Abstract
Objective
There has been a substantial decline in patients presenting for emergent and routine cardiovascular care in the United States after the onset of the coronavirus disease 2019 (COVID-19) pandemic. We sought to assess the risk of adverse clinical outcomes among patients undergoing coronary artery bypass graft (CABG) surgery during the 2020 COVID-19 pandemic period and compare the risks with those undergoing CABG before the pandemic in the year 2019.
Methods
A retrospective cross-sectional analysis of the TriNetX Research Network database was performed. Patients undergoing CABG between January 20, 2019, and September 15, 2019, contributed to the 2019 cohort, and those undergoing CABG between January 20, 2020, and September 15, 2020, contributed to the 2020 cohort. Propensity-score matching was performed, and the odds of mortality, acute kidney injury, stroke, acute respiratory distress syndrome, and mechanical ventilation occurring by 30 days were evaluated.
Results
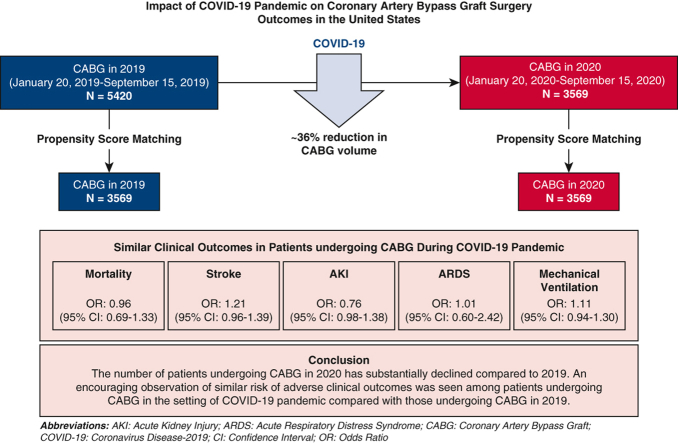
The number of patients undergoing CABG in 2020 declined by 35.5% from 5534 patients in 2019 to 3569 patients in 2020. After propensity-score matching, 3569 patient pairs were identified in the 2019 and the 2020 cohorts. Compared with those undergoing CABG in 2019, the odds of mortality by 30 days were 0.96 (95% confidence interval [CI], 0.69-1.33; P = .80) in those undergoing CABG in 2020. The odds for stroke (odds ratio [OR], 1.201; 95% CI, 0.96-1.39), acute kidney injury (OR, 0.76; 95% CI, 0.59-1.08), acute respiratory distress syndrome (OR, 1.01; 95% CI, 0.60-2.42), and mechanical ventilation (OR, 1.11; 95% CI, 0.94-1.30) were similar between the 2 cohorts.
Conclusions
The number of patients undergoing CABG in 2020 has substantially declined compared with 2019. Similar odds of adverse clinical outcomes were seen among patients undergoing CABG in the setting of COVID-19 compared with those in 2019.
Key Words: coronavirus disease 2019, coronary artery disease, coronary artery bypass graft, myocardial infarction
Abbreviations and Acronyms: CABG, coronary artery bypass grafting; CI, confidence interval; COVID-19, coronavirus disease 2019; EHR, electronic health record; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; OR, odds ratio; UAB, University of Alabama at Birmingham
Graphical abstract
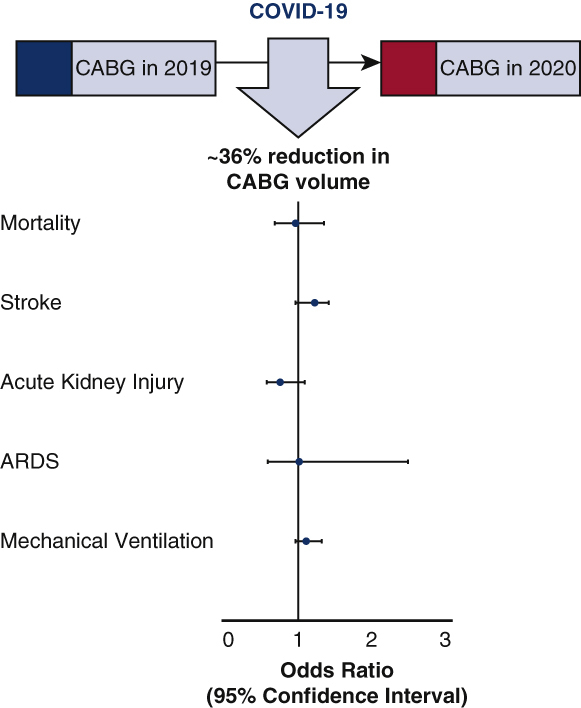
Impact of COVID-19 on CABG surgery volume and outcomes.
Central Message.
There has been a ∼36% decline in CABG volume in 2020 due to COVID-19. There has been no associated increase in adverse clinical outcomes among patients undergoing CABG during the COVID-19 pandemic.
Perspective.
Despite the reorganization of health care systems and patient reluctance to seek treatment, there has been no associated increase in adverse clinical outcomes among patients undergoing CABG during the COVID-19 pandemic. However, the clinical effects of the decline in CABG volume, including delays in CAD treatment, require further surveillance and investigation.
See Commentaries on pages 144 and 146.
