Abstract
Flavonoids are polyphenolic phytochemicals produced in fruits, nuts and vegetables and dietary consumption of these structurally diverse compounds is associated with multiple health benefits including increased lifespan, decreased cardiovascular problems and low rates of metabolic diseases. Preclinical studies with individual flavonoids demonstrate that these compounds exhibit anti-inflammatory and anticancer activities and they enhance the immune system. Their effectiveness in both chemoprevention and chemotherapy is associated with their targeting of multiple genes/pathways including nuclear receptors, the aryl hydrocarbon receptor (AhR), kinases, receptor tyrosine kinases and G protein-coupled receptors. However, despite the remarkable preclinical activities of flavonoids, their clinical applications have been limited and this is due, in part, to problems in drug delivery and poor bioavailability and these problems are being addressed. Further improvements that will expand clinical applications of flavonoids include mechanism-based precision medicine approaches which will identify critical mechanisms of action of individual flavonoids with optimal activities that can be used in combination therapies.
Keywords: Cancer, Nuclear receptor, Nuclear translocation, Cell signaling, Apoptosis
Introduction
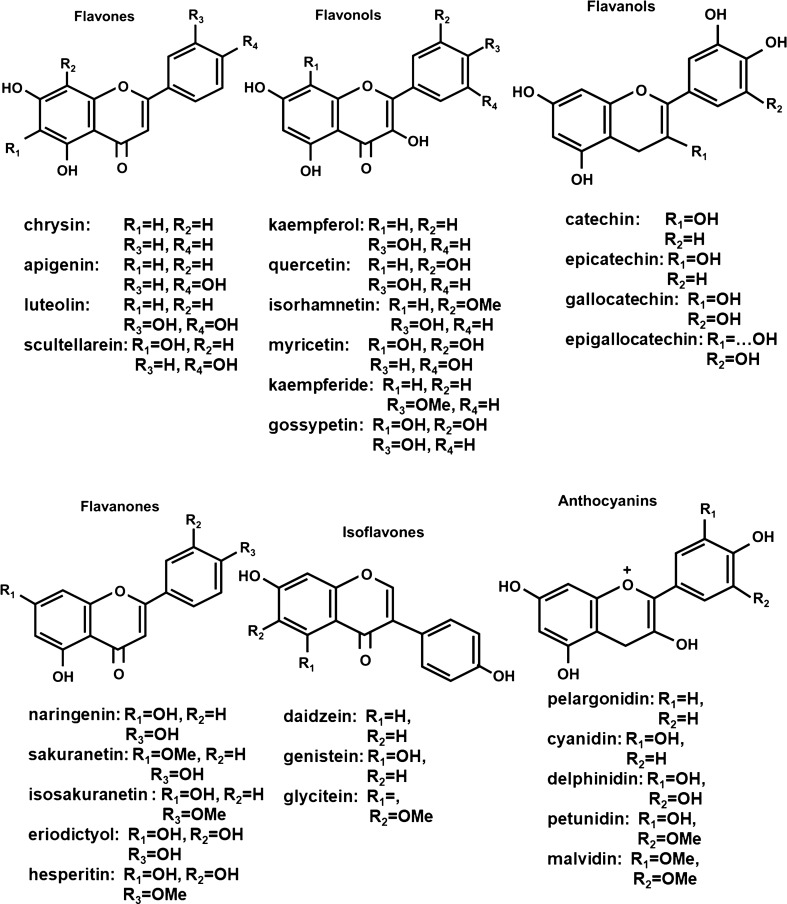
Flavonoids are polyphenolic phytochemicals produced in fruits, vegetables and grains and consumption of flavonoid-rich foods and nutriceuticals has been associated with a wide range of health benefits (rev. in [1–8]). Flavonoids contain a common phenylchromen-4-one scaffold which can be substituted with a phenyl ring at C2 or C3 to give the flavone and isoflavone backbone structure. Further modifications at C4 (a ketone group), C2–C3 (saturated or olefinic) plus hydroxy or methoxy substituents on the phenylchromen-4-one and phenyl rings results in formation of flavanones, flavanols, flavonols, flavones, anthocyanidins and isoflavones (Fig. 1). In addition, chalcones in which the ether ring of flavonoids has been cleaved are also considered to be members of the flavonoid family of plant polyphenolics. Flavonoids are synthesized from phenylalanine and malonyl—Co A [9, 10] and over 8000 individual flavonoids have been identified in plants [7]. Flavonoids are secondary metabolites that exhibit multiple functions in plants including their role in protecting against various internal and external stressors. Consumption of fruits and vegetables has long been associated with improved overall human health [11–14] and flavonoids have been recognized as one of the important classes of phytochemicals that enhance health benefits. Moreover, there is evidence for widespread use of individual and flavonoid mixtures as nutriceutical for maintaining health and for treatment of multiple diseases and aliments.
Fig. 1.
Structure of flavonoids and some individual members of each class
Effects of flavonoids on non-cancer and cancer endpoints in humans
Flavonoids have been extensively investigated for their effects on multiple non-cancer and cancer endpoints and PubMed lists over 123,000 publications dealing with these phytochemicals. Although detailed structure–activity and mechanistic studies on flavonoids are limited, the effects of these compounds have been attributed, in part to their activities as antioxidants, antimicrobial and antiviral activities, radical trapping agents and as inhibitors of key enzymes/factors such as cyclooxygenases, and acetylcholinesterase. Many studies report that flavonoids modulate expression of multiple genes and gene products that result in beneficial effects however, the mechanisms and specific polyphenolics associated with individual flavonoid-induced responses are not well defined. Among the over 123,000 citations on flavonoids, there are many primary and review articles on the health promoting effects of these compounds in several disease models of both prevention and intervention/therapeutics. Results of laboratory and preclinical studies would predict enormous health benefits from these compounds whereas human studies show modest and limited responses and some examples of effects in humans that are associated with flavonoid consumption including aging and selected disease are summarized below.
Aging and cardiovascular disease
Biological aging is a complex process that results in the temporal deterioration of cells due to the net accumulation of damage in multiple cell types and is due, in part, to age-dependent decreases in cell repair and maintenance pathways, enhanced stress, DNA damage, mitochondrial injury and inflammation [15]. The major diseases where age is a prominent risk factor include vascular disease and atherosclerosis, joint degeneration, metabolic diseases (obesity and diabetes), skin diseases, circulatory disease (hypertension, coronary artery disease), eye diseases (macular degeneration) and neurodegenerative diseases (Alzheimer’s, dementia and decreased cognitive-functions) [15]. The effects of flavonoid or polyphenolic intake on mortality as an age-dependent response has been investigated in several human studies [16–22]. A recent report on the Danish Diet Cancer and Health Cohort [16] of 56,048 participants showed that dietary intakes of approximately 500 mg/day of total flavonoids decreased overall mortality and subsequent higher intakes of up to 2000 mg/day did not further decrease mortality. The authors also reported similar effects on decreased mortality associated with dietary intakes of individual sub-classes of flavonoids including flavonols, flavanols, flavanones, flavones and anthocyanins. There was also evidence that dietary flavonoids provide some mitigation of alcohol and smoking-dependent higher rates of mortality. Interestingly there were some differences in the mortality studies in the various cohorts. For example, in the prospective Nurses’ Health Study II, a comparison between the lowest and highest consumers of total flavonoids was significant only in one model even though there was lower mortality rate in the high consumer group [22]. There was a significant increase in mortality of women in the high vs. low grapefruit consuming group whereas selected flavonoid/polyphenol-rich foods such as red wine, tea, peppers, blueberries and strawberries were associated with decreased mortality. The overall consensus from most studies is that dietary flavonoids significantly lower mortality rates and can provide some protection from factors that contribute to higher rates of mortality. Many of the reports on dietary flavonoids and mortality also examine the possible association with mortality from cardiovascular diseases [9, 16–18, 20]. A high intake of dietary flavonoids was also associated with decreased cardiovascular mortality and in the Danish cohort study this association was observed for individual sub-classes of flavonoids.
Diabetes
Diabetes is another aging-related disease which in recent years has significantly increased in many countries due to diet-induced obesity. A recent meta-analysis of 18 different prospective cohort studies on polyphenol exposure and the risk of type 2 diabetes [23] reported that by comparison of extreme quintiles of intake there was an inverse association for flavonoids, flavonols, flavan-3-ols, catechins, anthocyanidins and isoflavones. A similar inverse correlation was observed for dietary intake of flavonoids and the risk of gestational diabetes mellitus [24]. A recent review summarized past and current/ongoing clinical trials on effects of various flavonoids/polyphenols on diabetes and diabetic complications including nephropathy, retinopathy, neuropathy and cardiovascular complications [25]. Although there were some indications of benefit, effects of the clinically approved and recommended use of flavonoid mixtures for treatment of diabetes and its complications were minimal despite encouraging results from animal models and cell culture studies [25, 26]. Nevertheless, meta-analysis of clinical trials (primarily with supplements) showed that flavonols and isoflavones decreased body mass index, flavonols also decreased waist circumference whereas flavonoids, flavanones and anthocyanins were not inversely associated with markers of obesity [27]. Another report (single cohort) showed that other polyphenolics correlated with decreased body mass index [28], however, flavanols were not separated out in this study. Overall, the results suggest a possible role for flavonoids in ameliorating the effects of diabetes and in diabetes prevention but identification of specific sub-classes of flavonoids that are most efficacious requires further investigation.
Neurodegeneration
There are several studies showing that consumption of flavonoid and polyphenolic foods protects against some signs and markers of neurodegeneration including various dementias and Alzheimer disease. Both intervention studies with various flavonoid-enriched foods [29–33] and evidence from the beneficial effects of the Mediterranean diet [34–37] suggest a role for these phytochemicals in neurodegenerative disease prevention. For example, decreased development of Alzheimer’s dementia was associated with both strawberry and total flavonoid intake [38]. In the prospective Framingham Offspring Cohort study, individuals with the highest intake of flavonols, anthocyanins and flavonoid polymers had the lowest risk for Alzheimer disease and related dementias and this correlated changes in MRI indicators [39, 40].
Anti-inflammatory and immune cell effects
Inflammation plays an important role in many diseases and there is considerable evidence from in vitro and animal model studies that flavonoids inhibit multiple inflammatory pathways [41–43]. Many of the studies noted above are associated with flavonoid/polyphenol-mediated anti-inflammatory effects and this is confirmed in other reports [44–47]. For example, a clinical study with the citrus flavonoid hesperidin (500 mg/d for 3 weeks) decreased levels of several circulation markers of inflammation including c-reactive protein, serum amyloid A protein and soluble E-secretin [45]. Flavonoids and polyphenolics are immunomodulatory compounds and impact multiple immune cell types and the effects are highly variable and dependent on the compound, animal model and immune component [41, 43, 45, 48–51]. Reviews on the effects of flavonoids and related compounds on immune cell responses demonstrate their wide-ranging effects on immune cells and immune cell responses which include modulation of β cell and antibody production, enhancement of NK cell cytotoxicity, inhibition of Th17-dependent differentiation and NLRP3 inflammation and induction of CD8 + cells. Human intervention studies on the effects of flavonoid on immune responses give mixed results and these compounds are not routinely used for immune therapies.
