Abstract
Zooplankton is very sensitive to various agrochemicals including glyphosate herbicides which may arise from runoff in paddy fields. In this study, acute toxicity test of Glyphosate-Based Herbicides (GBHs) was conducted to Daphnia magna and Cyclops vicinus. Acute toxicity test was performed to both organisms at the Glyphosate concentrations of 20, 80, 160, 320, and 640 mg/L in exposure time of 12 h, 24 h, and 48 h. The mortality and morphology were observed to determine the LC50 and the effect of its morphology. The test showed that D. magna was more susceptible than C. vicinus. The LC50 of GBHs to D. magna and C. vicinus for its different exposure time were respectively show as follows: 76.67 mg/L and 207.89 mg/L (12 h); 36.2 mg/L and 159.8 mg/L (24 h); and 21.34 mg/L and 92.93 mg/L (48 h). There were no significant differences of the alteration of spin length, body length, and head length of D. magna to exposure of GBHs, except the head width. While body length alteration of C. vicinus was significantly different towards the increase in concentration.
Keywords: Glyphosate, Acute toxicity, Morphology alteration, Daphnia magna, Cyclops vicinus, Toxicology
Introduction
Agriculture is the sector that uses the most water in its activities, especially for irrigation. Around 70%, in some cases, up to 90% of the world's water needs are used for irrigation [1]. As the most water user sector, agricultural activities are a source of water pollution. The pollutant is in the form of residues of agrochemicals such as chemical fertilizers and pesticides on runoff to water bodies [2]. Pesticides are an important parts of agriculture to control various kinds of weeds, insects, and fungi [3]. Pesticides from agricultural runoff can contaminate surface and groundwaters [2]. Pesticide residues in the aquatic environment can poison and kill aquatic biota that could lead to decline of biodiversity. The poisoning can cause growth abnormalities, change the behavior and shapes so that population development is inhibited [4–6].
There are several classes of pesticides depending on the target organism, including herbicides, insecticides, fungicides, and nematicides. The emergence and development of herbicides easily control weeds easily and make important contributions to global food production [7]. Herbicides are chemicals used to eradicate weeds or inhibit wild plants that interfere with cultivation. The intensive use of herbicides can cause accumulation of chemical residues in the soil which can cause environmental pollution and endanger to other organisms and biological processes in the soil [8]. Glyphosate-Based Herbicides (GBHs) is a herbicide that has an active ingredient in the form of glyphosate. The GBHs is a type of herbicide that is widely used throughout the world [7, 8], including Indonesia. Glyphosate (N-(phosphonomethyl)glycine) is a broad-spectrum, non-selective herbicide, usually used to connect grass, weeds, and bushes. Glyphosate is included in the category of organophosphate pesticides. This herbicide can also be used for land preparation before planting during plant development and after harvest. Glyphosate depletes the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) needed for the synthesis of aromatic amino acids that are essential for plant survival [9–12].
Several studies have found glyphosate residues in water bodies and have the potential to cause physical changes in aquatic organisms [13, 14]. On the other hand, there is no standard for the minimum level of residual glyphosate active ingredients in rivers. Therefore, it is necessary to conduct a GBHs toxicity test to determine the safe limits of residues in rivers and LC50 which are expected to contribute as a basis for policy making considerations. Daphnia magna is a water invertebrate and an established model organism for toxicology studies [15] and testing methodology for this species is well developed [16]. However, copepods are a much higher contributor to zooplankton in many surface water, but they are not routinely used in toxicity tests [16]. There are no studies on the acute toxicity of GBHs against the freshwater copepod, Cyclops vicinus. Therefore purpose of this study is acute toxicity test and investigate the morphological alteration effect of D. magna and also C. vicinus.
Materials and methods
Test organism management
For this toxicity test, D. magna Straus, 1820 was obtained from cultures by the Institute of Biology, University of Szczecin, Poland, which was collected from Pond located in North Poland 53o44′47.6″N 17o31′51.6″E. During the cultivation, D. magna was fed green algae, namely Chorella sp. Chorella sp. powder was weighed as much as 0.3 g, gently stir in order to dissolve with water in the container. Aeration was also supplied to provide oxygen requirements for D. magna. The culture was run for 5 days prior to the experiment. C. vicinus Uljanin, 1875 were obtained from Odra river, Poland. C. vicinus was collected using a zooplankton net (mesh size 100 µm, d = 20 cm) and sorted from another zooplankton species using Zeiss Primo Vert reverse microscope (Germany). While C. vicinus without through a cultivation process. C. vicinus obtained from Odra river were sufficient in the amount needed and must have relatively the same size. A total of 180 specimens D. magna and C. vicinus were randomly assigned to give a loading of ten specimens per tank.
Acute bioassay
The acute 12 h, 24 h, and 48 h static bioassay was performed in the Hydrobiology Laboratorium, Biology Institute of Szczecin University based on SOP #2024; Revision 2.0; 09/24/90; US EPA Contract EP-W-09-031. The glyphosate-based herbicides used was from a brand named SUMIN ATUT 360 SL (360 g/L). The active ingredient was 360 g/L. It was produced by Adama Polska Sp. z o.o., the city of Warsaw, Poland. It was contained glyphosate in the form of isopropylamine salt (a compound from the group of aminophosphates) 360 g/L and 30.85% of detergent. Six different concentrations of glyphosate were made, namely 20 mg/L, 80 mg/L, 160 mg/L, 320 mg/L, 640 mg/L, and a control with no toxicant (0 mg/L). This concentration was made through dilution of the isopropylamine salt by using distilled water. Each concentration was prepared in 100 ml volume and replicated three times on both D. magna and C. vicinus.
