Abstract
Triple-negative breast cancer (TNBC) is characterized by poor outcome and the most challenging breast cancer type to treat worldwide. TNBC manifests distinct profile of mitochondrial functions, which dictates reprogrammed metabolism, fosters tumor progression, and notably serves as therapeutic targets. Mitochondrial microRNAs (mitomiRs) are a group of microRNAs that critically modulate mitochondrial homeostasis. By a pathway-centric manner, mitomiRs tightly orchestrate metabolic reprogramming, redox status, cell apoptosis, mitochondrial dynamics, mitophagy, mitochondrial DNA (mtDNA) maintenance, and calcium balance, leading to an emerging field of study in various cancer types, including TNBC. We herein review the recent insights into the roles and mechanism of mitomiRs in TNBC and highlight its clinical value in diagnosis and prognosis as well as vital advances on therapeutics of preclinical and clinical studies.
1. Introduction
Breast cancer (BC) is the most common cancer in women globally, accounting for about a quarter of female cancer [1, 2]. In spite of recent improvements in molecular and imaging diagnosis and treatments including hormone therapy, target therapy, chemotherapy, and radiotherapy, BC remains the leading cause of cancer death worldwide [1–3]. Based on the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor 2 (HER2), and proliferation marker protein Ki-67, BC can be categorized into four subtypes including luminal A, luminal B, HER2, and triple-negative breast cancer (TNBC) [4].
TNBC is tested negative for ER, PR, and HER2 by immunohistochemical staining, accounting for 15-20% of BC cases [5]. Advanced breast cancer comprises inoperable locally advanced breast cancer and metastatic (stage IV) breast cancer. As shown in Figure 1(a), the bone, the liver, and the lungs account, respectively, for about 67%, 40.8%, and 36.9% of the common metastatic sites, wherein basal-like BC (BLBC, accounting for 75% of the TNBC subtypes [6]) hits 40%, 35%, and 35%, respectively, of the metastatic BC [7]. More than 70% of the metastatic TNBC cases fails to survive after five years of diagnosis and exhibits worse prognosis than other BC subtypes [8]. Due to the lack of ER/PR/HER2 receptors, TNBC is unresponsive to endocrine and target therapy, such as tamoxifen, aromatase inhibitors, and/or anti-HER2-targeted therapies. As such, surgery, radiotherapy, and predominantly nonspecific chemotherapy (e.g., anthracycline and taxane regimens) remain the mainstay for management of these patients, yet with severe side effects worsening quality of life [9, 10]. Although the combination uses of chemotherapy regimens (e.g., capecitabine in conjunction with taxanes) have shown increased response rates, the multiagent approach led to increased toxicities [11, 12]. Therefore, treatments for TNBC have since a major challenge for oncologists due to the absence of unambiguous molecular targets.
Figure 1.
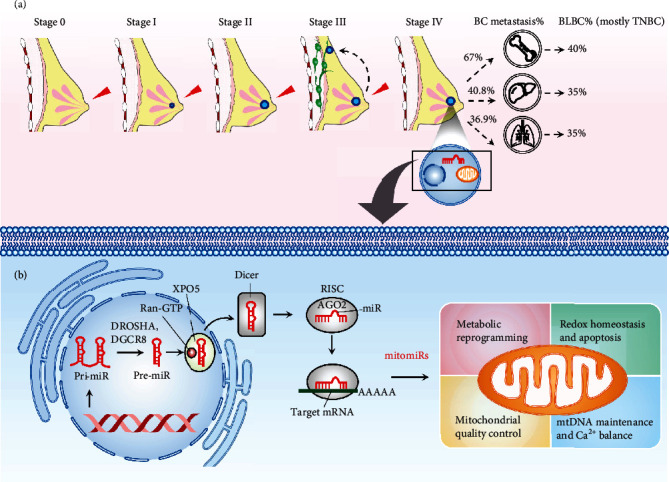
Graphic overview depicting the roles of mitomiRs in modulating mitochondrial homeostasis and its involvements in the scenario of TNBC progression. (a) TNBC stages classified by TNM system [7]. Advanced breast cancer comprises inoperable locally advanced breast cancer and metastatic (stage IV) breast cancer. The bone, the liver, and the lungs account, respectively, for about 67%, 40.8%, and 36.9% of the common metastatic sites, wherein basal-like BC (BLBC, accounting for 75% of the TNBC subtypes [6]) hits 40%, 35%, and 35%, respectively, of the metastatic BC [7]. (b) The mitomiR biogenesis and its impacts on mitochondrial dimensions. The precursor transcripts (pri-miR) are transcribed and posttranscriptionally cleaved by microprocessor (DROSHA and DGCR8) in the nucleus to liberate the pre-miR hairpin. The pre-miR is then exported to the cytoplasm by exportin 5 (XPO5) bound to guanosine 5′-triphosphate (Ran-GTP). In the cytoplasm, the DICER endoribonuclease cleaves the loop of the pre-miR to produce the mature miR. The resulting miR embeds in a groove of Argonaute (AGO) of the RNA-induced silencing complex (RISC) and ultimately targets a strand of mRNA by base pairing its 3′ untranslated region (UTR). When bound to an mRNA, RISC inhibits translation, yet the main effect is to degrade the mRNA through deadenylation. mitomiRs are a particular cluster of miRs that predominantly occupy a sphere of influence on dimensions of mitochondrial homeostasis, including metabolic reprogramming, redox homeostasis, mitochondrial quality control, mtDNA maintenance, and Ca2+ balance.
Mitochondria are dynamic organelles that produce energy through oxidative phosphorylation (OXPHOS) for the sophisticated biochemical reactions of a cell. Originated from eubacterial endosymbiosis, each of them has multiple copies of mitochondrial DNA (mtDNA), which is a circular, double-stranded DNA (16,569-base pairs in human) encoding 37 genes, including 13 proteins, 22 tRNAs, and 2 rRNAs [13]. Structurally, mitochondria have double-membrane system, composed of outer membrane and inner membrane, which compartmentalize intermembrane space, crista, and matrix where OXPHOS takes place to generate ATP [13]. More than intracellular powerhouses, mitochondria play a central role in controlling physiological process and cellular fate by orchestrating metabolism, redox status, apoptosis, dynamics, mitophagy (a mechanism selectively degrading mitochondria by autophagy), mtDNA maintenance, and calcium balance [14, 15]. More recently, approaches aiming at disturbances of cellular metabolism and mitochondrial functions for the treatment of cancer, including BC, have been emerging [15, 16].
MicroRNAs (miRs) are small, approximately 22-nucleotide noncoding RNAs. miR genes are derived from within the introns and are subject to similar types of epigenetic regulation as are the protein-coding genes [17]. As shown in Figure 1(b), a miR gene is initially transcribed by RNA polymerase II into a primary miRNA (pri-miRNA) and then processed in the nucleus by microprocessor complex comprising an endoribonuclease Drosha and a double-stranded RNA-binding protein DiGeorge syndrome critical region 8 (DGCR8) into a ~70 nt precursor miR (pre-miR) [18], which are then exported to the cytoplasm by an exportin 5/guanosine 5′-triphosphate (XPO5/RanGTP) complex. In the cytoplasm, the loop of a pre-miRNA is cleaved by the endonuclease Dicer to generate a mature miR [19]. The regulatory functions of a mature miR are accomplished by associating with the Argonaute (AGO) of the RNA-induced silencing complex (RISC) [20]. As such, a miR ultimately targets a strand of mRNA by base pairing its 3′ untranslated region (UTR) and negatively regulates mRNA expression in most cases. When bound to an mRNA, RISC inhibits translation, yet the main effect is to degrade the mRNA through deadenylation [17].
mitomiRs are a group of miRs that intimately regulate mitochondrial functions. mitomiRs can exert the repressive effect on gene expression to modulate mitochondrial functions by two ways: one major type of mitomiRs targets mRNAs in the cytoplasm; the other type of mitomiRs is imported into mitochondria to target mtDNA-encoded mRNAs. With regard to the gene origin of the mitomiRs, the vast majority of them are nuclear-encoded, while few of the mitomiRs are encoded by mtDNA (e.g., miR-1974, miR-1977, and miR-1978) [21].
Mounting evidence in recent years has noted the translational implication of mitomiRs in metabolic disorder, degenerative diseases, and cancer [22–24]. Their functions in modulating multiple aspects of mitochondrial homeostasis permit alterations in cancer metabolism, growth, metastasis, and sensitivity to clinical drugs. More importantly, mitomiRs' potential as a predictive panel and therapeutic targets is notably emerging. As such, we herein focus on the novel biological roles and mechanisms of mitomiRs on the mitochondria-centric metabolic network in the context of TNBC (Figure 1). Moreover, we highlight recent insights into the advanced understating of predictive value and therapeutic implication of mitomiRs.
2. Roles of mitomiRs in Regulating Metabolic Reprogramming of TNBC
Cancer cells are characterized by significant metabolic reprogramming which facilitate survival and rapid proliferation in a nutrient-deprived tumor microenvironment [25, 26]. Otto Warburg firstly identified that cancer cells maintain high levels of glycolysis for ATP production, regardless of oxygen availability, a phenomenon thereafter termed as the Warburg effect [27]. Since then, an increasing volume of study focused on cancer-associated metabolic reprogramming within crucial metabolic pathways, including altered metabolism of glucose, fatty acid, and amino acids as well as perturbed OXPHOS, in an attempt to find out metabolic susceptibilities during cancer progression. The potential preclinical/clinical reagents targeting metabolic reprogramming of TNBC have been reviewed (32296646). In this regard, we summarized a number of mitomiRs that have been reported to significantly regulate TNBC progression by acting to metabolic reprogramming (Table 1).
Table 1.
