Abstract
Purpose
Metabolic syndrome (MetS) comprises a cluster of risk factors for future cardiovascular and metabolic diseases. Only a few recent studies have reported the trend in the prevalence of MetS in youth. This study aimed to analyze trends in the prevalence of MetS and nutrient intake in the last 10 years and investigate the changes in MetS components among Korean children and adolescents.
Materials and Methods
We analyzed the data of 9513 children and adolescents aged 10–19 years from the 2008–2017 Korean National Health and Nutrition Examination Surveys. Diagnosis of MetS was based on the International Diabetes Federation (IDF) and modified National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) criteria.
Results
Based on the IDF criteria, MetS prevalence increased from 1.53% in 2008 to 3.19% in 2017 (p=0.007). Based on the NCEP-ATP III criteria, MetS prevalence increased from 2.18% in 2008 to 3.19% in 2017; however, the increase was not statistically significant. Daily calorie and fat intakes increased significantly during the study period. Among the risk factors that MetS comprises, the prevalence rates of central obesity, low high-density lipoprotein cholesterol levels, and high fasting glucose levels increased significantly.
Conclusion
Over the last 10 years, the prevalence of MetS has grown significantly with increasing calorie and fat intake in Korean children and adolescents. Central obesity and high-density lipoprotein cholesterol and fasting glucose levels have worsened. Therefore, active support and close monitoring are required to control MetS and prevent further increase in the prevalence of cardiovascular diseases.
Keywords: Metabolic syndrome, prevalence, children, nutrient, obesity
INTRODUCTION
Metabolic syndrome (MetS) comprises a cluster of risk factors for cardiovascular disease and type 2 diabetes mellitus.1,2,3 It is one of the major causes of death and has a high disease burden.4,5 MetS in childhood and adolescence increases the risk of various metabolic diseases in adulthood.6 To date, there are no definitive diagnostic criteria for MetS in children and adolescents. The two most commonly used definitions for MetS in children and adolescents are based on the criteria of the International Diabetes Federation (IDF) and the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III). Both criteria involve representative markers for central obesity, insulin resistance, high blood pressure (BP), and dyslipidemia.7
MetS is strongly associated with obesity. In adults, the prevalence of obesity and various components of MetS increased simultaneously by approximately 30–90% in more than 100 countries between 1990 and 2015.8 Since the 1980s, the prevalence of obesity among children and adolescents has increased 1.5 times in the United States (U.S.), and the prevalence of MetS has increased rapidly along with obesity.9,10 On the other hand, some studies have reported that since the 2000s, there has not been a significant increase in the prevalence of obesity among children and adolescents,11,12 and that the prevalence of MetS among children and adolescents in the U.S. also decreased slightly during that period. Based on the NCEP-ATP III criteria, the prevalence of MetS among American youths decreased from 7.3% in 1988–1994 to 6.5% in 2003–2006.13
In Korean children and adolescents, MetS prevalence in Korean children and adolescents fluctuated significantly during the 2000s.14 The prevalence of MetS reached its zenith in the early 2000s. According to the IDF criteria, MetS prevalence increased from 2.2% in 1998 to 3.6% in 2001 and then decreased to 1.8% in 2005.15 According to the NCEP-ATP III criteria, MetS prevalence increased from 4.0% in 1998 to 6.6% in 2005 and then decreased to 6.3% in 2007–2009.15,16 However, few studies have evaluated MetS prevalence from 2010 onward.
To address this gap, this study aimed to analyze the trend of MetS prevalence based on both the IDF and NCEP-ATP III criteria in the last 10 years, as well as the daily calorie and nutrient intakes among Korean children and adolescents. We further investigated changes in the prevalence rates of each of the risk factors composing MetS to elucidate trends in these components.
MATERIALS AND METHODS
Study design and subjects
We analyzed data obtained between 2008 and 2017 by the Korean National Health and Nutrition Examination Survey (KNHANES) conducted by the Korean Centers for Disease Control and Prevention (KCDC), which investigated children and adolescents aged 10–19 years. Since 1998, this cross-sectional and nationally representative annual survey has been performed to identify health behaviors in the Korean population, the current status of chronic diseases, and data concerning food and nutrient consumption. Using a multi-staged stratified sampling method based on age, sex, and geographic location, health surveys, examinations, and nutrition surveys were conducted for household members aged 1 year or older.17 Statistical weights were allocated on study selection to make the sample representative of the entire population.18 Nutrition survey was conducted through face-to-face interviews. Food intake questionnaire was designed as an open-ended survey for reporting the consumption of various dishes and foods using the 24-hour recall method with different measuring aids.
The KNHANES was approved by the KCDC, and written informed consent from statutory representatives was obtained for children enrolled in the present study. The study was approved by the Institutional Review Board of Yonsei University Gangnam Severance Hospital (IRB No. 3-2019-0217).
