Abstract
Surgery for inflammatory bowel diseases (IBD) management has passed through an important evolution over the last decades, with innovative strategies and new technologies, especially in minimally invasive surgery (MIS) approaches. MIS procedures for IBD include multiport laparoscopy, single-port surgery, robotics, and the use of transanal platforms. These approaches can be used in the surgical management of both Crohn's disease (CD) and ulcerative colitis (UC). There are significant peculiarities in the surgical field in CD and UC, and their perfect understanding are directly related to better outcomes in IBD patients, as a consequence of improvement in knowledge by IBD surgeons. Different strategies to train colorectal surgeons were developed worldwide, for better application of MIS, usually for malignant or non-IBD benign diseases. There is a significant lack of evidence in specific training strategies for MIS in the IBD field. In this review, the authors outline the importance of adequate surgical training in IBD MIS, by discussing the current evidence on different approaches and emphasizing the need for better training protocols included in multidisciplinary teams in IBD centers throughout the globe.
Keywords: Crohn's disease, ulcerative colitis, surgery, laparoscopy
Over the last decades, the incidence and prevalence of inflammatory bowel diseases (IBD), namely Crohn's disease (CD) and ulcerative colitis (UC), has dramatically risen in different parts of the world. 1 A remarkable evolution has occurred, mainly in the past 20 years, in medical therapy for IBD, based not only in the development of new biological agents and small molecules, but also in new targeted therapeutic strategies aiming mucosal and radiographic healing, which result in better outcomes for patients. 2 Surgical rates seem to be decreasing in the biological era in population-based studies, but if this is widely observed in real-world practice remains controversial. 3 Despite the significant improvement in medical therapy, surgery is still needed in a significant proportion of IBD patients. 4
Colorectal surgery in general has also undergone a revolution in recent decades, with the development and widespread adoption of laparoscopic surgery. Several studies demonstrated the superiority of laparoscopy compared with conventional open colorectal resections in several outcomes, mainly shorter hospital stay, better cosmesis, fewer complications, and reduced blood loss. 5 This was also observed in IBD surgery, where today, laparoscopic surgery is considered the gold standard approach. 6 Application of laparoscopy also had aided in the development of enhanced recovery pathways, which also contributes to favorable outcomes to IBD patients. 7 Different strategies to train colorectal surgeons were developed worldwide, for better application of minimally invasive surgery (MIS), usually for malignant or non-IBD benign diseases. 8
More recently, other minimally invasive techniques were developed in colorectal surgery, as the single-port approach, 9 the use of robotics, 10 and different transanal platforms. 11 These new surgical strategies are currently also used in the management of both CD and UC for specific procedures. As an example, patients with acute severe colitis can be operated by a single-port approach at the ileostomy site, having incision-less operations, with reduced pain and other known benefits of MIS. 12 Different case series from different parts of the world had demonstrated the feasibility, safety, and adequate application of different minimally invasive techniques in IBD. 13 14
IBD in general are challenging diseases to manage. Several confounding factors and specific characteristics usually are brought to the table when surgery is indicated. Patients are usually malnourished, receiving multiple immunosuppressive therapies, and require preoperative optimization for best outcomes. The transmural characteristics of CD, generally presenting at surgical indication with abscesses, internal or external fistulas, and stenosis, also constitute one of the challenges to minimally invasive approaches. There is an absolute lack of evidence in specific training strategies for MIS in the IBD field. Therefore, the aim of this review is to outline the importance of adequate surgical training in IBD MIS, by discussing the current evidence on different approaches and emphasizing the need for better training protocols included in multidisciplinary teams (MDTs) in IBD centers.
IBD Surgery as a Subspecialty
Currently, specific programs in IBD surgery are lacking worldwide. Several gastroenterology programs offer IBD advanced fellowships that mix clinical outpatient and inpatient practice, endoscopy, research, and some limited exposure to surgery. In IBD referral centers, surgeons constitute one of the essential parts of MDTs, and need to work close to gastroenterologists, radiologists, as well as with other specialties, aiming better outcomes for patients.
Hansraj and Kavic had described several reasons to consider IBD surgery as a specific subspecialty, with a dedicated training program. 15 Important issues of the profile of IBD surgery are: the need for high surgical volume in these diseases, which limits complication rates; experience with specific technical aspects which are demanded (e.g., stricturoplasties instead of tendency to resect); full disposition to participate in MDTs from IBD referral centers; and finally, familiarity with minimally invasive procedures to manage IBD patients. Indeed, with the lack of specific programs to IBD surgery in general, the question remains: how to train an IBD surgeon in minimally invasive techniques?
A survey in Spain with 192 surgeons from the Spanish Association of Surgery revealed interesting findings. 16 Only 48.5% of the hospitals have an IBD MDT and only 56.1% of the procedures are initially performed with laparoscopy. The third level hospitals do operate more cases than second level (57% vs. 24% have 3–5 procedures per month, p < 0.001). The majority of the hospitals operate less than 10 cases of UC per year, with approximately 3 pouch procedures in a year. The absolute majority of surgeons who replied the survey emphasized the need for national registries and centralization of IBD surgery. Impressive 45.3% of the IBD procedures in Spain are performed by general surgeons. Specific programs for surgical IBD seem to not exist in Spain. This snapshot of information from a developed country emphasizes the real need for better training not only in IBD surgery in general, but mostly in MIS for IBD.