Roles and mechanisms of mitomiRs in metabolic reprogramming.
| mitomiR | Function | Target gene | Mechanism of action | Outcome | PMID | Reference |
|---|---|---|---|---|---|---|
| Glucose metabolism | ||||||
| miR-29a | (-)TNBC | ARG2 | ↓AGR2→↓HIF-1α | ↑Apoptosis ↓Proliferation ↓Migration ↓Invasion ↓Tumor growth |
33223849 | Wang et al., 2020 [28] |
| miR-210 | (+)TNBC | GPD1L, CYGB | ↓GPD1L→↑HIF-1α→↑glycolysis ↓CYGB→↓p53→↑glycolysis |
↓Apoptosis ↑Tumor growth |
32908121 | Du et al., 2020 [29] |
| miR-140 | (-)TNBC | GLUT1 | ↓GLUT1→↓glycolysis | ↑OCR/↓ECAR ↓Proliferation ↓Tumor growth |
31184216 | He et al., 2019 [30] |
| let-7a | (-)TNBC | STAT3 | ↓STAT3→↓hnRNPA1→↓PKM2 →↓Glycolysis |
↓Proliferation | 30368881 | Yao et al., 2019 [31] |
| miR-155 | (+)TNBC | PIK3R1, FOXO3a | ↓PI3K/↓FOXO3a→↑cMYC →↑HK2/↑PKM2/↑LDHA →↑Glycolysis |
↑Tumor growth | 29527004 | Kim et al., 2018 [32] |
| miR-128 | (-)TNBC | INSR, IRS1 | ↓INSR/↓INS1→↓p-AKT/↓HK2/↓PFK →↓Glycolysis |
↓Mitochondrial bioenergetics ↓Proliferation ↓Tumor growth |
29116653 | Xiao et al., 2018 [33] |
| miR-342 | (-)TNBC | MCT1 | ↓MCT1→↓lactate uptake | ↓Proliferation ↓Migration |
30115973 | Romero-Cordoba et al., 2018 [34] |
| miR-30a | (-)TNBC | LDHA | ↓LDHA→↓glycolysis | ↑OCR/↓ECAR ↓Proliferation ↓Migration ↓Invasion ↓Tumor growth ↓Tumor metastasis |
28461244 | Li et al., 2017 [35] |
| miR-21 | (+)TNBC | CAB39L, SESN1 | ↓CAB39L, ↓SESN1→↓p-AMPK→↑mTOR | ↑Proliferation ↑Migration ↑Invasion ↑Tumor growth |
28698800 | Pulito et al., 2017 [36] |
| miR-340 | (-)TNBC | MCU | ↓MCU→↓[Ca2+]m→↓LDH →↓Glycolysis |
↓ATP production ↓Lactate production ↓Migration ↓Tumor metastasis |
29137386 | Yu et al., 2017 [37] |
| miR-101 | (-)TNBC | AMPK | ↓AMPK→↓glycolysis | ↓Proliferation | 27145268 | Liu et al., 2016 [38] |
| miR-18a | (-)TNBC | HIF1A | ↓HIF-1α→↓hypoxic gene expression | ↓Tumor growth ↓Tumor metastasis |
25069832 | Krutilina et al., 2014 [39] |
| miR-143 | (-)TNBC | HK2 | ↓HK2→↓glycolysis | ↑Apoptosis ↓Proliferation ↓Tumor growth |
22354042 | Jiang et al., 2012 [40] |
| miR-155 | (+)TNBC | mir-143 | ↑miR-155→↓C/EBPβ→ ↓miR-143→↑HK2→↑glycolysis |
↑Tumor growth | 22354042 | Jiang et al., 2012 [40] |
| Fatty acid metabolism | ||||||
| miR-1291 | (-)TNBC | ESRRA | ↓ERRα→↓CPT1C | ↓Proliferation ↓Tumor growth ↑TNBC sensitivity to 2-DG |
32641987 | Chen et al., 2020 [41] |
| let-7a | (-)TNBC | SCD | ↓SCD, ↓G6PD, ↓FASN, ↓ASSDHPPT →↑OCR/↑ECR/↑ΔΨm |
↓Proliferation ↑TNBC sensitivity to doxorubicin |
25669981 | Serguienko et al., 2015 [42] |
| miR-195 | (-)TNBC | ACACA, FASN, HMGCR | ↓Cellular triglyceride ↓Cellular cholesterol ↓ΔΨm |
↓EMT ↓Proliferation ↓Migration ↓Invasion |
26632252 | Singh et al., 2015 [43] |
| Glutamine/cystine metabolism | ||||||
| miR-27a | (-)TNBC | SLC7A11, CTH | ↓xCT→↓cystine uptake ↓CTH→↓cystine production |
↓Mammosphere formation ↓CSC markers ↑TNBC sensitivity to doxorubicin and paclitaxel |
32066826 | Ueda et al., 2020 [36] |
| OXPHOS | ||||||
| miR-4485 | (-)TNBC | 16S rRNA | ↓16S rRNA→↓ETC enzymes | ↑Cell death ↓Proliferation ↓Tumor growth |
28220193 | Sripada et al., 2017 [44] |
| miR-663 | (-)TNBC | UQCC2 | ↓UQCC2→↓complex III activity | ↑OXPHOS subunits ↓Invasion ↓Tumor growth |
29066618 | Carden et al., 2017 [45] |
(+): oncogenic; (-): tumor suppressor; “↑”: enhanced; “↓”: reduced; ΔΨm: mitochondrial membrane potential; AASDHPPT: 4-phosphopantetheinyl transferase; ACACA: acetyl-CoA carboxylase; AGR2: anterior gradient 2; [Ca2+]m: mitochondrial calcium; AMPK: AMP-activated protein kinase; CAB39L: calcium-binding protein 39-like; C/EBPβ: CCAAT-enhancer-binding protein β; CPT1C: carnitine palmitoyltransferase 1C; CTH: cystathionine gamma-lyase; CYGB: cytoglobin; ECAR: extracellular acidification rate; EMT: epithelial-mesenchymal transition; ERRα (encoded by ESRRA): estrogen-related receptor alpha; FASN: fatty acid synthase; FOXO3a: forkhead box O3; G6PD: glucose-6-phosphate dehydrogenase; GLUT1: glucose transporter 1; GPD1L: glycerol-3-phosphate dehydrogenase 1; HIF1A/HIF-1α: hypoxia-inducible factor 1-alpha; HK2: hexokinase 2; HMGCR: 3-hydroxy-3-methylglutaryl CoA reductase; INSR: insulin receptor; IRS1: insulin receptor substrate 1; LDHA: lactate dehydrogenase A; MCT1: monocarboxylate transporter 1; MCU: mitochondrial calcium uniporter; mtDNA: mitochondrial DNA; mt-ROS: mitochondrial reactive oxygen species; OCR: oxygen consumption rate; OXPHOS: oxidative phosphorylation; PFK: phosphofructokinase; PI3K: phosphoinositide 3-kinases; PKM2: pyruvate kinase M1/2; SCD: stearoyl-CoA desaturase; SESN1: sestrin-1; STAT3: signal transducers and activators of transcription 3; UQCC2: ubiquinol-cytochrome c reductase complex assembly factor 2; xCT (encoded by SLC7A11): cystine/glutamate transporter.
Several lines of study noted that mitomiRs perturb glycolysis-associated targets to inhibit TNBC. Jiang et al. have demonstrated that miR-155/miR-143 axis modulates tumor development by dictating glycolysis [40]. They showed that miR-155 and miR-143 have TNBC-promoting and TNBC-inhibiting effect, respectively. Mechanistically, tumorigenic inflammatory signals augment glycolysis through miR-155, which suppresses miR-143 by targeting CCAAT-enhancer-binding protein β (C/EBPβ). The resulting decrease in miR-143 leads to an unleashed targeting inhibition on hexokinase 2 (HK2), leading to an increase in HK2 and consequently an induced glycolysis [40]. miR-18a was demonstrated to inhibit TNBC growth and metastasis in both in vivo and in vitro models and expression of hypoxic genes [39]. The molecular basis of miR-18a lies in the direct binding to the HIF1A UTR [39]. Inhibition of TNBC proliferation in vitro can be achieved by the overexpression of miR-101, which exerts repressive effect on gene expression of AMPK [38]. Yu et al. have shown that miR-340 inhibits glycolysis through modulating mitochondrial calcium homeostasis in TNBC [37]. Specifically, miR-340 targets the UTR of mitochondrial calcium uniporter (MCU) gene and represses the expression to reduce tumor growth and metastasis [37]. Li et al. demonstrated that miR-30a inhibits glycolysis and enhances mitochondrial respiratory activity by targeting lactate dehydrogenase A (LDHA), whereby the growth and metastasis of TNBC is reduced [35]. miR-342 was reported to disturb glucose metabolism and lactate uptake of TNBC by targeting monocarboxylate transporter 1 (MCT1) [34]. miR-128 was shown to specifically inhibit insulin receptor (INSR) and insulin receptor substrate 1 (IRS1) to abate glycolytic signaling pathway and mitochondrial respiration, resulting in decreased tumor growth [33]. Both miR-148a and miR-152 were shown to directly target pyruvate kinase M1/2 (PKM2) to counteract glycolytic metabolism of TNBC cells, leading to decreased cancer cell proliferation and angiogenesis [46]. Yao et al. have identified that let-7a represses signal transducers and activators of transcription 3 (STAT3) expression level to subsequently mediate hnRNPA1/PKM2 signaling, leading to a decrease in glucose metabolism and TNBC proliferation [31]. miR-140 showed antiglycolytic and antiproliferative effect on TNBC through direct binding to glucose transporter 1 (GLUT1) [30]. Li et al. showed that miR34a inhibitor promotes cell proliferation and glucose uptake, indicating the anti-TNBC effect of miR34a [47]. More recently, Wang et al. demonstrated that miR-29a overexpression exerts inhibitory effect on proliferation, migration, and invasion of TNBC cells through targeting the anterior gradient 2 (ARG2)/HIF-1α axis [28]. Furthermore, circular RNA circPVT1 was identified as a miR sponge, leading to suppressing the expression of miR-29a and promoting in vivo tumor growth [28].
In addition to glucose metabolism, some mitomiRs act toward fatty acid metabolism to exert inhibitory effect on TNBC. For example, miR-195 was shown to target acetyl-CoA carboxylase (ACACA), fatty acid synthase (FASN), and 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR) to suppress epithelial-mesenchymal transition (EMT), proliferation, migration, and invasion [43]. Serguienko et al. have demonstrated that let-7a targets SCD (stearoyl-CoA desaturase) to reduce cell proliferation and sensitivity to doxorubicin [42]. More recently, Chen et al. reported that miR-1291 targets estrogen-related receptor alpha (ERRα) to inhibit the expression level of carnitine palmitoyltransferase 1C (CPT1C), causing a decrease in cell proliferation in vitro and tumor growth in vivo [41].
With regard to amino acid metabolism, Ueda et al. demonstrated that miR-27a play a suppressive role over TNBC by disturbing glutamine/cystine dynamics. Specifically, miR-27a directly bind to UTR of cystine/glutamate transporter (xCT) and cystathionine gamma-lyase (CTH), whereby cystine uptake and production, respectively, are compromised, leading to a decrease in mammosphere formation and cancer stem cell markers as well as an increase in sensitivity to doxorubicin and paclitaxel [36]. On the other hand, some mitomiRs were shown to curb TNBC by impeding OXPHOS. miR-4485 and miR-663 have been identified to hinder mitochondrial function by direct binding to the UTR of mtDNA-encoded 16S rRNA and ubiquinol-cytochrome c reductase complex assembly factor 2 (UQCC2), respectively [44, 45].
