Abstract
Sexual dimorphism may play a key role in the pathogenesis of diabetic kidney disease (DKD) and explain differences observed in disease phenotypes, responses to interventions, and disease progression between men and women with diabetes. Therefore, omitting the consideration of sex as a biological factor may result in delayed diagnoses and suboptimal therapies. This review will summarize the effects of sexual dimorphism on putative metabolic and molecular mechanisms underlying DKD, and the potential implications of these differences on therapeutic interventions. To successfully implement precision medicine, we require a better understanding of sexual dimorphism in the pathophysiologic progression of DKD. Such insights can unveil sex-specific therapeutic targets that have the potential to maximize efficacy while minimizing adverse events.
Keywords: diabetic kidney disease, sex-related differences, sexual dimorphism, diabetic nephropathy
INTRODUCTION
The call for individualized medicine including differences based on sex has become increasingly louder in recent years [1]. An ever-expanding mass of studies show that many diseases affect each sex differently. Despite these findings, women remain largely underrepresented as participants in biomedical research. In fact, there are fewer women than men represented in trials evaluating cardiovascular and kidney disease outcomes, the leading causes of morbidity and mortality in women [1, 2]. Furthermore, even when women are equally represented in research, the potential influences of sex (a biologic variable) and/or gender (a social construct) are often inadequately investigated [2]. Men and women not only differ in their risk factors and propensity for kidney disease, but also in multiple biological processes including aging, cell apoptosis, and the functioning of several homeostatic systems (e.g. blood pressure, fluid balance, and the hypothalamic-pituitary-adrenal axis) that could modify the progression of kidney disease [3–5]. The reasons for these differences are multifactorial and may relate to the presence or absence of a Y chromosome and sex differences in gene expression, mitochondrial genome inheritance, and neurohormonal activity [6, 7].
Male sex is associated with enhanced risk for progression of acute kidney injury (AKI) and chronic kidney disease (CKD) in human and animal models [8–15]. However, a higher prevalence of CKD is reported in women than men in the United States (15% vs 12%) [16]. Comparatively, from 2007 to 2017, global CKD prevalence increased by 28.2% in women, while only a 25.3% increase was reported in men [17]. It would be interesting to see if this CKD trend was the same in developing countries. In addition, a large metanalysis of sex-related differences in CKD did not demonstrate greater nephroprotection in women than men when adjusting for additional risk factors including hypertension and albuminuria [18]. Notably, most women in these studies were postmenopausal, thus these results could be influenced by the loss of estrogen-mediated nephroprotection [18]. In the United States, almost half of patients with end-stage kidney disease (ESKD) have concurrent diabetic kidney disease (DKD), with over 80% suffering from type 2 diabetes (T2D) [19]. Yet, the data on sex and DKD risk are inconsistent. Studies have reported either a higher risk in men [20–35], a higher risk in women [25, 35–43], or no significant sexual dimorphism [44–49] (Table 1).
Table 1.
Studies demonstrating higher DKD risk in men vs. studies demonstrating higher risk in women
| 1518 | X | 12 years | ESKD (creatinine measurements) and mortality | The male sex was a risk factor for the development of ESKD but not for mortality | |
| 122842 | X | 25.5 years (meta-analysis) | ESKD (need for renal replacement therapy) | The pooled RR indicated a 19% significantly lower risk of ESKD in women (RR, 0.81; 95% CI, 0.69–0.94; P = 0.007) | |
| 59 | X | 5.5 years | GFR decline (Cr-EDTA method) | Male gender independently predicted an enhanced decline in GFR at baseline (P < 0.002) | |
| 1185 | X | Cross-sectional | Albumin excretion rate (AER) increase | Men had greater albumin excretion rate, independent of other factors known to cause or be associated with elevated AER | |
| 3636 | X | Cross-sectional (phase 1); 2 years (phase 2; n=529) | Urinary albumin to creatinine concentration ratio (UAC/ACR) in microalbuminuric range | UAC indicating microalbuminuria was associated with male sex (p = 0.0350; relative risk = 1.16; 95% confidence interval: 1.01–1.32) | |
| 5032 | X | 15 years | Presence of albuminuria (urinary albumin concentration > 50 mg/l) and reduced glomerular filtration rate (eGFR Cockroft-Gault ≤ 60 ml/min) | Male sex was an additional independent risk factor for albuminuria | |
| 286 | X | 18 years | Persistent albuminuria (urinary albumin > 30mg/24 h in at least two of three consecutive samples, with an increase of at least 30% above the baseline level | Male sex was a significant predictor for the development of persistent microalbuminuria | |
| 27805 | X | 2.5 years | Presence of albuminuria (UAC>2.5 mg/mmol or AER>20μg/min in a random spot or 24-h urine collection, respectively) | Male sex was associated with the development of macroalbuminuria | |
| 11681 | X | 20 years | Cumulative risk of ESKD (GFR <10–15 ml/min) | The cumulative incidence of DKD after 30 years of duration of diabetes has a men predominance (4.1% [95% CI 3.1–5.3] vs. 2.5% [1.7–3.5]). The highest risk of ESKD was found in male subjects diagnosed at age 20–34 years (hazard ratio 3.0 [95% CI 1.5–5.7]). | |
| 4416 | X | 21 years | ESKD (dialysis treatment or having received a kidney transplant). | The risk of ESKD doubled in men compared with women when age at onset was ≥15 years | |
| 657 | X | Cross-sectional | Nephropathy: renal failure (patients on dialysis and/or status post-kidney transplant) or an AER >200 μg/min in two of the three timed urine samples (or in the absence of urine collections, serum creatinine >2 mg/dl). | An excess of diabetic renal disease in men occurred after 25 years of duration of diabetes. | |
| 191 | X | 5.8 years | Incipient diabetic nephropathy (persistent microalbuminuria: UAR>30mg/24 h) | Male sex was a risk factors for the development of incipient or overt diabetic nephropathy (2.6 (1.2 to 5.4); P < 0.02) | |
| 574 | X | 7.8 years | Diabetic nephropathy (ΔCr: creatinine percentage of the initial value of the same patient; UAE>30mg/24h) | Male sex was significantly associated with diabetic nephropathy (ΔCr) | |
| 1146 | X | 5.7 years | Nephropathy: eGFR (CKD-EPI) <60 ml/min and/or UAC ≥ 2.5 mg/mmol | Median yearly estimated glomerular filtration rate slope was higher in men. Male sex was an independent risk factor of steep estimated glomerular filtration rate decline [adjusted odds ratio = 1.33 (1.02–1.76), P = 0.04] | |
| 344 | X | 8.1 years | Decline in eGFR (% eGFR of the initial values, calculated through the Japanese eGFR-estimating equation) | The mean annual eGFR change was −3.5 ± 2.7%/year in females and −2.0 ± 2.2%/year in males (P < 0.001) | |
| 3024 | X | X | Cross-sectional | Sex differences in the prevalence of DKD (eGFR (CKD-EPI) <60 mL/min or sex‐specific microalbuminuria (urine albumin/creatinine ratio ≥25 mg/g for women or ≥17 mg/g for men)), or advanced DKD (eGFR (CKD-EPI) <30 ml/min) at study enrollment | Women of all ages had 28% decreased odds of DKD (OR 0.72, 95% CI 0.62–0.83) |
| 184,396 | 11 (65%) | 6 (35%) | 11.4 years | ||
| 5032 | X | 15 years | Presence of albuminuria (urinary albumin concentration > 50 mg/l) and reduced glomerular filtration rate (eGFR Cockroft-Gault ≤ 60 ml/min) | Female sex was an additional independent risk factor for renal impairment | |