Intestinal inflammation and endometriosis
Several recent reviews summarize studies showing that structurally-diverse flavonoids inhibit inflammatory bowel disease and related intestinal inflammation in laboratory animal models [52–55]. Salaritabar and coworkers reviewed and summarized effects of individual flavonoids on dextran sodium sulfate (DSS) and TNBS-and acetic acid-induced inflammation in rodent models of ulcerative colitis and Crohn’s disease respectively. In the former model flavonoid that inhibit inflammation include quercetin, rutin, kaempferol, daidzein, naringenin, hesperidin, anthocyanins (cranberry) apigenin, baicalein, luteolin, fisetin, epigallocalechin-3-gallate and oligonal. TNBS-induced intestinal inflammation is inhibited by many of the same flavonoids and also morin, genistein, diosmin, tangeritin, catechiu, grape extracts and thearubigin. Rape bee pollen which contains high levels of kaempferol blocked DSS-induced colitis in mice and this included inhibition of colon shortening and decreased weight, spleen swelling and improved inflammation [56, 57]. There were also decreases in inflammatory cytokines in colon tissue with a notable decrease in IL-1β. It was also shown that there were treatment related effects on the gut microbial population with enhanced expression of Lactobacillus and decreased Allobaculum and Bacteriodes. All of these laboratory studies indicate that flavonoids and phytochemical extracts enriched in flavonoids would play an important role in preventing and treating inflammatory diseases of the intestine. There is some evidence that the Mediterranean diet which is enriched in flavonoids improves inflammatory impacts in patients with Crohn’s disease and inflammatory bowel disease [58]. There was also evidence for a beneficial effect of dietary isoflavones in a cohort of Polish patients with ulcerative colitis in remission [59]. Other studies also show the beneficial effects of flavonoids [60–63], however, it was concluded “To date, clinical studies are scarce and further research with well controlled procedures and higher number of patients is essential to establish the potential therapeutic use of flavonoids” [54].
Several studies have also reported the effects of different classes of flavonoids as inhibitor of endometriosis which afflicts over 5.5 million women in the United States and 176 million worldwide. Flavonoids that inhibit pro-endometriotic pathways/genes include epigallocalechin-3-gallate, luteolin, glycosylated flavonoids from Melilotus offinalis, quercetin 3,6-dihydroxyflavone and chrysin [64–74]. For example, epigallocatechin-3-gallate (ECGC) exhibits antifibrotic properties in mouse models, established endometriotic cells and patient derived cells and this includes decreased invasion and inhibition of multiple fibrotic genes including α-smooth muscle actin [74]. One study reported that a cocktail of agents which included quercetin reduced some of the symptoms and serum markers of endometriosis (PGE2 and CA-125) [75], however, clinical applications of flavonoids for treatment of endometriosis are minimal despite promising preclinical laboratory studies.
Cancer
A pubmed search of flavonoids and cancer listed over 22,000 papers demonstrating the high level of interest and preclinical studies on the anticancer activities of these phytochemicals. Flavonoids have been widely characterized as anticancer agents via their inhibition of multiple pro-oncogenic pathways and genes in cancer cells and also to a lesser extent, their induction of tumor suppressor-like responses and genes [2, 4, 6, 12–14, 76–80]. Based on this abundance of data, results of clinical trials on the chemopreventive and chemotherapeutic effects of flavonoids are underwhelming. A recent update on flavonoids as cancer chemopreventive agents summarized results of case control and prospective studies which correlated flavonoid intake with risks for breast, lung, prostate, gastric, pancreatic head and neck, and colorectal cancers. Although there was some evidence showing that intake of total or specific flavonoids was associated with decreased risks for some cancers, these observations were not observed in all studies [14]. A large prospective study did not observe an association between flavonoid intake and colorectal cancer risk; and this was also observed in one case control study but in two other case–control reports, there was an inverse association between flavonoid intake and risks for colorectal cancer [81–84]. Despite the extensive data on the anticancer activities of flavonoids, their applications for cancer therapy are limited and this is related, in part, to the low bioavailability of these compounds. Therapeutic trials on the effects of genistein on prostate and colorectal cancer are underway and isoflavones in combination with other agents had no effect on advanced pancreatic cancer but may have impacted PSA levels in prostate cancer patients [77, 80, 85–88].
Human studies show some benefits of flavonoids on mortality and other diseases based primarily on long term consumption of foods enriched in these compounds. However, despite the broad range of effects of flavonoids on multiple disease related pathways in preclinical studies, the therapeutic effects and ongoing clinical applications of flavonoids are not extensive. This may be due, in part, to poor bioavailability of these compounds which should improve with development of improved delivery systems [87]. Future therapeutic benefits from flavonoids may also require a more mechanistic and precision medicine approaches where specifically targeted pathways/genes have been identified and structure–activity studies have focused on using flavonoids which exhibit optimal response-specific activities. The following section will identify and briefly discuss several specific flavonoid targets that are responsible for many of the flavonoid-induced therapeutic responses. With few exceptions, the individual flavonoids that optimally modulate specific intracellular targets have not been identified.
Mechanisms of action of flavonoids
One of the important underlying mechanisms of action of dietary flavonoids and related polyphenols is associated with their inhibition of oxidative stress and related downstream responses including inflammatory diseases. Flavonoids scavenge free radicals and their subsequent damage by forming relatively stable phenoxy radicals and also by metal chelation [15, 88]. In addition, flavonoids interact with multiple gene products to inhibit their specific actions and thereby directly modulate a limited response or for kinase inhibition, the interaction could impact multiple downstream pathways.
-
i.
Flavonoids as kinase inhibitors.
Although flavonoids directly bind many proteins and modulate their activities, their interactions with multiple kinases and subsequent effects on downstream kinases-dependent signaling have been extensively investigated (rev. in [89–94]). Genistein was among the first flavonoids identified as a receptor tyrosine kinase (RTK) inhibitor [94] and inhibited autophosphorylation of the epidermal growth factor receptor (EGFR) and it acts as a non-competitive inhibitor of histone H2B [95]. Subsequent studies show the genistein and many other flavonoids inhibit a diverse spectrum of kinases by direct interactions with these proteins and a few structure–activity studies have identified flavonoids with optimal activities. Hou and Kumamoto [91] summarized the binding of flavonoids to multiple kinases showing both similarities and differences with respect to their interactions with one or more sites in multiple kinases. For example, myricetin binds the ATP pocket of Akt1, MKK4, Fyn, P13Kγ, p38MAPK and JNK3; myricetin also binds MEK1 and JAK1. A recent study confirmed interactions of myricetin with the ATP binding sites of both p38MAPK and JNK3 in modeling studies. However, results of flavonoid-kinase docking studies show that among a series of flavonoids that bind the ATP sites of JNK3 (acacetin, velutin, chrysoeriol, luteolin and myricetin), the β ring of myricetin is oriented in the binding site in the opposite direction compared to the other flavonoids [93]. The structure-dependent binding of 16 flavonoids to 3 acidophilic Ser/Thr protein kinases, namely golgi apparatus casein kinase (G-CK), CK1 and CK2 has also been reported [96]. G-CK inhibition by flavonoids (≤ 40 µM) was minimal and some structure-dependent inhibitory effects of flavonoids on CK1 activity were observed. In contrast, at least six flavonoids inhibited CK2 with IC50 values ≤ 1 µM and the presence of both 7- and 4΄-hydroxyl groups was a common structural feature of the active flavonoids which appear to occupy the ATP binding pocket. This is observed as an underlying mechanism of flavonoids for inhibiting multiple tyrosine kinases. However, despite the extensive evidence showing that flavonoids inhibit multiple tyrosine kinases, the clinical applications of these compounds as targeted kinase inhibitors are minimal.
-
ii.
Flavonoid effects on membrane-bound receptors.
Several studies demonstrate that flavonoids modulate expression or activity of multiple RTKs including EGFRs, cMET, insulin-like growth factor receptor (IGFR), vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptors [92, 97–106]. Many publications show that flavonoids inhibit the function of RTKs and block downstream signaling pathways, however, there is limited data on the mechanisms of flavonoid-RTK interactions. There is evidence that flavonoids mimic ATP and interact with ATP binding sites of RTKs [92] and this is also observed for kinases. Structure–activity studies among several flavonoids identified hesperetin and naringenin as HER2 tyrosine kinase inhibitors through interactions which prevented ATP binding [99]. In contrast, apigenin modulated HER2/HER3-P13K interactions resulting in enhanced degradation of HER2 in breast cancer cells [99]. Catechins and particularly epigallocatechin -3-gallate (ECGC) are also highly effective RTK inhibitors and at least some of their activities are due to occupation of ATP binding sites. Thus, like kinases, RTK activities can be modified by flavonoids, however, the design of optimal flavonoids for kinase specific inhibition and clinical applications is minimal.
-
iii.
Flavonoid effects on G-protein coupled receptor.
G-protein—coupled receptors (GPCR) are seven transmembrane receptors and there are over 800 GPCRs that play diverse roles in vision, taste, smell, behavior, immune responses and the nervous system. Table 1 summarizes some of the GPCRs that are modulated by flavonoids [107–137] and demonstrates that these phytochemicals can potentially influence the large class of cell membrane receptors. Development of optimal flavonoid ligands for activating/inhibiting GPCR should be an important area of development since it is estimated that GPCRs are targets for approximately 50% of all drugs that are currently being used [138]. As illustrated in Table 1, several flavonoids interact with GPR30. Hormonal signaling traditionally involves hormone-dependent activation of nuclear hormone receptors, however, induction of estrogen (ER) signaling has also been linked to activation of kinases pathways and phosphorylation of the ER. It was shown that extra nuclear ER activity was due to the membrane bound GPR30 which subsequently activates downstream kinases. Geinstein was initially identified as a GPR30 ligand and structure–activity in PC12 cells have identified several flavonoids that activate this receptor and optimization of flavonoid targeting GPR30 and other membrane receptors could be important for diverse clinical applications.
-
iv.
Flavonoids and the Aryl Hydrocarbon Receptor (AhR).