Coronary artery disease remains a major cause of mortality in the United States despite the advances in the management of acute coronary syndrome and cardiovascular risk factors.1 Coronary artery bypass grafting (CABG) is the most common cardiac surgery performed worldwide and a life-saving procedure for patients with severe coronary artery disease.1 The coronavirus disease 2019 (COVID-19) pandemic resulted in a precipitous decline in hospitalizations for myocardial infarction and revascularization procedures.2, 3, 4 In addition, there had been a precautionary postponement of elective procedures, including CABG, as part of resource prioritization and infection control.5,6 However, delays in the performance of CABG have been previously associated with increased mortality.7 The procedural delays, combined with the patients' reluctance to seek medical attention for cardiac symptoms during the evolution of the pandemic, may impact the clinical outcomes in patients with coronary artery disease. In addition, clinicians and patients may be concerned about the effects of elective exposure to a hospital environment in the setting of a pandemic. We sought to evaluate the clinical outcomes among patients undergoing CABG in the COVID-19 pandemic period compared with those undergoing CABG in the same time period in 2019.
Methods
Data Source
We identified patients undergoing CABG at health care organizations contributing to the TriNetX Research Network (Cambridge, Mass).8, 9, 10, 11, 12, 13 The TriNetX Research Network database is a cloud-based Health Insurance Portability and Accountability Act of 1996–compliant deidentified longitudinal patient-level federated electronic health record (EHR) database.8, 9, 10, 11, 12, 13 The data are available to researchers at participating health care organizations and can be accessed at www.trinetx.com. The data integration is performed after clearance through local data warehouses and research data repositories, before incorporation into the TriNetX database. Data from inpatient, outpatient, and specialty services are contributed by the participating organization. The structured data are mapped to standard and controlled clinical terminology. Regular data quality assessment is performed to eliminate records that do not adhere to quality standards and basic formatting requirements for adequate data representation. The referential integrity of the data is ensured to ensure data comparability across several databases. Regular monitoring of the temporal trend in data volume is also monitored by the TriNetX software to ensure data validity. The stored and transmitted data in the database is deidentified at the patient and health care organization level. Further details of the database are included in Appendix 1. The study was conducted after obtaining approval from the University of Alabama at Birmingham (UAB) Institutional Review Board (Application Number: IRB-300005908). This investigation involved secondary data analysis of deidentified patient-level data, and informed consent was not required for this study. The UAB Institutional Review Board waived the requirement for informed consent.
Study Population
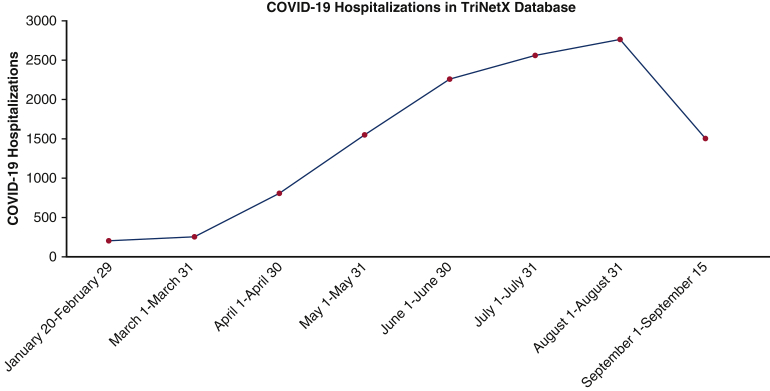
We evaluated the TriNetX Research Network EHR database to identify patients undergoing CABG (Current Procedural Terminology codes: 1006207, 1006199, 0210-0213) at 26 health care organizations in the United States (Table E1). Patients were grouped into those undergoing CABG during the COVID-19 period (January 20, 2020, to September 15, 2020) and those undergoing CABG in the same time period in 2019 (January 20, 2019, to September 15, 2019). Standard International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes were used to identify the respective phenotypes. The last patient included in the study period underwent CABG on September 15, 2020, and a follow-up period of 30 days (until October 15, 2020) was used in this study. We also tabulated the number of patients undergoing CABG in each comparative month from the 2019 and 2020 study periods and the total number of COVID-19 hospitalizations recorded in the TriNetX research database during the study period.
Measures and Outcomes
The baseline characteristics were identified for patient records up to 1 month before the index event (CABG). The primary study outcome was mortality occurring within 30 days of CABG. The secondary outcomes assessed included stroke (ICD-10: I60-I69), acute kidney injury (ICD-10: N17), acute respiratory distress syndrome (ICD-10 Code: J80),14 and mechanical ventilation (ICD-10 codes: 5A1945Z, 5A1955Z, 39.65; Current Procedural Terminology codes: 1022227, 31500, 0BH17EZ, 0BH18EZ) occurring within 30 days of CABG. Table E2 enlists the diagnosis codes for the various measures and outcomes.
Statistical Analysis
All statistical analyses were conducted using SAS, 9.4 (Cary, NC). The baseline characteristics of the study population were compared using descriptive statistics. Continuous data were summarized as median with interquartile range, and categorical data were summarized as counts and percentages. The categorical data were compared using the χ2 test, and continuous data were compared using the Wilcoxon rank-sum test. Month-wise CABG procedure counts in the 2 study periods were graphically tabulated. The month-wise COVID-19 hospitalizations recorded in the TriNetX research database were also graphically plotted. Logistic regression was used to develop propensity-score matching models to obtain a propensity score for each patient using and account for the differences in baseline characteristics.8 The model covariates included age, sex, race/ethnicity, body mass index, alcohol abuse (ICD-10: F10), asthma (J45), chronic obstructive pulmonary disease (J44.9), cerebrovascular disease (I60-I69), chronic kidney disease (N18), diabetes mellitus (E08-E13), heart failure (I50), hypertensive disease (I10-I16), ischemic heart disease (I20-I25), nicotine dependence (F17), and any neoplasm (C00-D49).8 Using a caliper of 0.1 pooled standard deviation, 1:1 nearest neighbor matching was performed. Logistic regression was used to estimate the odds of the study outcomes. The comparative risks of study outcomes were summarized using odds ratios (OR) with 95% confidence intervals (95% CI). The subgroup analysis of the aforementioned study outcomes was done in a single-center cohort of patients identified from the UAB and undergoing CABG in 2019 and 2020 during the specified time period to verify the veracity of the outcomes of the larger database on an institutional level. Statistical significance was determined by a 2-sided type I error of 0.05.
