Abstract
The coronavirus disease 2019 (COVID-19) pandemic has caused more than 100 million infections and 2 million deaths worldwide. In up to 20% of cases, COVID-19 infection can take a severe, life-threatening course. Therefore, preventive measures such as mask-wearing, hand hygiene, and social distancing are important. COVID-19 vaccines that use novel vaccine technology can prevent up to 95% of infections. However, the uncertainty regarding the efficacy and safety of vaccination in patients with autoimmune inflammatory rheumatic disease (AIIRD), who are immunocompromised due to underlying immune dysfunction and concomitant immunosuppressive treatment, warrants clear guidance. A task force of the Korean College of Rheumatology formulated a set of vaccination guidance based on the currently available data and expert consensus. The currently available COVID-19 vaccines are considered to be safe and effective. Every patient with AIIRD should receive one of the available COVID-19 vaccines unless contraindicated for medical reasons such as prior allergy/anaphylaxis to the COVID-19 vaccine or its components. Patients should continue immunosuppressive treatment for their underlying AIIRD, including biological and targeted synthetic disease-modifying anti-rheumatic drugs (b/tsDMARDs). Corticosteroids should be reduced to the lowest dose possible without aggravating the AIIRD. To improve the vaccine response, methotrexate can be withheld for 1–2 weeks after each vaccination, and the timing of rituximab and abatacept infusion should be adjusted if clinically acceptable. Rheumatologists should play a leading role in educating and vaccinating patients with AIIRD.
Keywords: COVID-19, Autoimmune Inflammatory Rheumatic Diseases, Vaccines, Immunosuppression
Graphical Abstract
INTRODUCTION
As of March 6, 2021, the coronavirus disease 2019 (COVID-19) pandemic has resulted in 116,061,296 infections and 2,580,050 deaths (https://coronavirus.jhu.edu/map.html). While most cases were mild to moderate, resembling a common cold or flu-like illness, as many as 20% of cases were life-threatening or fatal.1 The risk factors of severe complications or death include an older age and comorbidities such as diabetes or preexisting respiratory or cardiovascular disease.2 A proper antiviral immune response in the early phase of COVID-19 infection determines its clinical course and outcome.3
Autoimmune inflammatory rheumatic disease (AIIRD) is a heterogeneous group of autoimmune diseases primarily affecting the musculoskeletal organs and connective tissue. Each disease is characterized by a distinct type of immune dysfunction, with a unique inflammatory response and cytokine profile.4 In rheumatoid arthritis (RA), tumor necrosis factor (TNF)-α and interleukin (IL)-6 play a central role, whereas type 1 interferon (IFN) drives the systemic lupus erythematosus (SLE) pathogenesis.5 As IFN plays a crucial role in antiviral defense, including against COVID-19 infection, SLE patients might respond differently to COVID-19 infection than RA patients.6 In addition, underlying immune dysfunction and treatment-induced immunosuppression might increase the risk of COVID-19 complications in AIIRD patients.7,8
How AIIRD affects the infection rate and severity of COVID-19 has not been fully elucidated. The prevalence of COVID-19 among AIIRD patients was reported to be increased compared with the general population.9,10 The overall risk of hospitalization and death was not increased or was only slightly increased in AIIRD patients.10,11,12,13 However, an increased risk of COVID-19 complications was associated with concomitant use of corticosteroids (prednisolone equivalent of > 10 mg/day) and higher disease activity among AIIRD patients.14,15 Strikingly, biologic or targeted synthetic disease-modifying anti-rheumatic drugs (b/tsDMARDs) were associated with a reduced risk of severe COVID-19 infection, while conventional DMARDs did not influence infection severity.10 Therefore, it is important to continue optimal management of AIIRD and to take preventive measures, such as vaccination, to reduce the risk and the severity of COVID-19 infection.16
Several phase 3 clinical trials of COVID-19 vaccines demonstrated an efficacy of > 95% in preventing COVID-19 infection without an unusual safety signal (Table 1).17,18,19,20,21,22,23 Since AIIRD patients were not routinely included in the vaccination trials, it remains unclear how AIIRD and immunosuppressive therapy will affect the immune response to the COVID-19 vaccine in AIIRD patients. As such, uncertainty about the efficacy and safety of the COVID-19 vaccines, especially the novel mRNA vaccines and viral vector vaccines, in AIIRD patients could lead to hesitancy in both physicians and patients to provide broad COVID-19 vaccination coverage.24,25
Table 1. COVID-19 vaccines.
