Abstract
Background:
Low vitamin D levels along with high-intensity athletic training may put an athlete at increased risk for a stress fracture.
Purpose:
To assess whether supplementation with vitamin D is associated with a reduced risk of stress fractures in college athletes. We also assessed differences in vitamin D levels among athletes participating in outdoor versus indoor sports.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
The study participants included 802 National Collegiate Athletic Association Division I intercollegiate athletes (497 men and 305 women) on a sports team for at least 1 semester from 2012 to 2018. All athletes who had a baseline vitamin D level in their medical record were included. Athletes with vitamin D levels <40 ng/mL were given vitamin D supplements. We assessed differences in the rate of stress fracture among those who maintained or improved vitamin D levels to ≥40 ng/mL and those who did not, as well as differences in average baseline vitamin D levels by sport type (indoor vs outdoor).
Results:
The rate of stress fracture was 12% higher (95% CI, 6-19; P < .001) for those who remained low in vitamin D compared with those who were low at baseline but improved their vitamin D status to ≥40 ng/mL. The rate of stress fracture was also 12% higher (95% CI, 5-18; P < .001) for those who had low vitamin D levels compared with those who maintained normal levels. The mean baseline vitamin D values were significantly higher for men participating in outdoor sports versus indoor sports. For men, the mean vitamin D level was 5.7 ng/mL higher (95% CI, 0.9-10.5; P = .01) in outdoor athletes. For women, the mean vitamin D level was 3.7 ng/mL higher (95% CI, –0.58 to 8.03; P < .04) for outdoor versus indoor sports.
Conclusion:
Study results indicated that correcting low serum vitamin D levels reduces the risk of stress fracture. This study also presented evidence that athletes who participate in indoor sports may be at greater risk for vitamin D deficiency than those who compete in outdoor sports.
Keywords: stress fractures, epidemiology, student-athlete
There are over 460,000 National Collegiate Athletic Association (NCAA) student-athletes participating in college sports annually.15 As new college athletes begin practices, they may be exposed to more strenuous workouts that continue over their career, potentially leading to injuries including stress fractures. Stress fractures are bone injuries related to repeated mechanical stress on the bones over time that occur over a spectrum, from stress reactions (microdamage of the bone with edema) to stress fractures, where small bone fractures are visible on radiographs or magnetic resonance imaging (MRI). The prevalence of stress fractures in athletes ranges from 0.7% to 21%1,3 in published reports.
Data from the NCAA Injury Surveillance Program from 2004 to 2014, during which over 11 million athletes were studied, found the sports with the highest rates of stress fractures for women to be cross-country, gymnastics, and outdoor track.13 For men, it was cross-country running.18 Known risk factors for bone-related stress injuries include female sex, low bone density, female athlete triad (energy deficiency, menstrual irregularities, and low bone density), relative energy deficiency in sports, and other nutritional deficiencies.7 Stress fractures result in significant pain for the athlete, loss of training time, and loss in the ability to compete. With an average treatment duration of 6 to 8 weeks after diagnosis, a stress fracture may significantly limit an athlete’s participation in his or her respective sport.
Vitamin D is important for bone health, contributing to bone mineralization and calcium regulation. Low levels of vitamin D lead to significantly decreased levels of calcium absorption within the intestines.10 Decreased calcium absorption has been shown to increase parathyroid hormone levels, which enhance reabsorption of calcium within the kidneys but also activate osteoclasts that dissolve the collagen matrix in bone, increasing the risk of stress fractures.12 Vitamin D deficiency can stem from reduced skin synthesis of vitamin D from sunlight, insufficient intake, or inadequate absorption of dietary vitamin D. People with darker skin are known to have less absorption of vitamin D from sunlight.2,5
The prevalence of low vitamin D levels in athletes within 1 meta-analysis was 56%.9 Low vitamin D levels along with high-intensity athletic training may put an athlete at increased risk for a stress fracture.10 Supplementation with both calcium and vitamin D has been shown to reduce stress fractures among female military recruits.11 In a study of US Navy recruits, there was double the risk of stress fractures of the tibia and fibula in women with serum 25-hydroxyvitamin D concentrations <20 ng/mL compared with those with concentrations ≥40 ng/mL.4 Based on these data, we chose 40 ng/mL as the lower limit of the optimal level. Optimal serum concentrations of 25-hydroxyvitamin D have yet to be established. Although supplementation in athletes with low vitamin D has been recommended, whether it reduces the risk of stress fracture among college athletes has not been confirmed. The objective of this study was to assess whether low levels of serum vitamin D were associated with an increased risk of stress fractures in college athletes. Furthermore, would increasing low levels of vitamin D mitigate or reduce the increased risk? In addition, this study investigated differences in vitamin D levels by sport type (indoor vs outdoor), race, and sex.
