Abstract
There are limited data regarding whether mortality is higher in patients with non cystic fibrosis bronchiectasis (bronchiectasis) than in those without bronchiectasis. Using 2005–2015 data from the Korean National Health Insurance Service, we evaluated hazard ratio (HR) for all-cause mortality in the bronchiectasis cohort relative to the matched cohort. The effect of comorbidities over the study period on the relative mortality was also assessed. All-cause mortality was significantly higher in the bronchiectasis cohort than in the matched cohort (2505/100,000 vs 2142/100,000 person-years, respectively; P < 0.001). Mortality risk was 1.15-fold greater in the bronchiectasis cohort than in the matched cohort (95% confidence interval [CI] 1.09–1.22); mortality was greatest among elderly patients (HR = 1.17, 95% CI 1.10–1.25) and men (HR = 1.19, 95% CI 1.10–1.29). Comorbidities over the study period significantly increased the risk of death in the bronchiectasis cohort relative to the matched cohort: asthma (adjusted HR = 1.20, 95% CI 1.11–1.30), chronic obstructive pulmonary disease (adjusted HR = 1.24, 95% CI 1.15–1.34), pneumonia (adjusted HR = 1.50, 95% CI 1.39–1.63), lung cancer (adjusted HR = 1.85, 95% CI 1.61–2.12), and cardiovascular disease (adjusted HR = 1.34, 95% CI 1.23–1.45). In contrast, there were no significant differences in the risk of death in patients without bronchiectasis-related comorbidities and the matched cohort, except in the case of non-tuberculous mycobacterial infection. In conclusion, all-cause mortality was higher in patients with bronchiectasis cohort than those without bronchiectasis, especially in elderly patients and men. Comorbidities over the study period played a major role in increasing mortality in patients with bronchiectasis relative to those without bronchiectasis.
Subject terms: Prognosis, Risk factors, Epidemiology
Introduction
Non cystic fibrosis bronchiectasis (hereafter referred to as bronchiectasis) is a chronic respiratory disease being frequently encountered in daily practice1. Patients with bronchiectasis often suffer from persistent respiratory symptoms, decreased quality of life, and recurrent respiratory infection1. As the prevalence of bronchiectasis2–7 and associated health costs2,8,9 is increasing in many countries, this disease is becoming an emerging threat to global health10,11. However, unfortunately, these expectations are based on studies performed mostly in Western countries10,11. Thus, despite the general consideration that the prevalence of bronchiectasis is higher in Asian countries than in most Western countries, the epidemiology of bronchiectasis in most Asian countries is still lacking.
Reducing the mortality from bronchiectasis is one of the most important treatment outcomes of the disease. To achieve this outcome, the national mortality rate of bronchiectasis needs to be determined. It is also crucial to know the relative risk of mortality according to the presence or absence of bronchiectasis. This information would help decide whether a health policy is urgently needed to reduce mortality associated with bronchiectasis. However, limited information is available regarding the nationwide mortality due to bronchiectasis10. Furthermore, there is no known national representative data on mortality in patients with bronchiectasis in most Asian countries, including South Korea.
Comorbidities are known to increase the risk of mortality in patients with bronchiectasis12. These results have been well demonstrated by studies on a large number of bronchiectasis patients in Western countries12. However, since these comorbidities are also risk factors of mortality in the general population, it is unclear whether the comorbidities lead to higher mortality in bronchiectasis patients than in those without bronchiectasis. Given that improving survival is one of the major goals in treating chronic diseases, identifying the comorbidities associated with high mortality in patients with bronchiectasis relative to those without bronchiectasis is very important. However, limited data are available regarding this issue, especially in the Asian population.
Consequently, our principal aim was to compare the all-cause mortality in patients with bronchiectasis and those without bronchiectasis in Koreans. We also evaluated whether bronchiectasis-related comorbidities are associated with an increased mortality in patients with bronchiectasis relative to those without bronchiectasis.
Methods
Study population
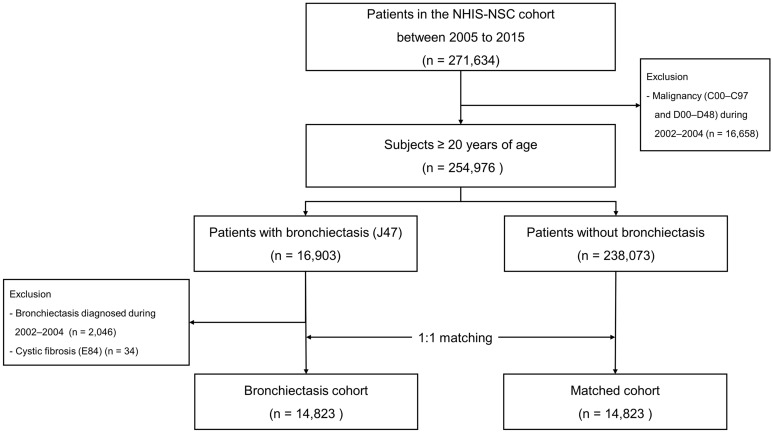
This study used data from the National Health Insurance Service-National Sample Cohort (NHIS-NSC), which is a retrospective population-based cohort that includes 2.2% of all Korean citizens13. The NHIS-NSC collects data regarding major and minor diagnoses using the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes, drug prescriptions, health examination outcomes, and mortality and the causes thereof13. As shown in Fig. 1, data regarding 271,634 patients aged ≥ 20 years were collected between January 2005 and December 2015. After exclusion of patients with any malignancy (ICD-10 diagnosis codes C00–C97 and D00–D48) during the washout period (January 2002–December 2004) (n = 16,658), 254,976 patients remained. Of these, 16,903 had bronchiectasis (ICD-10 diagnosis code J47). After the exclusion of patients with cystic fibrosis (ICD-10 diagnosis code E84) (n = 34) and patients diagnosed with bronchiectasis during washout period (January 2002–December 2004) (n = 2046), 14,823 incident bronchiectasis cases were identified. To establish a matched cohort, 1:1 matching was performed by age, sex, and Charlson Comorbidity Index (CCI) (baseline comorbidities were used to calculate CCI)14; accordingly, each patient with bronchiectasis was matched to a patient without bronchiectasis (Fig. 1)15. Patients were followed from the time diagnosed with bronchiectasis during the study period (January 2005–December 2015) until the date of death or until the end of the study period (December 31, 2015).
