Abstract
Background.
Endogenous opioids regulate pain, drug reward, and stress responses. We have previously shown reduced hypothalamic-pituitary-adrenal (HPA) responses to psychological stress and to opioid blockade among dependent smokers. In this study, we examined the extent to which biologically confirmed nicotine withdrawal alters endogenous opioid regulation of HPA axis functioning during rest and in response to acute stress.
Design and Methods.
Smokers were randomly assigned to one of two conditions; 24 hr withdrawal from all nicotine-containing products (n = 62) or smoking ad libitum (n = 44). A non-smoking comparison group (n = 43) was also included. Participants (85 males and 64 females) completed two acute stress sessions during which a placebo or 50 mg of naltrexone (opioid antagonist) were administered using a double-blind design. Blood and saliva samples (assayed for cortisol and adrenocorticotropic hormone, i.e., ACTH) and mood measures were obtained during a resting absorption period, after acute stress (public speaking, mental arithmetic, and cold pressor tasks), and during an extended recovery period.
Results.
Opioid blockade (naltrexone) was associated with increased ACTH and cortisol responses to stress, and tobacco withdrawal was associated with blunted hormonal responses. A pattern of sex differences also emerged, with women exhibiting reduced ACTH responses to stress and higher ACTH and plasma cortisol response to opioid blockade.
Conclusions.
Compared to ad libitum smoking, nicotine withdrawal is associated with blunted opioid modulation of the HPA axis. Sex may modulate these effects. Blunted endogenous opioid regulation may underlie an incentive process that reinforces smoking behavior and may warrant therapeutic attention.
Keywords: Endogenous opioid function, nicotine withdrawal, Stress, HPA, addiction
Introduction
Stress events normally activate several biological systems, including the HPA axis and the sympathetic nervous system (1–4). The HPA axis performs a central function in directing the neuroendocrine response to stress; and it plays a critical role as a mediator of stress effects on health (4–7). Endogenous opioids are naturally occurring, opiate-like substances that have been shown to regulate mood, pain, and the reinforcing properties of many drugs of addiction (8, 9). Endogenous opioids are released in response to stress and are directly involved in regulating the HPA stress response (10–13).
Regulation of the HPA stress response by the endogenous opioid system involves three points of interface (14, 15). These include direct inhibitory input from beta-endorphin neurons in the arcuate nucleus to the corticotropin-releasing factor (CRF) neurons in the hypothalamus, resulting in reduced ACTH release (16); decreased noradrenergic release from the locus coeruleus by opioid neurons within the brainstem (17–19); and indirect inhibitory effects of CRF activity by inhibiting the CRF-stimulatory norepinephrine neurons (17, 20). Opioid neurons also innervate the nucleus accumbens and prefrontal cortex, leading to increased dopamine release in the mesolimbic pathway (21–23). Blocking the endogenous opioid system using opioid antagonists disinhibits opioid regulation of the HPA axis. This results in increased HPA activity, evidenced by an acute increase in ACTH and cortisol production (24, 25). Opioid antagonists, such as intravenous naloxone or oral naltrexone, have been established as a test of the functional status of hypothalamic opioid tone (12, 26–28).
The stimulating effects of acute doses of nicotine on the HPA axis and on the endogenous opioid system have been documented in several laboratories (29–32). Nicotine’s effects on the HPA axis and on the endogenous opioid system are centrally mediated, although the specific pathways have not been fully elucidated. Nicotine stimulates opioidergic, dopaminergic, noradrenergic, and serotonergic neurotransmission (33, 34). It is possible that chronic nicotine exposure achieved through smoking alters these neuronal networks, leading to adaptation, including reduced opioid inputs to the paraventricular nucleus (PVN), which results in enhanced basal HPA activation and reduced stress response. Decreased opioid regulation may, then, lead to diminished effects of HPA axis opioid neurons, thereby attenuating HPA responses to opioid blockade challenges.
Smoking withdrawal may lead to acute changes in various neurobiological systems (35–37), though the characteristics and consequences of these changes are not well understood. Withdrawal may lead to a rebound increase in HPA and opioid activity; and the intensity of these changes may determine the emotional and behavioral difficulties that smokers encounter during smoking withdrawal. Decreased opioid regulation is one possible mechanism for altered HPA activity among chronic smokers; and this would be supported by diminished HPA axis regulation as a consequence of opioid blockade. Therefore, we hypothesized that smokers would show attenuated ACTH and cortisol responses to stress relative to non-smokers, and we expected that this attenuation would be most pronounced during nicotine withdrawal. We also hypothesized that, relative to non-smokers, smokers would exhibited diminished responses to opioid blockade, especially smokers experiencing acute tobacco withdrawal. To test these hypotheses, we examined endogenous opioid activity by assessing HPA responses to a 50 mg oral dose of the opioid antagonist naltrexone and to acute psychological stressors under distinct conditions: 1) ad libitum smoking and 2) smoking withdrawal compared to 3) non-smoking controls.
Because previous studies indicate sex differences in HPA responses to acute stress (38) and in HPA response to naltrexone (e.g., (39)), we also examined if sex modulates the effects of stress and opioid blockade in smokers and non-smokers. Females, compared to males, tend to have smaller cortisol responses to psychosocial stress (40, 41); therefore, we predicted that, relative to men, women would exhibit blunted stress responses. Previous studies also suggest that, compared to males, females exhibit enhanced responses to opioid blockade (42, 43). Furthermore, HPA stress responses of females are more heavily regulated by the endogenous opioid system (44–46). Thus, we predicted that, relative to men, women would exhibit pronounced responses to opioid blockade.
Methods
Design
To test our hypotheses, male and female non-smokers and smokers completed two acute stress laboratory assessment sessions: one following administration of placebo and one following naltrexone. Prior to the first assessment session, smokers were assigned randomly to one of two smoking conditions: ad libitum (smoking as usual, with the last cigarette smoked < 1 hour before the stress assessments), withdrawal (a period of smoking deprivation > 24 hours preceding the stress assessments); and non-smokers were included as a comparison group. To assess the impact of naltrexone and acute stress on stress hormones, blood and saliva samples were collected repeatedly within both assessment sessions (including samples before and after naltrexone/placebo administration as well as before and after exposure to an acute stress protocol). Subjective states were also measured repeatedly within both sessions (see below for details).
Participant Recruitment & Eligibility
Flyers posted around the campus community and online advertisements were used to recruit participants aged 18–75 years in the Minneapolis-St. Paul metropolitan area. Inclusion criteria were as follows: 1) no regular use of prescribed nor over-the-counter medications, except contraceptives; 2) no current diagnosis nor prior treatment of hypertension, renal or hepatic disease, nor cardiac or other chronic diseases (e.g., coronary heart disease, diabetes, neurological disorders, thyroid disorders, respiratory disorders); 3) no current nor history of major psychiatric disorders (e.g., depression, schizophrenia, alcohol and drug abuse); 4) no current opiate dependence, nor recent daily opiate use, nor use of any narcotic medication within 3 days before the study; 5) no pregnancy; 6) weight within ±30% of Metropolitan Life Insurance norms; 7) smokers needed to smoke at least 10 cigarettes per day for the past 2 years and they had to be disinterested in quitting smoking; and 8) non-smokers were required to have smoked less than 100 cigarettes in their lifetime and they could not have smoked for the previous 5 years. This study was approved by the Institutional Review Board (IRB) of the University of Minnesota.
During the medical screening, participants completed a demographic questionnaire, the Profile of Mood States questionnaire (POMS)(47), and the Perceived Stress Scale (PSS)(48). Participants were also asked about daily caffeine consumption and about average hours of sleep per day. In addition, smokers were asked about patterns of tobacco use (e.g., cigs/day) and they completed the Fagerström Test of Nicotine Dependence (FTND)(49).
A total of 209 individuals were enrolled after the on-site medical screening, of whom 171 attended the first laboratory session (72 smokers assigned to withdrawal, 50 ad lib smokers, and 49 non-smokers). There were no differences in demographics (age, sex ratio, education), body mass index (BMI), mood (PSS, POMS), nor smoking history (cigarettes per day, years of smoking, FTND) between those who dropped after the medical screening and those who participated in the first lab session (ps > .20). One-hundred and forty-nine of the 171 participants who completed the first lab also completed the second lab session. We found no differences in smoking condition assignment, demographics, mood, nor smoking history between those who completed both labs and those who only completed the first lab (ps > .05). Those who terminated after the first lab had higher BMIs than those who completed both labs, F(1, 168) = 5.21, p = .02. Only data from participants who completed both sessions were retained for analyses. The final sample (N = 149; 85 men and 64 women) consisted of 62 smokers assigned to the smoking withdrawal condition, 44 smokers assigned to the ad lib condition, and 43 non-smokers.
Procedure
Participants who met preliminary inclusion criteria assessed via a phone interview were invited to an on-site medical screening to examine height and weight and to confirm via interview the absence of contraindications to administration of naltrexone (including use of narcotic medications), current health conditions, and recent medical history. Participants were asked to read and sign the consent form before completing demographic, psychosocial, and smoking-related measures. Participants also reported daily caffeine consumption and average hours of sleep per day. Then, they were scheduled for two laboratory sessions, each of which lasted about 4 hours, during which participants were tested individually. The two lab sessions took place approximately 10 days apart; and all laboratory sessions started at approximately noon to control for diurnal variations in hormones. Before each session, participants were instructed to abstain from alcohol and analgesic medications for 24 hours. Randomization to smoking condition (ad libitum or withdrawal) took place at the end of the medical screening. Smokers who were assigned to the ad libitum smoking condition were asked to smoke cigarettes of their preferred brand at their own pace for 24 hours before each session and they were given a smoking break during the assessment sessions so that the time of last cigarette was < 1 hour before acute stress (see Figure 1). Smokers who were assigned to the smoking withdrawal condition were instructed to abstain from all tobacco and nicotine for 24 hours before each session and they were not allowed to use any nicotine products until the session was over. Non-smokers completed the same protocol except they were not randomized to a smoking condition.