Methods
Study Setting and Participants
We used a prospective cohort study design of NCAA Division I intercollegiate student-athletes at a single university from 2012 to 2018. All athletes competing on an athletic team completed a medical screening upon admission. At this examination, bloodwork was tested for total serum 25-hydroxyvitamin D. Those with low serum vitamin D levels (<40 ng/mL) were instructed to follow up with a team physician and were provided a vitamin D supplement. If the level was <20 ng/mL, vitamin D3 50,000 IUs weekly was given for 8 weeks, a commonly prescribed dosage.11 If the level measured 20 to 39 ng/mL, vitamin D3 30,000 IUs weekly was provided for 8 weeks. This dosage was similar to the Institute of Medicine’s recommendation of 4000 IUs daily for the tolerable upper limit of normal, but considerably less than the 10,000 IUs/day suggested by the Endocrine Society as the tolerable upper limit of normal.16 After the 8 weeks, athletes were invited back for a recheck of their levels. If they experienced a suspected stress reaction or fracture, they were seen by a university physician and the injury was reported in their medical record, as well as to the research team. A repeat measurement of the vitamin D level was ordered at the time of diagnosis with either stress fracture or reaction. A chart review of all study participants was completed to ensure that all stress-related bone injuries were captured. All student-athletes on a sports team for a minimum of 4 months, who consented to participate in the study and had a baseline vitamin D level in their medical record, were included in the study. Athletes with evidence of a prior stress fracture diagnosis were excluded. This study was approved by our institutional review board.
Measures
The primary outcome of interest was a diagnosed stress fracture as noted in the medical records, confirmed with a radiograph or MRI scan. The predictor variable was the most current vitamin D level before the diagnosis of stress fracture (continuous for regression), as documented in the athletes’ medical records. Vitamin D levels were also categorized (≥40 vs <40 ng/mL) to assess differences in the rate of stress fractures in athletes who improved their vitamin D levels.
Potential confounding variables were selected based on the literature. For female athletes, these included race (Black, White, other), oral contraceptives (yes/no), and body mass index (BMI; kg/m2 continuous variable); for male athletes, they included race and BMI. Race and oral contraceptive use information were self-reported; BMI was calculated based on the weight and height in the medical record from the baseline physical examination. The baseline questionnaire additionally asked athletes to self-report family history of osteoporosis, dairy product consumption, and, for women, amenorrhea (defined as missing more than 3 consecutive periods).
Statistical Analysis
Stata Version 16.0 (StataCorp) was used for all statistical analyses. The Fisher exact test was used to assess differences in the rate of stress fracture among 3 categories: (1) athletes who maintained vitamin D levels ≥40 ng/mL, (2) athletes with baseline vitamin D values <40 ng/mL and improved to ≥40 ng/mL, and (3) athletes with vitamin D values that remained <40 ng/mL throughout the study period. Logistic regression analysis was used to investigate the association between serum vitamin D levels (continuous variable) and stress fractures (yes/no). The most current serum vitamin D level before a diagnosed stress fracture or the most current vitamin D level reported (in the absence of a stress fracture) was used. Since female athletes are at higher risk for stress fractures than male athletes and there are different risk factors for women and men, separate regression models for were used for women and men. The number of observed stress fractures was small; therefore, it was not possible to adjust for potential confounding variables in the primary analysis.17 The assumptions for linearity in the log (odds) were met for continuous variables.
Mean baseline values of vitamin D were calculated for each sport. The mean values for all indoor and outdoor sports for male and female athletes were calculated, including mean baseline vitamin D levels by race for indoor and outdoor sports. Differences in average baseline vitamin D levels by sport type (indoor vs outdoor) for both men (equal variance) and women (unequal variance) were assessed by t tests.
Sensitivity Analysis
The outcome variable was broadened to include all suspected cases of stress reactions, confirmed stress reactions, and stress fractures, as documented in the medical records, and its relationship with serum vitamin D was assessed using logistic regression. Because the outcome variable number increased, it was possible to adjust for potential confounders in the sensitivity analysis. Based on the literature, race and BMI were adjusted for both sexes plus contraceptive use for women.