In contrast, some reports have explored that a group of mitomiRs responsible for metabolic reprogramming can act to promote the development of TNBC. In this regard, miR-21 was shown to exert its function by targeting calcium-binding protein 39-like (CAB39L) and sestrin-1 (SESN1) to promote proliferation and invasion of TNBC [48]. Kim et al. have reported that miR-155 promotes glycolysis and growth of TNBC by targeting an axis comprising phosphoinositide 3-kinase regulatory subunit 1, forkhead box O, and c-MYC (PI3R1-FOXO3a-cMYC axis) [32]. A recent report conducted by Du et al. identified that miR-210 exerts a positive effect in glucose metabolism, lactate production, extracellular acidification rate (ECAR), proliferation, and starvation-induced apoptosis. The molecular underpinnings were found to lie in direct binding of miR-210 to GPD1L and CYGB, whereby HIF-1α stabilization and p53 suppression can, respectively, be maintained [29]. An integrative network summarizing the roles and pathways of mitomiRs is illustrated in Figure 2.
Figure 2.
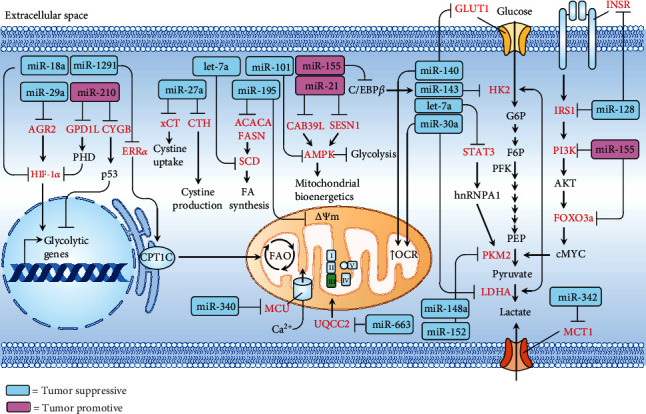
Integrative network depicting that tumor-suppressive (blue rectangle) or tumor-promotive (pink rectangle) mitomiRs act on specific target genes (red) that are involved in metabolic reprogramming. ΔΨm: mitochondrial membrane potential; ACACA: acetyl-CoA carboxylase; AGR2: anterior gradient 2; AMPK: AMP-activated protein kinase; CAB39L: calcium-binding protein 39-like; C/EBPβ: CCAAT-enhancer-binding protein β; CPT1C: carnitine palmitoyltransferase 1C; CTH: cystathionine gamma-lyase; CYGB: cytoglobin; ERRα: estrogen-related receptor alpha; F6P: fructose 6-phosphate; FASN: fatty acid synthase; G6P: glucose 6-phosphate; GLUT1: glucose transporter 1; GPD1L: glycerol-3-phosphate dehydrogenase 1; HIF-1α: hypoxia-inducible factor 1-alpha; HK2: hexokinase 2; INSR: insulin receptor; IRS1: insulin receptor substrate 1; LDHA: lactate dehydrogenase A; MCT1: monocarboxylate transporter 1; MCU: mitochondrial calcium uniporter; mtDNA: mitochondrial DNA; mt-ROS: mitochondrial reactive oxygen species; OCR: oxygen consumption rate; PEP: phosphoenolpyruvate; PFK: phosphofructokinase; PI3K: phosphoinositide 3-kinases; PKM2: pyruvate kinase M1/2; SCD: stearoyl-CoA desaturase; SESN1: sestrin-1; STAT3: signal transducers and activators of transcription 3; UQCC2: ubiquinol-cytochrome c reductase complex assembly factor 2; xCT: cystine/glutamate transporter.
3. Roles of mitomiRs in Redox Homeostasis and Cell Apoptosis
The increased demand for cellular ATP and accelerated oxidation of nutrient molecules trigger the generation of reactive oxygen species (ROS) derived from electron transport chain of mitochondria [49]. A moderate elevated ROS benefits cellular proliferation, whereas excessive ROS production can lead to oxidative damage to proteins, lipids, and DNA [50]. As such, intracellular ROS status plays a critical role in cell growth and survival. Indeed, a cluster of miRs functions to dictate the imbalance between the production of ROS and the activity of antioxidant defence system, resulting in the disruption of redox homeostasis and ultimately cancer cell apoptosis [22]. A study conducted by Eades et al. has demonstrated that miR-200a serves as repressor on TNBC cells by perturbing antioxidant defence mechanism [51]. Specifically, miR-200a directly targets Kelch-like ECH-associated protein 1 (KEAP1), leading to the NF-E2-related factor 2 (NRF2) stabilization and nuclear translocation to act forward the activation of Nrf2-dependent NAD(P)H-quinone oxidoreductase 1 (NQO1) gene transcription [51]. By targeting SCD, let-7a is able to alter fatty acid metabolism, leading to boosted mitochondrial activity and oxidative stress, as evidenced by an increase in oxygen consumption rate (OCR), ROS expression level, and the expression level of superoxide dismutase 2 (SOD2), thioredoxin reductase 1 (TXRND1), and heme oxygenase 1 (HO-1) [42]. Likewise, miR-27a was shown to disrupt glutathione- (GSH-) associated antioxidant mechanism by targeting xCT, which mediates the uptake of cystine, a precursor for GSH biosynthesis [36]. miR-324 has been reported to induce ROS level through inhibiting a driver of tumor progression, activated CDC42 kinase 1 (ACK1) [52]. In contrast, miR-373 plays an anti-ROS role in TNBC by targeting thioredoxin-interacting protein (TXNIP), which functions to inhibit antioxidant protein thioredoxin (Trx) by interacting with its redox-specific active cysteine residues [53]. The ROS-suppressing hallmark of miR-373/TXNIP acted to promote HIF-1α/twist family BHLH transcription factor 1 (TWIST1) pathway, leading to increased invasive behaviour of TNBC cells [53].
Furthermore, some mitomiRs induce ROS production by targeting genes involved in the integrity of mitochondria. In this regard, miR-4485 was demonstrated to translocate to mitochondria to exert direct binding activity to mtDNA-encoded 16S rRNA, resulting in a decrease in electron transport chain (ETC) enzyme activity and mitochondrial ATP production, as well as an increase in expression level of ROS [44]. The tumor-suppressive effect in vivo was verified by overexpression of miR-4485 in TNBC cells [44]. In addition, Ahir et al. recently identified that miR-34a is implicated in effectively suppressing growth and mammosphere formation of TNBC [54]. The inhibitory role of miR-34a on B cell lymphoma 2 (BCL-2) permits the formation of permeabilization pore on the outer membrane of the mitochondria (OMM), promoting the intracellular ROS production [54]. Table 2 summarizes the mitomiRs acting toward the regulation of redox homeostasis and the biological roles and mechanisms in TNBC.
Table 2.
Roles and mechanisms of mitomiRs in the perturbation of redox homeostasis.
| mitomiR | Function | Target gene | Mechanism of action | Outcome | PMID | Reference |
|---|---|---|---|---|---|---|
| miR-34a | (-)TNBC | BCL-2 | ↓BCL-2/↑BAX →↑ROS/↓ΔΨm |
↓Mammosphere formation ↓Tumor growth |
32319481 | Ahir et al., 2020 [54] |
| miR-27a | (-)TNBC |
SLC7A11
CTH NFE2L2 |
↓xCT, ↓CTH, ↓NRF2 →↑ROS, ↓autophagic flux |
↓Mammosphere formation ↓CSC markers ↑TNBC sensitivity to doxorubicin and paclitaxel |
32066826 | Ueda et al., 2020 [36] |
| miR-324 | (-)TNBC | ACK1 | ↓ACK1→↑ROS→ ↑DNA oxidative damage |
↓Proliferation ↓Tumor growth |
31751910 | Zhang et al., 2019 [52] |
| miR-4485 | (-)TNBC | 16S rRNA | ↓16S rRNA→↓ETC enzyme activity→↑ROS/↓ΔΨm | ↑Cell death ↓Proliferation ↓Tumor growth |
28220193 | Sripada et al., 2017 [44] |
| let-7a | (-)TNBC | SCD | ↓SCD→↑OCR→↑ROS | ↑SOD2, TXNRD1, HO-1 ↓Proliferation ↑Cell cycle arrest ↑TNBC sensitivity to doxorubicin |
25669981 | Serguienko et al., 2015 [42] |
| miR-373 | (+)TNBC | TXNIP | ↓TXNIP→↑TRX→↓ROS→↑HIF-1α→↑TWIST 1→↑miR-373 |
↑EMT ↑Migration and invasion ↑Tumor growth ↑Tumor metastasis |
26196741 | Chen et al., 2015 [53] |
| miR-200a | (-)TNBC | KEAP1 | ↓KEAP1→nuclear translocation of NRF2→↑NQO1 | ↑Antioxidant pathway ↓Anchorage-independent cell growth |
21926171 | Eades et al., 2011 [51] |
(+): oncogenic; (-): tumor suppressor; “↑”: enhanced; “↓”: reduced; ΔΨm: mitochondrial membrane potential; ACK1: activated CDC42 kinase 1; BAX: Bcl-2-associated X protein; BCL-2: B cell lymphoma 2; CTH: cystathionine gamma-lyase; CSCs: cancer stem cells; ETC: electron transport chain; HAX-1: HCLS1-associated protein X-1; HO-1: heme oxygenase 1; NQO1: NAD(P)H quinone dehydrogenase 1; NRF2 (encoded by NFE2L2): nuclear factor erythroid 2-related factor 2; ROS: reactive oxygen species; SCD: stearoyl-CoA desaturase; SOD2: superoxide dismutase mitochondrial; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; TNBCSC: triple-negative breast cancer stem cell; TRX: thioredoxin; TWIST1: twist family BHLH transcription factor 1; TXNIP: thioredoxin-interacting protein; TXNRD1: thioredoxin reductase 1; xCT (encoded by SLC7A11): cystine/glutamate transporter.