Measurements
The participants' weights were measured to the nearest 0.1 kg using a scale (GL-6000-20, G-tech, Seoul, Korea), and their heights and waist circumferences were measured to the nearest 0.1 cm using a stadiometer (Seca 225, Seca, Hamburg, Germany). Waist circumference was measured at the midpoint between the lower border of the rib cage and the iliac crest. Standard deviation scores of height, weight, and body mass index (BMI) were calculated using the lambda-mu-sigma method. BP was measured in the right arm using a standard mercury sphygmomanometer [Baumanometer Desk model 0320 in 2008–2012 and Baumanometer Wall Unit 33 (0850) in 2013–2017, W.A.Baum, New York, NY, USA] following the participants resting for 5 minutes in a sitting position. Two SBP and DBP readings were recorded at 5-minute intervals, and the mean BP was used for analysis. Blood samples were collected from participants who had fasted from 19.00 h on the day before the test. Laboratory measurements included serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, triglyceride (TG), high-density lipoprotein (HDL) cholesterol, and plasma fasting glucose levels.
Definition of MetS
Both the IDF and modified NCEP-ATP III criteria were used for the diagnosis of MetS in children and adolescents.7 The IDF criteria for diagnosing MetS include central obesity (waist circumference ≥90 cm in boys or ≥80 cm in girls, or ≥90th percentile for age and sex) and at least two of the following characteristics: 1) TG levels ≥150 mg/dL; 2) HDL cholesterol ≤40 mg/dL (or in adolescents aged over 16 years, ≤40 mg/dL in boys and ≤50 mg/dL in girls); 3) SBP ≥130 mm Hg or DBP ≥85 mm Hg; and 4) fasting glucose ≥100 mg/dL. The modified NCEP ATP-III criteria for diagnosing MetS include the presence of three or more of the following characteristics: 1) waist circumference ≥90th percentile for age and sex; 2) TG ≥110 mg/dL; 3) HDL cholesterol ≤40 mg/dL; 4) SBP or DBP ≥90th percentile for age, sex, and height; and 5) fasting glucose ≥110 mg/dL.19 The reference values for waist circumference and BP were based on growth charts published by the Korean Pediatric Society.20
Statistical analysis
SAS statistical software package version 9.3 (SAS Inc., Cary, NC, USA) was used for data analyses. We analyzed trends in the basic characteristics of study subjects and the prevalence rates of the components of MetS. One-way analysis of variance was used to compare the mean values of continuous variables. Rao-Scott chi-squared test was used to compare categorical variables, including the prevalence of MetS. Fisher's exact test was used to evaluate the trends of the components of MetS from 2008 to 2017. A p-value <0.05 was considered statistically significant.
RESULTS
Table 1 shows the demographic trends of subjects from 2008 to 2017. Body weight, waist circumference, and BMI increased from 2008, especially after 2013 (p<0.001). Height increased linearly (p=0.002). There was a significant increase in SBP (p<0.001) and fasting blood glucose levels after 2013 (p<0.001). Total cholesterol and HDL cholesterol levels increased significantly from 2008 to 2017 (p<0.001). Both AST and ALT levels gradually increased (p<0.025 and p<0.027, respectively).
Table 1. Trends of Basic Characteristics of Korean Children and Adolescents between 2008 and 2017.
| Variables | Total (n=9513) | 2008 (n=1239) | 2009 (n=1366) | 2010 (n=1076) | 2011 (n=935) | 2012 (n=889) | 2013 (n=962) | 2014 (n=732) | 2015 (n=772) | 2016 (n=789) | 2017 (n=753) | p value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (%) | 52.53 | 53.35 | 51.76 | 52.88 | 52.19 | 51.97 | 52.18 | 53.69 | 55.05 | 51.20 | 51.26 | |