Results
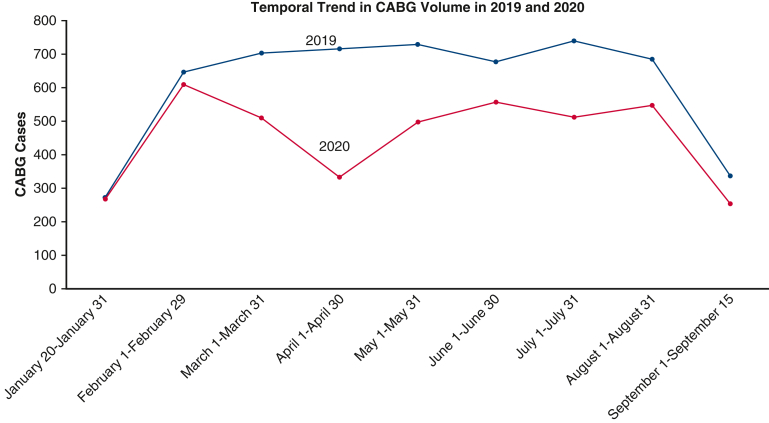
A total of 5534 patients underwent CABG in the 2019 study period, and 3569 patients underwent CABG in the 2020 study period. In the 2019 cohort, 13.8% (761) patients were from the Northeast, 9.8% (542) patients were from the Midwest, 66.0% (3653) patients were from the South, and 10.4% (578) patients were from the Western region. In the 2020 cohort, 12.1% (431) patients were from the Northeast, 12.4% (441) patients were from the Midwest, 63.7% (2272) patients were from the South, and 11.9% (425) patients were from the Western region. This amounted to a 35.5% decrease in CABG surgery volume. The baseline characteristics of the patients undergoing CABG in 2019 and 2020 before and after propensity-score matching are described in Table 1. The temporal trend in patients undergoing CABG in 2019 and 2020 are presented in Figure E1. The maximum decline in CABG procedures occurred in April 2020, and there was a gradual increase in CABG volume in the subsequent months (Figure E2). The month-wise COVID-19 hospitalization count in the database is depicted in Figure E3.
Table 1.
Baseline characteristics of the overall cohort
| Characteristics | Before propensity-score matching |
After propensity-score matching |
||||||
|---|---|---|---|---|---|---|---|---|
| CABG in 2020 (Total N = 3569) | CABG in 2019 (Total N = 5534) | P value | SMD | CABG in 2020 (Total N = 3569) | CABG in 2019 (Total N = 3569) | P value | SMD | |
| Demographics | ||||||||
| Age (Years) | 67 (60, 74) | 66 (59, 73) | .001 | 0.09 | 66 (59, 73) | 67 (59, 73) | .23 | 0.02 |
| Sex | ||||||||
| Male | 2664 (75.6%) | 4108 (74.2%) | .66 | 0.01 | 2664 (74.6%) | 2647 (74.2%) | .64 | 0.01 |
| Female | 905 (25.4%) | 1426 (25.8%) | 905 (24.4%) | 922 (25.8%) | ||||
| Race/ethnicity | ||||||||
| Non-Hispanic White | 2969 (83.2%) | 4517 (81.6%) | .51 | 0.04 | 2969 (83.2%) | 2957 (82.9%) | .76 | 0.005 |
| Non-Hispanic Black | 293 (8.2%) | 514 (9.3%) | 293 (8.2%) | 322 (9.0%) | ||||
| Non-Hispanic Asian | 91 (2.6%) | 151 (2.7%) | 91 (2.6%) | 92 (2.6%) | ||||
| Hispanic | 198 (5.6%) | 326 (5.9%) | 198 (5.6%) | 181 (5.1%) | ||||
| Other Non-Hispanic | 18 (0.4%) | 26 (0.4%) | 18 (0.4%) | 17 (0.4%) | ||||
| BMI, kg/m2 | 29.7 (22.6, 35.8) | 29.8 (23.1, 36.2) | .46 | 0.03 | 29.7 (22.8, 35.6) | 29.8 (23.4, 34.9) | .52 | 0.02 |
| Medical history | ||||||||
| Alcohol abuse | 146 (4.1%) | 234 (4.2%) | .75 | 0.007 | 146 (4.1%) | 147 (4.1%) | .95 | 0.001 |
| Asthma | 246 (6.9%) | 401 (7.3%) | .52 | 0.02 | 246 (6.9%) | 240 (6.7%) | .78 | 0.007 |
| Cerebrovascular disease | 1041 (29.2%) | 1711 (30.9%) | .08 | 0.04 | 1041 (29.2%) | 1054 (29.5%) | .73 | 0.008 |
| Chronic kidney disease | 794 (22.3%) | 1366 (24.7%) | .008 | 0.06 | 794 (22.3%) | 755 (21.2%) | .27 | 0.02 |
| Chronic obstructive pulmonary disease | 246 (6.9%) | 401 (7.3%) | .52 | 0.01 | 246 (6.9%) | 240 (6.7%) | .78 | 0.006 |
| Diabetes mellitus | 1645 (46.1%) | 2511 (45.4%) | .50 | 0.01 | 1645 (46.1%) | 1641 (46.0%) | .93 | 0.002 |
| Heart failure | 1471 (41.2%) | 2403 (43.4%) | .04 | 0.05 | 1471 (41.2%) | 1453 (40.7%) | .67 | 0.01 |
| Hypertensive diseases | 2463 (69.0%) | 3874 (70.0%) | .87 | 0.003 | 2463 (69.0%) | 2481 (69.5%) | .90 | 0.006 |
| Ischemic heart disease | 3396 (95.2%) | 5296 (95.7%) | .22 | 0.03 | 3396 (95.2%) | 3401 (95.3%) | .74 | 0.008 |
| Neoplasms | 691 (19.4%) | 1111 (20.1%) | .40 | 0.02 | 691 (19.4%) | 682 (19.1%) | .79 | 0.006 |
| Nicotine dependence | 778 (21.8%) | 1150 (20.8%) | .25 | 0.03 | 778 (21.8%) | 767 (21.5%) | .76 | 0.007 |
Continuous data are presented as median with interquartile range, and categorical data are presented as counts with percentage. CABG, Coronary artery bypass grafting; SMD, standardized mean difference; BMI, body mass index.
