Abstract
Salmonella enterica and Escherichia coli are bacteria of concern to veterinary public health and poultry health. Our research aimed to determine the factors associated with S. enterica and E. coli in commercial broiler chicken barns during the rest period between flocks to identify the best methods of sanitation for bacterial load reduction. This involved collecting samples from September 2015 to July 2016 from the floors of 36 barns before sanitation (baseline) and at 2 time intervals after sanitation, followed by microbiological and molecular analysis. A priori variables of interest included sanitation procedure (dry cleaning, wet cleaning, disinfection), sampling point (baseline, 2 d after sanitation, 6 d after sanitation), and flooring type (concrete, wood). The odds of detecting S. enterica were higher on wooden floors that were wet-cleaned than on concrete floors that were dry-cleaned, lower in the winter and spring than in the fall, and lower when samples were collected 2 d and 6 d after sanitation than at baseline. For E. coli, the concentration was higher on wooden floors than on concrete floors and in the summer than in the fall, and it was lower in postsanitation samples from disinfected barns than in presanitation samples from dry-cleaned barns and in the winter than in the fall. Among E. coli isolates, factors associated with the presence of qacEΔ1, a gene associated with resistance to quaternary ammonium compounds, included sanitation procedure, flooring type, cycle length, and the number of times per yr the barn is disinfected. Our findings highlight the importance of cleaning after litter removal, although the sanitation procedure chosen might differ depending on which pathogen is present and causing disease issues; dry cleaning appears to be preferable for S. enterica control, especially in barns with wooden floors, whereas disinfection appears to be preferable for E. coli reduction.
Key words: Salmonella enterica, Escherichia coli, sanitation, broiler chicken, Ontario
Introduction
Escherichia coli and Salmonella enterica are pathogens of concern to poultry and public health. Avian pathogenic E. coli is the cause of colibacillosis in chickens and has a major impact on the poultry industry worldwide (Ron, 2006; Stacy et al., 2014). The feces of healthy chickens are a possible reservoir for Avian pathogenic E. coli strains (Ewers et al., 2009; Kemmett et al., 2013). In humans, nontyphoidal S. enterica are a leading cause of foodborne gastrointestinal illnesses worldwide (Marcus, 2008; Chen et al., 2013). In poultry, infection with S. enterica can cause decreased production and mortality, resulting in economic losses to the producer (Park et al., 2011). S. enterica are found in a wide range of animal reservoirs, with broiler chicken flocks being a main reservoir and a common source of transmission to humans (Velge et al., 2005; Foley et al., 2008; Tabo et al., 2013). S. enterica contamination in broiler flocks has been attributed to farm structure and management, inadequate hygiene (Nayak et al., 2003; Liljebjelke et al., 2005; Gast, 2007), and contamination in the previous flock (Rose et al., 1999, 2003; Nayak et al., 2003; Cardinale et al., 2004; Liljebjelke et al., 2005; Gast, 2007; Volkova et al., 2010).
S. enterica are often difficult to control because of the fact that they can remain viable in the poultry environment for extended periods of time (Williams and Benson, 1978; Davies and Wray, 1996; Chen et al., 2013; Rajagopal and Mini, 2013) and the multiple potential contamination sources (Voss-Rech et al., 2015). Currently, the measures used to control S. enterica vary depending on the country. Although cleaning and disinfection has been shown to significantly reduce S. enterica contamination in broiler houses (Garber et al., 2003), the efficacy is often variable.
The Chicken Farmers of Canada's On-Farm Food Safety Assurance Program (Safe, Safer, Safest) dictates that chicken producers must remove all litter and dry-clean the barn after shipping each flock; furthermore, wet cleaning and disinfection must be conducted at least once per yr (Chicken Farmers of Canada, 2018). Common classes of disinfectants used in the commercial chicken industry include peroxides, phenolics, quaternary ammonium compounds (QACs), and aldehydes (Van Immerseel et al., 2009). Disinfectants applied at the optimal concentration have a lethal effect on the target bacteria, and the development of resistant mutants is unlikely (Karatzas et al., 2007). Common reasons for sublethal concentrations reaching target sites on bacteria include the presence of organic matter, incorrect application of the disinfectant (McLaren et al., 2011), water hardness, the presence of biofilm, and low temperatures (Taylor and Holah, 1996; Gradel et al., 2004; Lapidot et al., 2006). Sublethal concentrations create a selection pressure that, over time, drives the bacteria to acquire resistance genes or adapt in some way to the disinfectant agents (Hegstad et al., 2010). The frequent use and misuse of QACs for disinfecting barns has led to the emergence of resistant pathogenic and nonpathogenic bacteria (Langsrud et al., 2003; Tabata et al., 2003; Buffet-Bataillon et al., 2012). Genes associated with resistance to QACs, including qacEΔ1 and sugE(p), have been identified in E. coli isolates (Zou et al., 2014) as well as other gram-negative organisms (Ploy et al., 1998; Kücken et al., 2000; Li et al., 2010).