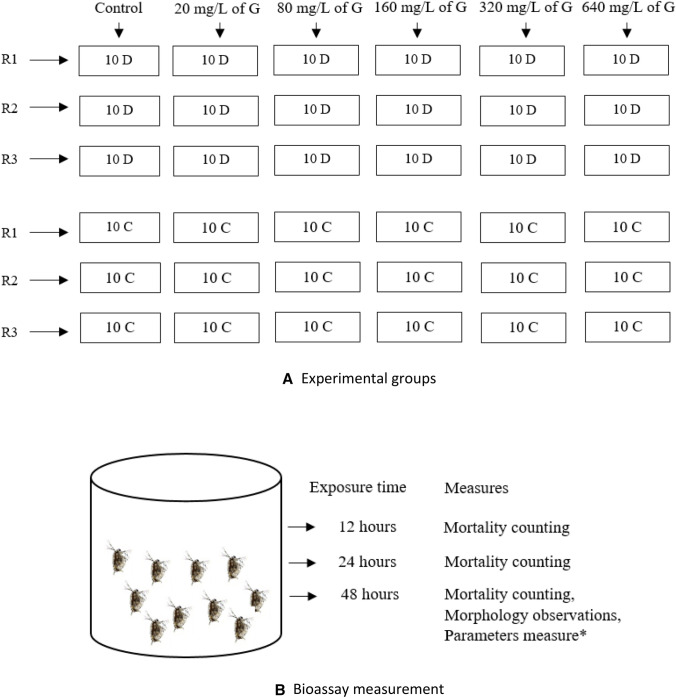
The ten individuals of D. magna and C. vicinus were introduced into each concentration in the container (Fig. 1). There were three replicates for each group. The exposure time are 12 h, 24 h, and 48 h. The experiments were kept at the same temperature without food. At the end of the test, the parameters of pH (power of hydrogen), temperature, EC (conductivity), TDS (Total Dissolved Solid), and DO (Dissolved Oxygen) were measured. Mortality was determined when the speciment did not respond to a repeated prodding [17].
Fig. 1.
Illustration of (a) experimental groups and (b) bioassay measurement. G (glyphosate), R (replicate), D (Daphnia magna), C (Cyclops vicinus), *(pH, temperature, EC, TDS, DO)
Morphological assays
Observations of the morphological response of D. magna and C. vicinus exposed to glyphosate were conducted after 48 h. The morphological indicators of D. magna were the spin length, body length, head width, and long head. While the morphological indicator of C. vicinus was body length. These indicators were observed using Zeiss Stereo microscope Discovery V12 (Germany). Each variable was measured from digital photographs, using the software Axio Vision. Responses were recorded if they differ from the controls.
Statistical analysis
The survival and mortality were counted for 12 h, 24 h, and 48 h. Because of there was partial mortality in any replicate, probit method was suitable used to calculate the LC50 [18]. The percentage of mortality was carried out for probit values against the logarithm of concentration using Microsoft excel. The regression analysis would give the equation based on intercept (b) and x (value) obtained. The LC50 was then calculated by substituting the probit value of 50% in the equation y = b + ax in which variable x is known and a = unknown and b = intercept. The anti-logarithm value of “a” was taken as the LC50 [17]. To determine the highest concentration value that does not cause an impact (NOEC), for this acute toxicity test the LC50 min/1000 was used [19]. The morphology indicators were analyzed using one-way analysis of variance (ANOVA). The significant difference was level at P = 0.05. Further, the linier regression linier was also done. Regression correlation is divided into six level classifications, namely: R2 = 0 (no correlation); R2 > 0–0.25 (very low correlation); R2 > 0.25–0.5 (moderate correlation); R2 > 0.5–0.75 (strong correlation); and R2 > 0.75–0.99 (very strong correlation); R2 = 1 (perfect correlation).
Results
Acute toxicity
In the present work, acute toxicity test with GBHs (in the form of 360 g/L isopylamine salt) was performed using D. magna and C. vicinus. Table 1 shows the mortality percentage of D. magna and C. vicinus based on the influence of exposure time and concentration variation. All D. magna died at a concentration of 160 mg/L with an exposure time of 48 h. Whereas C. vicinus died all at higher concentrations and longer exposure times, i.e. 640 mg/L and 48 h. Exposure time had a significant effect on the death of D. magna at a concentration of 160 mg/L with a significance value of 0.019. All D. magna had died at 320 mg/L and 640 mg/L. While on C. vicinus, exposure time had a significant effect on mortality at a concentration of 320 mg/L with a significance value of 0.009. At the highest concentration (640 mg/L), exposure time had no significant effect on death (with a significance value of 0.442) because almost all C. vicinus died. Concentration significantly affected the death of D. magna and C. vicinus, both at the time of exposure 12, 24, or 48 h (with an overall significance value of 0.000).
Table 1.