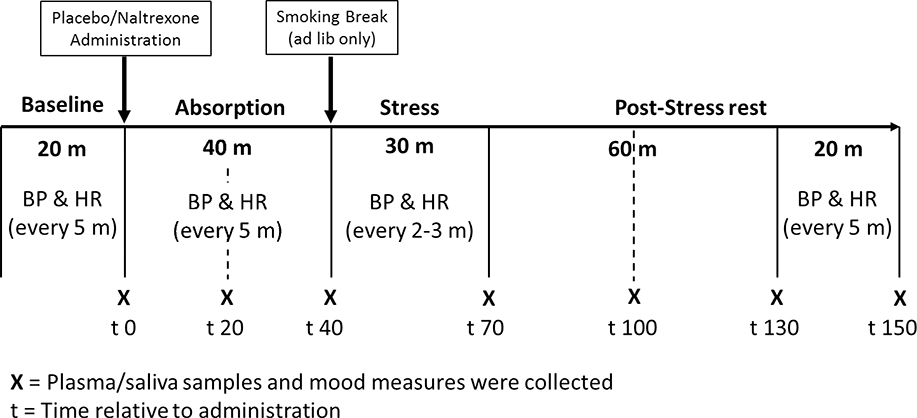
Figure 1.
Laboratory protocol
Figure 1. Laboratory protocol. Participants sat quietly and watched Planet Earth (nature documentary) for an initial baseline period (20 min), during which blood pressure (BP) and heart rate (HR) were obtained every five minutes. Then, participants completed mood and smoking withdrawal questionnaires and the first blood and saliva samples before ingesting a capsule containing either 50 mg of naltrexone or placebo at the start of a 40-minute ‘absorption’ period. During absorption, BP and HR were obtained at 5-minute intervals; and self-report measures as well as plasma and saliva samples were collected after 20- and 40-minutes during this time. After absorption, smokers in the ad libitum condition were asked to smoke one cigarette of their preferred brand. Then, all participants completed the stress tasks, during which BP and HR were collected every 2 to 3 minutes. Self-report measures and blood and saliva samples were collected after the stress tasks and then after 30-, 60-, and 80-minutes of post-stress rest during which they continued to watch Planet Earth. BP and HR were collected every 5 minutes during the last 20 minutes of the post-stress rest period.
Upon arrival to the lab sessions, participants completed a urine test to screen for drugs, including opioids. Females’ urine was also tested for pregnancy. A Bedfont Micro+ monitor (coVita, Haddonfield, NJ) was used to measure expired carbon monoxide (CO) in all smokers to verify smoking status (and withdrawal). Smokers in the withdrawal condition who had CO of 9ppm or higher were rescheduled. Participants were brought to a testing room and provided with a standard lunch. After lunch, an IV catheter was inserted and a blood pressure cuff was attached. As can be seen in Figure 1, the rest of the laboratory protocol consisted of seven periods: baseline, absorption 1, absorption 2, stress, post-stress rest 1, post-stress rest 2, and post-stress rest 3. After the baseline period, participants ingested a capsule containing either 50 mg of naltrexone or an identical placebo (one at each lab, in a random order) under double-blind conditions. Previous research in humans demonstrates the effectiveness of this dose in blocking the inhibitory effects of the endogenous opioid system on the HPA axis and demonstrates that this dose is adequate to produce nearly complete blockade (95%) of μ-opioid receptors (50, 51). The absorption period lasted 40 minutes to allow peak plasma concentration of the drug to be achieved (52). BP, HR, blood and saliva samples, self-reported mood, and smoking withdrawal questionnaires were collected at the end of each protocol period (see Figure 1).
Apparatus and measures
Acute stressors.
The stress protocol used in this study has been validated to induce hormonal, cardiovascular, and mood state changes in nicotine-dependent men and women (53–55). The stressor tasks were given in a fixed order and included a public speaking task, mental arithmetic, and a cold pressor test (CPT). For public speaking, the participant was given a topic and asked to prepare (4 min) and deliver (4 min) a speech that would be video recorded and evaluated by staff members. For the mental arithmetic task, the participant was given a three-digit starting number and they were asked to calculate the sum of the three digits and then add that sum to the three-digit number. They were asked to continue the series of calculations for 8 minutes. For the CPT, the participant was asked to immerse their dominant hand in a container filled with ice-water slurry (0–4°C).
Biological measures.
During both lab sessions, blood and saliva samples were collected once before drug (naltrexone or placebo) administration and six times thereafter (see Figure 1). Intravenous catheter was used to collect blood samples. Plasma samples were stored in −80 °C freezers and assayed for ACTH and cortisol at the Fairview Hospital (Minneapolis, MN) using chemiluminescent immunoassays with the Immulite® 2000 ACTH kit and the ADVIA Centaur® cortisol kit (Siemens Medical Solutions USA, Malvern, PA). Inter-assay coefficients of variation were <10% and <8% for plasma ACTH and plasma cortisol, respectively. Salivette® tubes (Sarstedt, Numbrecht, Germany) were used to collect saliva samples, which were stored in −20 °C freezers and assayed for cortisol using time-resolved fluorescence immunoassay (cortisol-biotin conjugation). The assay kits (IBL International) had a sensitivity of 0.4 nmol/L and inter- and intra-assay coefficients of variation were less than 10% and 12%, respectively. Cotinine was measured using enzyme linked immunoassays (DRG Diagnostics; inter- and intra-assay variability < 12%).
Self-report measures.
Distress and positive affect were measured using a questionnaire used in our previous studies (56), using response scales that ranged from 0 ‘Not at all’ to 7 ‘Very strong’. Distress consisted of the sum of responses to items of anxiety, irritability, impatience, and restlessness. Positive affect was determined by the sum of responses to items of cheerfulness, contentedness, calmness, controllability, and interest. Physical symptoms were scored by summing responses to items of headache, sweating, tremor, stomachache, drowsiness, fatigue, and coughing. Tobacco withdrawal symptoms and craving were measured by the Minnesota Nicotine Withdrawal Scale (MNWS) (57, 58). Smoking urges were measured by the Questionnaire on Smoking Urges-brief (QSU-B) (59, 60), which captures two aspects of smoking urges: strong desire to smoke (Factor 1) and anticipated relief from negative affect (Factor 2). At the end of each laboratory session, participants completed a form that included commonly reported side effects of naltrexone, including nausea, vomiting, agitation, anxiety, lightheadedness, dizziness, and sedation. This form has been used in previous studies in which naltrexone was administered (61).
Data analysis
Demographic, psychosocial, and smoking-related sample characteristics were analyzed using 3 (Smoking Group) x 2 (Sex) ANOVAs and chi-square tests. The primary hormonal outcome variables were ACTH and cortisol, all of which were log-transformed to meet normality assumptions. Hormonal, subjective stress (affect), and withdrawal measures were analyzed using a 3 Smoking Group (non-smokers, ad libitum smokers, withdrawal smokers) x 2 Sex (female, male) x 2 Drug Condition (placebo, naltrexone) x 7 Time (baseline, absorption 1, absorption 2, stress, post-stress rest 1, post-stress rest 2, post-stress rest 3) MANOVA. Significant three-way and four-way interactions including Time and Drug Condition were followed-up by creating an index of the difference in area under the curve (AUCΔ) between the two drug conditions. To do this, we first calculated AUC using the trapezoid method to reflect the stress response (between period 4 [immediately post-stress] and period 7 [after post-stress rest 3]) during the naltrexone and placebo sessions, respectively. Then, AUC of placebo was subtracted from that of naltrexone to create an index of the drug effect (AUCΔ). Finally, we used ANOVA to examine the effects of Smoking Group and Sex on AUCΔ.
Wilks’ Lambda is reported for all MANOVA procedures. All significant main effects of Smoking Group were followed-up with multiple comparison tests. Bonferroni correction was applied to simple effects tests; and p-values less than .05 were considered significant. Variation existed between sample size and degrees of freedom for reported results due to occasional missing data. SPSS version 24 (IBM Corp., Armonk, NY) was used for the data analysis.
Results
Participant characteristics
Participant characteristics are summarized in Table 1. Smokers and non-smokers did not differ in age, BMI, menstrual cycle phases during lab sessions, relationship status, race, daily caffeine consumption, average hours of sleep per day, nor total mood disturbance (POMS), ps > .05. Compared to non-smokers, smokers reported fewer years of education, F(2, 141) = 11.0, p < .001, η2 = .14. Smokers in both conditions had less educations than non-smokers (p < .001), but there was no difference between the two smoking conditions (p > .10). Smokers in the ad libitum condition had higher levels of perceived stress (PSS) than non-smokers, F(2, 142) = 7.75, p = .001, η2 = .10 (pairwise comparison: p < .001). There were no differences in PSS between the two smoking conditions (p = .16). Consistent with previous research (80), women reported higher levels of total mood disturbance than men, F(1, 141) = 6.04, p = .02, η2 = .04. There were no Smoking Group x Sex interactions for demographic nor psychosocial variables (ps > .05). On average, smokers in this study smoked 15.1 cigarettes per day (SD = 6.0) for 11.4 years (SD = 10.5). Men reported smoking more cigarettes per day than women, F(1, 100) = 4.36, p = .04, η2 = .04. Smokers in the two conditions (ad lib and withdrawal) did not differ in cigarettes per day, smoking duration, age when they started smoking, nor FTND scores (ps > .05). As expected, smokers in the withdrawal condition had lower CO (lab 1: F(1, 102) = 111, p < .001, η2 = .52; lab 2: F(1, 101) = 96.6, p < .001, η2 = .49) and lower cotinine (lab 1: F(1, 99) = 6.91, p = .01, η2 = .07; lab 2: F(1, 98) = 8.27, p = .005, η2 = .08) compared to smokers in the ad lib condition.
Table 1.
