Abstract
The COVID-19 pandemic has been a source of ongoing challenges and presents an increased risk of illness in group environments, including jails, long-term care facilities, schools, and residential college campuses. Early reports that the SARS-CoV-2 virus was detectable in wastewater in advance of confirmed cases sparked widespread interest in wastewater-based epidemiology (WBE) as a tool for mitigation of COVID-19 outbreaks. One hypothesis was that wastewater surveillance might provide a cost-effective alternative to other more expensive approaches such as pooled and random testing of groups. In this paper, we report the outcomes of a wastewater surveillance pilot program at the University of North Carolina at Charlotte, a large urban university with a substantial population of students living in on-campus dormitories. Surveillance was conducted at the building level on a thrice-weekly schedule throughout the university's fall residential semester. In multiple cases, wastewater surveillance enabled the identification of asymptomatic COVID-19 cases that were not detected by other components of the campus monitoring program, which also included in-house contact tracing, symptomatic testing, scheduled testing of student athletes, and daily symptom reporting. In the context of all cluster events reported to the University community during the fall semester, wastewater-based testing events resulted in the identification of smaller clusters than were reported in other types of cluster events. Wastewater surveillance was able to detect single asymptomatic individuals in dorms with resident populations of 150–200. While the strategy described was developed for COVID-19, it is likely to be applicable to mitigation of future pandemics in universities and other group-living environments.
Keywords: Wastewater, Epidemiology, SARS-CoV-2, Mitigation
Graphical abstract
1. Introduction
Wastewater-based epidemiology (WBE) has been a successful approach to address public health issues such as viral (Hellmér et al., 2014; Más Lago et al., 2003; McCall et al., 2020) and bacterial (Yan et al., 2018) disease outbreaks and substance abuse (Duvallet et al., 2020), with many other potential uses proposed (Choi et al., 2018). WBE approaches are often used at the community level to monitor the overall abundance of a viral or chemical signal and to guide public health interventions. Early in the COVID-19 pandemic, it was hypothesized that wastewater surveillance could be a cost-effective alternative to high-frequency diagnostic testing of populations (Hart and Halden, 2020), and could be used to guide more immediate and targeted interventions including testing of affected building populations. Many universities, e.g. (Abundis, 2020; Betancourt et al., 2020; Buczyner, 2020; Richtel, 2020) piloted this approach as part of their pandemic reopening strategy in Fall 2020, including UNC Charlotte.
UNC Charlotte is using a four-pronged approach that included wastewater surveillance, symptomatic testing, daily health checks, and in-house contact tracing. Student athletes are also tested at a mandated frequency. While it is not our purpose in this paper to describe the operational details of the other components of the campus response, the information gathered via in-house testing, contact tracing, and surveys does play an important role in how the wastewater surveillance data is used within the institution.
In designing the building-level wastewater surveillance program at UNC Charlotte, we identified six key areas in which methodological choices needed to be made: 1) where to sample, 2) how often to sample, 3) what type of sample to collect, 4) what type of sample concentration protocol to use, 5) what kind of RNA extraction protocol to use, and 6) what type of detection method to use. With approximately eight weeks to purchase equipment and implement the program once funding was secured, we were required to make initial choices about deployment of equipment and protocols based on relatively limited information. We describe outcomes of the fall 2020 program here, along with optimizations to initial methodological choices that were made during the semester.
Wastewater surveillance has been implemented on residential buildings only. A positive wastewater sample may trigger a surge testing event, in which all students are asked to stay in place and a testing team is sent to test everyone in the building. However, wastewater information is first considered in conjunction with other evidence. If a wastewater positive occurs in the absence of previously identified infected persons or known close contacts, then the affected student population is required to remain in their dormitory and to participate in surge testing.
UNC Charlotte's combined approach has proven successful at sustaining a de-densified population of students in university residence halls. The university has maintained the ability to offer a mix of instruction including in-person courses, without resorting to repeated mass-testing of the entire campus population. Wastewater monitoring has successfully provided early warning of the spread of COVID-19 in individual dormitory buildings, in some cases detecting a single presymptomatic individual in a dormitory population of 150+, and allowing for their isolation prior to further spread of the virus.
2. Methods
2.1. Residential wastewater collection
Nineteen on-campus sites at UNC Charlotte were chosen to cover 17 dormitories as well as the University's Greek Village (Fig. 1 ). Samples from dormitory sites were collected three times each week during the period of Sep. 28, 2020–Nov. 23, 2020.
Fig. 1.
Residential areas of UNC Charlotte Main Campus monitored in this study. Residential buildings are marked in yellow. Left panel: north campus residential areas. Right panel: South Village residential area.