| 3024 | X | X | Cross-sectional | Sex differences in the prevalence of DKD (eGFR (CKD-EPI) <60 mL/min or sex‐specific microalbuminuria (UAR≥25 mg/g for women or ≥17 mg/g for men)), or advanced DKD (eGFR (CKD-EPI) <30 ml/min) at study enrollment | Women had a greater prevalence of advanced DKD (OR 1.67, 95% CI 1.05–2.64) |
| 171 | X | X | Cross-sectional | ESKD Mississippi program enrolment (patients on dialysis and/or status post-kidney transplant) | Diabetic nephropathy accounted for 50.5% of ESKD cases among African American women, but for less than 20% among men. |
| 1464 | X | X | 3.1 years | Incident CKD (eGFR (CKD-EPI) <60 mL/min or sex‐specific microalbuminuria (UAR ≥25 mg/g for women or ≥17 mg/g for men)). | Women had an increased risk of incident CKD (sub-hazard ratio 1.37, 95% confidence interval (CI) 1.17, 1.60) compared with men. Sex differences in incident CKD were consistent across age groups and appeared to be driven by differences in the development of low eGFR rather than microalbuminuria. |
| 532 | X | 5 years | Hyperfiltration (99th percentile or higher of eGFR (≥140mL/min/1.73m2) or UAC ≥ 30μg/mg at 3 consecutive annual visits | Girls were disproportionally affected by DKD, with a 3-fold greater risk of developing hyperfiltration over 5 years compared to boys | |
| 1441 | X | 6.5 years | Time‐related risk of developing diabetic nephropathy (AER ≥ 40 mg/24 h, whether isolated or sustained) | Time to diabetic nephropathy was related to gender (P<0.01). Women had a greater risk for developing DKD | |
| 68 | X | Cross-sectional | Sex differences in the glomerular hemodynamic profile (Gomez’s equations) of healthy controls compared with patients with T1D with hyperfiltration and normofiltration | Women with T1D and hyperfiltration had higher efferent resistance and filtration fraction and lower effective renal plasma flow (ERPF) than their male counterparts | |
| 8114 | X | ≥ 15 years (ongoing when study was published) | Nephropathy (presence of ESKD, kidney failure, or repeated high urinary albumin levels) | The risk for developing nephropathy tends to be higher in females even if the difference is not statistically significant. T1D cases with onset between ages 5 and 14 yr had an increased complications risk | |
| 5000000 | X | X | Cross-sectional (meta-analysis) | Incidence of all stages of CKD (albuminuria or eGFR < 90 ml/min for more than 3 months), and incidence of ESKD only. | For the incidence of ESKD, the pooled women-to-men relative risk ratio was 1.38 (95 % CI 1.22, 1.55); p value was 0.114 and the I² statistic was 38.1 %, which denoted no significant heterogeneity between the studies |
| 494 | X | 5 years | Microalbuminuria (UAC ≥3.5 mg/mmol in boys or ≥4.0 mg/mmol in girls, but < 35 mg/mmol in two of three consecutive overnight collections) | The adjusted proportional probability (Cox model) of microalbuminuria was greater for girls (200%), after pubertal onset (310%), and with higher HbA1c (36% increase for every 1% increase in HbA1c). | |
| 5,020,340 | 8 (57%) | 6 (43%) | 8.2 years | ||
| 788 | X | 17.5 years | ESKD (starting dialysis or undergoing kidney transplant); macroalbuminuria (AER >200 μg/min (>300 mg/24 hours) in at least 2 of 3 validated timed biennial urine collections) | Both ESKD incidence and macroalbuminuria were higher in men within the 1950–64 cohort. However, within the 1965–80 cohort, incidence was higher in women | |
| 537 | X | 9 years | Progression to normoalbuminuria to micro and macroalbuminuria (urinary albumin excretion between 31–299 mg and ≥ 300 mg, respectively, per 24 h in at least two of three consecutive samples) | Sex was not a significant predictor of progression from normoalbuminuria to micro or macroalbuminuria | |
| 409 | X | 3 years | Doubling of the baseline serum creatinine concentration to at least 2.0 mg per deciliter | Sex was not associated with nephropathy progression | |
| 533 | X | X | 3 years | Prevalence and progression of CKD (creatinine increase or ACR in the albuminuric range) | CKD prevalence and progression rate were not significantly different between sexes, although more men had advanced-stage CKD than women |
| 20005 | X | 16.7 years | Cumulative incidence of ESKD (dialysis or kidney transplantation), accounting for death as a competing risk. | The risk of developing ESKD was lowest in patients whose diagnosis occurred at younger than 5 years or in later years. The risk did not differ significantly between sexes. | |
| 364 | X | 15 years | Incidence of ESKD (dialysis or death from DKD) | Sex did not predict ESKD in the Pima Indians cohort. | |
| 22,272 | 5 (71%) | 2 (29%) | 12.42 years |
In green, yellow, and grey are reported all the studies in which a higher risk in men, women or no differences between sexes were observed, respectively. Of note, Yu et al. [35] study was reported in both the green and yellow sections since different results were obtained: women were found at higher risk of advanced DKD while men had an higher prevalence of diabetic kidney disease (DKD). In the last line of each section is reported the total number of subjects included in the studies, the number of studies (% of total studies of the section) that enrolled patients with type 1 and type 2 diabetes and the arithmetic mean of all the follow-up periods. AER: albumin excretion rate; CI: confidence interval; CKD: chronic kidney disease; eGFR: estimated glomerular function rate; ESKD: end-stage kidney disease; OR: odds ratio; UAC: urinary albumin concentration; UAR: urine albumin creatinine ratio; T1D: type 1 diabetes; T2D: type 2 diabetes.
There are several potential reasons for these inconsistencies in the data on sexual dimorphism in DKD. One of the most compelling is that the tools used to assess DKD and CKD are often crude and subject to imprecision and inaccuracy. In fact, most studies estimate glomerular filtration rate (GFR) in lieu of gold standard measurements of kidney function. Indeed, endogenous filtration markers (e.g. serum creatinine and cystatin C) may differ in women and men [50]. In addition, there are multiple equations to estimate GFR, and some of them do not perform equivalently in men and women [51, 52]. Validated sex specific cut-offs for estimated GFR and albuminuria have not been extensively used [53]. Criteria for CKD staging may need to take into account the distribution of GFR by age and sex [51, 54]. Additionally, residual confounders such as the different impact of concomitant cardiovascular risk factors on DKD progression in men vs. women are not fully understood [51, 54, 55].
Even with the inconsistent data, several mechanisms have been proposed to explain the potential sexual dimorphism in DKD including differences in sex hormone concentrations [56–101], kidney hemodynamic function [39, 96–97, 102–112], adiponectin concentrations [113–121], oxidative stress [37, 38,100–101,122–126], activity and expression of membrane channels and water-electrolyte homeostasis within the kidney [127–131], and additional concomitant risk factors [132–133]. This review will outline the role of sex in the pathogenesis of DKD and discuss the potential effects of sexual dimorphism on existing and novel interventions to mitigate kidney injury (Figure 1).
Figure 1. Mechanisms of sexual dimorphism in DKD and potential therapeutic implications.
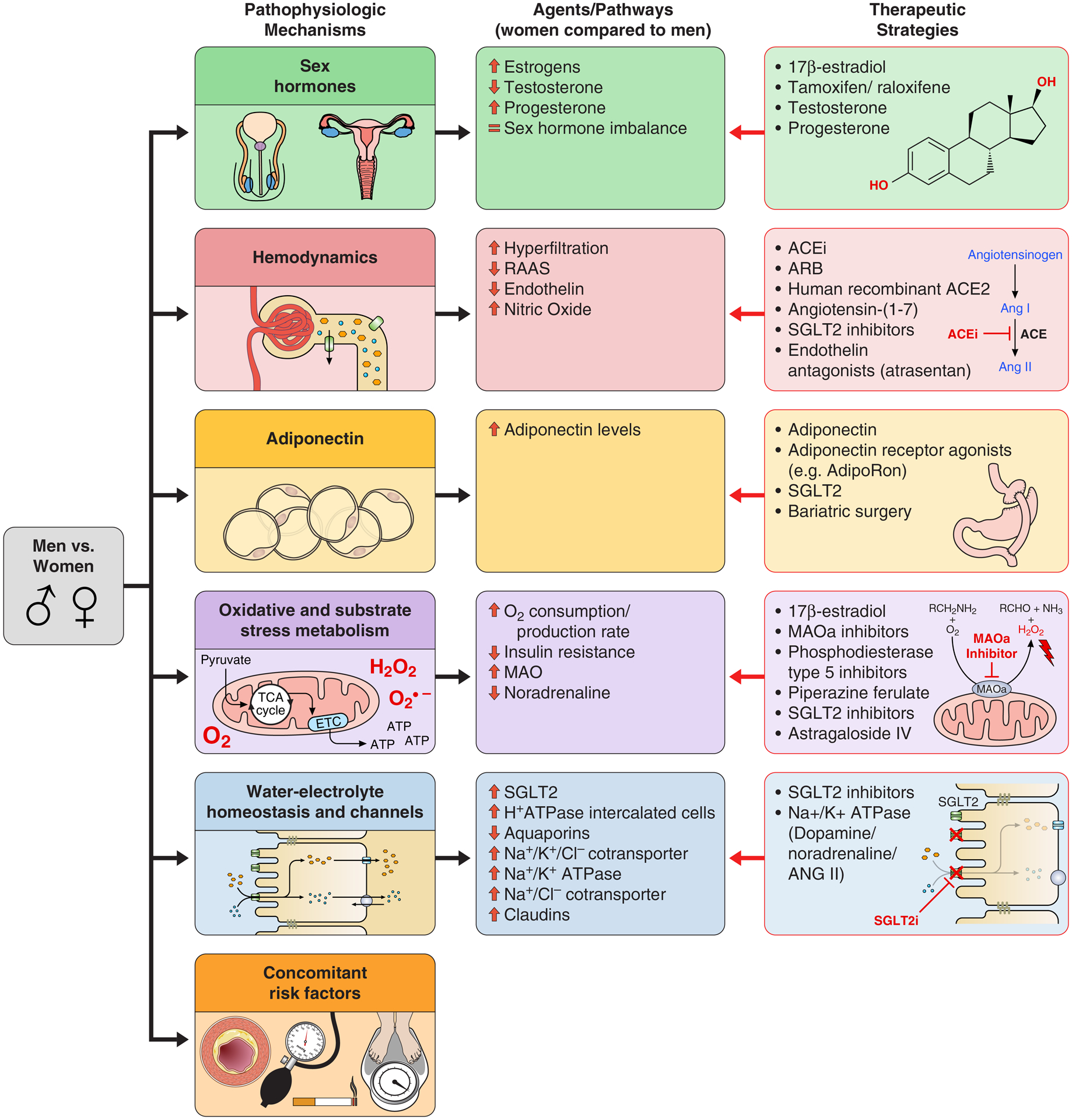
Abbreviations: ACEi: Angiotensin-converting enzyme inhibitors; ACE2: Angiotensin-converting enzyme 2; ARB: angiotensin receptor blocker; MAO: Monoamine oxidase; MAOa: Monoamine oxidase type A; PDE5: Phosphodiesterase type 5; RAAS: Renin-angiotensin-aldosterone system; SGLT2: Sodium glucose co-transporter 2.
