Abstract
Introduction:
Early identification of patients with novel corona virus disease 2019 (COVID-19) who may be at high mortality risk is of great importance.
Methods:
In this retrospective study, we included all patients with COVID-19 at Huanggang Central Hospital from January 23 to March 5, 2020. Data on clinical characteristics and outcomes were compared between survivors and nonsurvivors. Univariable and multivariable logistic regression were used to explore risk factors associated with in-hospital death. A nomogram was established based on the risk factors selected by multivariable analysis.
Results:
A total of 150 patients were enrolled, including 31 nonsurvivors and 119 survivors. The multivariable logistic analysis indicated that increasing the odds of in-hospital death associated with higher Sequential Organ Failure Assessment score (odds ratio [OR], 3.077; 95% confidence interval [CI]: 1.848-5.122; P < 0.001), diabetes (OR, 10.474; 95% CI: 1.554-70.617; P = 0.016), and lactate dehydrogenase greater than 245 U/L (OR, 13.169; 95% CI: 2.934-59.105; P = 0.001) on admission. A nomogram was established based on the results of the multivariable analysis. The AUC of the nomogram was 0.970 (95% CI: 0.947-0.992), showing good accuracy in predicting the risk of in-hospital death.
Conclusions:
This finding would facilitate the early identification of patients with COVID-19 who have a high-risk for fatal outcome.
Keywords: COVID-19, risk factors, predictive model, in-hospital death, mortality
The outbreak of coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread globally. On March 11, the World Health Organization (WHO) declared it as a public health emergency.1
The clinical manifestations of COVID-19 are wide in spectrum, which range from asymptomatic or mildly symptomatic infections to severe pneumonia, acute respiratory distress syndrome (ARDS), and respiratory failure. Patients with severe COVID-19 have a substantial risk of prolonged critical illness and death. However, most previous studies of COVID-19 focused primarily on epidemiological and clinical characteristics; only limited evidence is available on risk factors for poor clinical outcomes.2-5
Here, we present details of the clinical manifestations, laboratory findings, imaging features, and clinical outcomes of patients with COVID-19 admitted to Huanggang Central Hospital, in Hubei. We aim to identify risk factors associated with in-hospital death and construct a clinical risk model to predict the fatal outcome of patients with COVID-19 upon admission.
Patients and Methods
Study Participants
Between January 23, 2020, and March 5, 2020, all patients with confirmed and probable COVID-19 in Huanggang Central Hospital were admitted to the hospital. Our study enrolled 150 consecutive inpatients with confirmed COVID-19 who had a definite outcome (discharged or died) during this period. All patients were diagnosed with COVID-19 pneumonia according to the WHO interim guidance.6 Huanggang Central Hospital, a tertiary hospital with 2500 beds, was a designated hospital for patients with COVID-19. The patients were mainly from Huangzhou District and surrounding towns. The ethics committee of Huanggang Central Hospital approved this study and granted a waiver of informed consent from the study participants.
Data Collection
Data on epidemiological and demographic characteristics, underlying diseases, clinical manifestations, laboratory findings, chest computed tomography (CT) imaging, and outcomes of enrolled 150 patients with COVID-19 were obtained from electronic medical records. Test results at baseline rather than the worst value during hospitalization were used to predict clinical outcome. A team of experienced respiratory clinicians reviewed, abstracted, and cross-checked the data.
SARS-CoV-2 RT-PCR Test
Throat-swab samples were obtained from all patients on admission and tested for the presence of SARS-CoV-2 infection by using real-time reverse transcriptase-polymerase chain reaction assays (RT-PCR) according to a previously described protocol.7 Throat-swab specimens were obtained for RT-PCR re-examination every 2 to 3 d. For patients with repeated PCR tests, the first date of the result was recorded if the patients had consecutive negative results, while the latest result and date were recorded for patients who had inconsistent results of the consecutive tests.
Discharge
The criteria for discharge were absence of fever for at least 7 d, basically normal blood oxygen saturation without supplemental oxygen, substantial improvement in chest CT scans, and 2 consecutive throat-swab samples negative for SARS-CoV-2 RT-PCR test taken at least 24 h apart.
Definitions
Fever was defined as axillary temperature of at least 37.3°C. Sepsis and septic shock were defined according to the 2016 Third International Consensus Definition for Sepsis and Septic Shock.8 Acute kidney injury (AKI) was diagnosed according to the Kidney Disease Improving Global Guidelines (KDIGO).9 ARDS was diagnosed according to the Berlin Definition.10 Acute cardiac injury was diagnosed if the serum levels of cardiac biomarkers (eg, cardiac troponin I) were above the 99th percentile upper reference limit or if new abnormalities were shown in electrocardiography and echocardiography.7 The illness severity of COVID-19 was defined according to the Chinese management guideline for COVID-19 (version 6.0).11 Coagulopathy was defined as a 3-s extension of prothrombin time or a 5-s extension of activated partial thromboplastin time. Hypoproteinemia was defined as blood albumin of less than 25 g/L. Exposure history was defined as exposure to people with confirmed SARS-CoV-2 infection or residence or travel history in Wuhan in the last month, especially in the last 2 wk. The worldwide accepted pneumonia severity scoring systems such as CURB-65.12] and Pneumonia Severity Index (PSI)13] were used to assess pneumonia severity. Critical illness evaluation systems, including Sequential Organ Failure Assessment (SOFA)14] and Acute Physiology and Chronic Health Evaluation II (APACHE II),15 were used to assess disease severity.