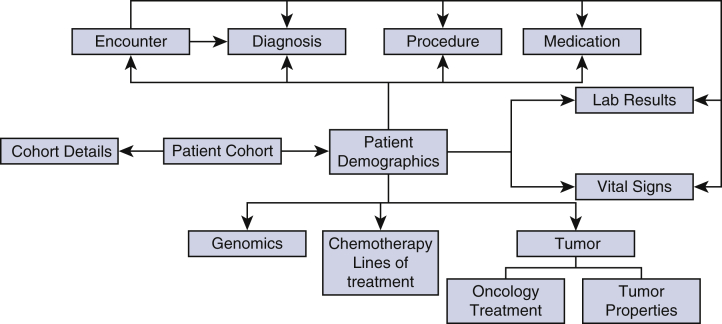
Figure E1.
Data tables relationship for TriNetX Research Network Database. This figure demonstrates the various raw data tables from the TriNetX Research Network database and their relationship.
Figure E2.
Temporal trend in CABG volume in 2019 and 2020. The curves in this figure demonstrates the temporal trend in monthly CABG surgery cases in the 2019 (blue) and 2020 (orange) study periods. CABG, Coronary artery bypass graft.
Figure E3.
Temporal trend in COVID-19 hospitalizations in the TriNetX Research Network Database. The curves in this figure demonstrates the temporal trend in monthly COVID-19 hospitalization at the hospitals in the TriNetX database. COVID-19, Coronavirus disease 2019.
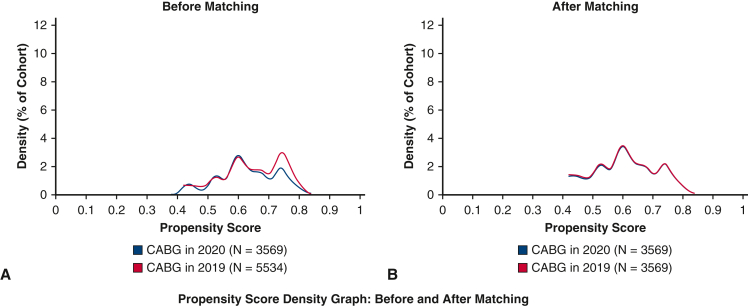
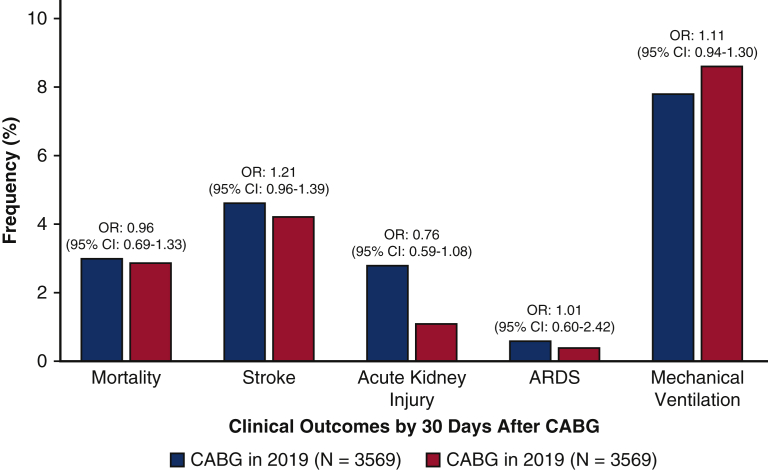
After propensity-score matching, 3569 CABG patient pairs were identified from the 2019 and 2020 cohorts (Figure 1). After propensity-score matching, the odds of mortality by 30 days (OR, 0.96; 95% CI, 0.69-1.33) were similar in the 2 cohorts (Figure 2, Table 2). The odds of stroke (OR, 1.21; 95% CI, 0.96-1.39), acute kidney injury (OR, 0.76; 95% CI, 0.59-1.08), acute respiratory distress syndrome (OR, 1.01; 95% CI, 0.60-2.42), and mechanical ventilation (OR, 1.11; 95% CI, 0.94-1.30) in the matched pairs were also similar between the patients undergoing CABG in 2019 and 2020 (Figure 2). There were only 8 patients who were identified with COVID-19 in the 30-day period following CABG. None of the 8 patients required mechanical ventilation. There was no mortality by 30 days in these 8 patients. In the secondary subgroup analysis restricted to the cohort identified from the UAB, there were 620 patients in the 2019 cohort and 470 patients in the 2020 cohort, amounting to a 25.2% decline in the CABG procedural volume. The mortality by 30 days and other secondary study outcomes were similar for the 2 time periods for the UAB cohort.
Figure 1.
Propensity score matching of patients undergoing CABG surgery in 2019 and 2020. The figure depicts the density function of the cohorts before (A) and after propensity-score matching (B). CABG, Coronary artery bypass graft.