Sex-related differences in the risk for DKD development and progression
To date, an unequivocal link between sex and DKD prevalence or progression has not been found. The influence of sex on the clinical course of DKD is actively under investigation. To synthesize a summary of studies evaluating sexual dimorphism in DKD, we performed a comprehensive literature review. The following key words were used to search the literature “diabetic kidney disease”, “diabetic renal disease”, “diabetic renal insufficiency”, “diabetic kidney insufficiency”, “diabetic kidney failure”, “diabetic renal failure”, “diabetic end stage renal disease”, “ diabetic end-stage kidney disease”, “diabetic ESKD”, “diabetic ESRD”, “diabetic proteinuria”, “diabetic albuminuria” and “sex”, “gender”, “men”, and “ women”. No search restrictions were imposed, and references were scanned to identify other potentially relevant studies. When referring to sexual dimorphism in human studies we use the terms men and women, and boys and girls as appropriate to distinguish between the sexes. In citing animal research, we instead use the terms males and females. From our literature search, we found 29 studies on sex-related differences in the prevalence and progression of DKD (Table 1). Most studies included individuals affected by type 1 diabetes (T1D) (18 studies, 60%), but 7 (23%) studies included participants with type 2 diabetes (T2D), and 5 (17%) studies included both individuals with T1D and T2D. Sixteen studies (50%) showed that men were at higher risk of developing DKD in both the T1D and T2D populations, while 10 studies (31%) showed that women were at higher risk and 6 studies (19%) showed no significant sex-related differences (Table 1). It should be noted that Yu et al reported men were at higher prevalence of DKD, while women were at higher risk of advanced DKD [35]. A majority of studies reviewed showed that men were at higher risk of DKD, even when considering the differences in overall duration of follow-up and the sizes of the populations examined. In addition, of the 10 studies with albuminuria as an independent outcome (8 [80%] with T1D), 6 studies (60%) showed a higher risk of albuminuria in men [23–27, 31], while only 2 (20%) demonstrated a higher risk in women [39, 43] and the remaining 2 showed no significant difference between sexes [44, 45]. These findings are consistent with non-diabetic CKD studies that also showed an increased prevalence and progression of kidney disease among men [8–16]. However, the larger meta-analysis of Shen et al. including 5,000,000 participants with either T1D or T2D showed a higher incidence of ESKD in women [42].
Possible explanations for these heterogeneous results may include key differences in the populations studied such as age, menopausal status for women, age of diabetes onset, diabetes duration, and presence of other comorbidities or risk factors for DKD progression, as well as differences in the study design including the equations used for calculating the GFR and variations in outcome measures. Indeed, epidemiologic data suggest that age of T1D onset predicts the risk of ESKD and mortality, with diagnoses around puberty conferring the greatest risk of future ESKD [28, 41, 43, 48]. Additionally, data also suggest that the risk of ESKD is equal in men and women if T1D is diagnosed during childhood, but there are sex differences if diabetes onset occurs peripubertally, although studies are split whether men or women are at higher risk [28, 29, 41, 43]. Indeed, the onset of T1D and T2D close to puberty has shown to magnify the risk for DKD development and progression in both sexes [56]. These findings support a key role for puberty and sex hormones in the development and progression of DKD. Menopause, the cessation of sex hormone production in older women, could also help us further define the role of sex hormones in the development of kidney disease and Yu et al. has demonstrated that women ≥60 years of age had a greater prevalence of advanced DKD compared to men [35]. Sex-differences are highly dependent on duration of diabetes, as the prevalence of DKD increases in men with T1D who have had diabetes for >25 years [28, 30]. This increase was not significant in women. Despite longer disease duration, childhood onset of T1D is protective against future development of DKD for both sexes, while pubertal onset of diabetes has a sex-dependent effect on DKD risk.
Men with either T1D or T2D appear to be at higher risk of developing DKD and especially albuminuria compared with women, particularly if they have had diabetes for >25 years. Yet, women have a higher prevalence of ESKD than men, which suggests a faster progression to ESKD in women vs. men but little has been conclusively shown. Post-menopausal women on the other hand are also at higher risk of DKD compared to men. However, it has to be noted that a majority of studies on sex-related differences on DKD did not consider the menopausal state as a variable and did not analyze the differences between pre- and post-menopausal women.
Pathophysiology of sex-related differences in DKD
1. Sex hormones
The expression of sex hormone receptors and concentrations of sex hormones are important to consider in the discussion of sex-related differences in DKD. Variable expression of sex hormone receptors has been shown in cells throughout the nephron including the glomerulus, proximal tubule, distal tubule, connecting tubule, and collecting duct, but the precise localization of these receptors is still under debate [57].
In the kidney, the androgen receptor (AR), a soluble nuclear receptor, is expressed in both men and women, but it is unknown exactly where this receptor is located [57]. The progesterone receptor is present in the cortex and medulla of the kidney, in both sexes [58]. Estrogen can bind either soluble intracellular receptors (ERα and ERβ), or recently discovered membrane bound receptors (mER). ERα is expressed in the kidney in both sexes, but it is still unclear if ERβ is present in men [57]. In animal kidney models, ERβ increases and ERα decreases during menopause, and there is a normalization of ERβ levels with replacement hormone therapy [58]. Conversely, an increase in ERα/ERβ ratio has been observed in diabetic rat models compared to healthy controls, with a normalization after 17β-estradiol supplementation through an increase in ERβ and a decrease in ERβ expression [59]. Supplementation with 17β-estradiol (E2) in animal models attenuates the development of DKD and reduces albuminuria, podocyte injury, glomerulosclerosis, and tubulointerstitial fibrosis through regulation of expression and signaling of the extracellular matrix and TGF-β [60–67]. In studies of both human and animal models with diabetes and menopause who were treated with either E2 or ER modulators (tamoxifen or raloxifene), there was an improvement in DKD progression [68–72]. In addition, recent data showed that women with pre-gestational diabetes who had preeclampsia had a 4–5 times increased long-term risk of end-stage renal disease or death [73] and preeclampsia has shown to be related to a decrease in estradiol concentration [74]. Estradiol metabolites concentrations can also be determinant for the progression of DKD. For example, 2-ethoxyestradiol and 2-methoxyestradiol have shown to be renoprotective, increasing renal blood flow and glomerular filtration, and decreasing albuminuria in diabetic ZSF1 rats [75].
Progesterone has also shown to play a role in DKD. Replacement of progesterone can ameliorate DKD in rat models of diabetes [76]. However, some studies suggest that high levels of progesterone could play a key role in the development of insulin resistance and gestational diabetes [77]. Progesterone also has a high affinity for the mineralocorticoid receptor and can antagonize its effects [57]. However, the overall antagonist or agonist activity of progesterone also depends on its metabolites. For example, 20alpha-DH-progesterone in vitro has demonstrated the strongest agonistic potency reaching 11.5% of aldosterone transactivation, while 17alpha-OH-progesterone has shown to be a strong mineral corticoid receptor antagonist [78].
Testosterone is one of multiple androgens synthesized by cytochrome P450 enzymes in the gonads, adrenal glands and, interestingly, also in the kidney [79]. Men with either T1D or T2D have lower concentrations of total and calculated free testosterone compared with healthy controls [80–83], and a decrease in serum testosterone predicts the development of macroalbuminuria [83]. However, elevated serum estradiol and testosterone are independent predictors of ESKD development in men with T1D [83]. Testosterone supplementation attenuates kidney injury in diabetic rat models [84]. In human studies, testosterone may attenuate the morbidity and mortality of cardiovascular disease in men with T2D, but there are no conclusive data for DKD in adults with T1D [85]. In men with androgen deficiency, testosterone supplementation improves arterial vasoreactivity, reduces proinflammatory cytokine levels, decreases triglycerides and total cholesterol concentrations, reduces visceral fat, and enhances glucose-stimulated insulin secretion [86–88]. Studies of testosterone action and replacement in women are scarce but observational data suggest that testosterone may have a cardioprotective role, particularly in menopausal women [89].
Low Sex Hormone-Binding Globulin (SHBG) has been found in men with T2D and young boys with T1D, and a correlation between lower concentrations and new onset microalbuminuria has been found in men with T1D [80, 83]. However, young men with childhood onset T1D have higher concentrations of SHBG and testosterone than their healthy counterparts [90].
Among individuals with diabetes, there is an imbalance in sex hormone concentrations as men demonstrate lower levels of total testosterone and higher levels of estradiol due to increased adipose-tissue driven testosterone aromatization when compared with non-diabetic controls [60, 83, 86, 91–93]. By contrast, women with diabetes have lower concentrations of estradiol and higher concentrations of total testosterone when compared to their non-diabetic counterparts [60, 83, 86, 91–93]. Interestingly, women with diabetes and albuminuria have lower estradiol concentrations than counterparts without albuminuria and healthy controls [94]. In addition, sex hormones play different roles in men and women. For example, estrogens have shown to regulate growth hormone (GH) secretion only in men [95] and GH can also play an important role in DKD development and progression [96].
Insulin sensitivity and secretion have also shown to be influenced by sex hormones [97, 98]. Pre-menopausal women demonstrated higher insulin sensitivity and postprandial insulin levels than men [97, 98]. However, women with T1D showed greater deficits in insulin sensitivity than men counterparts [99]. Women with T2D had a similar glucose metabolism than men with T2D [100]. Thus, in women with both types of diabetes insulin sensitivity and secretion were equal or worse than men counterpart, loosing the advantage in glucose metabolism seen in adults with normal glucose tolerance.
Sex hormone differences are theorized to influence the dimorphism of the renin-angiotensin-aldosterone-system (RAAS). The RAAS plays a key role in kidney hemodynamic function and disease progression [101]. In general, men have higher RAAS activity levels than women [102]. There are multiple possible sex hormone-related etiologies for this dimorphism. Estrogen promotes higher angiotensinogen levels and reduces angiotensin converting enzyme [103] activity, renin activity, angiotensin II receptor type 1 (AT1R) density, aldosterone secretion, angiotensin II (AT2) activity, and hemodynamic and excretory responses to AT2 [101, 104] (Figure 2). In addition, progesterone and aldosterone compete for the mineralocorticoid receptor, effecting the regulation of water and electrolytes. Androgens can induce renal vasoconstriction through increased RAAS activity [102, 104]. Furthermore, the role of the counter-regulatory arm of the RAAS, characterized by the ACE2-Angiotensin 1–7 axis, has not been thoroughly investigated. This arm is upregulated by estrogen and generally opposes the traditional pathway, leading to vasodilation, natriuresis, and anti-proliferative effects on vascular smooth muscle cells [102].
Figure 2.