| Age (yr) | 14.64 | 14.31 | 14.45 | 14.59 | 14.57 | 14.70 | 14.66 | 14.78 | 14.88 | 14.86 | 14.74 | |
| Height (cm) | 161.75 (0.14) | 160.65 (0.39) | 161.71 (0.40) | 161.52 (0.45) | 161.40 (0.50) | 162.09 (0.45) | 161.96 (0.37) | 161.55 (0.51) | 162.03 (0.49) | 162.45 (0.44) | 162.49 (0.48) | <0.001 |
| Weight (kg) | 52.88 (0.18) | 54.03 (0.49) | 54.72 (0.49) | 54.59 (0.54) | 54.87 (0.62) | 54.83 (0.56) | 55.34 (0.51) | 55.59 (0.70) | 57.06 (0.61) | 56.43 (0.65) | 55.98 (0.59) | 0.002 |
| BMI (kg/m2) | 20.85 (0.05) | 20.61 (0.12) | 20.64 (0.11) | 20.71 (0.14) | 20.72 (0.15) | 20.62 (0.13) | 20.82 (0.15) | 21.02 (0.18) | 21.49 (0.17) | 21.10 (0.18) | 20.96 (0.16) | <0.001 |
| WC (cm) | 70.25 (0.13) | 69.90 (0.36) | 69.55 (0.33) | 69.68 (0.41) | 69.88 (0.40) | 69.22 (0.35) | 69.61 (0.43) | 70.77 (0.47) | 72.64 (0.44) | 71.65 (0.48) | 70.16 (0.46) | <0.001 |
| WHtR | 0.34 (0.00) | 0.33 (0.00) | 0.34 (0.00) | 0.34 (0.00) | 0.34 (0.00) | 0.34 (0.00) | 0.34 (0.00) | 0.34 (0.00) | 0.35 (0.00) | 0.34 (0.00) | 0.34 (0.00) | <0.001 |
| SBP (mm Hg) | 108.83 (0.16) | 105.35 (0.43) | 109.04 (0.40) | 108.19 (0.58) | 107.96 (0.48) | 108.75 (0.45) | 108.90 (0.47) | 110.03 (0.53) | 110.24 (0.47) | 110.82 (0.48) | 109.90 (0.54) | <0.001 |
| DBP (mm Hg) | 66.87 (0.14) | 66.26 (0.45) | 69.10 (0.32) | 66.99 (0.47) | 66.77 (0.46) | 66.02 (0.57) | 66.18 (0.39) | 66.43 (0.46) | 66.91 (0.42) | 67.30 (0.46) | 66.68 (0.48) | 0.158 |
| Fasting glucose (mg/dL) | 89.98 (0.12) | 90.02 (0.28) | 88.54 (0.26) | 88.56 (0.29) | 88.58 (0.40) | 88.64 (0.35) | 90.17 (0.38) | 92.39 (0.71) | 91.36 (0.31) | 91.50 (0.33) | 91.05 (0.39) | <0.001 |
| Total cholesterol (md/dL) | 159.98 (0.39) | 157.75 (0.98) | 157.94 (1.14) | 157.93 (1.22) | 158.24 (1.44) | 159.20 (1.45) | 157.66 (0.98) | 159.40 (1.30) | 162.34 (1.16) | 165.33 (1.25) | 165.82 (1.29) | <0.001 |
| HDL cholesterol (mg/dL) | 51.22 (0.15) | 49.86 (0.35) | 49.53 (0.35) | 49.46 (0.37) | 51.45 (0.54) | 52.08 (0.57) | 52.16 (0.42) | 52.60 (0.48) | 52 (0.47) | 52.34 (0.49) | 51.45 (0.42) | <0.001 |
| LDL cholesterol (mg/dL) | 95.98 (0.80) | 89.76 (1.35) | 90.97 (1.48) | 94.67 (1.51) | 94.67 (1.84) | 106.62 (6.16) | 97.71 (4.75) | 102.87 (7.19) | 95.48 (1.11) | 108.50 (5.47) | 126.88 (8.42) | 0.088 |
| Triglycerides (mg/dL) | 85.98 (0.72) | 90.64 (2.53) | 87.19 (1.85) | 85.18 (2.09) | 91.06 (2.11) | 84.26 (2.52) | 84.65 (2.13) | 86.36 (2.48) | 86.87 (2.28) | 85.75 (2.12) | 88.14 (2.59) | 0.736 |
| AST (IU/L) | 19.02 (0.11) | 19.01 (0.30) | 18.72 (0.18) | 18.95 (0.30) | 18.90 (0.29) | 18.67 (0.32) | 18.30 (0.22) | 18.83 (0.38) | 19.93 (0.57) | 19.34 (0.39) | 19.70 (0.38) | 0.025 |
| ALT (IU/L) | 15.55 (0.21) | 15.48 (0.53) | 14.71 (0.33) | 15.32 (0.57) | 15.57 (0.76) | 14.48 (0.47) | 15.20 (0.46) | 15.09 (0.71) | 16.80 (1.05) | 16.63 (0.85) | 16.51 (0.87) | 0.027 |
| Creatinine (mg/dL) | 0.73 (0.00) | 0.77 (0.01) | 0.72 (0.01) | 0.71 (0.01) | 0.73 (0.01) | 0.73 (0.01) | 0.74 (0.01) | 0.74 (0.01) | 0.74 (0.01) | 0.73 (0.01) | 0.71 (0.01) | 0.156 |
| eGFR (mL/min/1.73 m2) | 68.84 (0.43) | 70.40 (1.22) | 67.23 (1.13) | 65.82 (1.11) | 67.78 (1.36) | 67.85 (1.60) | 70.06 (1.22) | 70.37 (1.72) | 71.62 (1.36) | 70.05 (1.49) | 67.92 (1.38) | 0.131 |
BMI, body mass index; WC, waist circumference; WHtR, waist-height ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate.
