi HH, Mahdi N & Almawi WY (2015) Serum IL-6, IL-10, and TNFalpha levels in pediatric sickle cell disease patients during vasoocclusive crisis and steady state condition. Cytokine, 72, 43–47. [DOI] [PubMed] [Google Scholar]
- Seixas E, Gozzelino R, Chora A, Ferreira A, Silva G, Larsen R, Rebelo S, Penido C, Smith NR, Coutinho A & Soares MP (2009) Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proceedings of the National Academy of Sciences, USA, 106, 15837–15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram N, Tailor A, Mendelsohn L, Wansapura J, Wang X, Higashimoto T, Pauciulo MW, Gottliebson W, Kalra VK, Nichols WC, Kato GJ & Malik P (2010) High levels of placenta growth factor in sickle cell disease promote pulmonary hypertension. Blood, 116, 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SC, Shacks SJ, Mitchell RA & Banks A (1995) Serum interleukin-6 levels in the steady state of sickle cell disease. Journal of Interferon and Cytokine Research, 15, 1061–1064. [DOI] [PubMed] [Google Scholar]
- Tordjman R, Delaire S, Plouët J, Ting S, Gau-lard P, Fichelson S, Roméo P & Lemarchandel V (2001) Erythroblasts are a source of angiogenic factors. Blood, 97, 1968–1974. [DOI] [PubMed] [Google Scholar]
- Vercellotti GM, Zhang P, Nguyen J, Abdulla F, Chen C, Nguyen P, Nowotny C, Steer CJ, Smith A & Belcher JD (2016) Hepatic overexpression of hemopexin inhibits inflammation and vascular stasis in murine models of sickle cell disease. Molecular medicine (Cambridge, Mass)., 22, 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichinsky E (2017) Chronic organ failure in adult sickle cell disease. Hematology Am Soc Hematol Educ Program, 2017, 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener FA, Eggert A, Boerman OC, Oyen WJ, Verhofstad A, Abraham NG, Adema G, van Kooyk Y, de Witte T & Figdor CG (2001) Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood, 98, 1802–1811. [DOI] [PubMed] [Google Scholar]
- Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ & Figdor CG (2003) Different faces of the heme-heme oxygenase system in inflammation. Pharmacological Reviews, 55, 551–571. [DOI] [PubMed] [Google Scholar]
- Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R & Prabhu SD (2010) Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation, 121, 1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Luo W, Wang J, Guo C, Wolffe SL, Wang J, Sun EB, Bradley KN, Campbell AD & Eitzman DT (2013) Paradoxical protection from atherosclerosis and thrombosis in a mouse model of sickle cell disease. British Journal of Haematology, 162, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mendelsohn L, Rogers H, Leitman S, Raghavachari N, Yang Y, Yau Y, Tallack M, Perkins A, Taylor J, Noguchi C & Kato G (2014) Heme-bound iron activates placenta growth factor in erythroid cells via erythroid Krüppel-like factor. Blood, 124, 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Yuan X, Schmidt PJ, Bresciani E, Samuel TK, Campagna D, Hall C, Bishop K, Calicchio ML, Lapierre A, Ward DM, Liu P, Fleming MD & Hamza I (2013) HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metabolism, 17, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Xi C, Thomas B & Pace BS (2018) Loss of NRF2 function exacerbates the pathophysiology of sickle cell disease in a transgenic mouse model. Blood, 131, 558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Similarity in GUSB expression in vehicle and haem-injected mice.
Fig S2. Basal PlGF expression is highly variable in Nrf2−/− mice.
Fig S3. Haem-induced PlGF expression in solid organs is not Nrf2 dependent.
Table SI. Rt-qPCR Taqman probes.