Campus wastewater could be readily accessed in two types of locations – inside buildings, directly from building plumbing cleanouts, or at external sites accessed via manholes. At external manhole sites, there was potential that wastewater could be significantly diluted by other building outflows, especially grey water from laundry rooms, showers, and other facilities. Early reports of detection of COVID-19 in wastewater (Medema et al., 2020; Peccia et al., 2020; Wu et al., 2020) were primarily based on settled influents at wastewater treatment plants, and less information was available about appropriate concentration methods for raw sewage from smaller sewersheds or individual buildings. We initially chose to avoid collection of sewage mixed with grey water, to avoid excessive dilution of input material. Initial collection sites (Phase I) were primarily internal building wastewater lines accessed via standard PVC caps, drilled and fitted with sealed barbed fittings on each side, to which a hand pump or an autosampler could be connected.
During the semester, after a detailed analysis of building blueprints and plumbing architecture was completed, collection sites were relocated to manhole access points (Phase II) to better aggregate wastewater from entire building populations. Autosamplers were anchored to concrete pads adjacent to the manhole sites to prevent theft or tampering, and collection tubes were anchored within the stream of wastewater flow using PVC guides.
ISCO GLS compact and Hach AS950 portable autosamplers are each loaded with sterilized and acid-washed PET screw-cap carboys in 1-gallon and 2.5-gallon volumes respectively. As many sites are located outdoors, sealed lead-acid AGM batteries are used as power sources for each sampler and recharged daily in between collection events. Vinyl suction tubing of 3/8″ inner diameter installed with low-flow stainless steel pickup strainers (ISCO) was used for all units at varying lengths of 15–25 ft depending on the depth required of the access points. Manhole locations allowed for direct visual placement of the weighted steel strainer within the flowing wastewater stream, and the additional capability of semi-permanently anchoring the strainer against the flow to maximize collection with 1-inch PVC pipe placed within the manhole. Cleanout pipes were of much smaller diameter, requiring the tapping of removable PVC access caps with barbed coupling fittings or bulkhead fittings to allow tubing attachment and sample flow through the cap. The anti-clog pickup holes located on the upper length of the strainer were blocked off with heat shrink tubing to allow suction from the single bottom orifice. This was necessary as cleanout pipes do not contain stagnant water or continuous wastewater flow; therefore the strainer is never submerged and must be placed vertically within the stack, resting the end of the strainer against the bottom of the pipe (Fig. 2 ). Composite sampling is set to collect over a 24-hour period, with individual 20 mL pulls intervaled at 10–30 min depending on the installation site type (manhole or cleanout).
Fig. 2.
Autosampler setup for surface manholes and cleanout cap locations.
2.2. Sample concentration and RNA extraction
Wastewater samples were concentrated using electronegative filtration following the method in (Ahmed et al., 2020; Haramoto et al., 2009) and as described in Ciesielski et al. (2021) with a slight modification. A 40 mL aliquot was taken from each sample, and the pH of the aliquot adjusted to 3.5–4.0 using concentrated HCl, followed by the addition of 100× MgCl2 hexahydrate (2.5 M) at a ratio of 1:100. As a process control, a known concentration of Bovine Coronavirus modified live vaccine (16445-3 Bovilis Coronavirus Calf Vaccine) was spiked into the composite sample at a ratio of 1:10000. Then the wastewater sample was passed through a 0.45 μm pore size, 47 mm diameter, electronegative filter (HA, Millipore) via a disposable filter funnel (Pall Corporation) using a vacuum filtration manifold. 40 mL of PBS buffer was subjected to the same treatment as a filtration method control. Filters were resuspended and/or stored in 1 mL of AVL lysis buffer with carrier RNA (Qiagen), vigorously vortexed and incubated for 10 min prior to RNA extraction. In cases where filters cannot be processed immediately, they were stored at −80 °C.
RNA was then extracted from a 200 μL aliquot of the resuspended filtrate using the QIAamp viral mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. RNA was eluted with 60 μL of AVE buffer. For infrequent cases where insufficient sample volume was collected for processing using the HA filtration/extraction approach, instead a direct extraction method, in which 1 mL of wastewater sample was mixed with 1 mL of AVL lysis buffer and 200 μL aliquots of the resulting mixture were then used in the extraction process.