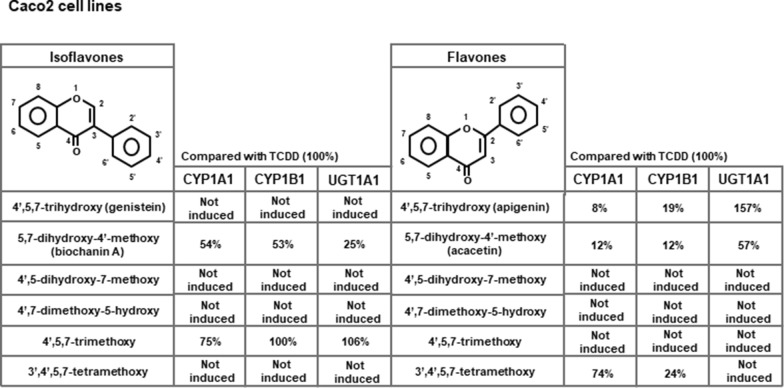
The AhR is a basic-helix-loop-helix transcription factor that forms an active nuclear heterodimer with the AhR nuclear translocator (Arnt) protein to activate gene expression [139]. The AhR was initially discovered as the intracellular receptor that mediates the biochemical and toxic effects induced by 2,3,7,8-tetrachlorodinezo-p-dioxin (TCDD) and structurally-related halogenated aromatics [139, 140]. However, subsequent studies demonstrate that the AhR plays an important role in maintaining cellular homeostasis and in pathophysiology and this receptor also binds structurally diverse compounds including health promoting phytochemicals such as indole-3-carbinol and flavonoids [140]. Extensive structure–activity studies demonstrate that different classes of flavonoids exhibit AhR activity as evidenced by their induction of AhR responsive CYP1A1 gene expression in cell lines and animal models [141]. However, recent studies on flavones and isoflavones demonstrate that the AhR activity of these compounds is compound-, response- and cell-context dependent [142, 143]. For example; Results illustrated in Fig. 2 show the differences between isomeric isoflavones and flavones as activators of AhR-responsive CYP1A1, CYP1B1 and UGT1A1 in Caco2 colon cancer cells. The 4΄,5,7-trimethoxy-isoflavone and 4΄,5,7-trimethoxyflavone; the latter compound was inactive as an inducer whereas the magnitude of the isoflavone-induced response was similar to that observed for TCDD in Caco2 cells. There are clearly major differences in the AhR activity of “isomeric” flavones and isoflavones even though the only structural difference involves the site of attachment of the phenyl ring at C1 or C2. The AhR activity of these compounds was also investigated in mouse hepatocytes (YAMC cells) and although TCDD was active, minimal activity was observed for flavonoids [142, 143].
These results suggest that flavonoids are selective AhR modulators (SAhRMs) that exhibit both AhR agonist or antagonist [140]. There are several examples of flavonoid-induced health promoting activities that are AhR-responsive and many of these are associated with the gastrointestinal tract and immune system where the AhR plays a key role. In models of intestinal inflammation anthocyanidins, cardamonin and alpinetin are protective and these responses are due, in part, to the AhR and enhanced T-regulatory cell functions by naringenin, baicalin and baicalein were AhR-dependent [144–149]. Although there is extensive evidence for a role of AhR in cancer, clinical applications of AhR-active flavonoids are not ongoing.
Table 1.
Flavonoid interactions/modulation of G-protein coupled receptors
| Receptor | Flavonoid | References |
|---|---|---|
| EP1 (prostaglandin receptor) | EGCG (antagonist) | [107] |
| 5-HT1A | Acacetin | [108] |
| Parathyroid hormone receptor 1 | Quercetin (ant) | [109] |
| Thromboxane receptors | Multiple | [110, 111] |
| P2Y12 | Luteolin conjugate | [111] |
| Cannabinoid receptors (CB) | Calechins, quercetin and anthocyanadins | [112–114] |
| Glucogen-like peptide—1 receptor |
Flavonoids myrcetin |
[115] |
| Opiod receptor |
Methoxyflavones ECGC |
[116–118] |
| Muscarinic acetylcholine receptor | Multiple flavonoids | [119] |
| Opsin | Multiple flavonoids | [120] |
| Calcium semsing receptor | Ligustroflavone | [121] |
| CXCR4 | Hesperidin (ant) | [122] |
| Free fatty acid receptor 1 (FFA1, GPR40) | Delphinidin | [123] |
| Muscarinic receptor | Polymethoxyflavones | [124] |
| Bitter taste receptors—TAS2R39 |
6-Methoxyflavones multiple |
[125] [126] |
| TAS2R39/14 | Isoflavones | [127] |
| TAS2R39/46 | Tangeretin, nobiletin and related compounds | [128, 129] |
| GPER (GPR30) |
Baicalein (ant) Genistein, daidzein ECGC, prunetin Icarin, genistein |
Fig. 2.
Summary of induction of AhR responsive CYP1A1, CYP1B1 and UGT1A1 gene expression (mRNA levels) in Caco2 cells by isomeric flavones and isoflavones with the same substitution patterns (142, 143)
Flavonoids and the estrogen receptor (ESR1/ERα and ESR2/ERβ)
There is extensive evidence that flavones/isoflavones and other flavonoids bind and activate/inactivate both ERα and ERβ [150–153]. Kuiper and coworkers examined binding of structurally diverse flavonoids to both ERα and ERβ, and the most active compounds were the isoflavones genistein and daidzein which preferentially bound ERβ compared to ERα [153]. With the exception of apigenin and kaempferol, most other flavonoids did not directly bind ERα and ERβ, however, among multiple studies, the estrogenic or antiestrogenic activities of flavonoids was highly variable [150–153]. The estrogenic activity of flavonoids and its impact on human health and particularly estrogen-related conditions have been extensively investigated with respect to their potential adverse vs. health promoting effects [153, 154]. Correlations between exposure to estrogenic flavonoids and enhanced disease are minimal, however, there is an extensive literature on the contributions of dietary isoflavones to improved health outcomes. Studies show that isoflavone consumption protects against metabolic diseases, enhances cognitive function, decreases risk of coronary heart disease and is associated with decreased risks from ovarian and breast cancers [155–161]. Most of these studies correlated health benefits with total isoflavone intake and there were also correlations with individual isoflavones daidzein and genistein; however, some decreased cancer risks also correlated with the intake of other flavonoids [160]. These results demonstrate that among all the flavonoid-mediated pathways, interactions with ER and possible GPR30 lead to some of the health benefits associated with intake of this class of phytochemicals.
Flavonoids and their interactions with other nuclear receptors
The nuclear receptor (NR) superfamily contains 48 members which include steroid hormone or endocrine receptors (including ERα and ERβ), heterodimeric receptors, adopted orphan receptors, enigmatic orphans and orphan receptors [162]. NRs play a key role in maintaining cellular homeostasis and pathophysiology and the complex roles and interactions of endogenous and exogenous ligands can impact human health. As indicated above, flavonoids (including isoflavones) bind and modulate ERα- and ERβ-mediated gene expression and downstream responses and most studies indicate that dietary flavonoids enhance health. Although most NRs bind low molecular weight compounds such as flavonoids, the activity of these compounds as ligands for NRs and their potential impacts on laboratory animal models and human health have not been extensively investigated. An in vitro screening assay for flavonoid-induced activation of NR-dependent reported genes confirmed interactions with ERα/ERβ but none of the 27 flavonoids exhibited glucocorticoid receptor or thyroid hormone receptor activity [163]. Apigenin activated progesterone receptor-mediated gene expression and blocked genistein induced estrogenic responses in the uterus thus demonstrating flavonoids with opposing activities [164]. Similar results were obtained with kaempferol which binds progesterone receptor A(PRA) and also inhibits genistein-induced estrogenic response in the rodent uterus [165]. Several studies showed that flavonoids exhibited both agonist and antagonist activities as ligands for peroxisome proliferator-activated receptor γ (PPARγ) [73, 166–171]. Genistein and daidzein exhibited PPARγ agonist pro-adipogenic activities in several cell lines [73] whereas apigetrin inhibited adipogenesis in 3T3-L1 cells and downregulated PPARγ [169]. Flavonoids also exhibited PPARγ agonist activities in cancer cells and mouse hepatocytes [172–174]. The liver X receptors (LXRα and LXRβ) are important for cholesterol metabolism and several flavonoids modulate LXR-dependent transactivation [175–177]. Structure–activity studies in Hela cells shows that quercetin (LXR α/β) and apigenin (LXRβ) are agonists whereas galangin and naringenin are antagonists [175]. The farnesoid X receptor (FXR) regulates the biosynthesis and circulation of bile acids and is a potential drug target for treating metabolic diseases. Several flavonoids act as FXR ligands and these include quercetin, EGCG, schaftoside and prenylflavonoids [178–182]. PXR and CAR are key receptors involved in the induction of drug metabolizing enzymes and flavonoids such as hydroxylated flavones, isorhamnetin, genistein, EGCG and alpinetin activate these receptors (primarily PXR) [183–187]. There is evidence of specificity among flavonoids for their differential activation of rat vs. human PXR [183, 187] and inhibition of inflammatory bowel disease in rodents by isorhamnetin was PXR-dependent [184, 187]. These studies demonstrate that NRs are prime targets of flavonoids and optimization of drug-target interactions should identify specific NR-interacting flavonoids that have potential clinical applications.
Mechanism-based applications of flavonoids—summary
There is strong evidence from human studies that flavonoids contribute to disease prevention and their overall antioxidant properties contribute to these health benefits. However, it is also evident from mechanistic studies that individual flavonoids and their mixtures modulate activity or expression of multiple genes and downstream responses. For example, Fig. 3 illustrates some of the responses reported for genistein, a major isoflavonoids component of soy-based foods which has been associated with many health benefits. Like many other flavonoids genistein interacts directly with multiple tyrosine kinases, G-protein coupled receptors and intracellular receptors. Thus, a clinical application of genistein which targets a single gene/pathway is complicated by its diverse activities and potential off-target effects. Moreover, many other flavonoids exhibit a similar pattern of effects on multiple pathways. There are also many examples of multiple flavonoids targeting a single pathway and Fig. 4 illustrates how DSS, TNBS and acetic acid-induced intestinal inflammation is inhibited by several flavonoids acting through different pathways. It is possible that some of these responses for which a specific pathway has not been identified, act through a gene/pathway which has not yet been identified. The successful development of flavonoids for clinical applications will require a more mechanistic precision medicine approach which could include the following;
Development of improved overall and tissue-specific delivery methods to enhance the overall bioavailability of flavonoids.
Response-specific mechanisms for inflammatory and age-related diseases need to be identified and utilized for chemoprevention and chemotherapy.
After identifying mechanism-based intracellular targets required for specific flavonoid-mediating responses, structure activity studies need to be carried out to identify the most active (optimal) flavonoid.
Chemopreventive and chemotherapeutic applications of flavonoids need to be maximized using combinations of active flavonoids.
Fig. 3.
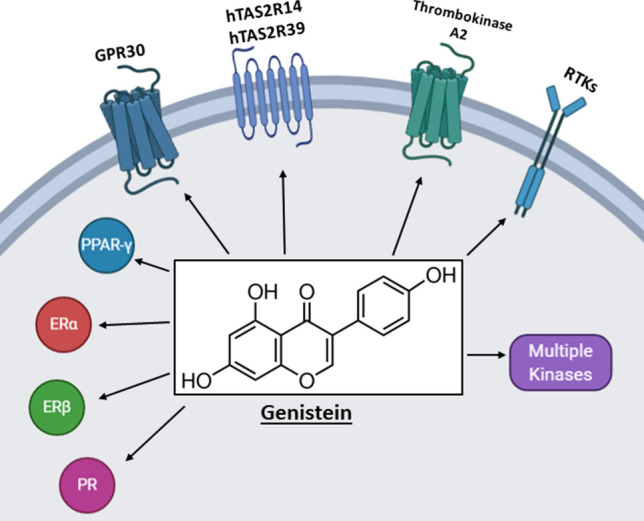
Genistein inhibits/activates multiple pathways including nuclear receptors, kinases, receptor tyrosine kinases and G-protein coupled receptors (89, 91–94, 110, 111, 126, 135, 150)
Fig. 4.