Percentage mortality of Daphnia magna and Cyclops vicinus exposed to glyphosate based herbicides
| Concentration (mg/L) | Species | Hours | ||
|---|---|---|---|---|
| 12 | 24 | 48 | ||
| 0 | D. magna | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| C. vicinus | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 20 | D. magna | 10.00 ± 17.32 | 46.67 ± 25.17 | 53.33 ± 25.17 |
| C. vicinus | 10.00 ± 17.32 | 10.00 ± 17.32 | 23.33 ± 15.28 | |
| 80 | D. magna | 23.33 ± 40.41 | 53.33 ± 25.17 | 70.00 ± 26.46 |
| C. vicinus | 16.67 ± 28.87 | 16.67 ± 28.87 | 26.67 ± 20.82 | |
| 160 | D. magna | 63.33 ± 11.55 | 83.33 ± 15.28 | 100.00 ± 0.00 |
| C. vicinus | 30.00 ± 0.00 | 36.67 ± 11.55 | 43.33 ± 11.55 | |
| 320 | D. magna | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| C. vicinus | 46.67 ± 5.77 | 50.00 ± 10.00 | 73.33 ± 5.77 | |
| 640 | D. magna | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| C. vicinus | 90.00 ± 10.00 | 93.33 ± 11.55 | 100.00 ± 0.00 | |
Table 2 presented the value of LC50 for both D. magna and C. vicinus of the exposure of GBHs at the period bioassay of 12 h, 24 h, and 48 h. The LC50 of D. magna for each exposure time were 76.67 h, 36.20 h, and 21.34 (48 h), so the NOEC estimated 0.021 mg/L. While the LC50 of C. vicinus for each exposure time were 207.89 (12 h), 159.81 (24 h), and 92.93 (48 h), so the NOEC estimated 0.09 mg/L.
Table 2.
The LC50 values of Daphnia magna and Cyclops vicinus exposed to glyphosate based herbicides
| Hours | Species | Equation for the regression analysis | LC50 |
|---|---|---|---|
| 12 | D. magna | y = 3.3246x–1.2657 | 76.67 |
| C. vicinus | y = 1.564x + 1.3749 | 207.89 | |
| 24 | D. magna | y = 2.4367x + 1.202 | 36.20 |
| C. vicinus | y = 1.8428x + 0.9393 | 159.81 | |
| 48 | D. magna | y = 2.4035x + 1.8052 | 21.34 |
| C. vicinus | y = 2.276x + 0.5205 | 92.93 |
Table 3 showed the environmental parameters of water as media exposure after 48 h period bioassay. The pH and DO decrease with increasing glyphosate concentration. While the conductivity and TDS increase with increasing concentration. This proved that concentration influences pH, conductivity, TDS, and DO. However, temperature is not affected by glyphosate concentration.
Table 3.
Physico-chemical parameters of the media exposure
| Gpyphosate concentration (mg/L) | Physico-chemicals parameters | ||||
|---|---|---|---|---|---|
| pH | Temperature (oC) | Conductivity (S/m) | TDS (mg/L) | DO (mg/L) | |
| 0 | 6.84 ± 0.03 | 22.4 ± 0.00 | 26.87 ± 0.95 | 0.02 ± 0.00 | 3.95 ± 0.14 |
| 20 | 6.04 ± 0.08 | 22.47 ± 0.06 | 54.40 ± 1.11 | 0.04 ± 0.00 | 3.13 ± 0.15 |
| 80 | 5.68 ± 0.18 | 22.3 ± 0.10 | 126.83 ± 1.76 | 0.08 ± 0.00 | 2.83 ± 0.06 |
| 160 | 5.20 ± 0.02 | 22.17 ± 0.12 | 157.90 ± 1.42 | 0.11 ± 0.01 | 2.55 ± 0.05 |
| 320 | 4.95 ± 0.02 | 21.97 ± 0.06 | 253.73 ± 8.40 | 0.16 ± 0.01 | 2.63 ± 0.04 |
| 640 | 4.83 ± 0.03 | 21.70 ± 0.35 | 424.83 ± 6.87 | 0.27 ± 0.01 | 2.49 ± 0.05 |
Morphology alterations
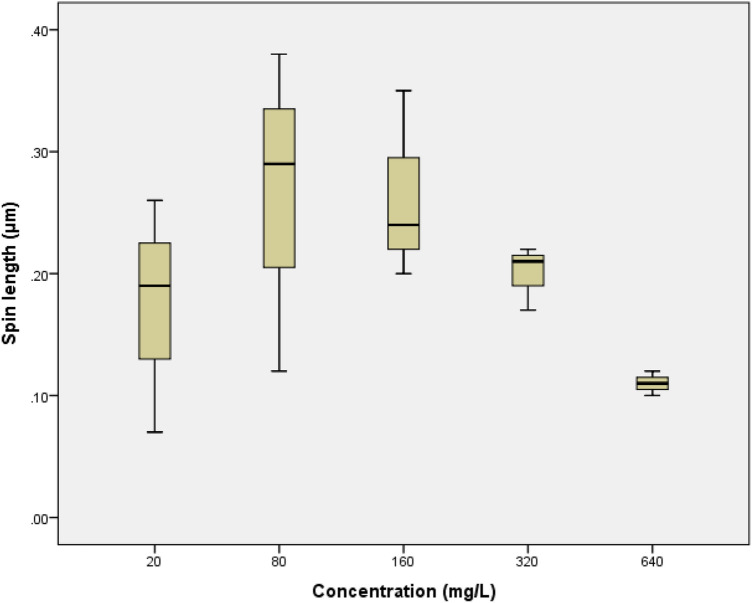
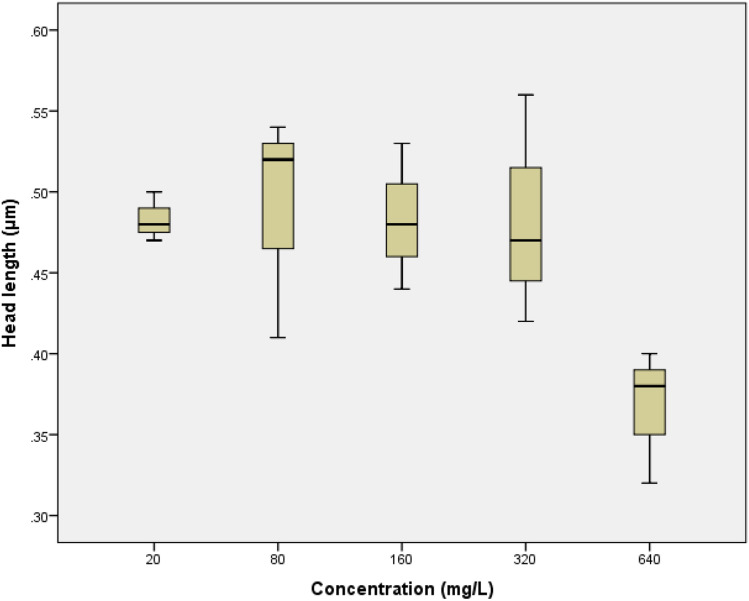
The boxplot charts of concentration correlation to changes in morphological indicators comprise spin length, body length, dead width, and head length. D. magna morphology alteration boxplot charts are shown in Fig. 2, 3, 4, 5. While for the boxplot chart of C. vicinus with body length indicator is shown in Fig. 6. There was no significant differences on concentration of glyphosate to the spin length of D. magna, with significance value of 0.441. At a concentration of 80 mg/L, it appeared that the spin length was longer than the concentration of 20 mg/L. However, at a concentration of 160 mg/L the effects of shortening of the spin length began to be seen and continued to decline to 640 mg/L.
