Abstract
We report a case of a patient who developed dialysis-dependent renal failure after the use of Canagliflozin. A 66-year old male with type 2 diabetes recovering from left knee septic arthritis at a rehabilitation facility, was admitted with oliguric acute kidney injury (AKI) five days after starting caniglifozin the SGLT2 inhibitor. The patient presented with hematuria, non-nephrotic proteinuria and creatinine of 6.8 mg/dL from a baseline of 1.1 to 1.3 mg/dL. There was no recent use of radiocontrast, intravenous immunoglobulins or colloids. The patient subsequently required hemodialysis. Due to recent antibiotic use (ampicillin-sulbactam), acute interstitial nephritis was considered as a differential diagnosis. Hence, a kidney biopsy was performed which documented the presence of osmotic nephropathy. The patient’s renal function returned to baseline after 2 weeks of hemodialysis. This case confirms an association of osmotic nephropathy with the use of Canagliflozin and discusses potential mechanisms. We recommend kidney biopsy for cases of severe AKI associated with SGLT2 inhibitors to better understand the relationship of this complication with the use of this class of medications.
Keywords: Acute renal failure, Canagliflozin, hemodialysis, SGLT2 inhibitors, osmotic nephropathy
Introduction:
SGLT2 inhibitors (the glifozins) have been considered a breakthrough in the management of diabetes, as recent clinical trials with this class demonstrate reduction in cardiovascular mortality1,2 and preservation of kidney function in type 2 diabetes3,4. SGLT2 inhibitors have several protective effects, including lowering systemic and glomerular pressure, stimulating natriuresis, reducing serum uric acid levels, and decreasing body weight5–7. Despite the benefits of SGLT2 inhibitors, there are concerns that SGLT2 inhibitors might increase risk of acute kidney injury (AKI). The Federal Drug Administration (FDA) received over 1200 reports of AKI between January 2013 and September 2016 of which 42 % were hospitalized and 16 individuals died.8 This was 1.7-fold higher than the reports the FDA received for AKI in type 2 diabetics receiving other medications and led to a warning on the risk for AKI in SGLT2 inhibitors by the FDA.
Lately the FDA data have been questioned, as there was no specified control group, and a propensity analysis suggested that SGLT2 inhibitors carry an overall reduced risk for AKI compared with people with type 2 diabetes with similar clinical characteristics over a follow-up of one to two years.9 Furthermore, some cases of AKI being reported may simply relate to the acute reduction in eGFR that occurs with initiation of SGLT2 inhibitors, likely due to the hemodynamic effect of SGLT2 inhibitors to reduce glomerular pressure.3,7 Other cases may be from volume depletion triggered by use of diuretics and ACE inhibitors that augment the salt and water losses associated with SGLT2-dependent osmotic diuresis.10
Unfortunately, very few subjects with SGLT2 inhibitor-associated AKI have undergone kidney biopsy, so it remains unclear whether there are specific kidney lesions induced by these drugs. Recently, a patient with AKI likely from SGLT2 inhibitors was reported to have the lesion of osmotic nephrosis.11 Here we report another case, and discuss its potential pathogenesis.