Statistical Analysis
Categorical variables were presented as numbers (percentages) and analyzed using χ2 test or Fisher’s exact test. Continuous variables with skewed distribution were shown as median (interquartile ranges) and analyzed using Mann-Whitney U test. If the missingness of continuous variables did not exceed 30%, the median was used to fill in. The variables, including baseline characteristics, laboratory findings, were excluded those with more than 30% missing values and unconfirmed indicators (eg, exposure, which was self-reported). Logistic regression was used for univariable and multivariable analyses to explore the risk factors associated with in-hospital death. Variables with P value <0.1 entered into logistic multivariable analysis. Continuous variables were dichotomized. It was determined that whether or not with multicollinearity among independent variables. Forward-stepwise regression was used to select variables. Area under curve (AUC), sensitivity, and specificity were analyzed using the receiver operating characteristic curve (ROC). A nomogram was established based on the results of multivariable analysis. The C-index, decision curve, and clinical impact curve were used to verify the nomogram. A 2-sided P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 25.0), R program (version 3.5.1), and Graphpad Prism (version 8.0).
Results
Demographics and Clinical Characteristics
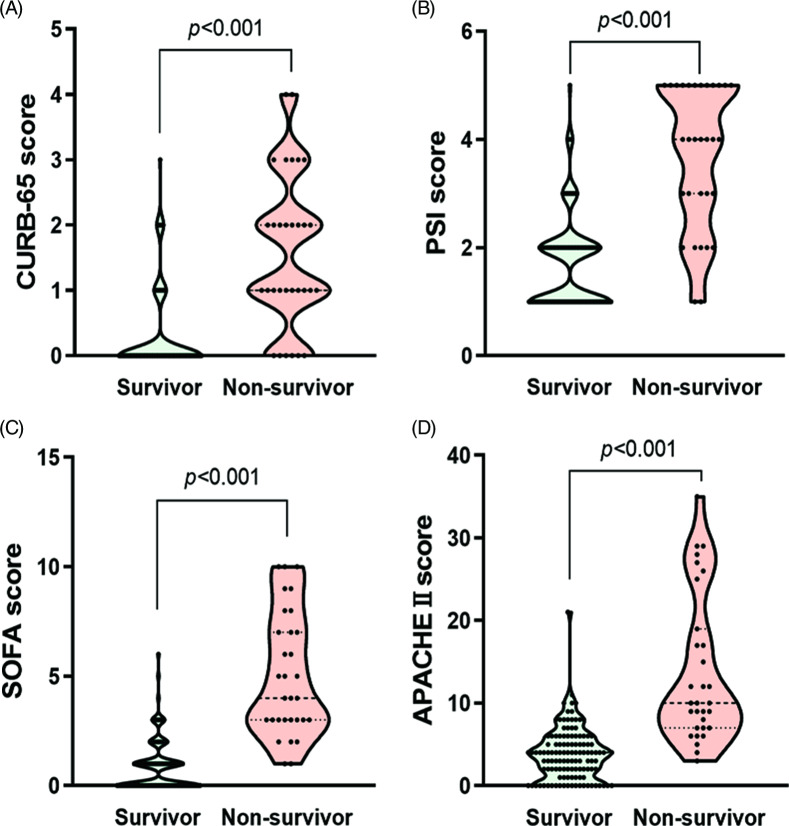
A total of 150 patients were enrolled in this study, including 31 dead patients and 119 discharged patients. As shown in Table 1, the nonsurvivors were older than the survivors (73 [IQR 62-79] y vs 48 [IQR 37-57]) y, P < 0.001). There were more male nonsurvivors (61.3%), while the survivors were dominated by females (58.0%). We noted that more nonsurvivors had hypertension, cardiovascular diseases, and cerebrovascular diseases than the survivors. The most common symptoms on admission were fever and cough, followed by fatigue. Dyspnea was more common in the nonsurvivors. Approximately one-third of the patients had a definite history of exposure before illness onset, and the median time from exposure to illness onset was 5.5 (IQR 4.8-10) d. General patients (83.2%) accounted for the majority in the survivor group, while severe and critical patients accounted for 93.5% in the nonsurvivor group. In addition, the nonsurvivors had higher CURB-65, PSI, SOFA, and APACHE II scores (Figure 1).
Table 1.
