Introduction
Microneedling, a minimally invasive procedure in which numerous epidermal and dermal microchannels are created through skin needling, has become a common and established treatment option for a plethora of dermatologic concerns, including skin rejuvenation, facial rhytides, and acne scarring. With studies supporting the efficacy of microneedling across many dermatologic applications as well as its minimal post-treatment recovery, this therapeutic option is generally well-regarded among both clinicians and patients. The limited side effects also augment its popularity with the most common - mild erythema, localized edema, and skin flaking - expected and self-resolving within 72 hours. However, microneedling is associated rarely with more concerning adverse effects. The procedure is disparate, including variable use of pre- and post-treatment topical cosmeceuticals, most of which aim to promote microneedling-induced wound healing and regeneration. With microneedling's creation of entry points into the skin, the possibility arises for development of an immune response to a penetrating material, whether from the needle or an antigenic topical agent. This adverse effect has been reported rarely in the literature with variable and not always favorable outcomes. We report a case of granulomatous dermatitis secondary to microneedling with almost complete resolution through use of oral methotrexate.
Case report
A 59-year-old female presented with an erythematous and pruritic facial eruption, which had appeared within 3 days of a facial microneedling treatment performed by an outside provider with a Rejuvapen (Refine USA, LLC) device. A cleanser and topical anesthetic were applied prior to treatment, and a Vitamin-C product and tinted sunscreen were subsequently applied. The eruption had persisted for several months despite numerous treatments, including topical corticosteroids, metronidazole cream, intralesional corticosteroids, cetirizine, minocycline, doxycycline, dapsone, and prednisone.
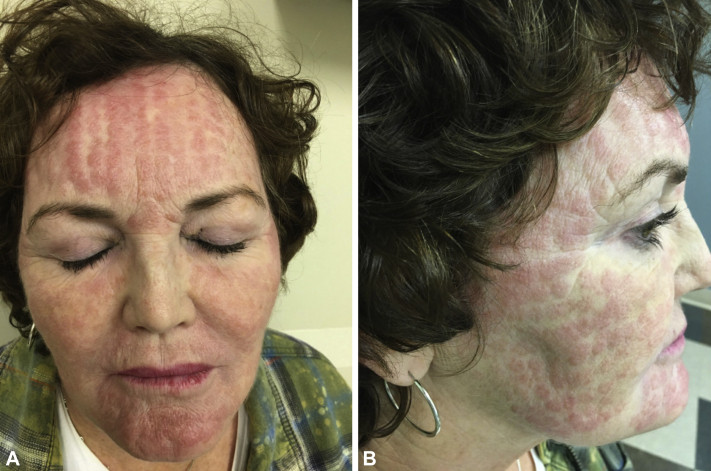
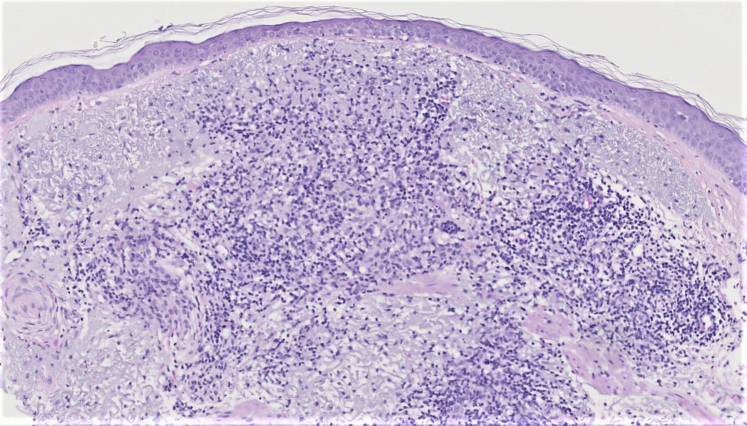
Physical exam revealed numerous and patterned pink erythematous thin papules on the forehead, temples, cheeks, and chin in a linear and regular configuration corresponding with sites of needle penetration (Fig 1 A and B). A 4-mm punch biopsy was obtained demonstrating a superficial interstitial and predominantly perivascular lymphohistiocytic inflammation (Fig 2). Stains for fungal and acid-fast organisms were negative, and polarizable material was not observed. A diagnosis of granulomatous dermatitis was made, and methotrexate 5 mg weekly (with folic acid supplementation) was initiated. This dose was increased to 15 mg weekly with gradual eradication of all granulomatous lesions over a 9-month total course. Interestingly, resolution of the granulomatous lesions revealed multiple discrete linear patterned pinpoint scars over the patient's forehead, temples, and cheeks (Fig 3), which the patient opted not to address.
