Abstract
Background/Objective
Subarachnoid hemorrhage (SAH) is a devastating neurological injury, further complicated by few available methods to objectively predict outcomes. With the recent shift in focus to neuroinflammation as a potential cause of adverse outcomes following SAH, we investigated the inflammasome-derived enzyme, caspase-1, as a potential biomarker for poor functional outcome.
Methods
SAH patients were recruited from a regional stroke referral center. Cerebrospinal fluid (CSF) samples from 18 SAH subjects were collected via an external ventricular drain and obtained as close as possible to admission (within 72 hours). For control subjects, we collected CSF from 9 patients undergoing lumbar puncture with normal CSF. Caspase-1 activity was measured using commercially available luminescence assays. SAH subjects were categorized at hospital discharge into those with good outcomes (Glasgow Outcome Scale, GOS, of 4–5) and poor outcomes (GOS of 1–3).
Results
CSF analysis demonstrated a nearly seven-fold increase in caspase-1 activity in SAH patients compared to controls (p<0.0001). Within the SAH group, 10 patients (55.6%) had good outcomes and 8 patients (44.4%) had poor outcomes. Mean caspase-1 activity in the poor outcome group was approximately three-times higher than the good outcome group (p=0.001). Caspase-1 activity was significantly correlated with GOS score (r=−0.705, p=0.001). Receiver operating characteristic curve analysis showed that caspase-1 activity can accurately differentiate between patients with good versus poor functional outcome (area under the curve 0.944, p=0.002).
Conclusions
Inflammasome-derived caspase-1 activity is elevated in the CSF of SAH patients compared to controls and higher levels correlate with worse functional outcome.
Keywords: Subarachnoid hemorrhage, inflammasome, caspase-1, neuroinflammation, cerebrospinal fluid, functional outcome
INTRODUCTION
Subarachnoid hemorrhage (SAH) is a life-threatening form of stroke, most commonly caused by the rupture of intracranial aneurysms. For SAH patients, prognosis is often poor, with a less than a 50% six-month survival rate.(1) Survivors are often faced with severe neurocognitive defects, functional disability, and diminished quality of life. Historically, cerebral vasospasm was believed to be the primary cause of delayed neurological deterioration (DND) and poor outcome. However, recent studies have suggested a multifactorial pathophysiology behind DND, as clinical trials that successfully reduced angiographic vasospasm did not improve patient functional outcome, morbidity, or mortality.(2,3)
Additionally, there is a lack of agreement on how to prognosticate outcome in SAH. The predictive models that are currently used, including modified Fisher scale, World Federation of Neurological Surgeons grade (WFNS), Hunt and Hess score, and Glasgow Coma Scale (GCS) obtained upon admission have demonstrated variable predictive value and are not entirely objective.(4–6) Ongoing research has focused on identifying biomarkers that might predict outcome in timely, objective, and cost-effective manners.(7) Biomarkers measured in the cerebrospinal fluid (CSF) are particularly useful in the setting of central nervous system (CNS) injuries due to their ability to reflect changes occurring within the brain parenchyma.(8) High-grade SAH patients typically have CSF diversion devices which allow for routine CSF sampling. Thus, the presence of external ventricular drains (EVDs) provide a unique opportunity to measure in real time the concentration of biochemical mediators that participate in signaling pathways associated with brain injury.
Recently, attention has turned to early brain injury (EBI), a complex pathological process that occurs within the first 72 hours after SAH, as a contributor to poor outcomes.(9) Mechanistically, EBI is characterized by neuroinflammation, blood-brain barrier dysfunction, microvascular dysregulation, cortical spreading depolarization, and cerebral hypoperfusion.(2) These processes are associated with neuronal cell death and set the stage for the development of DND and permanent neurologic damage. Among these various processes, neuroinflammation represents a key and modifiable contributor to EBI.(10) Neuroinflammation, as well as concurrent systemic inflammation, has been shown to be associated with neurologic deterioration in other diseases of the CNS, such as intracerebral hemorrhage (11–13) and ischemic stroke (14, 15). Such studies have suggested that early measurement of inflammatory makers such as neutrophil-to-lymphocyte ratios or matrix metalloproteases might be useful for predicting worsening of patient outcomes. We have previously reviewed the unique cellular and molecular inflammatory responses observed after SAH.(16)
Here, we focus on an important component of the innate immune response that has been less intensively studied in the context of SAH, the inflammasome. Inflammasomes are nod-like-receptor (NLR)-based multiprotein complexes that respond to damage-associated molecular patterns (DAMPs) and, upon activation, carry out pro-inflammatory responses. Caspase-1 is a key mediator of the inflammasome signaling cascade and a marker for inflammasome activity.(17,18)
Several studies have shown an upregulation of inflammasome proteins, such as caspase-1, following traumatic brain injury (TBI).(17,19,20) However, relatively few studies have investigated the role of caspase-1 in the context of SAH. We attempted to further understand the role of inflammasomes following SAH by measuring caspase-1 specifically because caspase-1 is the primary product of inflammasome formation and the obligatory enzyme for potentiating the downstream inflammatory response. We hypothesized that inflammasome activity is elevated in SAH patients and that it is associated with neurologic damage. To test our hypothesis, we measured caspase-1 activity in the CSF of SAH patients and determined its relationship to functional outcome.