Demographic and clinical characteristics of patients with COVID-19 on admission
| Total (n = 150) | Non-survivor (n = 31) | Survivor (n = 119) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 52 (39.5-66.5) | 73 (62-79) | 48 (37-57) | <0.001 |
| Sex, n (%) | ||||
| Male | 69 (46.0%) | 19 (61.3%) | 50 (42.0%) | 0.055 |
| Female | 81 (54.0%) | 12 (38.7%) | 69 (58.0%) | |
| Underlying diseases, n (%) | 54 (36.0%) | 23 (74.2%) | 31 (26.1%) | <0.001 |
| Hypertension | 25 (16.7%) | 14 (45.2%) | 11 (9.2%) | <0.001 |
| Diabetes | 17 (11.3%) | 11 (35.5%) | 6 (5.0%) | <0.001 |
| Cardiovascular disease | 12 (8.0%) | 9 (29.0%) | 3 (2.5%) | <0.001 |
| Cerebrovascular diseases | 10 (6.7%) | 7 (22.6%) | 3 (2.5%) | <0.001 |
| Chronic respiratory disease | 6 (4.0%) | 3 (9.7%) | 3(2.5%) | 0.195 |
| Chronic kidney disease | 2 (1.3%) | 2 (6.5%) | 0 (0.0%) | 0.042 |
| Malignancy | 3 (2.0%) | 2 (6.5%) | 1 (0.8%) | 0.109 |
| Others | 13 (8.7%) | 3 (9.8%) | 10 (8.4%) | 1.000 |
| Current smoker, n (%) | 12 (8.0%) | 2 (6.5%) | 10 (8.4%) | 1.000 |
| Exposure history, n (%) | 50 (33.3%) | 8 (25.8%) | 42 (35.3%) | 0.318 |
| Clinical characteristics | ||||
| Symptom, n (%) | ||||
| Fever (temperature ≥37·3°C) | 115 (76.7%) | 26 (83.9%) | 89 (74.8%) | 0.287 |
| Cough | 104 (69.3%) | 22 (71.0%) | 82 (68.9%) | 0.825 |
| Sputum production | 49 (32.7%) | 11 (35.5%) | 38 (31.9%) | 0.707 |
| Chest discomfort | 53 (35.3%) | 15 (48.4%) | 38 (31.9%) | 0.088 |
| Dyspnea | 21 (14.0%) | 11 (35.5%) | 10 (8.4%) | <0.001 |
| Fatigue | 86 (57.3%) | 27 (87.1%) | 59 (49.6%) | <0.001 |
| Myalgia | 42 (28.0%) | 6 (19.4%) | 36 (30.3%) | 0.229 |
| Diarrhea | 12 (8.0%) | 2 (6.5%) | 10 (8.4%) | 1.000 |
| Respiratory rate, breaths/min | 20 (19-21) | 21 (20-25) | 20 (19-21) | 0.016 |
| Pulse, beats/min | 80 (75-88) | 86 (74-106) | 80 (75-86) | 0.134 |
| Mean arterial pressure, mmHg | 93 (89-98) | 97 (90-108) | 93 (88-97) | 0.048 |
| Disease severity status, n (%) | ||||
| General | 101 (67.3%) | 2 (6.5%) | 99 (83.2%) | |
| Severe | 40 (26.7%) | 20 (64.5%) | 20 (16.8%) | <0.001 |
| Critical | 9 (6.0%) | 9 (29.0%) | 0 (0.0%) | |
| CURB-65 score, n (%) | ||||
| 0-1 | 126 (84.0%) | 16 (51.6%) | 110 (92.4%) | |
| 2-3 | 22 (14.7%) | 13 (41.9%) | 9 (7.6%) | <0.001 |
| 4-5 | 2 (1.3%) | 2 (6.5%) | 0 (0.0%) | |
| PSI score, n (%) | ||||
| I | 61 (40.7%) | 2 (6.5%) | 59 (49.6%) | |
| II-III | 65 (43.3%) | 10 (32.2%) | 55 (46.2%) | <0.001 |
| IV-V | 24 (16.0%) | 19 (61.3%) | 5 (4.2%) | |
| SOFA score | 1 (0-3) | 4 (3-7) | 1 (0-1) | <0.001 |
| APACHE||score | 4 (2-7.3) | 10 (7-19) | 4 (2-6) | <0.001 |
| Time from exposure to illness onset, days* | 5.5 (4.8-10) | 6 (4.3-12.3) | 5.5 (4.5-10) | 0.975 |
| Time from illness onset to hospital admission, days | 7 (5-10) | 7 (6-10) | 7 (5-10) | 0.286 |
Abbreviations: PSI, Pneumonia Severity Index; SOFA, Sequential Organ Failure Assessment; APACHE||, Acute Physiology and Chronic Health Evaluation.
Data available for 30 patients, including 4 non-survivors and 26 survivors.
Figure 1.
Comparison of pneumonia severity score and critical illness score between survivors and nonsurvivors. Violin diagram shows the higher CURB-65 score (A), PSI score (B), SOFA score (C), and APACHE II score (D) in the nonsurvivors. PSI, Pneumonia Severity Index; SOFA, Sequential Organ Failure Assessment; APACHE II, Acute Physiology and Chronic Health Evaluation II.