Data are expressed as weighted mean (SE).
Table 2 shows the trends of daily calorie intake and daily intakes of three macronutrients, namely carbohydrate, protein, and fat. The daily calorie and fat intakes increased significantly in both boys and girls during the study period (p<0.001). In boys, intakes of all three major nutrients increased, while in girls, only intakes of protein and fat increased.
Table 2. Trends of Calorie Intake and the Intakes of Three Major Nutrients in Korean Children and Adolescents between 2008 and 2017.
| Boys | Total (n=3600) | 2008 (n=487) | 2009 (n=508) | 2010 (n=423) | 2011 (n=369) | 2012 (n=346) | 2013 (n=349) | 2014 (n=253) | 2015 (n=299) | 2016 (n=305) | 2017 (n=261) | p value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy (kcal) | 2369.25 (19.75) | 2164.09 (53.02) | 2157.13 (37.50) | 2431.78 (67.06) | 2434.98 (59.21) | 2377.01 (62.06) | 2444.84 (66.80) | 2525.82 (83.42) | 2474.29 (66.99) | 2401.10 (58.85) | 2365.62 (66.07) | <0.001 |
| Carbohydrate (g) | 358.71 (2.85)) | 342.03 (8.19)) | 340.50 (5.86)) | 367.81 (9.23)) | 371.69 (8.82)) | 369.30 (8.62)) | 367.25 (8.91)) | 368.18 (12.25) | 356.74 (8.57)) | 347.78 (10.00) | 0.016 | |
| Protein (g) | 86.32 (0.95) | 76.16 (1.80) | 75.46 (1.43) | 87.57 (3.06) | 91.13 (3.71) | 88.43 (3.17) | 88.21 (3.23) | 92.84 (4.03) | 90.15 (2.95) | 90.13 (2.76) | 86.86 (3.10) | <0.001 |
| Fat (g) | 63.08 (0.85) | 52.79 (2.21) | 54.33 (1.69) | 66.07 (2.97) | 64.50 (2.41) | 61.20 (2.74) | 65.22 (2.96) | 70.72 (3.09) | 70.88 (2.84) | 64.34 (2.69) | 66.69 (2.88) | <0.001 |
| Girls | Total (n=3212) | 2008 (n=423) | 2009 (n=470) | 2010 (n=360) | 2011 (n=337) | 2012 (n=304) | 2013 (n=329) | 2014 (n=217) | 2015 (n=243) | 2016 (n=268) | 2017 (n=261) | p value |
| Girls | Total (n=3212) | 2008 (n=423) | 2009 (n=470) | 2010 (n=360) | 2011 (n=337) | 2012 (n=304) | 2013 (n=329) | 2014 (n=217) | 2015 (n=243) | 2016 (n=268) | 2017 (n=261) | p value |
| Energy (kcal) | 1855.48 (15.85) | 1736.79 (36.75) | 1759.62 (42.41) | 1906.57 (51.92) | 1872.33 (42.10) | 1911.84 (56.49) | 1889.54 (58.48) | 1927.08 (61.63) | 1936.15 (54.41) | 1832.71 (44.06) | 1816.54 (52.03) | 0.011 |
| Carbohydrate (g) | 285.02 (2.33) | 275.55 (5.68) | 281.29 (7.07) | 295.13 (7.46) | 292.41 (6.38) | 295.44 (7.69) | 282.80 (7.96) | 286.88 (8.99) | 292.17 (7.96) | 274.12 (6.99) | 274.54 (7.95) | 0.159 |
| Protein (g) | 66.21 (0.82) | 61.87 (2.00) | 61.00 (1.57) | 65.68 (2.18) | 66.73 (1.85) | 70.40 (3.28) | 67.11 (3.85) | 68.53 (3.10) | 69.09 (2.91) | 68.22 (2.36) | 65.21 (2.40) | 0.033 |
| Fat (g) | 49.07 (0.68) | 41.72 (1.64) | 44.04 (1.69) | 51.99 (2.54) | 48.09 (1.80) | 51.02 (2.38) | 51.33 (2.37) | 53.29 (2.85) | 52.37 (2.20) | 49.71 (1.95) | 49.45 (1.97) | <0.001 |
Data are expressed as weighted mean (SE).