2.3. Molecular detection of SARS-CoV-2
One-step quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was used to quantify SARS-CoV-2 RNA using the CDC N1 (Nucleocapsid) primer and probe set, 2019-nCoV CDC RUO Kit (Integrated DNA Technologies IDT, #10006713). All amplification reactions were carried out in a 20 μL reaction volume. The SARS-CoV-2 assay reaction includes 10 μL iTaq universal probes reaction mix (Bio-Rad laboratories, Hercules, CA), 0.5 μL iScript reverse transcriptase (Bio-Rad laboratories, Hercules, CA), 500 nM primers (IDT), 125 nM probe (IDT), and 5.0 μL of the extracted RNA template. Amplification and quantification were performed using a CFX96 qPCR thermocycler (Bio-Rad laboratories, Hercules, CA) following the thermal condition as: 25 °C for 2 min for initiation, reverse transcription at 50 °C for 15 min, and polymerase activation at 95ᴼC for 2 min followed by running 45 cycles at 95 °C for 3 s and 55 °C for 30 s (CDC qRT-PCR panel 2020). Control plasmid containing the complete nucleocapsid gene from 2019-nCoV (IDT, #10006625) and nuclease free water are used as positive and negative controls, respectively. All samples were run in triplicate, and for each sampling event a series of three positive and negative controls were included in the plate. For genome copy quantification, a standard curve was generated initially with plasmid based positive control from IDT, however, we later shifted to RNA based SARS-CoV-2 positive control (Ref-102860) from Twist Bioscience, CA, USA. A series of tenfold serial dilutions of the positive control, with concentrations ranging from 100,000 to 10 copies per reaction was used to generate a best fit curve with an R2 value of 0.998 (Supplementary Fig. 1). Calculated primer efficiency under these conditions met the standard requirements (Bustin et al., 2009).
The limit of detection (LoD) and limit of quantification (LoQ) of the assay and method was determined following previously described methods (Betancourt et al., 2021; Francy et al., 2012). The assay LoD was calculated by running an extended dilution series of RNA based positive control up to 1 copy/reaction in 6 replicates. The lowest concentration at which all the replicates were positive was considered as the LoD of the assay, converted to copies/L and considering the sample volume processed to obtain the LoD of the method. The LoQ, expressed as the minimum viral copies that can be quantified accurately, was determined by first calculating the Cq for LoQ (Cq LoQ) using the following equation: Cq LoQ = Cq LoD − 2(σCq LoD) where Cq LoD is the average Cq reported for LoD and σCqLoD is the standard deviation of the CqLoD. Then the CqLoQ is used to calculate copies/L using the standard curve for the sample volume processed.
A summary of the LoD and LoQ of the assays and the method applied is included in the supplementary file (Supplementary Table 1, Supplementary Fig. 1). However, there is still a possibility of detecting a RT-qPCR positive signal even if a small amount of template RNA is present (Ahmed et al., 2021). This is critical especially for building-level surveillance when the number of residents is small. For quality control, both the extraction blank and reagent blank are also amplified to identify any carryover contamination. RNA extraction and RT-qPCR analysis are performed in separate rooms to prevent cross-contamination. Environmental samples often have inhibitors that can inhibit the amplification reaction. Inhibition was detected by running the diluted RNA template along with the main sample to observe the effect on Cq value (Peccia et al., 2020). Bovine coronavirus is quantified according to the published protocol by Decaro et al. (2008). Primer and probe sequences are shown in Supplementary Table 2. The BCoV RT- qPCR assay contains 10 μL iTaq universal probes reaction mix (Bio-Rad), 0.5 μL iScript reverse transcriptase (Bio-Rad), 600 nM forward primer, 600 nM reverse primer, 200 nM probe, and 5.0 μL extracted RNA. All protocols used in the project are also described and regularly updated in public protocols at https://www.protocols.io/workspaces/unc-charlotte-covid-wbe.
3. Results and discussion
3.1. Reporting cycle and laboratory timeline
Using the protocols described in Methods, the wastewater surveillance laboratory is able to provide a readout of positive wastewater signal for campus sites within 26–30 h of sample collection (Fig. 3 ). The laboratory runs three weekly cycles beginning with the setup of autosamplers and initiation of a 24 h composite sampling protocol on Day 1. In the morning of Day 2, samples are collected and returned to the laboratory for processing. The major processing steps include an HA (electronegative) filtration process, which is the most time consuming of the processing protocols, followed by RNA extraction from the collected filter. On the morning of Day 3, qPCR detection is set up and performed, and the laboratory reports to the university administration with a target deadline of 2 pm approximately 30 h following sample collection. This allows sufficient time for consultation with the testing center and contact tracing groups before the end of the business day. University stakeholders can determine that a dormitory showing positive signal will be “locked down” overnight as early as 36 h after sample collection, and students tested beginning the following morning.
Fig. 3.
Weekly sample collection and processing timeline for the UNC Charlotte wastewater surveillance project.
3.2. Prevalence of wastewater positives during fall 2020
During the period from September 28, 2020 to November 23, 2020, the UNC Charlotte wastewater surveillance team collected and processed 332 out of an expected 475 wastewater samples from 19 building sites. Failure to collect samples generally occurred due to low flow at collection sites or problems with placement of autosampler intakes, and these issues were gradually corrected over the course of the semester. For a sample to be considered positive, SARS-CoV-2 must have been detected in all three sample replicates with mean Cq below 45. Of the samples collected, 40 were classified as positives, and 15 others were labeled suspicious due to positive signal appearing in only two qPCR replicates. In some cases these samples were reanalyzed and treated as positives if they were found to be reproducible. 16 of these positives were “new” positives, defined as the first positive wastewater test in a given building in greater than 10 days. 8 of these resulted in a wastewater surge test. 13 samples were “expected” positives due to the sampling of the isolation or quarantine buildings. Fig. 4 summarizes the sample collection and testing results, and detailed results are provided in Supplementary File 1.