Celentano et al described one of the only studies investigating training methods in MIS specifically in IBD. 17 In a period of 4 years (2009–2013), all laparoscopic procedures for IBD were classified into two groups after six main steps of the procedures were established. The six critical steps were: theater setup and laparoscopic access; dissection and division of vascular pedicle; mobilization of the colon; bowel division; specimen extraction; and anastomosis. In the first group ( n = 77), the trainee performed the whole procedure (all steps) with the presence of the unscrubbed trainer in the operating room. In the second group ( n = 74), the trainer did two or more critical steps of the procedure (usually the most challenging ones). Rates of reoperation, readmission, conversion, length of stay, and duration of the procedure, as well as postoperative morbidity, were prospectively recorded. There were no differences in postoperative morbidity, readmissions, blood loss, or hospital stay between the groups. Operating time was longer with the unscrubbed trainer (166.6 vs. 130.4 minutes). Selection bias, with trainers' intervention in more complex procedures (as there was no randomization) could justify these findings. The authors concluded that IBD laparoscopic resections performed by a supervised trainee were safe when compared with trainer-performed procedures, despite longer duration of the operations. This demonstrates that there is a need for better strategies in proctoring MIS for IBD.
Table 1 describes in detail some of the specific features that need to be considered in IBD surgery. It is important to emphasize that there is vast evidence considering the benefits of laparoscopy as compared with open procedures, but the experience in colorectal surgery training in MIS remains concentrated more deeply in colorectal cancer and other diseases, as diverticulosis and rectal prolapse, for example. The transmural characteristics and consequent complications of CD, such as contained perforation, abscesses, and fistulas need to be considered, as well as the important concept of bowel preserving surgery. In UC, few centers have robust experience with laparoscopic pouch surgery, and it is known that complication rates are inversely related to the volume of patients. These specific characteristics challenge the current status for MIS for IBD training worldwide.
Table 1. Specific needs of an IBD surgeon, focusing on minimally invasive techniques and characteristics of the diseases.
| IBD in general | Crohn's disease | Ulcerative colitis |
|---|---|---|
| Preoperative optimization regarding immunosuppression, anemia, and nutrition | Familiarity with procedures in the whole GI tract (upper GI, small and large bowel, anus) | Early involvement in admissions for acute severe UC to make prompt decisions |
| Performance of high volumes aiming for lower complication rates | Specific management of the mesentery due to friability | Use of MIS for subtotal colectomies |
| Proficiency with minimally invasive procedures | Documentation of length and characteristics of small bowel | Tailor surgical strategy between 1, 2, and 3 procedures, according to patients' specific characteristics |
| Involvement in MDTs from IBD referral centers | Use of MIS as initial techniques, aiming for less adhesions in future operations and preservation of abdominal wall integrity | Experience in pouch surgery and MIS pouch surgery, abdominally and transanally |
| Specific combined medical-surgical research in IBD | Familiarity with bowel sparing procedures (strictureplasties) and the need to avoid short bowel syndrome | Experience in redo pouch operations, as many complications can be referred to an IBD surgical specialist |
Abbreviations: GI, gastrointestinal; IBD, inflammatory bowel disease; MDT, multidisciplinary team; MIS, minimally invasive surgery; UC, ulcerative colitis.
Learning Curves and Surgical Volume in IBD Minimally Invasive Surgery
There is an obvious inverse relation between surgical experience and postoperative complications in abdominal surgery overall. The more experienced a surgeon has in a specific method, the more technically developed he/she will be, and a reduction in complication rates can be expected. This relation can also be applicable to IBD MIS.
Tekkis et al demonstrated in a retrospective study with 900 patients that the learning curve for colorectal laparoscopic surgery (in different diseases) in right-sided resections was estimated to be 55 cases, and in left-sided resections to be 62 cases, with risk-adjusted cumulative sum model. 18 One would expect that in ileocolic resections for CD, the numbers should be even increased, as previously described, due to the intrinsic challenging characteristics of complicated disease, often observed at the moment of surgical indication (multiple organ involvement, difficult mesentery, fistulas, abscesses, etc.). As a consequence, the expectation for the adequate number of cases for more complex procedures, as total colectomy and pouch operations for IBD, to define a learning curve for these specific situations, would be even greater in terms of number of cases needed to adequate expertise.
Nguyen and Steinhart, in a population-based study from Ontario, Canada, analyzed outcomes of surgery for CD in relation to volume. 19 All CD-related bowel resections from 1996 to 2009 were captured (2,842 patients). Overall early complication rates did not vary between different quartiles of surgeon volume. However, late postoperative hospitalizations due to complications were more common in surgeons with lower volume of procedures. Centralization of surgery for IBD was speculated, with referrals to more experienced IBD surgeons being suggested by the authors. Better studies analyzing the relation between volume of specific MIS procedures in IBD are warranted.
The European Crohn's and Colitis Organization (ECCO) consensus for surgery in UC emphasizes that surgeons with high volume tend to have better outcomes in pouch surgery for UC. 20 The guidelines state that results can be optimized in centers with at least 10 pouch procedures per year. However, the absolute number of centers or surgeons that fulfill these criteria worldwide is unknown. As previously reported by the Spanish survey, the majority of third level hospitals in Spain perform approximately 3 pouches per year for UC. 16 The Association of Coloproctology of Great Britain and Ireland pouch registry also demonstrated similar numbers. 21 In their most recent report (2017), 5,352 pouch procedures in 5,248 patients were described (81.4% for UC). Case volume varied widely in the 73 centers involved. Only 4 centers reported an average of at least 10 pouches per year over the past 5 years, which demonstrates the size of the problem in the U.K. Overall, 44 centers reported fewer than 10 cases in 5 years. The top 10 contributing sites accounted for 59.6% of all pouches included in the registry in the past 5 years. A significant increase in laparoscopic surgery was also noted in the same period. The results of this registry demonstrate the alarming small number of centers with higher volume in the surgical treatment of UC. This also emphasizes the clear need for better training strategies in MIS for IBD.
A study from the Cleveland Clinic (Cleveland, OH) demonstrated that a reduction in pelvic sepsis for pouches could be observed after 143 cases, and overall pouch morbidity after 239 cases of laparoscopic pouch operations for different diseases, including IBD. 22 There was a clear difference in learning curves between high- and low-volume surgeons in the same institution. These findings demonstrate that in a complex operation as the laparoscopic pouch, a high volume of operations is needed to reach expertise and better results.