| Vaccines | Producer | Category | Dosing schedule | Efficacy % (95% CI) | Anaphylaxis | Storage | Ref. |
|---|---|---|---|---|---|---|---|
| BNT162b2 | Pfizer-bioNtech | mRNA | 2 times | 95.0 (90.3–97.6) | 1:100,000 | −70°C | 17 |
| 0, 21 days | |||||||
| mRNA 1273 | Moderna | mRNA | 2 times | 94.1 (89.3–96.8) | 0.25:100,000 | −20°C | 18 |
| 0, 28 days | |||||||
| ChAdOx1/AZD1222 | Astra-Zeneca | Vector (chimpanzee adenovirus vector) | 2 times | 62.1 (41.0–75.7) for SD/SD | - | 2–8°C | 19 |
| 0, 8–12 weeks | 90.0 (67.4–97.0) for LD/SD | ||||||
| Gam-COVID-Vac (Sputnik V™) | Gamaleya Research Institute | Vector (adenovirus type 26 and type 5) | 2 times | 91.6 (85.6–95.2) | - | 2–8°C | 20 |
| 0, 21 days | |||||||
| Ad.26.COV2.S | Johnson & Johnson | Vector (adenovirus type 26 vector) | 1 time | 66.9 (59.0–73.4) | - | 2–8°C | 21 |
| NVX-CoV2373 | Novavax | Protein subunit (recombinant SARS-CoV-2 glycoprotein) | 2 times | 89.3 (75.2–95.4) | - | 2–8°C | 22 |
| 0, 21 days | 60.1 (19.9–80.1) for S Africa variant | ||||||
| CoronaVac | Sinovac | Inactivated COVID-19 virus | 2 times | 50.6 for all case, 83.7 for cases requiring medical treatment | - | 2–8°C | 23 |
| 0, 14 days |
COPVID-19 = coronavirus disease 2019, CI = confidence interval, LD = low dose, SD = standard dose.
This article aims to summarize the current knowledge of COVID-19 vaccines and the potential effects of AIIRDs and immunosuppressive treatment on the vaccine response, followed by clinical guidance on COVID-19 vaccination in patients with AIIRDs.
COVID-19 VACCINES
The major antigenic target of COVID-19 vaccines is the surface spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which binds to the angiotensin-converting enzyme 2 receptor on host cells and initiates viral entry into host cells.26
There are several categories of COVID-19 vaccines: mRNA vaccines, adenovirus vector vaccines, and protein subunit or inactivated virus vaccines.27 The mRNA vaccines contain mRNA encoding the spike protein of SARS-CoV-2 encapsulated in lipid nanoparticles. Vector vaccines use a replication-defective adenovirus that contains DNA encoding the SARS-CoV-2 viral spike protein. The viral DNA in the replication-defective adenovirus enters host nuclei without modifying the host genome. Both mRNA vaccines and modified adenovirus vectors instruct the host cells to produce the viral spike protein. Since mRNA vaccines and vector vaccines do not contain or induce infectious viral particles, they are considered non-live vaccines. Protein subunit vaccines contain purified viral spike proteins that were produced in vitro, while the inactivated virus vaccine contains killed SARS-CoV-2.27 All vaccines directly or indirectly deliver the spike protein of SARS-CoV-2 to the host immune system to induce a humoral and cellular immune response.
The mRNA vaccines, BNT162b2 (Pfizer, New York, NY, USA) and mRNA 1273 (Moderna, Cambridge, MA, USA), contain the mRNA in a lipid nanoparticle that contains polyethylene glycol (PEG) in the lipid film as a carrier. Both vaccines showed an efficacy of close to 95% and provided protection beginning approximately 10 days after the first dose. Nanoparticles are temperature-sensitive.17,18 The BNT162b2 and mRNA 1273 vaccines are required to be stored at −70°C and −20°C, respectively. Of note, allergic responses to PEG can result in rare anaphylactic reactions.28
The ChAdOx1/AZD1222 (AstraZeneca, Cambridge, UK), Ad.26.COV2.S (Johnson & Johnson, New Brunswick, NJ, USA), and Sputnik V (Gamaleya, Moscow, Russia) vaccines use adenovirus vectors, which are stable at low temperatures.19,20 Preexisting host immunity to adenovirus vectors could potentially interfere with and dampen the vaccine response.29
The efficacy of the AstraZeneca vaccine ranged from 62.1% to 90.0%, depending on the dose and dosing interval.19 Half a dose in the first injection followed by a second standard dose increased the vaccine efficacy from 62.1% to 90.0%. In a newer report, delaying the second dose to 12 weeks after the first injection increased the vaccine efficacy from 55.1% to 81.3%.19 This unusual relationship between vaccine efficacy and dosing interval merits further investigation. Sputnik V also showed an efficacy of 91.6%, without any major safety issues.20
NVX-CoV2373 (Novavax, Gaithersburg, MD, USA) and CoronaVac (Sinovac, Beijing, China) contain recombinant SARS-CoV-2 glycoprotein and inactivated COVID-19 virus, respectively.30,31 Efficacy and safety data on CoronaVac in well-designed clinical trials are pending.