Fig. 2.
The boxplot of the concentration increase towards spin length alteration on Daphnia magna
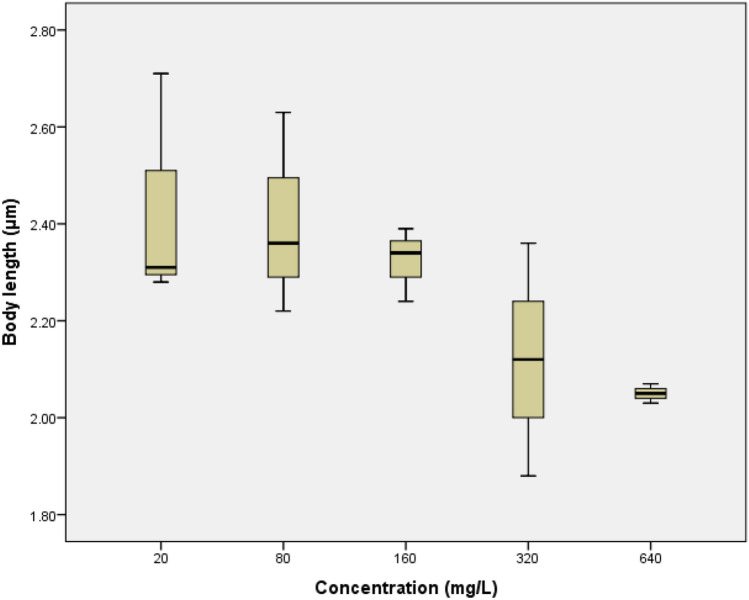
Fig. 3.
The boxplot of the concentration increase towards body length alteration on Daphnia magna
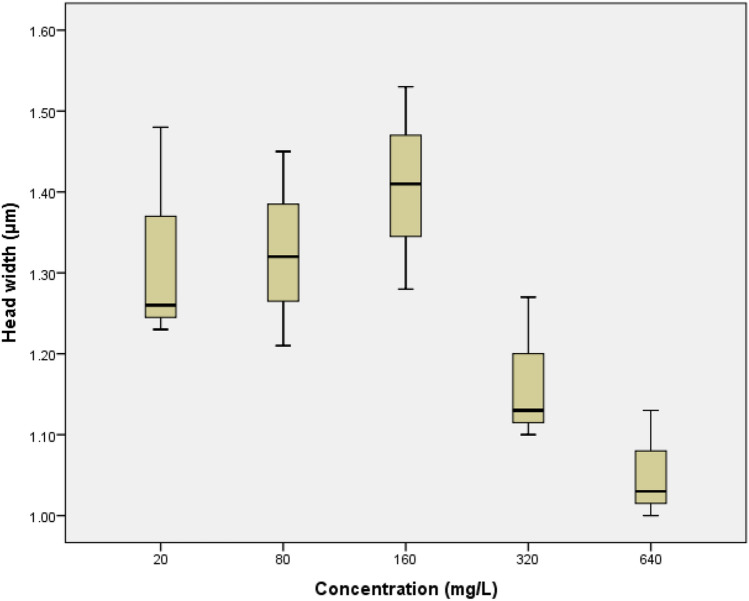
Fig. 4.
The boxplot of the concentration increase towards head width alteration on Daphnia magna
Fig. 5.
The boxplot of the concentration increase towards head length alteration on Daphnia magna
Fig. 6.
