Abstract
Purpose
To present a case of ulcerative colitis associated with teprotumumab treatment for thyroid eye disease.
Observations
A 46-year-old Indian female was treated with teprotumumab (Tepezza) for severe thyroid eye disease within 9 months of diagnosis. The patient noted progression of her disease on oral prednisone and demonstrated severe, debilitating proptosis accompanied by eye ache and dry eyes. After 5 infusions of teprotumumab over a four-month period, the patient developed bloody diarrhea and fecal urgency. These symptoms progressively worsened and after two additional treatments, she underwent a colonoscopy. This confirmed the diagnosis of ulcerative colitis (UC). Treatment with teprotumumab was halted prior to the administration of the 8th infusion; however, the patient continued to have severe gastrointestinal symptoms two months after her last treatment.
Conclusions and importance
Teprotumumab is an insulin-like growth factor-1 receptor (IGF-1R) inhibitor demonstrated to improve proptosis in patients with active thyroid eye disease. Most adverse events reported are mild or moderate in severity; however, inflammatory bowel disease (IBD) is a serious adverse event that can develop as a result of treatment.
Keywords: Teprotumumab, Tepezza, Graves' orbitopathy, Ulcerative colitis
1. Introduction
The etiology of thyroid eye disease has not been fully elucidated. Patients with active disease have been shown to have elevated levels of the insulin-like growth factor-1 receptor (IGF-1R) and orbital fibroblasts have been implicated in the pathogenesis of the disease.1, 2 Multiple treatment options have been well studied, including various courses of corticosteroid therapy, orbital radiotherapy, and monoclonal antibodies, including rituximab and tocilizumab.3,4 However, teprotumumab, an inhibitor of the IGF-IR, is the sole monoclonal antibody approved by the US Food and Drug Administration for the medical treatment of thyroid eye disease.5 Published studies of teprotumumab have documented adverse events, including muscle spasms, nausea, diarrhea, stomatitis and hearing loss; however, though inflammatory bowel disease (IBD) is a contraindication to its use, it has not been reported previously as an adverse event.6
2. Case report
A 46-year-old Indian female was referred to our clinic two months after being diagnosed with hyperthyroid Graves’ disease. She was initiated on oral methimazole therapy (5 mg daily) by her primary care provider. Her past ocular history was significant for high myopia with astigmatism. She was a non-smoker and occasionally consumed alcohol.
The patient's best corrected visual acuity was 20/20 in her right eye and 20/30 in her left eye. Her extraocular movements were intact bilaterally. Her CAS was 2. Thyroid stimulating immunoglobulin (TSI) was 404 (<140% normal). Her exam was significant for severe eyelid retraction bilaterally with the MRD1 measuring 5 mm in both eyes. Hertel measurements were 22 mm and 23 mm in her right eye and left eye, respectively. She was started on selenium 200 mcg daily, offered intravenous or oral prednisone, continued on daily methimazole, and asked to return to clinic in 6 weeks.
The patient returned for follow up 12 weeks later with complaints of worsening proptosis, dry eyes and ocular ache. MRD1 measured 6 mm in both eyes and Hertel measurements had increased to 26 mm in both right and left eyes. The patient had been placed on oral prednisone (30 mg daily) by her primary care physician without improvement after 8 weeks. Teprotumumab (Tepezza) treatment was discussed in detail. Her TSI was 449.
At the time of teprotumumab initiation, the patient demonstrated diplopia and restriction of up gaze bilaterally. Hertel measurements were 28 mm and 26 mm in her right eye and left eye, respectively. Visual acuity in both eyes was stable Her CAS was 6. She started on an initial dose of 10 mg/kg of body weight for the first dose, followed by 20 mg/kg for the remaining doses.
The patient experienced tolerable muscle spasms, subjective hearing impairment and gastrointestinal symptoms during each treatment. However after the 5th teprotumumab treatment, she developed hematochezia and fecal urgency. Her abdominal symptoms progressively worsened after two additional infusions. She underwent a CT scan (Fig. 1) and colonoscopy which established the diagnosis of ulcerative colitis (UC) (Fig. 2) Teprotumumab infusions were discontinued while the ulcerative colitis was managed. Of note, after the third and each subsequent treatment, the patient demonstrated gradual improved proptosis, extraocular motility and diplopia. Her TSI declined to 360. Improvement from presentation through treatment is demonstrated in Fig. 3. Despite discontinuation of teprotumumab, the patient continued to have severe gastrointestinal symptoms two months after the 7th treatment. She was treated with infliximab and prednisone. Her subjective hearing loss ultimately resolved..