Figure 1.
Flow chart of the study population. NHIS-NCS, National Health Insurance Service-National Sample Cohort.
Methodologically, adult (aged ≥ 20 years) patients with bronchiectasis were included in this study because there are substantial differences between bronchiectasis in adults and that in children/adolescents16. Plus, we determined January 2002–December 2004 as the washout period and excluded patients with bronchiectasis during the period to include incident cases. The reason why we constructed an incident bronchiectasis cohort was that a prevalent cohort might underestimate short-term mortality because the more serious cases die in the early phase of the disease and are not included in the calculation of deaths over the period of observation17. For a similar reason, we excluded patients with malignancies during the washout period since the prevalent malignancies might overestimate the mortality of the study population.
The Institutional Review Board of Hanyang University Hospital approved the study and waived the requirement for informed consent because NHIS-NSC data are de-identified (approval no. HYUH 2019-05-020). All methods were performed in accordance with the relevant guidelines and regulations.
Definition
Adult bronchiectasis was defined using the following criteria: (1) age ≥ 20 years and (2) at least one claim under ICD-10 code J476. Bronchiectasis-associated comorbidities were defined using the following ICD-10 codes: angina pectoris (I20), myocardial infarction (I21, I22, or I25.2), asthma (J45–J46), chronic obstructive pulmonary disease (COPD) (J42–J44, except J43.0 [unilateral emphysema]), cerebrovascular disease (G45–G46, I60–I69, or H34.0), depression (F32–F34), diabetes mellitus (E10–E14), high cholesterol level (E78), gastroesophageal reflux disease (K21), hypertension (I10–I15), heart failure (I43, I50, I09.9, I11.0, I25.5, I13.0, I13.2, I42.0, I42.5–I42.9, or P29.0), inflammatory bowel disease (K50–K51), non-tuberculous mycobacteria (NTM) infection (A31), osteoporosis (M80–M81), and rheumatological disease (M05, M06, M31.5, M32, M33, M34, M35.1, M35.3, or M36.0)15. Baseline comorbidities were assessed at the time of study enrolment; those were used to calculate CCI (see Table 1 and Supplementary Table S1). Comorbidities over the study period were assessed at the time of study enrolment as well as during follow-up period. As we excluded patients with malignancies diagnosed during the washout period, lung cancer (C34) plus other malignancies (C00–C97, except C34) were only assessed during the follow-up period.
Table 1.
Baseline patient characteristics.
| Bronchiectasis cohort (n = 14,823) |
Matched cohort (n = 14,823) |
P value | ||
|---|---|---|---|---|
| Age (years) | 58.7 ± 15.0 | 58.7 ± 15.0 | 0.951 | |
| Age group | ||||
| 20–29 years | 590 (4.0) | 587 (4.0) | 1.0 | |
| 30–39 years | 1257 (8.5) | 1255 (8.5) | ||
| 40–49 years | 2073 (14.0) | 2069 (14.0) | ||
| 50–59 years | 3257 (22.0) | 3268 (22.1) | ||
| 60–69 years | 3709 (25.0) | 3707 (25.0) | ||
| ≥ 70 years | 3937 (26.5) | 3937 (26.5) | ||
| Sex | 0.991 | |||
| Male | 7154 (48.3) | 7153 (48.3) | ||
| Female | 7669 (51.7) | 7670 (51.7) | ||
| Type of insurance | ||||
| Self-employed health insurance | 4216 (28.4) | 5597 (37.8) | 1.0 | |
| Employee health insurance | 9347 (63.1) | 9226 (62.2) | 1.0 | |
| Medical aid | 1260 (8.5) | – | ||
| Charlson Comorbidity Indexa | 2.92 ± 2.5 | 2.92 ± 2.5 | 0.988 | |
Data are presented as number (%) or mean with standard deviation.
aComorbidities at the time of enrolment were used.