METHODS
Study Population
SAH patients treated with EVDs were recruited from the University of Illinois Medical Center at Chicago. This study was conducted in accordance with the Declaration of Helsinki and approved by the local institutional review board prior to initiation. Written informed consent was required from all patients or designated proxies. Patients were eligible to participate if they were at least 18 years of age, had non-traumatic aneurysmal SAH confirmed by computed tomography (CT), aneurysm visualized by either digital subtraction angiography or noninvasive cerebral angiography, and presence of an EVD. Exclusion criteria were non-aneurysmal SAH, pregnancy, previous neurological disability (defined as preadmission modified Rankin Scale ≥2), or history of stroke, brain tumor, intracranial surgery, traumatic brain injury, previously treated cerebral aneurysm, or other vascular malformation. Control subjects were non-pregnant persons 18 years of age or older with no previous neurological disorders who underwent lumbar puncture or CSF drainage for evaluation of headache but had normal CSF analysis including chemistry, differential cell count, and cultures as well as normal brain magnetic resonance imaging (MRI).
Clinical Assessment
Subjects with SAH were prospectively followed during hospitalization. Our primary outcome was functional status at time of discharge, assessed via Glasgow Outcome Score (GOS). Good functional outcome was defined as GOS 4–5 and poor outcome was defined as GOS of 1–3.(21) Baseline characteristics, including age, sex, and laboratory data were measured across all groups. Within the SAH group, aneurysm location (anterior or posterior), aneurysm treatment (coiling vs. clipping), and initial modified Fisher scale, Hunt-Hess score, and Glasgow Coma Score were recorded. Additionally, complications during hospital stay such as angiographic vasospasm and DND were recorded. Angiographic vasospasm was determined via cerebral angiography and the impression of a neurointerventionalist. DND was defined as the development of new focal neurological deficits, decreased level of consciousness (persistent drop in GCS by ≥2 points), or both, with or without the presence of angiographic vasospasm.(22)
Cerebrospinal Fluid Collection and Analysis
CSF samples were collected between 2010–2019 as part of a larger University of Illinois NeuroRepository. Amongst collected samples, we selected those for patients that met our inclusion criteria, had CSF collected as close as possible to the time of admission (no later than 72 hours of symptom onset to correspond with the time window of EBI), following surgical clipping or endovascular coiling of the aneurysm, and those that were collected using the same sterile technique from the distal port of the EVD buretrol. Approximately 10 ml of CSF was collected from the distal port of the EVD, immediately placed on ice, and centrifuged at 1000g for 5 minutes at room temperature. Supernatants were collected, aliquoted into cryovials, and stored at −80°C until analysis.
Caspase-1 Activity
Caspase-1 activity was measured using the commercially available Caspase-Glo® 1 Inflammasome Assay (Promega Corporation, Madison, Wisconsin, USA).(23) Briefly, this assay uses Z-WEHD-aminoluciferin, a substrate upon which caspase-1 exerts its enzymatic activity. Cleavage of the amino-terminus of the Z-WEHD substrate by caspase-1 results in release of Ultra-Glo™ Recombinant Luciferase. Luminescence intensity was measured after 60 min of incubation using a VICTOR3 1420 Multilabel Plate Counter (Perkin Elmer, Shelton, Connecticut, USA) as counts per second (CPS). Data is plotted as CPS/μL of CSF.
Statistical Analysis
Descriptive variables are presented as either percentages, mean ± standard deviation (SD), or median and interquartile range (IQR). Continuous variables were analyzed by the Mann-Whitney U test. Categorical variables were compared by the Fisher Exact test or Chi Square test. Caspase-1 levels and GOS were correlated using the Spearman’s rank correlation coefficient. Logistic regression analysis was used to predict poor vs. good outcome in SAH patients. First, univariate analysis was conducted with each of the variables. Any variable with p<0.10 in univariate analysis was then entered into a multivariate logistic regression model. The presence of multicollinearity among independent variables was assessed using weighted linear regression and defined as a variance inflation factor ≥5 or tolerance of <0.20.(24) Receiver operating characteristic (ROC) curves were created for caspase-1 ability to distinguish poor vs. good outcome compared to commonly used clinical assessments. Youden’s index was calculated to determine optimal test cut-off scores and corresponding sensitivity and specificity. A p-value of <0.05 was considered statistically significant for all analyses. Statistical Package for the Social Sciences (Version 26, IBM® SPSS®, Chicago, Illinois, USA) software was used to conduct the analysis. Figures were made using GraphPad Prism (Version 8.3.0, La Jolla, California, USA).
RESULTS
We collected a total of 27 CSF samples, nine of which were obtained from control patients and 18 from SAH patients. Baseline characteristics are displayed in Table 1. The mean ages for the control and SAH group were 43.5 ± 14.6 years and 50.4 ± 9.9 years, respectively (p=0.106). There was no difference in sex between SAH patients and controls (p>0.999). SAH patients were then stratified into those with good or poor functional outcome based on GOS. Eight (44.4%) patients had a poor outcome (GOS 1–3) while ten (55.6%) had a good outcome (GOS 4–5). There was no difference in age, sex, aneurysm location, or aneurysm treatment between the two groups. Notably, the only clinical score on admission that was different between good and poor outcome groups was the GCS (p=0.034, Table 1). Good outcome patients had a median baseline GCS of 15 (14–15) while those with poor outcomes had a median baseline GCS of 10 (4–14). There were no differences in modified Fisher scale (p=0.475) or Hunt-Hess score (p=0.146) between groups. Additionally, there were no differences in the number of patients who developed angiographic vasospasm (p=0.638) or DND (p=0.153).
Table 1.