As seen, there is scarce data relating surgical volume to outcomes, specifically in IBD. Cases are spread among different centers and specialties (colorectal surgeons, general surgeons, acute care surgeons, etc.). However, it remains clear that no specific protocols for training in laparoscopic surgery or other minimally invasive procedures for IBD are currently standardized. If similar proctoring and training strategies from laparoscopic colorectal surgery could be replicated in IBD-related surgery, this remains to be studied. The role of a subspecialty as IBD surgery, represented by specific surgical groups of physicians in scientific associations, as the ECCO or the Crohn's and Colitis Foundation, needs to be developed to establish criteria to train younger surgeons in IBD MIS procedures.
Evidence with Special Minimally Invasive Techniques in IBD Surgery
Most of the solid evidence of MIS in IBD is based on multiport laparoscopy, and the advantages of the method in comparison with conventional surgery are widely published in the literature. 5 More recently, other minimally invasive techniques were developed in colorectal surgery, and started to be used in the surgical approach for CD and UC. In this section, we summarize the evidence for IBD with these specific methods.
Single-Port Surgery
Given the popularity and success of multiport laparoscopic for benign and malignant diseases, in an effort to push the minimally invasive envelope, early in the 21st century, single-incision laparoscopic surgery (SILS) colorectal procedures were developed. 23 Similar to traditional laparoscopy, these are technology-dependent procedures, specifically a smaller version of the hand-assist port which allowed for 3 or more 5 mm laparoscopic instruments to be inserted at the planned extraction site, which was typically a 4-cm periumbilical incision (terminal ileitis) or the nascent ileostomy site (total colectomy for UC or CD, proctocolectomy for UC or CD, if combined with transanal). The purported benefits of SILS compared with traditional laparoscopy were decreased pain from the lack of the additional ports, and marginally improved cosmesis for the same reason. 24 Of note, given the young age of many IBD patients who require surgery, IBD surgeons should not discount the potential cosmetic benefits, or lack thereof, of the surgical procedures that they offer to their patients. Several studies have in fact shown decreased pain scores in the IBD population who underwent SILS. 25 However, SILS procedures are technically demanding and are typically significantly longer in operative length compared with multiport laparoscopy. On the other hand, SILS procedures can be readily converted to multiport laparoscopy midstream.
Given the aforementioned technically demanding nature of SILS, with a limited access portal and field of view, the learning curve has been shown to be steep, varying from 50 cases for high-volume laparoscopic IBD surgeons to more than 250 cases for septic complications. 22 26 Specifically, the limited access requires ergonomic adjustment, such that the surgeon and trainee must stand “shoulder-to-shoulder” with their (typically) right arms extended. In addition, some surgeons who perform SILS prefer a zero-degree 5-mm flexible tip high-definition laparoscope, which demands an upfront capital investment but more importantly expertise in driving the camera, which is typically done by the attending surgeon. Thus, a “double-learning curve” exists for SILS to gain experience driving a laparoscope in a limited area at a time, while also operating in a limited field of view. Add the nuances of operating on the multiple phenotypes of IBD with fistulae, abscesses, and friable tissues and mesentery, and one can reasonably assume that SILS for IBD would be a procedure for which advanced IBD surgical training would be beneficial to overcome the steepest part of the learning curve. And just as hand-assisted laparoscopic surgery provided an avenue for non-laparoscopists to begin to extend their expertise and familiarity with laparoscopy, multiport laparoscopy and reduced-port laparoscopy can help trainees to become better prepared for the technical demands of SILS.
When critically examining the evidence in support of SILS for IBD, several themes, some of which are mentioned above, emerge. As more experience has been gathered globally with SILS colectomy and proctectomy for IBD, the difference in operative times has diminished to the point of nonsignificance. 27 So too the cost differences have for the most part evaporated. However, aside from cosmesis and slightly but statistically significantly reduced pain scores in the immediate days following surgery, no clinical advantage has been demonstrated. Thus, the choice to use multiport laparoscopy, reduced port laparoscopy, or SILS approaches for patients with IBD remains at the discretion and expertise of the operating surgeon and are all equally acceptable and may be considered somewhat interchangeable. Of note, some of the authors prefer reduced port 5-mm laparoscopy with a flexible tip laparoscope for most IBD cases, making a 4-cm periumbilical extraction incision first in the case of CD, and for UC with the ileostomy site serving as both the stapling port and extraction incision for total abdominal colectomy and ileal pouch-anal anastomosis (IPAA) construction. This approach, in our opinion, balances the costs (including time and education of trainees) and benefits of minimally invasive “straight” laparoscopic surgery. That being said, we feel it is crucial that IBD surgical trainees may be exposed to all these techniques, including robotic-assisted laparoscopic surgery. 28
Robotics and IBD
In contrast to the evolution, in terms of the learning curve, of laparoscopic surgery from hand-assisted to straight multiport laparoscopy, to reduced port laparoscopy, and finally SILS, robotic colorectal surgery demands an entirely different skill set and learning curve, but does afford an ergonomic advantage for the surgeon. 29 30 Although robotic-assisted laparoscopic surgery is an order of magnitude more complex than traditional laparoscopic surgery, the approach is more readily mastered in a stepwise approach with a learning curve measured in a few dozen, compared with several to many dozen of cases. 31 Trainees typically first complete an online curriculum to understand the technology and some of its limitations, then undergo a 2-day formal training at a centralized industry-sponsored course, then master the basics of bedside assistance to the operating surgeon with robot arm docking, suctioning, and retraction. Finally, dedicated “console time” completes the trainees' introduction to robotic-assisted colorectal surgery. For attending surgeons who are seeking new robotic credentialing and privileges, an additional final step is typically completion of several “proctored” cases with a specific surgeon who is considered an expert and past the learning curve, and then a departmental review of the surgeon's initial dozen or so first robotic cases. Of note, surgeons' initial robotic colorectal cases are typically selected to ease the steepness of the learning curve, such as starting with straightforward benign disease (such as short fibrotic stenosis ileal CD), then proceeding to more complex cases (such as sigmoid diverticulitis), then to more advanced benign disease (most phenotypes of IBD) and malignancies.