All-cause mortality was defined as all deaths during follow-up for up to 10 years after enrolment (January 2005–December 2015) irrespective of the cause of death. Causes of mortality were determined using data provided by Statistics Korea, an initiative of the Ministry of Strategy and Finance of South Korea. Causes of death were classified as one of the followings: (1) respiratory diseases (J00–J99); (2) cardiovascular diseases (I00–I99); (3) malignant neoplasms including lung cancer (C00–C97); (4) injury, poisoning, and external causes (S00–S99 and T00–T98); (5) endocrine diseases (E00–E90); (6) gastrointestinal diseases (K00–K93); (7) neurological diseases (G00–G99); (8) mental and behavioural disorders (F00–F99); (9) musculoskeletal and connective tissue diseases (M00–M99); and (10) miscellaneous.
Outcomes
Primary outcomes were the comparison of overall mortality in the bronchiectasis cohort compared to the matched cohort. Secondary outcomes were the impact of bronchiectasis-related comorbidities on mortality in patients with and without bronchiectasis.
Statistical analysis
The McNemar test was used to compare the baseline characteristics of the bronchiectasis and matched cohorts. The Kaplan–Meier method was used to generate survival curves, and the log-rank test was used to compare survival between the two groups. To evaluate the impact of bronchiectasis on mortality, mortality incidence rates (per 100,000 person-years) were compared between the bronchiectasis and matched cohorts using the normal approximation test for binomials. To determine hazard ratios (HR) for mortality, a Cox proportional hazards regression model was used, with adjustments for age, sex, insurance type, and CCI. For the evaluation of HR for each cause of mortality. In addition, to assess the effect of bronchiectasis-related comorbidities (asthma, COPD, pneumonia, NTM infection, lung cancer, and cardiovascular disease) on mortality in the bronchiectasis cohort versus the matched cohort, we used a Cox proportional hazard regression model with adjustment for age, sex, insurance type, and CCI. All statistical analyses were performed using SAS software ver. 9.4 (SAS Institute, Cary, NC, USA). All tests were two-sided and P-values < 0.05 were considered statistically significant.
Results
Baseline characteristics
The baseline characteristics of the bronchiectasis and the matched cohorts are summarised in Table 1. The mean age of the bronchiectasis cohort was 58.7 years, and 51.7% of the patients were women. There were no between-cohort differences in mean age, age group distribution, sex, insurance type, or CCI. Detailed comorbidity profiles at the time of study enrolment are summarised in Supplementary Table S1.
Mortality of the bronchiectasis cohort relative to the matched cohort
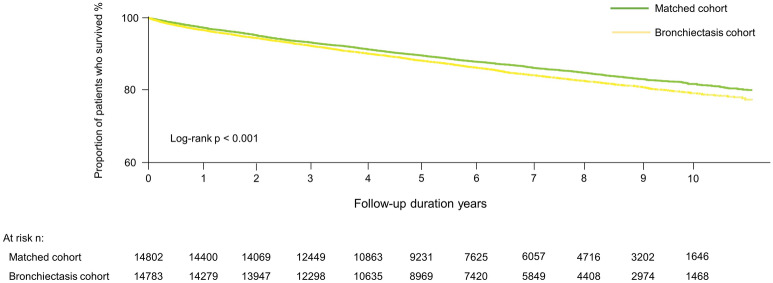
As shown in Supplementary Figure S1, estimated all-cause mortality was significantly higher in the bronchiectasis cohort than in the matched cohort (2505.1/100,000 person-years vs 2142.2/100,000 person-years, P < 0.001), consistent with the results of survival analysis (Fig. 2). During the follow-up period, mortality risk was 1.15-fold greater (95% confidence interval [CI] 1.09–1.22) for patients in the bronchiectasis cohort than for those in the matched cohort. HRs for mortality in the bronchiectasis cohort relative to the matched cohort were 1.17 (95% CI 1.10–1.25) in elderly patients (age ≥ 60 years) and 1.19 (95% CI 1.10–1.29) in male. However, there were no significant differences in mortality risk in the bronchiectasis cohort relative to the matched cohort with respect to younger patients (age < 60 years) (HR = 1.17, 95% CI 0.98–1.39) or women (HR = 1.10, 95% CI 0.99–1.21) (Table 2).
Figure 2.
Kaplan–Meier survival analysis of time to death by bronchiectasis status.
Table 2.
Mortality in the bronchiectasis cohort relative to the matched cohort.
| Total (N = 29,646) |
Male (n = 14,307) |
Female (n = 15,339) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. at risk | No. of death | IR (/100,000 PY) | HRa | 95% CI | No. at risk | No. of death | IR (/100,000 PY) | HRa | 95% CI | No. at risk | No. of death | IR (/100,000 PY) | HRa | 95% CI | |
| Overall | |||||||||||||||
| Matched | 14,823 | 2058 | 2142.2 | Ref | Ref | 7153 | 1250 | 2781.3 | Ref | Ref | 7670 | 808 | 1580.4 | Ref | Ref |
| BE | 14,823 | 2250 | 2505.1 | 1.15 | 1.09–1.22 | 7154 | 1405 | 3362.0 | 1.19 | 1.10–1.29 | 7669 | 845 | 1759.5 | 1.10 | 0.99–1.21 |
| Age group | |||||||||||||||
| < 60 years | |||||||||||||||
| Matched | 7179 | 246 | 485.8 | Ref | Ref | 3398 | 168 | 713.6 | Ref | Ref | 3781 | 78 | 287.8 | Ref | Ref |
| BE | 7177 | 274 | 569.7 | 1.17 | 0.98–1.39 | 3394 | 195 | 872.2 | 1.22 | 0.99–1.51 | 3783 | 79 | 306.9 | 1.05 | 0.77–1.44 |
| ≥ 60 years | |||||||||||||||
| Matched | 7644 | 1812 | 3989.0 | Ref | Ref | 3755 | 1082 | 5056.4 | Ref | Ref | 3889 | 730 | 3038.3 | Ref | Ref |
| BE | 7646 | 1976 | 4736.5 | 1.17 | 1.10–1.25 | 3760 | 1210 | 6226.5 | 1.21 | 1.12–1.32 | 3886 | 766 | 3437.2 | 1.12 | 1.01–1.24 |
Data are presented as risk ratios (95% confidence interval).
aUnadjusted hazard ratio.