To date, there have been no studies conducted in Canada, and very few worldwide, to determine the effect of sanitation on the presence of S. enterica and E. coli in commercial broiler barns. Thus, the objectives of this study were to 1) determine how S. enterica and E. coli are affected by the current barn cleaning and disinfection procedures recommended to broiler producers by the poultry industry in Ontario, with a special focus on sanitation procedures, rest period, and flooring type, and 2) identify factors associated with the presence of the QAC resistance genes qacEΔ1 and sugE(p) in E. coli isolates.
Materials and methods
Barn Recruitment and Sanitation Procedures
The study was originally designed as a field experiment, in which sections of the floors of 2-story commercial broiler chicken barns (with concrete flooring on the lower level and wooden flooring on the upper level) were to be subjected to 3 different sanitation procedures (described in the following part of the article). Under this design, 30 barns were required based on a confidence of 90%, power of 80%, 2 to 1 ratio (cleaning vs. no cleaning), and proportion of samples in the noncleaned and cleaned sections with E. coli concentrations ≥6 log of 60 and 15%, respectively. However, it was necessary to change the design to a cohort study with presanitation and postsanitation visits (described in the following part of the article) to accommodate the producers' cleaning procedures and schedules. With the existing budget, we were able to increase the number of barns to 36, targeting equal numbers in each sanitation group. Because of low enrollment of 2-story barns, barns with any number of stories were made eligible to participate. For logistical reasons, only barns in southern Ontario were considered for inclusion in the study. The number of barns enrolled per farm premise was limited to 1, although if a producer had more than 1 premise, 1 barn from each premise was eligible for enrollment.
Producers were recruited by field representatives of the Chicken Farmers of Ontario (provincial chicken marketing board) during farm visits for unrelated purposes, by research team members at producer meetings, by feed representatives, and by word-of-mouth by participating producers.
Upon recruitment, basic information was collected: barn dimensions, address, flock shipment date, and the sanitation procedure the producer planned to complete. At this time, a baseline sampling date was arranged or a subsequent contact date was set up. The study was conducted over an 11-mo period from September 2015 to July 2016.
The producer was asked to clean all stories of the barn using their normal protocol, and then the barn was categorized into 1 of 3 sanitation groups: dry cleaning only (hereafter referred to as dry cleaning); dry cleaning followed by wet cleaning (hereafter referred to as wet cleaning); or dry cleaning followed by wet cleaning followed by disinfection (hereafter referred to as disinfection). In Canada, regardless of the sanitation procedure used, the litter is removed from the barn after each flock before sanitation. Dry cleaning normally involves blowing the dust from the ceiling, walls, floor, and fans of the barn with a backpack blower after litter removal, with or without sweeping the floor afterwards. Wet cleaning normally involves power washing the barn with pressurized water (usually with a detergent) after the dry cleaning step. Disinfection normally involves spraying, foaming, or fogging the barn with a disinfectant after the wet cleaning step. If the sanitation procedure performed by the producer did not include all the normal steps (e.g., if the wet cleaning step was skipped before disinfection), the barn was categorized based on the final step of the sanitation procedure, as the final step would be expected to have the greatest impact on pathogen load. This also eliminated the need to create additional categories of unique sanitation procedures comprised of very few barns.
Questionnaire
A basic questionnaire, administered through a face-to-face interview with the producer, was used to gather data about the sanitation procedure. It included questions related to who performed the procedure (producer, employee, company); the areas cleaned (ceiling, walls, floor, feed lines and feed pans, water lines); products used and concentration (when applicable); method of application (backpack blower, sweep, or other for dry cleaning; spray or foam for wet cleaning; spray, foam, or fog for disinfection); relative water temperature (cold vs. warm or hot); use of a pressure washer (when applicable); and time (in h) between each major step in the procedure. Other questions included whether anyone entered the barn after the sanitation procedure had been completed and historical cleaning and disinfection (sanitation procedure used after the previous flock, number of times the barn is wet-cleaned per yr, number of times the barn is disinfected per yr). If a producer was unsure of the answer, the research team attempted to obtain the information in another way (e.g., reading the label on the disinfectant container and recording the active ingredient(s) listed). The flock and barn characteristics previously provided by the producer during barn enrollment, such as the cycle length (8, 9, 10, 12 wk), number of stories (1, 2, 3), and flooring type of each level (concrete, wood), were verified at the first visit.