In addition to the well-described training pathway detailed above, there are several aspects of robotic surgery which lend themselves to overcoming the learning curve. First, the control system is exceptionally well-designed in terms of the human interface, with ergonomics and ease of surgeon use being premier. Second, the system provides optimal three-dimensional visualization and high-definition images, both of which remove a layer of artificiality to the “interface” that traditional laparoscopy suffers from. Another benefit of the system is called “telestration” in which the supervising surgeon can literally illustrate lines and arrows, for example, on a flat touchscreen viewing monitor. Finally, and most importantly to trainees, is the availability of the “teaching console” which allows two surgeons to operate synchronously in tandem. This is especially helpful for the steepest part of the learning curve, when the supervising surgeon can set the stage for successful dissection by the trainee by optimizing retraction and exposure, while maintain the ability to intervene and assume the dissection as needed in cases of difficult anatomy, what can be frequent in IBD cases.
To date, and similar to SILS, multiple small case series of robotic surgery for IBD have been reported. Although a critical examination of the literature as a whole would suggest that robotic surgery offers few if any advantages over traditional MIS in terms of overall postoperative outcomes, several areas of incremental improvement standout. The first is the ability to perform totally intracorporeal ileocolic anastomosis after ileocolic resection for CD. 31 32 The advantage of this technique is that it allows the surgeon to place the specimen extraction incision remote from the anastomosis, typically as a Pfannenstiel incision, with arguably lower wound infection and hernia rates although that can be debatable. Intracorporeal strictureplasty for CD has also been reported, but without single-port access, this may result in more/larger incisions relative to reduced port laparoscopy or SILS.
The other is for pelvic dissection. Although in rectal cancer no advantage is seen, many surgeons who are familiar with robotic proctectomy feel very strongly that it gives a distinct advantage in the case of difficult pelvic exposure, as in the obese male pelvis, for example. The robotic stapler arm also gives the best approximation of a right-angled staple line when transecting the anorectal junction for an IPAA. 30 Whether these purported advantages are worth the significantly increase health care resources (direct and indirect costs) from a societal perspective remains to be seen. That being said, robotic-assisted laparoscopic surgery for IBD is here to stay, and as newer platforms are released and the field becomes more competitive, and costs go down, utilization is likely to increase. Finally, robotic surgery can be combined with both SILS, and also with transanal surgery, as outlined below. 33
Transanal Surgery in the IBD Field
The use of transanal platforms in the surgical management of UC and CD is described for specific procedures. The experience with transanal management of rectal cancer and transanal total mesorectal excision (TaTME) supported colorectal surgeons in application of this method in the management of IBD.
Proctectomies with full anorectal resection can be performed with the transanal approach. After initiating the perineal dissection in the intersphincteric plane, a transanal port can be placed in the perineal wound and the bottom-up dissection is performed. Different platforms from different manufacturers can be used (Gelpoint, Applied Medical; SILS Port, Medtronic). When there is no associated rectal dysplasia or neoplasia, a close rectal dissection can be used, whereas in cases with associated malignancy, a TaTME plane is suggested. Rectal excision is indicated in older patients with surgical indication for proctocolectomy in UC, where the sphincter function is impaired and there is no indication for a pouch procedure, due to the risk of fecal incontinence and poor functional status. It can also be indicated in CD cases, where proctectomy/proctocolectomy is indicated due to rectal fibrotic stenosis or severe perianal fistulizing disease, for example. The transanal approach can be used in proctocolectomies, where the colon can be resected with a combined abdominal approach, or as a single completion proctectomy, in staged procedures with previous colectomy. There can be cases of completion proctectomies when an exclusive transanal access can be used, without an abdominal team. The procedure is feasible and the decision of using or not an abdominal laparoscopic combined approach needs to be individualized.
The transanal approach for pouch procedures with IPAA was described in the management of UC and in familial adenomatous polyposis. 34 The rectal dissection is performed from below (bottom-up approach), after placing the transanal platform ( Fig. 1 ). A circumferential purse-string suture is made 3 cm above the dentate line, and the site of distal rectal resection is well identified ( Fig. 2 ). A circumferential rectal dissection is performed from below, following the close rectal dissection plane in patients with no dysplasia or neoplasia, with energy devices or monopolar cautery. The transanal team usually terminates the dissection with support of the laparoscopic abdominal team, and the specimen can be extracted transanally ( Fig. 3 ) or via the ileostomy site. The pouch is constructed by the abdominal team (usually via the ileostomy site), and then is sequentially attached to the anvil of a circular stapler and a Foley catheter ( Fig. 4 ). A purse-string suture is made in the distal margin of the anorectal cuff, and a single-stapling technique is applied after the pouch is directed to the pelvis from the abdomen, with support from the abdominal team for traction via Foley catheter and orientation of the pouch position. After firing the circular stapler ( Fig. 5 ), the IPAA can be reinforced with oversewing of the staple lines with interrupted stitches.
Fig. 1.