Fig. 4.
Overview of sample collection and detection outcomes over the 8-week on-campus session.
Over the course of the 8 week in-person semester, the number of observed positive or suspicious wastewater samples at each sampling event gradually increased. From a purely procedural point of view, this can be partly explained by improvement in our ability to collect samples at some sites by relocation or reconstruction of the site. But within the larger context of a gradual increase in positivity rates in Mecklenburg County, NC, the increase in cases on campus was also to be expected. The number of new daily positive cases in Mecklenburg County as a whole (“COVID-19 Data Releases”, n.d.) and the number of buildings detected as positive on each sampling event are correlated over this time frame (Fig. 5 ).
Fig. 5.
Total number of SARS-CoV-2 positive wastewater samples from UNCC dormitories (red, right axis) and daily new positive cases in Mecklenburg county (blue, left axis) from September 28 to November 23, 2020. Curves are fit to the data using Loess Smoothing. Pearson correlation coefficient between the two series is 0.769.
3.3. Wastewater-triggered surge testing events and outcomes
On September 24 and 25, students began arriving on campus. North Carolina had experienced a consistent level of coronavirus infection all summer without a dramatic spike in case numbers (Fowler and Jasper, n.d.). September 30, our second day of sampling, brought the first of many wastewater positives, while the campus as a whole was still largely free of known COVID-19 cases. This event gave the university an opportunity to shape its strategy for responding to future events (Fig. 6 ).
Fig. 6.
Timeline of campus actions after the first positive wastewater event on the UNC Charlotte campus, Sept. 30, 2020.
In mid-October, a cluster was detected via contact tracing, in the absence of any wastewater signal. This alerted us to the need to relocate some of our initial sampling sites to provide more complete coverage of large buildings. Despite early concerns about dilution, samples obtained at manhole sites have proven to be easily handled with our standard laboratory protocols and to be sufficiently concentrated to allow for successful detection of presymptomatic COVID-19 cases.
As the semester progressed, wastewater-triggered events became more frequent, both due to improved ability to collect samples and to increasing prevalence of COVID-19 in the community as a whole. In the week leading up to student move-out prior to the Thanksgiving holiday, four buildings had to be surge tested consecutively. Although reporting windows for the closely clustered dates in November overlapped, no building was surge tested twice during that time period. All subsequent cases were able to be connected by ordinary contact tracing and were not considered an independent detection event.
On only one occasion during this timeframe did a wastewater-triggered surge testing effort fail to identify any positive case. On November 4, three buildings (smaller buildings sampled as a pool at one manhole) were selected to be tested based on an ongoing positive wastewater signal. However, only 70% of students identified as living in those buildings complied with the testing request, and the source of the positive signal was not identified.
Table 1 shows outcomes of the eight wastewater-triggered surge tests for COVID-19 at UNC Charlotte during the in-person portion of fall semester 2020, Sept. 28–Nov. 23. The table reflects that on Nov. 18, the university reported the results of three separate surge tests at the same time with two of the buildings grouped as a cluster of five cases based on contact evidence. While the current analysis is based on the public record, we are working to secure IRB approval for access to the underlying testing and building occupancy data to improve accuracy of models developed using these data.
Table 1.
Outcomes of wastewater-triggered surge tests during fall 2020.
| NinerNotice date | Positive WW sample collected | Site ID | Site Cq | Percent tested | Positivity rate reported | Number cases |
|---|---|---|---|---|---|---|
| Oct. 2 | Sept. 30 | Building 4 | 42.3 | 96% | <1% | 1 |
| Oct. 28 | Oct. 26 | Building 13 | 39.0 | 95% | <1% | 1 |
| Nov. 4 | Nov. 2 | Group 3 | 35.8 | 70% | 0% | 0 |
| Nov. 11 | Nov. 9 | Building 7 | 33.8 | 85% | <1% | 3 |
| Nov. 16 | Nov. 13 | Building 14 | 36.2 | 82% | <2% | 2 |
| Nov. 18 | Nov. 13, | Building 1, | 35.7, | 86%, | <1%, | 1 |
| Nov. 16, | Building 4, | 30.6, | 77%, | <3%, | 3 or 2 | |
| Nov, 16, | Building 10 | 31.6 | 92% | <3% | 2 or 3 |
Reporting of positives on campus can be stratified as either WBE identified or identified via other means. Clusters detected in surge testing events triggered by wastewater surveillance were consistently smaller in size than clusters detected by other methods (Fig. 7 ). While this is based on only a small amount of publicly available data (n = 8 for both wastewater surge tests and non-wastewater clusters) for fall semester, it is an encouraging trend in that context. A t-test between the two groups finds that the difference is significant with a p-value of 0.000546. In addition to detecting presymptomatic cases, the wastewater program has been able to provide corroborative evidence for the existence of cases suggested by contact tracing, and resumption of negative wastewater signal in a building has also been interpreted as an indication that an outbreak in a building has been successfully contained.