BE, bronchiectasis; IR, incidence rate; PY, person-years; HR, hazard ratio; CI, confidence interval; Ref, reference.
Effects of bronchiectasis-related comorbidities on the risk of mortality in the bronchiectasis cohort relative to the matched cohort
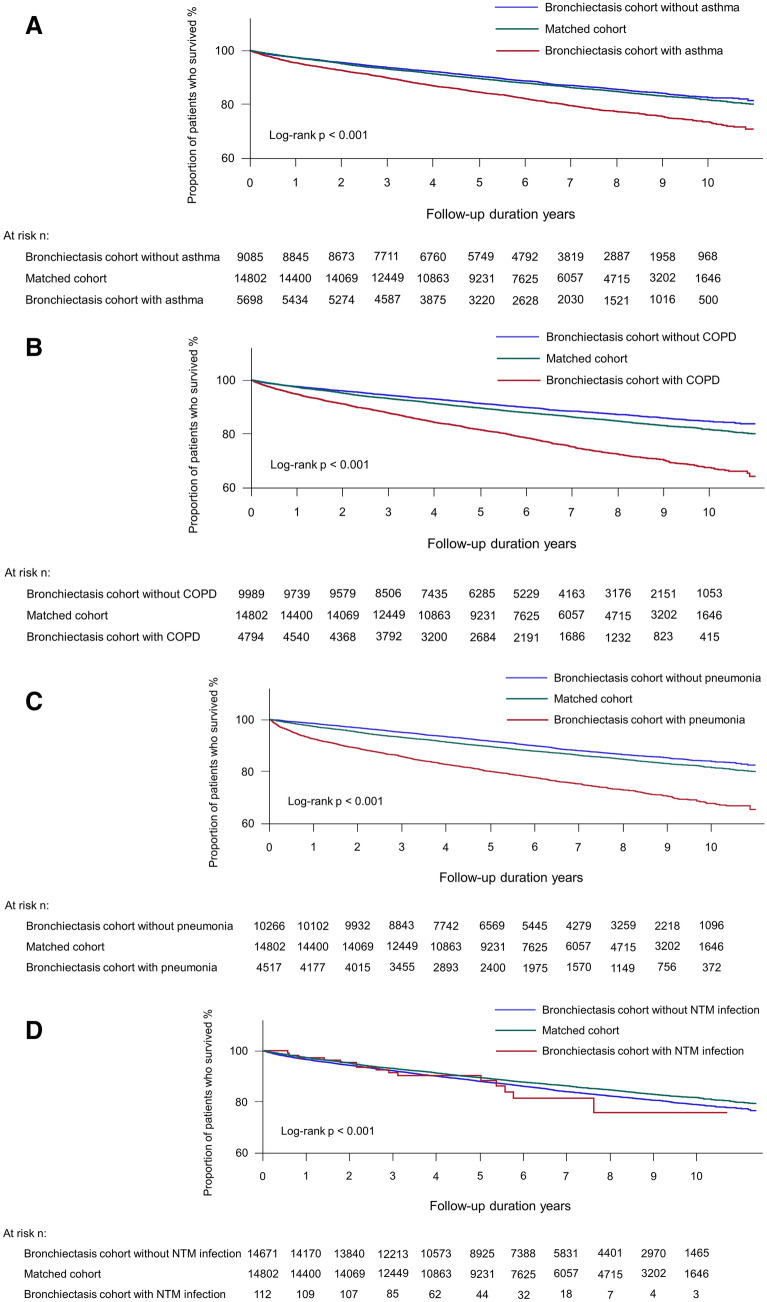
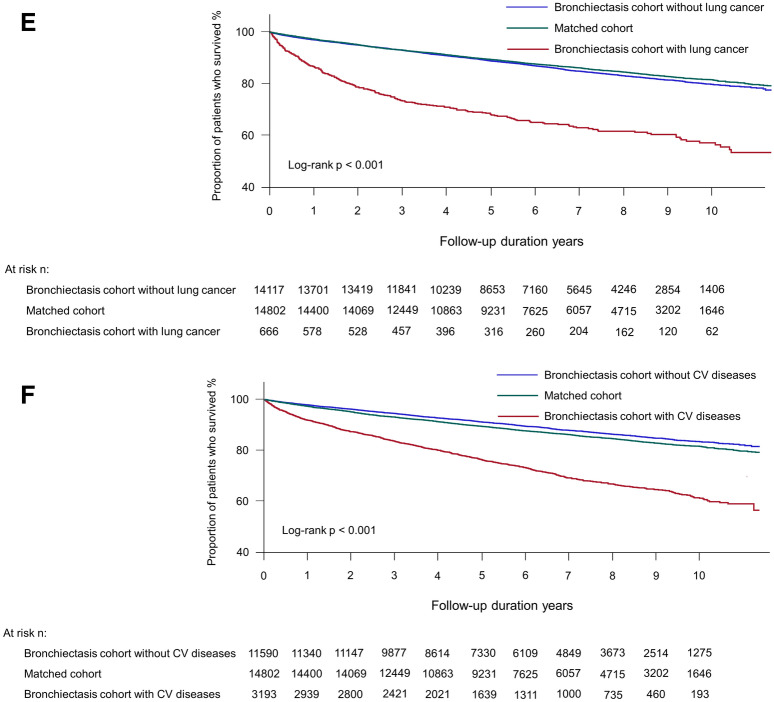
As shown in Table 3, the presence of the following bronchiectasis-related comorbidities—asthma (adjusted HR = 1.20, 95% CI 1.11–1.30), COPD (adjusted HR = 1.24, 95% CI 1.15–1.34), pneumonia (adjusted HR = 1.50, 95% CI 1.39–1.63), lung cancer (adjusted HR = 1.85, 95% CI 1.61–2.12), and cardiovascular disease (adjusted HR = 1.34, 95% CI 1.23–1.45)—significantly increased the risk of death in the bronchiectasis cohort relative to the matched cohort, in line with the survival analyses (asthma in Fig. 3A, COPD in Fig. 3B, pneumonia in Fig. 3C, lung cancer in Fig. 3E, and cardiovascular disease in Fig. 3F). However, patients with NTM infection in the bronchiectasis cohort did not have an increased risk of death compared to the matched cohort (adjusted HR = 1.07, 95% CI 0.64–1.78) (Fig. 3D).
Table 3.
The effects of comorbidities over the study period on the risk of mortality in the bronchiectasis cohort relative to the matched cohort.
| Number at risk | Mortality | ||||
|---|---|---|---|---|---|
| Number of death | Incidence rate (/100,000 PY) | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) | ||
| Asthma | |||||
| Matched cohort | 14,823 | 2058 | 2142.2 | Reference | Reference |
| Bronchiectasis cohort without asthma | 9107 | 1149 | 2021.0 | 0.93 (0.87–1.00) | 1.05 (0.97–1.13) |
| Bronchiectasis cohort with asthma | 5716 | 1101 | 3337.4 | 1.53 (1.42–1.65) | 1.20 (1.11–1.30) |
| COPD | |||||
| Matched cohort | 14,823 | 2058 | 2142.2 | Reference | Reference |
| Bronchiectasis cohort without COPD | 10,015 | 1121 | 1795.6 | 0.82 (0.77–0.89) | 1.02 (0.95–1.10) |