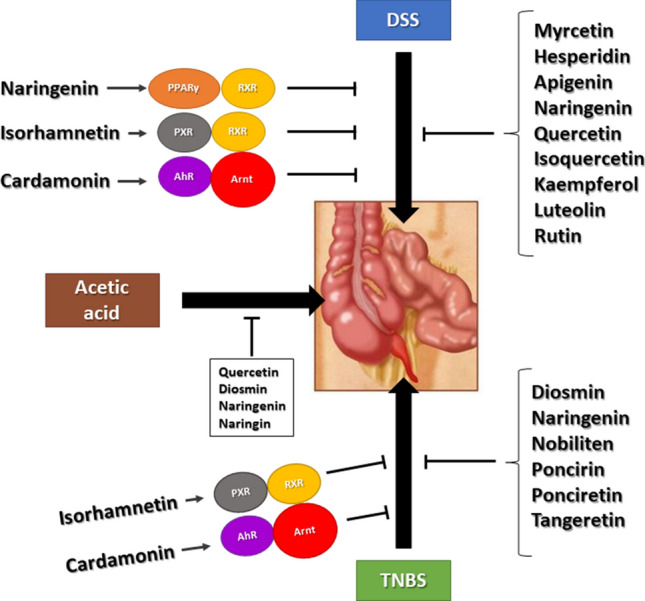
Flavonoids inhibit chemical induced gut inflammation in rodent models through multiple known and unknown pathways
Completion of the above will greatly facilitate turning the diverse and highly promising actions of flavonoids in multiple models of disease prevention into clinical applications which can be used alone and in drug combinations for treating non-cancer and cancer endpoints.
Acknowledgements
The financial assistance of the National Institutes of Health (P30-ES029607 and R01-AT010282), the Syd Kyle Chair endowment and Texas AgriLife are gratefully acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Anticancer efficacy of polyphenols and their combinations. Nutrients. 2016 doi: 10.3390/nu8090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Bi X, Yu B, Chen D. Isoflavones: anti-inflammatory benefit and possible caveats. Nutrients. 2016 doi: 10.3390/nu8060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kikuchi H, Yuan B, Hu X, Okazaki M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am J Cancer Res. 2019;9(8):1517–1535. [PMC free article] [PubMed] [Google Scholar]
- 5.Krizova L, Dadakova K, Kasparovska J, Kasparovsky T. Isoflavones. Molecules. 2019 doi: 10.3390/molecules24061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020 doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alseekh S, Perez de Souza L, Benina M, Fernie AR. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry. 2020;174:112347. doi: 10.1016/j.phytochem.2020.112347. [DOI] [PubMed] [Google Scholar]
- 8.Pei R, Liu X, Bolling B. Flavonoids and gut health. Curr Opin Biotechnol. 2020;61:153–159. doi: 10.1016/j.copbio.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Tohge T, Fernie AR. Combining genetic diversity, informatics and metabolomics to facilitate annotation of plant gene function. Nat Protoc. 2010;5(6):1210–1227. doi: 10.1038/nprot.2010.82. [DOI] [PubMed] [Google Scholar]
- 10.Perez de Souza L, Garbowicz K, Brotman Y, Tohge T, Fernie AR. The acetate pathway supports flavonoid and lipid biosynthesis in arabidopsis. Plant Physiol. 2020;182(2):857–869. doi: 10.1104/pp.19.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96(21):1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 12.Murillo G, Mehta RG. Cruciferous vegetables and cancer prevention. Nutr Cancer. 2001;41(1–2):17–28. doi: 10.1080/01635581.2001.9680607. [DOI] [PubMed] [Google Scholar]
- 13.Key KTJ. Fruit vegetables and cancer risk. Br J Cancer. 2011;104(1):6–11. doi: 10.1038/sj.bjc.6606032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Garcia C, Sanchez-Quesada C, Gaforio JJ. Dietary flavonoids as cancer chemopreventive agents: an updated review of human studies. Antioxidants (Basel) 2019 doi: 10.3390/antiox8050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Weng W, Gao R, Liu Y. New insights for cellular and molecular mechanisms of aging and aging-related diseases: herbal medicine as potential therapeutic approach. Oxid Med Cell Longev. 2019;2019:4598167. doi: 10.1155/2019/4598167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bondonno NP, Dalgaard F, Kyro C, Murray K, Bondonno CP, Lewis JR, Croft KD, Gislason G, Scalbert A, Cassidy A, et al. Flavonoid intake is associated with lower mortality in the danish diet cancer and health cohort. Nat Commun. 2019;10(1):3651. doi: 10.1038/s41467-019-11622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y. Je Y Flavonoid intake and mortality from cardiovascular disease and all causes: a meta-analysis of prospective cohort studies. Clin Nutr ESPEN. 2017;20:68–77. doi: 10.1016/j.clnesp.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Pounis G, Costanzo S, Bonaccio M, Di Castelnuovo A, de Curtis A, Ruggiero E, Persichillo M, Cerletti C, Donati MB, de Gaetano G, et al. Reduced mortality risk by a polyphenol-rich diet: an analysis from the Moli-sani study. Nutrition. 2018;48:87–95. doi: 10.1016/j.nut.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Medina-Remon A, Casas R, Tressserra-Rimbau A, Ros E, Martinez-Gonzalez MA, Fito M, Corella D, Salas-Salvado J, Lamuela-Raventos RM, Estruch R, et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: a substudy of the PREDIMED trial. Br J Clin Pharmacol. 2017;83(1):114–128. doi: 10.1111/bcp.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivey KL, Hodgson JM, Croft KD, Lewis JR, Prince RL. Flavonoid intake and all-cause mortality. Am J Clin Nutr. 2015;101(5):1012–1020. doi: 10.3945/ajcn.113.073106. [DOI] [PubMed] [Google Scholar]
- 21.Liu XM, Liu YJ, Huang Y, Yu HJ, Yuan S, Tang BW, Wang PG, He QQ. Dietary total flavonoids intake and risk of mortality from all causes and cardiovascular disease in the general population: a systematic review and meta-analysis of cohort studies. Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201601003. [DOI] [PubMed] [Google Scholar]
- 22.Ivey KL, Jensen MK, Hodgson JM, Eliassen AH, Cassidy A, Rimm EB. Association of flavonoid-rich foods and flavonoids with risk of all-cause mortality. Br J Nutr. 2017;117(10):1470–1477. doi: 10.1017/S0007114517001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rienks J, Barbaresko J, Oluwagbemigun K, Schmid M, Nothlings U. Polyphenol exposure and risk of type 2 diabetes: dose-response meta-analyses and systematic review of prospective cohort studies. Am J Clin Nutr. 2018;108(1):49–61. doi: 10.1093/ajcn/nqy083. [DOI] [PubMed] [Google Scholar]
- 24.Gao Q, Zhong C, Zhou X, Chen R, Xiong T, Hong M, Li Q, Kong M, Xiong G, Han W, et al. Inverse association of total polyphenols and flavonoids intake and the intake from fruits with the risk of gestational diabetes mellitus: a prospective cohort study. Clin Nutr. 2020 doi: 10.1016/j.clnu.2020.05.053. [DOI] [PubMed] [Google Scholar]
- 25.Caro-Ordieres T, Marin-Royo G, Opazo-Rios L, Jimenez-Castilla L, Moreno JA, Gomez-Guerrero C, Egido J. The coming age of flavonoids in the treatment of diabetic complications. J Clin Med. 2020 doi: 10.3390/jcm9020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao H, Ou J, Chen L, Zhang Y, Szkudelski T, Delmas D, Daglia M, Xiao J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit Rev Food Sci Nutr. 2019;59(20):3371–3379. doi: 10.1080/10408398.2018.1492900. [DOI] [PubMed] [Google Scholar]
- 27.Akhlaghi M, Ghobadi S, Mohammad Hosseini M, Gholami Z, Mohammadian F. Flavanols are potential anti-obesity agents, a systematic review and meta-analysis of controlled clinical trials. Nutr Metab Cardiovasc Dis. 2018;28(7):675–690. doi: 10.1016/j.numecd.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Tresserra-Rimbau A, Castro-Barquero S, Vitelli-Storelli F, Becerra-Tomas N, Vazquez-Ruiz Z, Diaz-Lopez A, Corella D, Castaner O, Romaguera D, Vioque J, et al. Associations between dietary polyphenols and type 2 diabetes in a cross-sectional analysis of the predimed-plus trial: role of body mass index and sex. Antioxidants (Basel) 2019 doi: 10.3390/antiox8110537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Field DT, Williams CM, Butler LT. Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol Behav. 2011;103(3–4):255–260. doi: 10.1016/j.physbeh.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Whyte AR, Williams CM. Effects of a single dose of a flavonoid-rich blueberry drink on memory in 8 to 10 y old children. Nutrition. 2015;31(3):531–534. doi: 10.1016/j.nut.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Alharbi MH, Lamport DJ, Dodd GF, Saunders C, Harkness L, Butler LT, Spencer JP. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur J Nutr. 2016;55(6):2021–2029. doi: 10.1007/s00394-015-1016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamport DJ, Pal D, Macready AL, Barbosa-Boucas S, Fletcher JM, Williams CM, Spencer JP, Butler LT. The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: an acute, randomised, placebo-controlled cross-over trial in healthy, young adults. Br J Nutr. 2016;116(12):2160–2168. doi: 10.1017/S000711451600430X. [DOI] [PubMed] [Google Scholar]
- 33.Scholey AB, French SJ, Morris PJ, Kennedy DO, Milne AL, Haskell CF. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol. 2010;24(10):1505–1514. doi: 10.1177/0269881109106923. [DOI] [PubMed] [Google Scholar]
- 34.Valls-Pedret C, Lamuela-Raventos RM, Medina-Remon A, Quintana M, Corella D, Pinto X, Martinez-Gonzalez MA, Estruch R, Ros E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. 2012;29(4):773–782. doi: 10.3233/JAD-2012-111799. [DOI] [PubMed] [Google Scholar]
- 35.Safouris A, Tsivgoulis G, Sergentanis TN, Psaltopoulou T. Mediterranean diet and risk of dementia. Curr Alzheimer Res. 2015;12(8):736–744. doi: 10.2174/1567205012666150710114430. [DOI] [PubMed] [Google Scholar]
- 36.Anastasiou CA, Yannakoulia M, Kosmidis MH, Dardiotis E, Hadjigeorgiou GM, Sakka P, Arampatzi X, Bougea A, Labropoulos I, Scarmeas N. Mediterranean diet and cognitive health: Initial results from the hellenic longitudinal investigation of ageing and diet. PLoS ONE. 2017;12(8):e0182048. doi: 10.1371/journal.pone.0182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berti V, Walters M, Sterling J, Quinn CG, Logue M, Andrews R, Matthews DC, Osorio RS, Pupi A, Vallabhajosula S, et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology. 2018;90(20):e1789–e1798. doi: 10.1212/WNL.0000000000005527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal AN, Mais DD. Sensitivity and specificity of alzheimer type II astrocytes in hepatic encephalopathy. Arch Pathol Lab Med. 2019;143(10):1256–1258. doi: 10.5858/arpa.2018-0455-OA. [DOI] [PubMed] [Google Scholar]