Host response to COVID-19 vaccines
Although vaccines trigger both cellular and humoral immunity, the post-vaccination antibody titer is often used as a surrogate marker for the successful generation of immunity.32 For example, a serum antibody ratio against influenza strain antigens of ≥ 1/40 is considered a protective response after seasonal influenza vaccination.33 Currently, the protective antibody titer for COVID-19 vaccination has not been defined. Recent data suggest that cellular immunity might be more important than humoral immunity in clearing SARS-COV-2 virus.3 A significant proportion (1–10%) of PCR-positive COVID-19 patients were seronegative (i.e., they tested negative for anti-COVID-19 antibodies using commercial test kits), suggesting that an early and robust T cell response against COVID-19 might clear the virus before a B cell response is mounted.34
Humoral and cellular immune memory declines with distinct kinetics after COVID-19 vaccination, and the circulating antibody titers do not necessarily represent T cell immune memory.35 Therefore, measuring both antiviral cellular and humoral immunity might be crucial to accurately assess protective immunity after COVID-19 vaccination.
IMMUNOMODULATORY THERAPY AND VACCINE RESPONSE DURING THE COVID-19 PANDEMIC
Immunomodulatory medications can impact: 1) AIIRD disease activity, 2) infection risk, and 3) the vaccine response of AIIRD patients. DMARDs suppress the host immune response and AIIRD disease activity, but not necessarily the vaccine response. For example, tocilizumab elicits a strong anti-inflammatory response without suppressing protective humoral responses after vaccination in patients with RA.36,37 By contrast, abatacept (ABA) and rituximab (RTX) decrease the humoral response to vaccines. A prednisolone equivalent of >10 mg/day was associated with a greater risk of COVID-19 infection,15 whereas b/tsDMARDs did not increase the COVID-19 infection risk.10,38 However, the effects of individual DMARDs on both the host vaccine response and the AIIRD disease activity need to be evaluated in future studies. As of now, the net benefit of DMARD continuation during the COVID-19 pandemic outweighs the associated risks.
Corticosteroids
The response to influenza vaccination was not suppressed by up to 20 mg/day of prednisone.39 A higher dose (prednisolone equivalent of > 20 mg/day) was associated with a blunted vaccine response.40 According to the Centers for Disease Control and Prevention (CDC) recommendations, a prednisolone equivalent of ≥ 20 mg/day for ≥ 2 weeks contraindicates the use of live-attenuated vaccines because of the risk of viral reactivation.41,42 As COVID-19 vaccines are non-live vaccines with no potential for viral reactivation in vivo, corticosteroids can be continued safely to control the underlying AIIRD. However, corticosteroid use should be kept as low as possible to decrease the risk of COVID-19 complications, since corticosteroids at a prednisone-equivalent dose of > 10 mg/day can increase the severity of COVID-19 infection.10,14,38
Conventional synthetic DMARDs
Methotrexate (MTX) decreases humoral responses to seasonal influenza vaccines and pneumococcal vaccines. In previous studies, temporary discontinuation of MTX for 2 weeks after vaccination increased the humoral vaccine response to seasonal influenza by > 20% without increasing the risk of flares.43,44,45
Data on the effects of hydroxychloroquine, sulfasalazine, and leflunomide on vaccine responses are scarce. Of note, hydroxychloroquine was found not to be beneficial in either the treatment or prevention of COVID-19 infection in SLE patients, and it should not be prescribed to prevent or treat COVID-19 infection.46
b/tsDMARDs
There was no increased risk of developing severe COVID-19 among patients on biological or synthetic DMARDs including Janus kinase (JAK) inhibitors compared with the general population.38,47 Blockade of TNF-α or IL-6 did not influence humoral immunity to influenza or pneumococcal vaccines.36,48 However, their effects on cellular immunity have been less studied. Since overproduction of TNF-α and IL-6 can drive a detrimental hyper-inflammatory response (cytokine storm) in severe COVID-19 cases, those medications should be continued.49
Activation of CD28 is critical for initial priming of naïve T cells by antigen presenting cells.50 Since ABA can significantly decrease the vaccine response,51,52 it should be administered at least 1 week after the initial dose (but not after the second dose) of COVID-19 vaccine.