Sample Collection
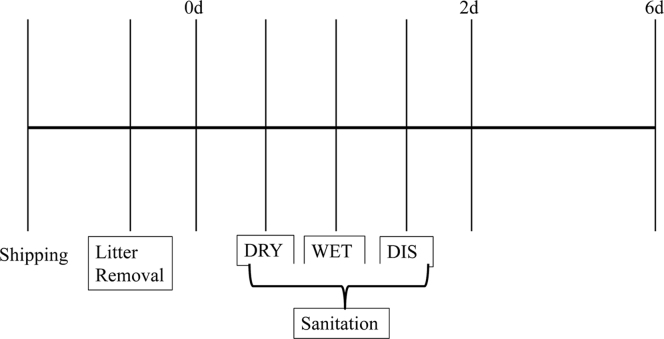
Samples were collected at 3 different time points (hereafter referred to as sampling point): 1) baseline, which was after litter removal and before any cleaning had taken place; 2) 2 d after the final step of the sanitation procedure had taken place; and 3) 6 d after the final step of the sanitation procedure had taken place (Figure 1). It should be noted that the elapsed time between the baseline and the 2-day and 6-day sampling points differed between sanitation procedures, as a full disinfection takes longer than a dry or wet clean.
Figure 1.
Timeline of major steps in the cleaning and sampling process in a study that investigated factors associated with the presence of S. enterica and the concentration of E. coli in samples collected from the floor(s) of commercial broiler chicken barns in Ontario during the rest period. DRY: Dry cleaning (dry cleaning only); WET: wet cleaning (dry cleaning followed by wet cleaning); DIS: disinfection (dry cleaning followed by wet cleaning followed by disinfection). 0d: baseline sampling; 2 d: sampling 2 d after the final step of the sanitation procedure; 6d: sampling 6 d after the final step of the sanitation procedure.
Before visiting the barn, and using the barn dimensions provided by the producer, each floor was divided into 4 equal sections. Within each section, a 1-m2 sampling area was generated based on a random width and length provided by Microsoft Office Excel 2007 (Microsoft Corporation, Redmond, WA). This made a total of 4 randomly selected 1-m2 sampling areas per floor, which allowed us to account for the variability and complexity of pathogens under field conditions. For multistory barns, each floor had 4 different sampling areas to reduce any potential bias of the spots chosen. Upon the first visit to the barn, after donning personal protective equipment (disposable boots, coveralls, and caps), the sampling areas were identified using a 230′ Laser Distance Measuring device (Johnson Level & Tool Mfg. Co., Inc., Mequon, WI). The sampling areas were marked with Fluorescent Red Orange Marking Spray Paint (Rust-Oleum Professional, Concord, ON, Canada) using a 1-m-long piece of metal, which was cleaned with Lysol wipes (Reckitt Benckiser Inc., Parsippany, NJ) immediately after exiting the barn.
Each 1-m2 sampling area was swabbed using a Hydrated-Sponge with 10-mL Dey-Engley Neutralizing Buffer (3M, St. Paul, MN), which had been transported to the barn in a cooler with an ice pack and had been given a unique code, representing the barn number, sampling point (baseline, 2 d, 6 d), and the sampling area number within the barn. These sponges came with individual, prepackaged gloves, which were used, then disposed of, after swabbing each area. Each sampling area was visually divided into 2 halves: 1 half was swabbed with 1 side of the sponge, and the other half was swabbed with the other side of the sponge. The swabbing was performed in a right to left direction, starting in the upper right hand corner. Once collected, the sponges were put back into the individual bags they came in, put into the cooler, and transported to the Animal Health Laboratory at the University of Guelph.
At the 2-day and 6-day postsanitation visits, the original sampling areas were used as a guide as to where to sample next. We did not use the same area because the prior sampling would have removed some of the bacteria from the floor; instead, we marked new 1-m2 areas, each time, adjacent to the original.