Results
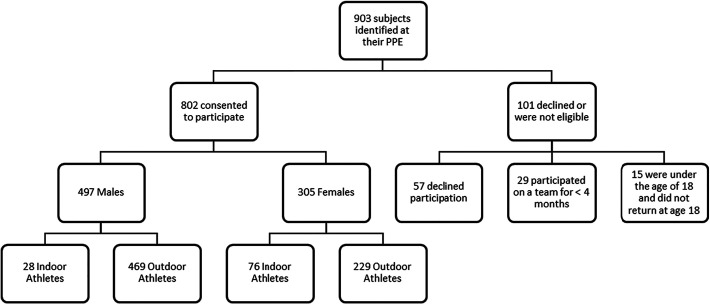
A total of 802 college athletes, 497 men and 305 women, consented to participate in the study. Totals included 57 athletes who declined participation and 29 who were not eligible because they had participated in their sport for <4 months. Fifteen student-athletes were younger than 18 years at the time of their preparticipation physical examination and did not return to enroll in the study upon turning 18 years of age. The study population is summarized in Figure 1. The mean baseline vitamin D level for men was 37.5 ng/mL, and for women it was 43.5 ng/mL. Baseline characteristics including sex, race, family history of osteoporosis, dairy consumption, and birth control use are summarized in Table 1.
Figure 1.
Study population flowchart. PPE, preparticipation physical exam.
Table 1.
Baseline Characteristics of College Athlete Study Participants, 2012 to 2018a
| Male Athletes (n = 497) | Female Athletes (n = 305) | |
|---|---|---|
| Age, y | 18.7 ± 1.2 | 18.6 ± 1.2 |
| BMI, kg/m2 | 25.5 ± 5 | 21.9 ± 3 |
| Baseline vitamin D, ng/mL | 37.5 ± 12.7 | 43.5 ± 14.7 |
| Race | ||
| Black | 111 (22) | 47 (15) |
| White | 270 (54) | 197 (65) |
| Other | 116 (24) | 61 (20) |
| Family history of osteoporosis | 1 (<1) | 6 (2) |
| Average daily servings of dairy | ||
| ≤2 | 225 (46) | 193 (63) |
| 3-5 | 238 (48) | 104 (34) |
| ≥6 | 31 (6) | 7 (2) |
| Birth control | ||
| Oral contraceptives | N/A | 124 (41) |
| Depo-Provera | N/A | 8 (3) |
| Progesterone | N/A | 7 (2) |
| None | N/A | 166 (54) |
| History of amenorrhea | N/A | 29 (10) |
aData are reported as mean ± SD or n (%). All variables have missing rates <5%. BMI, body mass index; N/A, medications or conditions that would only be applicable to females.
Table 2 lists baseline vitamin D levels by sport and by race. In addition, vitamin D levels by indoor versus outdoor sports were calculated. Male athletes had a mean difference in vitamin D that was 5.7 ng/mL higher for outdoor sports (95% CI, 0.9-10.5; P = .01). Female athletes had a mean difference in vitamin D that was 3.7 ng/mL higher for outdoor sports (95% CI, –0.6 to 8.0; P < .04 for a 1-sided t test). The mean baseline value of vitamin D was lowest among Black athletes for both indoor and outdoor sports.
Table 2.