Fig. 7.
Number of positive cases detected in wastewater-triggered surge testing events vs. number of cases in other reported campus clusters during the fall semester (Sept. 1-Nov. 23, 2020). Numbers are extracted from publicly available reports (Niner Notices, (“Latest NinerNotices,” n.d.)) made available to the University community and do not include students who reported late for required surge testing or cases that evaded surge testing.
Since the initial submission of this manuscript, several new publications or preprints describing university campus wastewater testing programs have become available. In an overview of WBE program operations at multiple universities (Harris-Lovett et al., 2021), the authors identify challenges similar to those we describe above. University of Arizona (Betancourt et al., 2021), Kenyon College (Barich and Slonczewski, 2021), and Hope College (Travis et al., 2021) have recently reported on WBE use case studies from their institutions. However, few of these initial reports have as yet included underlying data to provide a basis for comparison. As other efforts and their methods and accompanying data are reported, it will become easier to compare and assess consistency of results across institutions.
3.4. Future directions for campus wastewater surveillance
With the experience of Fall 2020 to build on, in Spring 2021, we have been able to shorten the wastewater processing cycle to provide same-day results. We have implemented surveillance sequencing as part of the UNC Charlotte COVID-19 monitoring program, and are sequencing banked wastewater and clinical samples as well as those collected throughout the spring. To inform sewershed modeling and provide a means of monitoring our non-resident population, we recently selected and added a group of “neighborhood” wastewater collection sites that capture larger areas of the campus. We have also added sampling sites at several non-residential buildings that are known to have experienced relatively high student traffic during the fall semester, such as the library, the student union, and the university recreation center. While data from these sites will not be directly actionable, in that the university administration can not specifically identify and test everyone who may have passed through those buildings, the neighborhood sites and high-traffic buildings will provide a better understanding of background levels of SARS-CoV-2 on the campus as a whole.
While we are generally satisfied with the performance of our extraction and detection steps for routine sample processing, continuing optimization of detection protocols is also a priority for the project. While the use of N1 as the sole indicator of the presence of SARS-CoV-2 is supported by the literature, the BioRad CFX96 platform permits multiplexing of N1 and N2 primer/probe sets, or multiplexing with components of other probe sets in use worldwide. As we look to generalize the building wastewater monitoring strategy to other scenarios, it is also of interest to multiplex SARS-CoV-2 primer/probe sets with process controls and with diagnostic primer/probe sets for other infectious diseases. Availability of low-cost sequencing also holds the promise of identifying individual strains which are spreading in the campus environment, and in connecting the history of wastewater positives with specific patient tests.
With widespread vaccination on the horizon in the U.S., the future of a COVID-19 specific wastewater monitoring program is likely not long-term, but its lifespan depends on how quickly vaccines are rolled out to the general population and adopted. National policy leaders have recently gone on record with statements (Kaplan, 2020) that vaccination rates above 75–80% will ultimately be needed to bring the COVID-19 pandemic to an end. It is predicted that even with gradual introduction of vaccines, some types of reopening policies may result in continued waves of outbreaks (Shayak and Sharma, 2020), and that vaccine hesitancy (Dror et al., 2020) may be a significant challenge in achieving sufficient vaccine coverage in the short term. As students and employees are vaccinated, it will be of great interest to follow the wastewater and other evidence streams on campus to detect when vaccines begin making an impact on spread, and at what point a resumption of in-person operations at normal density is safe. Another factor in the timeline of the COVID-19 pandemic is the emergence of new strains such as B.1.1.7 (“Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations”, 2020), their ability to evade antibodies elicited by current vaccines, and the ability of labs to adapt to detection and monitoring of multiple strains. However, the lessons learned from COVID-19 are applicable to monitoring of other pandemic diseases and potentially also to management of recurring seasonal outbreaks such as influenza, which may not be as urgently threatening as COVID-19, but still impact community health and operations.
4. Conclusions
In fall of 2020, UNC Charlotte implemented a wastewater testing program that provided actionable results for University administrators and quickly identified asymptomatic students in dormitories before infection spread.
Sampling entire buildings from exterior manholes provided the best coverage of the campus, and dilution by other sources did not negatively impact our ability to identify individual COVID-19 cases within building populations. When it is possible to use a single site to sample an entire building, this is adequate for the purposes of the program at current occupancy levels.