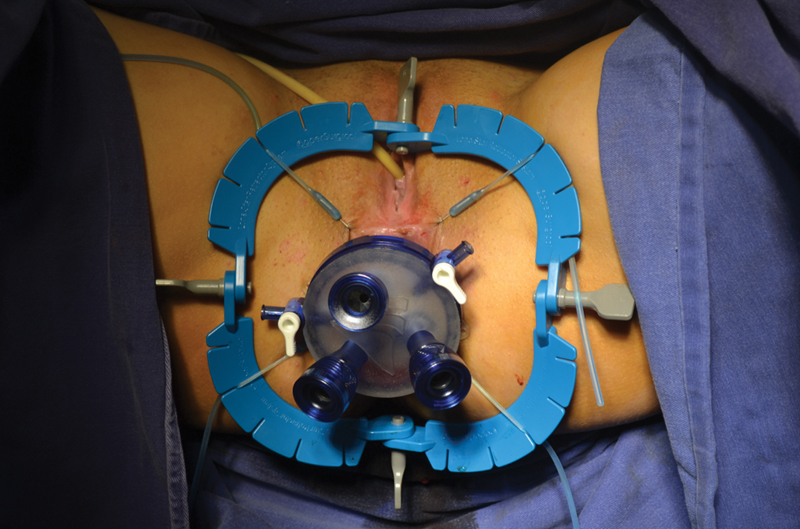
Port and trocars for transanal completion proctectomy.
Fig. 2.
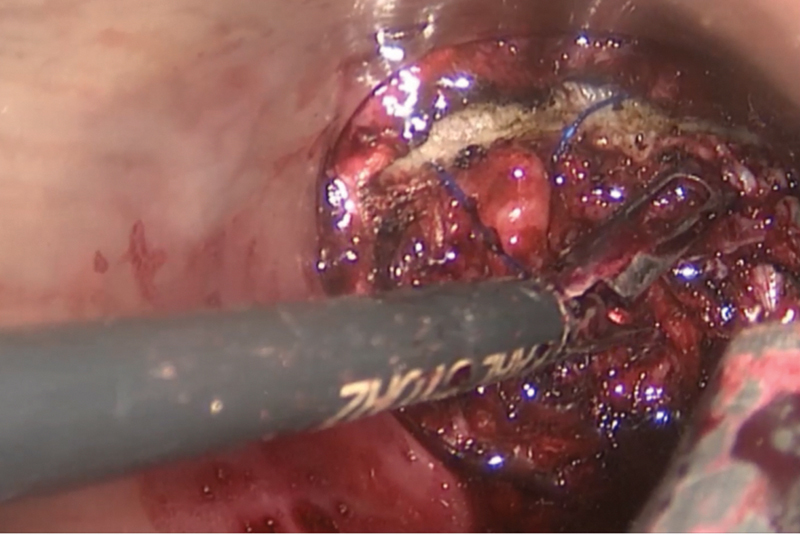
Beginning of dissection after definition of distal margin under direct vision.
Fig. 3.
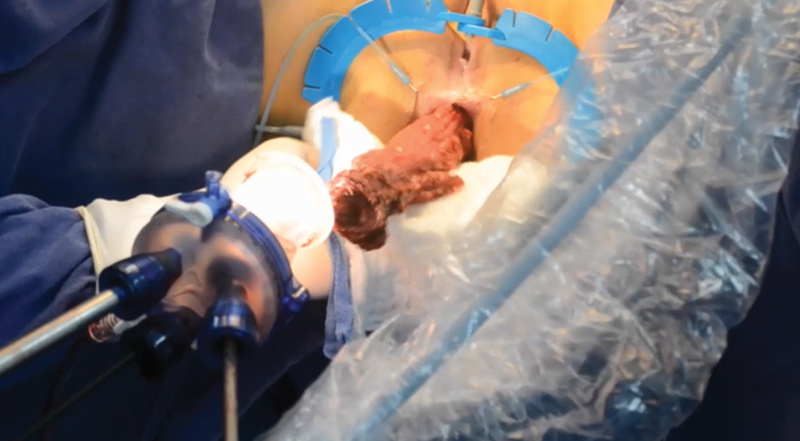
Transanal extraction of the specimen.
Fig. 4.
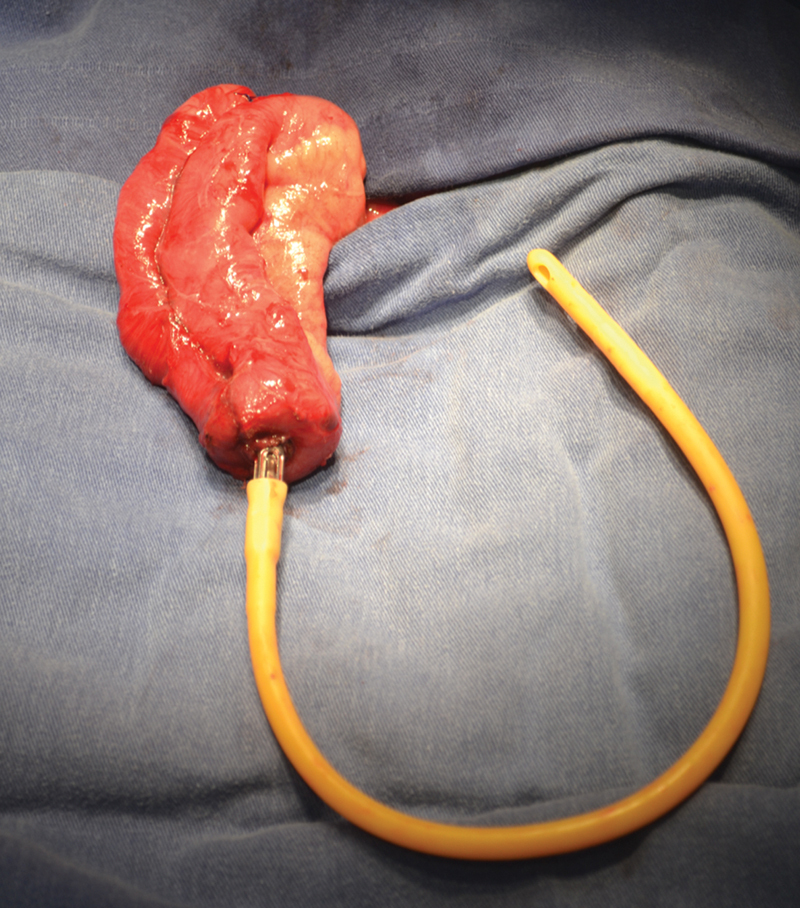
Pouch creation at stoma site, extensor, and anvil.