- 39.Shishtar E, Rogers GT, Blumberg JB, Au R, Jacques PF. Long-term dietary flavonoid intake and risk of Alzheimer disease and related dementias in the Framingham Offspring Cohort. Am J Clin Nutr. 2020;112(2):343–353. doi: 10.1093/ajcn/nqaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shishtar E, Rogers GT, Blumberg JB, Au R, DeCarli C, Jacques PF. Flavonoid intake and MRI markers of brain health in the framingham offspring cohort. J Nutr. 2020;150(6):1545–1553. doi: 10.1093/jn/nxaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferraz CR, Carvalho TT, Manchope MF, Artero NA, Rasquel-Oliveira FS, Fattori V, Casagrande R, Verri WA., Jr Therapeutic potential of flavonoids in pain and inflammation: mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules. 2020 doi: 10.3390/molecules25030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farzaei MH, Singh AK, Kumar R, Croley CR, Pandey AK, Coy-Barrera E, Kumar Patra J, Das G, Kerry RG, Annunziata G, et al. Targeting inflammation by flavonoids: novel therapeutic strategy for metabolic disorders. Int J Mol Sci. 2019 doi: 10.3390/ijms20194957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124. doi: 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- 44.Godeberge P. Daflon 500 mg in the treatment of hemorrhoidal disease: a demonstrated efficacy in comparison with placebo. Angiology. 1994;45(6 Pt 2):574–578. [PubMed] [Google Scholar]
- 45.Rizza S, Muniyappa R, Iantorno M, Kim JA, Chen H, Pullikotil P, Senese N, Tesauro M, Lauro D, Cardillo C, et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96(5):E782–792. doi: 10.1210/jc.2010-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cospite M. Double-blind, placebo-controlled evaluation of clinical activity and safety of Daflon 500 mg in the treatment of acute hemorrhoids. Angiology. 1994;45(6 Pt 2):566–573. [PubMed] [Google Scholar]
- 47.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32(6):421–427. doi: 10.1016/j.nutres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Martinez G, Mijares MR, De Sanctis JB. Effects of flavonoids and its derivatives on immune cell responses. Recent Pat Inflamm Allergy Drug Discov. 2019;13(2):84–104. doi: 10.2174/1872213X13666190426164124. [DOI] [PubMed] [Google Scholar]
- 49.Sharma S, Naura AS. Potential of phytochemicals as immune-regulatory compounds in atopic diseases: a review. Biochem Pharmacol. 2020;173:113790. doi: 10.1016/j.bcp.2019.113790. [DOI] [PubMed] [Google Scholar]
- 50.Margina D, Ungurianu A, Purdel C, Nitulescu GM, Tsoukalas D, Sarandi E, Thanasoula M, Burykina TI, Tekos F, Buha A, et al. Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem Toxicol. 2020;143:111558. doi: 10.1016/j.fct.2020.111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Focaccetti C, Izzi V, Benvenuto M, Fazi S, Ciuffa S, Giganti MG, Potenza V, Manzari V, Modesti A, Bei R. Polyphenols as immunomodulatory compounds in the tumor microenvironment: friends or foes? Int J Mol Sci. 2019 doi: 10.3390/ijms20071714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vezza T, Rodriguez-Nogales A, Algieri F, Utrilla MP, Rodriguez-Cabezas ME, Galvez J. Flavonoids in inflammatory bowel disease: a review. Nutrients. 2016;8(4):211. doi: 10.3390/nu8040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oteiza PI, Fraga CG, Mills DA, Taft DH. Flavonoids and the gastrointestinal tract: local and systemic effects. Mol Aspects Med. 2018;61:41–49. doi: 10.1016/j.mam.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Salaritabar A, Darvishi B, Hadjiakhoondi F, Manayi A, Sureda A, Nabavi SF, Fitzpatrick LR, Nabavi SM, Bishayee A. Therapeutic potential of flavonoids in inflammatory bowel disease: a comprehensive review. World J Gastroenterol. 2017;23(28):5097–5114. doi: 10.3748/wjg.v23.i28.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Musumeci L, Maugeri A, Cirmi S, Lombardo GE, Russo C, Gangemi S, Calapai G, Navarra M. Citrus fruits and their flavonoids in inflammatory bowel disease: an overview. Nat Prod Res. 2020;34(1):122–136. doi: 10.1080/14786419.2019.1601196. [DOI] [PubMed] [Google Scholar]
- 56.Algieri F, Rodriguez-Nogales A, Rodriguez-Cabezas ME, Risco S, Ocete MA, Galvez J. Botanical drugs as an emerging strategy in inflammatory bowel disease: a review. Mediators Inflamm. 2015;2015:179616. doi: 10.1155/2015/179616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen S, Zhao H, Cheng N, Cao W. Rape bee pollen alleviates dextran sulfate sodium (DSS)-induced colitis by neutralizing IL-1beta and regulating the gut microbiota in mice. Food Res Int. 2019;122:241–251. doi: 10.1016/j.foodres.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 58.Chicco F, Magri S, Cingolani A, Paduano D, Pesenti M, Zara F, Tumbarello F, Urru E, Melis A, Casula L, et al. Multidimensional impact of mediterranean diet on IBD patients. Inflamm Bowel Dis. 2020 doi: 10.1093/ibd/izaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skolmowska D, Glabska D, Guzek D, Lech G. Association between dietary isoflavone intake and ulcerative colitis symptoms in polish caucasian individuals. Nutrients. 2019 doi: 10.3390/nu11081936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biedermann L, Mwinyi J, Scharl M, Frei P, Zeitz J, Kullak-Ublick GA, Vavricka SR, Fried M, Weber A, Humpf HU, et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—an open pilot study. J Crohns Colitis. 2013;7(4):271–279. doi: 10.1016/j.crohns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Dryden GW, Lam A, Beatty K, Qazzaz HH, McClain CJ. A pilot study to evaluate the safety and efficacy of an oral dose of (-)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflamm Bowel Dis. 2013;19(9):1904–1912. doi: 10.1097/MIB.0b013e31828f5198. [DOI] [PubMed] [Google Scholar]
- 62.Kolacek M, Muchova J, Dvorakova M, Paduchova Z, Zitnanova I, Cierna I, Orszaghova Z, Szekyova D, Jajcaiova-Zednickova N, Kovacs L, et al. Effect of natural polyphenols (Pycnogenol) on oxidative stress markers in children suffering from Crohn's disease—a pilot study. Free Radic Res. 2013;47(8):624–634. doi: 10.3109/10715762.2013.807508. [DOI] [PubMed] [Google Scholar]
- 63.Kim H, Venancio VP, Fang C, Dupont AW, Talcott ST, Mertens-Talcott SU. Mango (Mangifera indica L.) polyphenols reduce IL-8, GRO, and GM-SCF plasma levels and increase Lactobacillus species in a pilot study in patients with inflammatory bowel disease. Nutr Res. 2020;75:85–94. doi: 10.1016/j.nutres.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Park S, Song G, Lim W. Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J Nutr Biochem. 2020;78:108328. doi: 10.1016/j.jnutbio.2019.108328. [DOI] [PubMed] [Google Scholar]
- 65.Park S, Lim W, Bazer FW, Whang KY, Song G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J Nutr Biochem. 2019;63:87–100. doi: 10.1016/j.jnutbio.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 66.Park S, Lim W, You S, Song G. Ameliorative effects of luteolin against endometriosis progression in vitro and in vivo. J Nutr Biochem. 2019;67:161–172. doi: 10.1016/j.jnutbio.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Wang CC, Xu H, Man GC, Zhang T, Chu KO, Chu CY, Cheng JT, Li G, He YX, Qin L, et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis. 2013;16(1):59–69. doi: 10.1007/s10456-012-9299-4. [DOI] [PubMed] [Google Scholar]
- 68.Yu MM, Zhou QM. 3,6-dihydroxyflavone suppresses the epithelial-mesenchymal transition, migration and invasion in endometrial stromal cells by inhibiting the Notch signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(12):4009–4017. doi: 10.26355/eurrev_201806_15287. [DOI] [PubMed] [Google Scholar]
- 69.Ilhan M, Ali Z, Khan IA, Tastan H, Kupeli Akkol E. The regression of endometriosis with glycosylated flavonoids isolated from Melilotus officinalis (L.) Pall. in an endometriosis rat model. Taiwan J Obstet Gynecol. 2020;59(2):211–219. doi: 10.1016/j.tjog.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Ding D, Cai X, Zheng H, Guo SW, Liu X. Scutellarin suppresses platelet aggregation and stalls lesional progression in mouse with induced endometriosis. Reprod Sci. 2019;26(11):1417–1428. doi: 10.1177/1933719118817661. [DOI] [PubMed] [Google Scholar]
- 71.Ilhan M, Ali Z, Khan IA, Tastan H, Kupeli Akkol E. Bioactivity-guided isolation of flavonoids from Urtica dioica L. and their effect on endometriosis rat model. J Ethnopharmacol. 2019;243:112100. doi: 10.1016/j.jep.2019.112100. [DOI] [PubMed] [Google Scholar]
- 72.Ryu S, Bazer FW, Lim W, Song G. Chrysin leads to cell death in endometriosis by regulation of endoplasmic reticulum stress and cytosolic calcium level. J Cell Physiol. 2019;234(3):2480–2490. doi: 10.1002/jcp.26770. [DOI] [PubMed] [Google Scholar]
- 73.Toh MF, Mendonca E, Eddie SL, Endsley MP, Lantvit DD, Petukhov PA, Burdette JE. Kaempferol exhibits progestogenic effects in ovariectomized rats. J Steroids Horm Sci. 2014;5(3):136. doi: 10.4172/2157-7536.1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsuzaki S, Darcha C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum Reprod. 2014;29(8):1677–1687. doi: 10.1093/humrep/deu123. [DOI] [PubMed] [Google Scholar]
- 75.Signorile PG, Viceconte R, Baldi A. Novel dietary supplement association reduces symptoms in endometriosis patients. J Cell Physiol. 2018;233(8):5920–5925. doi: 10.1002/jcp.26401. [DOI] [PubMed] [Google Scholar]
- 76.Del Bo C, Bernardi S, Marino M, Porrini M, Tucci M, Guglielmetti S, Cherubini A, Carrieri B, Kirkup B, Kroon P, et al. Systematic review on polyphenol intake and health outcomes: is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients. 2019 doi: 10.3390/nu11061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abbaszadeh H, Keikhaei B, Mottaghi S. A review of molecular mechanisms involved in anticancer and antiangiogenic effects of natural polyphenolic compounds. Phytother Res. 2019;33(8):2002–2014. doi: 10.1002/ptr.6403. [DOI] [PubMed] [Google Scholar]
- 78.Khater M, Greco F, Osborn HMI. Antiangiogenic activity of flavonoids: a systematic review and meta-analysis. Molecules. 2020 doi: 10.3390/molecules25204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, Busselberg D. Flavonoids in cancer and apoptosis. Cancers (Basel) 2018 doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bisol A, de Campos PS, Lamers ML. Flavonoids as anticancer therapies: a systematic review of clinical trials. Phytother Res. 2020;34(3):568–582. doi: 10.1002/ptr.6551. [DOI] [PubMed] [Google Scholar]