A thrice-weekly sampling met the University's monitoring needs, and both the wastewater surveillance team and the larger Testing, Tracing and Monitoring Task Force agreed that there is no apparent need to test wastewater at a higher frequency. However, shortening the time between the start of autosampling and reporting of results is considered a priority. Currently, autosamplers are started at approximately 8 a.m. on day one of the lab's three-day working cycle, and results are reported to the administration by 2 p.m. on day three. Surge testing of students requires preparation of personnel and materials, so that the earliest a round of testing will start after a positive report is generally the following morning. This can result in a positive signal from one case overlapping into a second and even third cycle of sampling, depending on how long it takes to test the affected areas and move infected students to isolation. There are two “pain points” in the current protocol that have the potential to shorten this timeframe – first, the length of the autosampling window, and second, the use of the well-validated but time-consuming HA filtration process for the concentration step. Optimizations for both of these steps are in progress.
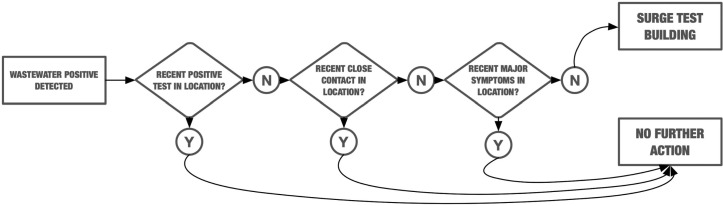
Finally, the university's mix of testing, self-reporting, contact tracing and wastewater sampling has created a wealth of potentially interesting metadata. Once remaining issues of student privacy and data access are resolved, these data will support modeling of the relationship of wastewater signal to COVID-19 prevalence on campus and in the surrounding region. Integration of wastewater results with building-level aggregate reporting of positive tests, close contacts and voluntary symptom reports will enable development of a wastewater dashboard for internal administrative use. We will test analytic approaches to determine if the implied decision tree used by administrators in choosing when to surge test can automatically make the same recommendations, and if not, what an accurate formal representation of that decision process would be (Fig. 8 ).
Fig. 8.
Decision tree representation of the process used by UNC Charlotte administrative group when deciding whether or not to follow up positive wastewater signal with surge testing.
In combination with these other information sources, wastewater surveillance proved to be sufficiently sensitive to detect cases before they seeded clusters, and was very cost-effective. During fall semester, our monitoring program tested 332 samples; if every collection had gone perfectly that total would have been 475. On the other hand, had the university chosen to surveil by testing the 3000-plus students in those buildings three times weekly, that would have amounted to over 9000 tests per week. It is important for those considering implementing wastewater surveillance on college campuses and in other group living situations to consider the context in which positive pathogen signals in wastewater will be evaluated, and especially how wastewater information will be combined with other information to decide when a positive result is actionable. Without insights from symptomatic testing, contact tracing and voluntary symptom reporting, positive wastewater samples would likely have triggered many more costly surge testing events at UNC Charlotte. As part of a comprehensive approach to pandemic mitigation, wastewater surveillance is a valuable tool for identifying undetected new or presymptomatic COVID-19 cases in dormitories. While time will tell whether the WBE approach is as useful in future pandemic scenarios with different viral transmission patterns, it is clear that this approach is worth refining. Development of wastewater surveillance infrastructure plans, analytics and decision-support tools for organizations using WBE to protect residents and workers will improve readiness for the next pandemic.
The following are the supplementary data related to this article.
Supplementary File 1: Wastewater monitoring laboratory qPCR report of complete data underlying Figure 4.
Supplementary information describing the qPCR protocol.
CRediT authorship contribution statement
Cynthia Gibas: Conceptualization, Project administration, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. Kevin Lambirth: Conceptualization, Investigation, Methodology, Writing – original draft. Neha Mittal: Investigation, Data curation, Supervision, Formal analysis, Methodology, Writing – original draft. Md Ariful Islam Juel: Investigation, Data curation, Methodology, Writing – original draft. Visva Bharati Barua: Investigation, Data curation, Supervision, Methodology, Writing – original draft. Lauren Roppolo Brazell: Investigation. Keshawn Hinton: Investigation. Jordan Lontai: Investigation. Nicholas Stark: Investigation. Isaiah Young: Investigation. Cristine Quach: Investigation. Morgan Russ: Investigation. Jacob Kauer: Investigation. Bridgette Nicolosi: Investigation. Don Chen: Investigation, Formal analysis. Srinivas Akella: Formal analysis, Writing – review & editing. Wenwu Tang: Software, Data curation, Writing – review & editing. Jessica Schlueter: Project administration, Methodology, Resources, Writing – review & editing. Mariya Munir: Conceptualization, Project administration, Funding acquisition, Resources, Methodology, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The UNC Charlotte wastewater group would like to thank Chancellor Sharon Gaber, Provost Joan Lorden, and Richard Tankersley, Vice Chancellor for Research and Economic Development and his team for strong institutional support of this project, Lawrence Mays, Chair of the Department of Bioinformatics and Genomics, and John Daniels, Chair of the Department of Civil and Environmental Engineering, for facilitating setup of the project in their research spaces, Rachel Noble and colleagues in the North Carolina Policy Collaboratory for early research into wastewater sample processing protocols that was leveraged in this project, Greg Cole and his team in Facilities Management for construction and plumbing support, Jennifer Gibas for the graphical abstract, and the Testing, Tracing and Monitoring Task Force (Robert Jones, Medical Director, Keith Carnes, Angelica Martins, Emily Stewart, Patrick Versace, and Susan Messina) for ongoing discussion of the institutional COVID-19 management strategy at UNC Charlotte.