Sample characteristics.
| Non-smokers (n=43) | Smokers (ad lib) (n=44) | Smokers (withdrawal) (n=62) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Female (n=22) | Male (n=21) | Female (n=17) | Male (n=27) | Female (n=25) | Male (n=37) | |
|
| ||||||
| Age (yr) | 33.9 (2.7) | 39.1 (2.7) | 32.2 (3.0) | 35.1 (2.4) | 33.0 (2.5) | 35.9 (2.1) |
| BMI (kg/m2) | 25.9 (1.2) | 26.8 (1.2) | 28.2 (1.4) | 27.4 (1.1) | 29.3 (1.2) | 24.8 (0.9) |
| Education (yr)a | 15.9 (0.5) | 14.7 (0.6) | 13.4 (0.6) | 12.6 (0.5) | 13.2 (0.5) | 13.2 (0.4) |
| Single (%) | 61.9 | 65.0 | 86.7 | 76.0 | 76.0 | 88.6 |
| Caucasian (%) | 81.0 | 71.4 | 68.8 | 76.9 | 58.3 | 75.7 |
| Menstrual phase matched at labs (%) | 46.2 | N/A | 54.5 | N/A | 66.7 | N/A |
| Caffeine (cups/day) | 0.8 (0.3) | 0.5 (0.3) | 0.7 (0.3) | 1.1 (0.3) | 1.0 (0.3) | 1.2 (0.2) |
| Sleep (hr/day) | 7.6 (0.2) | 7.6 (0.2) | 7.5 (0.3) | 7.1(0.2) | 7.4 (0.2) | 6.9 (0.2) |
| POMSb | 17.9 (5.0) | 3.3 (5.1) | 14.7 (5.8) | 13.3 (4.6) | 20.5 (4.7) | 7.2 (3.8) |
| PSSa | 16.8 (0.8) | 17.8 (0.8) | 20.8 (0.9) | 19.9 (0.7) | 19.8 (0.7) | 18.1 (0.6) |
| Cigarettes (daily)b | N/A | N/A | 14.9 (1.4) | 14.7 (1.1) | 12.1 (1.2) | 17.2(0.9) |
| Smoking Duration (yr) | N/A | N/A | 9.7 (2.6) | 10.3 (2.1) | 10.2 (2.2) | 13.8 (1.7) |
| Age Started Smoking (yr) | N/A | N/A | 15.1 (1.1) | 17.0 (0.9) | 15.5 (1.0) | 15.7 (0.8) |
| FTND | N/A | N/A | 5.6 (0.5) | 5.4 (0.4) | 4.6 (0.4) | 5.6 (0.4) |
| CO (ppm)c | ||||||
| Lab 1 | N/A | N/A | 18.6 (1.7) | 15.8 (1.3) | 2.4 (1.4) | 3.0 (1.1) |
| Lab 2 | N/A | N/A | 17.5 (1.7) | 15.7 (1.4) | 2.3 (1.4) | 2.8 (1.2) |
| Cotinine (ng/ml)c | ||||||
| Lab 1 | N/A | N/A | 197.5 (44.9) | 221.0 (34.1) | 83.5 (34.8) | 145.2 (28.6) |
| Lab 2 | N/A | N/A | 241.1 (47.6) | 221.2 (40.0) | 93.7 (35.7) | 152.9 (29.3) |
Unless indicated, entries show mean and standard error of the mean.
Notes:
Smoking Group effect was significant [Education: smokers in both conditions had fewer years of educations than non-smokers (p < .001); PSS: smokers in the ad libitum condition had higher levels of perceived stress than non-smokers. There were no difference in education or PSS between the two smoking conditions]
Sex effect was significant
Smoking Group effect was significant.
BMI = body mass index. POMS = Profile of Mood States questionnaire (range: 32– 97). PSS = Perceived Stress Scale (range: 10 – 30). FTND = Fagerström Test of Nicotine Dependence (range: 1–10). CO = carbon monoxide.
Hormonal measures
Table 2 and Figure 2 depicts estimated means and standard errors by sex and smoking group for all hormonal measures.
Table 2.
Hormonal measures.
| Non-smokers | Smokers (ad lib) | Smokers (withdrawal) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Female | Male | Female | Male | Female | Male | |||||||
|
| ||||||||||||
| Placebo | Naltrexone | Placebo | Naltrexone | Placebo | Naltrexone | Placebo | Naltrexone | Placebo | Naltrexone | Placebo | Naltrexone | |
|
| ||||||||||||
| ACTH a,b,c,d,e,f (pg/ml) | ||||||||||||
| Baseline | 19.2 (2.5) | 21.5 (2.4) | 17.7 (2.5) | 17.1 (2.4) | 20.1 (2.6) | 25.1 (2.1) | 19.5 (1.9) | 17.2 (1.8) | 20.9 (3.0) | 23.1 (2.9) | 20.1 (1.8) | 19.1 (1.7) |
| Absorption 1 | 17.1 (1.8) | 17.9 (2.0) | 13.7 (1.8) | 13.9 (2.0) | 15.5 (1.9) | 18.3 (2.1) | 15.0 (1.4) | 13.1 (1.5) | 20.9 (2.2) | 23.6 (2.4) | 16.1 (1.3) | 15.2 (1.4) |
| Absorption 2 | 16.6 (1.6) | 18.0 (3.0) | 13.6 (1.6) | 16.0 (3.0) | 14.0 (1.6) | 16.3 (3.2) | 13.8 (1.2) | 14.3 (2.3) | 19.0 (1.9) | 24.2 (3.6) | 14.7 (1.1) | 16.3 (2.1) |
| Stress | 26.8 (5.2) | 39.8 (11.8) | 25.9 (5.2) | 51.0 (11.8) | 29.3 (5.4) | 41.8 (12.3) | 27.0 (3.9) | 48.0 (8.9) | 26.8 (6.3) | 34.0 (14.2) | 26.7 (3.6) | 40.3 (8.2) |
| Post-stress 1 | 17.8 (2.5) | 34.1 (10.9) | 20.5 (2.5) | 36.4 (10.9) | 19.8 (2.6) | 33.2 (11.3) | 20.2 (1.9) | 29.6 (8.2) | 21.2 (3.0) | 25.8 (13.1) | 19.0 (1.8) | 34.2 (7.5) |
| Post-stress 2 | 15.2 (2.0) | 34.9 (8.9) | 15.5 (2.0) | 22.1 (8.9) | 16.5 (2.1) | 25.8 (9.3) | 16.2 (1.5) | 20.7 (6.7) | 20.9 (2.4) | 23.7 (10.7) | 16.1 (1.4) | 27.4 (6.2) |
| Post-stress 3 | 14.2 (2.2) | 39.7 (9.3) | 14.5 (2.2) | 19.2 (9.3) | 16.8 (2.3) | 24.8 (9.7) | 14.0 (1.7) | 15.1 (7.0) | 23.4 (2.7) | 23.9 (11.2) | 15.1 (1.5) | 26.4 (6.4) |
| Plasma cortisol a,b,c,e,f,g (ug/dl) | ||||||||||||
| Baseline | 11.7 (1.1) | 12.2 (0.8) | 8.6 (1.1) | 9.3 (0.8) | 11.8 (1.1) | 11.3 (0.8) | 9.4 (0.9) | 9.0 (0.6) | 7.9 (1.1) | 9.3 (0.8) | 10.6 (0.7) | 9.9 (0.5) |
| Absorption 1 | 10.2 (0.9) | 11.0 (0.7) | 7.0 (0.9) | 7.4 (0.7) | 9.4 (0.9) | 9.9 (0.8) | 7.5 (0.7) | 7.2 (0.6) | 7.6 (0.9) | 8.3 (0.7) | 8.4 (0.6) | 8.3 (0.5) |
| Absorption 2 | 8.9 (0.8) | 9.0 (0.8) | 6.0 (0.8) | 6.7 (0.8) | 8.3 (0.8) | 8.3 (0.8) | 6.2 (0.6) | 6.2 (0.6) | 6.4 (0.8) | 7.8 (0.8) | 7.6 (0.5) | 7.6 (0.5) |
| Stress | 10.2 (0.9) | 13.0 (1.3) | 12.2 (0.9) | 14.0 (1.3) | 9.4 (1.0) | 15.8 (1.4) | 10.3 (0.7) | 15.9 (1.1) | 8.6 (0.9) | 12.2 (1.3) | 10.2 (0.6) | 12.3 (0.9) |
| Post-stress 1 | 9.7 (1.0) | 15.4 (1.4) | 11.7 (1.1) | 16.3 (1.4) | 9.9 (1.1) | 16.7 (1.5) | 9.6 (0.9) | 15.3 (1.1) | 9.0 (1.1) | 13.4 (1.4) | 9.9 (0.7) | 12.7 (1.0) |
| Post-stress 2 | 8.3 (0.8) | 14.8 (1.2) | 9.0 (0.8) | 12.4 (1.2) | 7.9 (0.9) | 15.7 (1.2) | 7.6 (0.7) | 12.0 (1.0) | 7.5 (0.8) | 11.8 (1.2) | 8.2 (0.6) | 10.9 (0.8) |
| Post-stress 3 | 7.0 (0.7) | 14.5 (1.1) | 7.6 (0.8) | 10.9 (1.1) | 7.1 (0.8) | 13.3 (1.1) | 5.7 (0.6) | 9.2 (0.9) | 6.7 (0.8) | 10.7 (1.1) | 6.6 (0.5) | 9.4 (0.7) |
| Salivary cortisol a,c,e,f,g,h (ng/ml) | ||||||||||||
| Baseline | 3.0 (0.5) | 3.8 (0.5) | 2.8 (0.5) | 3.4 (0.5) | 3.0 (0.8) | 3.1 (0.7) | 4.3 (0.5) | 4.2 (0.5) | 2.8 (0.5) | 2.9 (0.5) | 3.8 (0.4) | 3.4 (0.4) |
| Absorption 1 | 2.7 (0.3) | 2.9 (0.3) | 2.2 (0.4) | 2.5 (0.3) | 2.7 (0.5) | 2.1 (0.5) | 2.6 (0.3) | 3.0 (0.3) | 2.3 (0.3) | 2.4 (0.3) | 2.7 (0.3) | 2.4 (0.3) |
| Absorption 2 | 2.4 (0.3) | 2.4 (0.3) | 2.0 (0.3) | 2.1 (0.3) | 1.8 (0.4) | 2.2 (0.4) | 2.1 (0.3) | 2.2 (0.3) | 2.1 (0.3) | 2.1 (0.3) | 2.2 (0.2) | 2.2 (0.2) |
| Stress | 2.5 (0.4) | 3.5 (1.1) | 2.9 (0.4) | 5.0 (1.2) | 2.0 (0.5) | 5.1 (1.7) | 2.8 (0.4) | 7.2 (1.1) | 2.1 (0.4) | 2.4 (1.1) | 3.1 (0.3) | 4.8 (0.9) |
| Post-stress 1 | 2.3 (0.6) | 3.9 (1.2) | 4.6 (0.6) | 8.2 (1.3) | 2.7 (0.9) | 6.0 (1.9) | 2.8 (0.6) | 6.7 (1.2) | 2.3 (0.6) | 3.0 (1.3) | 3.1 (0.5) | 4.2 (1.0) |
| Post-stress 2 | 1.5 (0.3) | 4.4 (0.9) | 2.2 (0.3) | 5.1 (1.0) | 2.0 (0.5) | 6.1 (1.4) | 1.9 (0.3) | 3.3 (0.9) | 2.0 (0.3) | 2.7 (0.9) | 2.1 (0.3) | 3.7 (0.7) |
| Post-stress 3 | 1.6 (0.2) | 5.2 (0.7) | 2.0 (0.3) | 4.0 (0.8) | 1.7 (0.4) | 5.8 (1.1) | 2.0 (0.2) | 4.0 (0.7) | 2.0 (0.3) | 2.5 (0.7) | 1.9 (0.2) | 3.2 (0.6) |
Entries show mean and standard error of the mean. Variable names with asterisks showed significant effect(s) for the following:
Drug effect
Sex × Drug interaction
Time effect
Smoking Group × Time interaction
Sex × Time interaction
Drug × Time interaction
Smoking Group × Drug × Time interaction
Smoking Group × Drug interaction.