Several lines of study have revealed a group of mitomiRs modulate the carcinogenesis and sensitivity to drugs of TNBC by targeting genes implicated in apoptotic signaling. Liu et al. have demonstrated that miR-101 play a positive role in the induction of TNBC apoptosis by targeting induced myeloid leukemia cell differentiation protein Mcl-1 (MCL-1), a prosurvival member of the Bcl-2 family [55]. Overexpression of miR-101 was shown to inhibit tumor growth and sensitize cells to paclitaxel-induced apoptosis [55]. Similarly, Zheng et al. demonstrated that miR-145 has a promotive role in receptor-interacting protein Fas-associated protein with death domain caspase-8 (RIP1-FADD-casp8) apoptosis signaling induced by TNF-α [56]. By targeting cellular inhibitor of apoptosis (cIAP1), miR-145 can exert TNBC-suppressive function, leading to the activation of the extrinsic pathway, whereby activated casp8 act to cleave BH3 interacting-domain death agonist (BID) to initiate the mitochondrial apoptotic pathway [56]. miR-233 has been shown to specifically suppress the expression of HCLS1-associated protein X-1 (HAX-1), an antiapoptotic protein that inhibits the activation of caspase-9 [57]. miR-223 overexpression potentiated proapoptotic effect induced by TNF-related apoptosis-inducing ligand (TRAIL) over triple-negative breast cancer stem cells (TNBCSCs) [57]. Patel el al. revealed that miR-15a/miR-16 overexpression in TNBC cells results in increased intrinsic pathway of apoptosis through direct of B cell-specific Moloney murine leukemia virus integration site 1 (BMI1), an oncogene involving mitochondrial homeostasis [58]. miR-20b was shown to target VEGF and cause deceased BCL-2 and increased BAX and activation of cell apoptosis, resulting in an inhibitory effect on proliferation, migration, and invasion of TNBC cells [59]. Both groups, Ahir et al. and Majzoub et al., have recently shown that BCL-2 is a specific target of miR-34a [54] and miR-125a/miR-181a [55]. These mitomiRs can exert suppressive effect on TNBC cells by inducing apoptosis as well as reducing cell migration [55] and tumor growth [54]. Conversely, miR-224 has been reported to be tumor-promotive in TNBC. Zhang et al. showed that miR-224 provides apoptotic resistance and enhances proliferation, migration, and invasion by targeting caspase-9 [60]. Collectively, the recent understandings of mitomiRs in targeting apoptosis pathway in the context of TNBC are listed in Table 3. The schematic sophisticated network encompassing tumor-suppressive/tumor-promotive mitomiRs targeting molecules central to redox homeostasis and apoptosis is illustrated in Figure 3.
Table 3.
Roles and mechanisms of mitomiRs in cell apoptosis.
| mitomiR | Function | Target gene | Mechanism of action | Effect on TNBC | PMID | Reference |
|---|---|---|---|---|---|---|
| miR-34a | (-)TNBC | BCL2 | ↓BCL-2→↑BAX | ↑Apoptosis ↓Migration ↓Mammosphere formation ↓Tumor growth |
32319481 | Ahir et al., 2020 [54] |
| miR-224 | (+)TNBC | CASP9 | ↓Caspase-9 | ↓Apoptosis ↑Proliferation ↑Migration ↑Invasion |
30886656 | Zhang et al., 2019 [60] |
| miR-125a/miR-181a | (-)TNBC | BCL2 | ↓BCL-2→↑BAX | ↑Phospho-p53 ↑PARP cleavage ↑Apoptosis |
31541355 | Majzoub et al., 2019 |
| miR-20b | (-)TNBC | VEGF | ↓BCL-2→↑BAX →Caspase-9/3 |
↑Apoptosis ↓Proliferation ↓Migration ↓Invasion |
28550685 | Lu et al., 2018 [59] |
| miR-15/16 | (-)TNBC | BMI1 | ↓BCL-2/↑BAX/↑BID →↓ΔΨm→↑caspase-9/3 |
↑Cell death ↓Proliferation ↓Migration |
27596816 | Patel et al., 2016 [58] |
| miR-223 | (-)TNBC | HAX1 | ↓HAX-1 | ↑TRAIL-induced apoptosis of TNBCSC | 27618431 | Sun et al., 2016 [57] |
| miR-145 | (-)TNBC | cIAP | ↓cIAP→↑FADD→ ↑Caspase-8/3→↑tBID |
↑TNF-α-induced apoptosis | 26733177 | Zheng et al., 2016 [56] |
| miR-101 | (-)TNBC | MCL1 | ↓MCL-1→↓ΔΨm | ↑Apoptosis ↓Proliferation ↓Tumor growth ↑TNBC sensitivity to paclitaxel |
26036638 | Liu et al., 2015 [55] |
2-DG: 2-deoxy-D-glucose; ΔΨm: mitochondrial membrane potential; BAX: Bcl-2-associated X protein; BCL-2: B cell lymphoma 2; BID: BH3 interacting-domain death agonist; CASP9: caspase-9; cIAP: cellular inhibitor of apoptosis; FADD: Fas-associated protein with death domain; HAX-1: HCLS1-associated protein X-1; MCL-1: induced myeloid leukemia cell differentiation protein Mcl-1; PARP: poly ADP-ribose polymerase; tBID: truncated BH3 interacting-domain death agonist; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; TNBCSC: triple-negative breast cancer stem cell; VEGF: vascular endothelial growth factor.
Figure 3.
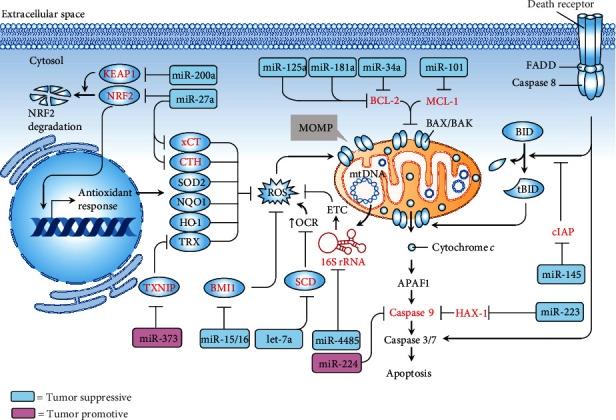
mitomiRs implicated in the regulatory network of redox homeostasis and the fate of apoptosis in the scenario of TNBC. Tumor-suppressive (blue rectangle) or tumor-promotive (pink rectangle) mitomiRs and their target genes (red) act on specific target genes (red). APAF1: apoptotic protease activating factor 1; BAX: Bcl-2-associated X protein; BCL-2: B cell lymphoma 2; BID: BH3 interacting-domain death agonist; BMI1: B cell-specific Moloney murine leukemia virus integration site 1; CTH: cystathionine gamma-lyase; cIAP: cellular inhibitor of apoptosis; ETC: electron transport chain; FADD: Fas-associated protein with death domain; HAX-1: HCLS1-associated protein X-1; HO-1: heme oxygenase 1; MCL-1: induced myeloid leukemia cell differentiation protein Mcl-1; MOMP: mitochondrial outer membrane permeabilization; mtDNA: mitochondrial DNA; NQO1: NAD(P)H quinone dehydrogenase 1; NRF2: nuclear factor erythroid 2-related factor 2; OCR: oxygen consumption rate; SCD: stearoyl-CoA desaturase; ROS: reactive oxygen species; SOD2: superoxide dismutase mitochondrial; tBID: truncated BH3 interacting-domain death agonist; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; TRX: thioredoxin; TWIST1: twist family BHLH transcription factor 1; TXNIP: thioredoxin-interacting protein; TXNRD1: thioredoxin reductase 1; xCT (encoded by SLC7A11): cystine/glutamate transporter.
4. Roles of mitomiRs in Regulating Mitochondrial Dynamics and Mitophagy
Mitochondria are highly dynamic organelles. The dynamic properties comprising fusion, fission, and degradation not only play critical role for their optimal function in energy generation but also have profound impact on human diseases, including cancer [61]. The multiple events of mitochondrial fusion require hydrolysis of GTP and mitochondrial membrane potential [62]. Mitofusins 1 and 2 (MFN1/2) are both GTP-hydrolyzing enzymes of the dynamin superfamily. They are located on the outer membrane of mitochondria (OMM) and required for OMM fusion [63]. The fusion of inner membrane of mitochondria (IMM) is mediated by another member of the dynamin superfamily, optic atrophy 1 (OPA1), located in IMM [64]. On the other hand, the mitochondrial fission largely relied on the activity of dynamin-related protein 1 (DRP1), a GTP-hydrolyzing enzyme [65, 66]. DRP1 is recruited from the cytosol to associate with corresponding receptors residing on OMM, including mitochondrial fission 1 protein (FIS1), mitochondrial fission factor (MFF), mitochondrial dynamics protein MID49 (MID49), and mitochondrial dynamics protein MID51 (MID51) [67–69]. Mitochondrial fission acts to promote segregation of damaged mitochondria, which subsequently facilitates mitochondrial fragments of the appropriate size for engulfment by autophagosomes. The degradation of dysfunctional mitochondria by autophagy, or mitophagy, is a major mechanism for mitochondrial quality control [61].
More recently, the emerging role of mitomiRs in TNBC progression via regulating the mitochondrial quality control machinery has been revealed (Figure 4). In this regard, Purohit et al. reported that miR-195 exerts anti-TNBC effect by targeting NFN2. The dysregulated mitochondrial dynamics was evidenced by decreased MFN2 and OPA1, increased DRP1, and enhanced mitochondrial fission. miR-195 also led to declined mitochondrial OXPHOS and bioenergetics and increased ROS level and cell apoptosis [70]. Zhang et al. demonstrated that overexpression of miR-1 exerts a tumor-suppressive effect by simultaneously targeting mitophagy-associated genes including MINOS1 (mitochondrial inner membrane organizing system 1), GPD2 (glycerol-3-phosphate dehydrogenase 2), and LRPPRC (leucine-rich pentatricopeptide-repeat containing), leading to an induction of mitophagy in breast cancer stem cells [71]. On the other hand, Hu et al. showed that miR-137 play a tumor-promotive role in breast cancer stem cells [72]. By direct targeting FUN14 domain containing 1 (FUNDC1), miR-137 repressed the mitophagy by reducing the expression of FUNDC1, NIP-3-like protein X (NIX), and LC3-II, resulting in an increase in mitochondrial biogenesis and bioenergetics and a decrease in ROS level and cell apoptosis [72]. Table 4 highlights the mitomiRs involved in mitochondrial dynamic and mitophagy.
Figure 4.