| Bronchiectasis cohort with COPD | 4808 | 1129 | 4123.0 | 1.90 (1.77–2.04) | 1.24 (1.15–1.34) |
| Pneumonia | |||||
| Matched cohort | 14,823 | 2058 | 2142.2 | Reference | Reference |
| Bronchiectasis cohort without pneumonia | 10,283 | 1162 | 1793.5 | 0.83 (0.77–0.89) | 0.91 (0.84–0.98) |
| Bronchiectasis cohort with pneumonia | 4540 | 1088 | 4347.4 | 1.98 (1.84–2.13) | 1.50 (1.39–1.63) |
| NTM infection | |||||
| Matched cohort | 14,823 | 2058 | 2142.2 | Reference | Reference |
| Bronchiectasis cohort without NTM infection | 14,711 | 2235 | 2503.2 | 1.15 (1.08–1.22) | 1.11 (1.05–1.19) |
| Bronchiectasis cohort with NTM infection | 112 | 15 | 2843.6 | 1.29 (0.78–2.14) | 1.07 (0.64–1.78) |
| Lung cancer | |||||
| Matched cohort | 14,823 | 2058 | 2142.2 | Reference | Reference |
| Bronchiectasis cohort without lung cancer | 14,150 | 2009 | 2325.1 | 1.07 (1.01–1.14) | 1.06 (0.99–1.13) |
| Bronchiectasis cohort with lung cancer | 673 | 241 | 7069.1 | 3.18 (2.78–3.65) | 1.85 (1.61–2.12) |
| Cardiovascular disease | |||||
| Matched cohort | 14,823 | 2058 | 2142.2 | Reference | Reference |
| Bronchiectasis cohort without cardiovascular disease | 11,609 | 1342 | 1847.1 | 0.86 (0.80–0.92) | 1.01 (0.94–1.08) |
| Bronchiectasis cohort with cardiovascular disease | 3214 | 908 | 5290.9 | 2.40 (2.22–2.60) | 1.34 (1.23–1.45) |
Data are presented as risk ratios (95% confidence interval).
Comorbidities, including asthma, COPD, pneumonia, NTM infection, and cardiovascular disease, were assessed at the time of study enrolment as well as during the follow-up period; lung cancer was assessed during the follow-up period.
PY, person-years; HR, hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; NTM, non-mycobacterial mycobacteria.
aAdjusted for age, sex, type of insurance, and Charlson Comorbidity Index.
Figure 3.