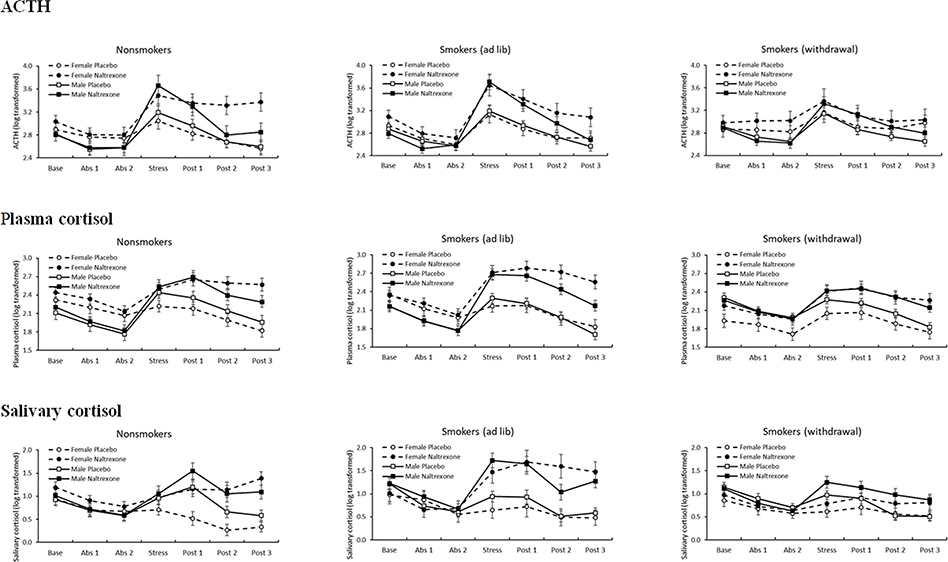
Figure 2.
Sex differences in drug effects on hormonal measures
Figure 2. Estimated means and standard error of the mean by sex and smoking group for ACTH, plasma cortisol, and salivary cortisol collected during rest before ingestion of the drug capsule, during rest absorption period, following acute stress, and during a recovery rest period.
Adrenocorticotropin.
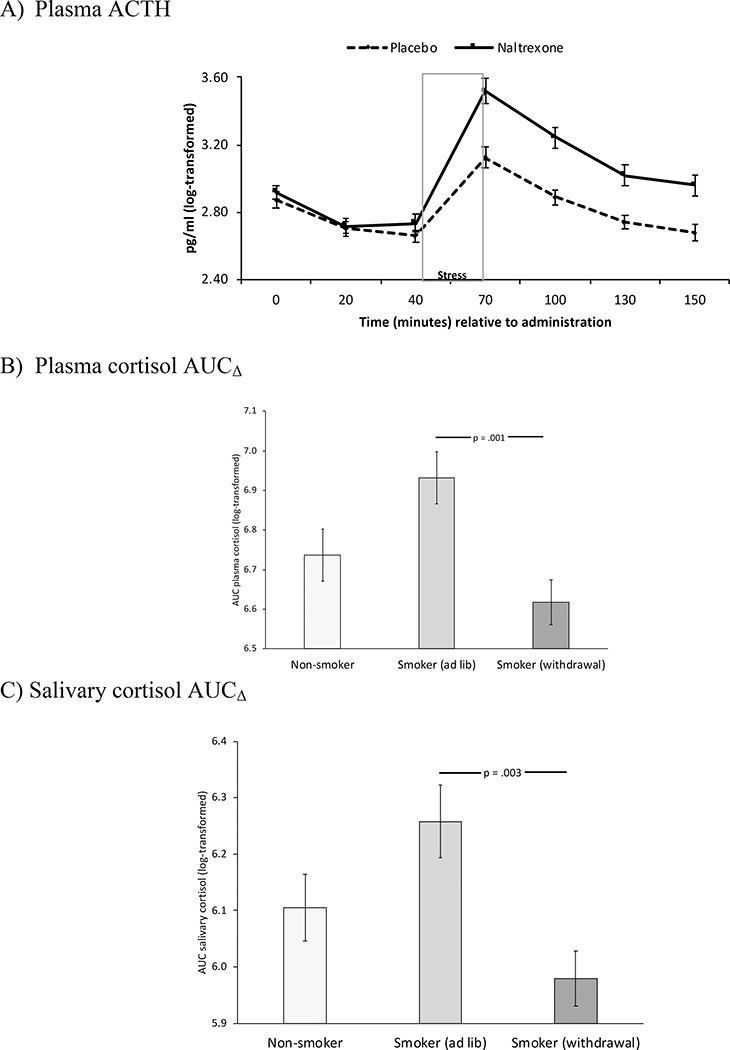
ACTH concentrations were higher during naltrexone labs, F(1, 91) = 36.2, p < .001, η2 = .29; and this effect was most pronounced among women (Drug x Sex), F(1, 91) = 4.35, p = .04, η2 = .05. All groups showed the expected ACTH increase in response to stress (Time), F(6, 86) = 45.8, p < .001, η2 = .76 (see Figure 2); however, there was evidence of Time x Drug, Time x Sex, and Time x Smoking Group effects. Specifically, ACTH responses to stress were greater after naltrexone than after placebo, F(6, 86) = 5.45, p < .001, η2 = .28 (see Figure 3A); and ACTH responses to stress were stronger among men than among women, F(6, 86) = 3.35, p = .005, η2 = .19. In addition, smokers in the withdrawal condition exhibited an attenuated ACTH stress response relative to those in the ad lib condition, F(12, 172) = 1.96, p = .03, η2 = .12. No other group differences were found.
Figure 3.
Hormonal measures
Figure 3. Estimated means and standard error of the mean for ACTH (3A), plasma cortisol (3B), and salivary cortisol (3C). Figure 3A depicts the Time x Drug interaction on ACTH. Figures 3B and 3C depict smoking group differences in the drug effect on cortisol, where AUCΔ indexes the drug effect (AUCnaltrexone - AUCplacebo) on cortisol AUC from immediately post-stress (period 4) to after the final rest (period 7).
Cortisol.
As seen in Figure 2, cortisol concentrations were higher during naltrexone labs (plasma cortisol: F(1, 118) = 76.9, p < .001, η2 = .39; salivary cortisol: F(1, 117) = 56.7p < .001,η2 = .34 ); and, for plasma cortisol, this effect was most pronounced among women (Drug x Sex), F(1, 118) = 7.95, p = .006, η2 = .06). We also found the expected Time x Sex interaction, F(plasma cortisol: F(6, 113) = 5.22, p < .001, η2 = .22; salivary cortisol: F(6, 112) = 5.08, p < .001, η2 = .21). Effects of naltrexone on the cortisol stress response differed across smoking groups, as indicated by a Smoking Group x Drug x Time interaction for both plasma cortisol, F(12, 226) = 2.14, p = .02, η2 = .10, and salivary cortisol, F(12, 224) = 1.90, p = .04, η2 = .09. A follow-up Smoking Group x Sex ANOVA conducted on AUCΔ revealed a main effect of Smoking Group for both plasma cortisol (F(2, 122) = 6.57, p = .002) and salivary cortisol (F(2, 128) = 5.85, p = .004), indicating greater drug-related cortisol responses in the ad lib condition than in the smoking withdrawal condition (see Figures 3B and 3C). No other group differences were found.
Self-report measures
Distress, positive affect, and physical symptoms.
Significant Smoking Group x Time interactions were found for distress (F(12, 248) = 2.12, p = .02, η2 = .09), positive affect (F(12, 242) = 2.01, p = .02, η2 = .09), and physical symptoms (F(12, 246) = 2.37, p = .007, η2 = .10; Table 3). Greater distress and physical symptoms during the baseline period were evident among smokers in the withdrawal condition relative to the other two groups, as indicated by follow-up comparisons at each period (Smoking Group effect at baseline, ps < .003). Similarly, baseline positive affect was lower in the smoking withdrawal condition than in the other two groups (ps ≤ .007). A Sex main effect indicated that, overall, women reported lower positive affect than men, F(1, 126) = 10.9, p = .001, η2 = .08.
Table 3.