Fig 1.
A, B, Granulomatous dermatitis secondary to microneedling. Numerous patterned, pink, erythematous, and thin papules on the forehead, temples, cheeks, and chin were observed in a linear and regular configuration, corresponding to the sites of needle penetration.
Fig 2.
Granulomatous dermatitis, punch biopsy. (Hematoxylin-eosin–stain; original magnification, x10.) Superficial, interstitial, and predominantly perivascular lymphohistiocytic inflammation with a background of solar elastosis.
Fig 3.
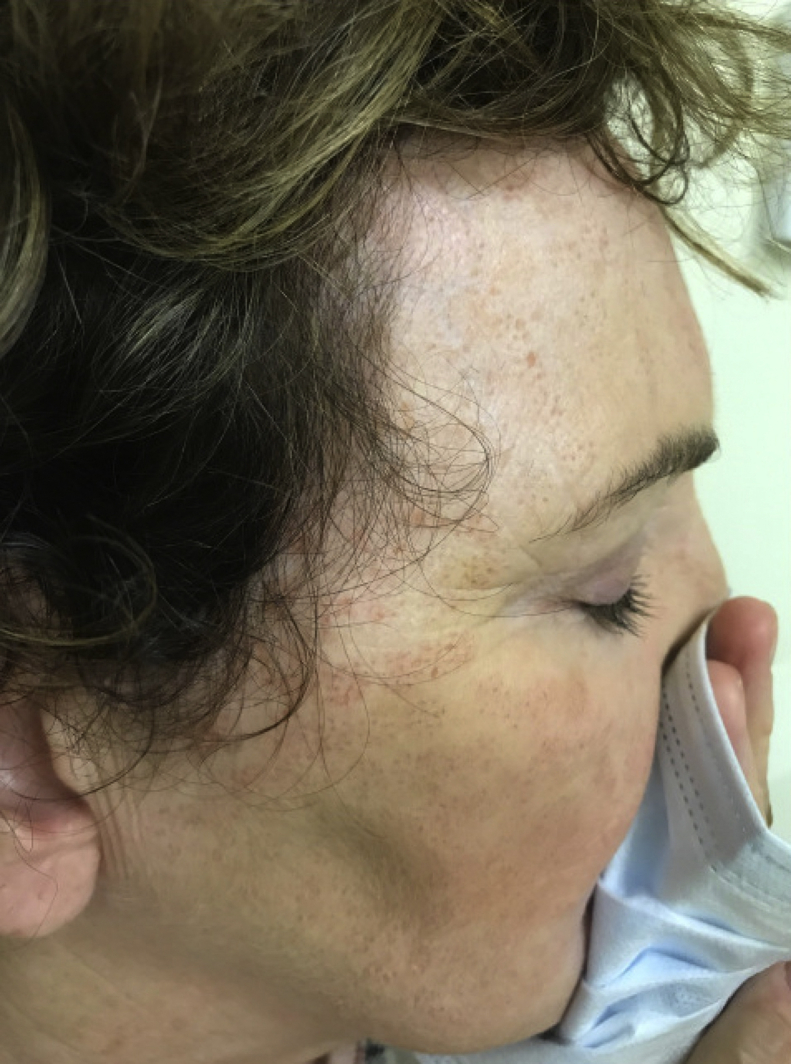
Resolved granulomatous dermatitis and residual tram-track scarring. Multiple discrete linear patterned pinpoint scars were observed.