RTX depletes B cells, and therefore it can substantially decrease humoral vaccine responses after vaccination.48,53 However, B cell depletion does not necessarily increase the risk of serious COVID-19 complications, since innate and cellular immunity remain intact to eliminate SARS-CoV-2.54 It is recommended that RTX be administered at least 1 month after vaccination.
The JAK inhibitor tofacitinib reduced the response to the PPSV-23 pneumococcal vaccine but not to the influenza vaccine.55 Baricitinib prevented the progression to severe COVID-19 pneumonia and increased the production of antibodies against the SARS-CoV-2 spike protein.56 Of note, AIIRD patients on IL-1, IL-6, or JAK inhibitors showed a low incidence of COVID-19, suggesting that b/tsDMARDs may inhibit the development of symptomatic/aggressive COVID-19 infection.9,14
Taken together, most DMARDs do not seem to be associated with more severe COVID-19 infection.57 Therefore, they should be continued in patients during the SARS-CoV-2 pandemic and during vaccination. However, in order to improve the vaccine response, the ABA, RTX, and MTX dosing schedule can be adjusted as clinically feasible.
TOWN HALL MEETING
Based on qualitative analysis of a town hall meeting involving seven rheumatologists of the Vaccine Task Force of the Korean College of Rheumatology (KCR) and six patient representatives (RA, ankylosing spondylitis, SLE, Sjogren' disease, and Crohn's disease), a list of key questions was identified (Table 2). Most questions addressed the safety and efficacy of COVID-19 vaccines in patients with AIIRD and the interaction between vaccination and b/tsDMARD use.
Table 2. Questions and concerns of patients with AIIRD about COVID-19 vaccination.
| • Is COVID-19 vaccination required for AIIRD patients? |
| • Are patients with AIIRD at a higher risk of COVID-19? |
| • Is the COVID-19 vaccine safe for patients with AIIRD? |
| • Can the COVID-19 vaccine aggravate AIIRD? |
| • What should I do if I develop a side effect after vaccination? |
| • Is vaccination safe while taking biological DMARDs? Should I withhold certain medications? |
| • Which vaccine should I choose among those available? |
| • Can a COVID-19 vaccine be administered together with other vaccines such as influenza or pneumococcal vaccines? |
| • Should I reschedule any planned medical procedure or surgery because of the COVID-19 vaccination? |
| • Should I consult my primary rheumatologist before the COVID-19 vaccination? |
COVID-19 = coronavirus disease 2019, AIIRD = autoimmune inflammatory rheumatic disease, DMARD = disease-modifying anti-rheumatic drug.
RECOMMENDATIONS
The overall benefits of vaccination outweigh any potential risks from the vaccine. Although complete vaccine protection is desired, even partial protection will prevent or reduce the severity of infection. Achieving herd immunity (i.e., ≥ 70% immunity in a population) is critical for containing COVID-19 spread and protecting patients at a high risk of COVID-19 complications who are not eligible for vaccination, such as patients who are allergic to components of the COVID-19 vaccine.58 Therefore, all patients and healthcare providers should receive the vaccination, according to the national guideline.
To facilitate a broad and timely COVID-19 vaccination response, a Vaccine Task Force, commissioned by the KCR, formulated clinical guidance for COVID-19 vaccination in patients with AIIRD based on the available data from previous vaccination trials in AIIRD patients, complemented by expert opinions. The recommendations were voted on using the Google Forms survey platform. The level of agreement was rated by 22 members of the Vaccination Task Force and the Committee of Health Policy of the KCR as follows: 1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, and 5 = strongly agree. All recommendations had a high level of agreement (Table 3). This guidance will be subject to frequent modifications as new evidence/data becomes available.
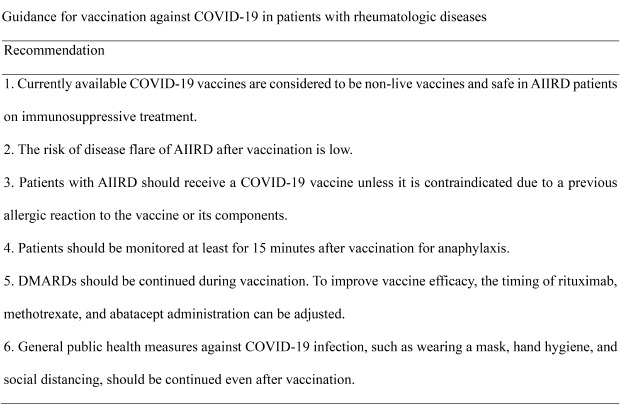
Table 3. Guidance for vaccination against COVID-19 in patients with rheumatologic diseases.