Summary of Sport Types and Baseline Vitamin D Levels by Sport and Sport Type (Indoor and Outdoor) and by Race for College Athletes, 2012 to 2018
| Male Athletes | Female Athletes | |||
|---|---|---|---|---|
| n (%) | Baseline Vitamin D, Mean ± SD | n (%) | Baseline Vitamin D, Mean ± SD | |
| Indoor sports | ||||
| Basketball | 28 (100) | 32.1 ± 10.6 | 24 (32) | 29.3 ± 14.0 |
| Gymnastics | N/A | N/A | 19 (25) | 42.6 ± 12.6 |
| Volleyball | N/A | N/A | 33 (43) | 47.9 ± 17.7 |
| All indoor sports | 28 (100) | 32.1 ± 10.6a | 76 (100) | 40.7 ± 17.2a |
| Race | ||||
| White | 9 (32) | 37.6 ± 12.3 | 40 (53) | 51.4 ± 15.5 |
| Black | 11 (39) | 27.1 ± 8.1 | 21 (27) | 25.7 ± 9.9 |
| Other | 8 (29) | 33.0 ± 9.3 | 15 (20) | 33.1 ± 7.2 |
| Outdoor sports | ||||
| Baseball | 99 (21) | 41.2 ± 9.0 | N/A | N/A |
| Cross-country/track and field | 80 (17) | 36.3 ± 10.8 | 73 (32) | 40.6 ± 13.4 |
| Football | 208 (44) | 33.2 ± 12.3 | N/A | N/A |
| Golf | 19 (4) | 43.4 ± 10.9 | 10 (4) | 39.8 ± 8.4 |
| Sand volleyball | N/A | N/A | 18 (8) | 51.7 ± 15.3 |
| Soccer | N/A | N/A | 36 (16) | 45.0 ± 11.7 |
| Softball | N/A | N/A | 34 (15) | 40.0 ± 12.5 |
| Swimming and diving | 40 (9) | 50.1 ± 15.1 | 42 (18) | 48.8 ± 11.4 |
| Tennis | 23 (5) | 44.9 ± 11.3 | 16 (7) | 53.3 ± 17.0 |
| All outdoor sports | 469 (100) | 37.9 ± 12.7a | 229 (100) | 44.4 ± 13.6a |
| Race | ||||
| White | 261 (56) | 43.5 ± 11.9 | 157 (69) | 48.8 ± 12.4 |
| Black | 100 (21) | 27.2 ± 8.6 | 26 (11) | 30.4 ± 8.9 |
| Other | 108 (23) | 34.0 ± 9.8 | 46 (20) | 37.8 ± 11.5 |
aOutdoor sports had a higher mean baseline vitamin D level than indoor sports for male (mean difference, 5.7; 95% CI, 0.9-10.5; P = .01) and female (mean difference, 3.7; 95% CI, –0.6 to 8.0; P = .04) athletes. N/A, not applicable.
Results of the Fisher exact test showed significant differences in the rate of stress fractures between athletes with low vitamin D levels (<40 ng/mL) who did not improve versus both those who improved their vitamin D level to ≥ 40 ng/mL and those who had maintained levels ≥ 40 ng/mL (Table 3). For athletes with low vitamin D levels who did not improve to ≥ 40 ng/mL, the rate of stress fracture was 12% higher (95% CI, 6%-19%) compared with those who had low levels at baseline but improved to levels ≥ 40 ng/mL, and the rate was also 12% higher (95% CI, 5%-18%) versus those who had maintained a level ≥ 40 ng/mL (P < .001 for both). There was no significant difference in the rate of stress fractures between those who improved their vitamin D levels to ≥ 40 ng/mL and those who had maintained a level ≥ 40 ng/mL (–0.76% difference; 95% CI, –2.2% to 0.7%). When comparing the rate of all stress reactions and stress fractures, there were also significant differences between groups. For athletes with low vitamin D levels who did not improve to ≥ 40 ng/mL, the rate of stress reactions was 19% higher (95% CI, 10%-27%) versus those with low levels who improved to ≥ 40 ng/mL, and it was also 19% higher (95% CI, 10%-28%) versus those who had maintained normal levels (P < .001 for both).
Table 3.
Difference in Rate of Stress Fractures and Stress Reactions Among College Athletes, 2012 to 2018, by Vitamin D Levels (Low in Vitamin D, Improved Vitamin D, or Normal Vitamin D)
| Male and Female Athletes Combined | Stress Fractures | Stress Reactions and Stress Fractures | ||||
|---|---|---|---|---|---|---|
| n (%) | % Difference (95% CI) | P Value | n (%) | % Difference (95% CI) | P Value | |
| “Stayed low” vs “low at baseline but improved” | ||||||
| <40 and improved to ≥40 ng/mL (n = 328) | 2 (0.6) | Reference | 21 (6.4) | Reference | ||
| <40 and stayed <40 ng/mL (n = 100) | 13 (13.0) | 12 (6-19) | <.001 | 25 (25.0) | 19 (10-27) | <.001 |
| “Stayed low” vs “maintained normal levels” | ||||||
| Maintained vitamin D ≥40 ng/mL (n = 366) | 5 (1.4) | Reference | 23 (6.3) | Reference | ||
| <40 and stayed <40 ng/mL (n = 100) | 13 (13.0) | 12 (5-18) | <.001 | 25 (25.0) | 19 (10-28) | <.001 |
| “Low at baseline but improved” vs “maintained normal levels” | ||||||
| Maintained vitamin D ≥40 ng/mL (n = 366) | 5 (1.4) | Reference | 23 (6.3) | Reference | ||
| <40 and improved to ≥40 ng/mL (n = 328) | 2 (0.6) | –0.76 (–2.2 to 0.7) | .32 | 21 (6.4) | 0.1 (–4 to 4) | .95 |
A significant association between vitamin D levels and stress fractures was not identified via logistic regression (the unadjusted odds ratio [OR] is reported in Table 4). The sensitivity analysis, which included both stress reactions and stress fractures, did detect a protective effect of higher vitamin D levels for combined stress reactions and stress fractures in men (OR, 0.94; 95% CI, 0.90-0.99). For women, the association between combined stress reactions and stress fractures and serum vitamin D levels was not significant (OR, 1.01; 95% CI, 0.96-1.06). Adjusting for race and BMI in the sensitivity analysis for men, the association remained significant (adjusted OR, 0.90; 95% CI, 0.84-0.97). Adjusting for race, BMI, and contraceptive use in the sensitivity analysis for women, the OR moved toward significance (adjusted OR, 0.91; 95% CI, 0.82-1.00) (Table 4).