Effects of estrogens on DKD pathophysiologic mechanisms
Sex hormones also play a regulatory role in oxidative stress. In animal studies, estradiol acts as an antioxidant and rats who have undergone oophorectomy demonstrate an increase in renal nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and the glomerulosclerosis index, a measure of the percentage of sclerosis in glomeruli [58]. Estradiol replacement reverses these changes [105, 106]. Conversely, androgens increase oxidative stress both systematically and in the kidney [58, 105, 106]. Sex hormone differences appear to play an integral role in the pathophysiology of sexual dimorphism in DKD and further exploration is needed to elucidate if treatments such as altering hormone levels could attenuate kidney injury caused by the hormone imbalances seen in T1D and T2D.
2. Kidney hemodynamic function
Kidney hemodynamic differences constitute another important facet of the effects of sexual dimorphism in DKD (Figure 3). One of the most studied and early events in the development of DKD is hyperfiltration [107]. We demonstrated with other research groups that adolescent girls with T1D or T2D have a higher prevalence of renal hyperfiltration compared to boys with diabetes [108, 109]. Škrtić and colleagues calculated kidney hemodynamic parameters by Gomez equations and found that women with T1D and hyperfiltration had higher efferent arteriolar resistance and lower effective renal plasma flow compared to men with T1D who also had hyperfiltration [39]. These pathophysiologic features lead to higher glomerular hydrostatic pressure in women which can worsen DKD. Another study in adolescents with T1D confirmed lower effective renal plasma flow during euglycemia and increased renal vascular resistance and filtration rates during clamped hyperglycemia in young women vs. young men [110]. The underlying mechanisms have not yet been fully explained but could involve sex differences in nitric oxide (NO) levels, since female animals have higher NO synthase activity that leads to an increase in NO which in turns causes vasodilation of the afferent arteriole [111, 112]. NO abnormalities have also been linked to DKD, with upregulation early in the development of diabetes and downregulation in advanced kidney disease [113]. Additionally, Studies have exhibited induction of NO synthesis by ER-mediated mechanisms [114].
Figure 3. Regulation of kidney hemodynamics.
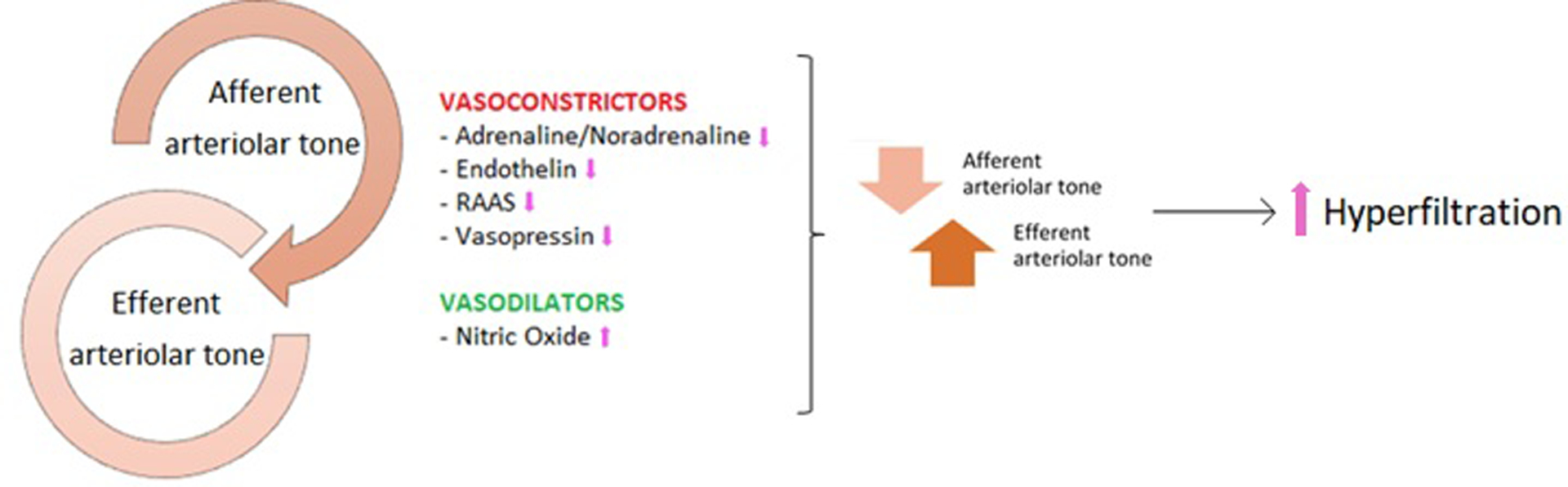
Kidney hemodynamics are regulated by the interplay between afferent and efferent arteriolar tone. Efferent vasoconstriction and afferent vasodilation increase intraglomerular pressure and glomerular filtration rate. The arrows indicate the changes in women compared to men.
When assessing kidney hemodynamics and filtration, it is important to consider the RAAS system. This system has demonstrated sex-dependent effects and plays a key role in kidney hemodynamics and disease progression [101]. Men have a higher sensitivity to AT2, greater cardiovascular protective effects in response to angiotensin-converting-enzyme inhibitors (ACEis) and AT2 receptor blockers (ARBs), and a better metabolic profile with lower AT2 receptor type II (AT2R) when compared to women [101, 102]. The distributions of AT2R and AT1R also differ between the sexes. As discussed previously, men have higher RAAS activity levels and this could explain the greater effect of both ACEis and ARBs in men [102].
Another important mechanism of sex-dimorphism in kidney hemodynamics is endothelin-1 (ET-1), a potent vasoconstrictor also produced by the kidney. Overall, ET-1 levels are higher in men than women and these levels increase due to a variety of conditions, including aging, diabetes and menopause in women [115]. However, in some clinical circumstances (e.g. pulmonary hypertension), women have shown better responses to type A endothelin receptor blockers [115]. These data are corroborated by the greater ET-1-induced vasoconstriction observed in female rats with diabetes [116]. Additionally, endothelin has shown sex hormone-responsive effects and testosterone treatment increases plasma endothelin concentrations in female animals [58].
Finally, copeptin, the C-terminal end of a vasopressin hormone precursor, correlates with a higher risk of development and progression of diabetes and DKD [117]. However, in the British Regional Heart Study, copeptin concentrations were associated with an increased risk of diabetes only in men [117].
3. Adiponectin
Adiponectin is an adipocytokine produced by adipocytes, skeletal and cardiac myocytes, and endothelial cells that plays an important role in inflammation and insulin sensitivity. Lower concentrations are associated with obesity, insulin resistance, and T2D [118]. Higher concentrations of this adipocytokine are found in women, suggesting a possible role of adiponectin in DKD sexual dimorphism [119]. In a longitudinal study of Pima Indians (n=1,069), adiponectin concentrations strongly correlated with serum creatinine and diabetes duration in participants with T2D after adjustment for age and sex [120]. Furthermore, lower levels of adiponectin predicted the onset of T2D [121–123]. Few studies have shown insulin-sensitizing, anti-atherogenic, and anti-inflammatory effects of adiponectin administration in humans and rodents [118]. However, Looker et al. found that adiponectin concentrations increased with macroalbuminuria and DKD, probably exerting a compensatory action against further progression of DKD [120]. In fact, adiponectin receptor agonism has shown to exert renoprotective effects in DKD [124]. In vitro experiments in human adipocytes from Horenburg et al. demonstrated that while adipocytes express AR and ER, both the expression and secretion of adiponectin are not affected by the presence of sex steroids, leading to the hypothesis of an additional serum factor that is responsive to sex hormones and is ultimately responsible for the sexual dimorphism of circulating adiponectin levels [125]. This hypothesis is further supported by a study in 1546 adults which also found that sex hormone regulation was not responsible for circulating adiponectin concentrations and did not explain the differences between the sexes [126]. In the same study, median serum adiponectin concentration was 50% higher in women than in men.
4. Oxidative stress
Oxidative stress is a key pathogenetic feature of many kidney diseases, including DKD, and there is evidence of higher degrees of oxidative stress in men than women [58]. Diabetes is intrinsically associated with an imbalance between O2 consumption and production rate in the kidney, leading to a relative state of hypoxia and ischemia [38, 40]. In addition, insulin resistance and hyperglycemia can worsen kidney hypoxia through ATP depletion due to mitochondrial dysfunction and AMP-activated protein kinase inhibition in glomerular and tubular cells [37, 38, 127]. Mitochondrial dysfunction can also lead to increased production of reactive oxygen species (ROS) [38]. Specifically in the kidney, hyperglycemia induces intracellular reactive-oxygen-species (ROS) in mesangial and tubular cells in a process involving intracellular glucose uptake and metabolism and advanced glycation end-products and cytokines including TGF-β1, and AT2 [128]. ROS are capable of subsequently activating transcription factors Nuclear Factor-Kappa B (NF-κB) and Activator-Protein-1 (AP-1) and upregulating extracellular matrix (ECM) expression which may lead to tubulointerstitial fibrosis [128]. Sex hormones play a regulatory role in oxidative stress. While estradiol acts as an antioxidant, androgens increase oxidative stress [58, 105, 106].
Noradrenaline has demonstrated associations with both worsened ischemia and ROS production in the kidney [129]. Male sex correlates with higher levels of noradrenaline at baseline and after kidney ischemic injury [5, 130, 131]. Animal models indicate that this sex dimorphism could be secondary to differences in monoamine oxidase type A (MAOa) concentrations and/or to the role of estradiol in reducing the effects of the renal sympathetic nervous system via NO production [131]. In addition, NO and ROS signaling cross-talk and NO-cGMP pathway interactions play a role in oxidative stress and insulin sensitivity and secretion [130]. Thus, NO is another important player of sex-related differences in oxidative stress.