The boxplot of the concentration increase towards body length alteration on Cyclops vicinus
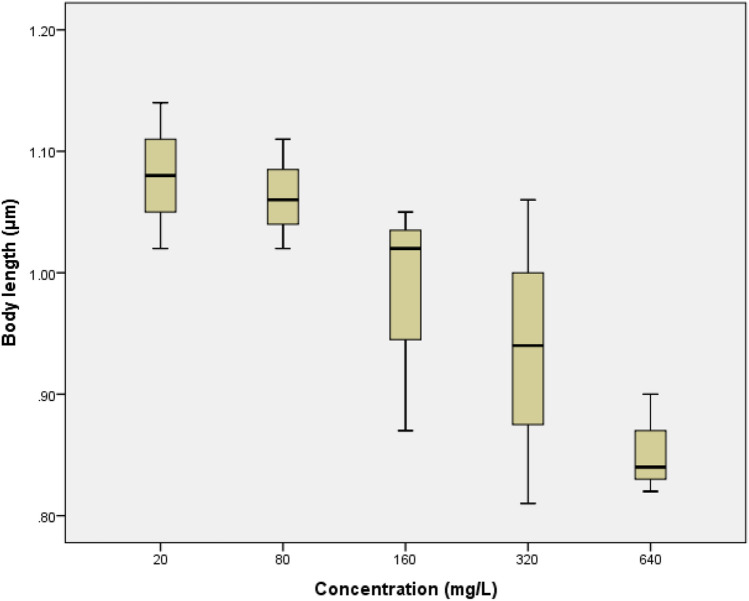
The difference in body length of D. magna after being exposed to glyphosate was not significant difference. The significance value of D. magna body length was 0.084 with the R2 value of 0.86. The boxplot shows that in the concentration of 20 mg/L until 160 mg/L almost had the same body length. Decrease in body length was only seen at a concentration of 320 mg/L and 640 mg/L. The head width alteration of D. magna had a significant difference with the value of 0.015. It can be seen in the Fig. 4 that at concentrations of 20–160 mg/L, the head width increased which indicates the growth of D. magna. However, at concentrations 320 mg/L and 640 mg/L there was an apparent decrease in head width.
The concentration of glyphosate to head length had no significant difference with the significance value of 0.071. While the R2 value was 0.76. The boxplot graph (Fig. 5) shows that at a concentration of 80, the head was longer than 20 mg/L, although after that there was a slight decrease to a concentration of 320 mg/L. At a concentration of 640 mg/L the head length was much shorter than at other concentrations. The morphological indicators of C. vicinus was the body length of C. vicinus. The significance value of body length alteration of C. vicinus was 0.024. It means there was the significant difference for the body length alteration. The boxplot (Fig. 6) shows a decrease in body length with each concentration.
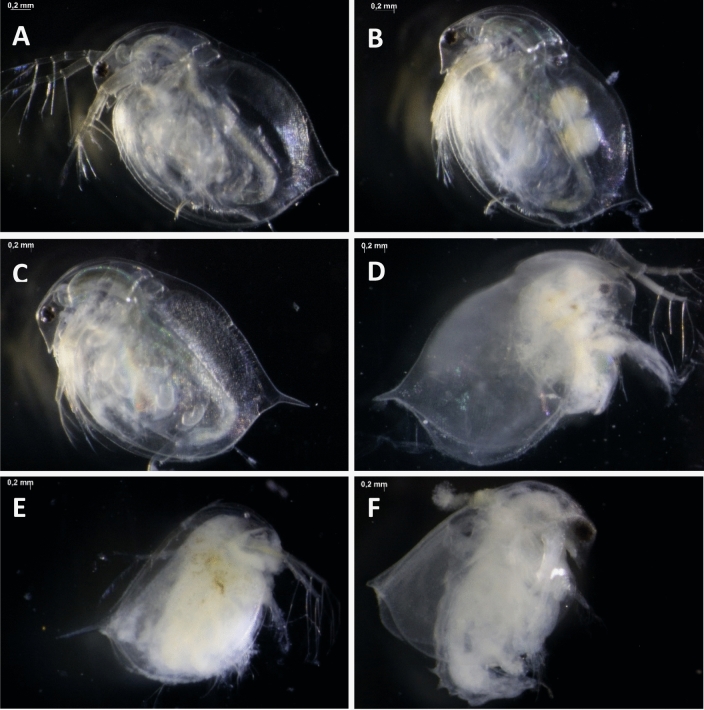
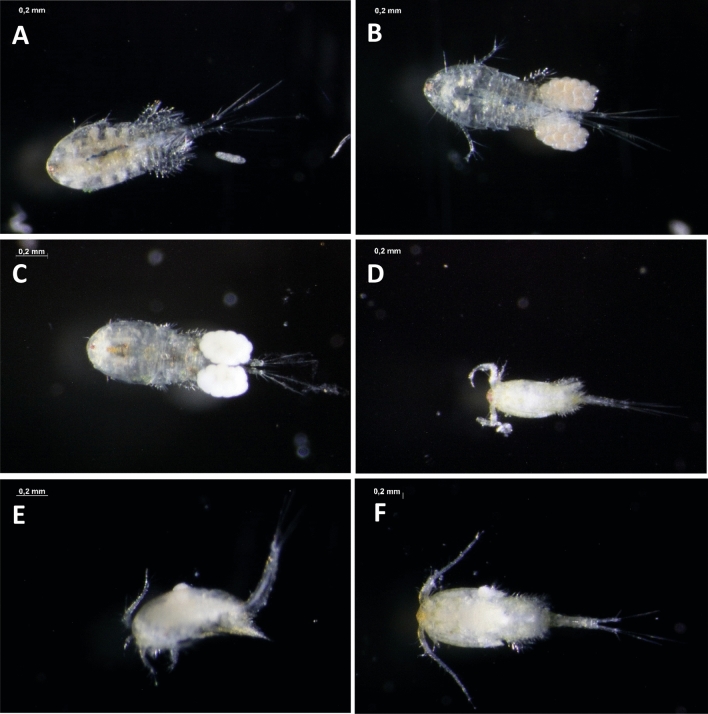
The alteration of morphological with the measuring of body length, head length, head width, and spin length of D. magna were quantitative data. Visually, there were different appearance of both D. magna and C. vicinus at each concentration. The difference visual of D. magna and C. vicinus in each concentration was presented in Fig. 7 and 8. D. magna changed color to slightly white with increasing concentration. In addition, the internal organs of D. magna with increasing concentration loose from the outer layer of the body. The control (Fig. 7a) is a control in which the color of daphnia was still clear and the internal organs were still attached to the outer layer. In Fig. 7b and c, it started to whiten a little but the internal organs had not been separated from the outer layer. Figure 7d and e show the colors get whiter and the internal organs shrink off the outer layer. At a concentration of 640 mg/L, the internal organs were loose and the color was also turbid white. C. vicinus changed color as does D. magna. However, the internal organs of the C. vicinus could not be separated from their outer layers. Figure 8a and b show the C. vicinus were still clear but in Fig. 8c it started to get whiter. Figure 8d, e, f were very murky white.
