Abstract
Pneumonia caused by severe acute respiratory syndrome coronavirus 2 emerged in China at the end of 2019. Because of the severe immunomodulation and lymphocyte depletion caused by this virus and the subsequent administration of drugs directed at the immune system, we anticipated that patients might experience fungal superinfection. We collected data from 186 patients who had coronavirus disease–associated pulmonary aspergillosis (CAPA) worldwide during March–August 2020. Overall, 182 patients were admitted to the intensive care unit (ICU), including 180 with acute respiratory distress syndrome and 175 who received mechanical ventilation. CAPA was diagnosed a median of 10 days after coronavirus disease diagnosis. Aspergillus fumigatus was identified in 80.3% of patient cultures, 4 of which were azole-resistant. Most (52.7%) patients received voriconazole. In total, 52.2% of patients died; of the deaths, 33.0% were attributed to CAPA. We found that the cumulative incidence of CAPA in the ICU ranged from 1.0% to 39.1%.
Keywords: SARS-CoV-2, Aspergillus, voriconazole, intensive care unit, aspergillosis, SARS-CoV-2, COVID-19, respiratory infections, severe acute respiratory syndrome coronavirus 2, coronavirus disease, zoonoses, viruses, coronaviruses, fungi
Cases of pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were first described in Wuhan, China, at the end of December 2019 (1). The infection rapidly spread, causing the coronavirus disease (COVID-19) pandemic (2).
Because SARS-CoV-2 and treatments such as dexamethasone or tocilizumab can impair the immune system, some researchers anticipated the possibility of fungal superinfection among COVID-19 patients (3–6). As of August 2020, researchers have documented COVID-19–associated pulmonary aspergillosis (CAPA) (7–9), invasive candidiasis (10), coccidioidomycosis (11), fusariosis (12), histoplasmosis (13), mucormycosis (14), pneumocystosis (15), and saccharomycosis (16). Varying cumulative rates of CAPA have been described, including rates of 0.7%–7.7% among COVID-19 patients (17,18), 2.5%–39.1% among ICU patients with COVID-19 (19,20), and 3.2%–29.6% among COVID-19 patients on mechanical ventilation (7,17). Many of these patients lack the concurrent conditions usually associated with invasive pulmonary aspergillosis (IPA) such as malignancies, neutropenia, or history of allogeneic stem cell or solid organ transplantation (21). Admission to the ICU or severe influenza are also risk factors for IPA in nonneutropenic patients (22–25). Reports of CAPA have been mostly limited to a few single-center studies; therefore, a comprehensive analysis of international distribution currently is lacking (4).
We analyzed reports in the literature (26–50; references 51–54in Appendix) and the FungiScope registry (reference 55 in Appendix) to describe baseline conditions, clinical management, and associated deaths in CAPA patients. This analysis also contextualizes the available cumulative incidences.
Methods
We conducted a retrospective analysis using clinical data of patients worldwide who received a CAPA diagnosis during March 1–August 31, 2020. Our analysis comprised data from the FungiScope registry and academic literature (Figure 1).
Figure 1.
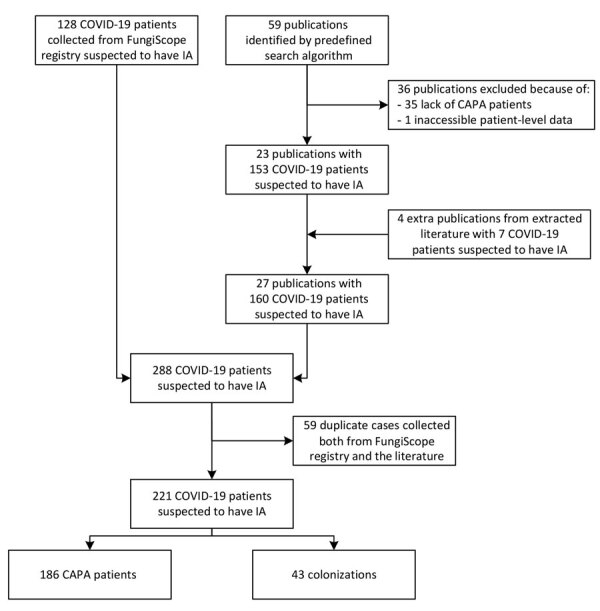
Enrollment process in study of patients with CAPA, March–August 2020. Patients were identified in the FungiScope registry and academic literature using the search string “(Aspergill*) AND (invasive OR putative OR probable OR infection OR case OR patient OR report) AND (COVID* OR corona* OR SARS-CoV-2) (Appendix Table 1). The initial 288 COVID-19 patients suspected to have IA were revised in a deduplication process; 59 double entries were identified. Only 1 report per patient was maintained. Thus, 221 individual COVID-19 patients suspected to have IA were assessed for CAPA. CAPA, COVID-19–associated pulmonary aspergillosis; COVID-19, coronavirus disease; EORTC/MSG, European Organization for Research and Treatment of Cancer/Mycoses Study Group; IA, invasive aspergillosis.
FungiScope (https://www.clinicaltrials.gov; National Clinical Trials identifier NCT01731353) is a global registry for emerging invasive fungal infections. FungiScope was approved by the local ethics committee of the University of Cologne, Cologne, Germany (study ID 05-102). The registry includes patients with invasive aspergillosis since 2019. FungiScope’s methods have been described previously (reference 55 in Appendix).
In addition, we conducted a literature search using the PubMed database (https://pubmed.ncbi.nlm.nih.gov) for suspected CAPA cases occurring in March–August 2020. We used the search string “(Aspergill*) AND (invasive OR putative OR probable OR infection OR case OR patient OR report) AND (COVID* OR corona* OR SARS-CoV-2),” which identified 59 published articles. We reviewed and extracted relevant data from each of the publications. When necessary, we contacted authors for additional details (Appendix).