Baseline characteristics of control and SAH patients stratified by outcome.
| Control | SAH |
|||||
|---|---|---|---|---|---|---|
| All | p-value | Good Outcome (GOS 4–5) | Poor Outcome (GOS 1–3) | p-value | ||
| Participants, n | 9 | 18 | - | 10 | 8 | - |
| Age, mean (SD) | 43.5 (14.6) | 50.4 (9.9) | 0.106 | 48.4 (7.5) | 52.8 (12.3) | 0.360 |
| Female Sex, n (%) | 5 (55.6) | 11 (61.1) | >0.999 | 6 (60.0) | 5 (62.5) | >0.999 |
| Anterior Aneurysm Location, n (%) | -- | 14 (77.8) | -- | 7 (70.0) | 7 (87.5) | 0.588 |
| Modified Fisher Scale, median (IQR) | -- | 3 (3–4) | -- | 3 (3–4) | 4 (3–4) | 0.475 |
| Hunt-Hess Score, median (IQR) | -- | 3 (2–5) | -- | 2.5 (2–3) | 3 (2–5) | 0.146 |
| Glasgow Coma Scale, median (IQR) | -- | 14 (6.75–15) | -- | 14.5 (14–15) | 10 (4–14) | 0.034 |
| Aneurysm Treatment, n (%) | ||||||
| Surgical Clipping | -- | 10 (55.6) | -- | 5 (50.0) | 5 (62.5) | 0.664 |
| Endovascular Coiling | 8 (44.4) | 5 (50.0) | 3 (37.5) | |||
| Angiographic Vasospasm, n (%) | -- | 12 (66.7) | -- | 6 (60.0) | 5 (62.5) | 0.638 |
| Delayed Neurologic Deterioration, n (%) | -- | 9 (50.0) | -- | 3 (30.0) | 6 (75.0) | 0.153 |
SAH: Subarachnoid Hemorrhage, GOS: Glasgow Outcome Score, SD: Standard Deviation, IQR: Interquartile Range
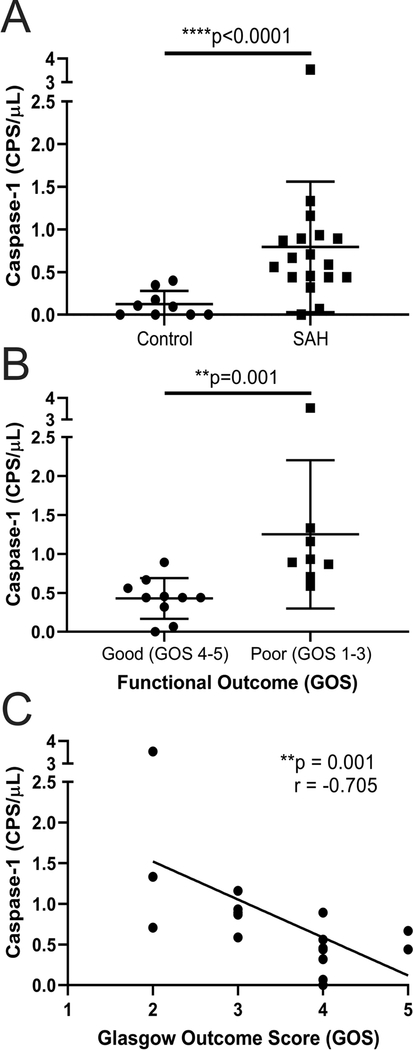
We next evaluated differences in CSF between SAH patients and controls. Compared to controls, SAH patients had higher red blood cells (RBCs), protein, glucose, and white blood cells (WBCs) in their CSF (all p<0.002, Table 2). The average caspase-1 activity in the control group was 0.12 CPS/μl while in the SAH group the activity levels were 0.79 CPS/μl, corresponding to an approximately seven-fold increase in caspase-1 activity in SAH patients compared to controls (p<0.0001, Figure 1A).
Table 2.
Admission cerebrospinal fluid labs in control and SAH Patients stratified by outcome.
| Lab, mean (SD) | Control n=9 | SAH | ||||
|---|---|---|---|---|---|---|
| All n=18 | p-value | Good Outcome (GOS 4–5) n=10 | Poor Outcome (GOS 1–3) n=8 | p-value | ||
| Red Blood Cells (cells/μL) | 2.88 (5.82) | 124,014.00 (202,079.73) | <0.0001 | 105,734.86 (160,131.04) | 142,293.14 (249,112.20) | 0.710 |
| Protein (mg/dL) | 30.75 (6.90) | 151.36 (170.52) | <0.0001 | 124.86 (105.60) | 177.86 (224.08) | >0.999 |
| Glucose (mg/dL) | 60.50 (6.99) | 90.71 (30.66) | 0.002 | 82.86 (29.02) | 98.57 (32.40) | 0.318 |
| White Blood Cells (cells/μL) | 1.00 (1.07) | 142.21 (165.35) | <0.0001 | 153.29 (216.71) | 131.14 (109.49) | 0.805 |
Figure 1.
A) Caspase-1 activity as measured in counts per second per microliter (CPS/μL) in our control group (n=9) compared to subarachnoid hemorrhage (SAH) group (n=18). Individual patient data is shown along with mean and standard deviation. B) Comparison of caspase-1 activity (CPS/μL) in our poor (Glasgow Outcome Score (GOS) 1–3, n=8) and good (GOS 4–5, n=10) outcome groups. Individual patient data is shown along with mean and standard deviation C) Caspase-1 activity (CPS/μL) is correlated with GOS in SAH (n=18).