Self-report measures of mood, withdrawal symptoms, and side effects.
| Non-smokers | Smokers (ad lib) | Smokers (withdrawal) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Female | Male | Female | Male | Female | Male | |||||||
|
| ||||||||||||
| Placebo | Naltrexone | Placebo | Naltrexone | Placebo | Naltrexone | Placebo | Naltrexone | Placebo | Naltrexone | Placebo | Naltrexone | |
|
| ||||||||||||
| Distress a,b,c | ||||||||||||
| Baseline | 3.0 (0.9) | 2.5 (1.0) | 1.8 (0.9) | 2.6 (1.0) | 3.8 (1.3) | 4.0 (1.3) | 2.8 (0.8) | 3.2 (0.9) | 7.0 (0.9) | 6.7 (0.9) | 5.7 (0.7) | 6.0 (0.8) |
| Absorption 1 | 2.6 (0.9) | 1.8 (1.0) | 1.5 (0.9) | 3.4 (1.0) | 3.6 (1.2) | 3.4 (1.3) | 2.9 (0.8) | 3.1 (0.9) | 6.5 (0.8) | 7.0 (0.9) | 5.5 (0.7) | 5.9 (0.7) |
| Absorption 2 | 3.3 (0.9) | 1.6 (1.0) | 2.2 (0.9) | 2.9 (1.0) | 5.2 (1.3) | 6.1 (1.4) | 3.6 (0.8) | 3.8 (0.9) | 6.3 (0.9) | 6.8 (0.9) | 5.8 (0.7) | 5.9 (0.8) |
| Stress | 5.2 (1.1) | 5.0 (1.1) | 6.1 (1.1) | 6.1 (1.1) | 5.6 (1.5) | 7.5 (1.5) | 5.7 (1.0) | 5.7 (1.0) | 8.3 (1.0) | 8.8 (1.0) | 7.3 (0.9) | 7.2 (0.8) |
| Post-stress 1 | 4.6 (1.2) | 3.4 (1.1) | 4.4 (1.2) | 4.1 (1.1) | 5.7 (1.7) | 6.9 (1.5) | 5.0 (1.1) | 5.2 (1.0) | 8.4 (1.1) | 8.2 (1.0) | 7.4 (0.9) | 6.8 (0.8) |
| Post-stress 2 | 4.4 (1.2) | 3.6 (1.1) | 4.3 (1.2) | 4.6 (1.1) | 6.0 (1.6) | 6.5 (1.4) | 5.9 (1.1) | 4.0 (1.0) | 7.5 (1.1) | 7.8 (1.0) | 6.1 (0.9) | 6.7 (0.8) |
| Post-stress 3 | 2.9 (1.2) | 2.8 (1.1) | 3.5 (1.2) | 4.0 (1.1) | 5.9 (1.6) | 5.6 (1.5) | 4.5 (1.1) | 3.7 (1.0) | 8.3 (1.1) | 7.1 (1.0) | 7.4 (0.9) | 7.2 (0.8) |
| Positive affect a,b,d | ||||||||||||
| Baseline | 14.3 (1.3) | 14.2 (1.5) | 20.3 (1.5) | 18.3 (1.6) | 15.4 (1.8) | 14.5 (2.0) | 18.8 (1.2) | 18.2 (1.4) | 13.3 (1.2) | 12.5 (1.4) | 16.2 (1.0) | 15.9 (1.1) |
| Absorption 1 | 14.7 (1.3) | 15.0 (1.5) | 19.0 (1.5) | 17.8 (1.6) | 15.5 (1.8) | 14.9 (2.0) | 18.6 (1.2) | 17.9 (1.4) | 12.0 (1.2) | 11.9 (1.4) | 16.7 (1.0) | 15.9 (1.1) |
| Absorption 2 | 14.0 (1.3) | 15.4 (1.4) | 18.8 (1.5) | 17.5 (1.6) | 15.1 (1.8) | 15.7 (2.0) | 17.8 (1.2) | 17.8 (1.4) | 11.6 (1.2) | 12.5 (1.4) | 16.1 (1.0) | 14.9 (1.1) |
| Stress | 10.0 (1.4) | 10.0 (1.5) | 13.4 (1.6) | 12.9 (1.7) | 12.2 (2.0) | 11.8 (2.1) | 16.0 (1.3) | 14.1 (1.4) | 10.1 (1.3) | 9.8 (1.4) | 14.7 (1.1) | 13.4 (1.2) |
| Post-stress 1 | 11.6 (1.4) | 12.6 (1.5) | 15.5 (1.5) | 15.1 (1.6) | 13.4 (1.9) | 11.8 (2.0) | 16.0 (1.3) | 15.8 (1.4) | 9.3 (1.3) | 10.9 (1.4) | 14.7 (1.1) | 13.2 (1.1) |
| Post-stress 2 | 12.4 (1.4) | 12.5 (1.5) | 16.5 (1.6) | 15.1 (1.7) | 13.0 (1.9) | 12.6 (2.1) | 15.0 (1.3) | 16.5 (1.4) | 11.2 (1.3) | 11.7 (1.4) | 15.8 (1.1) | 14.5 (1.2) |
| Post-stress 3 | 14.8 (1.5) | 13.8 (1.5) | 16.9 (1.6) | 16.8 (1.6) | 13.5 (2.0) | 13.1 (2.0) | 16.9 (1.4) | 17.4 (1.4) | 12.3 (1.4) | 12.5 (1.4) | 16.4 (1.1) | 14.4 (1.1) |
| Physical Symptoms a,b,d,e | ||||||||||||
| Baseline | 3.7 (1.0) | 3.2 (0.9) | 2.5 (1.0) | 2.3 (0.9) | 1.8 (1.2) | 3.0 (1.1) | 3.4 (0.9) | 3.7 (0.8) | 7.2 (1.0) | 6.0 (0.9) | 4.6 (0.7) | 4.3 (0.7) |
| Absorption 1 | 4.3 (1.0) | 3.0 (1.0) | 3.5 (1.0) | 2.7 (1.0) | 2.8 (1.3) | 3.7 (1.2) | 3.9 (0.9) | 4.6 (0.9) | 7.5 (1.0) | 6.5 (0.9) | 4.6 (0.8) | 4.8 (0.7) |
| Absorption 2 | 5.0 (1.1) | 3.6 (1.0) | 3.1 (1.1) | 3.9 (1.1) | 1.4 (1.3) | 3.8 (1.3) | 4.6 (1.0) | 4.8 (0.9) | 7.8 (1.0) | 7.4 (1.0) | 5.4 (0.8) | 5.5 (0.8) |
| Stress | 1.7 (0.8) | 1.9 (1.0) | 2.3 (0.8) | 1.6 (1.0) | 2.5 (1.0) | 4.1 (1.2) | 2.4 (0.7) | 3.6 (0.9) | 4.8 (0.8) | 5.2 (0.9) | 3.6 (0.6) | 4.4 (0.7) |
| Post-stress 1 | 1.4 (0.7) | 2.2 (0.9) | 2.0 (0.7) | 1.9 (0.9) | 2.0 (0.9) | 4.7 (1.1) | 2.2 (0.6) | 3.1 (0.8) | 4.2 (0.7) | 4.6 (0.9) | 2.6 (0.5) | 3.8 (0.7) |
| Post-stress 2 | 1.5 (0.7) | 2.1 (0.8) | 2.0 (0.8) | 3.0 (0.9) | 1.6 (0.9) | 2.3 (1.0) | 2.3 (0.7) | 2.6 (0.7) | 3.8 (0.7) | 4.0 (0.8) | 2.9 (0.6) | 3.4 (0.6) |
| Post-stress 3 | 2.7 (0.9) | 3.7 (0.9) | 1.9 (0.9) | 3.5 (0.9) | 2.5 (1.1) | 3.7 (1.1) | 2.7 (0.8) | 3.2 (0.8) | 5.1 (0.9) | 5.0 (0.9) | 3.1 (0.7) | 4.1 (0.7) |
| Withdrawal Symptoms a,b | ||||||||||||
| Baseline | N/A | N/A | N/A | N/A | 3.7 (1.5) | 4.2 (1.8) | 3.5 (1.0) | 4.4 (1.2) | 7.4 (1.0) | 7.6 (1.2) | 6.4 (0.9) | 6.7 (1.0) |
| Absorption 1 | N/A | N/A | N/A | N/A | 4.1 (1.6) | 3.4 (1.8) | 3.6 (1.1) | 4.5 (1.2) | 7.0 (1.1) | 8.3 (1.2) | 6.3 (0.9) | 6.6 (1.0) |
| Absorption 2 | N/A | N/A | N/A | N/A | 4.9 (1.7) | 5.5 (1.8) | 5.0 (1.1) | 5.0 (1.2) | 6.7 (1.1) | 7.8 (1.2) | 6.7 (1.0) | 6.8 (1.0) |
| Stress | N/A | N/A | N/A | N/A | 6.7 (2.0) | 9.5 (2.1) | 7.0 (1.4) | 7.6 (1.4) | 9.3 (1.4) | 10.8 (1.4) | 8.9 (1.2) | 9.8 (1.2) |
| Post-stress 1 | N/A | N/A | N/A | N/A | 6.4 (2.1) | 8.1 (2.0) | 6.0 (1.4) | 6.4 (1.4) | 8.8 (1.4) | 9.8 (1.4) | 9.6 (1.2) | 8.2 (1.2) |
| Post-stress 2 | N/A | N/A | N/A | N/A | 7.5 (1.9) | 7.5 (2.0) | 7.3 (1.3) | 5.5 (1.3) | 7.5 (1.3) | 9.6 (1.3) | 7.9 (1.1) | 8.5 (1.1) |
| Post-stress 3 | N/A | N/A | N/A | N/A | 8.1 (2.0) | 7.3 (2.1) | 6.1 (1.3) | 5.5 (1.4) | 9.0 (1.3) | 9.0 (1.4) | 9.2 (1.1) | 9.4 (1.2) |
| Craving a,b | ||||||||||||
| Baseline | N/A | N/A | N/A | N/A | 4.6 (0.5) | 3.7 (0.6) | 3.5 (0.4) | 4.0 (0.4) | 5.0 (0.4) | 4.1 (0.4) | 4.6 (0.3) | 4.4 (0.4) |
| Absorption 1 | N/A | N/A | N/A | N/A | 4.6 (0.5) | 4.4 (0.6) | 4.5 (0.4) | 4.7 (0.4) | 5.3 (0.4) | 4.6 (0.4) | 4.6 (0.3) | 4.4 (0.4) |
| Absorption 2 | N/A | N/A | N/A | N/A | 5.3 (0.5) | 5.1 (0.6) | 5.0 (0.4) | 5.0 (0.4) | 5.3 (0.4) | 4.4 (0.4) | 4.5 (0.3) | 4.4 (0.3) |
| Stress | N/A | N/A | N/A | N/A | 3.1 (0.6) | 2.9 (0.7) | 3.1 (0.4) | 3.4 (0.5) | 4.8 (0.4) | 4.5 (0.5) | 4.8 (0.3) | 4.4 (0.4) |
| Post-stress 1 | N/A | N/A | N/A | N/A | 3.8 (0.6) | 3.6 (0.6) | 3.8 (0.4) | 3.8 (0.4) | 5.2 (0.4) | 4.8 (0.5) | 4.8 (0.3) | 4.5 (0.4) |
| Post-stress 2 | N/A | N/A | N/A | N/A | 4.7 (0.6) | 4.0 (0.6) | 4.4 (0.4) | 4.0 (0.4) | 5.3 (0.4) | 4.8 (0.5) | 4.8 (0.3) | 4.7 (0.4) |
| Post-stress 3 | N/A | N/A | N/A | N/A | 4.5 (0.6) | 4.7 (0.6) | 4.5 (0.4) | 4.5 (0.4) | 5.4 (0.4) | 4.9 (0.5) | 4.8 (0.3) | 4.7 (0.4) |
| QSU-B F1 a,b | ||||||||||||
| Baseline | N/A | N/A | N/A | N/A | 35.6 (3.7) | 32.5 (3.4) | 32.2 (2.6) | 36.4 (2.3) | 37.8 (2.6) | 36.3 (2.4) | 35.5 (2.2) | 34.9 (2.0) |
| Absorption 1 | N/A | N/A | N/A | N/A | 38.1 (3.6) | 34.9 (3.5) | 35.0 (2.5) | 38.4 (2.4) | 36.5 (2.6) | 34.7 (2.5) | 34.9 (2.2) | 33.3 (2.1) |
| Absorption 2 | N/A | N/A | N/A | N/A | 41.4 (3.4) | 38.7 (3.4) | 37.1 (2.3) | 39.4 (2.3) | 35.3 (2.4) | 32.9 (2.4) | 34.0 (2.0) | 32.6 (2.0) |
| Stress | N/A | N/A | N/A | N/A | 25.2 (3.7) | 25.7 (3.8) | 27.6 (2.5) | 28.8 (2.6) | 35.8 (2.6) | 34.6 (2.7) | 34.6 (2.2) | 34.7 (2.2) |
| Post-stress 1 | N/A | N/A | N/A | N/A | 30.7 (3.7) | 32.8 (3.8) | 30.5 (2.5) | 28.3 (2.6) | 35.6 (2.6) | 34.1 (2.7) | 35.4 (2.2) | 34.4 (2.3) |