We reviewed each patient report using multiple diagnostic definitions. First, we evaluated the patients according to the consensus definition of Koehler et al. (reference 56 in Appendix); we classified the patients as having proven, probable, or possible CAPA. We used alternative definitions to evaluate patients who were nonclassifiable because of lack of essential information, such as the volume of saline recovered by nondirected bronchial lavage (NBL) fluid applied. We categorized the nonclassifiable patients as proven or probable according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria for invasive fungal infections (21) or as proven, putative, and colonized according to the AspICU algorithm for IPA in critically ill ICU patients by Blot et al. (23). We considered severe COVID-19 with acute respiratory distress syndrome (ARDS) to be a valid host criterion (i.e., acquired immunodeficiency) (8). We considered patients who met >1 definition to have CAPA; we categorized the rest as nonclassifiable.
We collected data on patients’ demographic characteristics and baseline conditions. We also collected data on abnormal radiographic images, mycologic evidence, signs and symptoms at CAPA diagnosis, site of infection, antifungal susceptibility testing, antifungal treatment, death at 6 and 12 weeks after CAPA diagnosis, and absolute death. In addition, we calculated the length of time between COVID-19 and CAPA diagnoses, CAPA diagnosis and most recent healthcare contact with the patient, ICU admission and CAPA diagnosis, and installation of mechanical ventilation and CAPA diagnosis. The contribution of CAPA to patient death (i.e., attributable mortality) was assessed by the treating medical team (Appendix Table 2). To determine the cumulative incidence of CAPA in the facilities included in the analysis, we asked each institution for 3 different denominators: the total number of COVID-19 patients, the number of COVID-19 patients admitted to the ICU, and the number of COVID-19 patients admitted to the ICU who needed mechanical ventilation during March–August 2020.
Statistical Analysis
We did not calculate an a priori sample size for this exploratory study. To analyze the demographic and clinical characteristics of patients with CAPA, we describe categorical variables using frequencies and percentages; we describe continuous variables using medians and interquartile ranges (IQRs). We used SPSS Statistics 25.0 (IBM, https://www.ibm.com) for statistical analyses.
Results
We identified 186 CAPA cases during March 1–August 31, 2020, in 17 different countries, according to European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria (21), Blot et al. algorithm (23), and Koehler et al. consensus definition (reference 56 in Appendix) (Figures 1, 2; Appendix Table 1). We identified 62 (33.3%) cases from literature, 45 (24.2%) from the FungiScope registry, and an additional 79 (42.5%) in both sources (Table 1). The median age among persons with CAPA was 68 years (IQR 59–73 years; range 15–87 years). Most (135; 72.6%) patients were men (Table 2).
Figure 2.
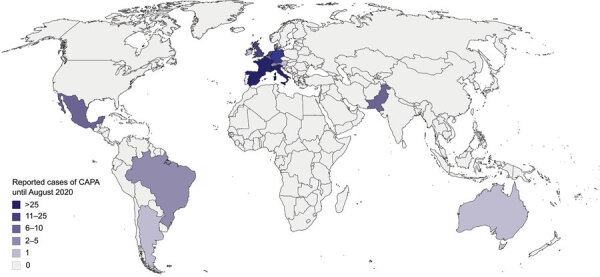
Global distribution of the 186 CAPA patients reported in the literature and FungiScope registry, March–August 2020. In total, 39 patients were from France, 36 from Italy, 26 from Spain, 23 from Germany, 14 from the Netherlands, 11 from the United Kingdom, 9 from Pakistan, 8 from Belgium, 6 from Mexico, 3 from Brazil, 3 from Switzerland, 2 from Denmark, 2 from Qatar, 1 from Argentina, 1 from Australia, 1 from Austria, and 1 from Ireland (Appendix Table 8). CAPA, COVID-19–associated pulmonary aspergillosis; COVID-19, coronavirus disease.
Table 1. Pathogens of 186 patients with coronavirus disease–associated pulmonary aspergillosis, March–August 2020*.
| Characteristic | No. (%) |
|---|---|
| Pathogens† | |
| Aspergillus fumigatus | 122 (65.6) |
| A. niger | 13 (7.0) |
| A. flavus | 10 (5.4) |
| A. terreus | 6 (3.2) |
| A. calidoustus | 1 (0.5) |
| A. lentulus | 1 (0.5) |
| A. nidulans | 1 (0.5) |
| A. penicillioides | 1 (0.5) |
| A. versicolor | 1 (0.5) |
| A. tubingensis | 1 (0.5) |
| Aspergillus spp. (culture)‡ | 1 (0.5) |
| Aspergillus spp. (serologic techniques) | 34 (18.3) |
| Other pathogens§ |
40 (21.5) |
| Case definition | |
| EORTC/MSG criteria (21) | |
| Proven | 7 (3.8) |
| Probable | 10 (5.4) |
| Nonclassifiable | 169 (90.9) |
| AspICU algorithm (23)¶ | |
| Proven | 7 (3.8) |
| Putative | 142 (76.3) |
| Colonization | 34 (18.3) |
| Nonclassifiable | 3 (1.6) |
| Consensus definition (reference 57 in Appendix) | |
| Proven | 7 (3.8) |
| Probable | 82 (44.1) |
| Possible | 19 (10.2) |
| Nonclassifiable¶# |
78 (41.9) |
| Mycologic evidence | |
| Culture** | 152 (81.7) |
| Microscopy†† | 3 (1.6) |
| Histologic techniques‡‡ | 7 (3.8) |
| PCR§§ | 43 (23.1) |
| Galactomannan test¶¶ | 113 (60.8) |
*Some patients had >1 pathogen or form of mycologic evidence. BAL, bronchoalveolar lavage; EORTC/MSG, European Organization for Research and Treatment of Cancer/Mycoses Study Group (21). †A total of 2 patients had A. fumigatus and A. niger coinfection, 1 patient had A. flavus and A. fumigatus coninfection, 1 patient had A. flavus and A. niger coinfection, 1 patient had A. fumigatus and A. terreus coinfection, and 1 patient had A. fumigatus and A. versicolor coinfection. ‡One patient had an Aspergillus spp. infection diagnosed by culture. No species determination was provided. Other patient samples were diagnosed as Aspergillus spp, using serologic techniques. §Small numbers of other pathogens were also retrieved from patient samples (Appendix Table 6). ¶AspICU method uses algorithm described by Blot et al. (23) for determining proven or putative aspergillosis in patients with influenza. #Up to 78 cases (41.9%) were considered nonclassifiable according to the definition (reference 56 in Appendix) because of lack of specific details about the type of aspiration performed. Of these, 75 (96.2%) were classified as putative according to the Blot et al. algorithm (23) and 3 (3.8%) as probable according to EORTC/MSG criteria (21). **Culture was used to analyze 50 BAL, 47 tracheal aspirate, 34 bronchial aspirate, 17 nondirected bronchial lavage, 3 sputum, 2 nonspecified lower respiratory tract, and 1 BAL and tracheal aspirate sample. ††Microscopy was used to analyze 1 BAL, 1 bronchial aspirate, and 1 tracheal aspirate sample. ‡‡Histologic techniques were used to analyze 7 lung tissue samples. §§PCR was used to analyze 16 BAL, 12 tracheal aspirate, 10 nondirected bronchial lavage, 3 bronchial aspirate, 1 lung tissue, and 1 serum sample. ¶¶Galactomannan tests were used to analyze 63 BAL, 30 serum or plasma, 22 nondirected bronchial lavage, 9 tracheal aspirate, 3 bronchial aspirate, and 1 sputum sample.