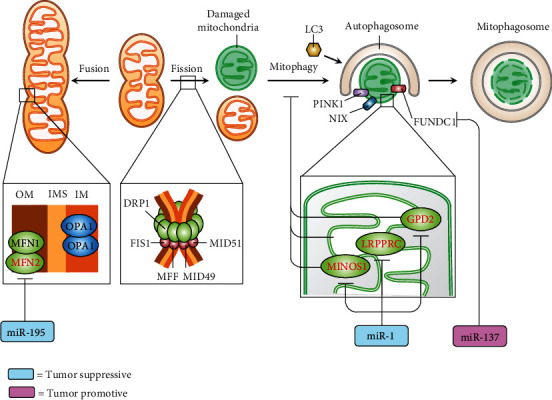
mitomiRs (miR-195, miR-1, and miR-137) regulate mitochondrial quality control machinery, e.g., mitochondrial dynamics and mitophagy. Tumor-suppressive (blue rectangle) or tumor-promotive (pink rectangle) mitomiRs and their target genes (red). DRP1: dynamin-related protein 1; FIS1: mitochondrial fission 1 protein; FUNDC1: FUN14 domain containing 1; GPD2: glycerol-3-phosphate dehydrogenase 2; LC3: microtubule-associated proteins 1A/1B light chain 3B; LDH: lactate dehydrogenase; LRPPRC: leucine-rich pentatricopeptide-repeat containing; MCU: mitochondrial calcium uniporter; MFF: mitochondrial fission factor; MFN: mitofusin; MID49/51: mitochondrial dynamics protein MID49/51; MINOS1: mitochondrial inner membrane organizing system 1; NIX: NIP-3-like protein X; OPA1: optic atrophy 1; PINK1: PTEN-induced kinase 1.
Table 4.
Roles and mechanisms of mitomiRs in mitochondrial homeostasis on dynamics, mitophagy, calcium transport, and mtDNA maintenance.
| mitomiR | Function | Target gene | Mechanism of action | Outcome | PMID | Reference |
|---|---|---|---|---|---|---|
| Mitochondrial dynamics | ||||||
| miR-195 | (-)TNBC | MFN2 | ↓MFN2 ↓OPA1 ↑DRP1 |
↑Mitochondrial fission ↓OCR ↓Mitochondrial ATP ↑ROS ↑Apoptosis |
30932749 | Purohit et al., 2019 [70] |
| Mitophagy | ||||||
| miR-137 | (+)BCSC | FUNDC1 | ↓FUNDC1/↓NIX/↓LC3-II →↓Mitophagy |
↑Mitochondrial biogenesis ↓ROS ↑ATP production ↓Apoptosis |
32945512 | Hu et al., 2020 [72] |
| miR-1 | (-)BCSC |
MINOS1
GPD2 LRPPRC |
↓MINOS1/↓GPD2/↓LRPPRC →↑Disorganized cristae →↑PINK1→↑LC3-II →↑Mitophagy |
Cell cycle arrest at G0/G1 ↓Tumor growth |
31765945 | Zhang et al., 2019 [71] |
| Mitochondrial calcium transport | ||||||
| miR-4488 | (-)TNBC | CX3CL1 | ↓MCU (TNBC)→ ↑miR-4488 EVs (TNBC)→ ↓CX3CL1 (ECs) |
↓Angiogenesis ↓Tumor metastasis ↑Survival time of mice |
33067576 | Zheng et al., 2020 [74] |
| miR-340 | (-)TNBC | MCU | ↓MCU→↓[Ca2+]m →↓LDH | ↓Glucose uptake ↓ATP production ↓Lactate production ↓Migration ↓Tumor metastasis |
29137386 | Yu et al., 2017 [37] |
| miR-195 | (-)TNBC | CYP27b1 | ↑[Ca2+]m ↑[Ca2+]C ↓ΔΨm |
↓EMT ↓Proliferation ↓Migration ↓Invasion |
26632252 | Singh et al., 2015 [43] |
| mtDNA maintenance | ||||||
| miR-499a | (+)TNBCSC | POLB | ↑POLB→↑mtDNA stability | ↑Tumorigenic genes ↑CSC genes ↑Proliferation |
33278391 | Manda et al., 2020 [76] |
| miR-128 | (-)TNBC | INSR, IRS1 | ↓INSR/↓INS1→↓mtDNA content | ↓Mitochondrial bioenergetics ↓Proliferation ↓Tumor growth |
29116653 | Xiao et al., 2018 [33] |
| miR-199a | (-)TNBC | TFAM | ↓TFAM→↓mtDNA content | ↑Cisplatin-induced apoptosis ↓Proliferation |
28126676 | Fan et al., 2017 [75] |
| miR-4485 | (-)TNBC | 16S rRNA | ↓16S rRNA→↓ETC enzyme activity→↓mitochondrial ATP | ↑Cell death ↓Proliferation ↓Tumor growth |
28220193 | Sripada et al., 2017 [44] |
(+): oncogenic; (-): tumor suppressor; “↑”: enhanced; “↓”: reduced; ΔΨm: mitochondrial membrane potential; BCSC: breast cancer stem cell; ↑[Ca2+]C: cytosolic calcium; [Ca2+]m: mitochondrial calcium; CX3CL1: C-X3-C motif chemokine ligand 1; CYP27B1: cytochrome P450 family 27, subfamily B, polypeptide 1; DRP1: dynamin-related protein 1; ECs: endothelial cells; EVs: extracellular vesicles; FUNDC1: FUN14 domain containing 1; GPD2: glycerol-3-phosphate dehydrogenase 2; INSR: insulin receptor; IRS1: insulin receptor substrate 1; LC3: microtubule-associated proteins 1A/1B light chain 3B; LDH: lactate dehydrogenase; LRPPRC: leucine-rich pentatricopeptide-repeat containing; MCU: mitochondrial calcium uniporter; MFN: mitofusin; MINOS1: mitochondrial inner membrane organizing system 1; NIX: NIP-3-like protein X; OPA1: optic atrophy 1; PINK1: PTEN-induced kinase 1; POLB: DNA polymerase beta; ROS: reactive oxygen species; TFAM: mitochondrial transcription factor A; UQCC2: ubiquinol-cytochrome C reductase core protein 2.
5. mitomiRs and Calcium Transport
A growing body of literature has revealed the implications of mitochondrial calcium uptake in tumorigenesis [73]. Indeed, some of the studies unravelled the roles of a cluster of mitomiRs in inhibiting TNBC progression by disturbing mitochondrial calcium uptake. Table 4 outlines the recent findings regarding this issue. Specifically, Singh et al. demonstrated that miR-195 acts toward increased mitochondrial calcium by targeting cytochrome P450 family 27, subfamily B, polypeptide 1 (CYP27B1), leading to dysfunctional mitochondria as evidenced by loss of mitochondrial membrane potential. They confirmed its antitumor role by showing that overexpression of miR-195 impedes EMT, proliferation, migration, and invasion of TNBC cells [43]. Yu et al. showed that miR-340 negatively regulates mitochondrial calcium uniporter (MCU) by direct interacting with MCU 3′UTR. Downregulated MCU was shown to inhibit tumor Warburg effect, leading to a significant decrease in cell migration and invasion in vitro and lung metastasis in vivo [37]. Interestingly, Zheng el al. revealed that knockdown of MCU induces generation and secretion of miR-4488-containing extracellular vesicles (EVs) from TNBC cells. The miR-4488-containing EVs uptaken by endothelial cells acted to target an angiogenic activator C-X3-C motif chemokine ligand 1 (CX3CL1), resulting in suppressed angiogenic activity. Furthermore, the administration of miR-4488-containing EVs inhibited tumor metastasis and increased survival time of tumor-bearing mice [74]. The schematic pipeline regarding the roles and mechanisms of mitomiRs directing at mitochondrial calcium balance is illustrated in Figure 5.
Figure 5.
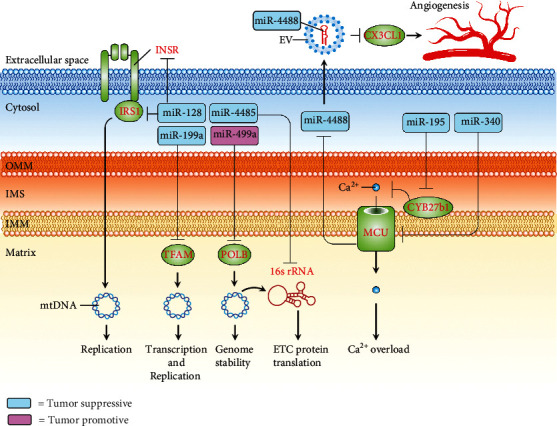
Schematic pipeline depicting mitomiRs that exert tumor-suppressive or tumor-promotive role on TNBC through acting on mechanisms involved in mtDNA maintenance or mitochondrial calcium uptake. Tumor-suppressive (blue rectangle) or tumor-promotive (pink rectangle) mitomiRs and their target genes (red). CX3CL1: C-X3-C motif chemokine ligand 1; CYP27B1: cytochrome P450 family 27, subfamily B, polypeptide 1; INSR: insulin receptor; IRS1: insulin receptor substrate 1; MCU: mitochondrial calcium uniporter; mtDNA: mitochondrial DNA; POLB: DNA polymerase beta; ROS: reactive oxygen species; TFAM: mitochondrial transcription factor A.
6. mitomiRs and mtDNA Maintenance
A line of research identified a group of mitomiRs functioning to modulate the maintenance of mtDNA in the context of TNBC (Table 4). Sripada et al. showed that miR-4485 suppresses tumor growth and cell proliferation of TNBC by targeting 16S rRNA encoded by mtDNA [44]. Fan et al. demonstrated that miR-199a directly targets mitochondrial transcription factor A (TFAM) and causes a significant decrease in mtDNA content, leading to reduced proliferation and potentiate proapoptotic effect induced by cisplatin [75]. In addition, Xiao et al. reported that miR-128 exerts a mtDNA-reducing effect through targeting INSR/IRS1, resulting in a decrease in mitochondrial bioenergetics, proliferation, and tumor growth of TNBC [33]. More recently, miR-499a was identified as a driver of TNBCSC development. In this regard, Manda et al. showed that miR-499a acts to mediate the suppression of DNA polymerase beta (POLB), which plays a master role in the repair and genome stability of mtDNA [76]. We summarized the mechanisms of mitomiRs acting on mtDNA maintenance of TNBC in Figure 5.
7. Prognostic Value, Therapeutic Implications, and Future Perspective
The expression of several mitomiRs can potentially be regarded as predictive, diagnostic, and prognostic biomarkers for TNBC (Table 5). A cluster of low-expressed mitomiRs is identified in tumor tissue [28, 33, 34, 44–47, 52, 55, 71, 77–79], while some of them are high-expressed [32, 53, 79, 80]. Of note, some mitomiRs showed clinical significance in predicting disease outcome. There are a group of mitomiRs enabling predicting survival time [33, 34, 45, 47, 77], while another set of them indicates advanced stage or metastasis [52, 53, 55]. Importantly, circulating mitomiRs identified as relevant biomarkers provide an opportunity to establish a rapid clinical panel which can be less invasive and more accessible [47, 48, 74, 79–81].