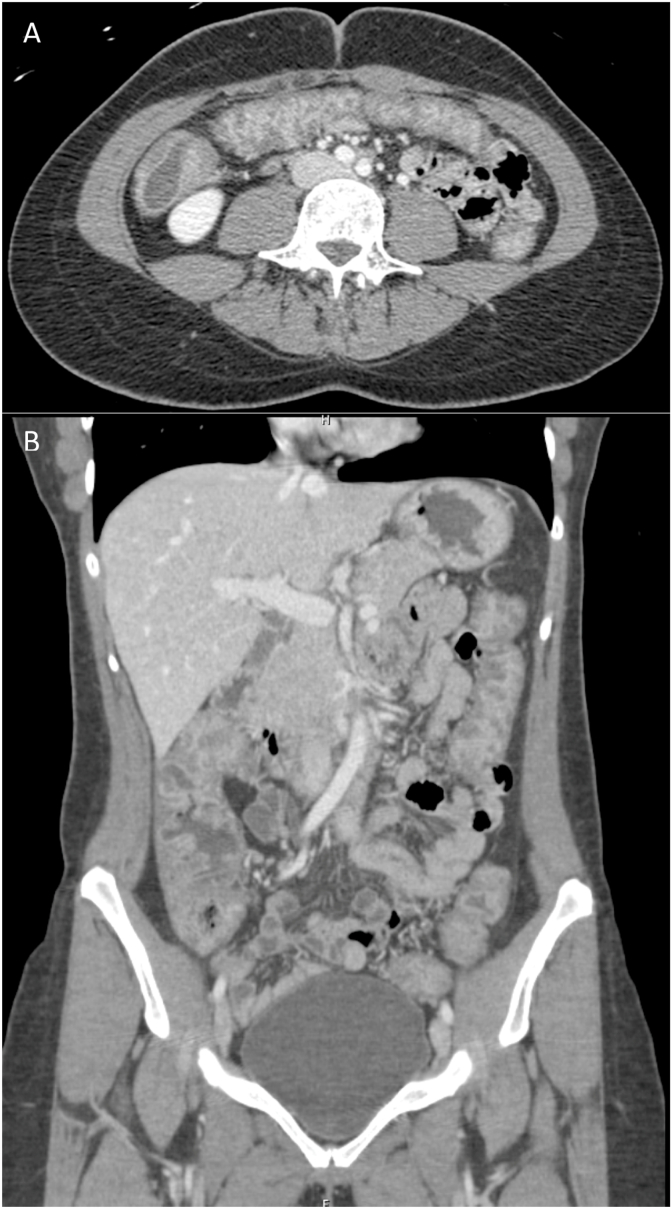
Fig. 1.
CT scan demonstrating diffuse colon wall thickening with increased mucosal enhancement consistent with ulcerative colitis (A axial, B coronal).
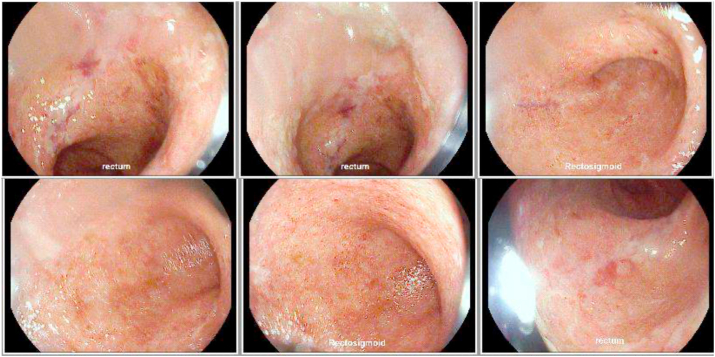
Fig. 2.
Flexible sigmoidoscopy demonstrating colitis, loss of vascular pattern and areas of shallow ulceration. (Images: Daniel Selvig MD).
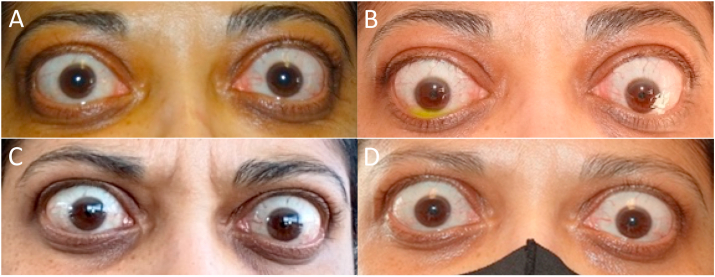
Fig. 3.