Kaplan–Meier survival analysis of the time to death in bronchiectasis patients with comorbidities, bronchiectasis patients without comorbidities, and those without bronchiectasis. (A) asthma, (B) COPD, (C) pneumonia, (D) NTM infection, (E) lung cancer, and (F) cardiovascular disease. COPD, chronic obstructive pulmonary disease; NTM, nontuberculous mycobacteria; CV, cardiovascular.
Patients in the bronchiectasis cohort without bronchiectasis-related comorbidities (asthma, COPD, pneumonia, lung cancer or cardiovascular disease) did not have an increased risk of death compared to the matched cohort, except in the case of NTM infection (adjusted HR = 1.11, 95% CI 1.05–1.19).
Causes of mortality
The common causes of mortality among patients in the bronchiectasis cohort were malignant neoplasms, including lung cancer (29.7%); respiratory diseases (19.8%); cardiovascular diseases (17.8%); and injury, poisoning, and external causes (7.3%). In comparison, the common causes of mortality among patients in the matched cohort were malignant neoplasms (37.2%); cardiovascular diseases (21.9%); respiratory diseases (7.5%); and injury, poisoning, and external causes (7.0%).
Discussion
We evaluated the impact of bronchiectasis on mortality using a longitudinal population-based cohort of Koreans. We found that patients with bronchiectasis were at increased risk of mortality compared to an age-, sex-, insurance type-, and CCI-matched population. The risks were highest in elderly patients and men. Bronchiectasis-related comorbidities (asthma, COPD, pneumonia, lung cancer, and cardiovascular diseases) could explain the increased risk of death in the bronchiectasis cohort relative to the matched cohort; however, patients in the bronchiectasis cohort without the comorbidities did not have increased risk of death compared to the matched cohort, except for NTM infection. The common causes of mortality in the bronchiectasis patients were malignant neoplasms, respiratory diseases, and cardiovascular diseases.
To the best of our knowledge, our study is the first to comprehensively analyse mortality in patients with bronchiectasis, compared to those without bronchiectasis, utilising a nationwide, representative, longitudinal, population-based cohort. Consistent with other authors, we found high mortality in patients with bronchiectasis12,18–23. However, in previous studies, the sample sizes were relatively small18–22, the mortality rate was not compared with that of controls12,18–23, or patients were only enrolled at a single centre20–22,24. Thus, data from previous studies were not generalisable. A cross-sectional study performed in the UK addressed these prior limitations by comparing mortality of bronchiectasis patients with that of the general population10. Although that analysis provided valuable insight regarding bronchiectasis mortality, the patients were not incident cases. Given the nature of a cross-sectional study, the study could only offer the mortality rate in a specified year in comparison with the general population10. Therefore, an important strength of our study was that we derived the bronchiectasis mortality rate by means of a large population-based longitudinal analysis of incident cases. Furthermore, we minimised selection bias by using nationally representative data.
We found that elderly patients and men with bronchiectasis were at higher risk of mortality than those without bronchiectasis. The analysis with the bronchiectasis cohort in this study revealed that older age18,19,21,23,25 and male sex10,19,23 were significantly associated with higher mortality, consistent with the findings in previous studies. Although the reasons for the elderly and male associations with poor outcomes are unclear, we may acquire insight from COPD mortality studies showing similar results. One study in the Netherlands found that mortality was higher for patients with COPD than for patients without COPD, especially among men and elderly patients26. A recent Korean study found an increasing HR trend for mortality in men with COPD compared to men without COPD27. Whether such findings are common among patients with chronic respiratory diseases is unclear. Further studies are needed.
Another interesting finding was that bronchiectasis-related comorbidities could explain the increased risk of death in the bronchiectasis cohort compared to the matched cohort, as the risk of death in bronchiectasis patients without bronchiectasis-related comorbidities was comparable to the risk of death in those without bronchiectasis. This finding suggests that bronchiectasis-related comorbidities, including asthma, COPD, pneumonia, lung cancer, and cardiovascular diseases, may play a major role in increased mortality in the bronchiectasis cohort relative to the matched cohort. As shown in the previous reports18,21–24, respiratory conditions were common causes of mortality among patients with bronchiectasis in the present study. Comorbid COPD and asthma (both of which are included in the Bronchiectasis Aetiology Comorbidity Index [BACI]) are significantly associated with higher mortality in patients with bronchiectasis21,23,28–31. In contrast, the bronchiectasis cohort without NTM infection showed increased mortality relative to the matched cohort; however, the bronchiectasis cohort with NTM infection did not show increased mortality relative to the matched cohort. This phenomenon may be explained by the fact that macrolide, a core drug used in NTM infection, exert a protective effect on the mortality of the bronchiectasis patients with NTM infection32. However, the number of bronchiectasis cohort with NTM infection was relatively small not enough to draw definite conclusions; future research is needed. Despite increasing evidence of higher mortality in patients with bronchiectasis who have other comorbid pulmonary diseases (compared to patients with bronchiectasis who do not have other comorbid pulmonary diseases), no evidence-based treatment strategy is yet available for such patients. Both our results and previous findings emphasise the urgent need for integrated treatment strategies for patients who have bronchiectasis and other pulmonary diseases.