Table 2. Characteristics of 186 patients with coronavirus disease–associated pulmonary aspergillosis, March–August 2020*.
| Patient characteristic | No. (%) |
|---|---|
| Sex | |
| F | 51 (27.4) |
| M |
135 (72.6) |
| Median age, y (IQR) |
68 (58–73) |
| COVID-19† | 186 (100.0) |
| Median length of treatment, d (IQR) | 7 (6–11) |
| Median time from COVID-19 diagnosis to CAPA, d (IQR) |
10 (5–16) |
| Intensive care unit stay | 182 (97.8) |
| Median length of stay before CAPA diagnosis, d (IQR) | 8 (3–14) |
| Acute respiratory distress syndrome | 180 (96.8) |
| Mechanical ventilation | 175 (94.1) |
| Median time on ventilation before CAPA diagnosis, d (IQR) |
7 (3–13) |
| Corticosteroid use |
98 (52.7) |
| Concurrent conditions | |
| Chronic cardiovascular disease | 94 (50.5) |
| Renal failure‡ | 74 (39.8) |
| Diabetes mellitus | 64 (34.4) |
| Obesity | 47 (25.3) |
| Chronic pulmonary disease | 40 (21.5) |
| Hematologic or oncologic disease§ | 21 (11.3) |
| Hematologic malignancy | 10 (5.4) |
| Solid tumor | 9 (4.8) |
| Hematologic disease | 2 (1.1) |
| Solid organ transplantation¶ | 4 (2.2) |
| Neutropenia |
2 (1.1) |
| Other baseline conditions and characteristics# | 70 (37.6) |
| Lung infection | 186 (100.0) |
| Image abnormalities of the lungs | 182 (97.8) |
| Computed tomography scan | 134 (72.0) |
| Radiograph |
88 (47.3) |
| Antifungal treatment | 137 (73.7) |
| Median length of treatment, d (IQR) | 16 (10–33) |
| Amphotericin B | 36 (19.4) |
| Liposomal | 23 (12.4) |
| Deoxycholate | 11 (5.9) |
| Lipid complex | 2 (1.1) |
| Echinocandins | 24 (12.9) |
| Anidulafungin | 10 (5.4) |
| Caspofungin | 13 (7.0) |
| Micafungin | 1 (0.5) |
| Ibrexafungerp | 1 (0.5) |
| Triazoles | 117 (62.9) |
| Voriconazole | 98 (52.7) |
| Isavuconazole | 23 (12.4) |
| Posaconazole | 4 (2.2) |
| Fluconazole |
1 (0.5) |
| Overall mortality | 97 (52.2) |
| <6 wks | 89 (47.8) |
| <12 wks | 93 (50.0) |
| Median time to death, d (IQR) |
9 (3–18) |
| Cause of death** | |
| CAPA | 32 (17.2) |
| COVID-19 | 51 (27.4) |
| Other |
36 (19.4) |
| Median length of observation from CAPA diagnosis, d (IQR) | 22 (7–42) |
*Values are no. (%), except as indicated. Some patients had >1 baseline condition or characteristic, image abnormality, or antifungal drug. CAPA, COVID-19–associated pulmonary aspergillosis; COVID-19, coronavirus disease. †By definition, all CAPA patients had COVID-19 (Appendix Table 3). ‡In total, 54 patients had acute renal failure, 18 had chronic renal failure, and 2 had nonspecified renal failure. §In total, 9 patients had hematologic malignancy: 3 had chronic leukemia, 3 had lymphoma, 2 had myelodysplastic syndrome, and 1 had acute leukemia. Eight patients had a solid tumor: 1 had breast cancer, 1 had carcinoma, 1 had cervical/uterine cancer, 1 had lung cancer, 1 had esophageal carcinoma, 1 had prostate cancer, 1 had testicular cancer, and 1 had urothelial carcinoma. Two patients had hematologic disease: 1 had acquired hemophilia type A and 1 had hemophagocytic lymphohistiocytosis. ¶In total, 3 patients had a kidney transplant, 1 had a liver transplant, and 1 had a lung transplant. #Small numbers of patients had other concurrent conditions and characteristics (Appendix Table 7). **In total, 32 patients died of CAPA or CAPA/COVID-19: 7 died of CAPA only; 25 died of CAPA and COVID-19. In addition, 26 died of COVID-19 only.