We next determined whether differences could be noted in caspase-1 levels amongst SAH patients when stratified by functional outcome at time of discharge. Average caspase-1 activity in poor outcome patients (n=8) was 1.25 CPS/μL while that in good outcome patients (n=10) was 0.43 CPS/μL, corresponding to a near three-fold increase in poor outcome patients compared to good outcome patients (p=0.001, Figure 1B). No differences in CSF RBC, WBC, protein, or glucose were detected between good and poor outcome patients (all p>0.05, Table 2). Additionally, there was a significant correlation between GOS and active caspase-1 levels (r=−0.705, p=0.001, Figure 1C), demonstrating that as caspase-1 activity in CSF increased, functional outcome worsened. Caspase-1 levels were not correlated with admission scores including Fisher score, Hunt-Hess score, or GCS and no differences in caspase-1 levels were detected in patients who received surgical clipping compared to endovascular coiling (all p>0.05). There was one poor outcome patient with a measured caspase-1 activity level of 3.53 CPS/μL. This patient met our inclusion criteria; however, even upon exclusion of this patient, the difference between results remained statistically significant.
In order to determine whether caspase-1 activity in the CSF of SAH patients could be used to predict functional outcome at time of discharge, we conducted logistic regression analysis. Univariate analysis showed that measured caspase-1 activity was able to predict poor functional outcome (odds ratio (OR) 2.746, 95% confidence interval (CI) 1.090–6.919, p=0.032). We next performed multivariate logistic regression, adjusting caspase-1 activity by the admission GCS, given that this was the only recorded baseline difference between our groups (Table 1). Multicollinearity was not observed. After adjusting for baseline GCS, caspase-1 remained an independent predictor of poor functional outcome after SAH (OR 3.110, 95% CI 1.057–9.153, p=0.039). This difference remained significant even when the potential outlier mentioned above was excluded. Furthermore, this finding remained significant even when caspase-1 activity levels were adjusted by GCS values recorded at time of CSF sample collection rather than upon admission.
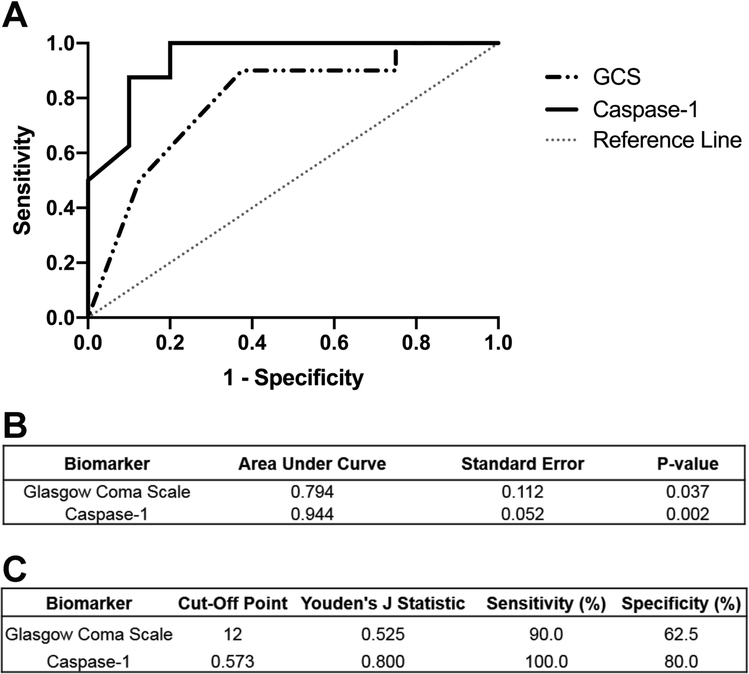
ROC curves were constructed for caspase-1 activity and baseline GCS (Figure 2A). Caspase-1 activity was able to significantly differentiate between those with good vs. poor functional outcome (area under the curve (AUC) = 0.944, p=0.002, Figure 2B). Notably, caspase-1 exhibited a better predictive value than GCS (AUC = 0.794, p = 0.037), a clinical scale that is frequently used to predict patient prognosis and which was the only clinical scale that differed at baseline in our cohort (Table 1). The optimal cut-point that optimizes sensitivity and sensitivity was calculated using the Youden’s Index (J=0.800) to be 0.573 CPS/μL. This calculated cut-point corresponded to a 100% sensitivity and 80% specificity for determining good vs. poor outcome after SAH (Figure 2C). We also calculated what cut-off would lead to 100% specificity for poor outcome and this was 0.913 CPS/μL, thus any patient with caspase-1 activity above this point would likely have a poor outcome.
Figure 2.
Receiver operating characteristic (ROC) curve of caspase-1 for the prediction of poor outcome. A) ROC curves were generated from caspase-1 levels in subarachnoid hemorrhage (SAH) patients (n=18, solid black line). ROC curve for GCS ability to predict outcome was plotted as well (dashed gray line). B) Caspase-1 had a better predictive ability (Area Under Curve (AUC) = 0.944) than did GCS (AUC = 0.794). C) Youden’s J Statistic yielded a cutoff value for capase-1 activity levels that would be indicative of poor outcome at discharge.
DISCUSSION
Our study demonstrates the potential for CSF levels of caspase-1 activity to serve as an objective biomarker for predicting functional outcome following aneurysmal SAH. Caspase-1 levels are elevated in the CSF of SAH patients compared to controls, are correlated with functional outcome, and have a better ability to predict poor outcome when compared to routinely used clinical scores.