Case Report:
A 66-year-old male presented to our hospital with oliguric acute kidney injury and possible need for hemodialysis on May 24th, 2018. On April 1st, 2018, he underwent a left total knee replacement and 3 weeks after surgery presented with pain and redness over the left knee. Left knee septic arthritis from group B streptococcus was diagnosed, and the patient was started on ampicillin-sulbactam and was admitted to the local rehabilitation facility for intravenous antibiotics and physical therapy. After 4 weeks he was switched to oral augmentin and the patient finished the six-week course of antibiotics two days prior to presenting to our hospital. The patient had a history of morbid obesity, type 2 diabetes mellitus and hypertension. The baseline serum creatinine ranged between 1.1 to 1.3 mg/dl, eGFR (CKD-EPI formula) of 56 to 60 ml/min/1.73m2, without microalbuminuria. While at the rehabilitation facility, the patient was started on Canagliflozin at 300 mg PO daily. The patient was on furosemide 80 mg and enalapril 5 mg PO daily. Five days after starting Canagliflozin, blood work revealed a serum creatinine of 6.8 mg/dl. Canagliflozin was discontinued on admission. See Table 1 for laboratory data. Two days after hospitalization, renal failure worsened, and hemodialysis was started. Prior to admission, the patient had history of mild diarrhea, 2 times a day, attributed to antibiotic use, but Clostridium difficile testing was negative. Detailed history obtained from the rehabilitation facility and thorough review of electronic records revealed no history of hypotension, no recent use of NSAID use, or other nephrotoxic agents such as intravenous contrast, intravenous immunoglobulins, or colloids. Given the temporal relationship of recent Group B streptococcus infection and antibiotic use, and severe acute kidney injury, a kidney biopsy was performed to rule out acute post-infectious glomerulonephritis or acute interstitial nephritis respectively. The biopsy showed glomeruli with mild mesangial sclerosis with 15–20 % tubulointerstitial fibrosis. The proximal tubules showed extensive vacuolization with foamy appearance and epithelial cell injury with cytoplasmic swelling and prominent cytoplasmic vacuolization (Figure 1, A). These features were suggestive of osmotic nephropathy. After continuing dialysis for 2 weeks, the patient recovered from renal failure and at discharge had a creatinine of 1.2 mg/dl.
Table 1.
Laboratory Data on Admission and Six months after discharge.
| On admission (on canagliflozin) |
6 months after discharge (off canagliflozin) |
|
|---|---|---|
| Height/Weight/BMI Vital signs |
Admission Height 70 inches / 386 pounds / 55 BMI Pulse regular, 88 per min; BP 139/80 |
|
| Sodium | 131 mEq/L | 139 mEq/L |
| Potassium | 5.5 mEq/L | 3.8 mEq/L |
| BUN | 52 mg/dL | 25 mg/dL |
| Creatinine | 6.8 mg/dL | 1.29 mg/dL |
| Serum glucose | 128 mg/dL | 200 mg/dL |
| Urine glucose | >500 mg/dL | N/A |
| Urine sodium/ FeNa | 32 mEq/L / 4.4 | N/A |
| Urine microscopy | 30–49 Rbc/hpf | 3–5 Rbc/hpf |
| Urine protein/creatinine | 2.0 gm/gm | undetectable |
| Urine microalbumin | undetectable | undetectable |
| AST/ALT/Bilirubin | 18 Iu/L; 22 Iu/L ; 0.8 mg/dL | |
N/A, not available.
Figure 1. Osmotic Nephrosis in a Patient with Canagliflozin-Mediated AKI.