Laboratory Findings and Imaging Features
Laboratory findings on hospital admission are summarized in Table 2. White blood cell counts and neutrophil counts were elevated, while lymphocyte counts and platelet counts were decreased in the nonsurvivor group compared with those in the survivor group. The nonsurvivors showed higher levels of total bilirubin, blood ureanitrogen (BUN), creatinine, fasting blood glucose, lactate dehydrogenase (LDH), and D-dimer, but lower albumin levels than the survivors. C-Reactive protein and procalcitonin (PCT) were also different between the nonsurvivors and survivors.
Table 2.
Laboratory and radiographic findings of patients with COVID-19 on admission
| Total (n = 150) | Non-survivor (n = 31) | Survivor (n = 119) | P-Value | |
|---|---|---|---|---|
| Laboratory findings | ||||
| Hematologic | ||||
| White blood cell counts, × 109 per L | 5.7 (4.0-8.2) | 9.1 (5.2-12.3) | 5.5 (3.9-7.2) | <0.001 |
| Lymphocyte counts, × 109 per L | 1.1 (0.7-1.6) | 0.6 (0.4-0.8) | 1.3 (0.9-1.7) | <0.001 |
| Neutrophil counts, × 109 per L | 3.8 (2.3-6.2) | 8.5 (4.1-10.9) | 3.5 (2.1-5.2) | <0.001 |
| Hemoglobin, g per L | 124.5 (117.0-136.0) | 128.0 (123.0-136.5) | 123.0 (116.0-136.0) | 0.298 |
| Hematocrit, % | 38.3 (35.6-41.9) | 37.9 (35.6-41.8) | 38.3 (35.6-42.2) | 0.913 |
| Platelet counts, × 109 per L | 191.5 (144.3-250.0) | 121.0 (104.0-193.5) | 198.0 (161.0-257.0) | <0.001 |
| Biochemical | ||||
| Aspartate aminotransferase, U/L | 19.4 (16.0-31.8) | 31.0 (20.5-50.0) | 18.0 (15.0-26.3) | <0.001 |
| Alanine aminotransferase, U/L | 19.0 (13.0-32.0) | 24.0 (19.5-34.0) | 17.0 (12.0-32.0) | 0.044 |
| Total bilirubin, μmol/L | 11.4 (8.3-17.5) | 19.3 (10.3-28.8) | 10.6 (8.1-15.6) | <0.001 |
| Albumin, g/L | 37.8 (34.6-41.7) | 33.3 (29.7-35.5) | 39.1 (35.9-42.2) | <0.001 |
| Globulin, g/L | 27.8 (25.1-30.8) | 30.3 (25.4-34.8) | 27.4 (25.1-29.9) | 0.018 |
| Urea nitrogen, mmol/L | 4.3 (3.2-6.2) | 9.1 (4.6-13.3) | 4.1 (3.1-5.0) | <0.001 |
| Creatinine, μmol/L | 73.5 (57.5-90.1) | 88.7 (72.9-108.0) | 70.2 (54.6-84.0) | 0.001 |
| Fasting blood glucose, mmol/L | 5.8 (5.0-7.0) | 7.8 (5.9-12.9) | 5.8 (4.8-6.4) | <0.001 |
| Lactate dehydrogenase, U/L | 208.5 (178.3-270.8) | 423.0 (208.5-571.0) | 208.5 (168.5-230.0) | <0.001 |
| Coagulation function | ||||
| Prothrombin time, s* | 12 (11.4-12.7) | 12.7 (12.0-13.6) | 11.9 (11.1-12.5) | <0.001 |
| Activated partial thromboplastin time, s* | 31.2 (29.3-33.0) | 31.0 (27.5-34.1) | 31.2 (29.5-32.8) | 0.595 |
| D-dimer, μg/L* | 196.0 (101.3-384.3) | 638.0 (258.5-5189.5) | 173.0 (88.5-264.0) | <0.001 |
| Infection-related indices | ||||
| Erythrocyte sedimentation rate, mm/h† | 29.0 (18.5-43.5) | 29.0 (28.5-44.5) | 29.0 (17.0-41.5) | 0.165 |
| C-Reactive protein, mg/L ‡ | 9.2 (1.0-47.3) | 57.6 (31.5-97.4) | 7 (0.5-30.8) | <0.001 |
| Procalcitonin, ng/mL, n (%)§ | ||||
| <0.5 | 77 (83.7%) | 8 (53.3%) | 69 (89.6%) | |
| 0.5-2 | 10 (10.9%) | 5 (33.3%) | 5 (6.5%) | 0.007 |
| >2 | 5 (5.4%) | 2 (13.3%) | 3 (3.9%) | |
| Imaging features | ||||
| Ground glass opacification, n (%) | 129 (86.0%) | 29 (93.5%) | 100 (84.0%) | 0.043 |
| Pleural effusion, n (%) | 10 (6.7%) | 7 (22.6%) | 3 (2.5%) | <0.001 |
| Unilateral pulmonary infiltration, n (%) | 26 (17.3%) | 1 (3.2%) | 25 (21.0%) | 0.020 |
| Bilateral pulmonary infiltration, n (%) | 124 (82.7%) | 30 (96.8%) | 94 (79.0%) | 0.020 |
Data available for 141 patients, missing for 9 survivors.