| Post-stress 2 | N/A | N/A | N/A | N/A | 33.5 (3.7) | 33.6 (3.7) | 34.0 (2.6) | 32.0 (2.6) | 36.1 (2.6) | 34.9 (2.6) | 34.7 (2.2) | 35.7 (2.2) |
| Post-stress 3 | N/A | N/A | N/A | N/A | 34.3 (3.7) | 36.5 (3.7) | 33.6 (2.5) | 33.6 (2.6) | 36.5 (2.6) | 34.7 (2.6) | 35.5 (2.2) | 36.2 (2.2) |
| QSU-B F2 a,b,f | ||||||||||||
| Baseline | N/A | N/A | N/A | N/A | 19.8 (3.2) | 19.5 (3.2) | 16.0 (2.2) | 18.0 (2.2) | 20.1 (2.2) | 19.6 (2.3) | 15.9 (1.9) | 15.4 (1.9) |
| Absorption 1 | N/A | N/A | N/A | N/A | 21.8 (3.3) | 21.9 (3.4) | 17.4 (2.3) | 19.1 (2.3) | 18.6 (2.4) | 17.7 (2.4) | 16.3 (2.0) | 14.6 (2.0) |
| Absorption 2 | N/A | N/A | N/A | N/A | 23.4 (3.3) | 23.6 (3.4) | 17.2 (2.3) | 18.9 (2.4) | 17.0 (2.3) | 17.1 (2.4) | 15.0 (2.0) | 14.4 (2.0) |
| Stress | N/A | N/A | N/A | N/A | 16.7 (3.2) | 16.1 (3.5) | 13.0 (2.2) | 15.6 (2.4) | 17.4 (2.3) | 17.3 (2.5) | 15.4 (1.9) | 15.8 (2.1) |
| Post-stress 1 | N/A | N/A | N/A | N/A | 19.8 (3.6) | 20.2 (3.5) | 15.8 (2.5) | 15.6 (2.4) | 18.2 (2.6) | 15.5 (2.5) | 16.7 (2.2) | 14.9 (2.1) |
| Post-stress 2 | N/A | N/A | N/A | N/A | 19.0 (3.6) | 18.8 (3.6) | 17.2 (2.5) | 17.6 (2.5) | 18.6 (2.6) | 15.9 (2.5) | 15.7 (2.2) | 15.0 (2.1) |
| Post-stress 3 | N/A | N/A | N/A | N/A | 22.2 (3.7) | 22.9 (3.6) | 17.4 (2.6) | 16.6 (2.5) | 19.1 (2.6) | 17.4 (2.6) | 16.4 (2.2) | 16.2 (2.2) |
| Side effects | 1.2 (0.6) | 2.8 (0.6) | 1.1 (0.6) | 1.9 (0.6) | 3.2 (0.7) | 3.1 (0.7) | 1.6 (0.5) | 1.9 (0.5) | 2.8 (0.5) | 3.2 (0.6) | 2.4 (0.4) | 3.0 (0.5) |
Entries show mean and standard error of the mean. Variable names with superscripts showed significant effects for the following:
Time effect
Smoking Group × Time interaction
Smoking Group effect
Sex effect
Sex × Time interaction
Smoking Group × Sex × Time interaction.
Tobacco craving and withdrawal symptoms.
Craving in the ad lib smoking group dropped significantly immediately after the absorption period, as indicated by a Smoking Group x Time interaction, F(6, 91) = 8.75, p < .001, η2 = .37. This was expected because smokers in the ad lib group smoked a cigarette immediately prior to the stress tasks to minimize withdrawal effects on the stress response.
Though all smokers showed increases in withdrawal symptoms (MNWS) in response to stress (F(6, 84) = 13.6, p < .001, η2 = .49; Table 3), smokers in the withdrawal condition experienced greater withdrawal symptoms than smokers in the ad lib smoking condition during pre-stress periods, as indicated by a Smoking Group x Time interaction, F(6, 84) = 2.22, p = .049, η2 = .14. A Smoking Group x Time interaction with the same pattern of effects was found for smoking urges (QSU-B Factor 1: F(6, 86) = 18.7, p < .001, η2 = .57; QSUB Factor 2: F(6, 86) = 6.80, p < .001, η2 = .32).
Side effects.
Reported side effects were greater after naltrexone than after placebo, F(1, 138) = 5.41, p = .02, η2 = .04 (see Table 3).
Discussion
This study demonstrated that 1) HPA responses to stress are greater after opioid blockade than after placebo in all groups; 2) opioid blockade is associated with enhanced plasma and salivary cortisol stress responses among ad lib smokers relative to smokers experiencing withdrawal; 3) tobacco withdrawal, compared to ad lib smoking, leads to attenuated HPA stress responses; 4) women exhibit reduced ACTH responses to stress compared to men; and 5) women have higher plasma cortisol than men during opioid blockade labs.
The results from our study suggest that the impact of long-term nicotine consumption emerges during stress. In total, these results indicate that cigarette smoking is associated with a significant disruption of the HPA axis response to stress and to opioid blockade; and these effects likely contribute to deterioration of mood and to enhanced reinforcement value of tobacco and craving during withdrawal. HPA hyporesponsiveness during tobacco withdrawal is a possible consequence of prolonged nicotine exposure, leading to long-term alterations of central dopaminergic, cholinergic, and opioidergic systems (62). Hyporesponsiveness to stress may also reflect a preexisting (i.e., prior to initiation of tobacco use) alteration in stress and emotion-related processing centers in the brain, such as in forebrain and limbic system functions (63–65).
The blunted HPA response to opioid blockade medication observed during smoking withdrawal, compared to smoking ad libitum, may reflect decreased opioid regulation leading to diminished HPA responses to opioid blockade. Reduced opioid tone may influence modulation of mesolimbic dopaminergic transmission, creating conditions that maintain smoking behavior or that complicate attempts to abstain. Reduced opioid regulation may also contribute to increased negative affect, especially during nicotine withdrawal. For example, reduced opioid stimulatory inputs to the dopaminergic system may lead to reduced basal dopamine activity, and this may enhance rewarding effects of dopamine-stimulating drugs, such as nicotine (66, 67). Consistent with these hypotheses are findings demonstrating that higher levels of circulating endogenous opioids are associated with greater emotional stability and positive mood after exercise (68, 69), while dysphoric states are usually associated with impaired endogenous opioid functions (70–72).
The normalization of opioid blockade responses during ad lib smoking relative to the abstinence condition, combined with smokers’ attenuated stress responses during withdrawal, confirms the impact of chronic exposure to nicotine on HPA activity and on related regulatory systems (73, 74). These altered responses may be relevant to maintaining tobacco use. For example, it is possible that a blunted stress response exacerbates withdrawal symptoms and craving and, therefore, may enhance the reinforcing effects of tobacco (75). Mechanistic work to define neuronal pathways mediating these relationships is still needed (32).
Cessation of nicotine delivery leads to changes in brain circuits underlying nicotine reinforcement (76); and preclinical studies indicate that nicotine withdrawal leads to insensitivity of the HPA axis to stress, even though HPA negative feedback mechanisms as well as expressions of glucocorticoid receptors (GR) and CRF mRNA in the hippocampus and the PVN are normal (77). In a relevant experiment, μ-opioid receptor binding and met-enkephalin concentrations were assessed after chronic nicotine administration (0.3 mg/kg nicotine; a dose relevant to human nicotine intake) in rats (78). Chronic nicotine significantly lowered met-enkephalin and upregulation of μ-opioid receptors in the striatum and midbrain, as compared to controls. Thus, it is possible that chronic exposure to nicotine among smokers modifies μ-opioid receptors, which, in-turn, alter regulation of opioid-HPA interactions.