Editor: Jay Gan
References
- Abundis, M., 2020. New Mexico Universities Use Wastewater Samples to Detect COVID-19 Early [WWW Document]. KOB. URL https://www.kob.com/albuquerque-news/new-mexico-universities-use-wastewater-samples-to-detect-covid-19-early/5921168/ (accessed 12.22.20).
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Tscharke B., Bertsch P.M., Bibby K., Bivins A., Choi P., Clarke L., Dwyer J., Edson J., Nguyen T.M.H., O’Brien J.W., Simpson S.L., Sherman P., Thomas K.V., Verhagen R., Zaugg J., Mueller J.F. SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: a temporal case study. Sci. Total Environ. 2021;761:144216. doi: 10.1016/j.scitotenv.2020.144216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barich, D., Slonczewski, J.L., 2021. Wastewater virus detection complements clinical COVID-19 testing to limit spread of infection at Kenyon college. bioRxiv. doi: 10.1101/2021.01.09.21249505. [DOI]
- Betancourt, W.W., Schmitz, B.W., Innes, G.K., Pogreba Brown, K.M., Prasek, S.M., Stark, E.R., Foster, A.R., Sprissler, R.S., Harris, D.T., Sherchan, S.P., Gerba, C.P., Pepper, I.L., 2020. Wastewater-based epidemiology for averting COVID-19 outbreaks on the University of Arizona campus. bioRxiv. doi: 10.1101/2020.11.13.20231340. [DOI]
- Betancourt W.Q., Schmitz B.W., Innes G.K., Prasek S.M., Pogreba Brown K.M., Stark E.R., Foster A.R., Sprissler R.S., Harris D.T., Sherchan S.P., Gerba C.P., Pepper I.L. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total Environ. 2021;779:146408. doi: 10.1016/j.scitotenv.2021.146408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczyner, B.M., 2020. Florida Atlantic University researchers testing wastewater for signs of COVID-19 [WWW document]. URL https://www.wptv.com/coronavirus/florida-atlantic-university-researchers-testing-wastewater-for-signs-of-covid-19 (accessed 12.22.20).
- Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., Vandesompele J., Wittwer C.T. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Choi P.M., Tscharke B.J., Donner E., O’Brien J.W., Grant S.C., Kaserzon S.L., Mackie R., O’Malley E., Crosbie N.D., Thomas K.V., Mueller J.F. Wastewater-based epidemiology biomarkers: past, present and future. Trends Analyt. Chem. 2018;105:453–469. [Google Scholar]
- Ciesielski M., Clerkin T., Blackwood A.D., Gonzalez R., Larson A., Thompson H., Noble R.T. Assessing sensitivity and reproducibility of two molecular workflows for the detection of SARS-CoV-2 in wastewater. J. Virol. Methods. 2021 doi: 10.1016/j.jviromet.2021.114230. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Data Releases [WWW Document], n.d. URL https://www.mecknc.gov/news/Pages/COVID-19-Data-Dashboard.aspx (accessed 12.22.20).
- Decaro N., Elia G., Campolo M., Desario C., Mari V., Radogna A., Colaianni M.L., Cirone F., Tempesta M., Buonavoglia C. Detection of bovine coronavirus using a TaqMan-based real-time RT-PCR assay. J. Virol. Methods. 2008;151:167–171. doi: 10.1016/j.jviromet.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror A.A., Eisenbach N., Taiber S., Morozov N.G., Mizrachi M., Zigron A., Srouji S., Sela E. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35:775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvallet C., Hayes B.D., Erickson T.B., Chai P.R., Matus M. Mapping community opioid exposure through wastewater-based epidemiology as a means to engage pharmacies in harm reduction efforts. Prev. Chronic Dis. 2020;17 doi: 10.5888/pcd17.200053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, H., Jasper, S., n.d. Coronavirus live updates: Here’s what to know in North Carolina on Aug. 28. Charlotte Observer.