Nearly all (182; 97.8%) patients were admitted to the ICU, most for ARDS (180; 96.8%) or mechanical ventilation (175; 94.1%). Other common baseline conditions and characteristics included corticosteroid administration (98; 52.7%), chronic cardiovascular disease (94; 50.5%), renal failure (74; 39.8%), diabetes mellitus (64; 34.4%), and obesity (47; 25.3%). Overall, 40 (21.5%) patients had chronic pulmonary disease (Table 2).
In total, 110 (59.1%) patients received either hydroxychloroquine (98; 52.7%) or chloroquine (12; 6.5%) for treatment of COVID-19. Sixty-eight (36.6%) patients received corticosteroids, mainly methylprednisolone monotherapy (26; 14.0%) or antivirals (67; 36.0%), especially lopinavir/ritonavir monotherapy (56; 30.1%). COVID-19 treatment had a median duration of 7 days before recovery or death (IQR 6–11 days; range 1–32 days) (Table 2; Appendix Table 3).
In 152 (81.7%) patients, CAPA was diagnosed a median of 10 days (IQR 5–16 days; range 0–51 days) after a positive respiratory sample for SARS-CoV-2 infection by reverse transcription PCR. Among all patients, Aspergillus fumigatus was the most frequently reported (122/152; 65.6%) pathogen. Six patients (3.2%) had cultures positive for >1 Aspergillus species. Samples mainly were from bronchoalveolar lavage (BAL) (50; 26.9%), tracheal aspirates (48; 25.8%), or bronchial aspirates (34; 18.3%). In 55 (29.6%) patients, culture was the only diagnostic tool that produced a positive result. Galactomannan (GM) levels were positive (i.e., optical density index ≥1.0) in samples from 113 (60.8%) patients, including BAL samples from 63 (33.9%) patients, serum or plasma from 29 (15.6%), and NBL from 22 (11.8%). Histologic techniques were used for diagnosis in 7 (3.8%) cases. Abnormal radiographic imaging was found in 182 (97.8%) patients, either in computed tomography scans (94; 50.5%), in chest radiographs (48; 25.8%), or both (40; 21.5%) (Table 2).
Overall, 30 (16.1%) patients provided samples for >1 antifungal susceptibility test, such as microdilution according to European Committee on Antimicrobial Susceptibility Testing guidelines (20; 10.8%) (reference 57 in Appendix), Etest (11; 5.9%), and Clinical and Laboratory Standards Institute microdilution procedures (1; 0.5%) (reference 58 in Appendix). The tests were predominantly performed on A. fumigatus (29; 15.6%) isolates, 3 of which had the TR34L98H resistance mutation in the cyp51A gene. One (0.5%) patient had voriconazole-resistant A. lentulus (MIC 2 µg/mL by EUCAST guidelines) (Appendix Table 4).
Of 186 CAPA patients, 49 (26.3%) patients did not receive mold-active antifungal therapy. The most common treatments were triazoles (117; 62.9%), especially voriconazole (98; 52.7%, including 79 patients for whom voriconazole was a first-line treatment) and isavuconazole (23; 12.4%). In total, 34 (19.4%) patients received amphotericin B, especially liposomal amphotericin B (23; 12.4%). Of the patients who received amphotericin B, 15 (65.2%) received it as first-line treatment. Antifungal treatment was administered for a median of 16 days before recovery or death (IQR 10–33 days; range 1–92 days) (Table 2; Appendix Table 5).
In total, 97 (52.2%) patients died, most (89; 47.8%) <6 weeks after CAPA diagnosis. In 32 (17.2%) patients, death was attributed to Aspergillus; including 25 (13.4%) patients who died of aspergillosis and COVID-19 infection. Patients were observed for a median of 22 days (IQR 7–42 days; range 0–144 days) after CAPA diagnosis; survivors were treated for a median of 40 days (IQR 28–50 days; range 1–144 days) and patients who died for a median of 9 days (IQR 3–18 days; range 0–144 days) (Table 2).
In total, 19 of 39 institutions provided denominators for cumulative incidence over the duration of the study period. The CAPA incidence among all COVID-19 patients ranged from 0.1%–9.7%. Among COVID-19 patients admitted to ICU, cumulative incidences ranged from 1.0%–39.1%. Among patients admitted to ICU who needed mechanical ventilation, cumulative incidences ranged from 1.1%–47.4% (Table 3).
Table 3. Cumulative incidences of CAPA in 19 facilities, March–August 2020*.