Neuroinflammation has recently been proposed as a key mediator for the observed EBI seen in SAH patients. As such, there has been a lot of interest in identifying novel markers of neuroinflammation that might be useful for evaluating the extent of EBI.(16) While current research has investigated many other potential biomarkers of neuroinflammation, the role of the inflammasome complex as a potentiator of EBI has remained largely unexplored. Due to its central role as a mediator of downstream pro-inflammatory effects of inflammasome activity, caspase-1 lends itself to be a potentially useful and early biomarker for prognosis. Following rupture of intracranial aneurysms, blood and blood products (e.g., hemin) that accumulate in the subarachnoid space will drive formation of inflammasome complexes that will in turn activate caspase-1, potentiating neuroinflammation and, in doing so, potentially worsening patient outcomes (Figure 3). This sequence of events might explain why higher levels of active caspase-1 are seen in the context of SAH and why caspase-1 might be used as a potential marker for higher levels of neuroinflammation in general.
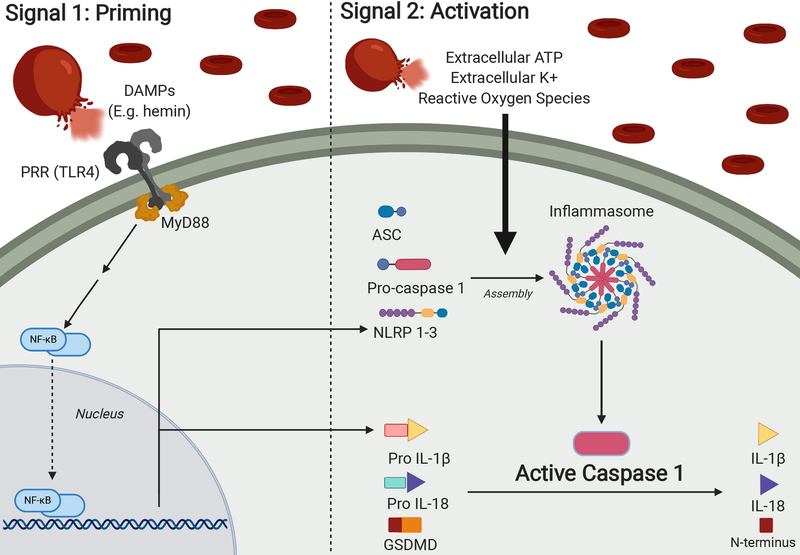
Figure 3.
The inflammasome complex and activation of caspase-1. Upon rupture of an intracranial aneurysm, blood rushes into the subarachnoid space causing increased intracranial pressure and reduced cerebral blood flow. As these extravasated red blood cells degrade, they release intracellular contents such as hemoglobin, hemin, bilirubin, potassium, adenosine triphosphate (ATP) and others.(48–50) In addition to driving formation of reactive oxygen species, these degraded blood products serve as damage-associated molecular patterns (DAMPs) that will drive a two-step process of priming and activation of the inflammasome complex.
In the priming step, DAMPs will be recognized by pattern recognition receptors (PRRs), which will signal to nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) to upregulate DNA transcription of Nucleotide-binding oligomerization domain, Leucine rich Repeat and Pyrin domain containing (NLRPs) NOD-like-receptors and pro-IL-1β.(51–55) There are various subtypes of inflammasomes expressed by multiple cell types in the CNS, each characterized by differing protein constitution, with NLRP3 in microglia being the most well-known.(51) NLRPs and pro- IL-1β are necessary components for inflammasome and caspase-1 activity, making their upregulation by NF-κB an important priming step.
A second activation signal, mediated by DAMPs derived from extravasated blood, will drive NLRPs, adapter protein apoptosis associated speck like protein containing a caspase recruitment domain (ASC) and pro-caspase-1 to form the multimeric inflammasome complex. The inflammasome can lead to the activation of caspase-1 by cleaving pro-caspase-1 into caspase-1, the active form of the enzyme, which will then in turn cleave other proinflammatory molecules into their respective active forms. There are two main effects of these proteins – inflammation, mediated by interleukins IL-1β and IL-18, and pyroptosis, mediated by the cleaved N-terminus of gasdermin D. Pyroptosis is a form of cell death involving the rupture of cell membranes and release of proinflammatory intracellular contents, making it distinct from the non-inflammatory cell death observed with apoptosis.(56,57) Figure created with BioRender.com.
Our results are in agreement with the work of others who have examined the role of the inflammasome complex in EBI. In a recent study done in TBI, it was observed that caspase-1 increases in the first 72 hours after trauma, particularly in individuals with poor outcome as measured by GOS.(25) A direct comparison between this study and ours is prevented by the pathogenic mechanisms that distinguish TBI and SAH as well as differing methodologies for measuring caspase-1. However, this data supports our claim that caspase-1 constitutes an early marker of poor outcome after brain injury. Only one other study has investigated inflammasome activity in the CSF after SAH specifically.(18) Similar to our findings, Wu et al. (2016) reported that known inflammasome proteins (Nod-like-receptor family pyrin domain containing 1 (NLRP1), apoptosis associated speck like protein containing a caspase recruitment domain (ASC) and caspase-1) were elevated in the CSF of SAH patients and that higher levels of these proteins were correlated with poor outcome three months after the SAH. Our study expands upon these findings by showing that not only is caspase-1 correlated to poor outcome, but it has an ability to predict outcome that exceeds that of currently used clinical scores. In addition, the study of Wu et al. (2016) used western blot, which only allows for relative semi-quantitative measurement of caspase-1. The caspase-1 luminescence assay used in our study allowed us to determine a quantitative cutoff point for those predicted to have a poor functional outcome. Additionally, the method reported here is a faster and more efficient technique which would be more easily translatable to a clinical setting compared to more traditional methods such as immunoblot analysis.