Fig. 5.
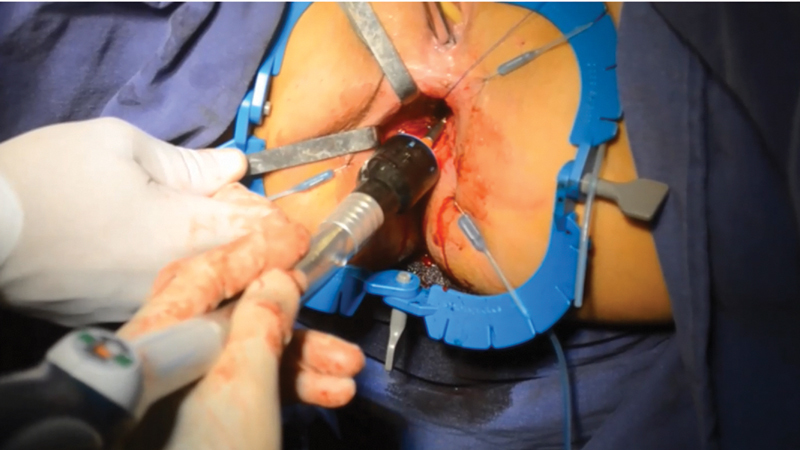
Single stapling anastomosis.
The feasibility of the transanal pouch was described in some technical descriptions and case series. A case series including 16 patients with the double single-port approach (abdominal and transanal) for UC was published by the St. Mark's hospital group. 34 The early surgical complication rate (up to 30 days after the procedure), was acceptable and comparable with other minimally invasive approaches. Five patients had minor complications (31.25%) and one patient had an anastomotic leakage 2 weeks after the procedure and was considered to have a major complication. All the cases from this series were operated with the TaTME approach, and close rectal dissection was not performed. The authors did not explore training issues with the technique, as with the limited number of cases, no learning curve could be estimated.
A retrospective multicentric comparison of the transanal pouch and single-stapling IPAA with laparoscopic transabdominal IPAA was recently published. 12 Ninety-seven patients with transanal IPAA were compared with 119 with transabdominal IPAA. Ninety-nine (46%) patients had a defunctioning ileostomy at time of pouch construction. The odds for postoperative morbidity were 0.52 times lower in the transanal IPAA group (95% confidence interval [0.29–0.92]; p = 0.026). In patients with morbidity, mean cumulative complication index of the transanal approach was 2.23 points lower than the transabdominal approach (95% confidence interval [6.64–3.36]; p = 0.13), which was not significant. The authors concluded that the transanal pouch with bottom-up approach is a safe and feasible procedure, suggesting lower morbidity. However, more solid and prospective comparative data are needed to better define criteria for this technique in comparison with the transabdominal proctectomy and double-stapling IPAA, which is still the most used approach worldwide.
One of the specific characteristics of the transanal pouch for UC is the close rectal dissection, where the mesorectal plane is not followed as in rectal cancer, and the mesorectal fat is left in the pelvis. The aim of the close rectal dissection is that there is less pelvic dead space, with the possibility of having smaller presacral sinuses in cases of IPAA anastomotic dehiscence, and of preventing small bowel adhesions to the sacral promontory which can lead to afferent limb syndrome. One prospective study demonstrated that the close rectal dissection was associated with lower complication rates as compared with TME. 35 On this basis, for benign diseases, where a rectal resection is required, close rectal dissection can be recommended in cases of UC, but given the possible involvement of the mesentery in the etiology of inflammation in CD may not be beneficial in the long term. The close rectal dissection (intramesorectal) approach seems technically less demanding than the TaTME, when performed transanally, with support of energy devices, what can make training of young surgeons faster as compared with TaTME.
There is no evidence of training programs for transanal techniques specifically for IBD in the literature. As previously stated, specific courses for TaTME approach for rectal cancer are being disseminated, and in some of them, the transanal pouch is also explored. Cadaveric dissections, animal models, and simulators can also be applied in transanal approach for IBD in the near future.
Future Trends in Training for MIS in IBD
As minimally invasive approaches to the management of IBD continue to evolve, so too must the manner in which training is provided in these techniques. Specifically, as we continue to refine the role for robotics, SILS, and transanal approaches to increasingly complex disease presentations, the training provided in these domains will need to be expanded as well.
SILS, transanal, and robotic technologies to IBD operations represent novel approaches that are not readily transferable from existing techniques. Trainees who are skilled at laparoscopic low-anterior resections of the rectum are unlikely to be able to immediately apply their knowledge to a transanal proctectomy, even though they are dissecting in the same planes using much the same instrumentation. Therefore, it will be important to foster the use of skilled trainers in a simulated environment prior to performance on patients. For experienced surgeons looking to adopt new technologies, this will likely require a short time to become familiar to the instrumentation and approach, as they already possess a robust understanding of the anatomy and surgical techniques. For more inexperienced trainees, however, the time to competency in the simulated environment will likely be substantially longer.
Perhaps the most important outcome of any training program is assuring that surgeons who complete the training are safe and competent. Although, as was noted earlier, specific case numbers have been suggested at which learning curves are overcome for some minimally invasive approaches, repetition does not imply competence. While the majority of trainees may be able to safely complete a straightforward single-incision resection after a predefined number of cases, there will be outliers who are unable to do so without significant more experience, just as there will be those who require many fewer repetitions to become competent. Additionally, just because a trainee is able to approach uncomplicated IBD through a novel approach does not mean they are ready to do so in more unusual or difficult situations. This begets the importance of assuring learners are appropriately credentialed prior to permitting independent practice.