| Country, site no. | CAPA cases, no. | Denominator, no. (% CAPA) |
Timeframe | ||
|---|---|---|---|---|---|
| COVID-19 patients | COVID-19 patients in ICU | COVID-19 patients on mechanical ventilation | |||
| Argentina, I | 2 | 673 (0.3) | 163 (1.2) | 69 (2.9) | Mar–Aug |
| Belgium, I | 4 | 274 (1.5) | 46 (8.7) | 32 (12.5) | Mar–Aug |
| Belgium, II | 4 | NA | 34 (11.8) | 20 (20.0) | Mar–Apr |
| France, I | 2 | 519 (0.4) | 113 (1.8) | 45 (4.4) | Mar–Aug |
| Germany, I | 1 | 83 (1.2) | 18 (5.6) | 15 (6.7) | Mar–Aug |
| Germany, II | 11 | 231 (4.8) | 64 (17.2) | 56 (19.6) | Mar–Aug |
| Germany, III | 9 | 93 (9.7) | 38 (23.7) | 27 (33.3) | Mar–Aug |
| Germany, IV | 7 | 123 (5.7) | 76 (9.2) | 57 (12.3) | Mar–Aug |
| Ireland, I | 3 | 181 (1.7) | 15 (20.0) | 14 (21.4) | Mar–Aug |
| Italy, I | 2 | 1,279 (0.2) | 196 (1.0) | 188 (1.1) | Mar–Aug |
| Italy, II | 8 | 1,055 (0.8) | 144 (5.6) | 142 (5.6) | Mar–Aug |
| Mexico, I | 6 | 312 (1.9) | 131 (4.6) | 115 (5.2) | Mar–Aug |
| Netherlands, I | 9 | NA | NA | 53 (17.0) | Apr |
| Netherlands, II | 6 | 483 (1.2) | 118 (5.1) | NA | Mar–Aug |
| Pakistan, I | 9 | 147 (6.1) | 23 (39.1) | 19 (47.4) | Mar–Apr |
| Spain, I | 8 | 1,543 (0.5) | 348 (2.3) | 146 (5.5) | Mar–Aug |
| Spain, II | 8 | 7,880 (0.1) | NA | NA | Mar–Aug |
| Spain, III | 10 | 5,890 (0.2) | NA | NA | Mar–Aug |
| Switzerland, I | 3 | NA | 118 (2.5) | 80 (3.8) | Mar–May |
| United Kingdom, I | 19 | 14,615 (0.1) | 257 (7.4) | 200 (9.5) | Mar–May |
| Total | 131 | 35,381 (0.4) | 1,902 (6.9) | 1,278 (10.3) | Mar–Aug |
*CAPA, COVID-19–associated pulmonary aspergillosis; COVID-19, coronavirus disease; ICU, intensive care unit; NA, not available.
Discussion
We described 62 CAPA cases in the literature, 45 in the FungiScope registry, and 79 in both that were diagnosed during March 1–August 31, 2020. Men had a higher (2.6:1) prevalence of CAPA than women. This finding corresponds with a meta-analysis of >3 million COVID-19 patients that showed that men were at increased risk for severe COVID-19 and therefore complications such as CAPA (reference 59 in Appendix).
Most (97.8%) patients were admitted to the ICU, mainly because of ARDS, need for mechanical ventilation, or both. We found that corticosteroid administration, chronic cardiovascular disease, renal failure, diabetes mellitus, and obesity were common characteristics among these patients. Approximately 1 in 5 patients had chronic pulmonary disease. Patients had many similarities to influenza-associated pulmonary aspergillosis (IAPA) patients from Schauwvlieghe et al. (22), including similar rates of mechanical ventilation (IAPA 90.0% vs. CAPA 94.1%), corticosteroid administration (IAPA 56.0% vs. CAPA 52.7%), baseline renal failure (IAPA 42.0% vs. CAPA 39.8%), obesity (IAPA 30.0% vs. CAPA 25.3%), and chronic pulmonary disease (IAPA 16.0% vs. CAPA 21.5%). IAPA patients had a higher proportion of malignancies (30.0% vs. 11.3%) and solid organ transplantation (13.0% vs. 2.7%); however, CAPA patients had a higher prevalence of diabetes mellitus (12.0% vs. 34.4%). In our study, 50.5% of patients had chronic cardiovascular disease. These differences in the distribution of baseline characteristics between IAPA and CAPA patients reflects the epidemiology of COVID-19, which is more common among those with chronic cardiovascular disease, whereas hematologic or oncologic malignancies (22) are more common among those with IAPA (reference 60 in Appendix). Only 2% of COVID-19 patients have cancer (reference 61 in Appendix).
Available guidelines for aspergillosis management recommend diagnostic procedures such as respiratory culture and galactomannan index of BAL samples (references 60,62 in Appendix). However, these procedures have a high risk for aerosolization; safety precautions should be used when handling samples from COVID-19 patients (references 63,64 in Appendix). The elevated risk for SARS-CoV-2 transmission and the initial recommendation against using bronchoscopy for COVID-19 diagnosis (references 63,64 in Appendix) might explain the low number of BAL tests used to diagnose CAPA in our study. Schauwvlieghe et al. (22) diagnosed IAPA by using BAL cultures in 63.0% of the patients and the galactomannan test in 88.0%. In the current study, BAL cultures tested positive for Aspergillus in 26.9% of COVID-19 patients; galactomannan tests were positive in 33.9% of patients. Alternative respiratory sample sources (e.g., bronchial aspirate, NBL, tracheal aspirate, and sputum) were used for cultures in 35.4% of IAPA patients (22) and 31.2% of CAPA patients. Alternative samples also were used for galactomannan tests in 17.2% of CAPA patients; if optical density index cutoff values were not standardized for alternative samples, clinicians used the values for BAL. Almost all (97.8%) patients had imaging abnormalities; however, many had only marginally typical features of aspergillosis, hampering the differential diagnosis of CAPA according to radiologic criteria.
Positive isolates were recovered from 81.7% of CAPA patients. Similar to IAPA patients, the most common (80.3%) pathogen was A. fumigatus (22). In total, 5 patients had azole-resistant infections: 4 A. fumigatus and 1 A. lentulus infection. We noted 2 patients who had a possible previous exposure to triazoles. The professions of these 2 patients involved exposure to fungicides and manipulated organic matter containing triazole-resistant A. fumigatus. Therefore, the treating teams hypothesized that workplace exposure might have contributed to these patients’ illness. We found a similar proportion of patients with previous azole exposure as Verweij et al. (reference 65 in Appendix); however, the proportion found by Verweij et al. should be considered with caution because of small sample size.
Triazoles, especially voriconazole, were the most frequently administered antifungal drugs: 52.7% of the study cohort and 71.5% of the patients on antifungal treatment received voriconazole. We found that voriconazole use was associated with decreased death. The first-line use of voriconazole in 79 (80.6%) of 98 patients aligns with current recommendations (references 56,60,62 in Appendix).