5. Membrane channels and Water-electrolyte homeostasis
Renal ion transport alterations have been related to DKD development and progression [132]. Animal models demonstrate sex-related differences in both membrane channels and water-electrolyte homeostasis, with females appearing to concentrate urine better than males in the setting of dehydration [133]. Two possible explanations for this finding include increased aquaporin 2 mobilization and expression and increased activity of the collecting duct intercalated cell vacuolar-type H+ pumping ATPase in female animals vs. males [133]. Veiras and colleagues found higher Na+-K+-Cl− cotransporter 2 and Na+-K+-ATPase protein densities along the kidney medulla and higher Na+-Cl− cotransporter, Claudin 7, and epithelial Na+ channel concentrations in the distal nephron and collecting duct of female mice [134]. These findings suggest an increased uptake of electrolytes in the kidneys in women vs. men, subsequently leading to increased reabsorption of water and more concentrated urine. Sabolic and colleagues also found both sex and species differences in the sodium glucose co-transporter 2 (SGLT2) transcripts with female rats having higher expression than male counterparts [135]. This finding could also suggest increased reabsorption of water in female animals as the reabsorption of sodium and glucose increases due to increased transporters. Contrastingly, male animals have higher expression of the aquaporin protein 1, a water transporter in the kidney [136]. These findings highlight the significant variability between the sexes in both kidney membrane channels and water-electrolyte homeostasis. More studies exploring human pathophysiology might be considered to fully identify the role of sex in both normal physiology and DKD.
6. Different impact of concomitant risk factors
Many cardiovascular risk factors such as hypertension, hyperuricemia, central obesity, and dyslipidemia can magnify the risk for DKD [34, 137]. In addition, these risk factors appear to have less deleterious effects in women than in men [137]. However, in individuals with diabetes, women appear to be paradoxically affected as they have higher risk of complications than men in the setting of positive risk factors, irrespective of diabetes type [34, 55]. In a meta-analysis (n= 5 162 654 participants), Wang et al. found that women with diabetes have a 58% greater risk of coronary heart disease (CHD) and a 13% greater risk of all-cause mortality when compared with men [138]. Hormone imbalances could serve as a possible explanation for these observed differences, but little is conclusively known.
Sexual Dimorphism and Therapies
The discussion of the DKD pathophysiology presents numerous therapeutic implications. Replacement of estradiol [58, 68–72], progesterone [76], and testosterone [86–88] have already shown promising results, improving DKD in both human and animal models through different pathways which involve almost all the pathophysiologic mechanisms previously shown.
SGLT2 inhibitors have emerged as a compelling therapy to protect kidney function in DKD. SGLT2 inhibitors ameliorate kidney hemodynamic dysfunction and reduce kidney injury across cardiovascular and kidney outcome trials in adults with T2D [139, 140]. These inhibitors may also regulate oxidative stress through a reduction of renal O2 consumption in the proximal tubular cells [139] and may play a role in the regulation of adiponectin [118, 141]. It is notable that SGLT2 inhibition has provided similar renoprotective effects in both sexes [142, 143], emphasizing the potential value of this class in the treatment of both sexes with DKD. Currently, SGLT2 inhibitors are rare as both a monotherapy and in a combination therapy, but there is much promise in its benefits [144]. Drugs targeting sex differences in SGLT2 expression, as well as aquaporin channels, claudins, and Na+/K+ ATPases, could also be helpful in the treatment of DKD. In particular, the Na+/K+ ATPase can be regulated by multiple different mechanisms, including adrenergic agonists/antagonists [145] and dopamine receptors [146].
Therapies acting on sex-differences in kidney hemodynamics could also be promising. Men and women respond differently to RAAS inhibitors, but in order to optimize therapeutic choices further controlled studies are needed [102]. In addition, there are promising experimental drugs. For example, in animal models human recombinant ACE2 and angiotensin-(1–7) somministration slow the progression of DKD [147]. Vasopressin V2 receptors (V2R) antagonists, such as lixivaptan, reduce albuminuria and hyperfiltration in diabetic animal models [148] and could be a therapeutic target as well. Finally, the “Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease” study, a double-blind, randomized, placebo-controlled trial of atrasentan, a selective endothelin receptor antagonist, has recently demonstrated a possible role for endothelin receptor antagonists in preserving renal function in individuals with T2D who are at risk of DKD [149].
There are also sex-specific therapies that could correct the oxidative imbalance present in diabetes. Estradiol replacement and monoamine oxidase (MAOa) inhibitors could potentially reverse diabetic oxidative imbalances as they influence redox homeostasis [58, 131]. Phosphodiesterase type 5 inhibitors (PDE-5), piperazine ferulate, astragaloside IV, bardoxolone and idebenone all attenuate oxidative stress and exert renoprotective effects in diabetes [150–154]. Treatment with Astragaloside IV reduces oxidative stress through inhibition of the TLR4/NFκB pathway, one of the main injury patterns of oxidative stress [151, 155]. In the randomized controlled Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes [152] trial, bardoxolone was associated with improvements in GFR, but no significant reductions were seen in ESKD or cardiovascular mortality in adults with T2D who had stage 4 CKD [152]. In addition to its antioxidative action, Idebenone increases insulin sensitivity both in vitro and in vivo [153], potentially relieving some stress on the kidney as glucose levels entering the organ decrease. Another potential therapeutic target involves adiponectin, which could also play a part in regulating sex-related differences in oxidative stress in DKD [117, 155]. For example, AdipoRon, an adiponectin receptor agonist, improves DKD in human glomerular endothelial cells and in diabetic mice models [156]. Indeed, mechanisms of action of this oral drug include the reduction of high-glucose–induced oxidative stress and lipotoxicity [156]. Thiazolidinediones and different bariatric surgery’s approaches have also been shown to increase adiponectin concentrations and exert nephroprotective effects [155, 157]. However, most animal and human studies involving antioxidant drugs do not include an equal number of men and women or male and female animals and sex is rarely considered as an independent variable. Thus, the effect of sexual dimorphism on these pathways remains unknown. However, it is reasonable to hypothesize that antioxidant drugs could have different effects in men vs. women.
There are potential implications of sexual dimorphism in other comorbidities which should also be considered in the development of therapies for DKD. Hypertension is a known risk factor for the progression of a variety of kidney diseases, and sex-related differences in the pathophysiology and therapeutic response of hypertension have been shown [158]. Men and women, for example, have differential responses to ACEi and ARB therapies in the treatment of hypertension [102]. Another example is represented by hyperuricemia, which has also demonstrated a key role in the pathogenesis of DKD [159]. In fact, hyperuricemic women respond better to febuxostat than to allopurinol compared to men [160]. Much remains to be learned about how the effects of the therapies mentioned potentially differ between the sexes. Strategies leveraging these therapies cannot be fully optimized until potential sex-related differences are fully explored.
Conclusions
In this review, we have described in detail the effects of sexual dimorphism on the metabolic and molecular mechanisms underlying DKD (Figure 1). Men with either T1D or T2D appear to be at higher risk of DKD than pre-menopausal women, and the sex-related discrepancy is magnified when diabetes duration exceeds 25 years. Post-menopausal women, in contrast, appear to be at a higher risk than both groups for DKD development. Additionally, pre- and post-menopausal women have a higher prevalence of ESKD than men. Concomitant risk factors such as hypertension appear to progress to end organ damage to a greater degree in women vs. men with diabetes. Childhood onset of T1D is a protective factor for both sexes, while pubertal onset of both diabetes types represents a risk factor for the development of DKD.
Yet, much remains unknown about the precise mechanisms of these sex-differences and how they relate to the progression of DKD. Consequently, further study of the sexual dimorphism in DKD is critical. Not only to advance our overall understanding, but also to develop targeted therapies that take into consideration the inherent differences in pathophysiology between the sexes. Though promising, the efficacy and value of the potential therapies explored in this review cannot be fully understood and put into clinical practice without further studies exploring the effects of sexual dimorphism. An integrated biological approach is necessary and possible research strategies include clinical phenotyping through the use of kidney clearance studies and functional imaging, as well as histopathological and molecular phenotyping through kidney biopsy analysis. Improvements in our understanding of the mechanisms underlying sex differences in DKD and other related comorbidities could serve as an important first step towards personalized precision medicine.