| Recommendation | Level of agreement |
|---|---|
| 1. Currently available COVID-19 vaccines are considered to be non-live vaccines and safe in AIIRD patients on immunosuppressive treatment. | 4.4 |
| 2. The risk of disease flare of AIIRD after vaccination is low. | 4.1 |
| 3. Patients with AIIRD should receive a COVID-19 vaccine unless it is contraindicated due to a previous allergic reaction to the vaccine or its components. | 4.7 |
| 4. Patients should be monitored at least for 15 minutes after vaccination for anaphylaxis. | 4.7 |
| 5. DMARDs should be continued during vaccination. To improve vaccine efficacy, the timing of rituximab, methotrexate, and abatacept administration can be adjusted. | 4.1 |
| 6. General public health measures against COVID-19 infection, such as wearing a mask, hand hygiene, and social distancing, should be continued even after vaccination. | 4.8 |
Currently available vaccines include Pfizer, Moderna, AstroZeneca, Novavax, and Sputnic V. Level of agreement: 1 = strongly disagree; 2 = disagree; 3 = neutral; 4 = agree; 5 = strongly agree.
COVID-19 = coronavirus disease 2019, AIIRD = autoimmune inflammatory rheumatic disease, DMARD = disease-modifying anti-rheumatic drug.
Clinical practice guidelines for vaccination of patients with AIIRD developed jointly by the Korean College of Rheumatology and the Korean Society of Infectious Diseases and the 2019 update of the European League Against Rheumatism (EULAR) recommendations for vaccination in adult patients with AIIRD should be followed for COVID-19 vaccination.41,59,60 The decision on COVID-19 vaccination should be shared between physicians (rheumatologists and primary care physicians) and patients. Ideally, the vaccination should be administered when the patient's AIIRD is in a quiescent state and before the commencement of immunosuppressive therapy, if clinically feasible. Patients should receive the available COVID-19 vaccine according to the government's policy and guidelines at their earliest convenience. Vaccination should not be delayed due to ongoing immunosuppressive therapy. Patients with AIIRD and their family members should receive a COVID-19 vaccine.
1. Currently available COVID-19 vaccines are considered to be non-live vaccines and generally safe in AIIRD patients on immunosuppressive treatment.
The mRNA, virus vector, protein subunit, and inactivated virus vaccines will not cause any SARS-CoV-2 infection since viral replication is not possible. Therefore, none of the currently available COVID-19 vaccines will cause COVID-19 infection in AIIRD patients on immunosuppressive treatment.
Common side effects of COVID-19 vaccine include injection site pain, redness and swelling, and a low-grade fever within 1–3 days after injection, and they generally resolve within 1–3 days of onset. These side effects might be stronger after the boost (second) injection and occur more often in younger persons compared with older persons (> 55 years of age).18,32
2. The risk of disease flare of AIIRD after vaccination is low.
Common vaccines do not aggravate the disease activity of AIIRD patients, although the effect of the COVID-19 vaccine on the AIIRD remains unclear.
3. Patients with AIIRD should receive a COVID-19 vaccine unless it is contraindicated due to a previous allergic reaction to the vaccine or its components.
An immediate, severe allergic reaction to a previous COVID-19 vaccine or its components are contraindications to COVID-19 vaccination.
Anaphylaxis can develop after mRNA vaccination in rare cases (incidence of 1:100,000 with BNT162b2) where an anti-PEG immune response is mounted.28 The COVID-19 mRNA vaccines are encapsulated in nanoparticles containing PEG. PEG is present in PEGylated medications and bowel preparation solution, and is used as a carrier in injectable drugs such as injectable methylprednisolone acetate. Patents with a prior history of allergy to PEG may not receive mRNA-based vaccines.61 Patients with previous PEG allergies, including allergies to mRNA vaccines, may still receive non-PEG vaccines.
Patients with an allergy to polysorbate 80 may not receive polysorbate 80-containing vaccines or mRNA vaccines because polysorbate is potentially cross-reactive to PEG and may cause anaphylactic reactions in those patients.62 Polysorbate 80 is a nonionic surfactant and emulsifier that is present in many vaccines such as hepatitis B, human papillomavirus, pneumococcal conjugate, and influenza vaccines, as well as in many foods. Due to its lower molecular weight, polysorbate is less likely to a trigger an allergic reaction.
4. Patients should be monitored at least for 15 minutes after vaccination for anaphylaxis.
All patients should be monitored for at least 15 minutes after vaccination. Patients with a history of immediate allergic response to a vaccine, injectable medications without PEG or polysorbate, food, drugs, venom, or latex; patients with a history of idiopathic anaphylaxis; and patients who develop itching and swelling confined to the injection site during their post-vaccination observation period should be monitored for at least 30 minutes to assess for the development of anaphylaxis. Full resuscitation with epinephrine should be available at all times.62
5. DMARDs should be continued during vaccination. To improve vaccine efficacy, the timing of RTX, MTX, and ABA administration can be adjusted.