We found a 50% mortality rate at 12 weeks after CAPA diagnosis. This finding is similar to the 51.0% mortality rate of IAPA patients in the same timeframe; however, these rates are almost 20 points higher than in other cohorts, such as aspergillosis patients with acute leukemia (33.8%) (reference 66 in Appendix). Nonetheless, in our study CAPA was attributed as the main reason for death in only 17.2% of the patients, whereas in Koehler et al. (reference 66 in Appendix), it was the main cause of death for 26.9% of patients with hematologic conditions.
We found an overall 6.9% cumulative incidence for CAPA among patients during the study period, although incidences varied by institution (1.0%–39.1% of CAPA patients admitted to ICU). In most facilities, the rates of CAPA were lower than those of IAPA (14%–19%) (reference 67 in Appendix). However, these ranges might vary according to diagnostic protocols in the different countries and healthcare facilities. Differences in screening practices for CAPA in COVID-19 patients might have affected detection rates and therefore our calculations of cumulative incidence (8). Further analyses are necessary to establish the geographic variance of this rate.
The first limitation of this study is that, because of the cross-sectional design of this study, we could not control for disease severity. Second, samples from the lower respiratory tract are the best way to differentiate between colonization and infection, but a low percentage of patients in this study had mycologic evidence from BAL culture or galactomannan tests. Third, we analyzed many cases from literature and could not contact certain authors for further details. In addition, institutions might not have documented all CAPA cases in the literature or FungiScope registry. Given the regional variability of the patient distribution, longitudinal studies might be a more appropriate tool to determine rates. Finally, because of the retrospective nature of the study, we could not retrieve the necessary clinical and diagnostic details of all patients. As a result, many patients were not classifiable according to the definitions used in this article, possibly contributing to an underdiagnosis of CAPA.
In conclusion, we described a large cohort of CAPA patients using cases from the literature and the FungiScope registry. CAPA occurs mostly in ICU patients on mechanical ventilation. We found that CAPA patients had high rates of chronic cardiovascular disease, renal failure, diabetes mellitus, and corticosteroid use. We also found that CAPA substantially contributed to a high death rate in COVID-19 patients, although cumulative incidence varied by treatment site. We believe that improved screening can identify and enable early treatment of CAPA.
Additional information on COVID-19–associated pulmonary aspergillosis.
Acknowledgments
This work was carried out as part of routine duties. FungiScope is supported by unrestricted grants from Amplyx Pharmaceuticals, Inc.; Basilea Pharmaceuticals; Cidara Therapeutics, Inc.; F2G Ltd.; Matinas BioPharma; Mundipharma International; Pfizer Inc.; and Scynexis, Inc. FungiScope has been supported in the past by unrestricted grants from Astellas Pharma Inc., Gilead Sciences Inc., and MSD Sharp & Dohme GmbH.
J.S. has received research grants from Basilea Pharmaceuticals International Ltd. and travel grants from the Meta-Alexander Foundation and German Society for Infectious Diseases, outside the context of the submitted work. C.G.V. has received grants and speaker fees from Gilead Sciences, Inc. and Merck Sharp & Dohme Corp., and speaker fees from Janssen Pharmaceuticals, Lilly, Novartis, and Pfizer Inc., outside the context of the submitted work. M.S. receives funding from the Medical Faculty of the University of Hamburg, Hamburg, Germany for clinical leave. F.H. received lecture and other honoraria from Correvio Pharma Corp., InfectoPharm Arzneimittel und Consilium GmbH, and Novartis, outside the context of the submitted work. K.F.P. is financially supported by the Coordination for the Improvement of Higher Education Personnel Foundation and Ministry of Education of Brazil (proposal no. 09/2020) and a nonfinancial scientific grant from IMMY, outside the context of the submitted work. D.R.G. has received honoraria from Stepstone Pharma GmbH and unconditional grants from MSD Italia Srl and Correvio Pharma Corp. J.F.M. reports grants from F2G Ltd. and Pulmocide, consultancy fees from Scynexis, Inc., and speaker fees from Gilead Sciences Inc., United Medical, and Teva Pharmaceutical Industries Ltd., outside the context of the submitted work. J.P.G. has participated in advisory boards and received speaker honoraria from Pfizer Inc. and Gilead Sciences Inc., outside the context of the submitted work. E.S. has received grants from the Philipp Schwartz Initiative of the Alexander von Humboldt Foundation. O.A.C. is financially supported by the German Federal Ministry of Research and Education; is funded by the Deutsche Forschungsgemeinschaft under Germany's Excellence Strategy (CECAD, EXC 2030 – 390661388); has received research grants from Actelion Pharmaceuticals Global, Amplyx Pharmaceuticals, Inc., Astellas Pharma Inc., Basilea Pharmaceutica International Ltd., Cidara Therapeutics, Inc., Da Volterra, F2G Ltd., Gilead Sciences Inc., Janssen Pharmaceuticals, The Medicines Company, Melinta Therapeutics, Merck Sharp & Dohme Corp., Octapharma AG, Pfizer Inc., and Scynexis, Inc.; is a consultant to Actelion Pharmaceuticals Global, Allecra Therapeutics GmbH, Amplyx Pharmaceuticals, Inc., Astellas Pharma Inc., Basilea Pharmaceutica International Ltd., BIOSYS USA LLC, Cidara Therapeutics, Inc., Da Volterra, Entasis Therapeutics, F2G Ltd., Gilead Sciences Inc., Matinas BioPharma Holdings, Inc., MedPace, Inc., The Menarini Group, Merck Sharp & Dohme Corp., Mylan Inc., Nabriva Therapeutics plc, NOXXON Pharma, Octapharma AG, Paratek Pharmaceuticals, Inc., Pfizer Inc., Pharmaceutical Solutions Industry, Roche Diagnostics, Scynexis, Inc., and Shionogi Inc.; and received lecture honoraria from Al-Jazeera Pharmaceutical Industries, Astellas Pharma Inc., Basilea Pharmaceutica International Ltd., Gilead Sciences Inc., Grupo Biotoscana, Merck Sharp & Dohme Corp., and Pfizer Inc., outside the context of the submitted work. P.K. has received nonfinancial scientific grants from Miltenyi Biotec GmbH and the Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases, and lecture honoraria from or is advisor to Akademie für Infektionsmedizin e.V., Ambu GmbH, Astellas Pharma Inc., European Confederation of Medical Mycology, Gilead Sciences Inc., Gesundheits und Pflegezentrum Rüsselsheim gemeinnützige GmbH, Merck Sharp & Dohme Corp., and University Hospital, Ludwig Maximilian University of Munich, and is advisor to Gilead Sciences Inc. and NOXXON N.V. outside the context of the submitted work.