- 81.Nimptsch K, Zhang X, Cassidy A, Song M, O'Reilly EJ, Lin JH, Pischon T, Rimm EB, Willett WC, Fuchs CS, et al. Habitual intake of flavonoid subclasses and risk of colorectal cancer in 2 large prospective cohorts. Am J Clin Nutr. 2016;103(1):184–191. doi: 10.3945/ajcn.115.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu M, Chen YM, Huang J, Fang YJ, Huang WQ, Yan B, Lu MS, Pan ZZ, Zhang CX. Flavonoid intake from vegetables and fruits is inversely associated with colorectal cancer risk: a case-control study in China. Br J Nutr. 2016;116(7):1275–1287. doi: 10.1017/S0007114516003196. [DOI] [PubMed] [Google Scholar]
- 83.Zamora-Ros R, Not C, Guino E, Lujan-Barroso L, Garcia RM, Biondo S, Salazar R, Moreno V. Association between habitual dietary flavonoid and lignan intake and colorectal cancer in a Spanish case-control study (the Bellvitge Colorectal Cancer Study) Cancer Causes Control. 2013;24(3):549–557. doi: 10.1007/s10552-012-9992-z. [DOI] [PubMed] [Google Scholar]
- 84.Zamora-Ros R, Guino E, Alonso MH, Vidal C, Barenys M, Soriano A, Moreno V. Dietary flavonoids, lignans and colorectal cancer prognosis. Sci Rep. 2015;5:14148. doi: 10.1038/srep14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, Forman JD, Cher ML, Powell I, Pontes JE, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59(1):1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 86.El-Rayes BF, Philip PA, Sarkar FH, Shields AF, Ferris AM, Hess K, Kaseb AO, Javle MM, Varadhachary GR, Wolff RA, et al. A phase II study of isoflavones, erlotinib, and gemcitabine in advanced pancreatic cancer. Invest New Drugs. 2011;29(4):694–699. doi: 10.1007/s10637-010-9386-6. [DOI] [PubMed] [Google Scholar]
- 87.Zhao J, Yang J, Xie Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: an overview. Int J Pharm. 2019;570:118642. doi: 10.1016/j.ijpharm.2019.118642. [DOI] [PubMed] [Google Scholar]
- 88.Castelli V, Grassi D, Bocale R, d’Angelo M, Antonosante A, Cimini A, Ferri C, Desideri G. Diet and brain health: which role for polyphenols? Curr Pharm Des. 2018;24(2):227–238. doi: 10.2174/1381612824666171213100449. [DOI] [PubMed] [Google Scholar]
- 89.Zhao L, Yuan X, Wang J, Feng Y, Ji F, Li Z, Bian J. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorg Med Chem. 2019;27(5):677–685. doi: 10.1016/j.bmc.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 90.Dong X, Zhou X, Jing H, Chen J, Liu T, Yang B, He Q, Hu Y. Pharmacophore identification, virtual screening and biological evaluation of prenylated flavonoids derivatives as PKB/Akt1 inhibitors. Eur J Med Chem. 2011;46(12):5949–5958. doi: 10.1016/j.ejmech.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 91.Hou DX, Kumamoto T. Flavonoids as protein kinase inhibitors for cancer chemoprevention: direct binding and molecular modeling. Antioxid Redox Signal. 2010;13(5):691–719. doi: 10.1089/ars.2009.2816. [DOI] [PubMed] [Google Scholar]
- 92.Teillet F, Boumendjel A, Boutonnat J, Ronot X. Flavonoids as RTK inhibitors and potential anticancer agents. Med Res Rev. 2008;28(5):715–745. doi: 10.1002/med.20122. [DOI] [PubMed] [Google Scholar]
- 93.Goettert M, Schattel V, Koch P, Merfort I, Laufer S. Biological evaluation and structural determinants of p38alpha mitogen-activated-protein kinase and c-Jun-N-terminal kinase 3 inhibition by flavonoids. Chem Bio Chem. 2010;11(18):2579–2588. doi: 10.1002/cbic.201000487. [DOI] [PubMed] [Google Scholar]
- 94.Chae HS, Xu R, Won JY, Chin YW, Yim H. Molecular targets of genistein and its related flavonoids to exert anticancer effects. Int J Mol Sci. 2019 doi: 10.3390/ijms20102420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262(12):5592–5595. doi: 10.1016/S0021-9258(18)45614-1. [DOI] [PubMed] [Google Scholar]
- 96.Lolli G, Cozza G, Mazzorana M, Tibaldi E, Cesaro L, Donella-Deana A, Meggio F, Venerando A, Franchin C, Sarno S, et al. Inhibition of protein kinase CK2 by flavonoids and tyrphostins. A structural insight. Biochemistry. 2012;51(31):6097–6107. doi: 10.1021/bi300531c. [DOI] [PubMed] [Google Scholar]
- 97.Larsen CA, Dashwood RH, Bisson WH. Tea catechins as inhibitors of receptor tyrosine kinases: mechanistic insights and human relevance. Pharmacol Res. 2010;62(6):457–464. doi: 10.1016/j.phrs.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh P, Bast F. Screening of multi-targeted natural compounds for receptor tyrosine kinases inhibitors and biological evaluation on cancer cell lines, in silico and in vitro. Med Oncol. 2015;32(9):233. doi: 10.1007/s12032-015-0678-8. [DOI] [PubMed] [Google Scholar]
- 99.Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 2004;279(6):4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 100.Elsayed HE, Ebrahim HY, Mohyeldin MM, Siddique AB, Kamal AM, Haggag EG, El Sayed KA. Rutin as a novel c-met inhibitory lead for the control of triple negative breast malignancies. Nutr Cancer. 2017;69(8):1256–1271. doi: 10.1080/01635581.2017.1367936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chandrika BB, Steephan M, Kumar TRS, Sabu A, Haridas M. Hesperetin and naringenin sensitize HER2 positive cancer cells to death by serving as HER2 tyrosine kinase inhibitors. Life Sci. 2016;160:47–56. doi: 10.1016/j.lfs.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 102.Fang J, Zhou Q, Shi XL, Jiang BH. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis. 2007;28(3):713–723. doi: 10.1093/carcin/bgl189. [DOI] [PubMed] [Google Scholar]
- 103.Hu W, Wu X, Tang J, Zhao G, Xiao N, Zhang L, Li S. Anti-cancer targets of formononetin and molecular mechanisms in osteosarcoma: findings of bioinformatic and experimental assays. J Cell Mol Med. 2019;23(5):3505–3511. doi: 10.1111/jcmm.14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singh P, Bast F. Screening and biological evaluation of myricetin as a multiple target inhibitor insulin, epidermal growth factor, and androgen receptor; in silico and in vitro. Invest New Drugs. 2015;33(3):575–593. doi: 10.1007/s10637-015-0240-8. [DOI] [PubMed] [Google Scholar]
- 105.Hariri BM, McMahon DB, Chen B, Freund JR, Mansfield CJ, Doghramji LJ, Adappa ND, Palmer JN, Kennedy DW, Reed DR, et al. Flavones modulate respiratory epithelial innate immunity: anti-inflammatory effects and activation of the T2R14 receptor. J Biol Chem. 2017;292(20):8484–8497. doi: 10.1074/jbc.M116.771949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sheng Y, Li W, Zhu F, Liu K, Chen H, Yao K, Reddy K, Lim DY, Oi N, Li H, et al. 3,6,2',4',5'-Pentahydroxyflavone, an orally bioavailable multiple protein kinase inhibitor, overcomes gefitinib resistance in non-small cell lung cancer. J Biol Chem. 2014;289(41):28192–28201. doi: 10.1074/jbc.M114.593475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang H, Wang M, Sun H, Zhu S, Jin J. Synergetic effect of EP1 receptor antagonist and (-)-epigallocatechin-3-gallate in hepatocellular carcinoma. Pharmacology. 2019;104(5–6):267–275. doi: 10.1159/000502076. [DOI] [PubMed] [Google Scholar]
- 108.Xiao WZ, Zhou WH, Ma Q, Cui WG, Mei QY, Zhao X. Serotonergically dependent antidepressant-like activity on behavior and stress axis responsivity of acacetin. Pharmacol Res. 2019;146:104310. doi: 10.1016/j.phrs.2019.104310. [DOI] [PubMed] [Google Scholar]
- 109.Li S, Pei Y, Wang W, Liu F, Zheng K, Zhang X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed Pharmacother. 2019;114:108839. doi: 10.1016/j.biopha.2019.108839. [DOI] [PubMed] [Google Scholar]
- 110.Navarro-Nunez L, Castillo J, Lozano ML, Martinez C, Benavente-Garcia O, Vicente V, Rivera J. Thromboxane A2 receptor antagonism by flavonoids: structure-activity relationships. J Agric Food Chem. 2009;57(4):1589–1594. doi: 10.1021/jf803041k. [DOI] [PubMed] [Google Scholar]
- 111.Xu H, Lu H, Zhu X, Wang W, Zhang Z, Fu H, Ma S, Luo Y, Fu J. Inhibitory effects of luteolin4'ObetaDglucopyranoside on P2Y12 and thromboxane A2 receptormediated amplification of platelet activation in vitro. Int J Mol Med. 2018;42(1):615–624. doi: 10.3892/ijmm.2018.3634. [DOI] [PubMed] [Google Scholar]
- 112.Korte G, Dreiseitel A, Schreier P, Oehme A, Locher S, Hajak G, Sand PG. An examination of anthocyanins' and anthocyanidins' affinity for cannabinoid receptors. J Med Food. 2009;12(6):1407–1410. doi: 10.1089/jmf.2008.0243. [DOI] [PubMed] [Google Scholar]
- 113.Korte G, Dreiseitel A, Schreier P, Oehme A, Locher S, Geiger S, Heilmann J, Sand PG. Tea catechins' affinity for human cannabinoid receptors. Phytomedicine. 2010;17(1):19–22. doi: 10.1016/j.phymed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 114.Refolo MG, D’Alessandro R, Malerba N, Laezza C, Bifulco M, Messa C, Caruso MG, Notarnicola M, Tutino V. Anti proliferative and pro apoptotic effects of flavonoid quercetin are mediated by cb1 receptor in human colon cancer cell lines. J Cell Physiol. 2015;230(12):2973–2980. doi: 10.1002/jcp.25026. [DOI] [PubMed] [Google Scholar]
- 115.Wootten D, Simms J, Koole C, Woodman OL, Summers RJ, Christopoulos A, Sexton PM. Modulation of the glucagon-like peptide-1 receptor signaling by naturally occurring and synthetic flavonoids. J Pharmacol Exp Ther. 2011;336(2):540–550. doi: 10.1124/jpet.110.176362. [DOI] [PubMed] [Google Scholar]
- 116.Tarawneh A, Leon F, Pettaway S, Elokely KM, Klein ML, Lambert J, Mansoor A, Cutler SJ. Flavonoids from perovskia atriplicifolia and their in vitro displacement of the respective radioligands for human opioid and cannabinoid receptors. J Nat Prod. 2015;78(6):1461–1465. doi: 10.1021/acs.jnatprod.5b00218. [DOI] [PubMed] [Google Scholar]
- 117.Ruiu S, Anzani N, Orru A, Floris C, Caboni P, Alcaro S, Maccioni E, Distinto S, Cottiglia F. Methoxyflavones from Stachys glutinosa with binding affinity to opioid receptors: in silico, in vitro, and in vivo studies. J Nat Prod. 2015;78(1):69–76. doi: 10.1021/np500671v. [DOI] [PubMed] [Google Scholar]