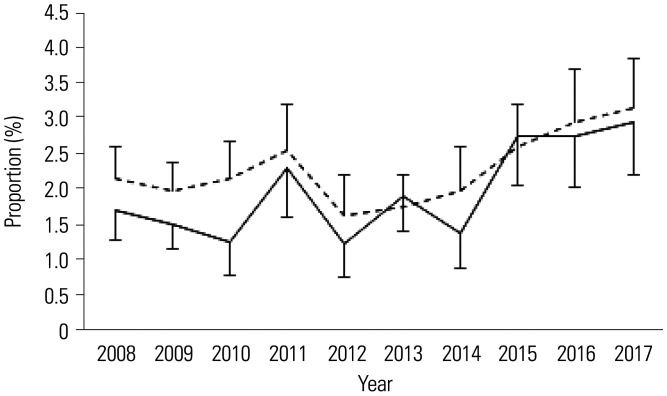
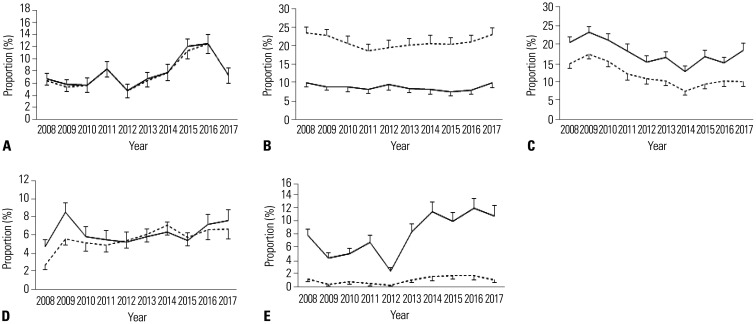
Table 3 shows the trends of the prevalence of MetS and its metabolic components among Korean children and adolescents between 2008 and 2017 according to the IDF and NCEP-ATP III criteria. Based on the IDF criteria, the prevalence of MetS increased from 1.53% in 2008 to 3.19% in 2017. The prevalence increased consistently after 2013, and the increase was significant (p=0.007). Based on the NCEP-ATP III criteria, the prevalence of MetS increased from 2.18% in 2008 to 3.19% in 2017; however, the increase was not statistically significant (p=0.448). Among the components of MetS, the prevalence rates of central obesity, low HDL cholesterol levels, and high fasting glucose levels significantly increased.
Table 3. Trends of the Prevalence of Metabolic Syndrome and Its Components in Korean Children and Adolescents between 2008 and 2017 according to the IDF and NCEP-ATP III Criteria.
| Variable | Total (n=9513) | 2008 (n=1239) | 2009 (n=1366) | 2010 (n=1076) | 2011 (n=935) | 2012 (n=889) | 2013 (n=962) | 2014 (n=732) | 2015 (n=772) | 2016 (n=789) | 2017 (n=753) | p value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IDF | 168 (1.77) | 19 (1.53) | 24 (1.76) | 11 (1.02) | 16 (1.71) | 9 (1.01) | 16 (1.66) | 9 (1.23) | 21 (2.72) | 19 (2.41) | 24 (3.19) | 0.007 | |
| Central obesity | 928 (9.78) | 122 (9.89) | 111 (8.15) | 87 (8.13) | 90 (9.64) | 64 (7.22) | 79 (8.23) | 73 (10.00) | 113 (14.64) | 112 (14.21) | 77 (10.23) | <0.001 | |
| Triglycerides ≥150 mg/dL | 718 (8.64) | 107 (9.53) | 111 (9.23) | 82 (8.74) | 64 (7.56) | 56 (7.21) | 70 (8.63) | 47 (8.22) | 54 (8.04) | 58 (8.08) | 69 (10.22) | 0.927 | |
| HDL ≤40–50 mg/dL* | 1464 (17.56) | 225 (20.04) | 273 (22.69) | 179 (19.08) | 136 (16.06) | 111 (14.29) | 130 (16.03) | 75 (13.11) | 108 (16.07) | 110 (15.32) | 117 (17.36) | 0.001 | |
| SBP ≥130 or DBP ≥85 mm Hg | 530 (5.59) | 48 (3.88) | 105 (7.70) | 54 (5.04) | 40 (4.29) | 37 (4.19) | 49 (5.10) | 45 (6.17) | 46 (6.00) | 50 (6.36) | 56 (7.44) | 0.159 | |
| FPG >100 mg/dL | 638 (7.69) | 84 (7.57) | 56 (4.69) | 42 (4.53) | 49 (5.80) | 36 (4.64) | 65 (8.03) | 65 (11.36) | 74 (11.01) | 81 (11.28) | 86 (12.74) | <0.001 | |
| NCEP-ATP III | 213 (2.24) | 27 (2.18) | 32 (2.34) | 24 (2.23) | 21 (2.25) | 13 (1.46) | 9 (1.11) | 9 (1.57) | 13 (1.93) | 21 (2.66) | 24 (3.19) | 0.448 | |
| Waist ≥90th percentile† | 917 (9.66) | 121 (9.81) | 107 (7.86) | 87 (8.13) | 90 (9.64) | 64 (7.22) | 77 (8.02) | 73 (10.00) | 109 (14.12) | 112 (14.21) | 77 (10.23) | <0.001 | |
| Triglycerides ≥ 110 mg/dL | 1763 (21.15) | 267 (23.78) | 277 (23.03) | 193 (20.58) | 153 (18.06) | 138 (17.76) | 169 (20.84) | 117 (20.45) | 137 (20.39) | 152 (21.17) | 160 (23.70) | 0.596 | |
| HDL <40 mg/dL | 1043 (12.51) | 172 (15.32) | 207 (17.21) | 140 (14.93) | 94 (11.10) | 85 (10.94) | 82 (10.11) | 47 (8.22) | 68 (10.12) | 78 (10.86) | 70 (10.39) | <0.001 | |
| BP ≥90th percentile‡ | 535 (5.64) | 34 (2.75) | 81 (5.94) | 48 (4.48) | 42 (4.51) | 48 (5.44) | 61 (6.35) | 53 (7.27) | 57 (7.43) | 53 (6.74) | 58 (7.70) | 0.037 | |
| FPG ≥110 mg/dL | 81 (0.98) | 13 (1.17) | 5 (0.42) | 5 (0.54) | 5 (0.59) | 3 (0.39) | 9 (1.11) | 9 (1.57) | 13 (1.93) | 10 (1.39) | 9 (1.33) | 0.050 | |
IDF, International Diabetes Federation; NCEP-ATP III, modified National Cholesterol Education Program-Adult Treatment Panel III; HDL, high-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose.