Figure 2.
Risk of adverse clinical outcomes after CABG surgery in 2019 and 2020. The figure depicts the frequency of the study outcomes in the propensity-score matched populations undergoing coronary artery bypass graft in 2019 and 2020. OR, Odds ratio; CI, confidence interval; CABG, coronary artery bypass graft; ARDS, acute respiratory distress syndrome.
Table 2.
Study outcomes after propensity-score matching in patients undergoing CABG
| Outcome (occurring by 30 d) | Event frequency |
Odds ratio (95% CI) | |
|---|---|---|---|
| CABG 2019 (N = 3569) | CABG 2020 (N = 3569) | ||
| Death | 3.0% | 2.9% | 0.96 (0.69-1.33) |
| Stroke | 4.6% | 4.2% | 1.21 (0.96-1.39) |
| Acute kidney injury | 2.8% | 1.1% | 0.76 (0.59-1.08) |
| ARDS | 0.6% | 0.4% | 1.01 (0.60-2.42) |
| Mechanical ventilation | 7.8% | 8.6% | 1.11 (0.94-1.30) |
CABG, Coronary artery bypass grafting; CI, confidence interval; ARDS, acute respiratory distress syndrome.
Discussion
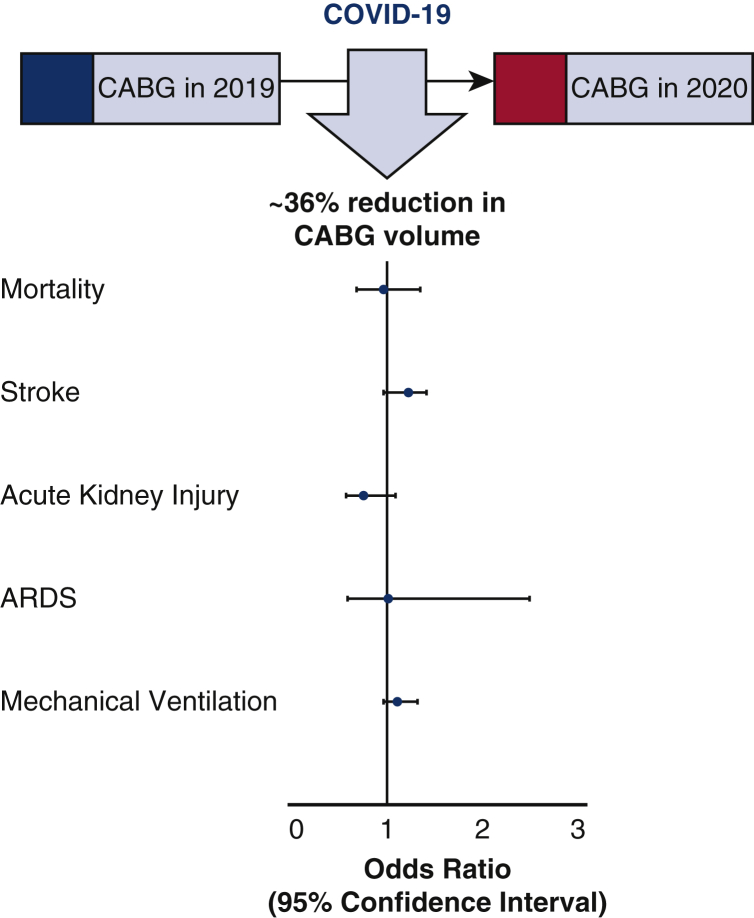
In this study, we observed that there was a ∼36% reduction in CABG procedure volume between 2019 and 2020 (Figure 3). The lowest number of CABG procedures occurred in April 2020, and there has been a gradual increase in the patients undergoing CABG in the setting of the pandemic. We observed that the risks of mortality and adverse clinical outcomes by 30 days following CABG were similar in patients undergoing CABG in 2019 and 2020 (Figure 4).
Figure 3.
Impact of COVID-19 on CABG surgery volume and outcomes. The figure depicts the forest plot of odds ratios with confidence intervals for the study outcomes in the propensity-score matched populations undergoing CABG in 2019 and 2020. COVID-19, Coronavirus disease 2019; CABG, coronary artery bypass graft; ARDS, acute respiratory distress syndrome.
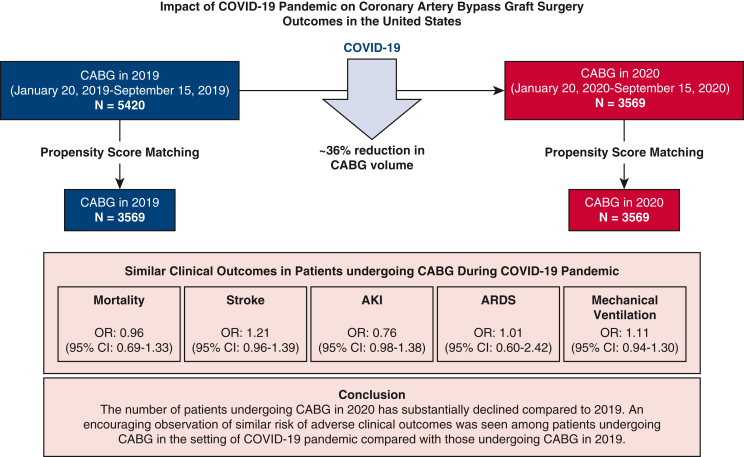
Figure 4.