Biography
Dr. Salmanton-García is an epidemiologist at University Hospital Cologne, Cologne. His primary research interests are invasive fungal infections, infectious diseases, epidemiology, and database management.
Footnotes
Suggested citation for this article: Salmanton-García J, Sprute R, Stemler J, Bartoletti M, Dupont D, Valerio M, et al. COVID-19–associated pulmonary aspergillosis, March–August 2020. Emerg Infect Dis. 2021 Apr [date cited]. https://doi.org/10.3201/eid2704.204895
These senior authors contributed equally to this article.
Members of this group are listed at the end of this article.
References
- 1.World Health Organization. Pneumonia of unknown cause. 2020 [cited 2021 Jun 23]. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/.
- 2.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036. 10.1136/bmj.m1036 [DOI] [PubMed] [Google Scholar]
- 3.Koehler P, Meis JF, Ostrosky-Zeichner L, Böll B, Hoenigl M, Cornely OA, et al. COVID-19/influenza–associated pulmonary aspergillosis—management. 2020 [cited 2020 May 30].. https://repository.publisso.de/resource/frl%3A6421494.
- 4.Beer KD, Jackson BR, Chiller T, Verweij PE, Van de Veerdonk FL, Wauters J. Does pulmonary aspergillosis complicate coronavirus disease 2019? Crit Care Explor. 2020;2:e0211. 10.1097/CCE.0000000000000211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costantini C, van de Veerdonk FL, Romani L. Covid-19–associated pulmonary aspergillosis: the other side of the coin. Vaccines (Basel). 2020;8:713. 10.3390/vaccines8040713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. J Fungi (Basel). 2020;6:91. 10.3390/jof6020091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–9. 10.1016/S2213-2600(20)30237-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koehler P, Cornely OA, Böttiger BW, Dusse F, Eichenauer DA, Fuchs F, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–34. 10.1111/myc.13096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellière S, Dudoignon E, Fodil S, Voicu S, Collet M, Oillic PA, et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2020;S1198-743X(20)30756-4. 10.1016/j.cmi.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerg Infect Dis. 2020;26:2694–6. 10.3201/eid2611.203504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang CC, Senining R, Kim J, Goyal R. An acute pulmonary coccidioidomycosis coinfection in a patient presenting with multifocal pneumonia with COVID-19. J Investig Med High Impact Case Rep. 2020;8:2324709620972244. 10.1177/2324709620972244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poignon C, Blaize M, Vezinet C, Lampros A, Monsel A, Fekkar A. Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID-19. Clin Microbiol Infect. 2020;26:1582–4. 10.1016/j.cmi.2020.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messina FA, Marin E, Caceres DH, Romero M, Depardo R, Priarone MM, et al. Coronavirus disease 2019 (COVID-19) in a patient with disseminated histoplasmosis and HIV—a case report from Argentina and literature review. J Fungi (Basel). 2020;6:275. 10.3390/jof6040275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2020;S0735-6757(20)30826-3. 10.1016/j.ajem.2020.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon AA, Berg DD, Brea EJ, Deutsch AJ, Kidia KK, Thurber EG, et al. A case of COVID-19 and Pneumocystis jirovecii coinfection. Am J Respir Crit Care Med. 2020;202:136–8. 10.1164/rccm.202003-0766LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventoulis I, Sarmourli T, Amoiridou P, Mantzana P, Exindari M, Gioula G, et al. Bloodstream infection by Saccharomyces cerevisiae in two COVID-19 patients after receiving supplementation of Saccharomyces in the ICU. J Fungi (Basel). 2020;6:98. 10.3390/jof6030098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202:132–5. 10.1164/rccm.202004-1038LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang SY, Lian JS, Hu JH, Zhang XL, Lu YF, Cai H, et al. Clinical characteristics of different subtypes and risk factors for the severity of illness in patients with COVID-19 in Zhejiang, China. Infect Dis Poverty. 2020;9:85. 10.1186/s40249-020-00710-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: An observational study from Pakistan. Mycoses. 2020;63:766–70. 10.1111/myc.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamoth F, Glampedakis E, Boillat-Blanco N, Oddo M, Pagani JL. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin Microbiol Infect. 2020;26:1706–8. 10.1016/j.cmi.2020.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71:1367–76. 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. ; Dutch-Belgian Mycosis study group. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–92. 10.1016/S2213-2600(18)30274-1 [DOI] [PubMed] [Google Scholar]
- 23.Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. ; AspICU Study Investigators. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. 10.1164/rccm.201111-1978OC [DOI] [PubMed] [Google Scholar]
- 24.Koehler P, Bassetti M, Kochanek M, Shimabukuro-Vornhagen A, Cornely OA. Intensive care management of influenza-associated pulmonary aspergillosis. Clin Microbiol Infect. 2019;25:1501–9. 10.1016/j.cmi.2019.04.031 [DOI] [PubMed] [Google Scholar]