Expert, direct supervision of a trainee applying a new minimally invasive technique to the operative management of IBD probably provides the most effective means to assure competence before independent practice. However, the limited number of experts in some of these procedures precludes having someone available at every institution where patients could benefit from these approaches. Telementoring provides an exciting opportunity to overcome these barriers. 36 In this model, an expert trainer is able to remotely participate as a supervisor. In the most robust system, the telementor is able to see both the laparoscopic view and has a live video feed of the operating room. They are also able to provide direct verbal feedback in real-time to the surgeon. After observing the surgeon complete several cases safely, though evaluation of a previously established metric, they may deem the surgeon competent to proceed independently. It may be valuable to have the telementor return for a follow-up assessment several months into the trainee's practice to assure they continue to perform satisfactorily. Less time-intensive models of telementoring can include the review of edited or unedited videos of a procedure, and this service is already commercially available in the United States. Furthermore, it has to be expected that, with the advent and widespread of 5G Internet connections, telementoring will be a growing way to train future IBD surgeons.
Given the ingenuity of the surgical community, and our drive to ever improve the care we provide, the next generation of minimally invasive approaches is certainly coming soon. It will be critical to continue to rigorously assess each new technology for effectiveness and safety prior to training the next generation in its application. Further, our community must remain vigilant in assuring practitioners adopting new technologies are effectively trained and assessed prior to beginning independent practice, even in highly specialistic fields as IBD surgery.
Footnotes
Conflict of Interest None declared.
References
- 1.Ng S C, Shi H Y, Hamidi N. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;6736(17):1–10. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2.REACT Study Investigators Khanna R, Bressler B, Levesque B G.Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial Lancet 2015386(10006):1825–1834. [DOI] [PubMed] [Google Scholar]
- 3.Olivera P, Spinelli A, Gower-Rousseau C, Danese S, Peyrin-Biroulet L. Surgical rates in the era of biological therapy: up, down or unchanged? Curr Opin Gastroenterol. 2017;33(04):246–253. doi: 10.1097/MOG.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 4.Rungoe C, Langholz E, Andersson M. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut. 2014;63(10):1607–1616. doi: 10.1136/gutjnl-2013-305607. [DOI] [PubMed] [Google Scholar]
- 5.Patel S V, Patel S VB, Ramagopalan S V, Ott M C. Laparoscopic surgery for Crohn's disease: a meta-analysis of perioperative complications and long term outcomes compared with open surgery. BMC Surg. 2013;13:14. doi: 10.1186/1471-2482-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggiori L, Panis Y. Surgical management of IBD--from an open to a laparoscopic approach. Nat Rev Gastroenterol Hepatol. 2013;10(05):297–306. doi: 10.1038/nrgastro.2013.30. [DOI] [PubMed] [Google Scholar]
- 7.Spinelli A, Bazzi P, Sacchi M.Short-term outcomes of laparoscopy combined with enhanced recovery pathway after ileocecal resection for Crohn's disease: a case-matched analysis J Gastrointest Surg 20131701126–132., discussion 132 [DOI] [PubMed] [Google Scholar]
- 8.Stefanidis D, Anderson-Montoya B, Higgins R V. Developing a coaching mechanism for practicing surgeons. Surgery. 2016;160(03):536–545. doi: 10.1016/j.surg.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Brockhaus A C, Sauerland S, Saad S. Single-incision versus standard multi-incision laparoscopic colectomy in patients with malignant or benign colonic disease: a systematic review, meta-analysis and assessment of the evidence. BMC Surg. 2016;16(01):71. doi: 10.1186/s12893-016-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renshaw S, Silva I L, Hotouras A, Wexner S D, Murphy J, Bhan C. Perioperative outcomes and adverse events of robotic colorectal resections for inflammatory bowel disease: a systematic literature review. Tech Coloproctol. 2018;22(03):161–177. doi: 10.1007/s10151-018-1766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Buck van Overstraeten A, Wolthuis A M, D'Hoore A. Transanal completion proctectomy after total colectomy and ileal pouch-anal anastomosis for ulcerative colitis: a modified single stapled technique. Colorectal Dis. 2016;18(04):O141–O144. doi: 10.1111/codi.13292. [DOI] [PubMed] [Google Scholar]
- 12.Van Overstraeten A DB, Mark-christensen ÃA, Wasmann K A. Transanal versus transabdominal minimally invasive (completion) proctectomy with ileal pouch-anal: a comparative study. Ann Surg. 2017;266(05):878–883. doi: 10.1097/SLA.0000000000002395. [DOI] [PubMed] [Google Scholar]
- 13.Holubar S D, Dozois E J, Privitera A, Pemberton J H, Cima R R, Larson D W. Minimally invasive colectomy for Crohn's colitis: a single institution experience. Inflamm Bowel Dis. 2010;16(11):1940–1946. doi: 10.1002/ibd.21265. [DOI] [PubMed] [Google Scholar]
- 14.Holubar S D, Privitera A, Cima R R, Dozois E J, Pemberton J H, Larson D W. Minimally invasive total proctocolectomy with Brooke ileostomy for ulcerative colitis. Inflamm Bowel Dis. 2009;15(09):1337–1342. doi: 10.1002/ibd.20914. [DOI] [PubMed] [Google Scholar]