Table 5.
Clinicopathological relevance of mitomiRs.
| mitomiRs | Specimen | Expression | Clinical relevance | PMID | Reference |
|---|---|---|---|---|---|
| miR-4488 | Serum EVs | ↓ | Low expression in TNBC patients | 33067576 | Zheng et al., 2020 [74] |
| miR-21 | Serum | ↓ | Decreased level in BC patients after metformin treatment | 28698800 | Pulito et al., 2017 [48] |
| miR-34a | Serum Tissue |
↓ | Low expression predicts poor OS of TNBC patients | 31616515 31897330 |
Li et al., 2019 [47] Kim et al., 2019 [77] |
| miR-29a | Tissue | ↓ | Low expression predicts poor OS of BC patients | 33223849 | Wang et al., 2020 [28] |
| miR-1 | Tissue | ↓ | Low expression in BC patients | 31765945 | Zhang et al., 2019 [71] |
| miR-324 | Tissue | ↓ | Low expression correlated with large tumor size and advanced TNM stage (III-IV) of BC patients | 31751910 | Zhang et al., 2019 [52] |
| miR-342 | Tissue | ↓ | Low expression predicts poor OS of TNBC patients | 30115973 | Romero-Cordoba et al., 2018 [34] |
| miR-128 | Tissue | ↓ | Low expression predicts poor OS and DFS of TNBC patients | 29116653 | Xiao et al., 2018 [33] |
| miR-125b | Tissue | ↓ | Low expression in BC tissue and TNBC cell line | 29434858 | Hu et al., 2018 |
| miR-4485 | Tissue | ↓ | Low expression in BC patients | 28220193 | Sripada et al., 2017 [44] |
| miR-663 | Tissue | ↓ | Low expression predicts poor OS of BC patients | 29066618 | Carden et al., 2017 [45] |
| miR-195 | Tissue | ↓ | Low expression in TNBC patients | 29183284 | Qattan et al., 2017 [79] |
| miR-148a, miR-152 | Tissue | ↓ | Low expression in TNBC tissue compared to adjacent normal tissue | 25703326 | Xu et al., 2015 [46] |
| miR-101 | Tissue | ↓ | Low expression associated with advanced TNM stage (III-IV) and lymph node infiltration of TNBC patients | 26036638 | Liu et al., 2015 [55] |
| miR-195 | Plasma | ↑ | High expression in TNBC patients | 29183284 | Qattan et al., 2017 [79] |
| miR-210 | Plasma | ↑ | High expression in TNBC patients | 29183284 | Qattan et al., 2017 [79] |
| miR-16 | Plasma | ↑ | High expression in TNBC patients | 29183284 | Qattan et al., 2017 [79] |
| miR-221 | Serum Tissue |
↑ | High expression in serum and tumor tissue of BC patients | 26503209 | Ye et al., 2016 [80] |
| miR-27a | Serum | ↑ | High expression in BC patients and in TNBC cell line | 26662313 | Zhou et al., 2016 [81] |
| miR-155 | Tissue | ↑ | High expression in tumor tissue indicates elevated glucose uptake of TNBC patients | 29527004 | Kim et al., 2018 [32] |
| miR-210 | Tissue | ↑ | High expression in TNBC patients | 29183284 | Qattan et al., 2017 [79] |
| miR-16 | Tissue | ↑ | High expression in TNBC patients | 29183284 | Qattan et al., 2017 [79] |
| miR-373 | Tissue | ↑ | High expression predicts lymph node metastasis of BC patients | 26196741 | Chen et al., 2015 [53] |
“↑”: enhanced; “↓”: reduced; BC: breast cancer; DFS: disease-free survival; EVs: extracellular vesicles; OS: overall survival; TNBC: triple-negative breast cancer; TNM: tumor, node, metastasis.
Recent insights into therapeutic approaches in preclinical model pave new paths to potential treatments for TNBC (Table 6). These strategies exert treatment effect by targeting various aspects implicated in mitochondrial homeostasis, including metabolic reprogramming, apoptosis, redox signaling, and mitochondrial calcium transport. Most common approaches employed genetic manipulation, including siRNA, shRNA, and antisense for mitomiR inhibition [28, 52, 59, 80]. Although some approaches conducted by virus-based gene delivery demonstrated treatment efficiency [33, 82], the potential adverse effects to humans must be taken into consideration in the future should they be registered for clinical trials. The use of chemotherapeutics combo could be promising but still need more advanced study, such as in vivo model (31541355). Nanoparticle-loaded mitomiRs provide a potential way to achieve highly efficient delivery into tumor (32319481). Interestingly, Ahir et al. demonstrated a mitomiR-promoting nanotechnology using copper oxide nanowire fabricated with folic acid (CuO-Nw-FA) that enables enhanced cellular uptake in TNBC cells without imparting significant toxicity in normal cellular system [83]. Oral hypoglycemic drug [48], natural products [76, 84], epigenetic modifier [51], and physical agent [85] have been shown to effectively inhibit TNBC cell proliferation. More notably, the notion of using mitomiR-containing EVs may offer a treatment strategy with advantage of high purity, specificity, and biodistribution as less safety concern [74]. Of note, some clinical trials utilizing mitomiR-based treatment for several types of cancer have been registered (Table 7). Unfortunately, due to the occurrence of immune-related serious adverse events, the trial using miR-34a was terminated (NCT01829971). The termination of anti-miR-155-based drug is due to business reasons, but not concerns regarding safety or lack of efficacy (NCT03713320). The miR-26-based approach, TargomiRs, may be a next glimmer of hope for cancer treatment. The first-in-human, phase 1 trial of TargomiR patients with malignant pleural mesothelioma has completed. The results showed acceptable safety profile and early signs of activity of TargomiRs in patients [86], supporting the feasibility of further study.
Table 6.
mitomiR-based therapeutics and targeting mechanisms in preclinical study of TNBC.
| Approach/reagent | Effecting mitomiR | Experimental model | PMID | Reference |
|---|---|---|---|---|
| Metabolic reprogramming | ||||
| siRNA-circPVT1 | ↑miR-29a | In vivo, in vitro | 33223849 | Wang et al., 2020 [28] |
| Lentivirus-miR-128 | ↑miR-128 | In vivo, in vitro | 29116653 | Xiao et al., 2018 [33] |
| Small molecule (metformin) | ↓miR-21 | In vitro, in vivo | 28698800 | Pulito et al., 2017 [48] |
| Apoptosis | ||||
| Nanoparticle-loaded miR-34a | ↑miR-34a | In vivo, ex ovo, in vitro | 32319481 | Ahir et al., 2020 [54] |
| Nanoparticle-loaded anti-miR-10b | ↓miR-10b | In vivo, ex ovo, in vitro | 32319481 | Ahir et al., 2020 [54] |
| Cisplatin+thiosemicarbazone compound 4 | ↑miR-125a, ↑miR-181a | In vitro | 31541355 | Majzoub et al., 2019 |
| shRNA-lncCAMTA1 | ↑miR-20b | In vitro | 28550685 | Lu et al., 2018 [59] |
| Anti-miR-221 | ↓miR-221 | In vitro | 26503209 | Ye et al., 2016 [80] |
| Redox signaling | ||||
| Natural product (ursolic acid) | ↓miR-499a | Ex ovo, in vitro | 33278391 | Manda et al., 2020 [76] |
| Physical agent (ultrasound) | ↑miR-200c | In vitro | 32313093 | Shi et al., 2020 [85] |
| Natural product (parthenolide) | ↑miR-29b | In vitro | 31313842 | De Blasio, 2019 [84] |
| shRNA-LINC00963 | ↑miR-324 | In vitro, in vivo | 31751910 | Zhang et al., 2019 [52] |
| Adenovirus-MDA-7 | ↓miR-221 | In vitro, in vivo | 30842276 | Pradhan et al., 2019 [82] |
| CuO-Nw-FA | ↑miR-425 | In vivo, ex ovo, in vitro | 26520043 | Ahir et al., 2016 [83] |
| Histone deacetylase inhibitor (SAHA) | ↑miR-200a | In vitro | 21926171 | Eades et al., 2011 [51] |
| Mitochondrial calcium transport | ||||
| Exosome | ↑miR-4488 | In vivo, in vitro | 33067576 | Zheng et al., 2020 [74] |
“↑”: enhanced; “↓”: reduced; circPVT1: circular RNA PVT1; CuO-Nw-FA: copper oxide nanowire conjugated with folic acid; lncRNA: long noncoding RNA; MITF: melanogenesis-associated transcription factor; SAHA: suberoylanilide hydroxamic acid.
Table 7.
Interventional clinical trials for mitomiRs.
| Intervention | Effecting mitomiR | Cancer type | Trial number | Phase (status) |
|---|---|---|---|---|
| Metabolic reprogramming | ||||
| Cobomarsen (MRG-106) | ↓miR-155 | T-cell lymphoma/mycosis fungoides | NCT03713320; NCT03837457 | Phase 2 (terminated) |
| TargomiRs | ↑miR-16 | Mesothelioma NSCLC |
NCT02369198 | Phase 1 (completed) |
| Apoptosis | ||||
| MRX-34 | ↑miR-34a | Liver cancer | NCT01829971 | Phase 1 (terminated) |
| Lymphoma | ||||
| SCLC | ||||
| NSCLC | ||||
| Melanoma | ||||
| Multiple myeloma | ||||
| Renal cell carcinoma | ||||
“↑”: enhanced; “↓”: reduced; NSCLC: non-small-cell lung cancer; SCLC: small cell lung cancer.
8. Conclusions
Due to the absence of unambiguous molecular targets for TNBC, its clinical treatment is still a challenging issue. The hallmarks of altered metabolism and mitochondrial fitness in TNBC provide an opportunity for advancing new diagnostic/prognostic tools and therapeutic approaches. mitomiRs are a subgroup of microRNAs that closely regulate mitochondrial functionality by targeting genes present in the cytosol or mitochondria. Growing evidence has elucidated its effectiveness and mechanism of action in the context of TNBC, making mitomiRs an emerging field of study. Indeed, the predictive, prognostic, and diagnostic value of a number of mitomiRs has been revealed. There is increasing novel mitomiR-based therapeutics aiming for efficient inhibition on tumor growth. It is important to note that some mitomiRs were qualified for the early stage of clinical trial. In spite of these inspiring advances, there is still a need to gain deeper insight into mitomiR-dictated mechanisms with respect to mitochondrial homeostasis, to develop accessible prediction panel with satisfactory sensitivity/specificity and to explore interventions suitable for clinical use. To summarize, mitomiRs represent attractive therapeutic targets for the treatment of TNBC.