Image of patient: A at presentation B prior to initiating treatment C after four teprotumumab treatments D after seven teprotumumab treatments. (Images: Rona Z Silkiss MD FACS).
The patient did not have a diagnosis of UC prior to the initiation of teprotumumab treatment, nor did she have any symptoms of the disease. However, on further questioning the patient revealed that two members of her family (a first degree relative and second degree relative) had been diagnosed with UC. Additionally, the patient had at least one episode of bilateral posterior uveitis of unknown etiology one year prior to the diagnosis of thyroid eye disease and almost two years prior to the diagnosis of UC. Systemic workup was negative at that time.
3. Discussion
Teprotumumab is a human monoclonal antibody that acts as an insulin-like growth factor-I (IGF-I) receptor inhibitor. The development of ulcerative colitis associated with teprotumumab treatment in a patient without a prior diagnosis of IBD has not been previously reported in the literature. The most common adverse events reported in the clinical trials were nausea (19%), muscle spasms (19%), diarrhea (14%), hyperglycemia (12%), alopecia (7%), dry skin (7%), dysgeusia (7%), headache (7%), hearing impairment (7%), and weight loss (7%).2 The majority of these events were mild and resolved while the patients continued to receive treatment. One patient with a recent diagnosis of ileitis and colitis was diagnosed and treated for IBD while receiving treatment with teprotumumab.2 As such, known IBD is a contraindication to the use of teprotumumab.
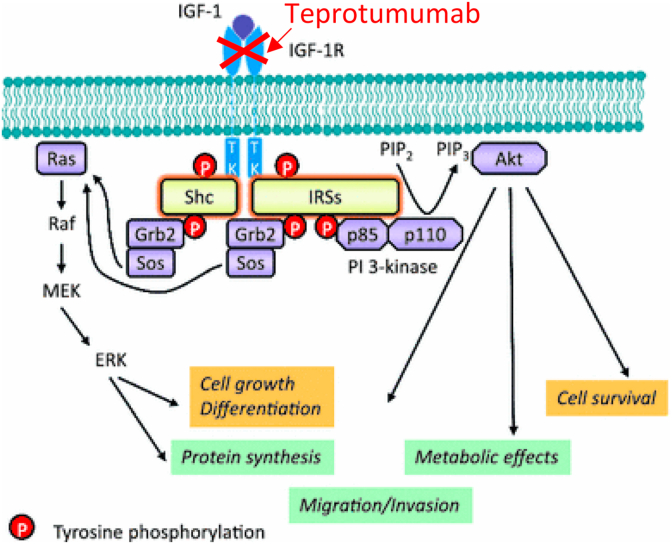
Although the etiology of IBD remains unknown, patients with active disease present with reduced serum levels of IGF-1.7, 8 This is hypothesized to be secondary to gastrointestinal dysfunction and acquired growth hormone (GH) resistance.9 This is possibly induced by circulating inflammatory cytokines that are elevated with chronic inflammation.8 In the normal setting, IGF-1 is stimulated by GH to promote cell proliferation, somatic growth, and anabolic cellular processes. Animal models of intestinal inflammation have suggested that IGF-1 may promote mucosal repair during inflammation. Studies have also shown that IGF-I derived from intestinal mesenchymal cells regulates the growth and function of neighboring intestinal epithelial cells.6, 10 It can be postulated, therefore, that inhibition of IGF-1R would result in decreased levels of serum and gastrointestinal IGF-1. (Fig. 411) Decreased availability of IGF-1 induced by teprotumumab inhibition of IGF-1R may mimic the conditions found in patients with IBD and lead to similar gastrointestinal symptoms and signs, especially if there is a familial predisposition.
Fig. 4.
Inhibition of IGF-1R leads to intestinal inflammation.
The fact that the patient’s gastrointestinal symptoms improved after treatment with infliximab is a significant one. Infliximab is a monoclonal antibody against TNF-F061, which is a proinflammatory cytokine that has been shown to be implicated in GH resistance by decreasing hepatic growth hormone receptor synthesis, and, subsequently, hepatic IGF-1 production.12, 13 This is important given that IGF-1 is produced primarily by the liver via stimulation by GH.14 Studies have shown that TNF-F061 is a key factor in the induction and maintaining of the inflammatory process in patients with IBD.15 Therefore, the successful reduction of symptoms after the initiation of infliximab may result from the suppression of TNF-F061 and the subsequent restoration of the normal GH/IGF-1 axis and serum IGF-1 levels.16
Given our patient's family history of UC and personal history of posterior uveitis, it is possible that she may have had yet to be diagnosed UC prior to the initiation of teprotumumab that was exacerbated by the treatment.