Fig. 7.
The visual appeareance of Daphnia magna after exposure. a (control), b (20 mg/L), c (80 mg/L), d (160 mg/L), e (320 mg/L), and f (640 mg/L)
Fig. 8.
The visual appeareance of Cyclops vicinus after exposure. a (control), b (20 mg/L), c (80 mg/L), d (160 mg/L), e (320 mg/L), and f (640 mg/L)
Discussion
Acute toxicity
The mortality data indicated that GBHs at higher concentration had a detrimental effect on the survival of D. magna and C. vicinus. Typically, as the concentration of the GBHs increased, the mortality increased significantly (p < 0.05) for each of period of bioassay (12 h, 24 h, and 48 h) both in D. magna and C. vicinus (Table 1). The increased mortality as the concentration toxicant increased could be due to the effect of stressed and/or alteration of the various organs/systems (electrolytes, hematological, hispathology, enzymes and metabolites) [17]. As Kish [15] did a study of D. magna with the exposure of GBHs, he monitored heart rates of adult D. magna in slow motion for 10 s under a stereo microscope. Kish [15] reported that the 7–100% concentrations of GBHs reduced heart rates about 50% after approximately 1–5 min of exposure before killing them in less than 10 min. The GBHs that used by Kish [15] was Roundup® that had 480 g/L active compound of glyphosate. For 7–100% concentration of GBHs, so the glyphosate active compound concentration are 25.2–360 mg/L. The value of 7% (25.2 mg/L glyohosate active compound) is close to LC50 of D. magna with the 24 h period bioassay.
This study found the LC50 of 48 h GBHs exposure was 21.34 mg/L. The estimated NOEC was 0.021 mg/L. There were some works with the different value. They were Sarigül Z and Bekcan S [20], Alberdi et al. [21], and Folmar et al. [22] that reported LC50 of GBHs exposure to D. magna for 48 h were 0.012 mg/L, 61.750 mg/L, and 3.000 mg/L, respectively. There were some factors that caused the differences of LC50 value. Environmental factors such as physic and chemical parameters were also affected the life of C. vicinus. Environmental factors like relative humidity, pH, temperature, conductivity, TDS, and DO affect the life of D. magna [23]. Besides that, the different brand of GBHs has the different toxicity because of the other component of the herbicides, which is the kind of detergent that functions as surfactant [24].
At all the same concentrations and periods of bioassay, D. magna speciments died more than C. vicinus. There had been no previous study about GBHs acute toxicity to C. vicinus species. Toxicity test using freshwater copepod Cyclops sp. is a new method. The first toxicity study using Cyclops sp. was done by Marus, Elphick and Bailey [16] on Total Dissolved Solid (TDS) exposure. According to this LC50 result, it shows that D. magna was more sensitive than C. vicinus. The 48 h LC50 of C. vicinus exposure to GBHs was 92.93 mg/L, so the NOEC estimated 0.092 mg/L.
Studies had shown that when water quality is affected by toxicants, any physiological changes will be reflected in the values of one or more of the hematological parameters [25]. Based on the measurements (Table 3), pH of the media exposure were in the range of 4.83 ± 0.03–6.84 ± 0.03. The optimum pH is between 7.2 and 8.5 but the acceptable pH for most species is 6.5–9.5 [26]. Based on pH measurement of media exposure, only the control that was in the range of acceptable pH with the value of 6.84 ± 0.03. The 20–640 mg/L concentration were 6.04 ± 0.08 to 4.83 ± 0.03 of pH value. The higher concentration of glyphosate, the lower pH measured. That was happened because glyphosate is a weak acid. As an ionizable substance, glyphosate provides higher toxicity in a low pH environment, where a higher proportion of molecules is present in a neutral state, where moving through the biomembrane is facilitated, compared to glyphosate ions [12, 27].
The temperature had not significant difference between the control with the range concentrations. This result was contrast with the study of Micah et al. [28] that reported the higher glyphosate concentration, the temperature would increase. The room temperature in the laboratory also affected the temperature of the water (media exposure). Therefore, the temperature change was not significantly different. The conductivity of media exposure were higher with the higher concentration. The increase of the conductivity was significant, it was in accordance with the study by Lautenschlager and Schaertl [29] that studied about electrical conductivity of five concentrations of two glyphosate-containing herbicides. They reported that conductivity increased for each 1% of the increasing of herbicide concentration. The higher concentration, the higher the TDS measured. Both conductivity and TDS increased significantly. This was convenient with the study by Micah et al. [28] who reported that there was a positive correlation between glyphosate concentration, TDS, and electrical conductivity. Conductivity and TDS are correlated and usually expressed by a simple equation: TDS = k EC (in 25 °C) [30].