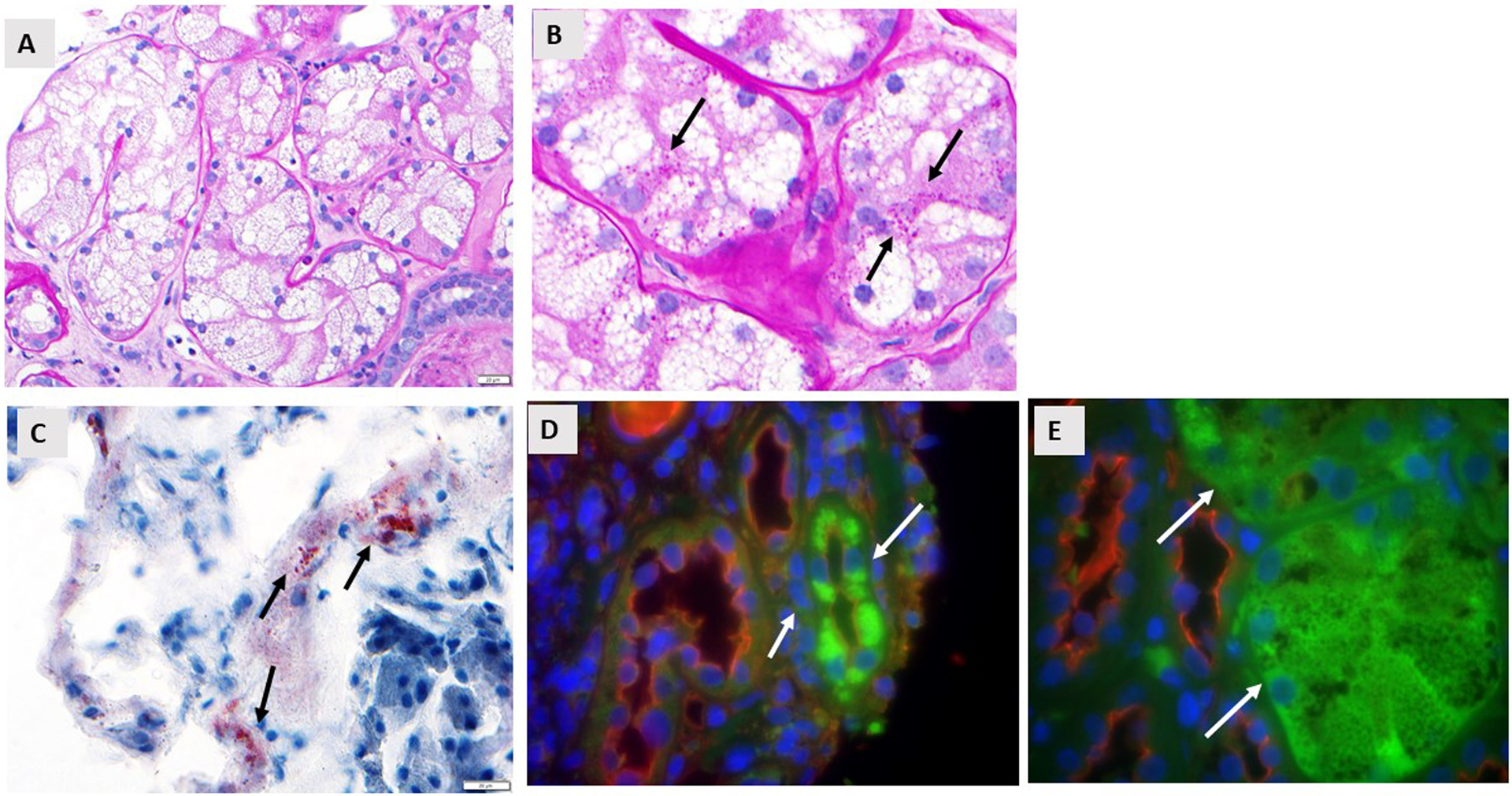
A. PAS stain shows vacuolated cytoplasm of proximal tubular cells consistent with severe osmotic nephrosis (40x magnification). B. PAS stain at high power showing the presence of small glycogen deposition (pink dots, arrows) in the cytoplasm of the injured proximal tubular epithelium (100x). Documentation this was glycogen was shown by diastase staining. C. Oil Red O staining shows the presence of rare positive lipid deposits in some proximal tubules (arrows, 40x). D. Immunofluorescence of some proximal tubules for aldose reductase (white arrows 40x). E. As in D) but for fructokinase (white arrows, 100x).
Discussion:
We report a case of dialysis-dependent AKI due to ‘osmotic nephrosis’ in a patient receiving the SGLT2 inhibitor, Canagliflozin. Features resembling osmotic nephrosis has been previously reported in a patient receiving dapaglifozin.11 In our case, one could argue that concomitant use of furosemide, and enalapril may have caused volume depletion and intra-renal hemodynamic changes predisposing to kidney injury. However, the diffuse and significant features of osmotic nephropathy was the predominant lesion. The dose of Canagliflozin used in our patient was also in accordance to the prescribing guidelines. Our patient did not receive intravenous contrast, immunoglobulins, mannitol or any other agent known to cause osmotic nephropathy. The significance of these findings is that subjects with severe AKI from the glifozins are not commonly biopsied, and the cause is generally attributed to volume depletion from an osmotic diuresis which is a known consequence of SGLT2 inhibition.