Table 4.
Association Between Serum Vitamin D Levels and Stress Fractures Among College Athletes, 2012 to 2018a
| Stress Fractures | Stress Reactions and Stress Fractures | Unadjusted | Sensitivity | Adjusted Sensitivity | |
|---|---|---|---|---|---|
| Male athletes (n = 497) | 8 (2) | 31 (6) | 0.98 (0.92-1.03) | 0.94 (0.90-0.99) | 0.90 (0.84-0.97) |
| Female athletes (n = 305) | 13 (4) | 41 (13) | 0.99 (0.94-1.04) | 1.01 (0.96-1.06) | 0.91 (0.82-1.00) |
aData are presented as n (%) or odds ratio (95% CI). For male athletes, adjusted for race and body mass index (BMI); for female athletes, adjusted for race, BMI, and oral contraceptive use. Sensitivity results are for stress reactions and stress fractures.
Discussion
In this population of NCAA athletes, we found evidence of a protective association of serum vitamin D levels against stress fractures. The rate of stress fracture was significantly higher for athletes with low vitamin D levels than for athletes with initial vitamin D levels in the normal range or those who improved to the normal range. This suggests that athletes who correct insufficient vitamin D levels may be reducing their risk for stress fractures. It is important to note that the unadjusted logistic regression did not find evidence of a protective effect of vitamin D levels for stress fractures. This is not surprising given the low number of stress fractures within the sample size, limiting the power to detect significance while controlling for potential confounding variables.
Athletes involved in swimming and diving, an outdoor sport at our university, had the highest mean vitamin D value for men and the second highest for women, behind tennis. These sports require minimal equipment or uniforms that may hinder vitamin D conversion by sunlight. Black athletes had the lowest mean values of vitamin D, indicating that they are at higher risk for vitamin D deficiency, regardless of sport type (indoor vs outdoor).
Considerations for Vitamin D Supplements
Some athletes with low baseline vitamin D levels who were provided vitamin D supplements reported not taking them regularly or not taking them at all. When considering supplementation for college athletes low in vitamin D, it is important to consider the context of when and how the vitamin D supplement is given for maximum effectiveness. Providing education about the role of vitamin D in bone health may be beneficial. It may be prudent for schools that provide vitamin D–fortified juices or other vitamin D–rich foods to help promote adequate vitamin D levels. In addition, clinicians should be mindful of the possibility of vitamin D toxicity, although the risk appears minimal. For example, there are studies where patients took up to 30,000 IU/day for extended periods of time without apparent toxicity.8 In addition, no cases of toxicity have been reported in patients with levels <200 ng/mL.9 In our study, there were no vitamin D levels above 100 ng/mL at baseline or after supplementation.
Comparison With Other Studies
Other studies have shown similar outcomes, supporting the use of vitamin D in preventing stress fractures. In a retrospective chart review of adults (mean age, 43 years) who experienced stress fractures, over 80% had serum vitamin D levels <40 ng/mL.14 A recent study of professional American football players found that players with low vitamin D levels were more likely to have a history of bone fracture.13 Adolescent girls (aged 9-15 years) assessed through the Growing Up Today Study had lower stress fracture risk with higher reported dietary vitamin D intake (both vitamin D consumption and stress fractures were reported by parents).19 The prevalence of vitamin D deficiency found among college athletes in this study population was similar to the rates reported in other populations of athletes in the United States.6
Strengths and Limitations
The prospective study design, including the use of medical records and measurable vitamin D levels, added to the strength of this study. The study included a variety of sports for both men and women, providing insight into vitamin D levels for athletes and particular sports where athletes may generally have lower vitamin D levels, such as basketball, football, track, and cross-country.