The authors recommend a careful and detailed history of autoimmune disease prior to initiating teprotumumab therapy as the drug may exacerbate or illicit inflammatory bowel disease.
Patient consent
The patient has consented to publication of the case both written and orally.
Funding
This report was supported by the California Pacific Medical Center, San Francisco, CA.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Declaration of competing interest
The following authors have no financial disclosures: MS, RZS.
Acknowledgements
None.
References
- 1.Smith TJ, Hegedüs L, Douglas R. Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves’ orbitopathy. Best Pract Res Clin Endocrinol Metabol. 2012;26(3):291–302. doi: 10.1016/j.beem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith TJ, Kahaly GJ, Ezra DG. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761. doi: 10.1056/NEJMoa1614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silkiss RZ, Reier A, Coleman M, Lauer S. Rituximab for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2010;26(5):310–314. doi: 10.1097/IOP.0b013e3181c4dfde. [DOI] [PubMed] [Google Scholar]
- 4.Silkiss RZ, Paap MK, Roelofs KA, Weis E. Treatment of corticosteroid-resistant thyroid eye disease with subcutaneous tocilizumab. Can J Ophthalmol. 2021;56(1):66–70. doi: 10.1016/j.jcjo.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Douglas RS, Kahaly GJ, Patel A. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341–352. doi: 10.1056/NEJMoa1910434. [DOI] [PubMed] [Google Scholar]
- 6.Theiss AL, Fruchtman S, Lund PK. Growth factors in inflammatory bowel disease: the actions and interactions of growth hormone and insulin-like growth factor-I. Inflamm Bowel Dis. 2004;10(6):871–880. doi: 10.1097/00054725-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Hjortebjerg R, Thomsen KL, Agnholt J, Frystyk J. The IGF system in patients with inflammatory bowel disease treated with prednisolone or infliximab: potential role of the stanniocalcin-2/PAPP-A/IGFBP-4 axis. BMC Gastroenterol. 2019;19(1):83. doi: 10.1186/s12876-019-1000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsanos KH, Tsatsoulis A, Christodoulou D, Challa A. Reduced serum insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 levels in adults with inflammatory bowel disease. Growth Hormone IGF Res. 2001;11(6):364–367. doi: 10.1054/ghir.2001.0248. [DOI] [PubMed] [Google Scholar]
- 9.Michalak A., Mosińska P., Fichna J. Common links between metabolic syndrome and inflammatory bowel disease: current overview and future perspectives. Pharmacol Rep. 2016;68(4):837–846. doi: 10.1016/j.pharep.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Simmons JG, Pucilowska J, Lund PK. Autocrine and paracrine actions of intestinal fibroblast-derived insulin-like growth factors. Am J Physiol Gastrointest Liver Physiol. 1999;276(4):G817–G827. doi: 10.1152/ajpgi.1999.276.4.G817. [DOI] [PubMed] [Google Scholar]
- 11.Girnita L, Worrall C, Takahashi S. Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci. 2014;71(13):2403–2427. doi: 10.1007/s00018-013-1514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf M, Bohm S, Kreymann MB, Kreymann G. Proinflammatory cytokines interleukin-1b and tumor necrosis factor a inhibit growth hormone stimulation of insulin-like growth factor I synthesis and growth hormone receptor mRNA levels in cultured rats liver cells. Eur J Endocrinol. 1996;135:729–737. doi: 10.1530/eje.0.1350729. [DOI] [PubMed] [Google Scholar]
- 13.Fan J, Char D, Bagby GI, Gelato MC, Lang CH. Regulation of insulin-like growth factor-I and IGF-binding proteins by tumor necrosis factor. Am J Physiol. 1995;269:1204–1212. doi: 10.1152/ajpregu.1995.269.5.R1204. [DOI] [PubMed] [Google Scholar]
- 14.Baxter RC. The somatomedins: insulin-like growth factors. Adv Clin Chem. 1986;25:49–115. doi: 10.1016/s0065-2423(08)60124-9. [DOI] [PubMed] [Google Scholar]
- 15.Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 16.Gentilucci V, Caviglia R, Picardi A. Infliximab reverses growth hormone resistance associated with inflammatory bowel disease. Aliment Pharmacol. Therapeut. 2005;21(9):1063–1071. doi: 10.1111/j.1365-2036.2005.02449.x. [DOI] [PubMed] [Google Scholar]