Our findings that smokers in the withdrawal condition experienced higher levels of negative affect, withdrawal symptoms, and smoking urges as well as lower levels of positive affect during baseline periods support the validity of the tobacco withdrawal protocol. Furthermore, findings from these measures were consistent with previous studies examining the time course of withdrawal symptoms and the relationship between tobacco withdrawal and stress response (55, 79).
Limitations of this study include its cross-sectional nature, which precludes determination of whether the observed opioid-HPA alterations among smokers were the result of chronic tobacco use or whether the alterations predisposed individuals to initiate (or maintain) smoking. In addition, because smokers in this study were not interested in quitting, results may not represent the entire smoking population. This study also had relatively small subgroups in follow-up tests for higher-order interaction effects.
This study also has several strengths, such as its large sample of smokers, well-controlled manipulation of smoking withdrawal, inclusion of multiple methods of assessment, and use of a double-blind and repeated measures design. Furthermore, the novel and important results obtained from this study justify a need for additional research to examine interactions of the HPA-endogenous opioid systems within the context of smoking cessation and relapse. For example, future research should extend the current findings to examine the extent to which alterations in endogenous opioid system regulation of the stress response predict smoking relapse and the extent to which such alterations are normalized after long-term smoking abstinence.
Our findings are also consistent with previous studies documenting sex differences in trait negative mood (80) and in HPA responses to opioid antagonists (46) and to stress (38, 81). However, we did not find evidence of sex as a moderator of the observed hormonal differences smoking groups. Nevertheless, it is important to account for a growing literature indicating that compared to their male counterparts, female tobacco users are more likely to smoke to manage negative affect (82, 83), to show distress after being exposed to stimuli that are specific to smoking, and to have difficulty quitting tobacco (84–86). Also, whereas changes in HPA stress responses are predictive of early smoking relapse in men, trait negative mood and withdrawal symptoms are risk factors for smoking relapse in women (87, 88). These observations, combined with research documenting the role of endogenous opioids in mood regulation, drug reinforcement, and regulation of HPA stress responses (8, 9)(10–13), indicate a need for further examination of the potential role of sex hormones in the interplay between the endogenous opioid system and the HPA axis.
In summary, this study demonstrated that opioid blockade was associated with enhanced plasma and salivary cortisol stress responses during ad lib smoking compared to short-term smoking withdrawal. This study also demonstrated that, compared to men, women exhibited reduced ACTH responses to stress; women also experienced higher levels of ACTH during opioid blockade labs. These results suggest that treatment strategies in the context of addiction may benefit from targeting stress-response regulation systems, including the endogenous opioid system.
Acknowledgements
We would like to thank the following individuals for their help with collecting (Barbara Gay, Elizabeth Ford, Dayna Schleppenbach, Soni Rraklli Uccellini, Angie Forsberg) and managing (Jie Gooder) the data for this study. Nikki Neumann, Christopher Schweiger, and Dan Vuicich helped with the conducting the assays.
Funding
This research was supported in part by grants to the first author from the National Institute of Health (R01DA016351 and R01DA027232).
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
References
- 1.Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. AnnNYAcadSci. 1995;771:1–18. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. The brain is an important target of adrenal steroid actions. A comparison of synthetic and natural steroids. Ann N Y Acad Sci. 1997;823:201–13. [DOI] [PubMed] [Google Scholar]
- 3.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. [DOI] [PubMed] [Google Scholar]
- 4.al’Absi M, Arnett DK. Adrenocortical responses to psychological stress and risk for hypertension. Biomed Pharmacother. 2000;54(5):234–44. [DOI] [PubMed] [Google Scholar]
- 5.Chrousos GP, Gold PW. The Concepts of Stress and Stress System Disorders - Overview of Physical and Behavioral Homeostasis. Jama-Journal of the American Medical Association. 1992;267(9):1244–52. doi: DOI 10.1001/jama.267.9.1244. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–9. [DOI] [PubMed] [Google Scholar]
- 7.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. EndocrRev 2000;21:55–89. [DOI] [PubMed] [Google Scholar]
- 8.Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. ProgNeuropsychopharmacolBiolPsychiatry. 2001;25:729–41. [DOI] [PubMed] [Google Scholar]
- 9.Martin del Campo AF, Dowson JH, Herbert J, Paykel ES. Effects of naloxone on diurnal rhythms in mood and endocrine function: a dose-response study in man. Psychopharmacology (Berl). 1994;114(4):583–90. [DOI] [PubMed] [Google Scholar]
- 10.Rushen J, Schwartze N, Ladewig J, Foxcroft G. Opioid modulation of the effects of repeated stress on A.C.T.H., cortisol, prolactin, and growth hormone in pigs. Physiology and Behavior. 1993;53:923–8. [DOI] [PubMed] [Google Scholar]
- 11.Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. AnnuRevNeurosci. 1984;7:223–55. [DOI] [PubMed] [Google Scholar]
- 12.Wand GS, Schumann H. Relationship between plasma adrenocorticotropin, hypothalamic opioid tone, and plasma leptin. J ClinEndocrinolMetab. 1998;83(6):2138–42. [DOI] [PubMed] [Google Scholar]
- 13.Morris M, Salmon P, Steinberg H, Sykes EA, Bouloux P, Newbould E, McLoughlin L, Besser GM, Grossman A. Endogenous opioids modulate the cardiovascular response to mental stress. Psychoneuroendocrinology. 1990;15(3):185–92. [DOI] [PubMed] [Google Scholar]
- 14.Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–58. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Avila CA, Oncken C, Van Kirk J, Wand G, Kranzler HR. Adrenocorticotropin and cortisol responses to a naloxone challenge and risk of alcoholism. BiolPsychiatry. 2002;51:652–8. [DOI] [PubMed] [Google Scholar]
- 16.Hellbach S, Gartner P, Deicke J, Fischer D, Hassan AH, Almeida OF. Inherent glucocorticoid response potential of isolated hypothalamic neuroendocrine neurons. FASEB J. 1998;12:199–207. [DOI] [PubMed] [Google Scholar]
- 17.Valentino RJ, Van Bockstaele E. Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology (Berl). 2001;158:331–42. [DOI] [PubMed] [Google Scholar]
- 18.Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. ProgNeurobiol. 1998;56:237–67. [DOI] [PubMed] [Google Scholar]
- 19.Christie MJ. Mechanisms of opioid actions on neurons of the locus coeruleus. ProgBrain Res 1991;88:197–205. [DOI] [PubMed] [Google Scholar]
- 20.Jackson RV, Grice JE, Jackson AJ, Hockings GI. Naloxone-induced ACTH release in man is inhibited by clonidine. ClinExpPharmacolPhysiol 1990;17:179–84. [DOI] [PubMed] [Google Scholar]
- 21.Herkenham M, Edley SM, Stuart J. Cell clusters in the nucleus accumbens of the rat, and the mosaic relationship of opiate receptors, acetylcholinesterase and subcortical afferent terminations. Neuroscience. 1984;11:561–93. [DOI] [PubMed] [Google Scholar]
- 22.Devine DP, Leone P, Wise RA. Mesolimbic dopamine neurotransmission is increased by administration of mu-opioid receptor antagonists. EurJPharmacol. 1993;243:55–64. [DOI] [PubMed] [Google Scholar]
- 23.Cowen MS, Lawrence AJ. The role of opioid-dopamine interactions in the induction and maintenance of ethanol consumption. ProgNeuropsychopharmacolBiolPsychiatry. 1999;23:1171–212. [DOI] [PubMed] [Google Scholar]
- 24.Naber D, Pickar D, Davis GC, Cohen RM, Jimerson DC, Elchisak MA, Defraites EG, Kalin NH, Risch SC, Buchsbaum MS. Naloxone effects on beta-endorphin, cortisol, prolactin, growth hormone, HVA and MHPG in plasma of normal volunteers. Psychopharmacology (Berl). 1981;74(2):125–8. Epub 1981/01/01. doi: 10.1007/bf00432677. [DOI] [PubMed] [Google Scholar]
- 25.King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotransformation of oral naltrexone: preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002;26(6):778–88. Epub 2002/05/15. doi: 10.1016/s0893-133x(01)00416-x. [DOI] [PubMed] [Google Scholar]
- 26.Munro CA, Oswald LM, Weerts EM, McCaul ME, Wand GS. Hormone responses to social stress in abstinent alcohol-dependent subjects and social drinkers with no history of alcohol dependence. Alcohol ClinExpRes. 2005;29:1133–8. [DOI] [PubMed] [Google Scholar]
- 27.Uhart M, Oswald L, McCaul ME, Chong R, Wand GS. Hormonal responses to psychological stress and family history of alcoholism. Neuropsychopharmacology. 2006;31(10):2255–63. [DOI] [PubMed] [Google Scholar]
- 28.Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The Micro-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31:204–11. [DOI] [PubMed] [Google Scholar]
- 29.Mendelson JH, Goletiani N, Sholar MB, Siegel AJ, Mello NK. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;33(4):749–60. Epub 2007/05/16. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- 30.Hadjiconstantinou M, Neff NH. Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology. 2011;60(7–8):1209–20. Epub 2010/11/22. doi: 10.1016/j.neuropharm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Kishioka S, Kiguchi N, Kobayashi Y, Saika F. Nicotine effects and the endogenous opioid system. J Pharmacol Sci. 2014;125(2):117–24. Epub 2014/05/31. [DOI] [PubMed] [Google Scholar]
- 32.alʼAbsi M Stress and Addiction: When a Robust Stress Response Indicates Resiliency. Psychosom Med 2018;80(1):2–16. doi: 10.1097/PSY.0000000000000520.PubMed PMID: 28834923; PMCID: PMC5741515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu Y, Matta SG, Brower VG, Sharp BM. Norepinephrine secretion in the hypothalamic paraventricular nucleus of rats during unlimited access to self-administered nicotine: An in vivo microdialysis study. JNeurosci. 2001;21(22):8979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pontieri FE, Colangelo V, La Riccia M, Pozzilli C, Passarelli F, Orzi F. Psychostimulant drugs increase glucose utilization in the shell of the rat nucleus accumbens. Neuroreport. 1994;5:2561–4. [DOI] [PubMed] [Google Scholar]