Acknowledgments
The authors would like to thank James Waddell for his assistance with the proofreading and revision of this article. This research was funded by the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-442-001-MY3, MOST 109-2314-B-442-001, and MOST 109-2314-B-075B-002), National Health Research Institutes (NHRI-109BCCO-MF-202015-01), and Show Chwan Memorial Hospital, Taiwan (SRD-109023, SRD-109024, SRD-109025, and RD107063).
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Fitzmaurice C., Abate D., Abbasi N., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017. JAMA Oncology. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Torre L. A., Siegel R. L., Ward E. M., Jemal A. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiology, Biomarkers & Prevention. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 4.Goldhirsch A., Wood W. C., Coates A. S., Gelber R. D., Thurlimann B., Senn H. J. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Annals of Oncology. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabbri F., Salvi S., Bravaccini S. Know your enemy: genetics, aging, exposomic and inflammation in the war against triple negative breast cancer. Seminars in Cancer Biology. 2020;60:285–293. doi: 10.1016/j.semcancer.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Angius A., Cossu-Rocca P., Arru C., et al. Modulatory role of microRNAs in triple negative breast cancer with basal-like phenotype. Cancers. 2020;12(11):p. 3298. doi: 10.3390/cancers12113298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harbeck N., Penault-Llorca F., Cortes J., et al. Breast cancer. Nature Reviews. Disease Primers. 2019;5(1):p. 66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 8.Bonotto M., Gerratana L., Poletto E., et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. The Oncologist. 2014;19(6):608–615. doi: 10.1634/theoncologist.2014-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenton J. D., Carey L. A., Ahmed A. A., Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? Journal of Clinical Oncology. 2005;23(29):7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 10.Jitariu A. A., Cimpean A. M., Ribatti D., Raica M. Triple negative breast cancer: the kiss of death. Oncotarget. 2017;8(28):46652–46662. doi: 10.18632/oncotarget.16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchini G., Balko J. M., Mayer I. A., Sanders M. E., Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nature Reviews. Clinical Oncology. 2016;13(11):674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conlin A. K., Seidman A. D. Taxanes in breast cancer: an update. Current Oncology Reports. 2007;9(1):22–30. doi: 10.1007/BF02951422. [DOI] [PubMed] [Google Scholar]
- 13.Andrews R. M., Kubacka I., Chinnery P. F., Lightowlers R. N., Turnbull D. M., Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nature Genetics. 1999;23(2):p. 147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 14.Tait S. W., Green D. R. Mitochondrial regulation of cell death. Cold Spring Harbor Perspectives in Biology. 2013;5(9) doi: 10.1101/cshperspect.a008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues T., Ferraz L. S. Therapeutic potential of targeting mitochondrial dynamics in cancer. Biochemical Pharmacology. 2020;182:p. 114282. doi: 10.1016/j.bcp.2020.114282. [DOI] [PubMed] [Google Scholar]
- 16.Reda A., Refaat A., Abd-Rabou A. A., et al. Role of mitochondria in rescuing glycolytically inhibited subpopulation of triple negative but not hormone-responsive breast cancer cells. Scientific Reports. 2019;9(1):p. 13748. doi: 10.1038/s41598-019-50141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodall G. J., Wickramasinghe V. O. RNA in cancer. Nature Reviews. Cancer. 2021;21(1):22–36. doi: 10.1038/s41568-020-00306-0. [DOI] [PubMed] [Google Scholar]
- 18.Carthew R. W., Sontheimer E. J. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito K., Ishizuka A., Siomi H., Siomi M. C. Processing of pre-microRNAs by the Dicer-1-Loquacious complex in Drosophila cells. PLoS Biology. 2005;3(7, article e235) doi: 10.1371/journal.pbio.0030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S., Lian S. L., Moser J. J., et al. Identification of GW182 and its novel isoform TNGW1 as translational repressors in Ago2-mediated silencing. Journal of Cell Science. 2008;121(24):4134–4144. doi: 10.1242/jcs.036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandiera S., Ruberg S., Girard M., et al. Nuclear outsourcing of RNA interference components to human mitochondria. PLoS One. 2011;6(6, article e20746) doi: 10.1371/journal.pone.0020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu C., Zhou D., Wang Q., et al. Crosstalk of microRNAs and oxidative stress in the pathogenesis of cancer. Oxidative Medicine and Cellular Longevity. 2020;2020:13. doi: 10.1155/2020/2415324.2415324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortega M. A., Fraile-Martinez O., Guijarro L. G., et al. The regulatory role of mitochondrial microRNAs (MitomiRs) in breast cancer: translational implications present and future. Cancers. 2020;12(9):p. 2443. doi: 10.3390/cancers12092443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purohit P. K., Saini N. Mitochondrial microRNA (MitomiRs) in cancer and complex mitochondrial diseases: current status and future perspectives. Cellular and Molecular Life Sciences. 2021;78(4):1405–1421. doi: 10.1007/s00018-020-03670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeBerardinis R. J., Chandel N. S. Fundamentals of cancer metabolism. Science Advances. 2016;2(5, article e1600200) doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlova N. N., Thompson C. B. The emerging hallmarks of cancer metabolism. Cell Metabolism. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koppenol W. H., Bounds P. L., Dang C. V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nature Reviews. Cancer. 2011;11(5):325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Huang K., Shi L., Zhang Q., Zhang S. <p>CircPVT1 promoted the progression of breast cancer by regulating MiR-29a-3p-mediated AGR2-HIF-1α pathway</p>. Cancer Management and Research. 2020;Volume 12:11477–11490. doi: 10.2147/CMAR.S265579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Y., Wei N., Ma R., Jiang S., Song D. A miR-210-3p regulon that controls the Warburg effect by modulating HIF-1α and p53 activity in triple-negative breast cancer. Cell Death & Disease. 2020;11(9):p. 731. doi: 10.1038/s41419-020-02952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He Y., Deng F., Zhao S., et al. Analysis of miRNA-mRNA network reveals miR-140-5p as a suppressor of breast cancer glycolysis via targeting GLUT1. Epigenomics. 2019;11(9):1021–1036. doi: 10.2217/epi-2019-0072. [DOI] [PubMed] [Google Scholar]
- 31.Yao A., Xiang Y., Si Y. R., et al. PKM2 promotes glucose metabolism through a let-7a-5p/Stat3/hnRNP-A1 regulatory feedback loop in breast cancer cells. Journal of Cellular Biochemistry. 2019;120(4):6542–6554. doi: 10.1002/jcb.27947. [DOI] [PubMed] [Google Scholar]
- 32.Kim S., Lee E., Jung J., et al. MicroRNA-155 positively regulates glucose metabolism via PIK3R1-FOXO3a-cMYC axis in breast cancer. Oncogene. 2018;37(22):2982–2991. doi: 10.1038/s41388-018-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao M., Lou C., Xiao H., et al. MiR-128 regulation of glucose metabolism and cell proliferation in triple-negative breast cancer. The British Journal of Surgery. 2018;105(1):75–85. doi: 10.1002/bjs.10646. [DOI] [PubMed] [Google Scholar]
- 34.Romero-Cordoba S. L., Rodriguez-Cuevas S., Bautista-Pina V., et al. Loss of function of miR-342-3p results in MCT1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Scientific Reports. 2018;8(1):p. 12252. doi: 10.1038/s41598-018-29708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L., Kang L., Zhao W., et al. miR-30a-5p suppresses breast tumor growth and metastasis through inhibition of LDHA-mediated Warburg effect. Cancer Letters. 2017;400:89–98. doi: 10.1016/j.canlet.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Ueda S., Takanashi M., Sudo K., Kanekura K., Kuroda M. miR-27a ameliorates chemoresistance of breast cancer cells by disruption of reactive oxygen species homeostasis and impairment of autophagy. Laboratory Investigation. 2020;100(6):863–873. doi: 10.1038/s41374-020-0409-4. [DOI] [PubMed] [Google Scholar]
- 37.Yu C., Wang Y., Peng J., et al. Mitochondrial calcium uniporter as a target of microRNA-340 and promoter of metastasis via enhancing the Warburg effect. Oncotarget. 2017;8(48):83831–83844. doi: 10.18632/oncotarget.19747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu P., Ye F., Xie X., et al. mir-101-3p is a key regulator of tumor metabolism in triple negative breast cancer targeting AMPK. Oncotarget. 2016;7(23):35188–35198. doi: 10.18632/oncotarget.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krutilina R., Sun W., Sethuraman A., et al. MicroRNA-18a inhibits hypoxia-inducible factor 1α activity and lung metastasis in basal breast cancers. Breast Cancer Research. 2014;16(4):p. R78. doi: 10.1186/bcr3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang S., Zhang L. F., Zhang H. W., et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. The EMBO Journal. 2012;31(8):1985–1998. doi: 10.1038/emboj.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y., Zhou Y., Han F., et al. A novel miR-1291-ERRα-CPT1C axis modulates tumor cell proliferation, metabolism and tumorigenesis. Theranostics. 2020;10(16):7193–7210. doi: 10.7150/thno.44877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serguienko A., Grad I., Wennerstrom A. B., et al. Metabolic reprogramming of metastatic breast cancer and melanoma by let-7a microRNA. Oncotarget. 2015;6:2451–2465. doi: 10.18632/oncotarget.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh R., Yadav V., Kumar S., Saini N. MicroRNA-195 inhibits proliferation, invasion and metastasis in breast cancer cells by targeting FASN, HMGCR, ACACA and CYP27B1. Scientific Reports. 2015;5(1):p. 17454. doi: 10.1038/srep17454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sripada L., Singh K., Lipatova A. V., et al. hsa-miR-4485 regulates mitochondrial functions and inhibits the tumorigenicity of breast cancer cells. Journal of Molecular Medicine (Berlin, Germany) 2017;95(6):641–651. doi: 10.1007/s00109-017-1517-5. [DOI] [PubMed] [Google Scholar]