Impact of COVID-19 Pandemic on CABG surgery outcomes in the United States. This figure describes the decline in the number of patients undergoing CABG surgery in the prepandemic (2019) and the pandemic period (2020). After propensity-score matching, 3569 patient-pairs were identified, and the odds of the study outcomes were similar between the patients undergoing CABG in 2019 and 2020. COVID-19, Coronavirus disease 2019; CABG, coronary artery bypass graft; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; OR, odds ratio; CI, confidence interval.
The COVID-19 pandemic has presented unique challenges for cardiac surgery services.15 The health care policies were revised during the COVID-19 pandemic to conserve and prioritize resources. The personnel, equipment, and critical care service limitations due to the pandemic were presumed to impact the clinical outcomes in patients who underwent CABG.6 We noted a substantial reduction in CABG volume in the database compared with the 2019 volume. Previous investigations have noted a reduction in patients presenting with myocardial infarction2 and ∼50% to 80% decline in the volume of cardiac surgery during the pandemic.9,10 The temporal decline in patients presenting with acute myocardial infarction indicated that patients might be avoiding medical care while being symptomatic at home, thereby potentially worsening clinical outcomes.2,4 However, we did not observe an increased risk of mortality, acute respiratory distress syndrome, or need for mechanical ventilation in the patients undergoing CABG during the pandemic period. Providers are likely to either include only those patients who were at very high risk and needed CABG or only those patients who were relatively healthier to survive the likelihood of developing severe COVID-19 subsequent to CABG. The observed results may also stem from a careful patient selection by providers in the anticipated likelihood of developing COVID-19 during the postoperative period. The observed decline in CABG volume may also be a result of patient hesitancy in seeking care due to the fear of contracting COVID-19. The declined volume may also be a result of health care organizations delaying emergent procedures to reserve resources for the care of the patients with severe COVID-19. Further research is needed to assess the impact of the COVID-19 pandemic and lockdown on cardiovascular risk factors, diseases, and procedural outcomes.
These findings may be reassuring for apprehensive clinicians and patients requiring CABG during the COVID-19 pandemic. Following the resumption of procedures, the provision of peri- and postoperative cardiac surgical care in the setting of a pandemic requires strict infection control measures for the protection of patients and health care personnel.11, 12, 13 While the reassuring findings are encouraging, continued comprehensive COVID-19 infection control protocol implementation and COVID-19 screening of patients and health care personnel and proactive management of coronary artery disease patients is needed.12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Our data do not provide information regarding the COVID-19 infection rates among health care workers taking care of patients who underwent CABG. Given that very few patients were diagnosed with COVID-19 in the postoperative period highlights the effectiveness of preoperative screening and strict infection control measures. The findings from our study may help guide the conversation at the time of consenting of patients prior to CABG.22
Our study findings may be limited by the use of administrative EHR data to identify the study outcomes. There may be undercoding of prevalent medical conditions such as ischemic heart disease in the EHR data. These limitations of EHR data have been described previously.23, 24, 25, 26 Given the observational nature of the study, there may also be residual confounding in our analysis due to unmeasured confounders. There may have been changes in the coding practices for cardiovascular procedures over time that cannot be accounted for in these data. The decline in CABG volume noted in our study may have been more profound in regions in which the impact of COVID-19 was more widespread. Since the TriNetX database deidentifies patients at the individual and organizational level, we are unable to ascertain a clustering effect of COVID-19 on CABG volume. We were also unable to assess the disease severity, hemodynamic and echocardiographic parameters, and preoperative risk metrics such as the Society of Thoracic Surgeons risk calculator score, due to limited patient-level information available in the patient EHRs. However, this study still provides important contemporary information on the impact of COVID-19 on CABG procedure volume and associated clinical outcomes.
Conclusions
There has been a substantial decline in CABG volume during the COVID-19 pandemic. However, the risk of developing adverse clinical outcomes after CABG has not been affected by the pandemic. These findings may be reassuring for patients requiring cardiovascular care in the setting of the pandemic.
Conflict of Interest Statement
Dr Kuranz is an employee of TriNetX, Inc. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
We thank the University of Alabama at Birmingham Center for Clinical and Translational Science for providing access to the TriNetX (Cambridge, Mass) global healthcare research network. We would like to thank the design and analytics team of the TriNetX healthcare network.
Footnotes
This work was supported by the National Institutes of Health Mentored Patient-Oriented Research Award [5K23HL146887-02] to Dr Arora.
Appendix 1. Methods
The TriNetX Research Network database is a federated health research database that integrates the electronic health records of nearly 59 million patients. The diagnoses, procedures, vital signs, and laboratory results are linked to a unique deidentified patient ID. The deidentified patient ID is linked with a unique deidentified encounter ID. The variables available in the dataset are present as categorical (for diagnoses, procedures, and medication) or continuous (laboratory results and vital signs).
The data in TriNetX datasets are as follows:
-
•
primarily from health care organization (HCO)'s electronic medical record systems; and
-
•
collected for the primary purpose of providing care to patients.
The data in TriNetX datasets are not.
-
•
claims data, data primarily collected for the purposes of billing; or
-
•
data collected for the purposes of randomized clinical trials.
The majority of the HCOs are large academic medical institutions with both inpatient and outpatient facilities. Most of these HCOs are adult acute-care hospitals with multiple facilities and locations. All HCOs are currently located within the United States. The individual HCOs provide TriNetX with both inpatient and outpatient data. The data they provide is representative of the entire patient population at the HCO. Thus, the data from each HCO represent a combination of data from hospitals, urgent care centers, and primary care clinics. Most HCOs provide an average of seven years of historical data.
TriNetX typically receives data from HCOs and other data providers in 1 of 2 ways:
-
1.
TriNetX obtains data directly from an HCO's research repository (eg, i2b2) into the TriNetX environment.
-
2.
A HCO or data provider sends TriNetX data extracts in the form of CSV files.