Osmotic nephrosis is generally associated with the infusion of hyperosmotic agents (such as radiocontrast, mannitol, sucrose-containing immunoglobulins) or hyperoncotic agents (colloids) but can also be observed with severe hyperglycemia and glycosuria.12–14 A favored hypothesis is that the injury is due to pinocytosis with uptake into lysosomes, but osmotic and/or oncotic have not been completely ruled out.12 Acute kidney injury may be subtle and subclinical or severe. Clinical features develop with a week of exposure to the substance known to cause osmotic nephropathy. Although usually reversible in patients who do not have underlying severe chronic kidney disease, about 40 % patients may need renal replacement therapy during the acute phase. The kidney biopsy is characterized by diffuse “clear-cell” transformation of proximal tubular epithelial cells with isometric fine vacuolization of the cytoplasm.
A similar proximal tubular lesion can be observed in uncontrolled diabetes mellitus (often in cases of diabetic ketoacidosis or hyperglycaemic hyperosmolar nonketotic coma ) and is called the Armanni Ebstein lesion15. This lesion is likely mediated by hyperosmolality, and can be associated with both glycogen and lipid-containing vacuoles16. Vacuolization with glycogen can also be observed in Fanconi syndromes associated with glycosuria.17
Inhibition of the SGLT2 transporter is expected to increase urinary glucose concentrations by several fold in the proximal tubular lumen where 70% of the glomerular filtrate is absorbed. This could increase osmotic pressure for both proximal and distal tubular segments while also leading to enhanced glucose uptake in the S3 segment of the proximal tubule that does not express the SGLT2 transporter. We suggest that the etiology might be due to hyperosmolality–induced aldose reductase expression with the subsequent generation of sorbitol and fructose from the glucose that is taken up into the cells. Fructokinase, the enzyme metabolizing fructose, is present in all three segments of the proximal tubule with the highest concentration in the S3 segment.18 Fructose metabolism by fructokinase preferentially produces glycogen and lipid that might be protective in situations of mild hypoxia or injury. However, persistent activation leads to oxidative stress, uric acid accumulation, tubular injury, and local inflammation.19 Consistent with these observations, we observed de novo expression of aldose reductase in proximal tubules along with some fructokinase expression as well as glycogen deposition, and even rare lipid accumulation (Figure 1, C).
Further studies are needed to better understand the risk factors for the development of osmotic nephrosis with SGLT2 blockade. For example, this might be a greater problem for agents that block both SGLT1 and SGLT2, as this may lead to greater osmotic effects on distal tubules. Although speculative, we suggest that low-grade activation of this pathway may be reno-protective and account for less renal injury in recent studies with SGLT2 inhibitors, while excessive activation of these pathways may lead to severe AKI are also being observed.
In summary, osmotic nephrosis should be considered in the differential of AKI in the patient on SGLT2 inhibitors. We recommend a kidney biopsy in any individual who develops prolonged AKI (>5 days) or dialysis-dependent AKI that persists despite holding the SGLT2 inhibitor. Such a clinical presentation is unlikely due to a reduction in glomerular pressure or the effects of sodium and water depletion. Further studies are needed to understand how common this complication is, the mechanisms of its development, and its long-term consequences.
Footnotes
- Peter Bjornstad: PB has acted as a consultant for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi, Novo Nordisk and Horizon Pharma. PB serves on the advisory board of XORTX.
- Richard Johnson, Miguel Lanaspa and Carlos Roncal-Jimenez: have equity with Colorado Research Partners LLC that is making inhibitors of fructose metabolism.
- All the authors did NOT receive any funding toward the production of the manuscript.