- 118.Panneerselvam M, Tsutsumi YM, Bonds JA, Horikawa YT, Saldana M, Dalton ND, Head BP, Patel PM, Roth DM, Patel HH. Dark chocolate receptors: epicatechin-induced cardiac protection is dependent on delta-opioid receptor stimulation. Am J Physiol Heart Circ Physiol. 2010;299(5):H1604–1609. doi: 10.1152/ajpheart.00073.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swaminathan M, Chee CF, Chin SP, Buckle MJ, Rahman NA, Doughty SW, Chung LY. Flavonoids with M1 muscarinic acetylcholine receptor binding activity. Molecules. 2014;19(7):8933–8948. doi: 10.3390/molecules19078933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ortega JT, Parmar T, Jastrzebska B. Flavonoids enhance rod opsin stability, folding, and self-association by directly binding to ligand-free opsin and modulating its conformation. J Biol Chem. 2019;294(20):8101–8122. doi: 10.1074/jbc.RA119.007808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feng R, Ding F, Mi XH, Liu SF, Jiang AL, Liu BH, Lian Y, Shi Q, Wang YJ, Zhang Y. Protective effects of ligustroflavone, an active compound from ligustrum lucidum, on diabetes-induced osteoporosis in mice: a potential candidate as calcium-sensing receptor antagonist. Am J Chin Med. 2019;47(2):457–476. doi: 10.1142/S0192415X1950023X. [DOI] [PubMed] [Google Scholar]
- 122.Xia R, Xu G, Huang Y, Sheng X, Xu X, Lu H. Hesperidin suppresses the migration and invasion of non-small cell lung cancer cells by inhibiting the SDF-1/CXCR-4 pathway. Life Sci. 2018;201:111–120. doi: 10.1016/j.lfs.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 123.Hidalgo J, Teuber S, Morera FJ, Ojeda C, Flores CA, Hidalgo MA, Nunez L, Villalobos C, Burgos RA. Delphinidin reduces glucose uptake in mice jejunal tissue and human intestinal cells lines through FFA1/GPR40. Int J Mol Sci. 2017 doi: 10.3390/ijms18040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung LY, Yap KF, Goh SH, Mustafa MR, Imiyabir Z. Muscarinic receptor binding activity of polyoxygenated flavones from Melicope subunifoliolata. Phytochemistry. 2008;69(7):1548–1554. doi: 10.1016/j.phytochem.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 125.Roland WS, Gouka RJ, Gruppen H, Driesse M, van Buren L, Smit G, Vincken JP. 6-methoxyflavanones as bitter taste receptor blockers for hTAS2R39. PLoS ONE. 2014;9(4):e94451. doi: 10.1371/journal.pone.0094451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roland WS, van Buren L, Gruppen H, Driesse M, Gouka RJ, Smit G, Vincken JP. Bitter taste receptor activation by flavonoids and isoflavonoids: modeled structural requirements for activation of hTAS2R14 and hTAS2R39. J Agric Food Chem. 2013;61(44):10454–10466. doi: 10.1021/jf403387p. [DOI] [PubMed] [Google Scholar]
- 127.Roland WS, Vincken JP, Gouka RJ, van Buren L, Gruppen H, Smit G. Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39. J Agric Food Chem. 2011;59(21):11764–11771. doi: 10.1021/jf202816u. [DOI] [PubMed] [Google Scholar]
- 128.Kuroda Y, Ikeda R, Yamazaki T, Ito K, Uda K, Wakabayashi K, Watanabe T. Activation of human bitter taste receptors by polymethoxylated flavonoids. Biosci Biotechnol Biochem. 2016;80(10):2014–2017. doi: 10.1080/09168451.2016.1184558. [DOI] [PubMed] [Google Scholar]
- 129.Batenburg AM, de Joode T, Gouka RJ. Characterization and modulation of the bitterness of polymethoxyflavones using sensory and receptor-based methods. J Agric Food Chem. 2016;64(12):2619–2626. doi: 10.1021/acs.jafc.5b05833. [DOI] [PubMed] [Google Scholar]
- 130.Shang D, Li Z, Zhu Z, Chen H, Zhao L, Wang X, Chen Y. Baicalein suppresses 17-beta-estradiol-induced migration, adhesion and invasion of breast cancer cells via the G protein-coupled receptor 30 signaling pathway. Oncol Rep. 2015;33(4):2077–2085. doi: 10.3892/or.2015.3786. [DOI] [PubMed] [Google Scholar]
- 131.Luo LJ, Liu F, Lin ZK, Xie YF, Xu JL, Tong QC, Shu R. Genistein regulates the IL-1 beta induced activation of MAPKs in human periodontal ligament cells through G protein-coupled receptor 30. Arch Biochem Biophys. 2012;522(1):9–16. doi: 10.1016/j.abb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 132.Kajta M, Rzemieniec J, Litwa E, Lason W, Lenartowicz M, Krzeptowski W, Wojtowicz AK. The key involvement of estrogen receptor beta and G-protein-coupled receptor 30 in the neuroprotective action of daidzein. Neuroscience. 2013;238:345–360. doi: 10.1016/j.neuroscience.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 133.Moreno-Ulloa A, Mendez-Luna D, Beltran-Partida E, Castillo C, Guevara G, Ramirez-Sanchez I, Correa-Basurto J, Ceballos G, Villarreal F. The effects of (-)-epicatechin on endothelial cells involve the G protein-coupled estrogen receptor (GPER) Pharmacol Res. 2015;100:309–320. doi: 10.1016/j.phrs.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khan K, Pal S, Yadav M, Maurya R, Trivedi AK, Sanyal S, Chattopadhyay N. Prunetin signals via G-protein-coupled receptor, GPR30(GPER1): Stimulation of adenylyl cyclase and cAMP-mediated activation of MAPK signaling induces Runx2 expression in osteoblasts to promote bone regeneration. J Nutr Biochem. 2015;26(12):1491–1501. doi: 10.1016/j.jnutbio.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 135.Qiao C, Ye W, Li S, Wang H, Ding X. Icariin modulates mitochondrial function and apoptosis in high glucose-induced glomerular podocytes through G protein-coupled estrogen receptors. Mol Cell Endocrinol. 2018;473:146–155. doi: 10.1016/j.mce.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 136.Chen Y, Wang J, Hong DY, Chen L, Zhang YY, Xu YN, Pan D, Fu LY, Tao L, Luo H, et al. Baicalein has protective effects on the 17beta-estradiol-induced transformation of breast epithelial cells. Oncotarget. 2017;8(6):10470–10484. doi: 10.18632/oncotarget.14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Du ZR, Feng XQ, Li N, Qu JX, Feng L, Chen L, Chen WF. G protein-coupled estrogen receptor is involved in the anti-inflammatory effects of genistein in microglia. Phytomedicine. 2018;43:11–20. doi: 10.1016/j.phymed.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 138.Hauser AS, Chavali S, Masuho I, Jahn LJ, Martemyanov KA, Gloriam DE, Babu MM. Pharmacogenomics of GPCR drug targets. Cell. 2018;172(1–2):41–54 e19. doi: 10.1016/j.cell.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Avilla MN, Malecki KMC, Hahn ME, Wilson RH, Bradfield CA. The Ah receptor: adaptive metabolism, ligand diversity, and the xenokine model. Chem Res Toxicol. 2020;33(4):860–879. doi: 10.1021/acs.chemrestox.9b00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Safe S, Jin UH, Park H, Chapkin RS, Jayaraman A. Aryl hydrocarbon receptor (AHR) ligands as selective AHR modulators (SAhRMs) Int J Mol Sci. 2020 doi: 10.3390/ijms21186654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xue Z, Li D, Yu W, Zhang Q, Hou X, He Y, Kou X. Mechanisms and therapeutic prospects of polyphenols as modulators of the aryl hydrocarbon receptor. Food Funct. 2017;8(4):1414–1437. doi: 10.1039/c6fo01810f. [DOI] [PubMed] [Google Scholar]
- 142.Park H, Jin UH, Orr AA, Echegaray SP, Davidson LA, Allred CD, Chapkin RS, Jayaraman A, Lee K, Tamamis P, et al. Isoflavones as Ah receptor agonists in colon-derived cell lines: structure-activity relationships. Chem Res Toxicol. 2019;32(11):2353–2364. doi: 10.1021/acs.chemrestox.9b00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jin UH, Park H, Li X, Davidson LA, Allred C, Patil B, Jayaprakasha G, Orr AA, Mao L, Chapkin RS, et al. Structure-dependent modulation of aryl hydrocarbon receptor-mediated activities by flavonoids. Toxicol Sci Off J Soc Toxicol. 2018;164(1):205–217. doi: 10.1093/toxsci/kfy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang K, Lv Q, Miao YM, Qiao SM, Dai Y, Wei ZF. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem Pharmacol. 2018;155:494–509. doi: 10.1016/j.bcp.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 145.Miao Y, Lv Q, Qiao S, Yang L, Tao Y, Yan W, Wang P, Cao N, Dai Y, Wei Z. Alpinetin improves intestinal barrier homeostasis via regulating AhR/suv39h1/TSC2/mTORC1/autophagy pathway. Toxicol Appl Pharmacol. 2019;384:114772. doi: 10.1016/j.taap.2019.114772. [DOI] [PubMed] [Google Scholar]
- 146.Biagioli M, Carino A, Fiorucci C, Annunziato G, Marchiano S, Bordoni M, Roselli R, Giorgio CD, Castiglione F, Ricci P, et al. The aryl hydrocarbon receptor (AhR) mediates the counter-regulatory effects of pelargonidins in models of inflammation and metabolic dysfunctions. Nutrients. 2019 doi: 10.3390/nu11081820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang HK, Yeh CH, Iwamoto T, Satsu H, Shimizu M, Totsuka M. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J Agric Food Chem. 2012;60(9):2171–2178. doi: 10.1021/jf204625y. [DOI] [PubMed] [Google Scholar]
- 148.Bae MJ, Shin HS, See HJ, Jung SY, Kwon DA, Shon DH. Baicalein induces CD4(+)Foxp3(+) T cells and enhances intestinal barrier function in a mouse model of food allergy. Sci Rep. 2016;6:32225. doi: 10.1038/srep32225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu W, Chen X, Yu J, Xiao Y, Li Y, Wan S, Su W, Liang D. Baicalin modulates the Treg/Teff balance to alleviate uveitis by activating the aryl hydrocarbon receptor. Biochem Pharmacol. 2018;154:18–27. doi: 10.1016/j.bcp.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 150.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 151.Han DH, Denison MS, Tachibana H, Yamada K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci Biotechnol Biochem. 2002;66(7):1479–1487. doi: 10.1271/bbb.66.1479. [DOI] [PubMed] [Google Scholar]
- 152.Choi SY, Ha TY, Ahn JY, Kim SR, Kang KS, Hwang IK, Kim S. Estrogenic activities of isoflavones and flavones and their structure-activity relationships. Planta Med. 2008;74(1):25–32. doi: 10.1055/s-2007-993760. [DOI] [PubMed] [Google Scholar]