Data available for 111 patients, including 21 non-survivors and 90 survivors.
Data available for 143 patients, including 25 non-survivors and 118 survivors.
Data available for 93 patients, including 15 non-survivors and 78 survivors.
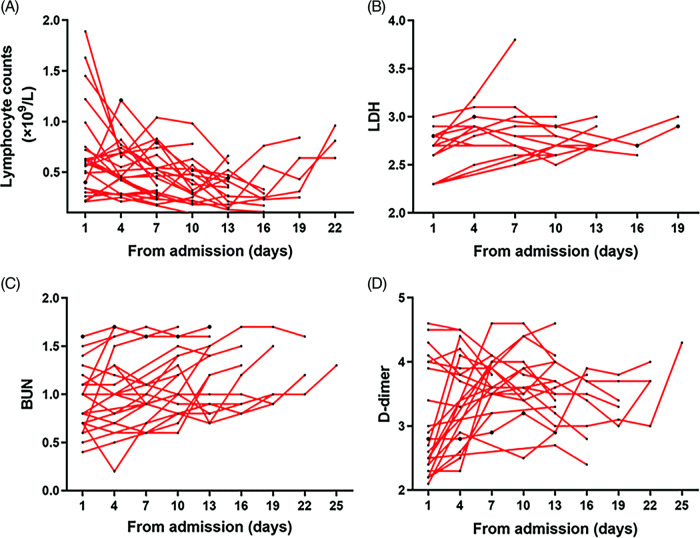
During the period from admission to death, laboratory indicators showed a dynamic change in the nonsurvivors, especially in the levels of lymphocyte counts, LDH, BUN, and D-dimer (Figure 2). The serial biomarker results were based on a very small subcohort because most patients were not continuously monitored during hospitalization.
Figure 2.
Temporal changes in laboratory markers from illness onset to death in nonsurvivors. (A) Line chart shows a dynamic decrease in lymphocyte counts after hospitalization. (B) LDH, (C) BUN, (D) D-dimer values basically show an upward trend throughout the clinical course. Lymphocyte counts, BUN, and D-dimer were obtained from 24 nonsurvivors. LDH was obtained from 15 nonsurvivors. LDH, BUN, and D-dimer values were log10-transformed for analysis, due to the wide range of variation. LDH, lactate dehydrogenase; BUN, blood urea nitrogen.
The most common imaging features were ground glass opacification (86.0%) and bilateral pulmonary infiltration (82.7%). Pleural effusion (22.6% vs 2.5%, P < 0.001) occurred more frequently in the nonsurvivors.
Clinical Outcomes
As shown in Table 3, of the 150 patients, sepsis was the most frequently observed complication (37.3%), followed by respiratory failure (26.7%). All of the nonsurvivors experienced sepsis. The common complications of the nonsurvivors included respiratory failure (93.5%), ARDS (83.9%), coagulopathy (77.4%), acidosis (64.5%), septic shock (61.3%), and AKI (61.3%). For the nonsurvivors, the median time from illness onset to respiratory failure was 9 (interquartile ration [IQR], 7-10) d; the median time from illness onset to ARDS was 10.5 (IQR 9-12) d; the median time from illness onset to septic shock was 18 (IQR, 14-23) d; the median time from illness onset to AKI was 16 (IQR, 10-22) d.
Table 3.
Clinical outcomes
| Total (n = 150) | Non-survivor (n = 31) | Survivor (n = 119) | P-Value | |
|---|---|---|---|---|
| Complication, n (%) | ||||
| Sepsis | 56 (37.3%) | 31 (100.0%) | 25 (21.0%) | <0.001 |
| Septic shock | 19 (12.7%) | 19 (61.3%) | 0 (0.0%) | <0.001 |
| Respiratory failure | 40 (26.7%) | 29 (93.5%) | 11 (9.2%) | <0.001 |
| ARDS | 26 (17.3%) | 26 (83.9%) | 0 (0.0%) | <0.001 |
| Acute cardiac injury | 15 (10.0%) | 15 (48.4%) | 0 (0.0%) | <0.001 |
| Heart failure | 13 (8.7%) | 12 (38.7%) | 1 (0.8%) | <0.001 |
| Acute kidney injury | 21 (14.0%) | 19 (61.3%) | 2 (1.7%) | <0.001 |
| Acidosis | 20 (13.3%) | 20 (64.5%) | 0 (0.0%) | <0.001 |
| Hypoproteinemia | 20 (13.3%) | 18 (58.1%) | 2 (1.7%) | <0.001 |
| Coagulopathy | 26 (17.3%) | 24 (77.4%) | 2 (1.7%) | <0.001 |
| Time from illness onset to respiratory failure, days | 9 (6.3-11) | 9 (7-10) | 6 (3-17) | 0.637 |
| Time from illness onset to ARDS, days | – | 10.5 (9-12) | – | – |
| Time from illness onset to septic shock, days | – | 18 (14-23) | – | – |
| Time from illness onset to acute kidney injury, days | – | 16 (10-22) | – | – |
| Time from illness onset to death or discharge, days | 24 (18-30) | 22 (13-25) | 25 (19-30) | 0.019 |
| Hospital length of stay, days | 16 (12-21) | 13 (6-17) | 17 (12-23) | 0.002 |
| Duration of viral shedding after COVID-19 onset, days | 7 (4-10) | 12 (7.8-15) | 6 (4-10) | <0.001 |
Abbreviations: ARDS, acute respiratory distress syndrome.