Based on the measurement results, the DO range from the highest concentration to the control is in the range of 2.49 ± 0.05—3.95 ± 0.14. The DO range recommended by Ebert [26] is > 3 mg/L. That range is very suitable for both D. magna and Cyclops because DO is needed for metabolic processes in their body. From the suggestion, the acceptable concentration was on 20 mg/L and control with the DO values of 3.13 ± 0.15 and 3.95 ± 0.14, respectively. Dissolved oxygen content is an important indicator of water quality, because DO is needed to oxidize organic or inorganic materials [23].
Morphology alterations
Active ingredient of glyphosate detected in surface waters giving the potential to alter the physiology of aquatic organisms [13]. Acute effect of glyphosate to morphological alteration of fish had been studied [31]. Bengtsson et al. [32] studied Daphnia pulex exposed to pure glyphosate either through contaminated water or contaminated diet had variable rates of glyphosate uptake, with higher body burden resulting from water column exposure (50 mg/g dry weight vs. 13 mg/g dry weight).
The alteration visual of D. magna and C. vicinus from 20 to 640 mg/L concentration were the changing colour, from transparent to be more murky white. This result was convenient with the study of Becaro et al. [33] about the exposure of AgNP toxicant to D. magna. The study reported the dark coloration observed in the lines of the intestine indicates that these organisms ingested the solution. From the study, we can conclude that the murky white colour showed the ingested glyphosate toxicant to their organs. The other hand, the inner organ of D. magna were smaller and it moved out of the out layer. The alteration of C. vicinus was the same with D. magna, but the C. vicinus looks like more tolerant.
The findings showed that the toxicant effects on the D. magna and C. vicinus increased as the GBHs concentration of exposure and the longer period of exposure. The NOEC of GBHs of D. magna and C. vicinus were 0.021 mg/L and 0.092 mg/L, respectively. The alteration morphology occurred in both D. magna and C. vicinus as the concentration of GBHs increases. The test showed that D. magna was more susceptible than C. vicinus.
Acknowledgements
The authors are grateful for the financial support from the Ministry of Research and Higher Education of the Republic of Indonesia with Master leading to Ph.D. scholarship (PMDSU), the agreement letter number 821/PKS/ITS/2019. Furthermore, the authors are also grateful for the PROM Programme 2019–International Scholarship Exchange of Ph.D. Candidates that was co-financed by the University of Szczecin and the European Social Fund under the Knowledge Education Development Operational Programme.
Compliance with ethical standards
Conflict of interest
Authors declare that there is no conflict of interest.
References
- 1.Schreinemachers P, Tipraqsa P. Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy. 2012;37:616–626. doi: 10.1016/j.foodpol.2012.06.003. [DOI] [Google Scholar]
- 2.Aktar W, Sengupta D, Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol. 2009;2:1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandya IY. Pesticides and their applications in agriculture. Asian J Appl Sci Technol. 2018;2:894–900. [Google Scholar]
- 4.Halappa R, David M. Behavioural responses of the freshwater fish, Cyprinus carpio (Linnaeus) following sublethal exposure to chlorpyrifos. Turk J Fish Aquat Sci. 2009;9:233–238. doi: 10.4194/trjfas.2009.0218. [DOI] [Google Scholar]
- 5.Santiasih I, Sulistiyaning H, Hermana J. The effects of particulate matters inhalation exposures of prallethrin and d-phenothrin mixture in mice (Mus musculus) against exhaled carbon dioxide concentration. Toxicol Res. 2020;36:59–67. doi: 10.1007/s43188-019-00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santiasih I, Titah HS, Hermana J. Carboxylesterase concentration in mouse exposed to particulate matters on inhalation exposure of prallethrin and d-phenothrin mixture. E3S Web Conf. 2019;125:04006. doi: 10.1051/e3sconf/201912504006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Jiang C, Huang H, Wei S, Huang Z, Wang H, Zhao D, Zhang C. Characterization of Eleusine indica with gene mutation or amplification in EPSPS to glyphosate. Pestic Biochem Physiol. 2017;143:201–206. doi: 10.1016/j.pestbp.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Widowati T, Cinta R, Ginting B, Widyastuti U, Ardiwinata N. Herbisida Glifosat Dan Paraquat Dari Rizosfer. Biopropal Industri. 2017;8:63–70. [Google Scholar]
- 9.Arango L, Buddrus-Schiemann K, Opelt K, Lueders T, Haesler F, Schmid M, Ernst D, Hartmann A. Effects of glyphosate on the bacterial community associated with roots of transgenic Roundup Ready® soybean. Eur J Soil Biol. 2014;63:41–48. doi: 10.1016/j.ejsobi.2014.05.005. [DOI] [Google Scholar]
- 10.Gomes MP, Le Manach SG, Moingt M, Smedbol E, Paquet S, Labrecque M, Lucotte M, Juneau P. Impact of phosphate on glyphosate uptake and toxicity in willow. Amsterdam: Elsevier; 2016. [DOI] [PubMed] [Google Scholar]