If supported by further research with larger cohorts and longer periods of patient follow-up, our findings might give physicians a better ability to predict functional outcome in SAH patients. Measuring caspase-1 in the CSF, as opposed to serum levels, is of particular use due to its ability to serve as a marker for what is occurring within the brain. The recently described glymphatic system provides a conduit for CSF to enter the brain parenchyma along paravascular routes and assists in the clearance of waste products, mediated by aquaporin-4.(26) This system has been studied in preclinical models of SAH ranging from rodent to non-human primates.(27–30) These studies suggest a mechanism by which blood products within the CSF are able to contact the CNS parenchyma. From there, blood products may activate microglia, initiate inflammasome signaling, promote peripheral leukocyte infiltration, and potentiate neurotoxicity.(31) Release of inflammasome mediators within the brain parenchyma can then exit along glymphatic routes to be measured within the CSF, offering clinicians a unique, less-invasive manner to assess molecular and cellular biomarkers underlying changes within the brain tissue. Future studies should investigate alterations in glymphatic system activity in SAH patients as well as methods to enhance clearance of toxic blood products to confirm whether this is translatable to humans.
A remaining question related to our work is how caspase-1, normally found in the intracellular compartment, could be present in the extracellular CSF. In addition to cellular damage inflicted by aneurysmal SAH, one possibility is secretion of caspase-1 in the form of extracellular vesicles (EVs). EVs are secreted membrane vesicles that have varied composition depending on the age, metabolic status, cell type, and disease state of the cell of origin.(32) It has been theorized that EVs play an important role in intercellular signaling and may potentiate inflammatory and coagulation responses.(33) Previous studies have shown caspase-1 within EVs secreted into CSF following TBI or spinal cord injury,(34) though this finding has not yet been observed in SAH. Secretion via EVs might be the mechanism by which caspase-1, or the inflammasome complex itself, is released from cells following inflammasome activation in response to blood products. Other theories suggest that caspase-1 and IL-1β are packaged into secretory lysosomes that fuse with plasma membranes, thereby allowing their release into the extracellular space. (35, 36) Another potential mechanism explaining the presence of caspase-1 in the extracellular space involves caspase-1’s role in promoting a unique form of programmed cell-death, termed pyroptosis. A key feature that distinguishes pyroptosis from the more well-known apoptosis is that pyroptosis involves the rupture of plasma membranes and release of pro-inflammatory cytokines – a process that is distinct from the non-inflammatory packaging of cellular contents into shedding vesicles seen in apoptosis. This pro-inflammatory form of cell death offers another potential explanation for how caspase-1 may reach the extracellular space. (37)
Further research might help elaborate the role that inflammasomes have in SAH, namely, whether the inflammasome and its pro-inflammatory effects may be playing a causative role towards driving EBI. Previous studies have focused on inhibiting the effects the cytokines IL-1β and IL-18, which are produced by active caspase-1, with the goal of reducing inflammation in other disease states. (38–40) Some studies in animal models of TBI have shown that disrupted inflammasome signaling can ameliorate neurologic impairment,(41,42) further supporting the idea that the inflammasome might be a useful target for future therapies. A known selective and reversible inhibitor of caspase-1, VX-765, has already shown efficacy in animal models of epilepsy, Alzheimer disease, and multiple sclerosis.(43–45) Additionally, this drug was used in a phase II clinical trial for the treatment of epilepsy and demonstrated a relatively acceptable safety profile (NCT01048255).(46) Whether or not inhibition of caspase-1 is efficacious in animal models of SAH remains to be seen, and future studies are warranted to determine potential therapeutic effect.
Even if caspase-1 only has a correlative – as opposed to causative – relationship with functional outcome, this finding could still be of practical use to clinicians caring for SAH patients. Some of the currently used prognostic tools, including Hunt-Hess and GCS, may be confounded by the use of sedatives, making prognostication difficult in such patients. Notably, CSF red blood cell and total protein levels were not correlated with functional outcome in our study (Table 2), suggesting that elevated CSF caspase-1 may reflect inflammasome activation derived from the brain parenchyma rather than a result of hemorrhage into the subarachnoid space alone.(47) The identification of measurable biomarkers of outcome may allow objective prognostication of patients and facilitate addressing goals of care early in the course of the disease. The luciferase assay that we used is quick, reproducible, and easy to implement in a clinical laboratory with a turnaround time of 90–120 minutes.
While this study has provided greater insight into the role of neuroinflammation in SAH, there are some limitations. First, although the sample size is relatively small, our study supports the proof of concept that quantification of early inflammation in CSF of SAH patients can add objective prognostic information. With a larger sample size, additional baseline differences may be uncovered which would then be included in the multivariate logistic regression analysis in addition to the baseline difference in admission GCS reported here. We recommend that further multi-institutional, prospective studies in larger patient cohorts are warranted to fully address the role of caspase-1 in association with poor outcomes after SAH. Second, inclusion criteria for this study in order to collect CSF required the presence of an EVD. Therefore, our conclusions are limited to higher-grade SAH patients who required an EVD as part of their standard of care. Third, while our study focused on caspase-1 as a proxy for inflammasome activity, future studies should attempt direct measurement of other inflammasome proteins such as ASC and NLRP and/or the downstream cytokines created by caspase-1 such as IL-1β and IL-18. Finally, while our outcomes were measured by GOS at time of discharge, in future studies we intend to track our patients’ recovery and assess the relationship between caspase-1 activity and long-term outcomes.