Notably, we found that cardiovascular diseases (not included in the BACI) in bronchiectasis were associated with higher mortality in patients with bronchiectasis relative to those without bronchiectasis. Previous studies showed that approximately 20% of deaths among patients with bronchiectasis were of cardiovascular origin21,23. Because the risk of coronary heart disease and stroke is higher in patients with bronchiectasis than in the general population33,34, such comorbidities require close attention in patients with bronchiectasis. Although we excluded patients diagnosed with malignancies at the time of enrolment, malignancy (especially lung cancer) was an important cause of mortality in patients with bronchiectasis, as shown in a previous study24. When we consider that many bronchiectasis patients had COPD35, a well-known risk factor for lung cancer36, we suspect that bronchiectasis may increase the risk of lung cancer37. Hence, surveillance for lung cancer may be beneficial in patients with bronchiectasis who have risk factors for lung cancer, including a history of smoking, tuberculosis, or COPD.
Our study had several limitations. First, the study only included the evaluation of Korean patients; therefore, our data may not be generalisable to other ethnic groups or populations. Second, the study may not have included patients with bronchiectasis who had mild symptoms because the ICD-10 code was used for the diagnosis of bronchiectasis. Plus, given the nature of study using ICD-10 codes, some of the study population might have been misclassified as having bronchiectasis. Third, this study did not include mortality factors evaluated in prior studies (e.g., smoking status21, body mass index18, or microbiological data21,38,39) because the NHIS-NCS database lacks such data.
In conclusion, all-cause mortality was significantly higher in patients with bronchiectasis than in those without bronchiectasis, especially in elderly patients and men. Bronchiectasis-related comorbidities played a major role in increased mortality in the bronchiectasis cohort relative to the matched cohort.
Supplementary Information
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, Information and Communications Technologies (No. 2020R1F1A1070468 and 2021M3E5D1A0101517621 to H. Lee; 2019R1G1A1008692 to H. Choi) and the Korea Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 202014X08-03). The funders had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. Part of this article was presented in the form of an abstract at the American College of Chest Physicians Annual Meeting 2020.
Author contributions
Conception and design: H.C., B.Y., J.W.S., H.L.; Data analysis: H.C., B.Y., Y.J.K., S.S., Y.S.J., Y.K., H.Y.P., J.W.S., H.L.; Data interpretation and manuscript writing: H.C., B.Y., S.W.R., Y.M.O., S.J.C., Y.Y., D.W.P., T.S.P., J.Y.M., S.H.K., T.H.K., H.J.Y., J.W.S., H.L.; H.L. is the guarantor of the manuscript.
Data availability
All data extracted in this study are included in the current article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hayoung Choi, Bumhee Yang, Jang Won Sohn and Hyun Lee.
Contributor Information
Jang Won Sohn, Email: jwsohn@hanynag.ac.kr.
Hyun Lee, Email: namuhanayeyo@hanyang.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-86407-8.
References
- 1.Chalmers JD, Chang AB, Chotirmall SH, Dhar R, McShane PJ. Bronchiectasis. Nat. Rev. Dis. Primers. 2018;4:45–45. doi: 10.1038/s41572-018-0042-3. [DOI] [PubMed] [Google Scholar]
- 2.Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin. Pulm. Med. 2005;12:205–209. doi: 10.1097/01.cpm.0000171422.98696.ed. [DOI] [Google Scholar]
- 3.Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142:432–439. doi: 10.1378/chest.11-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ringshausen FC, et al. Bronchiectasis in Germany: A population-based estimation of disease prevalence. Eur. Respir. J. 2015;46:1805–1807. doi: 10.1183/13993003.00954-2015. [DOI] [PubMed] [Google Scholar]
- 5.Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron. Respir. Dis. 2017;14:377–384. doi: 10.1177/1479972317709649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, H. et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur. Respir. J.54, 1900194 (2019). [DOI] [PubMed]
- 7.Aliberti S, et al. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm. Med. 2020;20:15. doi: 10.1186/s12890-020-1050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joish VN, Spilsbury-Cantalupo M, Operschall E, Luong B, Boklage S. Economic burden of non-cystic fibrosis bronchiectasis in the first year after diagnosis from a US health plan perspective. Appl. Health Econ. Health Policy. 2013;11:299–304. doi: 10.1007/s40258-013-0027-z. [DOI] [PubMed] [Google Scholar]
- 9.Goeminne PC, et al. The economic burden of bronchiectasis—known and unknown: A systematic review. BMC Pulm. Med. 2019;19:54. doi: 10.1186/s12890-019-0818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quint JK, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: A population-based cohort study. Eur. Respir. J. 2016;47:186–193. doi: 10.1183/13993003.01033-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seitz AE, et al. Trends and Burden of Bronchiectasis-Associated Hospitalizations in the United States, 1993–2006. Chest. 2010;138:944–949. doi: 10.1378/chest.10-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonnell MJ, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: An international multicentre cohort study. Lancet Respir. Med. 2016;4:969–979. doi: 10.1016/S2213-2600(16)30320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 14.Brusselaers N, Lagergren J. The Charlson Comorbidity Index in Registry-based Research. Methods Inf. Med. 2017;56:401–406. doi: 10.3414/ME17-01-0051. [DOI] [PubMed] [Google Scholar]