In theory, the underlying immune dysfunction and treatment-associated immunosuppression can blunt the vaccine response in AIIRD patients taking immunomodulatory medications. Preferably, the vaccination should be administered before beginning immunosuppressive therapy. DMARDs should be continued during vaccination since withholding DMARDs can increase disease activity, which is associated with worse COVID-19 infection severity and outcomes. Of note, use of b/tsDMARDs may be associated with less severe COVID-19 infection and a decreased mortality rate.14
MTX, ABA, and RTX can negatively influence the vaccine response. To increase the vaccine response, the timing of those medications can be adjusted if clinically feasible. Temporary discontinuation of methotrexate for 1–2 weeks after each vaccine dose can be considered. The next cycle of RTX should be started 4 weeks after the second vaccine dose. Abatacept should be given 1 week after the first vaccine dose and should be continued after the second vaccine dose. There are not sufficient data on the effect of cyclophosphamide or JAK inhibitors on the vaccine response to make a concrete recommendation. However, intravenous cyclophosphamide should be given 1 week after each vaccine dose. JAK inhibitors may be withheld for 1 week after each vaccine dose.
6. General public health measures against COVID-19 infection, such as wearing a mask, hand hygiene, and social distancing, should be continued even after vaccination.
To date, there is no evidence that vaccines can prevent COVID-19 transmission completely. Therefore, patients should continue all public health measures, including wearing a mask, hand hygiene, and social distancing, to decrease both asymptomatic and symptomatic COVID-19 transmission.
CONCLUDING REMARKS
Several effective COVID-19 vaccines with novel mechanisms of action are currently available. COVID-19 vaccines considered to be safe, with a safety profile consistent with other common non-live vaccines. All patients with AIIRD should receive the vaccine unless contraindicated due to a previous allergy to vaccines or their components. In general, immunomodulatory therapy should be safely continued during vaccination. If clinically feasible, MTX, RTX, and ABA should be withheld temporarily during vaccination.
The current vaccination guidance by the vaccination task force of the KCR is subject to frequent updates and modifications as new data on vaccination safety and efficacy in patients with AIIRD become available. As of now, physicians who care for AIIRD patients should play an active role in educating and vaccinating AIIRD patients to protect both the AIIRD patients and the public. Finally, this guidance has been developed to help physicians manage COVID-19 vaccination for AIIRD patients according to their best clinical judgment.
ACKNOWLEDGMENTS
The authors thank the patient representatives for their participation in the town hall meeting.
Footnotes
Funding: This work was supported by the Korean College of Rheumatology (2021).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Park JK, Lee EB, Shin K, Sung YK, Kim TH, Back HJ.
- Data curation: Park JK, Lee EB, Sung YK, Choi BY, Back HJ.
- Formal analysis: Lee EB, Kim TH, Kwon SR, Lee MS, Hong SJ, Lee SS, Back HJ.
- Supervision: Lee EB, Shin K, Sung YK, Kim TH, Kwon SR, Lee MS, Hong SJ, Choi BY, Lee SS, Back HJ.
- Writing - original draft: Park JK, Lee EB, Shin K, Sung YK, Kim TH, Kwon SR, Lee MS, Hong SJ, Choi BY, Lee SS, Back HJ.