Disclosures:
PB has acted as a consultant for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi, Novo Nordisk, AstraZeneca, Lilly USA and Horizon Pharma. PB serves on the advisory board of XORTX and Boehringer Ingelheim. All support was outside the submitted work. NN has previously consulted for Antares Pharma, Inc. D.Z.I.C has received honoraria from Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, Abbvie, Janssen, Bayer, Prometic, BMS and Novo-Nordisk and has received operational funding for clinical trials from Boehringer Ingelheim-Lilly, Merck, Janssen, Sanofi, AstraZeneca and Novo-Nordisk. RJ has consulted with Horizon, AstraZeneca, and has equity with XORTX Therapeutics and Colorado Research Partners LLC. DvR has acted as a consultant and received honoraria from Boehringer Ingelheim and Lilly, Merck, Novo Nordisk, MSD, Sanofi and AstraZeneca and has received research operating funds from Boehringer Ingelheim-Lilly Diabetes Alliance, AstraZeneca and MDS. All honoraria are paid to his employer (Amsterdam University Medical Center). FP, IM, KLT, RN, MP, and KJN have nothing to disclose. All authors approved of the final manuscript.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Putting gender on the agenda. Nature, 2010. 465(7299): p. 665. [DOI] [PubMed] [Google Scholar]
- 2.Mazure CM and Jones DP, Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Womens Health, 2015. 15: p. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tower J, Sex-specific regulation of aging and apoptosis. Mech Ageing Dev, 2006. 127(9): p. 705–18. [DOI] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F, Gender differences in glucose homeostasis and diabetes. Physiol Behav, 2018. 187: p. 20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goel N, et al. , Sex differences in the HPA axis. Compr Physiol, 2014. 4(3): p. 1121–55. [DOI] [PubMed] [Google Scholar]
- 6.Kim AM, Tingen CM, and Woodruff TK, Sex bias in trials and treatment must end. Nature, 2010. 465(7299): p. 688–9. [DOI] [PubMed] [Google Scholar]
- 7.Pan JS and Sheikh-Hamad D, Mitochondrial dysfunction in acute kidney injury and sex-specific implications. Med Res Arch, 2019. 7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrero JJ, Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res, 2010. 33(5): p. 383–92. [DOI] [PubMed] [Google Scholar]
- 9.Cobo G, et al. , Sex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysis. Clin Sci (Lond), 2016. 130(14): p. 1147–63. [DOI] [PubMed] [Google Scholar]
- 10.Silbiger S and Neugarten J, Gender and human chronic renal disease. Gend Med, 2008. 5 Suppl A: p. S3–S10. [DOI] [PubMed] [Google Scholar]
- 11.Neugarten J, Acharya A, and Silbiger SR, Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol, 2000. 11(2): p. 319–29. [DOI] [PubMed] [Google Scholar]
- 12.Evans M, et al. , The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis, 2005. 46(5): p. 863–70. [DOI] [PubMed] [Google Scholar]
- 13.Eriksen BO and Ingebretsen OC, The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int, 2006. 69(2): p. 375–82. [DOI] [PubMed] [Google Scholar]
- 14.Neugarten J, Golestaneh L, and Kolhe NV, Sex differences in acute kidney injury requiring dialysis. BMC Nephrol, 2018. 19(1): p. 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrero JJ, et al. , Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol, 2018. 14(3): p. 151–164. [DOI] [PubMed] [Google Scholar]
- 16.Prevention., C.f.D.C.a., Chronic Kidney Disease in the United States, 2019. 2019, US Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- 17.Fraser SDS and Roderick PJ, Kidney disease in the Global Burden of Disease Study 2017. Nat Rev Nephrol, 2019. 15(4): p. 193–194. [DOI] [PubMed] [Google Scholar]
- 18.Jafar TH, et al. , The rate of progression of renal disease may not be slower in women compared with men: a patient-level meta-analysis. Nephrol Dial Transplant, 2003. 18(10): p. 2047–53. [DOI] [PubMed] [Google Scholar]
- 19.Saran R, et al. , US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis, 2019. 73(3 Suppl 1): p. A7–a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skupien J, et al. , Variations in Risk of End-Stage Renal Disease and Risk of Mortality in an International Study of Patients With Type 1 Diabetes and Advanced Nephropathy. Diabetes Care, 2019. 42(1): p. 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong W, et al. , Sex-specific association between type 1 diabetes and the risk of end-stage renal disease: a systematic review and meta-analysis. Endocrine, 2020. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen P, et al. , Progression of diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int Suppl, 1999. 71: p. S101–5. [DOI] [PubMed] [Google Scholar]
- 23.Sibley SD, et al. , Gender and elevated albumin excretion in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort: role of central obesity. Am J Kidney Dis, 2006. 47(2): p. 223–32. [DOI] [PubMed] [Google Scholar]
- 24.Mangili R, et al. , Arterial hypertension and microalbuminuria in IDDM: the Italian Microalbuminuria Study. Diabetologia, 1994. 37(10): p. 1015–24. [DOI] [PubMed] [Google Scholar]
- 25.Retnakaran R, et al. , Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes, 2006. 55(6): p. 1832–9. [DOI] [PubMed] [Google Scholar]
- 26.Hovind P, et al. , Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ, 2004. 328(7448): p. 1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raile K, et al. , Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care, 2007. 30(10): p. 2523–8. [DOI] [PubMed] [Google Scholar]
- 28.Mollsten A, et al. , Cumulative risk, age at onset, and sex-specific differences for developing end-stage renal disease in young patients with type 1 diabetes: a nationwide population-based cohort study. Diabetes, 2010. 59(7): p. 1803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harjutsalo V, et al. , Sex-related differences in the long-term risk of microvascular complications by age at onset of type 1 diabetes. Diabetologia, 2011. 54(8): p. 1992–9. [DOI] [PubMed] [Google Scholar]
- 30.Orchard TJ, et al. , Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes, 1990. 39(9): p. 1116–24. [DOI] [PubMed] [Google Scholar]
- 31.Gall MA, et al. , Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ, 1997. 314(7083): p. 783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravid M, et al. , Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med, 1998. 158(9): p. 998–1004. [DOI] [PubMed] [Google Scholar]
- 33.de Hauteclocque A, et al. , The influence of sex on renal function decline in people with Type 2 diabetes. Diabet Med, 2014. 31(9): p. 1121–8. [DOI] [PubMed] [Google Scholar]
- 34.Kajiwara A, et al. , Sex Differences in the Renal Function Decline of Patients with Type 2 Diabetes. J Diabetes Res, 2016. 2016: p. 4626382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu MK, et al. , Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: the pathways study. Am J Nephrol, 2012. 36(3): p. 245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crook ED, et al. , Endstage renal disease owing to diabetic nephropathy in Mississippi: an examination of factors influencing renal survival in a population prone to late referral. J Investig Med, 2001. 49(3): p. 284–91. [DOI] [PubMed] [Google Scholar]
- 37.Yu MK, Katon W, and Young BA, Associations between sex and incident chronic kidney disease in a prospective diabetic cohort. Nephrology (Carlton), 2015. 20(7): p. 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjornstad P and Cherney DZ, Renal Hyperfiltration in Adolescents with Type 2 Diabetes: Physiology, Sex Differences, and Implications for Diabetic Kidney Disease. Curr Diab Rep, 2018. 18(5): p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, et al. , Factors predictive of nephropathy in DCCT Type 1 diabetic patients with good or poor metabolic control. Diabet Med, 2003. 20(7): p. 580–5. [DOI] [PubMed] [Google Scholar]
- 40.Skrtic M, et al. , Influence of sex on hyperfiltration in patients with uncomplicated type 1 diabetes. Am J Physiol Renal Physiol, 2017. 312(4): p. F599–F606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monti MC, et al. , Familial risk factors for microvascular complications and differential male-female risk in a large cohort of American families with type 1 diabetes. J Clin Endocrinol Metab, 2007. 92(12): p. 4650–5. [DOI] [PubMed] [Google Scholar]
- 42.Shen Y, et al. , Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: a systematic review and meta-analysis. Endocrine, 2017. 55(1): p. 66–76. [DOI] [PubMed] [Google Scholar]
- 43.Schultz CJ, et al. , Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Oxford Regional Prospective Study Group. Diabetes Care, 1999. 22(3): p. 495–502. [DOI] [PubMed] [Google Scholar]
- 44.Costacou T, et al. , Sex differences in the development of kidney disease in individuals with type 1 diabetes mellitus: a contemporary analysis. Am J Kidney Dis, 2011. 58(4): p. 565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossing P, Hougaard P, and Parving HH, Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care, 2002. 25(5): p. 859–64. [DOI] [PubMed] [Google Scholar]
- 46.Breyer JA, et al. , Predictors of the progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy. The Collaborative Study Group. Kidney Int, 1996. 50(5): p. 1651–8. [DOI] [PubMed] [Google Scholar]
- 47.Okada K, et al. , Sex differences in the prevalence, progression, and improvement of chronic kidney disease. Kidney Blood Press Res, 2014. 39(4): p. 279–88. [DOI] [PubMed] [Google Scholar]
- 48.Finne P, et al. , Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA, 2005. 294(14): p. 1782–7. [DOI] [PubMed] [Google Scholar]
- 49.Nelson RG, et al. , Determinants of end-stage renal disease in Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus and proteinuria. Diabetologia, 1993. 36(10): p. 1087–93. [DOI] [PubMed] [Google Scholar]
- 50.Inker LA, et al. , Performance of glomerular filtration rate estimating equations in a community-based sample of Blacks and Whites: the multiethnic study of atherosclerosis. Nephrol Dial Transplant, 2018. 33(3): p. 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glassock RJ and Winearls C, Screening for CKD with eGFR: doubts and dangers. Clin J Am Soc Nephrol, 2008. 3(5): p. 1563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Froissart M, et al. , Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol, 2005. 16(3): p. 763–73. [DOI] [PubMed] [Google Scholar]