- 35.Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol Psychiatry. 1998;43(7):525–30. doi: 10.1016/S0006-3223(97)00423-X. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert DG, McClernon FJ, Rabinovich NE, Dibb WD, Plath LC, Hiyane S, Jensen RA, Meliska CJ, Estes SL, Gehlbach BA. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: relations to depressive traits, nicotine exposure, and dependence. ExpClinPsychopharmacol. 1999;7(4):427–43. [DOI] [PubMed] [Google Scholar]
- 37.al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73(3):267–78. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 1999;61(2):154–62. [DOI] [PubMed] [Google Scholar]
- 39.Roche DJ, King AC, Cohoon AJ, Lovallo WR. Hormonal contraceptive use diminishes salivary cortisol response to psychosocial stress and naltrexone in healthy women. Pharmacol Biochem Behav. 2013;109:84–90. Epub 2013/05/16. doi: 10.1016/j.pbb.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Childs E, Dlugos A, De Wit H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 2010;47(3):550–9. Epub 2010/01/15. doi: 10.1111/j.1469-8986.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reschke-Hernández AE, Okerstrom KL, Edwards AB, Tranel D. Sex and stress: Men and women show different cortisol responses to psychological stress induced by the Trier Social Stress Test and the Iowa Singing Social Stress Test. Journal of Neuroscience Research. 2017;95(1–2):106–14. doi: 10.1002/jnr.23851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lovallo WR, King AC, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Naltrexone effects on cortisol secretion in women and men in relation to a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Psychoneuroendocrinology. 2012;37(12):1922–8. Epub 2012/05/08. doi: 10.1016/j.psyneuen.2012.04.006. PubMed PMID: 22575355; PMCID: PMC3449011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66(2):198–206. [DOI] [PubMed] [Google Scholar]
- 44.Klein LC, Jamner LD, Alberts J, Orenstein MD, Levine L, Leigh H. Sex differences in salivary cortisol levels following naltrexone administration. Journal of Applied Biobehavioral Research. 2000;5(2):144–53. doi: 10.1111/j.1751-9861.2000.tb00070.x. [DOI] [Google Scholar]
- 45.Lovallo WR, King AC, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Naltrexone effects on cortisol secretion in women and men in relation to a family history of alcoholism: Studies from the Oklahoma Family Health Patterns Project. Psychoneuroendocrinology. 2012;37(12):1922–8. doi: 10.1016/j.psyneuen.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roche DJO, Childs E, Epstein AM, King AC. Acute HPA axis response to naltrexone differs in female vs. male smokers. Psychoneuroendocrinology. 2010;35(4):596–606. doi: 10.1016/j.psyneuen.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 48.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 49.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991;86(9):1119–27. Epub 1991/09/01. [DOI] [PubMed] [Google Scholar]
- 50.al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosomatic Medicine. 2004;66(2):198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- 51.Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME. Differences in delta- and mu-Opioid Receptor Blockade Measured by Positron Emission Tomography in Naltrexone-Treated Recently Abstinent Alcohol-Dependent Subjects. Neuropsychopharmacology. 2008;33:653–65. [DOI] [PubMed] [Google Scholar]
- 52.King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ. Hypothalamic-Pituitary-Adrenocortical (HPA) Axis Response and Biotransformation of Oral Naltrexone. Preliminary Examination of Relationship to Family History of Alcoholism. Neuropsychopharmacology. 2002;26(6):778–88. [DOI] [PubMed] [Google Scholar]
- 53.al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34(3):266–75. [DOI] [PubMed] [Google Scholar]
- 54.al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology, Biochemistry and Behavior. United States: 2003. p. 401–10. [DOI] [PubMed] [Google Scholar]
- 55.al’Absi M, Nakajima M, Grabowski J. Stress response dysregulation and stress-induced analgesia in nicotine dependent men and women. Biol Psychol. 2013;93(1):1–8. doi: 10.1016/j.biopsycho.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology Biochemistry and Behavior. 2003;74(2):401–10. [DOI] [PubMed] [Google Scholar]
- 57.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–94. Epub 1986/03/01. [DOI] [PubMed] [Google Scholar]
- 58.Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob Control. 1998;7(1):92–3. Epub 1998/08/26.PubMed PMID: 9706762; PMCID: Pmc1759641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86(11):1467–76. Epub 1991/11/01. [DOI] [PubMed] [Google Scholar]
- 60.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. Epub 2001/03/22. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 61.King AC, Meyer PJ. Naltrexone alteration of acute smoking response in nicotine-dependent subjects. PharmacolBiochemBehav. 2000;66:563–72. [DOI] [PubMed] [Google Scholar]
- 62.Koob GF, Le MM. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–8. [DOI] [PubMed] [Google Scholar]
- 63.Robledo P, Koob GF. Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. BehavBrain Res. 1993;55:159–66. [DOI] [PubMed] [Google Scholar]
- 64.Chong RY, Uhart M, Wand GS. Endogenous opiates, addiction, and stress response. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. p. 85–104. [Google Scholar]
- 65.Lovallo W Individual differences in response to stress and risk for addiction. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. p. 265–84. [Google Scholar]
- 66.Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci. 2015;38(4):217–25. Epub 2015/01/29. doi: 10.1016/j.tins.2015.01.002. PubMed PMID: 25637939; PMCID: PMC4385443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruijnzeel AW. kappa-Opioid receptor signaling and brain reward function. Brain Res Rev. 2009;62(1):127–46. Epub 2009/10/07. doi: 10.1016/j.brainresrev.2009.09.008. PubMed PMID: 19804796; PMCID: 2787673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zorrilla EP, DeRubeis RJ, Redei E. High Self-Esteem, Hardiness And Affective Stability Are Associated With Higher Basal Pituitary-Adrenal Hormone Levels. Psychoneuroendocrinology. 1995;20:591–601. [DOI] [PubMed] [Google Scholar]
- 69.Wildmann J, Kruger A, Schmole M, Niemann J, Matthaei H. Increase of circulating beta-endorphin-like immunoreactivity correlates with the change in feeling of pleasantness after running. Life Sci. 1986;38:997–1003. [DOI] [PubMed] [Google Scholar]
- 70.Burnett FE, Scott LV, Weaver MG, Medbak SH, Dinan TG. The effect of naloxone on adrenocorticotropin and cortisol release: evidence for a reduced response in depression. J AffectDisord. 1999;53(3):263–8. [DOI] [PubMed] [Google Scholar]
- 71.Facchinetti F, Fioroni L, Martignoni E, Sances G, Costa A, Genazzani AR. Changes of opioid modulation of the hypothalamo-pituitary-adrenal axis in patients with severe premenstrual syndrome. PsychosomMed. 1994;56:418–22. [DOI] [PubMed] [Google Scholar]
- 72.Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000;23:411–8. [DOI] [PubMed] [Google Scholar]
- 73.al’Absi M, Wittmers LE, Erickson J, Hatsukami DK, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–10. [DOI] [PubMed] [Google Scholar]
- 74.Gilbert DG, Meliska CJ, Plath LC. Noise stress does not modulate effects of smoking/nicotine on ·-endorphin, cortisol, ACTH, glucose, and mood. Psychopharmacology. 1997;130:197–202. [DOI] [PubMed] [Google Scholar]
- 75.Steptoe A, Ussher M. Smoking, cortisol and nicotine. International Journal of Psychophysiology. 2006;59:228–35. [DOI] [PubMed] [Google Scholar]
- 76.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–9. [DOI] [PubMed] [Google Scholar]
- 77.Semba J, Wakuta M, Maeda J, Suhara T. Nicotine withdrawal induces subsensitivity of hypothalamic-pituitary-adrenal axis to stress in rats: implications for precipitation of depression during smoking cessation. Psychoneuroendocrinology. 2004;29:215–26. [DOI] [PubMed] [Google Scholar]
- 78.Wewers ME, Dhatt RK, Snively TA, Tejwani GA. The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Res. 1999;822(1–2):107–13. [DOI] [PubMed] [Google Scholar]
- 79.Morrell HE, Cohen LM, al’Absi M. Physiological and psychological symptoms and predictors in early nicotine withdrawal. Pharmacol Biochem Behav. 2008;89(3):272–8. doi: 10.1016/j.pbb.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 80.Kret ME, De Gelder B. A review on sex differences in processing emotional signals. Neuropsychologia. 2012;50(7):1211–21. Epub 2012/01/08. doi: 10.1016/j.neuropsychologia.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 81.Liu JJW, Ein N, Peck K, Huang V, Pruessner JC, Vickers K. Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. Psychoneuroendocrinology. 2017;82:26–37. Epub 2017/05/10. doi: 10.1016/j.psyneuen.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Ferguson SG, Frandsen M, Dunbar MS, Shiffman S. Gender and stimulus control of smoking behavior. Nicotine Tob Res. 2015;17(4):431–7. Epub 2015/03/13. doi: 10.1093/ntr/ntu195. PubMed PMID: 25762752; PMCID: PMC4432397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl). 2002;163:194–201. [DOI] [PubMed] [Google Scholar]
- 84.McKee SA, Weinberger AH. Innovations in translational sex and gender-sensitive tobacco research. Nicotine Tob Res. 2015;17(4):379–81. doi: 10.1093/ntr/ntu335.PubMed PMID: 25762746; PMCID: 4481708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith PH, Kasza KA, Hyland A, Fong GT, Borland R, Brady K, Carpenter MJ, Hartwell K, Cummings KM, McKee SA. Gender differences in medication use and cigarette smoking cessation: results from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2015;17(4):463–72. doi: 10.1093/ntr/ntu212.PubMed PMID: 25762757; PMCID: 4402353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, McKee SA. Sex/gender differences in smoking cessation: A review. Prev Med. 2016;92:135–40. Epub 2016/10/30. doi: 10.1016/j.ypmed.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.al’Absi M Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. International Journal of Psychophysiology. Netherlands 2006. p. 218–27. [DOI] [PubMed] [Google Scholar]
- 88.Nakajima M, al’Absi M, Dokam A, Alsoofi M, Khalil NS, Al Habori M. Gender Differences in Patterns and Correlates of Khat and Tobacco Use. Nicotine Tob Res 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]