Data are expressed as n (weighted %).
*Aged less than 16 years. Aged over 16 years: HDL cholesterol levels ≤40 mg/dL (male) and HDL cholesterol levels ≤50 mg/dL (female), †Adjusted for age and sex, ‡Adjusted for age, sex, and height.
Fig. 1 shows the increasing trend of MetS prevalence among Korean children and adolescents between 2008 and 2017 according to both the IDF and NCEP ATP-III criteria. The prevalence rates of central obesity, low HDL levels, and high fasting blood glucose levels increased during the study period (Fig. 2). However, the prevalence of high TG levels showed no significant change. Low HDL cholesterol and high fasting blood glucose levels had higher prevalence rates based on the IDF criteria than based on the NCEP-ATP III criteria.
Fig. 1. Trends of the prevalence of metabolic syndrome among Korean children and adolescents between 2008 and 2017 according to both the IDF and NCEP-ATP III criteria (Solid line: IDF, Dotted line: NCEP-ATP III). IDF, International Diabetes Federation; NCEP-ATP III, modified National Cholesterol Education Program-Adult Treatment Panel III.
Fig. 2. Trends of the prevalence rates of metabolic syndrome components among Korean children and adolescents between 2008 and 2017 according to both the IDF and NCEP-ATP III criteria. (A) Central obesity. (B) Elevated triglycerides. (C) Low high-density lipoprotein cholesterol. (D) Elevated blood pressure. (E) Elevated fasting plasma glucose (Solid line: IDF, Dotted line: NCEP-ATP III). IDF, International Diabetes Federation; NCEP-ATP III, modified National Cholesterol Education Program-Adult Treatment Panel III.
DISCUSSION
This study analyzed the trends in the prevalence of MetS and its metabolic components among Korean children and adolescents in the past 10 years based on both the IDF and NCEP-ATP III criteria. We found that based on the IDF criteria, the prevalence of MetS increased from 1.53% in 2008 to 3.19% in 2017, and based on the NCEP-ATP III criteria, it increased from 2.18% in 2008 to 3.19% in 2017; the daily calorie and fat intakes also significantly increased. MetS prevalence generally increased during the study period, especially after 2013. The components of MetS, including central obesity, low HDL cholesterol levels, and high fasting plasma glucose levels, also significantly increased over time.
There is some controversy regarding the trend of MetS over time mainly due to the same diagnostic criteria, and the definitions are not consistently used for MetS. Based on the NCEP ATP-III criteria, the age-adjusted prevalence of MetS in the U.S. decreased from 7.3% in 1988–1994 to 6.7% in 1999–2002 and 6.5% in 2003–2006 according to the National Health and Nutrition Examination Survey (NHANES).13 Another study involving the same subjects but using different cut-off values suggested that approximately 73.2% of the participants had at least one component of MetS, with the estimated MetS prevalence being 10.1% in individuals aged between 12 and 19 years according to the U.S. NHANES 2000–2010.21 Using the data of 2330 Korean adolescents (aged 10–18 years) from 2010 to 2012, the prevalence rates of MetS based on the NCEP-ATP III and IDF criteria were 5.7% and 2.1%, respectively.15 In this study, the two most frequently used definitions, the IDF and NCEP-ATP III criteria, showed similar results for the prevalence of MetS. However, among the components of MetS, low HDL cholesterol and high fasting plasma glucose showed higher prevalence rates with the use of the IDF criteria than with the use of the NCEP-ATP III criteria, since the criteria were different. The diagnostic criteria for MetS in children and adolescents are still being revised to develop a more precise and useful diagnostic tool. The IDF, for instance, revised the cut-off point and age group in 2007.22 Considering these different criteria for diagnosing MetS, different approaches and detailed analyses should be considered to obtain a more accurate prediction of the risk.