- 53.Neugarten J and Golestaneh L, Influence of Sex on the Progression of Chronic Kidney Disease. Mayo Clin Proc, 2019. 94(7): p. 1339–1356. [DOI] [PubMed] [Google Scholar]
- 54.Ricardo AC, et al. , Sex-Related Disparities in CKD Progression. J Am Soc Nephrol, 2019. 30(1): p. 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maric-Bilkan C, Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin Sci (Lond), 2017. 131(9): p. 833–846. [DOI] [PubMed] [Google Scholar]
- 56.Lane PH, Diabetic kidney disease: impact of puberty. Am J Physiol Renal Physiol, 2002. 283(4): p. F589–600. [DOI] [PubMed] [Google Scholar]
- 57.Khalil R, et al. , Sex steroids and the kidney: role in renal calcium and phosphate handling. Mol Cell Endocrinol, 2018. 465: p. 61–72. [DOI] [PubMed] [Google Scholar]
- 58.Yanes LL, Sartori-Valinotti JC, and Reckelhoff JF, Sex steroids and renal disease: lessons from animal studies. Hypertension, 2008. 51(4): p. 976–81. [DOI] [PubMed] [Google Scholar]
- 59.Wells CC, et al. , Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med, 2005. 2(4): p. 227–37. [DOI] [PubMed] [Google Scholar]
- 60.Taylor SR, Meadowcraft LM, and Williamson B, Prevalence, Pathophysiology, and Management of Androgen Deficiency in Men with Metabolic Syndrome, Type 2 Diabetes Mellitus, or Both. Pharmacotherapy, 2015. 35(8): p. 780–92. [DOI] [PubMed] [Google Scholar]
- 61.Dixon A and Maric C, 17beta-Estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-beta protein expression and signaling. Am J Physiol Renal Physiol, 2007. 293(5): p. F1678–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Catanuto P, et al. , 17 beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int, 2009. 75(11): p. 1194–1201. [DOI] [PubMed] [Google Scholar]
- 63.Mankhey RW, Bhatti F, and Maric C, 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol, 2005. 288(2): p. F399–405. [DOI] [PubMed] [Google Scholar]
- 64.Mankhey RW, et al. , 17beta-Estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. Am J Physiol Regul Integr Comp Physiol, 2007. 292(2): p. R769–77. [DOI] [PubMed] [Google Scholar]
- 65.Doublier S, et al. , Testosterone and 17beta-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int, 2011. 79(4): p. 404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keck M, et al. , Hormonal status affects the progression of STZ-induced diabetes and diabetic renal damage in the VCD mouse model of menopause. Am J Physiol Renal Physiol, 2007. 293(1): p. F193–9. [DOI] [PubMed] [Google Scholar]
- 67.Irsik DL, et al. , Renoprotective impact of estrogen receptor-alpha and its splice variants in female mice with type 1 diabetes. Am J Physiol Renal Physiol, 2018. 315(3): p. F512–F520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karl M, et al. , Differential effects of continuous and intermittent 17beta-estradiol replacement and tamoxifen therapy on the prevention of glomerulosclerosis: modulation of the mesangial cell phenotype in vivo. Am J Pathol, 2006. 169(2): p. 351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dixon A, et al. , Renoprotective effects of a selective estrogen receptor modulator, raloxifene, in an animal model of diabetic nephropathy. Am J Nephrol, 2007. 27(2): p. 120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szekacs B, et al. , Postmenopausal hormone replacement improves proteinuria and impaired creatinine clearance in type 2 diabetes mellitus and hypertension. BJOG, 2000. 107(8): p. 1017–21. [DOI] [PubMed] [Google Scholar]
- 71.Hadjadj S, et al. , Effect of raloxifene -- a selective oestrogen receptor modulator -- on kidney function in post-menopausal women with Type 2 diabetes: results from a randomized, placebo-controlled pilot trial. Diabet Med, 2007. 24(8): p. 906–10. [DOI] [PubMed] [Google Scholar]
- 72.Agarwal M, et al. , The relationship between albuminuria and hormone therapy in postmenopausal women. Am J Kidney Dis, 2005. 45(6): p. 1019–25. [DOI] [PubMed] [Google Scholar]
- 73.Sandvik MK, et al. , Are adverse pregnancy outcomes risk factors for development of end-stage renal disease in women with diabetes? Nephrol Dial Transplant, 2010. 25(11): p. 3600–7. [DOI] [PubMed] [Google Scholar]
- 74.Berkane N, et al. , From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr Rev, 2017. 38(2): p. 123–144. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, et al. , 2-Methoxyestradiol and 2-ethoxyestradiol retard the progression of renal disease in aged, obese, diabetic ZSF1 rats. J Cardiovasc Pharmacol, 2007. 49(1): p. 56–63. [DOI] [PubMed] [Google Scholar]
- 76.Al-Trad B, Ashankyty IM, and Alaraj M, Progesterone ameliorates diabetic nephropathy in streptozotocin-induced diabetic Rats. Diabetol Metab Syndr, 2015. 7: p. 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Branisteanu DD and Mathieu C, Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol Metab, 2003. 14(2): p. 54–6. [DOI] [PubMed] [Google Scholar]
- 78.Quinkler M, et al. , Agonistic and antagonistic properties of progesterone metabolites at the human mineralocorticoid receptor. Eur J Endocrinol, 2002. 146(6): p. 789–99. [DOI] [PubMed] [Google Scholar]
- 79.Quinkler M, et al. , The human kidney is a progesterone-metabolizing and androgen-producing organ. J Clin Endocrinol Metab, 2003. 88(6): p. 2803–9. [DOI] [PubMed] [Google Scholar]
- 80.Stellato RK, et al. , Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care, 2000. 23(4): p. 490–4. [DOI] [PubMed] [Google Scholar]
- 81.Fukui M, et al. , Low serum testosterone concentration in middle-aged men with type 2 diabetes. Endocr J, 2007. 54(6): p. 871–7. [DOI] [PubMed] [Google Scholar]
- 82.Barrett-Connor E, Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med, 1992. 117(10): p. 807–11. [DOI] [PubMed] [Google Scholar]
- 83.Maric C, et al. , Association between testosterone, estradiol and sex hormone binding globulin levels in men with type 1 diabetes with nephropathy. Steroids, 2010. 75(11): p. 772–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manigrasso MB, et al. , Combined inhibition of aromatase activity and dihydrotestosterone supplementation attenuates renal injury in male streptozotocin (STZ)-induced diabetic rats. Am J Physiol Renal Physiol, 2012. 302(9): p. F1203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang KS, et al. , Effects of testosterone supplementation therapy on lipid metabolism in hypogonadal men with T2DM: a meta-analysis of randomized controlled trials. Andrology, 2018. 6(1): p. 37–46. [DOI] [PubMed] [Google Scholar]
- 86.Traish AM, et al. , The dark side of testosterone deficiency: III. Cardiovascular disease. J Androl, 2009. 30(5): p. 477–94. [DOI] [PubMed] [Google Scholar]
- 87.Wortham M and Sander M, High T Gives beta Cells a Boost. Cell Metab, 2016. 23(5): p. 761–3. [DOI] [PubMed] [Google Scholar]
- 88.Fink J, Matsumoto M, and Tamura Y, Potential application of testosterone replacement therapy as treatment for obesity and type 2 diabetes in men. Steroids, 2018. 138: p. 161–166. [DOI] [PubMed] [Google Scholar]
- 89.Davis SR and Wahlin-Jacobsen S, Testosterone in women--the clinical significance. Lancet Diabetes Endocrinol, 2015. 3(12): p. 980–92. [DOI] [PubMed] [Google Scholar]
- 90.Danielson KK, Drum ML, and Lipton RB, Sex hormone-binding globulin and testosterone in individuals with childhood diabetes. Diabetes Care, 2008. 31(6): p. 1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ding EL, et al. , Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA, 2006. 295(11): p. 1288–99. [DOI] [PubMed] [Google Scholar]
- 92.Grossmann M, et al. , Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab, 2008. 93(5): p. 1834–40. [DOI] [PubMed] [Google Scholar]
- 93.Salonia A, et al. , Sexual function and endocrine profile in fertile women with type 1 diabetes. Diabetes Care, 2006. 29(2): p. 312–6. [DOI] [PubMed] [Google Scholar]
- 94.Reckelhoff JF and Maric C, Sex and gender differences in cardiovascular-renal physiology and pathophysiology. Steroids, 2010. 75(11): p. 745–6. [DOI] [PubMed] [Google Scholar]
- 95.Birzniece V, et al. , Disparate Effect of Aromatization on the Central Regulation of GH Secretion by Estrogens in Men and Postmenopausal Women. J Clin Endocrinol Metab, 2019. 104(7): p. 2978–2984. [DOI] [PubMed] [Google Scholar]
- 96.Nishad R, et al. , Growth hormone induces Notch1 signaling in podocytes and contributes to proteinuria in diabetic nephropathy. J Biol Chem, 2019. 294(44): p. 16109–16122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goossens GH, Jocken JWE, and Blaak EE, Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol, 2020. [DOI] [PubMed] [Google Scholar]
- 98.Basu A, Dube S, and Basu R, Men Are from Mars, Women Are from Venus: Sex Differences in Insulin Action and Secretion. Adv Exp Med Biol, 2017. 1043: p. 53–64. [DOI] [PubMed] [Google Scholar]
- 99.Millstein RJ, et al. , Sex-specific differences in insulin resistance in type 1 diabetes: The CACTI cohort. J Diabetes Complications, 2018. 32(4): p. 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tura A, et al. , Sex- and age-related differences of metabolic parameters in impaired glucose metabolism and type 2 diabetes compared to normal glucose tolerance. Diabetes Res Clin Pract, 2018. 146: p. 67–75. [DOI] [PubMed] [Google Scholar]
- 101.Miller JA, et al. , Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol, 2006. 17(9): p. 2554–60. [DOI] [PubMed] [Google Scholar]
- 102.White MC, Fleeman R, and Arnold AC, Sex differences in the metabolic effects of the renin-angiotensin system. Biol Sex Differ, 2019. 10(1): p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Agrawal M, Spencer HJ, and Faas FH, Method of LDL Cholesterol Measurement Influences Classification of LDL Cholesterol Treatment Goals. Journal of Investigative Medicine, 2010. 58(8): p. 945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Komukai K, Mochizuki S, and Yoshimura M, Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol, 2010. 24(6): p. 687–98. [DOI] [PubMed] [Google Scholar]
- 105.Kafami M, et al. , The effects of estradiol and testosterone on renal tissues oxidative after central injection of angiotensin II in female doca - salt treated rats. Horm Mol Biol Clin Investig, 2018. 37(3). [DOI] [PubMed] [Google Scholar]
- 106.Reed DK and Arany I, Sex hormones differentially modulate STAT3-dependent antioxidant responses during oxidative stress in renal proximal tubule cells. In Vivo, 2014. 28(6): p. 1097–100. [PubMed] [Google Scholar]