TriNetX requires at least 1 nondemographic fact for a patient record for it to be counted in the database. The referential integrity is maintained by ensuring that all patient records are organized with a deidentified patient ID and an encounter ID, which are linked with the diagnosis codes, procedure codes, and medication codes. This allows all patient records from the different health care organizations to be stored in a standardized format that can be made available to researchers for use.
TriNetX maps the data to a standard and controlled set of clinical terminologies. The data are then transformed into a proprietary data schema. This transformation process includes an extensive data quality assessment that includes “data cleaning.” This process rejects records that do not meet the TriNetX quality standards of having the electronic medical record data in the proper format to be accepted as a valid patient record.
The database currently contributes to the National Institutes of Health-National Center for Advancing Translational Sciences' National COVID Cohort Collaborative,E1 which aims to combine the electronic health records of all US health care organizations. The TriNetX database has also partnered with the Food & Drug Administration's Office of Surveillance and EpidemiologyE2 and the Sentinel Operations Center, led by the Harvard Pilgrim Health Care Institute,E3 to provide real-world surveillance data for epidemiologic research. The work from this database has been cited in the Centers for Disease Control and Prevention policy guidelinesE4 regarding COVID-19 in children.
There are several HCOs, including the University of Alabama at Birmingham, all of which are in the United States, which contributed to the data in this study. Due to contractual data-use agreement limitations and to ensure patient privacy, the TriNetX Research Network cannot share all of the details of the individual participant health care organizations. Table E1 enlists the health care organizations that publicly acknowledge the partnership with TriNetX Research Network.
Table E1.
The health care organizations that publicly acknowledge the partnership with TriNetX Research Network
Table E2.
Diagnosis codes used in the study analyses
| Characteristic | CPT/ICD/LOINC codes |
|---|---|
| CABG | CPT: 1006207 (Combined arterial-venous grafting for coronary bypass) CPT: 1006199 (Venous grafting only for coronary artery bypass) CPT: 1006216 (Arterial grafting for coronary artery bypass) CPT: 0210-0213 (Coronary artery bypass) |
| COVID-19 diagnosis | U07.1 (ICD-10 COVID-19)—Lab confirmed COVID-19 94309-2 (LOINC: SARS-CoV-2 [COVID-19] RNA [Presence] in unspecified specimen by NAA with probe detection) [Positive] 94315-9 (SARS-related coronavirus E gene [Presence] in unspecified specimen by NAA with probe detection) [Positive] 94316-7 (SARS-CoV-2 (COVID-19) N gene [Presence] in unspecified specimen by NAA with probe detection) [Positive] 94500-6 (SARS-CoV-2 (COVID-19) RNA [Presence] in respiratory specimen by NAA with probe detection) [Positive] 94533-7 (SARS-CoV-2 (COVID-19) N gene [Presence] in respiratory specimen by NAA with probe detection) [Positive] 94534-5 (SARS-CoV-2 (COVID-19) RdRp gene [Presence] in respiratory specimen by NAA with probe detection) [Positive] 94502-2 (SARS-related coronavirus RNA [Presence] in respiratory specimen by NAA with probe detection) [Positive] 94559-2 (SARS-CoV-2 (COVID-19) ORF1ab region [Presence] in respiratory specimen by NAA with probe detection) [Positive] 41458-1 (SARS coronavirus RNA [Presence] in unspecified specimen by NAA with probe detection) [Positive] |
| Mortality | “Deceased” (known deceased documented) |
| Mechanical ventilation | 31500 (CPT: Intubation, endotracheal, emergency procedure) 5A1945Z (ICD-10: Respiratory ventilation, 24-96 consecutive hours) 5A1955Z (ICD-10: Respiratory ventilation, greater than 96 consecutive hours) 0BH17EZ (ICD-10: Insertion of endotracheal airway into trachea, via natural or artificial opening) 0BH18EZ (ICD-10: Insertion of endotracheal airway into trachea, via natural or artificial opening endoscopic) 1022227 (CPT: ECMO/ECLS provided by physician) 39.65 (ICD-9: Extracorporeal membrane oxygenation [ECMO]) |
| Stroke | ICD-10: I60-I69 |
| Acute kidney injury | ICD-10: N17 |
| ARDS | ICD-10: J80 |
| Alcohol abuse | ICD-10: F10 |
| Asthma | ICD-10: J45 |
| COPD | ICD-10: J44.9 |
| Chronic kidney disease | ICD-10: N18 |
| Diabetes mellitus | ICD-10: E08-E13 |
| Heart failure | ICD-10: I50 |
| Hypertensive disease | ICD-10: I10-I16 |
| Ischemic heart disease | ICD-10: I20-I25 |
| Nicotine dependence | ICD-10: F17 |
| Neoplasm | ICD-10: C00-D49 |
The individual codes chosen for defining the study population and for the definition of study outcomes are described in the table. CPT, Current Procedural Terminology; ICD, International Classification of Diseases; LOINC, Logical Observation Identifiers Names and Codes; CABG, coronary artery bypass grafting; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; NAA, nucleic acid amplification; ECMO, extracorporeal membrane oxygenation; ECLS, extracorporeal life support; ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease.