The median time from illness onset to discharge was 25 (IQR, 19-30) d, whereas the median time to death was 22 (IQR 13-25) d. The median hospital durations of stay were 17 (IQR, 12-23) d for the survivors and 13 (IQR, 6-17) d for the nonsurvivors, respectively. The median duration of viral shedding of the nonsurvivors were longer than that of the survivors (12 [IQR, 7.8-15] d vs 6 [IQR, 4-10]) d; P < 0.001), even the virus was continuously detectable until death in 17 nonsurvivors.
Risk Factors for In-hospital Death
On the univariable analysis, the risk factors associated with in-hospital death were age, hypertension, diabetes, and cardiovascular disease. The elevated respiratory rate, PSI score, SOFA score, APACHE II score, white blood cell counts, total bilirubin, BUN, creatinine, LDH, D-dimer, and the decreased white blood cell counts, lymphocyte counts, platelet counts, and albumin were also associated with in-hospital death (Table 4).
Table 4.
Risk factors for in-hospital mortality
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value | OR | 95% CI | P-Value | |
| Demographics | ||||||
| Age, years | ||||||
| <65 | 1 (Ref) | |||||
| ≥65 | 11.783 | 4.768—29.119 | <0.001 | |||
| Underlying diseases | ||||||
| Hypertension | 8.806 | 3.156—20.716 | <0.001 | |||
| Diabetes | 10.358 | 3.439—31.196 | <0.001 | 10.474 | 1.554—70.617 | 0.016 |
| Cardiovascular disease | 15.818 | 3.964—63.118 | <0.001 | |||
| Clinical characteristics | ||||||
| Respiratory rate, breaths per min | ||||||
| ≤24 | 1 (Ref) | |||||
| >24 | 5.000 | 1.783—14.021 | 0.002 | |||
| PSI score* | 4.128 | 2.600—6.553 | <0.001 | |||
| SOFA score* | 3.212 | 2.079—4.961 | <0.001 | 3.077 | 1.848—5.122 | <0.001 |
| APACHEII score* | 1.502 | 1.268—1.779 | <0.001 | |||
| Laboratory findings | ||||||
| White blood cell counts, × 109 per L | ||||||
| 4—10 | 1 (Ref) | |||||
| <4 | 0.081 | 0.026—0.248 | <0.001 | |||
| >10 | 0.056 | 0.014—0.231 | <0.001 | |||
| Lymphocyte counts, × 109 per L | ||||||
| ≥0.8 | 1 (Ref) | |||||
| <0.8 | 12.676 | 5.011—32.068 | <0.001 | |||
| Platelet counts, × 109 per L | ||||||
| ≥100 | 1 (Ref) | |||||
| <100 | 6.900 | 1.812—26.273 | 0.005 | |||
| Total bilirubin, μmol/L | ||||||
| ≤20 | 1 (Ref) | |||||
| >20 | 8.359 | 3.321—21.042 | <0.001 | |||
| Albumin, g/L | ||||||
| ≥35 | 1 (Ref) | |||||
| <35 | 7.589 | 3.195—18.025 | <0.001 | |||
| Urea nitrogen, mmol/L | ||||||
| <11 | 1 (Ref) | |||||
| ≥11 | 18.413 | 4.672—72.560 | <0.001 | |||
| Creatinine, μmol/L | ||||||
| ≤133 | 1 (Ref) | |||||
| >133 | 11.250 | 2.068—61.213 | 0.005 | |||
| Lactate dehydrogenase, U/L | ||||||
| ≤245 | 1 (Ref) | 13.169 | 2.934—59.105 | 0.001 | ||
| >245 | 22.927 | 7.918—66.384 | <0.001 | |||
| D-dimer, μg/L | ||||||
| ≤500 | 1 (Ref) | |||||
| >500 | 9.107 | 3.700—22.417 | <0.001 | |||
Abbreviations: PSI, Pneumonia Severity Index; SOFA, Sequential Organ Failure Assessment; APACHE||, Acute Physiology and Chronic Health Evaluation||.
Per 1 unit increase.
The multivariable logistic analysis indicated that higher SOFA score (odds ratio [OR], 3.077; 95% confidence interval [CI]: 1.848-5.122; P < 0.001), history of diabetes (OR, 10.474; 95% CI: 1.554-70.617; P = 0.016), and LDH greater than 245 U/L (OR, 13.169; 95% CI: 2.934-59.105; P = 0.001) were risk factors for in-hospital death (Table 4). The predictive equation for in-hospital death: logit(P) = −5.594 + 1.124 × SOFA + 2.578 × LDH ([LDH ≤ 245] = 0, [LDH > 245] = 1) + 2.349 × diabetes (without diabetes = 0, with diabetes = 1), P = elogit(P) /1 + elogit(P).
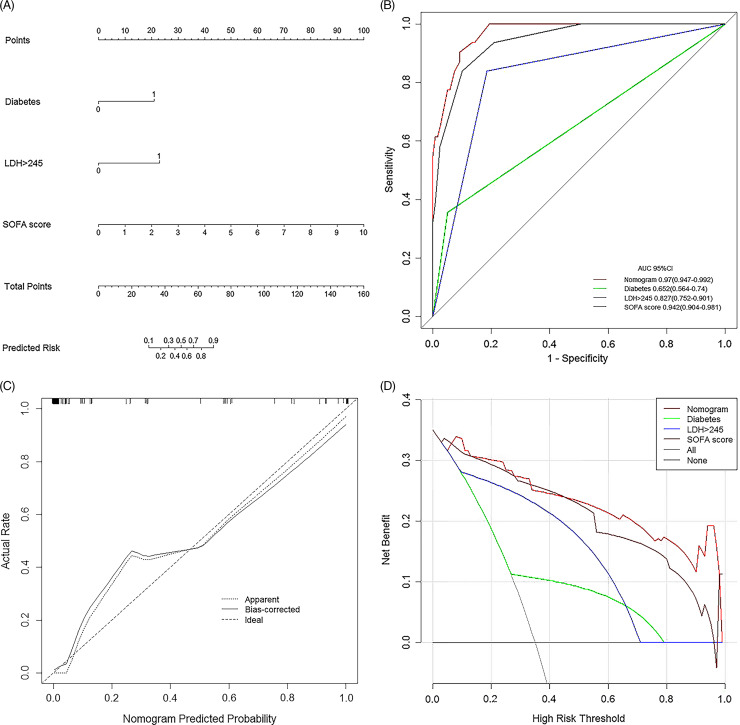
Development and Validation of a Nomogram for Predicting In-hospital Death
The independent predictors were used to establish a nomogram, and the points of each variable are shown in Figure 3A. The ROC curve analysis revealed that the AUC of the nomogram was 0.970 (95% CI: 0.947-0.992), showing good accuracy in predicting the risk of in-hospital death (Figure 3B). The sensitivity was 90.3%, and the specificity was 90.8%. The favorable calibration curve indicated that the prediction by the nomogram was highly consistent with the actual observation (Figure 3C). Moreover, the clinical impact curve (Figure 3D) indicated that the nomogram had good net benefits for the identification of the fatal outcome of patients with COVID-19.
Figure 3.
Prediction of in-hospital death of patients with COVID-19. A, Prognostic nomogram for predicting in-hospital death risk of patients with COVID-19. Prognostic patient’s value is located on each variable axis, and a line is drawn upward to determine the number of point nomogram for predicting in-hospital death risk of patients with COVID-2019. B, Area under the receiver operating characteristic curve (AUC) of SOFA score, diabetes, LDH, and the nomogram were 0.942, 0.827, 0.652, and 0.970, respectively. Calibration curve (C) and clinical impact curve of the nomogram (D), in which the predicted probability of in-hospital death was highly consistent with the actual observation and had good net benefit.
Fifty-two of 512 hospitalized patients with COVID-19 between March 5, 2020, and May 1, 2020, including all 12 dead patients and 40 discharged patients who were randomly selected according to hospitalization number, were enrolled to perform an external validation. The AUC of the nomogram was 0.923 (95% CI: 0.828-1.000), and the predictive accuracy was 0.942. The model was confirmed to be reliable.
Discussion
Most of patients with COVID-19 present with mild flu-like symptoms and recover quickly. However, many severe patients show rapid progression and develop multiple organ dysfunction, even death, indicating that recognition of risk factors are essential to identify those potentially needing critical care and management at an early stage. Several studies focused on risk factors associated with poor prognosis, including elder age, comorbidities, lymphopenia, D-dimer greater than 1 μg/L, elevated CRP, high LDH, high hypersensitive troponin I, high interleukin-6, hyperglycemia, and hypoproteinemia. However, these clinical and laboratory indicators are independent,2,5,16-18 which cannot comprehensively reflect the prognosis of COVID-19.
To our knowledge, no mature and reliable scoring system has been established to assess the risk of death in hospital with COVID-19. SOFA score is a low-cost, 2-min bedside clinical tool introduced to facilitate early recognition of sepsis and multi-organ dysfunction. SOFA score is a consistent and convincing tool that can be used across different patient populations and clinical settings. In the current study, we determined that sepsis occurred in 37.3% of patients with COVID-19 due to viral infection. We also identified SOFA score as an independent risk factor for death in adults who were hospitalized due to COVID-19.
Reports from the Chinese Center for Disease Control and Prevention from 44,672 confirmed cases of COVID-19 showed that patients with diabetes had a 3-fold higher overall case-fatality rate than those without diabetes (7.3% vs 2.3%, respectively).19 According to our results, we believed that increasing odds of death in hospital was associated with history of diabetes. The elevated LDH was confirmed as a predictive biomarker of death, consistent with those in previous reports.16,20 Furthermore, our study developed a nomogram model based on risk factors selected by multivariable logistic regression analysis to accurately predict fatal outcomes of patients with COVID-19. The nomogram model performed well in predicting in-hospital death, supported by external validation.
Few studies reported the long shedding of SARS-CoV-2 RNA, especially in severe patients.2,21 Among male patients, delayed admission to hospital after illness onset, and invasive mechanical ventilation were associated with prolonged SARS-CoV-2 RNA shedding.22 In the current study, we found that the duration of SARS-CoV-2 viral shedding had median values of 6 d in the survivor group and 12 d in the nonsurvivor group; 17 patients were nucleic acid positive until death. Although most studies have used qualitative or quantitative PCR tests as a diagnostic marker for infectious SARS-CoV-2, caution is required when applying such data to assess the duration of viral shedding and infection potential because PCR does not distinguish between infectious virus and noninfectious nucleic acid.
COVID-19 mainly injures the respiratory system, and some patients rapidly progress to ARDS. We found that the incidence of ARDS was 17.3%, and the median time from illness onset to ARDS was 10.5 (IQR, 9-12) d, consistent with previous reports.2,23 At present, the mechanisms of COVID-19-related ARDS remain unclear. The entry of pathogenic SARS-CoV-2 in humans leads to the activation of inflammatory cells, specifically CD4 lymphocytes, which subsequently transform into T helper 1 (Th1) cells. Activated inflammatory cells (Th1 cells and macrophages) enter the pulmonary circulation and induce cytokines (ie, “cytokine storm”), which lead to rapid and wide-spread damage of the pulmonary epithelium and alveolar cells.
A recent study of the single-cell transcriptome analysis found that ACE2 genes were significantly expressed in podocytes and proximal convoluted tubules as potential hosts cells targeted by SARS-CoV-2; this work suggests that the kidney might be an important target organ for SARS-CoV-2.24 A retrospective cohort study reported that mortality was higher in COVID-19 with AKI versus COVID-19 patients without AKI (60.5% vs 27.4%), and AKI was an independent predictor of mortality.25 Our study revealed that AKI incidence rates of 14.0% in all hospitalized patients and 61.3% in the nonsurvivors. These data provide robust evidence to support that patients with COVID-19 should be closely monitored for the development of AKI and measures taken to prevent it.
This study has several limitations. First, our study was conducted in 1 hospital, thereby potentially limiting the generalizability to hospital settings, especially in terms of the demographic characteristics of the patient population and the external validation of the prediction models. Second, missing data on some variables, such as erythrocyte sedimentation rate (ESR), PCT, and cardiac troponin I, may cause bias in the estimation and reduce the representativeness of the samples. Finally, interpretation of our findings might be limited by the small sample size.
In conclusion, we found that high SOFA score, history of diabetes, and LDH greater than 245 U/L were risk factors for in-hospital death of adult patients with COVID-19. The nomogram proposed in our study objectively predicted the prognosis of patients with COVID-19.
Author Contributions
Yuanyuan Niu, Zan Zhan, and Jianfeng Li contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interests.
References
- 1. World Health Organization. Coronavirus disease 2019 (COVID-19): situation report–37. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200226-sitrep-37-covid-19.pdf?sfvrsn=6126c0a4_2. Accessed February 26, 2020.
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. https://www.who.int/publications-detail/clinical-management-of-severeacute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected. Accessed March 5, 2020.
- 7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 10. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 11. National Health Commission of the People’s Republic of China. Chinese management guideline for COVID-19 (version 6.0). http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a832/6dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817. Accessed February 19, 2020.
- 12. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402 [DOI] [PubMed] [Google Scholar]
- 13. Lim WS, van der Eerden MM, Laing R, et al. Defining community-acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vincent J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 15. Knaus WA, Draper EA, Wagner DP, et al. APACHE||: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 16. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71(16):2089–2098. doi: 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J, Yu M, Tong S, et al. Predictive factors for disease progression in hospitalized patients with coronavirus disease 2019 in Wuhan, China. J Clin Virol. 2020;127:104392. doi: 10.1016/j.jcv.2020.104392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (Covid-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 20. Cecconi M, Piovani D, Brunetta E, et al. Early predictors of clinical deterioration in a cohort of 239 patients hospitalized for covid-19 infection in Lombardy, Italy. J Clin Med. 2020;9(5):1548. doi: 10.3390/jcm9051548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis. 2020;71(16):2249–2251. doi: 10.1093/cid/ciaa460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis. 2020;71:799–806. doi: 10.1093/cid/ciaa351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan XW, Xu D, Zhang H, et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46(6):1114–1116. doi: 10.1007/s00134-020-06026-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kolhe NV, Fluck RJ, Selby NM, et al. Acute kidney injury associated with COVID-19: a retrospective cohort study. PLoS Med. 2020;17(10):e10003406. doi: 10.1371/journal.pmed.1003406 [DOI] [PMC free article] [PubMed] [Google Scholar]