- 45.Carden T., Singh B., Mooga V., Bajpai P., Singh K. K. Epigenetic modification of miR-663 controls mitochondria-to-nucleus retrograde signaling and tumor progression. The Journal of Biological Chemistry. 2017;292(50):20694–20706. doi: 10.1074/jbc.M117.797001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Q., Liu L. Z., Yin Y., et al. Regulatory circuit of PKM2/NF-κB/miR-148a/152-modulated tumor angiogenesis and cancer progression. Oncogene. 2015;34(43):5482–5493. doi: 10.1038/onc.2015.6. [DOI] [PubMed] [Google Scholar]
- 47.Li Z., Gong X., Zhang W., et al. Inhibition of miRNA-34a promotes triple negative cancer cell proliferation by promoting glucose uptake. Experimental and Therapeutic Medicine. 2019;18:3936–3942. doi: 10.3892/etm.2019.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pulito C., Mori F., Sacconi A., et al. Metformin-induced ablation of microRNA 21-5p releases Sestrin-1 and CAB39L antitumoral activities. Cell discovery. 2017;3(1):p. 17022. doi: 10.1038/celldisc.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. Journal of Bioenergetics and Biomembranes. 1999;31(4):347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 50.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature Reviews. Drug Discovery. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 51.Eades G., Yang M., Yao Y., Zhang Y., Zhou Q. miR-200a regulates Nrf2 activation by targeting _Keap1_ mRNA in breast cancer cells. The Journal of Biological Chemistry. 2011;286(47):40725–40733. doi: 10.1074/jbc.M111.275495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang N., Zeng X., Sun C., et al. LncRNA LINC00963 promotes tumorigenesis and radioresistance in breast cancer by sponging miR-324-3p and inducing ACK1 expression. Molecular Therapy-Nucleic Acids. 2019;18:871–881. doi: 10.1016/j.omtn.2019.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Chen D., Dang B. L., Huang J. Z., et al. MiR-373 drives the epithelial-to-mesenchymal transition and metastasis via the miR-373-TXNIP-HIF1alpha-TWIST signaling axis in breast cancer. Oncotarget. 2015;6:32701–32712. doi: 10.18632/oncotarget.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahir M., Upadhyay P., Ghosh A., et al. Delivery of dual miRNA through CD44-targeted mesoporous silica nanoparticles for enhanced and effective triple-negative breast cancer therapy. Biomaterials Science. 2020;8(10):2939–2954. doi: 10.1039/d0bm00015a. [DOI] [PubMed] [Google Scholar]
- 55.Liu X., Tang H., Chen J., et al. MicroRNA-101 inhibits cell progression and increases paclitaxel sensitivity by suppressing MCL-1 expression in human triple-negative breast cancer. Oncotarget. 2015;6(24):20070–20083. doi: 10.18632/oncotarget.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng M., Wu Z., Wu A., Huang Z., He N., Xie X. MiR-145 promotes TNF-α-induced apoptosis by facilitating the formation of RIP1-FADDcaspase-8 complex in triple-negative breast cancer. Tumour Biology. 2016;37(7):8599–8607. doi: 10.1007/s13277-015-4631-4. [DOI] [PubMed] [Google Scholar]
- 57.Sun X., Li Y., Zheng M., Zuo W., Zheng W. MicroRNA-223 increases the sensitivity of triple-negative breast cancer stem cells to TRAIL-induced apoptosis by targeting HAX-1. PLoS One. 2016;11(9, article e0162754) doi: 10.1371/journal.pone.0162754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel N., Garikapati K. R., Ramaiah M. J., Polavarapu K. K., Bhadra U., Bhadra M. P. miR-15a/miR-16 induces mitochondrial dependent apoptosis in breast cancer cells by suppressing oncogene BMI1. Life Sciences. 2016;164:60–70. doi: 10.1016/j.lfs.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 59.Lu P., Gu Y., Li L., Wang F., Yang X., Yang Y. Long noncoding RNA CAMTA1 promotes proliferation and mobility of the human breast cancer cell line MDA-MB-231 via targeting miR-20b. Oncology Research. 2018;26(4):625–635. doi: 10.3727/096504017X14953948675395. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Zhang L., Zhang X., Wang X., He M., Qiao S. MicroRNA-224 promotes tumorigenesis through downregulation of caspase-9 in triple-negative breast cancer. Disease Markers. 2019;2019:9. doi: 10.1155/2019/7378967.7378967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan D. C. Mitochondrial dynamics and its involvement in disease. Annual Review of Pathology. 2020;15(1):235–259. doi: 10.1146/annurev-pathmechdis-012419-032711. [DOI] [PubMed] [Google Scholar]
- 62.Legros F., Lombes A., Frachon P., Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Molecular Biology of the Cell. 2002;13(12):4343–4354. doi: 10.1091/mbc.e02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koshiba T., Detmer S. A., Kaiser J. T., Chen H., McCaffery J. M., Chan D. C. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305(5685):858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 64.Mishra P., Carelli V., Manfredi G., Chan D. C. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metabolism. 2014;19(4):630–641. doi: 10.1016/j.cmet.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pagliuso A., Cossart P., Stavru F. The ever-growing complexity of the mitochondrial fission machinery. Cellular and Molecular Life Sciences. 2018;75(3):355–374. doi: 10.1007/s00018-017-2603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jimah J. R., Hinshaw J. E. Structural insights into the mechanism of dynamin superfamily proteins. Trends in Cell Biology. 2019;29(3):257–273. doi: 10.1016/j.tcb.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loson O. C., Song Z., Chen H., Chan D. C. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Molecular Biology of the Cell. 2013;24(5):659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Osellame L. D., Singh A. P., Stroud D. A., et al. Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission. Journal of Cell Science. 2016;129(11):2170–2181. doi: 10.1242/jcs.185165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Otera H., Miyata N., Kuge O., Mihara K. Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. The Journal of Cell Biology. 2016;212(5):531–544. doi: 10.1083/jcb.201508099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Purohit P. K., Edwards R., Tokatlidis K., Saini N. MiR-195 regulates mitochondrial function by targeting mitofusin-2 in breast cancer cells. RNA Biology. 2019;16(7):918–929. doi: 10.1080/15476286.2019.1600999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang S., Liu C., Zhang X. Mitochondrial damage mediated by miR-1 overexpression in cancer stem cells. Molecular Therapy-Nucleic Acids. 2019;18:938–953. doi: 10.1016/j.omtn.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu Q., Yuan Y., Wu Y., Huang Y., Zhao Z., Xiao C. MicroRNA‑137 exerts protective effects on hypoxia‑induced cell injury by inhibiting autophagy/mitophagy and maintaining mitochondrial function in breast cancer stem‑like cells. Oncology Reports. 2020;2020:p. 1. doi: 10.3892/or.2020.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peruzzo R., Costa R., Bachmann M., Leanza L., Szabo I. Mitochondrial metabolism, contact sites and cellular calcium signaling: implications for tumorigenesis. Cancers. 2020;12(9):p. 2574. doi: 10.3390/cancers12092574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng X., Lu S., He Z., et al. MCU-dependent negative sorting of miR-4488 to extracellular vesicles enhances angiogenesis and promotes breast cancer metastatic colonization. Oncogene. 2020;39(46):6975–6989. doi: 10.1038/s41388-020-01514-6. [DOI] [PubMed] [Google Scholar]
- 75.Fan X., Zhou S., Zheng M., Deng X., Yi Y., Huang T. MiR-199a-3p enhances breast cancer cell sensitivity to cisplatin by downregulating TFAM (TFAM) Biomedicine & Pharmacotherapy. 2017;88:507–514. doi: 10.1016/j.biopha.2017.01.058. [DOI] [PubMed] [Google Scholar]
- 76.Mandal S., Gamit N., Varier L., Dharmarajan A., Warrier S. Inhibition of breast cancer stem-like cells by a triterpenoid, ursolic acid, via activation of Wnt antagonist, sFRP4 and suppression of miRNA-499a-5p. Life Sciences. 2021;265:p. 118854. doi: 10.1016/j.lfs.2020.118854. [DOI] [PubMed] [Google Scholar]
- 77.Kim D., Lee J., Kang J., et al. Notch1 in tumor microvascular endothelial cells and tumoral miR-34a as prognostic markers in locally advanced triple-negative breast cancer. Journal of Breast Cancer. 2019;22(4):562–578. doi: 10.4048/jbc.2019.22.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu G., Zhao X., Wang J., et al. miR-125b regulates the drug-resistance of breast cancer cells to doxorubicin by targeting HAX-1. Oncology Letters. 2017;15:1621–1629. doi: 10.3892/ol.2017.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qattan A., Intabli H., Alkhayal W., Eltabache C., Tweigieri T., Amer S. B. Robust expression of tumor suppressor miRNA's let-7 and miR-195 detected in plasma of Saudi female breast cancer patients. BMC Cancer. 2017;17(1):p. 799. doi: 10.1186/s12885-017-3776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye Z., Hao R., Cai Y., Wang X., Huang G. Knockdown of miR-221 promotes the cisplatin-inducing apoptosis by targeting the BIM-Bax/Bak axis in breast cancer. Tumour Biology. 2016;37(4):4509–4515. doi: 10.1007/s13277-015-4267-4. [DOI] [PubMed] [Google Scholar]
- 81.Zhou S., Huang Q., Zheng S., Lin K., You J., Zhang X. miR-27a regulates the sensitivity of breast cancer cells to cisplatin treatment via BAK-SMAC/DIABLO-XIAP axis. Tumour Biology. 2016;37(5):6837–6845. doi: 10.1007/s13277-015-4500-1. [DOI] [PubMed] [Google Scholar]
- 82.Pradhan A. K., Bhoopathi P., Talukdar S., et al. MDA-7/IL-24 regulates the miRNA processing enzyme DICER through downregulation of MITF. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(12):5687–5692. doi: 10.1073/pnas.1819869116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahir M., Bhattacharya S., Karmakar S., et al. Tailored-CuO-nanowire decorated with folic acid mediated coupling of the mitochondrial-ROS generation and miR425-PTEN axis in furnishing potent anti-cancer activity in human triple negative breast carcinoma cells. Biomaterials. 2016;76:115–132. doi: 10.1016/j.biomaterials.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 84.De Blasio A., Di Fiore R., Pratelli G., et al. A loop involving NRF2, miR-29b-1-5p and AKT, regulates cell fate of MDA-MB-231 triple-negative breast cancer cells. Journal of Cellular Physiology. 2019;235(2):629–637. doi: 10.1002/jcp.29062. [DOI] [PubMed] [Google Scholar]
- 85.Shi D., Guo L., Sun X., et al. UTMD inhibit EMT of breast cancer through the ROS/miR-200c/ZEB1 axis. Scientific Reports. 2020;10(1):p. 6657. doi: 10.1038/s41598-020-63653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Zandwijk N., Pavlakis N., Kao S. C., et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. The Lancet Oncology. 2017;18(10):1386–1396. doi: 10.1016/S1470-2045(17)30621-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