- Writing - review & editing: Park JK, Lee EB, Shin K, Sung YK, Kim TH, Kwon SR, Lee MS, Hong SJ, Choi BY, Lee SS, Back HJ.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MJ, Lee EB, Song YW, Park JK. Profile of common inflammatory markers in treatment-naïve patients with systemic rheumatic diseases. Clin Rheumatol. 2020;39(10):2899–2906. doi: 10.1007/s10067-020-05049-9. [DOI] [PubMed] [Google Scholar]
- 5.Schett G, Elewaut D, McInnes IB, Dayer JM, Neurath MF. How cytokine networks fuel inflammation: Toward a cytokine-based disease taxonomy. Nat Med. 2013;19(7):822–824. doi: 10.1038/nm.3260. [DOI] [PubMed] [Google Scholar]
- 6.McNab F, Mayer-Barber K, Sher A, Wack A, O'Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15(2):87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52(1):53–61. doi: 10.1093/rheumatology/kes305. [DOI] [PubMed] [Google Scholar]
- 8.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 9.Hyrich KL, Machado PM. Rheumatic disease and COVID-19: epidemiology and outcomes. Nat Rev Rheumatol. 2021;17(2):71–72. doi: 10.1038/s41584-020-00562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218946. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 11.Emmi G, Bettiol A, Mattioli I, Silvestri E, Di Scala G, Urban ML, et al. SARS-CoV-2 infection among patients with systemic autoimmune diseases. Autoimmun Rev. 2020;19(7):102575. doi: 10.1016/j.autrev.2020.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarzi-Puttini P, Marotto D, Caporali R, Montecucco CM, Favalli EG, Franceschini F, et al. Prevalence of COVID infections in a population of rheumatic patients from Lombardy and Marche treated with biological drugs or small molecules: a multicentre retrospective study. J Autoimmun. 2021;116:102545. doi: 10.1016/j.jaut.2020.102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordtz R, Lindhardsen J, Soussi BG, Vela J, Uhrenholt L, Westermann R, et al. Incidence and severeness of COVID-19 hospitalisation in patients with inflammatory rheumatic disease: a nationwide cohort study from Denmark. Rheumatology (Oxford) 2020:keaa897. doi: 10.1093/rheumatology/keaa897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasseli R, Mueller-Ladner U, Hoyer BF, Krause A, Lorenz HM, Pfeil A, et al. Older age, comorbidity, glucocorticoid use and disease activity are risk factors for COVID-19 hospitalisation in patients with inflammatory rheumatic and musculoskeletal diseases. RMD Open. 2021;7(1):e001464. doi: 10.1136/rmdopen-2020-001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zucchi D, Tani C, Elefante E, Stagnaro C, Carli L, Signorini V, et al. Impact of first wave of SARS-CoV-2 infection in patients with Systemic Lupus Erythematosus: Weighting the risk of infection and flare. PLoS One. 2021;16(1):e0245274. doi: 10.1371/journal.pone.0245274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaccines and related biological products advisory committee meeting. [Updated 2021]. [Accessed February 13, 2021]. https://www.fda.gov/media/146219/download.
- 22.Novavax creating tomorrow's vaccine today: announcement of UK and South Africa trial results. [Updated 2021]. [Accessed February 13, 2021]. https://www.novavax.com/sites/default/files/2021-01/UK-SouthAfrica-Trial-Results--FINAL.pdf.
- 23.Sinovac announces phase III results of its COVID-19 vaccine. [Updated 2021]. [Accessed February 13, 2021]. http://www.sinovac.com/?optionid=754&auto_id=922.
- 24.Rosenbaum L. Escaping catch-22 - overcoming Covid vaccine hesitancy. N Engl J Med. 2021 doi: 10.1056/NEJMms2101220. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 25.Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021 doi: 10.1016/S2468-2667(21)00012-8. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brisse M, Vrba SM, Kirk N, Liang Y, Ly H. Emerging concepts and technologies in vaccine development. Front Immunol. 2020;11:583077. doi: 10.3389/fimmu.2020.583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner PJ, Ansotegui IJ, Campbell DE, Cardona V, Ebisawa M, El-Gamal Y, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021;14(2):100517. doi: 10.1016/j.waojou.2021.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thacker EE, Timares L, Matthews QL. Strategies to overcome host immunity to adenovirus vectors in vaccine development. Expert Rev Vaccines. 2009;8(6):761–777. doi: 10.1586/erv.09.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh EE, Frenck RW, Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis. 2013;207(6):974–981. doi: 10.1093/infdis/jis935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarzkopf S, Krawczyk A, Knop D, Klump H, Heinold A, Heinemann FM, et al. Cellular immunity in COVID-19 convalescents with PCR-confirmed infection but with undetectable SARS-CoV-2-specific IgG. Emerg Infect Dis. 2021;27(1):122–129. doi: 10.3201/2701.203772. [DOI] [PubMed] [Google Scholar]
- 35.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapetanovic MC, Kristensen LE, Saxne T, Aktas T, Mörner A, Geborek P. Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis. Arthritis Res Ther. 2014;16(1):R2. doi: 10.1186/ar4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bingham CO, 3rd, Rizzo W, Kivitz A, Hassanali A, Upmanyu R, Klearman M. Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: results of a randomised controlled trial (VISARA) Ann Rheum Dis. 2015;74(5):818–822. doi: 10.1136/annrheumdis-2013-204427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jovani V, Calabuig I, Peral-Garrido ML, Tovar-Sugrañes E, López-González MD, Bernabeu P, et al. Incidence of severe COVID-19 in a Spanish cohort of 1037 patients with rheumatic diseases treated with biologics and JAK-inhibitors. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218152. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 39.Inoue S, Shibata Y, Takabatake N, Igarashi A, Abe S, Kubota I. Influence of corticosteroid therapy on the serum antibody response to influenza vaccine in elderly patients with chronic pulmonary diseases. EXCLI J. 2013;12:760–765. [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer L, Gerstel PF, Poncet A, Siegrist CA, Laffitte E, Gabay C, et al. Pneumococcal polysaccharide vaccination in adults undergoing immunosuppressive treatment for inflammatory diseases--a longitudinal study. Arthritis Res Ther. 2015;17(1):151. doi: 10.1186/s13075-015-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furer V, Rondaan C, Heijstek MW, Agmon-Levin N, van Assen S, Bijl M, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(1):39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 42.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 43.Park JK, Lee MA, Lee EY, Song YW, Choi Y, Winthrop KL, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76(9):1559–1565. doi: 10.1136/annrheumdis-2017-211128. [DOI] [PubMed] [Google Scholar]
- 44.Park JK, Lee YJ, Shin K, Ha YJ, Lee EY, Song YW, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77(6):898–904. doi: 10.1136/annrheumdis-2018-213222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JK, Choi Y, Winthrop KL, Song YW, Lee EB. Optimal time between the last methotrexate administration and seasonal influenza vaccination in rheumatoid arthritis: post hoc analysis of a randomised clinical trial. Ann Rheum Dis. 2019;78(9):1283–1284. doi: 10.1136/annrheumdis-2019-215187. [DOI] [PubMed] [Google Scholar]
- 46.Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freites Nuñez DD, Leon L, Mucientes A, Rodriguez-Rodriguez L, Font Urgelles J, Madrid García A, et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(11):1393–1399. doi: 10.1136/annrheumdis-2020-217984. [DOI] [PubMed] [Google Scholar]
- 48.Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66(7):1016–1026. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]
- 49.Winthrop KL, Mariette X. To immunosuppress: whom, when and how? That is the question with COVID-19. Ann Rheum Dis. 2020;79(9):1129–1131. doi: 10.1136/annrheumdis-2020-218694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webb LM, Walmsley MJ, Feldmann M. Prevention and amelioration of collagen-induced arthritis by blockade of the CD28 co-stimulatory pathway: requirement for both B7-1 and B7-2. Eur J Immunol. 1996;26(10):2320–2328. doi: 10.1002/eji.1830261008. [DOI] [PubMed] [Google Scholar]
- 51.Alten R, Bingham CO, 3rd, Cohen SB, Curtis JR, Kelly S, Wong D, et al. Antibody response to pneumococcal and influenza vaccination in patients with rheumatoid arthritis receiving abatacept. BMC Musculoskelet Disord. 2016;17(1):231. doi: 10.1186/s12891-016-1082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crnkic Kapetanovic M, Saxne T, Jönsson G, Truedsson L, Geborek P. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15(5):R171. doi: 10.1186/ar4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westra J, van Assen S, Wilting KR, Land J, Horst G, de Haan A, et al. Rituximab impairs immunoglobulin (Ig)M and IgG (subclass) responses after influenza vaccination in rheumatoid arthritis patients. Clin Exp Immunol. 2014;178(1):40–47. doi: 10.1111/cei.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker D, Roberts CA, Pryce G, Kang AS, Marta M, Reyes S, et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol. 2020;202(2):149–161. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winthrop KL, Silverfield J, Racewicz A, Neal J, Lee EB, Hrycaj P, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75(4):687–695. doi: 10.1136/annrheumdis-2014-207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bronte V, Ugel S, Tinazzi E, Vella A, De Sanctis F, Canè S, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130(12):6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santos CS, Férnandez XC, Moriano Morales C, Álvarez ED, Álvarez Castro C, López Robles A, et al. Biological agents for rheumatic diseases in the outbreak of COVID-19: friend or foe? RMD Open. 2021;7(1):e001439. doi: 10.1136/rmdopen-2020-001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity. 2020;52(5):737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seo YB, Moon SJ, Jeon CH, Song JY, Sung YK, Jeong SJ, et al. The practice guideline for vaccinating Korean patients with autoimmune inflammatory rheumatic disease. Infect Chemother. 2020;52(2):252–280. doi: 10.3947/ic.2020.52.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seo MR, Kim JW, Park EJ, Jung SM, Sung YK, Kim H, et al. Recommendations for the management of patients with systemic rheumatic diseases during the coronavirus disease pandemic. Korean J Intern Med. 2020;35(6):1317–1332. doi: 10.3904/kjim.2020.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384(7):643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banerji A, Wickner PG, Saff R, Stone CA, Jr, Robinson LB, Long AA, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.12.047. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]