CONCLUSIONS
The inflammasome-derived protein caspase-1 is elevated in the CSF of SAH patients as compared to controls. Additionally, caspase-1 levels correlate with worse functional outcomes as measured by GOS. Caspase-1 activity measured within 72 hours of patient admission was a better predictor of prognosis compared to baseline GCS recorded at admission. Elevated caspase-1 activity in CSF was associated with poor outcome, even after adjusting for baseline differences; however, it should be noted that the sample size was small and our results should be confirmed using a large cohort before they are used in clinical practice. We believe that further investigations into caspase-1 are warranted in attempts to better understand how this objective, reliable, and easily acquired biomarker might serve clinicians managing SAH.
Details page.
The manuscript complies with all instructions to authors
Authorship requirements have been met and the final manuscript was approved by all authors
This manuscript has not been published elsewhere and is not under consideration by another journal
This manuscript adheres to ethical guidelines and ethical approvals (IRB) and use of informed consent. Informed consent was obtained from all individual participants included in the study.
Mr. Hirsch has nothing to disclose. Mr. Geraghty has nothing to disclose. Mr. Katz has nothing to disclose. Dr. Testai has nothing to disclose
Acknowledgments
The authors acknowledge Melissa N. Lara-Angulo, DNP, Maureen Hillman, RN, and the nurses of the Neuroscience Intensive Care unit of the University of Illinois for their assistance in the collection of CSF samples for this study. We also acknowledge Cory Reiter and Dr. Jeffrey A. Loeb, MD/PhD for their support of this project.
Sources of Funding: Mr. Hirsch received a Student Scholarship in Cerebrovascular Disease and Stroke from the American Heart Association. Mr. Geraghty receives grant support from the National Institute of Neurological Disorders and Stroke (Grant No. 1F31NS105525–01A1). Dr. Testai receives private donations from Louis and Christine Friedrich.
Footnotes
Disclosure: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Lantigua H, Ortega-Gutierrez S, Schmidt JM, et al. Subarachnoid hemorrhage: Who dies, and why? Crit Care. 2015;19:309–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: Beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep. 2017;19(12):50. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald RL, Higashida RT, Keller E, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011;10(7):618–625. [DOI] [PubMed] [Google Scholar]
- 4.Maragkos GA, Enriquez-Marulanda A, Salem MM, et al. Proposal of a grading system for predicting discharge mortality and functional outcome in patients with aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;121:e500–e510. [DOI] [PubMed] [Google Scholar]
- 5.Jaja BN, Cusimano MD, Etminan N, et al. Clinical prediction models for aneurysmal subarachnoid hemorrhage: A systematic review. Neurocrit Care. 2013;18(1):143–153. [DOI] [PubMed] [Google Scholar]
- 6.Kapapa T, Tjahjadi M, Konig R, Wirtz CR, Woischneck D. Which clinical variable influences health-related quality of life the most after spontaneous subarachnoid hemorrhage? Hunt and Hess scale, Fisher score, World Federation of Neurosurgeons score, Brussels coma score, and Glasgow coma score compared. World Neurosurg. 2013;80(6):853–8. [DOI] [PubMed] [Google Scholar]
- 7.Hong CM, Tosun C, Kurland DB, Gerzanich V, Schreibman D, Simard JM. Biomarkers as outcome predictors in subarachnoid hemorrhage--a systematic review. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals 2014;19:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agoston DV, Shutes-David A, Peskind ER. Biofluid biomarkers of traumatic brain injury. Brain Injury. 2017;31:1195–203. [DOI] [PubMed] [Google Scholar]
- 9.Cahill J, Zhang JH. Subarachnoid hemorrhage: Is it time for a new direction? Stroke. 2009;40:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Oliveira MAL, Macdonald RL. Neuroinflammation as a target for intervention in subarachnoid hemorrhage. Front Neurol. 2018;9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017;16;8(34):57489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Napoli M, Slevin M, Popa-Wagner A, Singh P, Lattanzi S, Divani A. Monomeric C-Reactive Protein and Cerebral Hemorrhage: From Bench to Bedside. Front Immunol. 2018;9:1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lattanzi S, Di Napoli M, Ricci S, Divani A. Matrix Metalloproteinases in Acute Intracerebral Hemorrhage. Neurotherapeutics. 2020;17:484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yong-Lin L, Jie-Kai L, Han-Peng Y, et al. High Neutrophil-to-Lymphocyte Ratio Predicts Hemorrhagic Transformation in Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis. Int J Hypertens. 2020;2020:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maestrini I, Tagzirt M, Gautier S, et al. Analysis of the association of MPO and MMP-9 with stroke severity and outcome. Neurology. 2020;95:97–108. [DOI] [PubMed] [Google Scholar]
- 16.Geraghty JR, Davis JL, Testai FD. Neuroinflammation and microvascular dysfunction after experimental subarachnoid hemorrhage: Emerging components of early brain injury related to outcome. Neurocrit Care. 2019;31:373–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerr N, Lee SW, Perez-Barcena J, et al. Inflammasome proteins as biomarkers of traumatic brain injury. PLoS One. 2018;13:e0210128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Q, Wang XL, Yu Q, et al. Inflammasome proteins in cerebrospinal fluid of patients with subarachnoid hemorrhage are biomarkers of early brain injury and functional outcome. World Neurosurg. 2016;94:472–9. [DOI] [PubMed] [Google Scholar]
- 19.Adamczak S, Dale G, de Rivero Vaccari, J P, Bullock MR, Dietrich WD, Keane RW. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: Clinical article. J Neurosurg. 2012;117:1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman LC, Ting JP. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016;136 Suppl 1:29–38. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Murray GD, Steyerberg EW, et al. Effects of Glasgow Outcome Scale misclassification on traumatic brain injury clinical trials. J Neurotrauma. 2008;25:641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien M, Moehring D, Munoz-Planillo R, et al. A bioluminescent caspase-1 activity assay rapidly monitors inflammasome activation in cells. J Immunol Methods. 2017;447:1–13. [DOI] [PubMed] [Google Scholar]