- 11.Yadav AK, Srivastava P, Kumar N, Abbassi R, Mishra BK. Constructed wetland-microbial fuel cell: an emerging integrated technology for potential industrial wastewater treatment and bio-electricity generation. Constr Wetl Ind Wastewater Treat. 2018 doi: 10.1002/9781119268376.ch22. [DOI] [Google Scholar]
- 12.Chłopecka M, Mendel M, Dziekan N, Karlik W. Glyphosate affects the spontaneous motoric activity of intestine at very low doses. In vitro study. Pestic Biochem Physiol. 2014;113:25–30. doi: 10.1016/j.pestbp.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Annett R, Habibi HR, Hontela A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J Appl Toxicol. 2014;34:458–479. doi: 10.1002/jat.2997. [DOI] [PubMed] [Google Scholar]
- 14.Klátyik S, Takács E, Mörtl M, Földi A, Trábert Z, Ács É, Darvas B, Székács A. Dissipation of the herbicide active ingredient glyphosate in natural water samples in the presence of biofilms. Int J Environ Anal Chem. 2017;97:901–921. doi: 10.1080/03067319.2017.1373770. [DOI] [Google Scholar]
- 15.Kish A, Duan K, Leanna K (2017) The impact of RounduP® on Daphnia magna (2017 AAAS Annual Meeting). https://aaas.confex.com/aaas/2017/webprogram/Paper20104.html. Accessed 31 Jan 2020
- 16.Marus EM, Elphick JR, Bailey HC. A new toxicity test using the freshwater copepod Cyclops vernalis. Bull Environ Contam Toxicol. 2015;95:357–362. doi: 10.1007/s00128-015-1592-7. [DOI] [PubMed] [Google Scholar]
- 17.Aghoghovwia OA, Izah SC. Toxicity of glyphosate based herbicides to fingerlings of Heterobranchus bidorsalis. Int J Avian Wildl Biol. 2018;3:397–400. doi: 10.15406/ijawb.2018.03.00127. [DOI] [Google Scholar]
- 18.Biesinger KE, Williams LR, Schalie van der S (1987) Procedures for conducting daphnia magna toxicity bioassays. EPA/600/8-87/011. Environmental Monitoring and Support Laboratory. Cincinnati, OH
- 19.Connon RE, Geist J, Werner I. Effect-based tools for monitoring and predicting the ecotoxicological effects of chemicals in the aquatic environment. Sensors (Basel) 2012;12:12741–12771. doi: 10.3390/s120912741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarigül Z, Bekcan S. Acute toxicity of the herbicide glyphosate on Daphnia magna. Turk J Agric For. 2009;15:204–208. [Google Scholar]
- 21.Alberdi JL, Sàenz ME, Di Marzio WD, Tortorelli MC. Comparative acute toxicity of two herbicides, paraquat and glyphosate, to Daphnia magna and D. spinulata. Bull Environ Contam Toxicol. 1996;57:229–235. doi: 10.1007/s001289900180. [DOI] [PubMed] [Google Scholar]
- 22.Folmar LC, Sanders HO, Julin AM. Toxicity of the herbicide glyphosate and several of its formulations to fish and aquatic invertebrates. Arch Environ Contam Toxicol. 1979;8:269–278. doi: 10.1007/BF01056243. [DOI] [PubMed] [Google Scholar]
- 23.Edwin T, Ihsan T, Pratiwi W. Acute toxicity test of metal lead (Pb), chromium (Cr) and cobalt (Co) on Daphnia magna. Jurnal Teknik Lingkungan UNAND. 2017;14:33–40. [Google Scholar]
- 24.Mcfalls J, Yi Y, Li M, Senseman S, Storey B (2015) Evaluation of generic and branded herbicides: Technical Report. Texas
- 25.Van Vuren JHJ. The effects of toxicants on the haematology of Labeo umbratus (Teleostei: Cyprinidae) Comp Biochem Physiol C Comp Pharmacol Toxicol. 1986;83:155–159. doi: 10.1016/0742-8413(86)90029-0. [DOI] [PubMed] [Google Scholar]
- 26.Ebert D (2005) Ecology, epidemiology, and evolution of parasitism in Daphnia [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Books
- 27.Schweizer M, Brilisauer K, Triebskorn R, Forchhammer K, Köhler H-R. How glyphosate and its associated acidity affect early development in zebrafish (Danio rerio) PeerJ. 2019;7:e7094. doi: 10.7717/peerj.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micah AD, Yusuf A, Umar R, Mohammed NA. Acute effect of glyphosate on water quality parameters and some antioxidant response of heteroclarias fingerlings. FUW Trends Sci Technol J. 2016;1:111–114. [Google Scholar]
- 29.Lautenschlager RA, Schaertl GR. Electrical conductivity of five concentrations of two glyphosate-containing herbicides. South J Appl For. 1991;15:85–88. doi: 10.1093/sjaf/15.2.85. [DOI] [Google Scholar]
- 30.Rusydi AF. Correlation between conductivity and total dissolved solid in various type of water: a review. IOP Conf Ser Earth Environ Sci. 2018;118:012019. doi: 10.1088/1755-1315/118/1/012019. [DOI] [Google Scholar]
- 31.Auta J, Ogueji EO (2008) Acute toxicity and behavioural effects of chlorpyrifosethyl pesticide to juveniles of Clarias gariepinus Teugels. In: 22nd Annual conference of the Fisheries Society of Nigeria (FISON), 12–16 Nov 2007, Kebbi, Nigeria, pp 264–272. http://aquaticcommons.org/23253/
- 32.Bengtsson G, Hansson LA, Montenegro K. Reduced grazing rates in Daphnia pulex caused by contaminants: implications for trophic cascades. Environ Toxicol Chem. 2004;23:2641–2648. doi: 10.1897/03-432. [DOI] [PubMed] [Google Scholar]
- 33.Becaro AA, Jonsson CM, Puti FC, Siqueira MC, Mattoso LHC, Correa DS, Ferreira MD. Toxicity of PVA-stabilized silver nanoparticles to algae and microcrustaceans. Environ Nanotechnol Monit Manag. 2015;3:22–29. doi: 10.1016/j.enmm.2014.11.002. [DOI] [Google Scholar]