- Francy D.S., Stelzer E.A., Bushon R.N., Brady A.M.G., Williston A.G., Riddell K.R., Borchardt M.A., Spencer S.K., Gellner T.M. Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal wastewaters. Water Res. 2012;46:4164–4178. doi: 10.1016/j.watres.2012.04.044. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Katayama H., Ito T., Ohgaki S. Development of virus concentration methods for detection of koi herpesvirus in water. J. Fish Dis. 2009;32:297–300. doi: 10.1111/j.1365-2761.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- Harris-Lovett, S., Nelson, K., Beamer, P., Bischel, H.N., Bivins, A., Bruder, A., Butler, C., Camenisch, T.D., Long, S.K.D., Karthikeyan, S., Larsen, D.A., Meierdiercks, K., Mouser, P., Pagsuyoin, S., Prasek, S., Radniecki, T.S., Ram, J.L., Roper, D.K., Safford, H., Sherchan, S.P., Shuster, W., Stalder, T., Wheeler, R.T., Korfmacher, K.S., 2021. Wastewater surveillance for SARS-CoV-2 on college campuses: Initial efforts, lessons learned and research needs. medRxiv. doi: 10.1101/2021.02.01.21250952. [DOI] [PMC free article] [PubMed]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80:6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, T., 2020. How many people need to get COVID-19 vaccine to control the pandemic? [WWW Document]. Yahoo Finance. URL https://finance.yahoo.com/news/many-people-covid-19-vaccine-170519346.html?guccounter=1&guce_referrer=aHR0cHM6Ly9kdWNrZHVja2dvLmNvbS8&guce_referrer_sig=AQAAADPbpSEJ04eI6PwR9icw-JKV13LY5q611PPIEuvgKI8En92Q9FQWF3BuduKZKweEcUTkvMqG0loYnrX0feqlh2OeGIL0x7DFWAQPIQibN-M1yF3IFT8-zvaVQCcQLKeab-q1Iabhb2EfM4Qn_xrfgu7BWkhYn21os4d6Cjz5vlCO (accessed 12.23.20).
- Más Lago P., Gary H.E., Jr., Pérez L.S., Cáceres V., Olivera J.B., Puentes R.P., Corredor M.B., Jímenez P., Pallansch M.A., Cruz R.G. Poliovirus detection in wastewater and stools following an immunization campaign in Havana. Cuba. Int. J. Epidemiol. 2003;32:772–777. doi: 10.1093/ije/dyg185. [DOI] [PubMed] [Google Scholar]
- McCall C., Wu H., Miyani B., Xagoraraki I. Identification of multiple potential viral diseases in a large urban center using wastewater surveillance. Water Res. 2020;184:116160. doi: 10.1016/j.watres.2020.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38:1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations [WWW Document], 2020. URL https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563?fbclid=IwAR0iMoSdo3lk1ZRTHgeMSffj5T1eD4stkPyzKlMgqbi3YZtTVIGkQN_A5mQ (accessed 12.23.20).
- Richtel M. Apps; The New York Times: 2020. Looking to Reopen, Colleges Become Labs for Coronavirus Tests and Tracking. [Google Scholar]
- Shayak, B., Sharma, M.M., 2020. COVID-19 spreading dynamics with vaccination – allocation strategy, return to normalcy and vaccine hesitancy. bioRxiv. doi: 10.1101/2020.12.10.20247049. [DOI]
- Travis, S.A., Best, A.A., Bochniak, K.S., Dunteman, N.D., Fellinger, J., Folkert, P.D., Koberna, T., Kopek, B.G., Krueger, B.P., Pestun, J., Pikaart, M.J., Sabo, C., Schuitema, A.J., 2021. Providing a safe, in-person, residential college experience during the COVID-19 pandemic. bioRxiv. doi: 10.1101/2021.03.02.21252746. [DOI] [PMC free article] [PubMed]
- Wu, F.Q., Xiao, A., Zhang, J.B., Gu, X.Q., Lee, W.L., Kauffman, K., Hanage, W.P., Matus, M., Ghaeli, N., Endo, N., Duvallet, C., Moniz, K., Erickson, T.B., Chai, P.R., Thompson, J., Alm, E.J., 2020. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. bioRxiv. doi: 10.1101/2020.04.05.20051540. [DOI] [PMC free article] [PubMed]
- Yan T., O’Brien P., Shelton J.M., Whelen A.C., Pagaling E. Municipal wastewater as a microbial surveillance platform for enteric diseases: a case study for Salmonella and Salmonellosis. Environ. Sci. Technol. 2018;52:4869–4877. doi: 10.1021/acs.est.8b00163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1: Wastewater monitoring laboratory qPCR report of complete data underlying Figure 4.
Supplementary information describing the qPCR protocol.