- 24.Geraghty JR, Lara-Angulo MN, Spegar M, Reeh J, Testai FD. Severe cognitive impairment in aneurysmal subarachnoid hemorrhage: Predictors and relationship to functional outcome. J Stroke Cerebrovasc Dis. 2020;29:105027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez-Bárcena J, Crespí C, Frontera G, et al. Levels of caspase-1 in cerebrospinal fluid of patients with traumatic brain injury: correlation with intracranial pressure and outcome [published online ahead of print, 2020 May 1]. J Neurosurg. 2020;1–6. [DOI] [PubMed] [Google Scholar]
- 26.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pu T, Zou W, Feng W, et al. Persistent malfunction of glymphatic and meningeal lymphatic drainage in a mouse model of subarachnoid hemorrhage. Exp Neurobiol. 2019;28:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golanov EV, Bovshik EI, Wong KK, et al. Subarachnoid hemorrhage - induced block of cerebrospinal fluid flow: Role of brain coagulation factor III (tissue factor). J Cereb Blood Flow Metab. 2018;38:793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goulay R, Flament J, Gauberti M, et al. Subarachnoid hemorrhage severely impairs brain parenchymal cerebrospinal fluid circulation in nonhuman primate. Stroke. 2017;48:2301–5. [DOI] [PubMed] [Google Scholar]
- 30.Luo C, Yao X, Li J, et al. Paravascular pathways contribute to vasculitis and neuroinflammation after subarachnoid hemorrhage independently of glymphatic control. Cell Death Dis. 2016;7:e2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Wang L, Xu H, Xing L, Zhuang Z, Zheng Y, Li X, Wang C, Chen S, Guo Z, Liang Q, Wang Y. Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat Commun. 2020;11:3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiter CR, Bongarzone ER. The role of vesicle trafficking and release in oligodendrocyte biology. Neurochem Res. 2020;45(3):620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lackner P, Dietmann A, Beer R, et al. Cellular microparticles as a marker for cerebral vasospasm in spontaneous subarachnoid hemorrhage. Stroke. 2010;41:2353–7. [DOI] [PubMed] [Google Scholar]
- 34.de Rivero Vaccari, J P, Brand F, Adamczak S, et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136 Suppl 1:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monteleone M, Stow JL, Schroder K. Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine. 2015;74:213–8 [DOI] [PubMed] [Google Scholar]
- 36.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999. May;10(5):1463–75. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009. February;7(2):99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zahid A, Li B, Kombe AJK, Jin T, Tao J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol. 2019;10:2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowarski R, Jackson R, Gagliani N, et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 2015;163:1444–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25:469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akamatsu Y, Pagan VA, Hanafy KA. The role of TLR4 and HO-1 in neuroinflammation after subarachnoid hemorrhage. J Neurosci Res. 2020;98:549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao X, Liu S, Ding W, et al. TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J Neuroimmunol. 2017;310:38–45. [DOI] [PubMed] [Google Scholar]
- 43.Flores J, Noël A, Foveau B, Lynham J, Lecrux C, LeBlanc AC. Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nat Commun. 2018;9:3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKenzie BA, Mamik MK, Saito LB, et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci U S A. 2018;115:E6065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: A summary of the eleventh eilat conference (EILAT XI). Epilepsy Res. 2013;103:2–30. [DOI] [PubMed] [Google Scholar]
- 46.Vertex P 2011. Vertex Announces Completion of Phase 2 Study of VX-765 in People with Epilepsy who did not Respond to Previous Treatment. Available at investors.vrtx.com/releasedetail.cfm?ReleaseID=555967. Accessed May 4, 2020
- 47.Nadkarni NA, Maas MB, Batra A, et al. Elevated cerebrospinal fluid protein is associated with unfavorable functional outcome in spontaneous subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2020;29:104605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pyne-Geithman GJ, Morgan CJ, Wagner K, et al. Bilirubin production and oxidation in CSF of patients with cerebral vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:1070–7. [DOI] [PubMed] [Google Scholar]
- 49.Kinfe TM, Chaudhry SR, Muhammad S, et al. Role of damage associated molecular pattern molecules (DAMPs) in aneurysmal subarachnoid hemorrhage (aSAH). Int J Mol Sci. 2018;19:2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucke-Wold BP, Logsdon AF, Manoranjan B, et al. Aneurysmal subarachnoid hemorrhage and neuroinflammation: A comprehensive review. Int J Mol Sci. 2016;17:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Rivero Vaccari, Juan Pablo, Dietrich WD, Keane RW. Activation and regulation of cellular inflammasomes: Gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab. 2014;34:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canesin G, Hejazi SM, Swanson KD, Wegiel B. Heme-derived metabolic signals dictate immune responses. Front Immunol. 2020;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman HM, Wanderer AA. Inflammasome and IL-1beta-mediated disorders. Curr Allergy Asthma Rep. 2010;10:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13:148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun. 2013;28:54–62. [DOI] [PubMed] [Google Scholar]
- 56.Chen S, Mei S, Luo Y, Wu H, Zhang J, Zhu J. Gasdermin family: A promising therapeutic target for stroke. Transl Stroke Res. 2018;9:555–63. [DOI] [PubMed] [Google Scholar]
- 57.Fang Y, Gao S, Wang X, et al. Programmed cell deaths and potential crosstalk with blood-brain barrier dysfunction after hemorrhagic stroke. Front Cell Neurosci. 2020;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]