- 15.Hansraj N, Kavic S M. Surgery for Crohn's disease: an emerging surgical specialty. Inflamm Bowel Dis. 2015;21(11):E28–E29. doi: 10.1097/MIB.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Guillén L, Blanco-Antona F, Millán-Scheiding M. Estado de la cirugía de la enfermedad inflamatoria en España. Resultado de una encuesta nacional. Cir Esp. 2016;94(10):560–568. doi: 10.1016/j.ciresp.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Celentano V, Finch D, Forster L, Robinson J M, Griffith J P. Safety of supervised trainee-performed laparoscopic surgery for inflammatory bowel disease. Int J Colorectal Dis. 2015;30(05):639–644. doi: 10.1007/s00384-015-2147-4. [DOI] [PubMed] [Google Scholar]
- 18.Tekkis P P, Senagore A J, Delaney C P, Fazio V W. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg. 2005;242(01):83–91. doi: 10.1097/01.sla.0000167857.14690.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen G C, Steinhart A H. The impact of surgeon volume on postoperative outcomes after surgery for Crohn's disease. Inflamm Bowel Dis. 2014;20(02):301–306. doi: 10.1097/01.MIB.0000438247.06595.b9. [DOI] [PubMed] [Google Scholar]
- 20.European Crohn's and Colitis Organisation (ECCO) . Øresland T, Bemelman W A, Sampietro G M. European evidence based consensus on surgery for ulcerative colitis. J Crohn's Colitis. 2015;9(01):4–25. doi: 10.1016/j.crohns.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Association of Coloproctology of Great Britain and Ireland (ACPGBI) Inflammatory Bowel Disease Clinical Advisory Group, commentators in the 2017 ACPGBI Ileoanal Pouch Report, and Ileoanal Pouch Registry contributors . Worley G HT, Fearnhead N S, Brown S R, Acheson A G, Lee M J, Faiz O D. Review of current practice and outcomes following ileoanal pouch surgery: lessons learned from the Ileoanal Pouch Registry and the 2017 Ileoanal Pouch Report. Colorectal Dis. 2018;20(10):913–922. doi: 10.1111/codi.14316. [DOI] [PubMed] [Google Scholar]
- 22.Rencuzogullari A, Stocchi L, Costedio M, Gorgun E, Kessler H, Remzi F H. Characteristics of learning curve in minimally invasive ileal pouch-anal anastomosis in a single institution. Surg Endosc. 2017;31(03):1083–1092. doi: 10.1007/s00464-016-5068-6. [DOI] [PubMed] [Google Scholar]
- 23.Leblanc F, Makhija R, Champagne B J, Delaney C P. Single incision laparoscopic total colectomy and proctocolectomy for benign disease: initial experience. Colorectal Dis. 2011;13(11):1290–1293. doi: 10.1111/j.1463-1318.2010.02448.x. [DOI] [PubMed] [Google Scholar]
- 24.de Groof E J, Buskens C J, Bemelman W A. Single-port surgery in inflammatory bowel disease: a review of current evidence. World J Surg. 2016;40(09):2276–2282. doi: 10.1007/s00268-016-3509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvello M, de Groof E J, de Buck van Overstraeten A. Single port laparoscopic ileocaecal resection for Crohn's disease: a multicentre comparison with multi-port laparoscopy. Colorectal Dis. 2018;20(01):53–58. doi: 10.1111/codi.13777. [DOI] [PubMed] [Google Scholar]
- 26.Keller D S, Flores-Gonzalez J R, Ibarra S, Haas E M. Review of 500 single incision laparoscopic colorectal surgery cases - lessons learned. World J Gastroenterol. 2016;22(02):659–667. doi: 10.3748/wjg.v22.i2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda K, Nagahara H, Shibutani M. The feasibility and short-term clinical outcomes of single-incision laparoscopic surgery for patients with complex Crohn's disease. Surg Today. 2018;48(02):242–247. doi: 10.1007/s00595-017-1581-z. [DOI] [PubMed] [Google Scholar]
- 28.Rencuzogullari A, Gorgun E, Costedio M. Case-matched comparison of robotic versus laparoscopic proctectomy for inflammatory bowel disease. Surg Laparosc Endosc Percutan Tech. 2016;26(03):e37–e40. doi: 10.1097/SLE.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 29.Aradaib M, Neary P, Hafeez A, Kalbassi R, Parvaiz A, Riordain D O. Safe adoption of robotic colorectal surgery using structured training: early Irish experience. J Robot Surg. 2019;13(05):657–662. doi: 10.1007/s11701-018-00911-0. [DOI] [PubMed] [Google Scholar]
- 30.Lightner A L, Kelley S R, Larson D W. Robotic platform for an IPAA. Dis Colon Rectum. 2018;61(07):869–874. doi: 10.1097/DCR.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 31.Shaw D D, Wright M, Taylor L. Robotic colorectal surgery learning curve and case complexity. J Laparoendosc Adv Surg Tech A. 2018;28(10):1163–1168. doi: 10.1089/lap.2016.0411. [DOI] [PubMed] [Google Scholar]
- 32.Scaringi S, Giudici F, Zambonin D, Ficari F, Bechi P. Totally robotic intracorporeal side-to-side isoperistaltic strictureplasty for Crohn's disease. J Minim Access Surg. 2018;14(04):341–344. doi: 10.4103/jmas.JMAS_212_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juo Y Y, Luka S, Obias V. Single-incision robotic colectomy (SIRC): current status and future directions. J Surg Oncol. 2015;112(03):321–325. doi: 10.1002/jso.23935. [DOI] [PubMed] [Google Scholar]
- 34.Leo C A, Samaranayake S, Perry-Woodford Z L. Initial experience of restorative proctocolectomy for ulcerative colitis by transanal total mesorectal rectal excision and single-incision abdominal laparoscopic surgery. Colorectal Dis. 2016;18(12):1162–1166. doi: 10.1111/codi.13359. [DOI] [PubMed] [Google Scholar]
- 35.Bartels S AL, Gardenbroek T J, Aarts M. Short-term morbidity and quality of life from a randomized clinical trial of close rectal dissection and total mesorectal excision in ileal pouch-anal anastomosis. Br J Surg. 2015;102(03):281–287. doi: 10.1002/bjs.9701. [DOI] [PubMed] [Google Scholar]
- 36.Camacho D R, Schlachta C M, Serrano O K, Nguyen N T. Logistical considerations for establishing reliable surgical telementoring programs: a report of the SAGES Project 6 Logistics Working Group. Surg Endosc. 2018;32(08):3630–3633. doi: 10.1007/s00464-018-6093-4. [DOI] [PubMed] [Google Scholar]