Contributor Information
Gautam Phadke, Sanford Health, University of North Dakota School of Medicine, Fargo, ND.
Amit Kaushal, Sanford Health, University of North Dakota School of Medicine, Fargo, ND.
Dean R Tolan, Department of Biology, Boston University, Boston MA..
Kai Hahn, B.Braun Medical Care AG Nephrology and Dialysis Center Hochfelden, Zurich, Switzerland..
Thomas Jensen, University of Colorado Anschutz Medical Campus, Aurora CO..
Petter Bjornstad, University of Colorado Anschutz Medical Campus, Aurora CO.
Carlos Roncal-Jimenez, University of Colorado Anschutz Medical Campus, Aurora CO.
Ana Andres Hernando, University of Colorado Anschutz Medical Campus, Aurora CO.
Miguel A Lanaspa, University of Colorado Anschutz Medical Campus, Aurora CO.
Mariam Priya Alexander, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN.
Richard J Johnson, University of Colorado Anschutz Medical Campus, Aurora CO.
References
- 1.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 2.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380(4):347–357. [DOI] [PubMed] [Google Scholar]
- 3.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323–334. [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295–2306. [DOI] [PubMed] [Google Scholar]
- 5.Weir MR. The kidney and type 2 diabetes mellitus: therapeutic implications of SGLT2 inhibitors. Postgrad Med. 2016;128(3):290–298. [DOI] [PubMed] [Google Scholar]
- 6.Storgaard H, Gluud LL, Bennett C, et al. Benefits and Harms of Sodium-Glucose Co-Transporter 2 Inhibitors in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(11):e0166125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skrtic M, Yang GK, Perkins BA, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia. 2014;57(12):2599–2602. [DOI] [PubMed] [Google Scholar]
- 8.Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: Analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis. 2017;27(12):1108–1113. [DOI] [PubMed] [Google Scholar]
- 9.Nadkarni GN, Ferrandino R, Chang A, et al. Acute Kidney Injury in Patients on SGLT2 Inhibitors: A Propensity-Matched Analysis. Diabetes Care. 2017;40(11):1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perlman A, Heyman SN, Stokar J, Darmon D, Muszkat M, Szalat A. Clinical Spectrum and Mechanism of Acute Kidney Injury in Patients with Diabetes Mellitus on SGLT-2 Inhibitors. Isr Med Assoc J. 2018;20(8):513–516. [PubMed] [Google Scholar]
- 11.Pleros C, Stamataki E, Papadaki A, et al. Dapagliflozin as a cause of acute tubular necrosis with heavy consequences: a case report. CEN Case Rep 2018;7:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51(3):491–503. [DOI] [PubMed] [Google Scholar]
- 13.Moreau JF, Droz D, Sabto J, et al. Osmotic Nephrosis Induced by Water-Soluble Triiodinated Contrast Media in Man. A Retrospective Study of 47 Cases. Radiology. 1975;115(2):329–336. [DOI] [PubMed] [Google Scholar]
- 14.Ahsan N, Palmer BF, Wheeler D, Greenlee RG, Jr., Toto RD. Intravenous immunoglobulin-induced osmotic nephrosis. Arch Intern Med. 1994;154(17):1985–1987. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie S, Waugh D. The pathology of Armanni-Ebstein diabetic nephropathy. Am J Pathol. 1957;33(6):1035–1057. [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen JL, Hansen TP. Lipids in the proximal tubules of the kidney in diabetic coma. Am J Forensic Med Pathol. 2000;21(4):416–418. [DOI] [PubMed] [Google Scholar]
- 17.Bendon RW, Hug G. Glycogen accumulation in the pars recta of the proximal tubule in Fanconi syndrome. Pediatr Pathol. 1986;6(4):411–429. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama T, Kosugi T, Gersch M, et al. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Renal Physiol. 2010;298(3):F712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cirillo P, Gersch MS, Mu W, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20(3):545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]