- 25.Verweij PE, Rijnders BJA, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46:1524–35. 10.1007/s00134-020-06091-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández NB, Cáceres DH, Beer KD, Irrazabal C, Delgado G, Farias L, et al. Ventilator-associated pneumonia involving Aspergillus flavus in a patient with coronavirus disease 2019 (COVID-19) from Argentina. Med Mycol Case Rep. 2020; Epub ahead of print. 10.1016/j.mmcr.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma A, Hofmeyr A, Bansal A, Thakkar D, Lam L, Harrington Z, et al. COVID-19 associated pulmonary aspergillosis (CAPA): An Australian case report. Med Mycol Case Rep. 2020; Epub ahead of print. 10.1016/j.mmcr.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prattes J, Valentin T, Hoenigl M, Talakic E, Reisinger AC, Eller P. Invasive pulmonary aspergillosis complicating COVID-19 in the ICU - A case report. Med Mycol Case Rep. 2020; Epub ahead of print. 10.1016/j.mmcr.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutsaert L, Steinfort N, Van Hunsel T, Bomans P, Naesens R, Mertes H, et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020;10:71. 10.1186/s13613-020-00686-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarrazyn C, Dhaese S, Demey B, Vandecasteele S, Reynders M, Van Praet JT. Incidence, risk factors, timing and outcome of influenza versus Covid-19 associated putative invasive aspergillosis. Infect Control Hosp Epidemiol. 2020;1–7; Epub ahead of print. 10.1017/ice.2020.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemos DRQ, D’Angelo SM, Farias LABG, Almeida MM, Gomes RG, Pinto GP, et al. Health system collapse 45 days after the detection of COVID-19 in Ceará, Northeast Brazil: a preliminary analysis. Rev Soc Bras Med Trop. 2020;53:e20200354. 10.1590/0037-8682-0354-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helleberg M, Steensen M, Arendrup MC. Invasive aspergillosis in patients with severe COVID-19 pneumonia. Clin Microbiol Infect. 2021;27:147–8. 10.1016/j.cmi.2020.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–9. 10.1016/S2213-2600(20)30237-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaize M, Mayaux J, Nabet C, Lampros A, Marcelin AG, Thellier M, et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26:1636–7. 10.3201/eid2607.201603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupont D, Menotti J, Turc J, Miossec C, Wallet F, Richard JC, et al. Pulmonary aspergillosis in critically ill patients with Coronavirus Disease 2019 (COVID-19). Med Mycol. 2021;59:110–4. 10.1093/mmy/myaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangneux JP, Bougnoux ME, Dannaoui E, Cornet M, Zahar JR. Invasive fungal diseases during COVID-19: We should be prepared. J Mycol Med. 2020;30:100971. 10.1016/j.mycmed.2020.100971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghelfenstein-Ferreira T, Saade A, Alanio A, Bretagne S, Araujo de Castro R, Hamane S, et al. Recovery of a triazole-resistant Aspergillus fumigatus in respiratory specimen of COVID-19 patient in ICU - A case report. Med Mycol Case Rep. 2020; Epub ahead of print. 10.1016/j.mmcr.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lescure FX, Bouadma L, Nguyen D, Parisey M, Wicky PH, Behillil S, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. 10.1016/S1473-3099(20)30200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schein F, Muñoz-Pons H, Mahinc C, Grange R, Cathébras P, Flori P. Fatal aspergillosis complicating severe SARS-CoV-2 infection: A case report. J Mycol Med. 2020;30:101039. 10.1016/j.mycmed.2020.101039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lahmer T, Rasch S, Spinner C, Geisler F, Schmid RM, Huber W. Invasive pulmonary aspergillosis in severe coronavirus disease 2019 pneumonia. Clin Microbiol Infect. 2020;26:1428–9. 10.1016/j.cmi.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohamed A, Hassan T, Trzos-Grzybowska M, Thomas J, Quinn A, O’Sullivan M, et al. Multi-triazole-resistant Aspergillus fumigatus and SARS-CoV-2 co-infection: A lethal combination. Med Mycol Case Rep. 2020 Jun 26; Epub ahead of print. 10.1016/j.mmcr.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antinori S, Rech R, Galimberti L, Castelli A, Angeli E, Fossali T, et al. Invasive pulmonary aspergillosis complicating SARS-CoV-2 pneumonia: A diagnostic challenge. Travel Med Infect Dis. 2020;38:101752. 10.1016/j.tmaid.2020.101752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. ; PREDICO study group. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2020;ciaa1065; Epub ahead of print. 10.1093/cid/ciaa1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruno G, Fabrizio C, Buccoliero GB. COVID-19-associated pulmonary aspergillosis: adding insult to injury. Lancet Microbe. 2020;1:e106. 10.1016/S2666-5247(20)30063-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meijer EFJ, Dofferhoff ASM, Hoiting O, Buil JB, Meis JF. Azole-resistant COVID-19–associated pulmonary aspergillosis in an immunocompetent host: a case report. J Fungi (Basel). 2020;6:79. 10.3390/jof6020079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19–associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202:132–5. 10.1164/rccm.202004-1038LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Biesen S, Kwa D, Bosman RJ, Juffermans NP. Detection of invasive pulmonary aspergillosis in COVID-19 with nondirected BAL. Am J Respir Crit Care Med. 2020;202:1171–3. 10.1164/rccm.202005-2018LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: An observational study from Pakistan. Mycoses. 2020;63:766–70. 10.1111/myc.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdalla S, Almaslamani MA, Hashim SM, Ibrahim AS, Omrani AS. Fatal coronavirus disease 2019–associated pulmonary aspergillosis; a report of two cases and review of the literature. IDCases. 2020;22:e00935. 10.1016/j.idcr.2020.e00935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falces-Romero I, Ruiz-Bastián M, Díaz-Pollán B, Maseda E, García-Rodríguez J; SARS-CoV-2 Working Group. Isolation of Aspergillus spp. in respiratory samples of patients with COVID-19 in a Spanish tertiary care hospital. Mycoses. 2020;63:1144–8. 10.1111/myc.13096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information on COVID-19–associated pulmonary aspergillosis.