- 153.Cipolletti M, Solar Fernandez V, Montalesi E, Marino M, Fiocchetti M. Beyond the antioxidant activity of dietary polyphenols in cancer: the modulation of estrogen receptors (ERs) signaling. Int J Mol Sci. 2018 doi: 10.3390/ijms19092624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dominguez-Lopez I, Yago-Aragon M, Salas-Huetos A, Tresserra-Rimbau A, Hurtado-Barroso S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: a review. Nutrients. 2020 doi: 10.3390/nu12082456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ding M, Franke AA, Rosner BA, Giovannucci E, van Dam RM, Tworoger SS, Hu FB, Sun Q. Urinary isoflavonoids and risk of type 2 diabetes: a prospective investigation in US women. Br J Nutr. 2015;114(10):1694–1701. doi: 10.1017/S0007114515003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ding M, Pan A, Manson JE, Willett WC, Malik V, Rosner B, Giovannucci E, Hu FB, Sun Q. Consumption of soy foods and isoflavones and risk of type 2 diabetes: a pooled analysis of three US cohorts. Eur J Clin Nutr. 2016;70(12):1381–1387. doi: 10.1038/ejcn.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakamoto M, Otsuka R, Nishita Y, Tange C, Tomida M, Kato Y, Imai T, Sakai T, Ando F, Shimokata H. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur J Clin Nutr. 2018;72(10):1458–1462. doi: 10.1038/s41430-017-0061-2. [DOI] [PubMed] [Google Scholar]
- 158.Ma L, Liu G, Ding M, Zong G, Hu FB, Willett WC, Rimm EB, Manson JE, Sun Q. Isoflavone Intake and the risk of coronary heart disease in US men and women: results from 3 prospective cohort studies. Circulation. 2020;141(14):1127–1137. doi: 10.1161/CIRCULATIONAHA.119.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee AH, Su D, Pasalich M, Tang L, Binns CW, Qiu L. Soy and isoflavone intake associated with reduced risk of ovarian cancer in southern Chinese women. Nutr Res. 2014;34(4):302–307. doi: 10.1016/j.nutres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 160.Cutler GJ, Nettleton JA, Ross JA, Harnack LJ, Jacobs DR, Jr, Scrafford CG, Barraj LM, Mink PJ, Robien K. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa Women's Health Study. Int J Cancer J Int Cancer. 2008;123(3):664–671. doi: 10.1002/ijc.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee SA, Shu XO, Li H, Yang G, Cai H, Wen W, Ji BT, Gao J, Gao YT, Zheng W. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women's Health Study. Am J Clin Nutr. 2009;89(6):1920–1926. doi: 10.3945/ajcn.2008.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sonoda J, Pei L, Evans RM. Nuclear receptors: decoding metabolic disease. FEBS Lett. 2008;582(1):2–9. doi: 10.1016/j.febslet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takeuchi S, Takahashi T, Sawada Y, Iida M, Matsuda T, Kojima H. Comparative study on the nuclear hormone receptor activity of various phytochemicals and their metabolites by reporter gene assays using Chinese hamster ovary cells. Biol Pharm Bull. 2009;32(2):195–202. doi: 10.1248/bpb.32.195. [DOI] [PubMed] [Google Scholar]
- 164.Dean M, Austin J, Jinhong R, Johnson ME, Lantvit DD, Burdette JE. The flavonoid apigenin is a progesterone receptor modulator with in vivo activity in the uterus. Horm Cancer. 2018;9(4):265–277. doi: 10.1007/s12672-018-0333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hall JM, Powell HA, Rajic L, Korach KS. The role of dietary phytoestrogens and the nuclear receptor ppargamma in adipogenesis: an in vitro study. Environ Health Perspect. 2019;127(3):37007. doi: 10.1289/EHP3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li T, Yan F, Meng X, Wang J, Ting Kam RK, Zeng X, Liu Z, Zhou H, Yang F, Ren R, et al. Improvement of glucocorticoid-impaired thymus function by dihydromyricetin via up-regulation of PPARgamma-associated fatty acid metabolism. Pharmacol Res. 2018;137:76–88. doi: 10.1016/j.phrs.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 167.Xu LJ, Yu MH, Huang CY, Niu LX, Wang YF, Wu CZ, Yang PM, Hu X. Isoprenylated flavonoids from Morus nigra and their PPAR gamma agonistic activities. Fitoterapia. 2018;127:109–114. doi: 10.1016/j.fitote.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 168.Cao H, Liu J, Shen P, Cai J, Han Y, Zhu K, Fu Y, Zhang N, Zhang Z, Cao Y. Protective effect of naringin on DSS-induced ulcerative colitis in mice. J Agric Food Chem. 2018;66(50):13133–13140. doi: 10.1021/acs.jafc.8b03942. [DOI] [PubMed] [Google Scholar]
- 169.Singh AK, Raj V, Keshari AK, Rai A, Kumar P, Rawat A, Maity B, Kumar D, Prakash A, De A, et al. Isolated mangiferin and naringenin exert antidiabetic effect via PPARgamma/GLUT4 dual agonistic action with strong metabolic regulation. Chem Biol Interact. 2018;280:33–44. doi: 10.1016/j.cbi.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 170.Hadrich F, Sayadi S. Apigetrin inhibits adipogenesis in 3T3-L1 cells by downregulating PPARgamma and CEBP-alpha. Lipids Health Dis. 2018;17(1):95. doi: 10.1186/s12944-018-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang D, Li Z, Chen B, Fang G, Sun X, Li F, Xu H, Chen Y, Ding W. Naringin protects against steroidinduced avascular necrosis of the femoral head through upregulation of PPARgamma and activation of the Notch signaling pathway. Mol Med Rep. 2018;17(2):3328–3335. doi: 10.3892/mmr.2017.8247. [DOI] [PubMed] [Google Scholar]
- 172.Jung Y, Kim JC, Choi Y, Lee S, Kang KS, Kim YK, Kim SN. Eupatilin with PPARalpha agonistic effects inhibits TNFalpha-induced MMP signaling in HaCaT cells. Biochem Biophys Res Commun. 2017;493(1):220–226. doi: 10.1016/j.bbrc.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 173.Takashina Y, Manabe A, Tabuchi Y, Cyanidin IA. Increases the expression of Mg(2+) Transport carriers mediated by the activation of PPARalpha in colonic epithelial MCE301 cells. Nutrients. 2019 doi: 10.3390/nu11030641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Suzuki M, Nakamura F, Taguchi E, Nakata M, Wada F, Takihi M, Inoue T, Ohta S, Kawachi H. 4',6-dimethoxyisoflavone-7-O-beta-D-glucopyranoside (wistin) is a peroxisome proliferator-activated receptor alpha (PPARalpha) agonist in mouse hepatocytes. Mol Cell Biochem. 2018;446(1–2):35–41. doi: 10.1007/s11010-018-3270-7. [DOI] [PubMed] [Google Scholar]
- 175.Fouache A, Zabaiou N, De Joussineau C, Morel L, Silvente-Poirot S, Namsi A, Lizard G, Poirot M, Makishima M, Baron S, et al. Flavonoids differentially modulate liver X receptors activity-Structure-function relationship analysis. J Steroid Biochem Mol Biol. 2019;190:173–182. doi: 10.1016/j.jsbmb.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 176.Haque MW, Bose P, Siddique MUM, Sunita P, Lapenna A, Pattanayak SP. Taxifolin binds with LXR (alpha & beta) to attenuate DMBA-induced mammary carcinogenesis through mTOR/Maf-1/PTEN pathway. Biomed Pharmacother. 2018;105:27–36. doi: 10.1016/j.biopha.2018.05.114. [DOI] [PubMed] [Google Scholar]
- 177.Luo T, Miranda-Garcia O, Sasaki G, Wang J, Shay NF. Genistein and daidzein decrease food intake and body weight gain in mice, and alter LXR signaling in vivo and in vitro. Food Funct. 2018;9(12):6257–6267. doi: 10.1039/c8fo01718b. [DOI] [PubMed] [Google Scholar]
- 178.Zhang G, Liu S, Tan W, Verma R, Chen Y, Sun D, Huan Y, Jiang Q, Wang X, Wang N, et al. Synthesis and biological evaluations of chalcones, flavones and chromenes as farnesoid x receptor (FXR) antagonists. Eur J Med Chem. 2017;129:303–309. doi: 10.1016/j.ejmech.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Waizenegger J, Lenze D, Luckert C, Seidel A, Lampen A, Hessel S. Dose-dependent induction of signaling pathways by the flavonoid quercetin in human primary hepatocytes: a transcriptomic study. Mol Nutr Food Res. 2015;59(6):1117–1129. doi: 10.1002/mnfr.201400764. [DOI] [PubMed] [Google Scholar]
- 180.Li G, Lin W, Araya JJ, Chen T, Timmermann BN, Guo GL. A tea catechin, epigallocatechin-3-gallate, is a unique modulator of the farnesoid X receptor. Toxicol Appl Pharmacol. 2012;258(2):268–274. doi: 10.1016/j.taap.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu M, Zhang G, Song M, Wang J, Shen C, Chen Z, Huang X, Gao Y, Zhu C, Lin C, et al. Activation of farnesoid X receptor by schaftoside ameliorates acetaminophen-induced hepatotoxicity by modulating oxidative stress and inflammation. Antioxid Redox Signal. 2020;33(2):87–116. doi: 10.1089/ars.2019.7791. [DOI] [PubMed] [Google Scholar]
- 182.Yang L, Broderick D, Campbell Y, Gombart AF, Stevens JF, Jiang Y, Hsu VL, Bisson WH, Maier CS. Conformational modulation of the farnesoid X receptor by prenylflavonoids: Insights from hydrogen deuterium exchange mass spectrometry (HDX-MS), fluorescence titration and molecular docking studies. Biochem Biophys Acta. 2016;1864(12):1667–1677. doi: 10.1016/j.bbapap.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lau AJ, Chang TK. 3-Hydroxyflavone and structural analogues differentially activate pregnane X receptor: Implication for inflammatory bowel disease. Pharmacol Res. 2015;100:64–72. doi: 10.1016/j.phrs.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 184.Dou W, Zhang J, Li H, Kortagere S, Sun K, Ding L, Ren G, Wang Z, Mani S. Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J Nutr Biochem. 2014;25(9):923–933. doi: 10.1016/j.jnutbio.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li Y, Ross-Viola JS, Shay NF, Moore DD, Ricketts ML. Human CYP3A4 and murine Cyp3A11 are regulated by equol and genistein via the pregnane X receptor in a species-specific manner. J Nutr. 2009;139(5):898–904. doi: 10.3945/jn.108.103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li X, Li S, Chen M, Wang J, Xie B, Sun Z. (-)-Epigallocatechin-3-gallate (EGCG) inhibits starch digestion and improves glucose homeostasis through direct or indirect activation of PXR/CAR-mediated phase II metabolism in diabetic mice. Food Funct. 2018;9(9):4651–4663. doi: 10.1039/c8fo01293h. [DOI] [PubMed] [Google Scholar]
- 187.Yu Z, Yue B, Ding L, Luo X, Ren Y, Zhang J, Mani S, Wang Z, Dou W. Activation of PXR by Alpinetin contributes to abrogate chemically induced inflammatory bowel disease. Front Pharmacol. 2020;11:474. doi: 10.3389/fphar.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]