- 15.Lee, H. et al. Increased mortality in patients with corticosteroid-dependent asthma: A nationwide population-based study. Eur. Respir. J.54, 1900804 (2019). [DOI] [PubMed]
- 16.Chang, A. B. et al. Task Force report: European Respiratory Society guidelines for the management of children and adolescents with bronchiectasis. Eur. Respir. J. (2021). [DOI] [PubMed]
- 17.Logroscino G, Hesdorffer DC. Methodologic issues in studies of mortality following epilepsy: Measures, types of studies, sources of cases, cohort effects, and competing risks. Epilepsia. 2005;46(Suppl 11):3–7. doi: 10.1111/j.1528-1167.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- 18.Onen ZP, et al. Analysis of the factors related to mortality in patients with bronchiectasis. Respir. Med. 2007;101:1390–1397. doi: 10.1016/j.rmed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Loebinger MR, et al. Mortality in bronchiectasis: A long-term study assessing the factors influencing survival. Eur. Respir. J. 2009;34:843–849. doi: 10.1183/09031936.00003709. [DOI] [PubMed] [Google Scholar]
- 20.Goeminne PC, Scheers H, Decraene A, Seys S, Dupont LJ. Risk factors for morbidity and death in non-cystic fibrosis bronchiectasis: A retrospective cross-sectional analysis of CT diagnosed bronchiectatic patients. Respir. Res. 2012;13:21. doi: 10.1186/1465-9921-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goeminne PC, Nawrot TS, Ruttens D, Seys S, Dupont LJ. Mortality in non-cystic fibrosis bronchiectasis: A prospective cohort analysis. Respir. Med. 2014;108:287–296. doi: 10.1016/j.rmed.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Ellis HC, Cowman S, Fernandes M, Wilson R, Loebinger MR. Predicting mortality in bronchiectasis using bronchiectasis severity index and FACED scores: A 19-year cohort study. Eur. Respir. J. 2016;47:482–489. doi: 10.1183/13993003.01312-2015. [DOI] [PubMed] [Google Scholar]
- 23.Keistinen T, Saynajakangas O, Tuuponen T, Kivela SL. Bronchiectasis: An orphan disease with a poorly-understood prognosis. Eur. Respir. J. 1997;10:2784–2787. doi: 10.1183/09031936.97.10122784. [DOI] [PubMed] [Google Scholar]
- 24.Sin S, et al. Mortality risk and causes of death in patients with non-cystic fibrosis bronchiectasis. Respir. Res. 2019;20:271. doi: 10.1186/s12931-019-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respir. Med. 2010;104:981–985. doi: 10.1016/j.rmed.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Afonso ASM, Verhamme KMC, Sturkenboom MCJM, Brusselle GGO. COPD in the general population: Prevalence, incidence and survival. Respir. Med. 2011;105:1872–1884. doi: 10.1016/j.rmed.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Park, H. Y. et al. Impact of chronic obstructive pulmonary disease on mortality: A large national cohort study. Respirology25, 726–734 (2019). [DOI] [PubMed]
- 28.Martinez-Garcia MA, et al. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140:1130–1137. doi: 10.1378/chest.10-1758. [DOI] [PubMed] [Google Scholar]
- 29.Wedzicha JA, Hurst JR. Structural and functional co-conspirators in chronic obstructive pulmonary disease exacerbations. Proc. Am. Thorac. Soc. 2007;4:602–605. doi: 10.1513/pats.200707-106TH. [DOI] [PubMed] [Google Scholar]
- 30.Ip MS, So SY, Lam WK, Yam L, Liong E. High prevalence of asthma in patients with bronchiectasis in Hong Kong. Eur. Respir. J. 1992;5:418–423. [PubMed] [Google Scholar]
- 31.De Soyza A, et al. Bronchiectasis rheumatoid overlap syndrome is an independent risk factor for mortality in patients with bronchiectasis: A Multicenter Cohort Study. Chest. 2017;151:1247–1254. doi: 10.1016/j.chest.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Chalmers JD, et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: An individual participant data meta-analysis. Lancet Respir. Med. 2019;7:845–854. doi: 10.1016/S2213-2600(19)30191-2. [DOI] [PubMed] [Google Scholar]
- 33.Navaratnam V, et al. Bronchiectasis and the risk of cardiovascular disease: A population-based study. Thorax. 2017;72:161–166. doi: 10.1136/thoraxjnl-2015-208188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleh AD, Kwok B, Brown JS, Hurst JR. Correlates and assessment of excess cardiovascular risk in bronchiectasis. Eur. Respir. J. 2017;50:1701127. doi: 10.1183/13993003.01127-2017. [DOI] [PubMed] [Google Scholar]
- 35.Polverino, E. et al. The overlap between bronchiectasis and chronic airway diseases: State of the art and future directions. Eur. Respir. J.52, 1800328 (2018). [DOI] [PubMed]
- 36.Young RP, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 37.Chung WS, Lin CL, Hsu WH, Kao CH. Increased risk of lung cancer among patients with bronchiectasis: A nationwide cohort study. QJM. 2016;109:17–25. doi: 10.1093/qjmed/hcu237. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Garcia MA, et al. Predicting high risk of exacerbations in bronchiectasis: The E-FACED score. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:275–284. doi: 10.2147/COPD.S121943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann. Am. Thorac. Soc. 2015;12:1602–1611. doi: 10.1513/AnnalsATS.201506-333OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data extracted in this study are included in the current article.