- 107.Tonneijck L, et al. , Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J Am Soc Nephrol, 2017. 28(4): p. 1023–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bjornstad P, et al. , Insulin Sensitivity and Diabetic Kidney Disease in Children and Adolescents With Type 2 Diabetes: An Observational Analysis of Data From the TODAY Clinical Trial. Am J Kidney Dis, 2018. 71(1): p. 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lovshin JA, et al. , Hyperfiltration, urinary albumin excretion, and ambulatory blood pressure in adolescents with Type 1 diabetes mellitus. Am J Physiol Renal Physiol, 2018. 314(4): p. F667–F674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cherney DZ, Sochett EB, and Miller JA, Gender differences in renal responses to hyperglycemia and angiotensin-converting enzyme inhibition in diabetes. Kidney Int, 2005. 68(4): p. 1722–8. [DOI] [PubMed] [Google Scholar]
- 111.Slyvka Y, et al. , Antioxidant diet and sex interact to regulate NOS isoform expression and glomerular mesangium proliferation in Zucker diabetic rat kidney. Acta Histochem, 2016. 118(2): p. 183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Neugarten J, et al. , Sex hormones and renal nitric oxide synthases. J Am Soc Nephrol, 1997. 8(8): p. 1240–6. [DOI] [PubMed] [Google Scholar]
- 113.Prabhakar S, et al. , Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol, 2007. 18(11): p. 2945–52. [DOI] [PubMed] [Google Scholar]
- 114.Xiao S, et al. , Effects of estradiol and its metabolites on glomerular endothelial nitric oxide synthesis and mesangial cell growth. Hypertension, 2001. 37(2 Pt 2): p. 645–50. [DOI] [PubMed] [Google Scholar]
- 115.Gohar EY and Pollock DM, Sex-Specific Contributions of Endothelin to Hypertension. Curr Hypertens Rep, 2018. 20(7): p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matsumoto T, et al. , Gender differences in vascular reactivity to endothelin-1 (1–31) in mesenteric arteries from diabetic mice. Peptides, 2008. 29(8): p. 1338–46. [DOI] [PubMed] [Google Scholar]
- 117.Muscogiuri G, et al. , Water intake keeps type 2 diabetes away? Focus on copeptin. Endocrine, 2018. 62(2): p. 292–298. [DOI] [PubMed] [Google Scholar]
- 118.Achari AE and Jain SK, Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int J Mol Sci, 2017. 18(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Christen T, et al. , Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides, 2018. 107: p. 25–31. [DOI] [PubMed] [Google Scholar]
- 120.Looker HC, et al. , Adiponectin concentrations are influenced by renal function and diabetes duration in Pima Indians with type 2 diabetes. J Clin Endocrinol Metab, 2004. 89(8): p. 4010–7. [DOI] [PubMed] [Google Scholar]
- 121.Lindsay RS, et al. , Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet, 2002. 360(9326): p. 57–8. [DOI] [PubMed] [Google Scholar]
- 122.Daimon M, et al. , Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese Population: the Funagata study. Diabetes Care, 2003. 26(7): p. 2015–20. [DOI] [PubMed] [Google Scholar]
- 123.Spranger J, et al. , Adiponectin and protection against type 2 diabetes mellitus. Lancet, 2003. 361(9353): p. 226–8. [DOI] [PubMed] [Google Scholar]
- 124.Kim Y and Park CW, Mechanisms of Adiponectin Action: Implication of Adiponectin Receptor Agonism in Diabetic Kidney Disease. Int J Mol Sci, 2019. 20(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Horenburg S, et al. , Influence of sex hormones on adiponectin expression in human adipocytes. Horm Metab Res, 2008. 40(11): p. 779–86. [DOI] [PubMed] [Google Scholar]
- 126.Laughlin GA, Barrett-Connor E, and May S, Sex-specific determinants of serum adiponectin in older adults: the role of endogenous sex hormones. Int J Obes (Lond), 2007. 31(3): p. 457–65. [DOI] [PubMed] [Google Scholar]
- 127.Friederich M, et al. , Diabetes-induced up-regulation of uncoupling protein-2 results in increased mitochondrial uncoupling in kidney proximal tubular cells. Biochim Biophys Acta, 2008. 1777(7–8): p. 935–40. [DOI] [PubMed] [Google Scholar]
- 128.Lee HB, et al. , Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol, 2003. 14(8 Suppl 3): p. S241–5. [DOI] [PubMed] [Google Scholar]
- 129.Tanaka R, et al. , Protective effect of 17beta-estradiol on ischemic acute kidney injury through the renal sympathetic nervous system. Eur J Pharmacol, 2012. 683(1–3): p. 270–5. [DOI] [PubMed] [Google Scholar]
- 130.Poolsup N, Suksomboon N, and Aung N, Effect of phosphodiesterase-5 inhibitors on glycemic control in person with type 2 diabetes mellitus: A systematic review and meta-analysis. J Clin Transl Endocrinol, 2016. 6: p. 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tanaka R, et al. , Sex differences in ischaemia/reperfusion-induced acute kidney injury depends on the degradation of noradrenaline by monoamine oxidase. Clin Exp Pharmacol Physiol, 2017. 44(3): p. 371–377. [DOI] [PubMed] [Google Scholar]
- 132.Spires D, Manis AD, and Staruschenko A, Ion channels and transporters in diabetic kidney disease. Curr Top Membr, 2019. 83: p. 353–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nair AV, et al. , Sex-dependent differences in water homeostasis in wild-type and V-ATPase B1-subunit deficient mice. PLoS One, 2019. 14(8): p. e0219940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Veiras LC, et al. , Sexual Dimorphic Pattern of Renal Transporters and Electrolyte Homeostasis. J Am Soc Nephrol, 2017. 28(12): p. 3504–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sabolic I, et al. , Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol, 2012. 302(8): p. C1174–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Herak-Kramberger CM, et al. , Sex-dependent expression of water channel AQP1 along the rat nephron. Am J Physiol Renal Physiol, 2015. 308(8): p. F809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gerdts E and Regitz-Zagrosek V, Sex differences in cardiometabolic disorders. Nat Med, 2019. 25(11): p. 1657–1666. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y, et al. , Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med, 2019. 17(1): p. 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gilbert RE, SGLT2 inhibitors: beta blockers for the kidney? Lancet Diabetes Endocrinol, 2016. 4(10): p. 814. [DOI] [PubMed] [Google Scholar]
- 140.Perkovic V, et al. , Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med, 2019. 380(24): p. 2295–2306. [DOI] [PubMed] [Google Scholar]
- 141.Wu P, et al. , Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effect of SGLT2 Inhibitor on Blood Leptin and Adiponectin Level in Patients with Type 2 Diabetes. Horm Metab Res, 2019. 51(8): p. 487–494. [DOI] [PubMed] [Google Scholar]
- 142.Mahmoud AN, et al. , Does Gender Influence the Cardiovascular Benefits Observed with Sodium Glucose Co-Transporter-2 (SGLT-2) Inhibitors? A Meta-Regression Analysis. Cardiol Ther, 2017. 6(1): p. 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Radholm K, et al. , Effects of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes in women versus men. Diabetes Obes Metab, 2020. 22(2): p. 263–266. [DOI] [PubMed] [Google Scholar]
- 144.Le P, et al. , Use of Antihyperglycemic Medications in U.S. Adults: An Analysis of the National Health and Nutrition Examination Survey. Diabetes Care, 2020. 43(6): p. 1227–1233. [DOI] [PubMed] [Google Scholar]
- 145.Taub M, Gene Level Regulation of Na,K-ATPase in the Renal Proximal Tubule Is Controlled by Two Independent but Interacting Regulatory Mechanisms Involving Salt Inducible Kinase 1 and CREB-Regulated Transcriptional Coactivators. Int J Mol Sci, 2018. 19(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang X, et al. , Reduction of renal dopamine receptor expression in obese Zucker rats: role of sex and angiotensin II. Am J Physiol Renal Physiol, 2010. 299(5): p. F1164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cassis P, et al. , Addition of cyclic angiotensin-(1–7) to angiotensin-converting enzyme inhibitor therapy has a positive add-on effect in experimental diabetic nephropathy. Kidney Int, 2019. 96(4): p. 906–917. [DOI] [PubMed] [Google Scholar]
- 148.El Boustany R, et al. , Antagonism of vasopressin V2 receptor improves albuminuria at the early stage of diabetic nephropathy in a mouse model of type 2 diabetes. J Diabetes Complications, 2017. 31(6): p. 929–932. [DOI] [PubMed] [Google Scholar]
- 149.Heerspink HJL, et al. , Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet, 2019. 393(10184): p. 1937–1947. [DOI] [PubMed] [Google Scholar]
- 150.Yang YY, et al. , Piperazine ferulate ameliorates the development of diabetic nephropathy by regulating endothelial nitric oxide synthase. Mol Med Rep, 2019. 19(3): p. 2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leng B, et al. , Astragaloside IV Suppresses High Glucose-Induced NLRP3 Inflammasome Activation by Inhibiting TLR4/NF-kappaB and CaSR. Mediators Inflamm, 2019. 2019: p. 1082497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Zeeuw D, et al. , Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med, 2013. 369(26): p. 2492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dassano A, Loretelli C, and Fiorina P, Idebenone and T2D: A new insulin-sensitizing drug for personalized therapy. Pharmacol Res, 2019. 139: p. 469–470. [DOI] [PubMed] [Google Scholar]
- 154.Tripathi AS, Mazumder PM, and Chandewar AV, Sildenafil, a phosphodiesterase type 5 inhibitor, attenuates diabetic nephropathy in STZ-induced diabetic rats. J Basic Clin Physiol Pharmacol, 2016. 27(1): p. 57–62. [DOI] [PubMed] [Google Scholar]
- 155.de Luis DA, et al. , Adiponectin gene variant RS rs266729: Relation to lipid profile changes and circulating adiponectin after bariatric surgery. Surg Obes Relat Dis, 2018. 14(9): p. 1402–1408. [DOI] [PubMed] [Google Scholar]
- 156.Kim Y, et al. , The Adiponectin Receptor Agonist AdipoRon Ameliorates Diabetic Nephropathy in a Model of Type 2 Diabetes. J Am Soc Nephrol, 2018. 29(4): p. 1108–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zha D, Wu X, and Gao P, Adiponectin and Its Receptors in Diabetic Kidney Disease: Molecular Mechanisms and Clinical Potential. Endocrinology, 2017. 158(7): p. 2022–2034. [DOI] [PubMed] [Google Scholar]
- 158.Reckelhoff JF, Sex Differences in Regulation of Blood Pressure. Adv Exp Med Biol, 2018. 1065: p. 139–151. [DOI] [PubMed] [Google Scholar]
- 159.Mauer M and Doria A, Uric Acid and Diabetic Nephropathy Risk. Contrib Nephrol, 2018. 192: p. 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chohan S, et al. , Women with gout: efficacy and safety of urate-lowering with febuxostat and allopurinol. Arthritis Care Res (Hoboken), 2012. 64(2): p. 256–61. [DOI] [PubMed] [Google Scholar]