Since MetS is strongly related to obesity, the prevalence of MetS tends to increase as the severity of obesity increases. In China, the prevalence of MetS increased from 2.3% in 2004– 2010 to 3.2% in 2011–2014 based on the NCEP-ATP III criteria.23 The prevalence of overweight and obesity among urban children and adolescents in China was comparable to that in developed countries, and it further increased from 2010 to 2015.24 Increases in the prevalence of overweight and obesity among Korean adolescents were found between 1998 and 2001; thereafter, the trends stabilized.22 During the same period, based on the IDF criteria, the prevalence of MetS in 4164 Korean adolescents was 2.2% in 1998, 3.6% in 2001, and 1.8% in 2005.16 In other words, the prevalence of MetS decreased despite the consistent prevalence of obesity among Korean youths. One explanation for the declining MetS prevalence was an increase in physical activity. The Health Plan 2010 was started by the Korean Ministry of Health and Welfare in the early 2000s for national health improvement, and included adolescent education regarding the importance of physical activity and nutrition. Its outcomes were evaluated in 2010. As a result of the campaign, the rate of moderate physical activity increased from 7.1% in 2005 to 10.2% in 2008 (https://khealth.or.kr/healthplan).
During the study period, the prevalence of MetS increased from 2008, especially after 2013. This study also showed that calorie intake increased over the study period, and among the three macronutrients, fat intake showed the fastest increase. This observation may help explain the increasing prevalence of MetS in Korea, as demonstrated in this study. Physical activity or sedentary behavior may also affect the prevalence of obesity, but these parameters were not analyzed in this study.25 Another study showed that the prevalence of extreme obesity increased, although the prevalence of childhood obesity in Korea has remained stable since the early 2000s based on data of 19593 subjects aged 2–19 years from the KNHANES conducted in 2001–2014.26 As the prevalence of extreme obesity increased, the prevalence of MetS also increased, even when the prevalence of obesity was maintained.27 This could be another possible explanation for the increase in the prevalence of MetS among Korean children and adolescents and the discrepancy between the increases in the prevalence rates of obesity and MetS.
This study had some limitations. First, since it was based on the KNHANES, the findings in this study might not be generalizable to other populations. Second, the factors associated with MetS, such as sedentary behavior and type of food, were not considered in this study. Therefore, this study only described the associations and not the causality. Third, we could not assess the effect of exercise, one of the most important factors affecting MetS, as we did not consider physical activity in this study. Fourth, puberty and pubertal timing are important factors that affect MetS; however, the KNHANES data did not include information about these factors. Nevertheless, this study also had some strengths. It was based on nationally representative data collected over 10 years in Korea. The authors analyzed the annual data and each component of MetS to elucidate the relationship between the prevalence of MetS and its components. Further studies are required to determine possible explanations for the observed trends in MetS among children and adolescents.
In conclusion, the prevalence rates of MetS, as well as those of central obesity, low HDL cholesterol, and high fasting glucose, increased in Korean children and adolescents between 2008 and 2017; in addition, the calorie and fat intakes also increased significantly. These findings suggest that more active support and close monitoring for children and adolescents are required to control MetS and prevent further metabolic diseases.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Hyun Wook Chae, Jae Hyun Kim, and Seong Ik Park.
- Data curation: Hyun Wook Chae, Jae Hyun Kim, Seong Ik Park, and Junghwan Suh.
- Formal analysis: Hye Sun Lee, Seong Ik Park, and Kyungchul Song.
- Investigation: Seong Ik Park, Kyungchul Song, Jun Suk Oh, and Youngha Choi.
- Methodology: Hyun Wook Chae, Jae Hyun Kim, Junghwan Suh, and Seong Ik Park.
- Project administration: Hyun Wook Chae, Jae Hyun Kim, and Junghwan Suh.
- Resources: Seong Ik Park, Kyungchul Song, Junghwan Suh, and Han Saem Choi.
- Software: Seong Ik Park, Hye Sun Lee, Jun Suk Oh, and Youngha Choi.
- Supervision: Hyun Wook Chae, Jae Hyun Kim, Junghwan Suh, Ahreum Kwon, and Ho-Seong Kim.
- Validation: Hyun Wook Chae, Jae Hyun Kim, Ahreum Kwon, and Ho-Seong Kim.
- Visualization: Seong Ik Park, Junghwan Suh, and Han Saem Choi.
- Writing—original draft: Seong Ik Park, Hyun Wook Chae, and Jae Hyun Kim.
- Writing—review & editing: Hyun Wook Chae, Jae Hyun Kim, Seong Ik Park, and Junghwan Suh.