Discussion
Though considered a safe and effective treatment for a myriad of dermaologic conditions,1 microneedling has been reported rarely to cause facial granulomatous reactions.2, 3, 4, 5, 6 These reactions are secondary to skin penetration of an antigenic topical product or needle material, in some cases supported by positive patch testing.2, 3, 4, 5, 6 Theoretically any of the topicals associated with the procedure, particularly those not intended for intradermal use, could be causative. We speculate that the most likely agent is the nickel in the needle or the Vitamin-C cosmeceutical, as both have been reported prior in the literature. Our patient's eruption differed from prior cases in that it was recalcitrant to all reported efficacious therapies, including topical, intralesional, and oral corticosteroids as well as oral tetracyclines. Novel to the literature, treatment with methotrexate was utilized with almost complete resolution. Methotrexate has been used anecdotally with improvement of granulomatous reactions secondary to dermal filler, the more common cause of such reactions in cosmetic dermatology.7 In our patient's case, improvement of the granulomatous lesions unmasked “tram-track” scars, another previously reported uncommon adverse effect of microneedling. Postulated etiologies of tram-track scarring include the use of a larger needling device or stronger pressure. In our case, chronic inflammation at the insertion sites may have contributed to or accentuated the resultant scars.8
These adverse events, though uncommon, are significant as they may leave patients seeking treatment for cosmetic reasons with disfiguring results. Additionally, these events are likely underreported as the majority of treatments are performed without physician supervision and some even by patients at home.2 Careful selection of topical products used in conjunction with microneedling may mitigate the risk of granulomatous dermatitis. As microneedling is widely recommended by dermatologists but minimally regulated, it is imperative that providers diligently communicate its potential risks with patients. This case emphasizes the adverse event of granulomatous dermatitis in the setting of microneedling and suggests a novel therapeutic option for such events.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
References
- 1.Alster T.S., Graham P.M. Microneedling: a review and practical guide. Dermatol Surg. 2018;44(3):397–404. doi: 10.1097/DSS.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 2.Soltani-Arabshahi R., Wong J.W., Duffy K.L., Powell D.L. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150(1):68–72. doi: 10.1001/jamadermatol.2013.6955. [DOI] [PubMed] [Google Scholar]
- 3.Yadav S., Dogra S. A cutaneous reaction to microneedling for postacne scarring caused by nickel hypersensitivity. Aesthet Surg J. 2016;36(4):NP168–NP170. doi: 10.1093/asj/sjv229. [DOI] [PubMed] [Google Scholar]
- 4.Fucci-da-Costa A.P.C., Camasmie H.R. Drug delivery after microneedling: report of an adverse reaction. Dermatol Surg. 2018;44(4):593–594. doi: 10.1097/DSS.0000000000001250. [DOI] [PubMed] [Google Scholar]
- 5.Pratsou P., Gach J. Severe systemic reaction associated with skin microneedling therapy in 2 sisters: a previously unrecognized potential for complications? J Am Acad Dermatol. 2013;68(Suppl 1):AB219. [Google Scholar]
- 6.Moshiri A., Solotoff S., Nguyen C., Elenitsas R. Facial granulomatous dermatitis after microneedling therapy. Abstract presented at: 24th World Congress of Dermatology; June 10-15, 2019; Milan, Italy. https://www.wcd2019milan-dl.org/abstract-book/documents/abstracts/03-aesthetic-cosmetic-dermatology/facial-granulomatous-dermatitis-after-microneedling-1869.pdf Accessed March 1, 2021. Available at:
- 7.Styperek A., Bayers S., Beer M., Beer K. Nonmedical-grade injections of permanent fillers: medical and medicolegal considerations. J Clin Aesthet Dermatol. 2013;6(4):22–29. [PMC free article] [PubMed] [Google Scholar]
- 8.Pahwa M., Pahwa P., Zaheer A. “Tram track effect” after treatment of acne scars using a microneedling device. Dermatol Surg. 2012;38(7 Pt 1):1107–1108. doi: 10.1111/j.1524-4725.2012.02441.x. [DOI] [PubMed] [Google Scholar]