This study was limited to athletes from a single university who consented to participate. The low number of stress fractures reported during the study period limited the adjustment for potential confounding variables in the logistic analysis. It is important to recognize that questionnaires may not accurately capture information such as dietary intake and family history of disease. Although the most recent vitamin D level recorded before the reported stress fracture was used, the length of time that the athlete’s vitamin D status was within the normal range was not considered. We also could not verify vitamin D levels for participants who did not return for follow-up testing; therefore, athletes who only had a baseline vitamin D level were assumed to keep that same vitamin D level over time.
Conclusion
Athletes who had normal serum vitamin D levels or who improved their serum vitamin D levels to the normal range had a significantly lower rate of stress fractures. This suggests that it is possible that athletes who correct low serum vitamin D levels may reduce stress fracture risk. This study also presents evidence that athletes who play indoor sports may be at greater risk for vitamin D deficiency than those who compete in outdoor sports. Larger prospective studies are warranted to determine risk differences and appropriate vitamin D levels to promote overall bone health for an active population.
Footnotes
Final revision submitted May 4, 2020; accepted May 20, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from University of Arizona (protocol No. 1200000480R006).
References
- 1. Abbott A, Bird ML, Wild E, et al. Part I: epidemiology and risk factors for stress fractures in female athletes. Phys Sportsmed. 2020;48(1):17–24. [DOI] [PubMed] [Google Scholar]
- 2. Angeline ME, Gee AO, Shindle M, Warren RF, Rodeo SA. The effects of vitamin D deficiency in athletes. Am J Sports Med. 2013;41(2):461–464. [DOI] [PubMed] [Google Scholar]
- 3. Bennell KL, Brukner PD. Epidemiology and site specificity of stress fractures. Clin Sports Med. 1997;16(2):179–196. [DOI] [PubMed] [Google Scholar]
- 4. Burgi AA, Gorham ED, Garland CF, et al. High serum 25-hydroxyvitamin D is associated with a low incidence of stress fractures. J Bone Miner Res. 2011;26(10):2371–2377. [DOI] [PubMed] [Google Scholar]
- 5. Cannell JJ, Hollis BW, Sorenson MB, Taft TN, Anderson JJ. Athletic performance and vitamin D. Med Sci Sports Exerc. 2009;41(5):1102–1110. [DOI] [PubMed] [Google Scholar]
- 6. Farrokhyar F, Tabasinejad R, Dao D, et al. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45(3):365–378. [DOI] [PubMed] [Google Scholar]
- 7. Goolsby MA, Boniquit N. Bone health in athletes. Sports Health. 2017;9(2):108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85(1):6–18. [DOI] [PubMed] [Google Scholar]
- 9. Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008;3(5):1535–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142–146. [DOI] [PubMed] [Google Scholar]
- 11. Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135(11):2739S–2748S. [DOI] [PubMed] [Google Scholar]
- 12. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 13. Maroon JC, Mathyssek CM, Bost JW, et al. Vitamin D profile in National Football League players. Am J Sports Med. 2015;43(5):1241–1245. [DOI] [PubMed] [Google Scholar]
- 14. Miller JR, Dunn KW, Ciliberti LJ, Jr, Patel RD, Swanson BA. Association of vitamin D with stress fractures: a retrospective cohort study. J Foot Ankle Surg. 2016;55(1):117–120. [DOI] [PubMed] [Google Scholar]
- 15. National Collegiate Athletic Association. Student-athletes. Accessed November 1, 2020. http://www.ncaa.org/student-athletes
- 16. Ogan D, Pritchett K. Vitamin D and the athlete: risks, recommendations, and benefits. Nutrients. 2013;5(6):1856–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. [DOI] [PubMed] [Google Scholar]
- 18. Rizzone KH, Ackerman KE, Roos KG, Dompier TP, Kerr ZY. The epidemiology of stress fractures in collegiate student-athletes, 2004-2005 through 2013-2014 academic years. J Athl Train. 2017;52(10):966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sonneville KR, Gordon CM, Kocher MS, et al. Vitamin D, calcium, and dairy intakes and stress fractures among female adolescents. Arch Pediatr Adolesc Med. 2012;166(7):595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]