References
- 1.Head S.J., Milojevic M., Taggart D.P., Puskas J.D. Current practice of state-of-the-art surgical coronary revascularization. Circulation. 2017;136:1331–1345. doi: 10.1161/CIRCULATIONAHA.116.022572. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman T.J., Wilson M.A., Chiu S.T., Penny B.W., Chepuri V.B., Waggoner J.W., et al. Case rates, treatment approaches, and outcomes in acute myocardial infarction during the coronavirus disease 2019 pandemic. JAMA Cardiol. 2020;5:1–6. doi: 10.1001/jamacardio.2020.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon M.D., McNulty E.J., Rana J.S., Leong T.K., Lee C., Sung S.H., et al. The COVID-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 4.Mafham M.M., Spata E., Goldacre R., Gair D., Curnow P., Bray M., et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George I., Salna M., Kobsa S., Deroo S., Kriegel J., Blitzer D., et al. The rapid transformation of cardiac surgery practice in the coronavirus disease 2019 (COVID-19) pandemic: insights and clinical strategies from a center at the epicenter. J Thorac Cardiovasc Surg. 2020;160:937–947.e2. doi: 10.1016/j.jtcvs.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel V., Jimenez E., Cornwell L., Tran T., Paniagua D., Denktas A.E., et al. Cardiac surgery during the coronavirus disease 2019 pandemic: perioperative considerations and triage recommendations. J Am Heart Assoc. 2020;9:e017042. doi: 10.1161/JAHA.120.017042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobolev B.G., Fradet G., Hayden R., Kuramoto L., Levy A.R., FitzGerald M.J. Delay in admission for elective coronary-artery bypass grafting is associated with increased in-hospital mortality. BMC Health Serv Res. 2008;8:185. doi: 10.1186/1472-6963-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison S.L., Fazio-Eynullayeva E., Lane D.A., Underhill P., Lip G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17:e1003321. doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S., Khan A., Chowdhry M., Bilal M., Kochhar G.S., Clarke K. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a multicenter research network study. Gastroenterology. 2020;159:1575–1578.e4. doi: 10.1053/j.gastro.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S., Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159:768–771.e763. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkhouli M., Nanjundappa A., Annie F., Bates M.C., Bhatt D.L. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95:1613–1620. doi: 10.1016/j.mayocp.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turk M.A., Landes S.D., Formica M.K., Goss K.D. Intellectual and developmental disability and COVID-19 case-fatality trends: TriNetX analysis. Disabil Health J. 2020;13:100942. doi: 10.1016/j.dhjo.2020.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parcha V., Kalra R., Bhatt S.P., Berra L., Arora G., Arora P. Trends and geographic variation in acute respiratory failure and acute respiratory distress syndrome mortality in the United States. Chest. 2021;159:1460–1472. doi: 10.1016/j.chest.2020.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudino M., Chikwe J., Hameed I., Robinson N.B., Fremes S.E., Ruel M. Response of cardiac surgery units to COVID-19: an internationally-based quantitative survey. Circulation. 2020;142:300–302. doi: 10.1161/CIRCULATIONAHA.120.047865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salenger R., Etchill E.W., Ad N., Matthew T., Alejo D., Whitman G., et al. The surge after the surge: cardiac surgery post-COVID-19. Ann Thorac Surg. 2020;110:2020–2025. doi: 10.1016/j.athoracsur.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed Abdel Shafi A., Hewage S., Harky A. The impact of COVID-19 on the provision of cardiac surgical services. J Card Surg. 2020;35:1295–1297. doi: 10.1111/jocs.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chikwe J., Gaudino M., Hameed I., Robinson N.B., Bakaeen F.G., Menicanti L., et al. Committee recommendations for resuming cardiac surgery activity in the SARS-CoV-2 era: guidance from an international cardiac surgery consortium. Ann Thorac Surg. 2020;110:725–732. doi: 10.1016/j.athoracsur.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood D.A., Mahmud E., Thourani V.H., Sathananthan J., Virani A., Poppas A., et al. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: from the North American Society Leadership. J Am Coll Cardiol. 2020;75:3177–3183. doi: 10.1016/j.jacc.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haft J.W., Atluri P., Ailawadi G., Engelman D.T., Grant M.C., Hassan A., et al. Adult cardiac surgery during the COVID-19 pandemic: a tiered patient triage guidance statement. Ann Thorac Surg. 2020;110:697–700. doi: 10.1016/j.athoracsur.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafi A.M.A., Harky A. Consenting in cardiac surgery during COVID-19: are the risks quantifiable? J Card Surg. 2020;35:2883–2886. doi: 10.1111/jocs.14993. [DOI] [PubMed] [Google Scholar]
- 23.Engelman D.T., Lother S., George I., Funk D.J., Ailawadi G., Atluri P., et al. Adult cardiac surgery and the COVID-19 pandemic: aggressive infection mitigation strategies are necessary in the operating room and surgical recovery. Ann Thorac Surg. 2020;110:707–711. doi: 10.1016/j.athoracsur.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The European Society for Cardiology ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESCCOVID-19-Guidance Available at:
- 25.Kalra R., Patel N., Doshi R., Arora G., Arora P. Evaluation of the incidence of new-onset atrial fibrillation after aortic valve replacement. JAMA Intern Med. 2019;179:1122–1130. doi: 10.1001/jamainternmed.2019.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalra R., Parcha V., Patel N., Bhargava A., Li P., Arora G., et al. Implications of atrial fibrillation among patients with atherosclerotic cardiovascular disease undergoing noncardiac surgery. Am J Cardiol. 2020;125:1836–1844. doi: 10.1016/j.amjcard.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
E-References
- National Center for Advancing Translational Sciences National COVID Cohort Collaborative (N3C) https://covid.cd2h.org/N3C Available at:
- Food and Drug Administration FDA reporter TriNetX. https://fdareporter.com/organizations/646531868-trinetx Available at:
- Cision PR Newswire TriNetX signs agreement with FDA Sentinel Program. https://www.prnewswire.com/news-releases/trinetx-signs-agreement-with-fda-sentinel-program-301092609.html Available at:
- Centers for Disease Control and Prevention School decision-making tool for parents, caregivers, and guardians. https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/decision-tool.html Available at: