Abstract
Urbanization, pollution and the modification of natural landscapes are characteristics of modern society, where the change in human relations with the environment and the impact on biodiversity are environmental determinants that affect the health-disease relationship. The skin is an organ that has a strong interface with the environment and, therefore, the prevalence patterns of dermatoses may reflect these environmental changes. In this article, aspects related to deforestation, fires, urbanization, large-scale agriculture, extensive livestock farming, pollution and climatic changes are discussed regarding their influence on the epidemiology of skin diseases. It is important that dermatologists be aware of their social responsibility in order to promote sustainable practices in their community, in addition to identifying the impacts of environmental imbalances on different dermatoses, which is essential for the prevention and treatment of these diseases.
Keywords: Climatic variations, Environment, Pollution, Radiation, Sustainability, Urbanization
Introduction
Since its inception around 100-200 thousand years ago, the history of Homo sapiens has comprised a strong interaction with the environment, especially from migrations outside Africa, where climatic, geographical and vegetation contingencies imposed adaptive pressures that resulted in much of the diversity of the species.1 Without adapting to the different environmental challenges, humankind would not have occupied the entire planet.
Therefore, environmental determinants have influenced both the evolution of the species and the health of humans.2 For instance, the most accepted hypothesis for the differentiation of skin tones is due to the evolutionary gain resulting from the skin synthesis of vitamin D by UVB radiation and the folate photolysis by UVA radiation, selecting lighter skin colors in low-latitude regions.3, 4, 5 Geographic isolation, associated with genetic drift and sexual selection, were also important in defining the characteristics of major human groups.6 However, this genotypic variability has implicated not only in different phenotypes, but also in the development of different physiological responses, leading to disease propensities, immunologic, metabolic and therapeutic responses.7, 8, 9, 10, 11, 12, 13, 14
While the Homo sapiens species evolved, it intensively interacted with the environment, interfering in the health-disease relationship. Human hunter and gatherer groups (such as Pygmies and Amerindian groups) caused a discreet environmental impact, as they had a shorter life expectancy and were more exposed to environmental problems, such as accidents caused by animals, floods, infestations, zoonoses and dietary restrictions (e.g., long periods of drought).15
From the moment Homo sapiens acquired a certain domain of agriculture, fishing and domestication of animals, they began to settle into territories, establishing the first population centers. This required greater use of natural resources and modification of the local environment. Thus, there was a gain in longevity, protection against natural hazards and the possibility of territorial expansion.16
With the development of industrialization and changes in the means of production, there have been demographic explosions, urbanization and migratory flows to urban areas. These factors resulted in great environmental impact, causing air, soil and water pollution, in addition to the unsustainable consumption of natural resources.17, 18 From a medical point of view, the different forms of work have led to the emergence of occupational diseases and globalized transportation has disseminated infectious diseases, such as syphilis and AIDS, in addition to favoring pandemics such as the Black Plague, influenza and the disease caused by the new coronavirus (COVID-19).19, 20, 21, 22, 23, 24, 25
The historical condition of humankind reinforces its bilateral relationship with the environment, as well as establishes sociological and economic relations and determines specific health conditions. Considering the high degree of interaction between human skin and the external environment, Dermatology especially reflects the changes in the environment. The main impacts of environmental changes in the specialty will be discussed below.
Environmental degradation
Deforestation
Both urban and rural expansion modify natural landscapes, restrict native vegetation coverage, modify the geography, water and waste flow, with a direct impact on biodiversity.26, 27, 28, 29
Deforestation, pasture development, crops and underground resources exploration have been historically associated with the emergence of arboviruses, zoonoses and other infectious diseases that arise in outbreaks or endemically, depending on how the deforestation is carried out. As forests are reduced (or become unbalanced) and the reservoirs of certain diseases are extinct, humans become involved in their natural cycle.2, 29, 30, 31
Examples that emerged from this imbalance: malaria epidemics after the construction of the Panama Canal, mining and the construction of railroads in the northern region of Brazil; the yellow fever epidemic on the coast of northeastern Brazil during the 17th century sugarcane expansion and the rabies outbreak on the island of Marajó (2018), Brazil, after agricultural expansion.32, 33, 34, 35, 36 Likewise, the recent COVID-19 pandemic originated in an industrialized area in China (Wuhan), possibly caused by human interaction with contaminated bats as a result of restrictions in their ecosystem, which should alert humankind about the emergence of environmental issues as a priority in the sustainable development of modern society.25, 37, 38
In Dermatology, American tegumentary leishmaniasis (ATL) is caused by protozoa of the genus Leishmania, which causes skin ulcers (Fig. 1) and can affect mucous membranes in later stages. It is transmitted by mosquitoes of the genus Phlebotomus, and has a zoonotic cycle in mammals, especially marsupials and rodents.39, 40, 41 In Brazil, the description of ATL was first reported during the construction of the Northwest Railway, in the beginning of the 20th century. This railroad was designed to transport the coffee production from the countryside of the Brazilian states of São Paulo and Mato Grosso. This entire area has large populations of sandflies, and the disease received the name of “Bauru ulcer” because Bauru is an important city in that region.36
Figure 1.

American cutaneous leishmaniasis. Facial ulcer, with an erythematous, infiltrated border and granular bottom; in a farmer from the Tietê river valley.
The incidence of ATL has been increasing in the last 30 years in practically all states of Brazil, with outbreaks being described in the Southeast, Midwest, Northeast and Amazon regions. Most cases of ATL are associated with the predatory process of colonization, road construction, new population centers and expansion of agricultural activities.42, 43
Deforestation can cause the migration of infectious agents into vectors in urban areas, favoring epidemics. This has been observed with yellow fever, previously transmitted by the Haemagogus mosquito in wild areas, which showed phases of intense transmission in the cities by the Aedes aegypti.44 Similarly, Aedes albopictus, the other disseminator of viral diseases originally from Asia and Africa, was recently introduced in Brazil and is also a vector of dengue fever, zika and chikungunya.45, 46 Deforestation and the depletion of natural reservoirs is the main explanation for the occurrence of autochthonous cases of ATL in metropolitan areas, Chagas disease and (human) rabies caused by attacks of hematophagous bats in cities.47, 48, 49, 50, 51, 52, 53, 54
The “fogo selvagem” subtype of pemphigus foliaceus differs from the type described by Cazenave in that it affects younger patients (under 45 years old) and has an endemic characteristic (between longitudes 45°–60 °W and latitudes 5°–25 °S, and at altitudes between 500–800 m). Endemic pemphigus foliaceus is an autoimmune bullous dermatosis characterized by an erythematous-desquamative rash with exulcerations due to the rupture of fragile bullous lesions with a craniocaudal distribution, with photosensitivity and without mucosal involvement (Fig. 2). The pathogenesis of the disease is associated with epitope spreading, in which repeated exposure to insect bites (Simulium nigrimanum) would increase the production of pathogenic antibodies of the IgG4 subclass that lead to the recognition of the EC1 and EC2 domains of Desmoglein-1.55, 56 During the 20th century, there was a great increase in the incidence of the disease along areas of deforestation in the countryside of Brazil, especially in the states of São Paulo, Mato Grosso, Goiás and Minas Gerais, following large river basins.57
Figure 2.

Fogo selvagem or endemic pemphigus foliaceus. Extensive exulcerations with hematic crusts on the back in a young resident of the Tietê river valley.
Territorial and biodiversity restrictions promoted by deforestation affect the reproduction of large predators, which demand a greater food load. Moreover, the recent description of attacks on humans by wild animals (e.g., monkeys, jaguars, anteaters, coatis) can be justified by food restrictions that affect the animals and their forced proximity to urban centers.58, 59, 60
The numerical restriction (or extinction) of predators may also explain the proliferation and increased incidence of accidents with scorpions, especially in the Northeast and Southeast regions of Brazil.61, 62
The occurrence of spotted fever outbreaks in the countryside of the state of São Paulo is attributed to the increase in the population of capybaras and other natural reservoirs of Rickettsia sp., which are protected by the prohibition of hunting. Also, with the decrease in the number of predators such as large felines, the populations of capybaras have increased exponentially and this has contributed to the spread of infected ticks (mainly Amblyomma sp.) spreading the disease to areas where domestic animals and the human population live.31, 63, 64
As a result of the same environmental imbalance, Lyme disease in the American continent is caused mainly by the spirochete Borrelia burgdorferi (sensu lato) and transmitted by tick bites. It originally occurs among wild animals, being described in European and North American deer populations. However, it is also present in Brazil, affecting both deer and capybaras, whichspread the disease to domestic animals and humans when living close to urban centers. The disease has an early cutaneous manifestation (erythema chronicum migrans), which can trigger sclerodermiform reactions and is potentially severe (Fig. 3).65 The Brazilian variant of Lyme disease (borreliosis-like illness, or Baggio-Yoshinari syndrome) needs further studies, especially since its incidence seems to be underestimated considering the number of clinical manifestations that may not be exactly the same as classic Lyme disease (European or North American).66, 67, 68, 69, 70
Figure 3.
(A), Erythema chronicum migrans as the early manifestation of borreliosis-like illness. (B), Remission of clinical picture after treatment with doxycycline (Kindly provided by Prof. Sinésio Talhari).
Brazil has the largest freshwater network in the world. Due to this fact, the main national energy matrix was developed based on the construction of hydroelectric power plants, especially starting from the 1950s, leading to the flooding of more than 34,000 km2.71 Large water dams, however, promote profound and damaging changes to the geography and the riverside ecosystem. The influence of the changes in the local microclimate (temperature and rainfall) will be discussed later. Changes in the aquatic fauna, both by reducing river flows and by the non planned introduction of fish species, are environmental determinants of health problems.72, 73, 74
Freshwater stingrays, for instance, are native animals from the North and Midwest regions of Brazil. After the construction of rainwater dams, natural barriers were reduced. This favored the mobilization of fish downstream the Paraná river, precipitating severe accidents involving fishermen and bathers in the Southeastern region, including the state of São Paulo and the Tietê river (Fig. 4). 75 Similarly, piranhas have settled in dammed areas and have caused a series of accidents among bathers in the summer season (Fig. 5).76, 77
Figure 4.
Freshwater stingrays (Potamotrygon sp.) associated with severe accidents with bathers and fishermen at the Paraná River basin. Detail of the serrated stinger. Skin lesions due to stingray accidents: lower-limb ulcers on an extensive livedoid base (< 72 h) that develops into necrosis and eschar (> 7 days).
Figure 5.
River resort (municipality of Adolfo-SP, 21°09'56"S, 49°43'03"W) at a reservoir area of the Tietê River. Accidents due to piranha attacks on bathers: punched-out ulcers in the foot. Details of the adult animal and the triangular teeth (Serrasalmus maculatus).
Finally, deforestation has also changed the regional rainfall cycle, where the impact on biodiversity extends beyond the deforested area. In the beginning of the 20th century, the city of São Paulo was known as the “land of drizzle” due to the intense rainfall resulting from the surrounding dense Atlantic forest and its rich hydrography. Currently, it is the largest megalopolis in Latin America, and the extensive urban “heat island” has substantially modified the region's microclimate. The progressive deforestation of the Amazon region is also noticeable with the reduction of the pluviometric index in the Northern region of Brazil that has occurred in the last 50 years.78
A process of deforestation of small areas used by indigenous and quilombola populations is the burning of vegetation (coivaras), which consists in a controlled and restricted use of fire. The fire is limited by firebreaks, which are deforested areas to interrupt the continuity of combustion, causing minimal environmental damage. However, the recent fires in extensive areas of the Brazilian Pantanal and the Amazon rainforest (as in Australia and Argentina) are attributed both to spontaneous combustion during drought periods and to the deforestation practice by residents, mainly for the expansion of pastures and agriculture.79
The reduction of rainfall favors the spread of fire outbreaks, which in addition to the massive air pollution and damage to biodiversity, promote the rapid migration of wild animals and disease vectors fleeing their burning habitat. In this context, the chances of accidents with animals and the transmission of zoonoses in urban areas near the burned regions increase.
Large-scale agriculture
The production of food for the planet’s current population demand depends on the improvement of production techniques and this includes the mechanization of crops, genetic engineering and the use of pesticides.
Since the 1950s, pesticides have been gradually included in Brazilian agriculture. However, the training of farmers for their use and management has not accompanied this trend, causing damage to human health and the environment. The lack of individual protection when handling the pesticides favors skin and respiratory toxicity.80, 81
Occupational or industrial exposure to chlorinated hydrocarbons (dinitrophenol, pentachlorophenol) leads to chloracne-like acneiform rashes (Fig. 6).19 The handling of organophosphates, carbamates, pyrethroids and dipyridyls leads to the development of allergic and irritant contact dermatitis.80 Exposure to pesticides has also been identified as a risk factor for some types of skin cancer, including squamous cell carcinoma and melanoma. Arsenic is believed to be the main carcinogen involved in this process.82, 83
Figure 6.
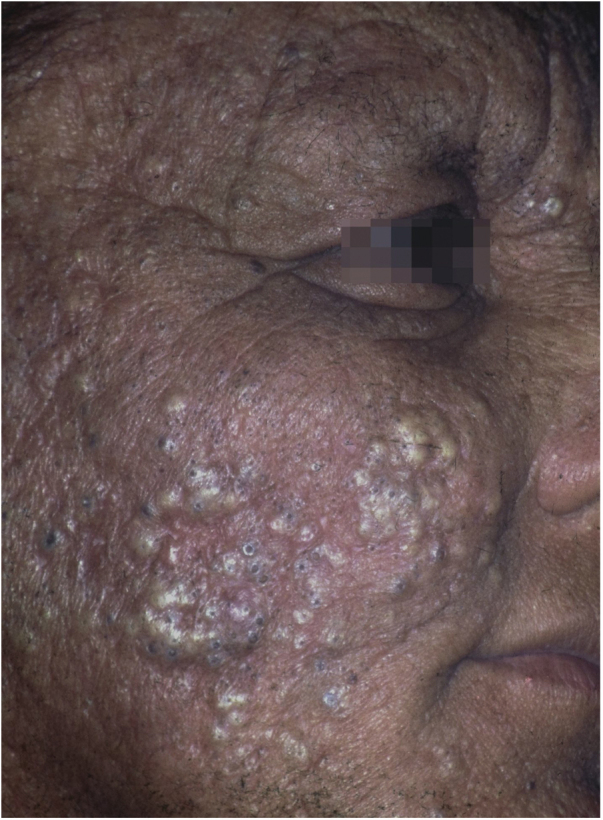
Chloracne. Farmer from the Tietê river valley with extensive papulopustular eruption with comedones. He reports unprotected handling of pesticides containing hexachlorobenzene (banned from use in Brazil in the 1980s). Three other family members were affected.
The mechanization of agriculture has greatly reduced the direct contact of farmers with the land and vegetation, which, in addition to requiring fewer professionals to perform the same activity, has also required greater professional qualification in the field. In the context of Dermatology, besides reducing occupational accidents and those caused by poisonous animals, the mechanization has also promoted a reduction in cases of deep mycoses in rural areas.84 The incidence of paracoccidioidomycosis has been steadily declining in the last 30 years, also because there is the interference of air humidity, water reservoirs and atmospheric pressure on the viability of fungi in the soil. States in the Amazon region (e.g. Rondônia), still maintain the most significant indicators due to a more most recent development of the agricultural activity.85, 86, 87
Genetic modifications of seeds (transgenic plants), the cultivation of non-native species, large-scale agriculture and extensive livestock farming demand deforestation and promote an important reduction in biodiversity, favoring the emergence of health problems.
Urbanization
The Brazilian demographic transition of the last century was characterized by rural-urban population migration, industrialization and the modification of the age pyramid. Few municipalities, however, showed a planned and sustainable development, resulting in problems related to housing, access to health, drinking water and basic sanitation (Fig. 7).88, 89 This situation constitutes a major challenge for public health policies because it depends on the knowledge and modification of its social determinants, instead of just promoting diagnoses and offering medications.90
Figure 7.

Non planned urbanization process in an indigenous village in the upper Solimões region, state of Amazonas (Kindly provided by Prof. Sinésio Talhari).
Large urban agglomerations as well as the need for means of mass transportation (e.g., trains, buses) constitute challenges for the control of diseases with respiratory transmission such as tuberculosis and COVID-19; their progression in large cities is more evident than in municipalities with low population density.91, 92
In Dermatology, ectoparasitoses are mainly influenced by urban agglomerations and poor sanitary conditions. The prevalence of scabies in the slums in the Brazilian Northeast region affects up to 8.8% of residents, while pediculosis can affect 43.4%.93 As a complicating factor, the indiscriminate use of pyrethroids to treat pediculosis of the scalp allowed the emergence of resistant strains of Pediculus humanus var. capitis, resulting in greater difficulty in infestation control and in restricting the epidemic (Fig. 8).94, 95
Figure 8.

Pediculus humanus capitis. Causal agent of pediculosis of the scalp, endemic in large urban agglomerations.
Sexually-Transmitted Infections (STIs), especially syphilis, Human Papillomavirus (HPV) anogenital warts, Human Immunodeficiency Virus (HIV), gonorrhea, non-gonococcal urethritis (e.g., Chlamydia sp.) and genital herpes have shown an increased incidence in the last two decades in several countries.96, 97 The economic development associated with urbanization favors the increase of prostitution and more sexual intercourse, maximizing the risk of STI transmission.2, 98, 99 The formation of new communities of workers for the construction of hydroelectric power plants, mining parks or planned cities (e.g., Brasilia, Teresina and Palmas) have historically accompanied an increase in firearm violence and STIs.100, 101, 102, 103
Some animals previously found only in rural areas have adapted to cities due to the lack of predators and to obtain food supplies from produced by humans such as the Loxosceles spider, which cause severe accidents with ulcer formation and kidney damage in its victims (Fig. 9).104 Certain cities such as Curitiba, in the southern region of Brazil, record thousands of accidents annually.105 Another example is the proliferation of pigeons from Europe and North Africa (Columba livia) in urban areas, increasing the risk of systemic mycoses such as cryptococcosis and infestations such as gamasoidosis, which have been recorded in several parts of the country.106, 107, 108, 109
Figure 9.

Skin ulcer with necrotic eschar formation derived from an accident caused by a spider of the genus Loxosceles (>96 h). Example of an adult brown spider (Loxosceles sp.).
The non planned urbanization of municipalities promotes important changes in the natural landscape, with an impact that goes beyond biodiversity and affects the local microclimate. The systematic drainage of water courses associated with extensive waterproofing of the soil and reduction of the vegetation cover, modify both the thermal stabilization capacity of water bodies and the terrestrial albedo (solar energy reflection coefficient). These elements promote a significant increase in temperature, amplified by the reduction of air circulation resulting from the successive construction of skyscrapers and by the production of heat from human activity (e.g., automobile traffic). The formation of these heat islands resultant from urbanization can change the temperature in the center of an urban area by more than 6 °C in relation to the adjacent rural area, with an important decrease in humidity and a reduction in the dispersion of air pollutants, which can favor several risks to human health.110, 111, 112, 113, 114, 115
Fig. 10 shows the effect the heat island inflicted on air temperature and humidity in the last 70 years in the metropolitan area of the municipality of São Paulo (Latitude: 23°32'56”S, Longitude: 46°38'20”W; altitude: 745 m), compared to the small rural municipality of São Simão (Latitude: 21°28'41”S, Longitude: 47°33'3”W; altitude: 663 m). There has been a consistent and progressive increase in the average temperature (+ 3.2 °C) and a reduction in the relative humidity of the air (-10%) in the metropolitan area, while the temperature and humidity naturally oscillated during the temporal series in the rural municipality.
Figure 10.
Temporal series (1960 to 2019) of monthly temperature averages (A and B) and relative humidity (C and D) in the municipalities of São Paulo-SP and São Simão-SP. (Source: INMET).
The modification of the terrestrial albedo by construction of buildings and the paving of soil also promotes greater reflection of ultraviolet radiation, which potentially worsens photoinduced dermatoses, such as melasma, rosacea, lupus erythematosus and the skin field cancerization.84, 116
Large cities (especially megalopolises) create vast islands of heat and air pollution, which are primarily associated with diseases linked to hypersensitivity, such as asthma, conjunctivitis and atopic dermatitis.117, 118, 119 The specific effects of air pollution on the skin will be discussed below.
Heat islands also interfere with the activity and reproduction of insects and arachnids. In Curitiba, the number of accidents by brown spiders showed a seasonal behavior, especially when the surface temperature exceeded 30 °C.120
The effect of heat on cultural rites linked to clothing (suits, synthetic clothes, closed shoes) and the manner of working (e.g., poorly ventilated production lines), promotes sweating and increased sebum production, which favor bacterial and fungal infections.
Urbanization, when associated with the lack of control of proliferation of stray domestic animals such as dogs and cats, in parallel with the lack of predators in the urban environment, also favors the emergence of zoonoses. Sporotrichosis is a subcutaneous mycosis caused by the fungus Sporothrix schenckii, more frequently found in countries with tropical or subtropical climates. In the skin, it manifests mainly as ulcers and ascending nodular lymphangitis (Fig. 11).121
Figure 11.

Cutaneous-lymphatic sporotrichosis, ulcer on the left hand dorsum, accompanied by ascending nodular lymphangitis in a young adult male who “adopted” an infected street animal. Picture detail showing the cat with an ulcer on the nose, a characteristic representation of feline sporotrichosis.
In Brazil, sporotrichosis of occupational origin, caused by trauma with vegetables (“gardener’s disease”) had its incidence reduced to the detriment of contamination by animals, which is on the rise among young adults, especially due to scratches or bites by contaminated cats, the main agents involved in this zoonotic chain.122 Felines have a high fungal load due to the habit of scratching trees, traveling long distances and engaging in fights, which favors contamination. The access of non-neutered cats to the streets, the abandonment or sacrifice of sick animals by the guardian, inadequate disposal of carcasses, associated with the lack of integration between the Epidemiological Surveillance and the Zoonosis Control Center of the municipalities are acts of negligence that contribute to the dynamic of disease transmission. Zoonotic sporotrichosis has been described in several states in Brazil and has become endemic in the Southeastern Region of the country, especially in Rio de Janeiro, in the last 20 years.123
Similarly, as a result of great urban mobility (such as international travel), lack of predators and emergence of resistance to pyrethroids, usually employed in residential pest control, bedbug (Cimex lectularius) bite epidemics have been described in several urban centers around the world.124, 125 Cimidiasis clinically manifests as pruritic edematous papules, especially on the extremities, which can assume a linear aspect (the bug’s “breakfast, lunch and dinner”), typical of fleas and bedbugs.126, 127 An additional concern regarding skin hypersensitivity reactions is the possibility that bedbugs can be vectors of other infectious diseases, evidence that is not yet a consensus among researchers.128, 129, 130
Pollution
One of the most damaging effects of modernity is the compromising of our environment (soil, air and water) with residues from human production. Environmental changes do not occur in isolation in the community. Deforestation, reduction of biodiversity, urbanization and pollution of the environment are usually shown to be interrelated as marks of modern human activity. Water contamination, noise pollution and waste disposal are of the utmost importance in public health.131, 132, 133, 134, 135
From the dermatological viewpoint, the skin is affected by particulate air pollution and volatile gases, especially nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), and carbon dioxide (CO2). The main mediators of air pollution in the skin are the Aryl Hydrocarbon receptors (AhR) present in all skin structures, which are activated by aromatic hydrocarbons such as dioxins, widely present in vehicular smoke.136, 137
Air pollution contributes to extrinsic skin aging, and mostly derives from the burning of fossil fuels and industrial activity in urban centers. Skin that is submitted to intense air pollution has a barrier deficit, with less squalene production, in addition to oxidative consumption of tocopherol and the formation of lentigos and wrinkles.136, 138 In the dermis, particulate pollution can induce inflammatory phenotypes in fibroblasts, with increased metalloprotein (MMP-1, MMP-3) synthesis, reduced collagen (COL1A1, COL1A2), and elastin synthesis.139
The specific damage from air pollution depends on the type of pollutant, the skin integrity and the intensity of exposure.140 Aromatic hydrocarbons promote different epithelial stimuli; in addition to the abovementioned ones, there is the formation of epoxy and diols which bind directly to DNA, promoting epigenetic changes in cell growth, potentiating the development of neoplasms, especially if there is joint stimulation with ultraviolet radiation.141, 142, 143
In addition to aging and carcinogenesis, air pollution favors the development of inflammatory dermatoses, such as eczema and acne. Aromatic hydrocarbons, especially dioxins, are known to induce acne mediated by AhR activation, acting on sebocytes, endothelium and epidermis.143, 144, 145 Individuals who traveled to areas with high air pollution levels reported an inflammatory acne outbreak. In Beijing, demand for acne care has been correlated with higher indicators of air pollution.144
The incidence of atopic dermatitis is influenced by low humidity, temperature and pollutants. Particulate pollution consists of numerous salts, heavy metals and aromatic hydrocarbons, which penetrate the skin through hair follicles and acrosyringea complex. Sweat increases the transepidermal penetration, promoting oxidative damage and inducing an inflammatory response in the dermis and epidermis, which culminates in damage to the barrier function.110, 137 In a four-year Chinese temporal series, the demand for eczema care was associated with daily air pollution indicators.146 Pollution is one of the factors responsible for the increased incidence of atopic dermatitis in Europe, which occurs more significantly in urban centers than in rural communities.131, 140
Forest fires, whether accidental or controlled fires aiming deforestation for agriculture, promote, in addition to deforestation, a reduction in biodiversity, reduction in soil water content and launch a large amount of gases and particles into the air. This shows that changes in the environment are not only measurable due to their direct effects, but also on the entire ecosystem as well.
In addition to the ozone that pollutes the atmosphere, promoting oxidative damage to the skin with a reduction in microflora and consumption of tocopherol, ozone also naturally forms in the stratosphere, around 20 to 30 km above sea level depending on the reaction of solar ultraviolet radiation with atmospheric oxygen.147 It plays a major role in completely blocking UVC radiation emitted by the sun (extremely mutagenic) and approximately 90% of UVB radiation. This equilibrium between ozone synthesis and degradation in the stratosphere ensures that a tolerable amount of mutagenic radiation reaches the planet surface.148
The ozone layer that circumscribes the Earth is not homogeneous, and has thinner areas, especially at the poles. There is an intense discussion about the cyclical variation in the configuration of the ozone layer and its degradation by air pollutants. Atmospheric emissions of halogenated compounds such as chlorofluorocarbon (CFC), halon, hydrochlorofluorocarbon (HFC), methyl bromide, carbon tetrachloride (CTC), methyl chloroform and hydrobromofluorocarbons (HBFC) have been associated with the reduction of the ozone layer in the atmosphere.148 The use of these compounds in soft drinks, propellants and foams has been reduced since the 1990s worldwide, with the expectation of complete interruption of use in the next 30 years.
It is estimated that a 1% reduction in the ozone layer promotes an increase of 2% in the incidence of UVB and, consequently, an increase of 2% in the incidence of skin cancer.149 In fact, in inhabited regions with lower concentrations of stratospheric ozone, higher rates of cutaneous and mucous neoplasms (basal cell carcinoma, squamous cell carcinoma and melanoma) are recorded in humans and animals, demanding greater stringency in photoprotection strategies.150, 151, 152, 153, 154
Climatic changes
Climate can be defined as the set of atmospheric changes in a certain region of the planet such as temperature, precipitation and winds, with patterns that tend to be repeated in a certain period of time (e.g., it is hot and rainy in January, in the city of Rio de Janeiro). Conventionally, the climatic behavior is evaluated in 30-year periods, and the main characteristic of the climate is its natural variability according to the years, with a complex interaction between the factors that determine it. It is important to differentiate “climate” from “meteorological weather”, which considers the state of the local atmosphere at a given time (e.g., today has been the hottest day of the year in the city of São Paulo).
The variation in the temperature of the oceans (the El Niño and La Niña phenomena and the decadal oscillation of the Pacific Ocean), solar activity (solar cycles), orbital trajectory (more circular or more elliptical), Earth’s axis inclination, volcanic activity and gravitational changes (lunar cycles) are the main global climate modifiers.155, 156, 157, 158, 159, 160 Its main determinants in a given region comprise the latitude, altitude, continentality (distance from the coast), cloud density, ocean temperatures, sea currents, vegetation/urbanization (albedo), water masses and geography.111, 115, 161, 162 This justifies the immense variability of climatic characteristics around the planet.
During the evoltion of humankind several global climate changes were recorded, such as small ice ages in Europe between 540 and 550 and between 1350 and 1850 due to less solar activity, changes in the Earth’s orbit and greater volcanic activity.163, 164, 165 There have also been significant warming periods, such as the Roman Warm Period (between 250 BC to 400 C.E.), and the medieval period (between 800 and 1200 C.E.) detected in the northern hemisphere. All of these changes resulted in social, economic and health impacts on the population.
There is currently an intense discussion with geopolitical repercussions on whether the focal environmental changes promoted by human activity can influence global climate, to the detriment of the evident abovementioned loco-regional changes and the natural variability of the planet’s climate cycles. However, this specific discussion goes beyond the scope of this article.
There is a clear seasonality in the incidence of dermatological diseases: psoriasis shows lower prevalence or severity in summer, due to ultraviolet radiation. However, there is a higher incidence of staphylococcal infections, actinic keratoses and accidents by venomous animals due to the type of leisure activities practiced during this time of the year. In contrast, there are more respiratory infections during the winter, when immunological imbalance favors leprosy reactions.166, 167, 168 It is therefore expected that climatic changes may interfere with the incidence of dermatoses.
Humidity and the increase in temperature constitute factors known to influence the rate of reproduction and activity of mosquitoes, the main vectors of infectious diseases.169, 170 A temporal series showed that the incidence of ATL in the Amazon region was strongly influenced by warm temperatures and changes in rainfall patterns caused by El Niño.171
In another series, in Peru, with 3,294 cases (2004–2007), the incidence of viral warts, actinic keratosis, rosacea and eczema was influenced by the climatic phenomena of the Pacific (El Niño / La Niña).172 These studies show that changes in regional microclimates promoted by deforestation, flooding/damming, agglomerations and urban heat islands are potential environmental determinants in the incidence of dermatoses.
In conclusion, environmental changes have an impact on human health/disease association, included in an ecological context. Currently, urbanization, large-scale agriculture, pollution of the environment and deforestation are the environmental determinants that should have the greatest impact on the incidence of dermatoses.173 Dermatologists should be aware of their social responsibility in order to promote sustainable practices in their community, in addition to identifying the environmental imbalances that favor each dermatosis, which is crucial for the prevention and treatment of these diseases.
Financial support
None declared.
Authors' contributions
Vidal Haddad Junior: Study concept, writing and approval of the final manuscript.
Adriana Lúcia Mendes: Study concept, writing and approval of the final manuscript.
Carolina Chrusciak Talhari: Study concept, writing and approval of the final manuscript.
Hélio Amante Miot: Study concept, writing and approval of the final manuscript.
Conflicts of interest
None declared.
Footnotes
How to cite this article: Haddad Junior V, Mendes AL, Talhari CC, Miot HA. Impact of environmental changes on Dermatology. An Bras Dermatol. 2021;96:210–23.
Study conducted at the Department of Dermatology, Faculty of Medicine, Universidade Estadual Paulista, Botucatu, SP, Brazil.
References
- 1.Tattersall I. Out of Africa: modern human origins special feature: human origins: out of Africa. Proc Natl Acad Sci USA. 2009;106:16018–16021. doi: 10.1073/pnas.0903207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellwanger J.H., Kulmann-Leal B., Kaminski V.L., Valverde-Villegas J.M., da Veiga A.B.G., Spilki F.R. Beyond diversity loss and climate change: Impacts of Amazon deforestation on infectious diseases and public health. An Acad Bras Cienc. 2020;92 doi: 10.1590/0001-3765202020191375. [DOI] [PubMed] [Google Scholar]
- 3.Rees J.L., Harding R.M. Understanding the evolution of human pigmentation: recent contributions from population genetics. J Invest Dermatol. 2012;132:846–853. doi: 10.1038/jid.2011.358. [DOI] [PubMed] [Google Scholar]
- 4.Yuen A.W., Jablonski N.G. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39–44. doi: 10.1016/j.mehy.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Jablonski N.G., Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 6.Jablonski N.G., Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8962–8968. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flores C., Ma S.F., Pino-Yanes M., Wades M.S., Pérez-Méndez L., Kittles R.A. African ancestry is associated with asthma risk in African Americans. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto P., Salgado C.G., Santos N., Alencar D.O., Santos S., Hutz M.H. Polymorphisms in the CYP2E1 and GSTM1 genes as possible protection factors for leprosy patients. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalueza-Fox C., Rompler H., Caramelli D., Stäubert C., Catalano G., Hughes D. A melanocortin 1 receptor allele suggests varying pigmentation among Neanderthals. Science. 2007;318:1453–1455. doi: 10.1126/science.1147417. [DOI] [PubMed] [Google Scholar]
- 10.Sortica Vde A., Ojopi E.B., Genro J.P., Callegari-Jacques S., dos Santos A.R., de Moraes M.O. Influence of genomic ancestry on the distribution of SLCO1B1, SLCO1B3 and ABCB1 gene polymorphisms among Brazilians. Basic Clin Pharmacol Toxicol. 2012;110:460–468. doi: 10.1111/j.1742-7843.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- 11.Suarez-Kurtz G., Pena S.D. Pharmacogenomics in the Americas: the impact of genetic admixture. Curr Drug Targets. 2006;7:1649–1658. doi: 10.2174/138945006779025392. [DOI] [PubMed] [Google Scholar]
- 12.D’Elia M.P., Brandao M.C., de Andrade Ramos B.R., da Silva M.G., Miot L.D.B.M., dos Santos S.E.M.B. African ancestry is associated with facial melasma in women: a cross-sectional study. BMC Med Genet. 2017;18:17. doi: 10.1186/s12881-017-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschini N., Chasman D.I., Cooper-DeHoff R.M., Arnett D.K. Genetics, ancestry, and hypertension: implications for targeted antihypertensive therapies. Curr Hypertens Rep. 2014;16:461. doi: 10.1007/s11906-014-0461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suarez-Kurtz G., Botton M.R. Pharmacogenetics of coumarin anticoagulants in Brazilians. Expert Opin Drug Metab Toxicol. 2015;11:67–79. doi: 10.1517/17425255.2015.976201. [DOI] [PubMed] [Google Scholar]
- 15.Prous A. Zahar; Rio de Janeiro: 2006. O Brasil antes dos brasileiros: a pré-história do nosso país. [Google Scholar]
- 16.Feldens F. 1th ed. Editora Univates; Lajeado: 2018. O Homem a Agricultura a História. [Google Scholar]
- 17.Kroll M., Bharucha E., Kraas F. Does rapid urbanization aggravate health disparities? Reflections on the epidemiological transition in Pune, India. Glob Health Action. 2014;7:23447. doi: 10.3402/gha.v7.23447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi-Espagnet A., Goldstein G.B., Tabibzadeh I. Urbanization and health in developing countries: a challenge for health for all. World Health Stat Q. 1991;44 185-44. [PubMed] [Google Scholar]
- 19.Alchorne Ade O., Alchorne M.M., Silva M.M. Occupational dermatosis. An Bras Dermatol. 2010;85:137–145. doi: 10.1590/s0365-05962010000200003. [DOI] [PubMed] [Google Scholar]
- 20.Mas-Coma S., Jones M.K., Marty A.M. COVID-19 and globalization. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan L., Ogunwole S.M., Cooper L.A. Historical Insights on Coronavirus Disease 2019 (COVID-19), the 1918 Influenza Pandemic, and Racial Disparities: Illuminating a Path Forward. Ann Intern Med. 2020 doi: 10.7326/M20-2223. 173-474-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shanks G.D. COVID-19 versus the 1918 influenza pandemic: different virus, different age mortality patterns. J Travel Med. 2020 doi: 10.1093/jtm/taaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohn S.K., Jr Epidemiology of the Black Death and successive waves of plague. Med Hist Suppl. 2008:74–100. [PMC free article] [PubMed] [Google Scholar]
- 24.Bossak B.H. AIDS and the Black Death. Qjm. 2007;100:144–145. doi: 10.1093/qjmed/hcl145. [DOI] [PubMed] [Google Scholar]
- 25.Skorka P., Grzywacz B., Moron D., Lenda M. The macroecology of the COVID-19 pandemic in the Anthropocene. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flies E., Clarke L., Brook B.W., Jones P. Urbanisation reduces the abundance and diversity of airborne microbes-but what 1 does that mean for our health? A systematic review. Sci Total Environ. 2020:1–8. doi: 10.1016/j.scitotenv.2020.140337. [DOI] [PubMed] [Google Scholar]
- 27.Wang R., Wu J., Yiu K.F., Shen P., Lam P.K.S. Long-term variation in phytoplankton assemblages during urbanization: A comparative case study of Deep Bay and Mirs Bay, Hong Kong, China. Sci Total Environ. 2020;745 doi: 10.1016/j.scitotenv.2020.140993. [DOI] [PubMed] [Google Scholar]
- 28.Corlett R.T. The Anthropocene concept in ecology and conservation. Trends Ecol Evol. 2015;30:36–41. doi: 10.1016/j.tree.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Gibb R., Redding D.W., Chin K.Q., Donnelly C.A., Blackburn T.M., Newbold T. Zoonotic host diversity increases in human-dominated ecosystems. Nature. 2020;584 doi: 10.1038/s41586-020-2562-8. 392-02. [DOI] [PubMed] [Google Scholar]
- 30.Walsh J.F., Molyneux D.H., Birley M.H. Deforestation: effects on vector-borne disease. Parasitology. 1993;106(Suppl):S55–75. doi: 10.1017/s0031182000086121. [DOI] [PubMed] [Google Scholar]
- 31.Pignatti M.G. Health and environment: emergent diseases in Brazil. Ambient Soc. 2004;7:133–147. [Google Scholar]
- 32.Hurtado L.A., Caceres L., Chaves L.F., Calzada J.E. When climate change couples social neglect: malaria dynamics in Panama. Emerg Microbes Infect. 2014;3:e27. doi: 10.1038/emi.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarcho S. Ferreyra da Rosa on yellow fever in Pernambuco. Bull N Y Acad Med. 1972;48:1343–1345. [PMC free article] [PubMed] [Google Scholar]
- 34.Hofman M.P.G., Hayward M.W., Heim M., Marchand P., Holandsen J.M., Urbano F. Right on track? Performance of satellite telemetry in terrestrial wildlife research. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira A.S., Casseb L.M.N., Barbosa T.F.S., Begot A.L., Brito R.M.O., Vasconcelos P.F.C. Rabies Virus in Bats, State of Para, Brazil, 2005-2011. Vector Borne Zoonotic Dis. 2017;17:576–581. doi: 10.1089/vbz.2016.2010. [DOI] [PubMed] [Google Scholar]
- 36.Benchimol J.L., Silva A.F.C.d. Railroads, disease, and tropical medicine in Brazil under the First Republic. História, Ciências. Saúde – Manguinhos. 2008;15:719–762. doi: 10.1590/s0104-59702008000300009. [DOI] [PubMed] [Google Scholar]
- 37.Tollefson J. Why deforestation and extinctions make pandemics more likely. Nature. 2020;584:175–176. doi: 10.1038/d41586-020-02341-1. [DOI] [PubMed] [Google Scholar]
- 38.Carlson C.J., Chipperfield J.D., Benito B.M., Telford R.J., O’Hara R.B. Don’t gamble the COVID-19 response on ecological hypotheses. Nat Ecol Evol. 2020;4:1155. doi: 10.1038/s41559-020-1279-2. [DOI] [PubMed] [Google Scholar]
- 39.Gontijo B., de Carvalho Mde L. [American cutaneous leishmaniasis] Rev Soc Bras Med Trop. 2003;36:71–80. doi: 10.1590/s0037-86822003000100011. [DOI] [PubMed] [Google Scholar]
- 40.Ashford R.W. Leishmaniasis reservoirs and their significance in control. Clin Dermatol. 1996;14:523–532. doi: 10.1016/0738-081x(96)00041-7. [DOI] [PubMed] [Google Scholar]
- 41.Roque A.L., Jansen A.M. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int J Parasitol Parasites Wildl. 2014;3:251–262. doi: 10.1016/j.ijppaw.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ECSd Vale, Furtado T. Tegumentary leishmaniasis in Brazil: a historical review related to the origin, expansion and etiology. An Bras Dermatol. 2005;80:421–428. [Google Scholar]
- 43.Lima A.P., Minelli L., Teodoro U., Comunello É. Tegumentary leishmaniasis distribution by satellite remote sensing imagery, in Paraná State, Brazil. An Bras Dermatol. 2002;77:681–692. [Google Scholar]
- 44.Couto-Lima D., Madec Y., Bersot M.I., Campos S.S., Motta M.A., dos Santos F.B. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci Rep. 2017;7:4848. doi: 10.1038/s41598-017-05186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrett A.D., Monath T.P. Epidemiology and ecology of yellow fever virus. Adv Virus Res. 2003;61:291–315. doi: 10.1016/s0065-3527(03)61007-9. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson R.L. Yellow fever: ecology, epidemiology, and role in the collapse of the Classic lowland Maya civilization. Med Anthropol. 1995;16:269–294. doi: 10.1080/01459740.1994.9966118. [DOI] [PubMed] [Google Scholar]
- 47.Bernardes Filho F., Bonatto D.C., Martins G., Maier Lde M., Nery J.A., Azulay-Abulafia L. Occurrence of two autochthonous cases of American cutaneous leishmaniasis in the neighborhood of Caju, city of Rio de Janeiro, Brazil. An Bras Dermatol. 2014;89:848–850. doi: 10.1590/abd1806-4841.20143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carneiro F.R.O., Amin G.A., Cruz L.B.P., Daher B.A. Urban American cutaneous leishmaniasis. An Bras Dermatol. 2018;93:156–158. doi: 10.1590/abd1806-4841.20186713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gil J.F., Nasser J.R., Cajal S.P., Juarez M., Acosta M., Cimino R.O. Urban transmission of American cutaneous leishmaniasis in Argentina: spatial analysis study. Am J Trop Med Hyg. 2010;82:433–440. doi: 10.4269/ajtmh.2010.09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Passos V.M., Falcao A.L., Katz N. Urban American cutaneous leishmaniasis in the Metropolitan Region of Belo Horizonte, Minas Gerais State, Brazil. Mem Inst Oswaldo Cruz. 1990;85:243–244. doi: 10.1590/s0074-02761990000200018. [DOI] [PubMed] [Google Scholar]
- 51.Schneider M.C., Aron J., Santos-Burgoa C., Uieda W., Ruiz-Velazco S. Common vampire bat attacks on humans in a village of the Amazon region of Brazil. Cad Saude Publica. 2001;17:1531–1536. doi: 10.1590/s0102-311x2001000600025. [DOI] [PubMed] [Google Scholar]
- 52.Sousa A.D.S.J., Palacios V., Miranda C.D.S., da Costa R.J.F., Catete C.P., Chagasteles E.J. Space-temporal analysis of Chagas disease and its environmental and demographic risk factors in the municipality of Barcarena, Para, Brazil. Rev Bras Epidemiol. 2017;20:742–755. doi: 10.1590/1980-5497201700040015. [DOI] [PubMed] [Google Scholar]
- 53.Mendes Wda S., Silva A.A., Neiva R.F., Costa N.M., de Assis M.S., Vidigal P.M.O. An outbreak of bat-transmitted human rabies in a village in the Brazilian Amazon. Rev Saude Publica. 2009;43:1075–1077. doi: 10.1590/s0034-89102009005000073. [DOI] [PubMed] [Google Scholar]
- 54.Benicio E., Cordeiro M., Monteiro H., Moura M.A.S., Oliveira C., Gadelha E.P.N. Sustained Presence of Cutaneous Leishmaniasis in Urban Manaus, the Largest Human Settlement in the Amazon. Am J Trop Med Hyg. 2015;93:1208–1213. doi: 10.4269/ajtmh.14-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoki V., Lago F., Yamazaki M.H., Santi C.G., Maruta C.W. Significance of epitope spreading in the pathogenesis of pemphigus vulgaris and foliaceus. An Bras Dermatol. 2008;83:157–161. [Google Scholar]
- 56.Li N., Aoki V., Hans-Filho G., Rivitti E.A., Diaz L.A. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem) J Exp Med. 2003;197:1501–1510. doi: 10.1084/jem.20022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell I., Reis V., Aoki V., Cunha P., Filho G.H., Alves G. Endemic pemphigus foliaceous. An Bras Dermatol. 2001:13–33. [Google Scholar]
- 58.Neto M.F., Garrone Neto D., Haddad V., Jr Attacks by jaguars (Panthera onca) on humans in central Brazil: report of three cases, with observation of a death. Wilderness Environ Med. 2011;22:130–135. doi: 10.1016/j.wem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Bittner G.C., Ritter Hans N., Hans Neto G., Morais M.O., Hans Filho G., Haddad V., Jr Coati (Nasua nasua) attacks on humans: case report. Wilderness Environ Med. 2010;21:349–352. doi: 10.1016/j.wem.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Haddad V., Jr, Assuncao M.C., de Mello R.C., Duarte M.R. A fatal attack caused by a lowland tapir (Tapirus terrestris) in southeastern Brazil. Wilderness Environ Med. 2005;16:97–100. doi: 10.1580/pr29-04.1. [DOI] [PubMed] [Google Scholar]
- 61.Chippaux J.P. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: from obvious facts to contingencies. J Venom Anim Toxins Incl Trop Dis. 2015;21:13. doi: 10.1186/s40409-015-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reckziegel G.C., Pinto Junior V.L. Analysis of the scorpionism in Brazil from 2000 to 2010. Rev Pan-Amaz Saude. 2014;5:67–68. [Google Scholar]
- 63.de Lima V.L., Figueiredo A.C., Pignatti M.G., Modolo M. [Spotted fever in the town of Pedreira, Sao Paulo state, Brazil. The relationship between the occurrence of cases and human parasitism by ixodid ticks] Rev Soc Bras Med Trop. 1995;28:135–137. doi: 10.1590/s0037-86821995000200010. [DOI] [PubMed] [Google Scholar]
- 64.Santos M., Haddad Júnior V., Ribeiro-Rodrigues R., Talhari S. Borreliose de Lyme. An Bras Dermatol. 2010;85:930–938. doi: 10.1590/s0365-05962010000600029. [DOI] [PubMed] [Google Scholar]
- 65.Santos M., Ribeiro-Rodrigues R., Talhari C., Ferreira L.C., Zelger B., Talhari S. Presence of Borrelia burgdorferi "Sensu Lato" in patients with morphea from the Amazonic region in Brazil. Int J Dermatol. 2011;50:1373–1378. doi: 10.1111/j.1365-4632.2011.05081.x. [DOI] [PubMed] [Google Scholar]
- 66.LoGiudice K., Ostfeld R.S., Schmidt K.A., Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vien V.P., Bassi R., Maxim T., Bogoch I.I. Lyme disease vs Baggio-Yoshinari syndrome in a returned traveller from Brazil. J Travel Med. 2017;24 doi: 10.1093/jtm/tax055. [DOI] [PubMed] [Google Scholar]
- 68.Gouveia E.A., Alves M.F., Mantovani E., Oyafuso L.K., Bonoldi V.L., Yoshinari N.H. Profile of patients with Baggio-Yoshinari Syndrome admitted at "Instituto de Infectologia Emilio Ribas". Rev Inst Med Trop Sao Paulo. 2010;52:297–303. doi: 10.1590/s0036-46652010000600003. [DOI] [PubMed] [Google Scholar]
- 69.Yoshinari N.H., Mantovani E., Bonoldi V.L., Marangoni R.G., Gauditano G. [Brazilian lyme-like disease or Baggio-Yoshinari syndrome: exotic and emerging Brazilian tick-borne zoonosis] Rev Assoc Med Bras (1992) 2010;56:363–369. doi: 10.1590/s0104-42302010000300025. [DOI] [PubMed] [Google Scholar]
- 70.Talhari S., de Souza Santos M.N., Talhari C., Ferreira L.C.L., Silva Jr R.M., Zelger B. Borrelia Burgdorferi "sensu lato" in Brazil: Occurrence confirmed by immunohistochemistry and focus floating microscopy. Acta Trop. 2010;115:200–204. doi: 10.1016/j.actatropica.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 71.Oliveira N.C.C. The Great Acceleration and Hydroelectric Dam Building in Brazil. Varia Historia. 2018;34:315–346. [Google Scholar]
- 72.de Queiroz A.R., Motta-Veiga M. [Analysis of the social and health impacts of large hydroelectric plants: lessons for a sustainable energy management] Cien Saude Colet. 2012;17:1387–1398. doi: 10.1590/s1413-81232012000600002. [DOI] [PubMed] [Google Scholar]
- 73.Giongo C.R., Mendes J.M.R., Santos F.K. Development, health and environment: contradictions in the construction of dams. Serv Soc Soc. 2015:501–522. [Google Scholar]
- 74.Haines A., Smith K.R., Anderson D., Epstein P.R., McMichael A.J., Roberts I. Policies for accelerating access to clean energy, improving health, advancing development, and mitigating climate change. Lancet. 2007;370:1264–1281. doi: 10.1016/S0140-6736(07)61257-4. [DOI] [PubMed] [Google Scholar]
- 75.Garrone Neto D., Haddad Junior V. [Stingrays in rivers in southeastern Brazil: occurrence localities and impact on the population] Rev Soc Bras Med Trop. 2010;43:82–88. doi: 10.1590/s0037-86822010000100018. [DOI] [PubMed] [Google Scholar]
- 76.Haddad V., Jr, Sazima I. Piranha attacks in dammed streams used for human recreation in the State of Sao Paulo, Brazil. Rev Soc Bras Med Trop. 2010;43:596–598. doi: 10.1590/s0037-86822010000500027. [DOI] [PubMed] [Google Scholar]
- 77.Haddad V., Jr, Sazima I. Piranha attacks on humans in southeast Brazil: epidemiology, natural history, and clinical treatment, with description of a bite outbreak. Wilderness Environ Med. 2003;14:249–254. doi: 10.1580/1080-6032(2003)14[249:paohis]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 78.Amorim T.X., Senna M.C.A., Cataldi M. Impactos do desmatamento progressivo da Amazônia na precipitação do Brasil. Rev Bras Climatol. 2019;24:151–174. [Google Scholar]
- 79.Leonel M. O uso do fogo: o manejo indígena e a piromania da monocultura. Estudos Avançados. Estudo Av. 2000;14:231–250. [Google Scholar]
- 80.Ribas P.P., Matsumura A.T.S. A química dos agrotóxicos: impacto sobre a saúde e meio ambiente. Revista Liberato. 2009;10:149–158. [Google Scholar]
- 81.Spiewak R. Pesticides as a cause of occupational skin diseases in farmers. Ann Agric Environ Med. 2001;8:1–5. [PubMed] [Google Scholar]
- 82.Dennis L.K., Lynch C.F., Sandler D.P., Alavanja M.C. Pesticide use and cutaneous melanoma in pesticide applicators in the agricultural heath study. Environ Health Perspect. 2010;118:812–817. doi: 10.1289/ehp.0901518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kennedy C., Bajdik C.D., Willemze R., Bouwes Bavinck J.N. Chemical exposures other than arsenic are probably not important risk factors for squamous cell carcinoma, basal cell carcinoma and malignant melanoma of the skin. Br J Dermatol. 2005;152:194–197. doi: 10.1111/j.1365-2133.2005.06411.x. [DOI] [PubMed] [Google Scholar]
- 84.Miguel L.M.Z., Jorge M.F.S., Rocha B., Miot H.A. Incidence of skin diseases diagnosed in a public institution: comparison between 2003 and 2014. An Bras Dermatol. 2017;92:423–425. doi: 10.1590/abd1806-4841.20175793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vieira Gde D., Alves Tda C., Lima S.M., Camargo L.M., Sousa C.M. Paracoccidioidomycosis in a western Brazilian Amazon State: clinical-epidemiologic profile and spatial distribution of the disease. Rev Soc Bras Med Trop. 2014;47:63–68. doi: 10.1590/0037-8682-0225-2013. [DOI] [PubMed] [Google Scholar]
- 86.Hrycyk M.F., Garcia Garces H., Bosco S.M.G., de Oliveira S.L., Marques S.A., Bagagli E. Ecology of Paracoccidioides brasiliensis, P. lutzii and related species: infection in armadillos, soil occurrence and mycological aspects. Med Mycol. 2018;56:950–962. doi: 10.1093/mmy/myx142. [DOI] [PubMed] [Google Scholar]
- 87.Restrepo A. The ecology of Paracoccidioides brasiliensis: a puzzle still unsolved. Sabouraudia. 1985;23:323–334. [PubMed] [Google Scholar]
- 88.Vasconcelos A.M.N., Gomes M.M.F. Demographic transition: the Brazilian experience. Epidemiol Serv Saude. 2012;21:539–548. [Google Scholar]
- 89.Libânio P.A.C., Chernicharo C.A.d.L., Nascimento N.d.O. The water quality dimension: an evaluation of the relationship between social, water availability, water services and public health indicators. Eng Sanit Amb. 2005;10:219–228. [Google Scholar]
- 90.Buss P.M., Pellegrini Filho A. Health and its social determinants. Physis. 2007;17:77–93. [Google Scholar]
- 91.Lima S.V.M.A., Rocha J.V.M., de Araujo K.C.G.M., Nunes M.A.P., Nunes C. Determinants associated with areas with higher tuberculosis mortality rates: an ecological study. Trop Med Int Health. 2020;25:338–345. doi: 10.1111/tmi.13349. [DOI] [PubMed] [Google Scholar]
- 92.Maroko A.R., Nash D., Pavilonis B.T. COVID-19 and Inequity: a Comparative Spatial Analysis of New York City and Chicago Hot Spots. J Urban Health. 2020;97:461–470. doi: 10.1007/s11524-020-00468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heukelbach J., Wilcke T., Winter B., Feldmeier H. Epidemiology and morbidity of scabies and pediculosis capitis in resource-poor communities in Brazil. Br J Dermatol. 2005;153:150–156. doi: 10.1111/j.1365-2133.2005.06591.x. [DOI] [PubMed] [Google Scholar]
- 94.Hemingway J., Miller J., Mumcuoglu K.Y. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89–96. doi: 10.1046/j.1365-2915.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 95.Larkin K., Rodriguez C.A., Jamani S., Fronza G., Roca-Acevedo G., Sanchez A. First evidence of the mutations associated with pyrethroid resistance in head lice (Phthiraptera: Pediculidae) from Honduras. Parasit Vectors. 2020;13:312. doi: 10.1186/s13071-020-04183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Avelleira J.C.R., Bottino G. Syphilis: diagnosis, treatment and control. An Bras Dermatol. 2006;81:111–126. [Google Scholar]
- 97.Gerbase A.C., Rowley J.T., Mertens T.E. Global epidemiology of sexually transmitted diseases. Lancet. 1998;351(Suppl 3):2–4. doi: 10.1016/s0140-6736(98)90001-0. [DOI] [PubMed] [Google Scholar]
- 98.Boon M.E., van Ravenswaay Claasen H.H., van Westering R.P., Kok L.P. Urbanization and the incidence of abnormalities of squamous and glandular epithelium of the cervix. Cancer. 2003;99:4–8. doi: 10.1002/cncr.10924. [DOI] [PubMed] [Google Scholar]
- 99.Ressler R.W., Waters M.S., Watson J.K. Contributing factors to the spread of sexually transmitted diseases: The case of welfare. Am J Econ Sociol. 2006;65:943–961. [Google Scholar]
- 100.Figueiredo A.C.P., Saraiva L.J.C. A prostituição em grandes projetos na Amazônia: o impacto do grande capital nos fluxos de mão de obra na UHE Belo Monte. Nova Revista Amazônica. 2018;6:69–77. [Google Scholar]
- 101.White J.H. The drowned prostitute: national development, public morality, and the shifting geography of sexual commerce in Alto Parana, Paraguay, 1974-1982. J Lat Amer Geogr. 2013:125–149. [Google Scholar]
- 102.Neto V.J., dos Santos J.C. The frontier violence practices: study on diamond mining in Juína, MT (1987-1994) História: Debates e Tendências. 2018;18:214–228. [Google Scholar]
- 103.Dos Santos J.C., Castravechi L.A. Relações de trabalho na Amazônia Mato-Grossense: o uso da violência contra peões e garimpeiros. Élisée-Revista de Geografia da UEG. 2014;3:26–48. [Google Scholar]
- 104.Málaque C.M.S.a, Castro-Valencia J.E., Cardoso J.L.C., FranÇa F.O.d.S., Barbaro K.C., Hui W.F. Clinical and epidemiological features of definitive and presumed loxoscelism in Sao Paulo. Brazil. Rev Inst Med Trop Sao Paulo. 2002;44:139–143. doi: 10.1590/s0036-46652002000300005. [DOI] [PubMed] [Google Scholar]
- 105.Marques-da-Silva E., Souza-Santos R., Fischer M., Rubio G. Loxosceles spider bites in the state of Paraná, Brazil: 1993-2000. J Venom Anim Toxins Incl Trop Dis. 2006;12:110–123. [Google Scholar]
- 106.de Oliveira Alves A., Bernardes Filho F. Gamasoidosis (bird mite dermatitis): A case series in a family. Pediatr Neonatol. 2018;59:102–103. doi: 10.1016/j.pedneo.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki C.M.P., Stolf H.O., Camargo R.M.P.d., Haddad V., Jr Gamasoidose ou dermatite por ácaros aviários: relato de caso. Diagn Tratamento. 2014;19:74–76. [Google Scholar]
- 108.Costa A.K., Sidrim J.J., Cordeiro R.A., Brilhante R.S., Monteiro A.J., Rocha M.F. Urban pigeons (Columba livia) as a potential source of pathogenic yeasts: a focus on antifungal susceptibility of Cryptococcus strains in Northeast Brazil. Mycopathologia. 2010;169:207–213. doi: 10.1007/s11046-009-9245-1. [DOI] [PubMed] [Google Scholar]
- 109.Spina-Tensini T., Muro M.D., Queiroz-Telles F., Strozzi I., Moraes S.T., Petterle R.R. Geographic distribution of patients affected by Cryptococcus neoformans/Cryptococcus gattii species complexes meningitis, pigeon and tree populations in Southern Brazil. Mycoses. 2017;60:51–58. doi: 10.1111/myc.12550. [DOI] [PubMed] [Google Scholar]
- 110.Zhou B., Rybski D., Kropp J.P. The role of city size and urban form in the surface urban heat island. Sci Rep. 2017;7:4791. doi: 10.1038/s41598-017-04242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gui X., Wang L., Yao R., Yu D., Li C. Investigating the urbanization process and its impact on vegetation change and urban heat island in Wuhan, China. Environ Sci Pollut Res Int. 2019;26:30808–30825. doi: 10.1007/s11356-019-06273-w. [DOI] [PubMed] [Google Scholar]
- 112.Mariani L., Parisi S.G., Cola G., Lafortezza R., Colangelo G., Sanesi G. Climatological analysis of the mitigating effect of vegetation on the urban heat island of Milan, Italy. Sci Total Environ. 2016;569-570:762–773. doi: 10.1016/j.scitotenv.2016.06.111. [DOI] [PubMed] [Google Scholar]
- 113.Susca T., Gaffin S.R., Dell’osso G.R. Positive effects of vegetation: urban heat island and green roofs. Environ Pollut. 2011;159:2119–2126. doi: 10.1016/j.envpol.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 114.Costa D.F.d., Silva H.R., Peres L.d.F. Identification of urban heat islands in Ilha Solteira - SP municipality using geotecnologies. Eng Agr. 2010;30:974–985. [Google Scholar]
- 115.Lemes M.d.C.R., Reboita M.S., Torres R.R. Mudança no uso e cobertura da terra na bacia do rio tietê e seus impactos na temperatura da superfície (TS) Rev Bras Climatol. 2020;27:224–240. [Google Scholar]
- 116.Schalka S., Steiner D., Ravelli F.N., Steiner T., Terena A.C., Marçon C.R. Brazilian consensus on photoprotection. An Bras Dermatol. 2014;89:1–74. doi: 10.1590/abd1806-4841.20143971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wong L.P., Alias H., Aghamohammadi N., Aghazadeh S., Nik Sulaiman N.M. Physical, Psychological, and Social Health Impact of Temperature Rise Due to Urban Heat Island Phenomenon and Its Associated Factors. Biomed Environ Sci. 2018;31:545–550. doi: 10.3967/bes2018.074. [DOI] [PubMed] [Google Scholar]
- 118.Heaviside C., Macintyre H., Vardoulakis S. The Urban Heat Island: Implications for Health in a Changing Environment. Curr Environ Health Rep. 2017;4:296–305. doi: 10.1007/s40572-017-0150-3. [DOI] [PubMed] [Google Scholar]
- 119.Tan J., Zheng Y., Tang X., Guo C., Li L., Song G. The urban heat island and its impact on heat waves and human health in Shanghai. Int J Biometeorol. 2010;54:75–84. doi: 10.1007/s00484-009-0256-x. [DOI] [PubMed] [Google Scholar]
- 120.Pinto L.R., de Assis Mendonça F., Araújo W.M. A influência das variações térmicas nos acidentes loxoscélicos em Curitiba/PR. Rev Bras Climatol. 2017;5:55–69. [Google Scholar]
- 121.Orofino-Costa R., Macedo P.M., Rodrigues A.M., Bernardes-Engemann A.R. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606–620. doi: 10.1590/abd1806-4841.2017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cordeiro F.N., Bruno C.B., Paula C.D., Motta Jde O. Familial occurrence of zoonotic sporotrichosis. An Bras Dermatol. 2011;86:S121–4. doi: 10.1590/s0365-05962011000700032. [DOI] [PubMed] [Google Scholar]
- 123.da Silva M.B., Costa M.M., Torres C.C., Galhardo M.C.G., do Valle A.C.F., Magalhães M.A.F.M. [Urban sporotrichosis: a neglected epidemic in Rio de Janeiro, Brazil] Cad Saude Publica. 2012;28:1867–1880. doi: 10.1590/s0102-311x2012001000006. [DOI] [PubMed] [Google Scholar]
- 124.Romero A., Potter M.F., Potter D.A., Haynes K.F. Insecticide resistance in the bed bug: a factor in the pest’s sudden resurgence? J Med Entomol. 2007;44:175–178. doi: 10.1603/0022-2585(2007)44[175:IRITBB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 125.Criado P.R., Belda Junior W., Criado P.R.F., Vasconcelos e Silva R., Vasconcellos C. Bedbugs (Cimicidae infestation): the worldwide renaissance of an old partner of human kind. Braz J Infect Dis. 2011;15:74–80. doi: 10.1016/s1413-8670(11)70144-1. [DOI] [PubMed] [Google Scholar]
- 126.Criado P.R., Criado R.F. Bedbugs (Heteroptera, Cimicidae): an etiology of pruritus to be remembered. An Bras Dermatol. 2011;86:163–164. doi: 10.1590/s0365-05962011000100028. [DOI] [PubMed] [Google Scholar]
- 127.Peres G., Yugar L.B.T., Haddad Junior V. Breakfast, lunch, and dinner sign: a hallmark of flea and bedbug bites. An Bras Dermatol. 2018;93:759–760. doi: 10.1590/abd1806-4841.20187384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salazar R., Castillo-Neyra R., Tustin A.W., Borrini-Mayori K., Naquira C., Levy M.Z. Bed bugs (Cimex lectularius) as vectors of Trypanosoma cruzi. Am J Trop Med Hyg. 2015;92:331–335. doi: 10.4269/ajtmh.14-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leulmi H., Bitam I., Berenger J.M., Lepidi H., Rolain J.M., Almeras L. Competence of Cimex lectularius Bed Bugs for the Transmission of Bartonella quintana, the Agent of Trench Fever. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taylor P., Morrison J. Cimex lectularis as a vector of Hepatitis B. Cent Afr J Med. 1980;26:198–200. [PubMed] [Google Scholar]
- 131.Manisalidis I., Stavropoulou E., Stavropoulos A., Bezirtzoglou E. Environmental and Health Impacts of Air Pollution: A Review. Front Publ Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dzhambov A.M., Dimitrova D.D. Urban green spaces’ effectiveness as a psychological buffer for the negative health impact of noise pollution: a systematic review. Noise Health. 2014;16:157–165. doi: 10.4103/1463-1741.134916. [DOI] [PubMed] [Google Scholar]
- 133.Gozzo F., Poupaert J.H. Xenoestrogens, pollution & health: a critical review. J Pharm Belg. 1998;53:278–286. [PubMed] [Google Scholar]
- 134.Newell K., Kartsonaki C., Lam K.B.H., Kurmi O.P. Cardiorespiratory health effects of particulate ambient air pollution exposure in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Planet Health. 2017;1:e368–e380. doi: 10.1016/S2542-5196(17)30166-3. [DOI] [PubMed] [Google Scholar]
- 135.Triassi M., Alfano R., Illario M., Nardone A., Caporale O., Montuori P. Environmental pollution from illegal waste disposal and health effects: a review on the "triangle of death". Int J Environ Res Public Health. 2015;12:1216–1236. doi: 10.3390/ijerph120201216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Krutmann J., Liu W., Li L., Pan X., Crawford M., Sore G. Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J Dermatol Sci. 2014;76:163–168. doi: 10.1016/j.jdermsci.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 137.Mancebo S.E., Wang S.Q. Recognizing the impact of ambient air pollution on skin health. J Eur Acad Dermatol Venereol. 2015;29:2326–2332. doi: 10.1111/jdv.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schikowski T., Huls A. Air Pollution and Skin Aging. Curr Environ Health Rep. 2020;7:58–64. doi: 10.1007/s40572-020-00262-9. [DOI] [PubMed] [Google Scholar]
- 139.Park S.Y., Byun E.J., Lee J.D., Kim S., Kim H.S. Air Pollution, Autophagy, and Skin Aging: Impact of Particulate Matter (PM10) on Human Dermal Fibroblasts. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19092727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Drakaki E., Dessinioti C., Antoniou C.V. Air pollution and the skin. Front Envir Sci. 2014;2 [Google Scholar]
- 141.Fernandes A.O., Banerji A.P. Inhibition of benzopyrene-induced forestomach tumors by field bean protease inhibitor(s) Carcinogenesis. 1995;16:1843–1846. doi: 10.1093/carcin/16.8.1843. [DOI] [PubMed] [Google Scholar]
- 142.Kelfkens G., de Gruijl F.R., van der Leun J.C. Tumorigenesis by short-wave ultraviolet A: papillomas versus squamous cell carcinomas. Carcinogenesis. 1991;12:1377–1382. doi: 10.1093/carcin/12.8.1377. [DOI] [PubMed] [Google Scholar]
- 143.Xu F., Yan S., Wu M., Li F., Song W., Zhao J. Ambient ozone pollution as a risk factor for skin disorders. Br J Dermatol. 2011;165:224–225. doi: 10.1111/j.1365-2133.2011.10349.x. [DOI] [PubMed] [Google Scholar]
- 144.Liu W., Pan X., Vierkotter A., Guo Q., Wang X., Wang Q. A Time-Series Study of the Effect of Air Pollution on Outpatient Visits for Acne Vulgaris in Beijing. Skin Pharmacol Physiol. 2018;31:107–113. doi: 10.1159/000484482. [DOI] [PubMed] [Google Scholar]
- 145.Krutmann J., Moyal D., Liu W., Kandahari S., Lee G.S., Nopadon N. Pollution and acne: is there a link? Clin Cosmet Investig Dermatol. 2017;10:199–204. doi: 10.2147/CCID.S131323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li A., Fan L., Xie L., Ren Y., Li L. Associations between air pollution, climate factors and outpatient visits for eczema in West China Hospital, Chengdu, south-western China: a time series analysis. J Eur Acad Dermatol Venereol. 2018;32:486–494. doi: 10.1111/jdv.14730. [DOI] [PubMed] [Google Scholar]
- 147.He Q.C., Tavakkol A., Wietecha K., Begum-Gafur R., Ansari S.A., Polefka T. Effects of environmentally realistic levels of ozone on stratum corneum function. Int J Cosmet Sci. 2006;28:349–357. doi: 10.1111/j.1467-2494.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 148.Slaper H., Velders G.J., Daniel J.S., de Gruijl F.R., van der Leun J.C. Estimates of ozone depletion and skin cancer incidence to examine the Vienna Convention achievements. Nature. 1996;384:256–258. doi: 10.1038/384256a0. [DOI] [PubMed] [Google Scholar]
- 149.Goldsmith L.A. Skin effects of air pollution. Otolaryngol Head Neck Surg. 1996;114:217–219. doi: 10.1016/S0194-59989670169-9. [DOI] [PubMed] [Google Scholar]
- 150.Moan J., Dahlback A. The relationship between skin cancers, solar radiation and ozone depletion. Br J Cancer. 1992;65:916–921. doi: 10.1038/bjc.1992.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee S.G., Ko N.Y., Son S.W., Bae H.J., Ha J.S., Pak H.Y. The impact of ozone depletion on skin cancer incidence in Korea. Br J Dermatol. 2013;169:1164–1165. doi: 10.1111/bjd.12472. [DOI] [PubMed] [Google Scholar]
- 152.Abarca J.F., Casiccia C.C. Skin cancer and ultraviolet-B radiation under the Antarctic ozone hole: southern Chile, 1987-2000. Photodermatol Photoimmunol Photomed. 2002;18:294–302. doi: 10.1034/j.1600-0781.2002.02782.x. [DOI] [PubMed] [Google Scholar]
- 153.Madronich S., de Gruijl F.R. Stratospheric ozone depletion between 1979 and 1992: implications for biologically active ultraviolet-B radiation and non-melanoma skin cancer incidence. Photochem Photobiol. 1994;59:541–546. doi: 10.1111/j.1751-1097.1994.tb02980.x. [DOI] [PubMed] [Google Scholar]
- 154.Diffey B.L. Stratospheric ozone depletion and the risk of non-melanoma skin cancer in a British population. Phys Med Biol. 1992;37:2267–2279. doi: 10.1088/0031-9155/37/12/008. [DOI] [PubMed] [Google Scholar]
- 155.Kerr R.A. Milankovitch Climate Cycles Through the Ages: Earth’s orbital variations that bring on ice ages have been modulating climate for hundreds of millions of years. Science. 1987;235:973–974. doi: 10.1126/science.235.4792.973. [DOI] [PubMed] [Google Scholar]
- 156.Hakkinen S., Rhines P.B. Decline of subpolar North Atlantic circulation during the 1990s. Science. 2004;304:555–559. doi: 10.1126/science.1094917. [DOI] [PubMed] [Google Scholar]
- 157.Minnis P., Harrison E.F., Stowe L.L., Gibson G.G., Denn F.M., Doelling D.R. Radiative climate forcing by the mount pinatubo eruption. Science. 1993;259:1411–1415. doi: 10.1126/science.259.5100.1411. [DOI] [PubMed] [Google Scholar]
- 158.Carslaw K.S., Harrison R.G., Kirkby J. Cosmic rays, clouds, and climate. Science. 2002;298:1732–1737. doi: 10.1126/science.1076964. [DOI] [PubMed] [Google Scholar]
- 159.Tollefson J. Climate change: The case of the missing heat. Nature. 2014;505:276–278. doi: 10.1038/505276a. [DOI] [PubMed] [Google Scholar]
- 160.Mochizuki T., Ishii M., Kimoto M., Chikamato Y., Watanabe M., Nozawa T. Pacific decadal oscillation hindcasts relevant to near-term climate prediction. Proc Natl Acad Sci USA. 2010;107:1833–1837. doi: 10.1073/pnas.0906531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carslaw K. Atmospheric physics: Cosmic rays, clouds and climate. Nature. 2009;460:332–333. doi: 10.1038/460332a. [DOI] [PubMed] [Google Scholar]
- 162.Singer S.F. Global climate controversy. Jama. 1996;276:373–374. [PubMed] [Google Scholar]
- 163.Riley P., Lionello R., Linker J.A., Owens M.J. The State of the Solar Wind, Magnetosphere, and Ionosphere During the Maunder Minimum. Proc Int Astron Union. 2018;13:247–250. doi: 10.1017/S1743921318001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eddy J.A. The maunder minimum. Science. 1976;192:1189–1202. doi: 10.1126/science.192.4245.1189. [DOI] [PubMed] [Google Scholar]
- 165.Solanki S.K., Schussler M., Fligge M. Evolution of the Sun’s large-scale magnetic field since the Maunder minimum. Nature. 2000;408:445–447. doi: 10.1038/35044027. [DOI] [PubMed] [Google Scholar]
- 166.Brito L.A.R., Nascimento A., Marque C., Miot H.A. Seasonality of the hospitalizations at a dermatologic ward (2007-2017) An Bras Dermatol. 2018;93:755–758. doi: 10.1590/abd1806-4841.20187309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang X., Towers S., Panchanathan S., Chowell G. A population based study of seasonality of skin and soft tissue infections: implications for the spread of CA-MRSA. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Andrade P.R., Pinheiro R.O., Sales A.M., Ximena I.R., de Mattos B.M.G., Ozório M.M. Type 1 reaction in leprosy: a model for a better understanding of tissue immunity under an immunopathological condition. Expert Rev Clin Immunol. 2015;11:391–407. doi: 10.1586/1744666X.2015.1012501. [DOI] [PubMed] [Google Scholar]
- 169.Viana D.V., Ignotti E. The ocurrence of dengue and weather changes in Brazil: A systematic review. Rev Bras Epidemiol. 2013;16:240–256. doi: 10.1590/S1415-790X2013000200002. [DOI] [PubMed] [Google Scholar]
- 170.Kovats R.S., Bouma M.J., Hajat S., Worrall E., Haines A. El Nino and health. Lancet. 2003;362:1481–1489. doi: 10.1016/S0140-6736(03)14695-8. [DOI] [PubMed] [Google Scholar]
- 171.Ferreira de Souza R.A., Andreoli R.V., Toshie Kayano M., Lima Carvalho A. American cutaneous leishmaniasis cases in the metropolitan region of Manaus, Brazil: association with climate variables over time. Geospat Health. 2015;10:314. doi: 10.4081/gh.2015.314. [DOI] [PubMed] [Google Scholar]
- 172.Gutierrez E.L., Galarza C., Ramos W., Mendoza M., Smith M.E., Ortega-Loayza A.G. Influence of climatic factors on the medical attentions of dermatologic diseases in a hospital of Lima, Peru. An Bras Dermatol. 2010;85:461–468. doi: 10.1590/s0365-05962010000400007. [DOI] [PubMed] [Google Scholar]
- 173.Gouveia N. Saúde e meio ambiente nas cidades: os desafios da saúde ambiental. Saude Soc. 1999;8:49–61. [Google Scholar]