- Approval of final manuscript: all authors.
References
- 1.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 2.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 4.Ighbariya A, Weiss R. Insulin Resistance, Prediabetes, Metabolic Syndrome: what should every pediatrician know? J Clin Res Pediatr Endocrinol. 2017;9:49–57. doi: 10.4274/jcrpe.2017.S005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno G, Merletti F, Biggeri A, Bargero G, Ferrero S, Runzo C, et al. Metabolic syndrome as a predictor of all-cause and cardiovascular mortality in type 2 diabetes: the Casale Monferrato Study. Diabetes Care. 2004;27:2689–2694. doi: 10.2337/diacare.27.11.2689. [DOI] [PubMed] [Google Scholar]
- 6.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Yadav D, Mishra M, Joseph AZ, Subramani SK, Mahajan S, Singh N, et al. Status of antioxidant and lipid peroxidation in type 2 diabetic human subjects diagnosed with and without metabolic syndrome by using NCEP-ATPIII, IDF and WHO criteria. Obes Res Clin Pract. 2015;9:158–167. doi: 10.1016/j.orcp.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 8.GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 10.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141:e20173459. doi: 10.1542/peds.2017-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S, Jang HC, Park KS, Cho SI, Lee MG, Joung H, et al. Changes in metabolic syndrome in American and Korean youth, 1997-2008. Pediatrics. 2013;131:e214–e222. doi: 10.1542/peds.2012-0761. [DOI] [PubMed] [Google Scholar]
- 14.Kang HT, Lee HR, Shim JY, Shin YH, Park BJ, Lee YJ. Association between screen time and metabolic syndrome in children and adolescents in Korea: the 2005 Korean National Health and Nutrition Examination Survey. Diabetes Res Clin Pract. 2010;89:72–78. doi: 10.1016/j.diabres.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, So WY. Prevalence of metabolic syndrome among Korean adolescents according to the national cholesterol education program, Adult Treatment Panel III and International Diabetes Federation. Nutrients. 2016;8:588. doi: 10.3390/nu8100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park MJ, Boston BA, Oh M, Jee SH. Prevalence and trends of metabolic syndrome among Korean adolescents: from the Korean NHANES Survey, 1998-2005. J Pediatr. 2009;155:529–534. doi: 10.1016/j.jpeds.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y. The Korea National Health and Nutrition Examination Survey (KNHANES): current status and challenges. Epidemiol Health. 2014;36:e2014002. doi: 10.4178/epih/e2014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement: executive summary. Crit Pathw Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Lee CG, Choi JM, Moon JS, Choe BK, Son CS, Yang SW, et al. 2005 Korean national survey of children and adolescents to establish the reference standard of growth and blood pressure. Final report. Seoul: Ministry of Health and Welfare; 2006. [Google Scholar]
- 21.Miller JM, Kaylor MB, Johannsson M, Bay C, Churilla JR. Prevalence of metabolic syndrome and individual criterion in US adolescents: 2001–2010 National Health and Nutrition Examination Survey. Metab Syndr Relat Disord. 2014;12:527–532. doi: 10.1089/met.2014.0055. [DOI] [PubMed] [Google Scholar]
- 22.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents-an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 23.Ye P, Yan Y, Ding W, Dong H, Liu Q, Huang G, et al. Prevalence of metabolic syndrome in Chinese children and adolescents: a meta-analysis. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36:884–888. [PubMed] [Google Scholar]
- 24.Zhang J, Li X, Hawley N, Zheng Z, Zou Z, Tan L, et al. Trends in the prevalence of overweight and obesity among Chinese school-age children and adolescents from 2010 to 2015. Child Obes. 2018;14:182–188. doi: 10.1089/chi.2017.0309. [DOI] [PubMed] [Google Scholar]
- 25.Albert Pérez E, Mateu Olivares V, Martínez-Espinosa RM, Molina Vila MD, Reig García-Galbis M. New insights about how to make an intervention in children and adolescents with metabolic syndrome: diet, exercise vs. changes in body composition. A systematic review of RCT. Nutrients. 2018;10:878. doi: 10.3390/nu10070878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nam HK, Kim HR, Rhie YJ, Lee KH. Trends in the prevalence of extreme obesity among Korean children and adolescents from 2001 to 2014. J Pediatr Endocrinol Metab. 2017;30:517–523. doi: 10.1515/jpem-2016-0456. [DOI] [PubMed] [Google Scholar]
- 27.Cho WK, Han K, Ahn MB, Park YM, Jung MH, Suh BK, et al. Metabolic risk factors in Korean adolescents with severe obesity: results from the Korea National Health and Nutrition Examination Surveys (K-NHANES) 2007-2014. Diabetes Res Clin Pract. 2018;138:169–176. doi: 10.1016/j.diabres.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]