Laboratory Analysis
At the Animal Health Laboratory, the swabs were aseptically transferred to a filter stomacher bag, and buffered peptone water (BPW) was added to a 1 in 10 dilution. Each bag was then massaged by hand and serially diluted to a dilution of 10−4; the stomacher bag is considered the 10−1 dilution, so we further diluted 10−2 to 10−4 with 9-mL PBS tubes. To determine the presence or absence of S. enterica, the BPW bags were incubated in an O2 atmosphere at 35°C for 18 to 24 h. Incubated BPW was then used to inoculate Brilliant Green Sulfa (BGS) agar and xylose lysine tergitol (XLT4) agar. The BGS and XLT4 agars were incubated at 35°C for 24 and 48 h. From the incubated BPW, 0.1 mL was transferred to a 10-mL Rappaport-Vassiliadis (RV) broth tube, and 1 mL was transferred to a 9-mL tube of Hajna Tetrathionate (HTT) broth. The RV and HTT broths were incubated at 42°C for 18 to 24 h. After incubation, the RV and HTT broths were plated on BGS and XLT4 agar, and the plates were incubated at 35°C for 24 and 48 h. All BGS and XLT4 plates were examined at 24 and 48 h of incubation for Salmonella-suspicious growth. All suspect colonies were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Serotyping and phagetyping was performed in the OIE Reference Centre for Salmonellosis, National Microbiology Laboratory at Guelph, Public Health Agency of Canada.
To obtain the E. coli concentration, 1 mL from each of the PBS serial dilution tubes was transferred to each of 4 E. coli Compact Dry count plates (Alere ULC, Ottawa, ON, Canada). The plates were incubated in an O2 atmosphere at 35°C for 24 h. After incubation, blue colonies only were enumerated using a Québec colony counter (Reichert, Inc., Depew, NY).
In order to determine the presence of the 2 genes that are associated with resistance to QACs, qacEΔ1 and sugE(p), a subset of E. coli isolates were subjected to PCR. This involved preparing a mastermix that contained 875 μL of water, 100 μL of 10× buffer, 20 μL of deoxyribonucleotide triphosphates with a final concentration of 200 μmol, and 2.5 μL of each primer with a final concentration of 250 nmol each (5′-AATCCATCCCTGTCGGTGTT-3′ and 5′-CGCAGCGACTTCCACGATGGGGAT-3′ for qacEΔ1 and 5′-GTCTTACGCCAAGCATTATCACTA-3′ and 5′-CAAGGCTCAGCAAACGTGC-3′ for sugE(p)), for a total volume of 1,000 μL. Then, 1.25 U of Taq polymerase was added to 25 μL of mastermix, and 24 μL of the mixture was transferred into a clean PCR tube where 1 μL of template solution was added. Template solution was prepared by boiling 1 loopful of E. coli in 500 mL of sterile water for 15 min; the boiled lysate was centrifuged for 3 min, and only the supernatant was used. The PCR conditions used were denaturation, 35 cycles at 95°C for 30 s; annealing, qacEΔ1 at 56°C for 25 s and sugE(p) at 57°C for 25 s; and extension, 68°C for 1 min, with the final extension step at 68°C for 5 min. Products were analyzed by electrophoresis on 2.0% agarose gels.
Statistical Analysis: Multilevel Logistic Regression Models
Laboratory and questionnaire data were entered manually into Microsoft Office Excel 2007 (Microsoft Corporation), where they were visually inspected for errors and coded if necessary. Once coded, the data were imported into STATA IC 13 (StataCorp, College Station, TX) for statistical analyses. The hierarchical structure of our models included samples within floors within visits within barn. There were 4 samples collected for microbiological analysis per floor, as many as 3 floors sampled per visit (depending on the number of stories), and 3 visits per barn. Sampling point was a fixed effect in the models indicating when the sample was collected relative to sanitation. However, the random effect for visit captured the weather or any factors present in the barn on the day of sampling.
Using data from all 3 sanitation groups, univariable logistic regression models, with random effects for barn, visit, and floor, were created to screen independent variables; it should be noted that explanatory variables specific to only wet cleaning and disinfection (e.g., relative water temperature, product used) were not used for this analysis because not all barns were wet-cleaned or disinfected, and therefore did not have observations. Variables that had a P value ≤ 0.20 on univariable analysis were considered for inclusion in a multivariable model. Sanitation procedure, sampling point, and flooring type were considered for further analysis regardless of significance on univariable screening, as they were a priori variables of interest. Collinearity between independent variables that met the screening criterion was assessed using Spearman's rank correlation coefficient. When 2 variables were deemed to be highly collinear (ρ ≥ |0.8|), the variable with the lowest Akaike information criterion value was considered for further analysis. Lowess curves were used to assess linearity between the log odds of the outcome (presence/absence of S. enterica) and continuous variables that met the screening criterion. If the linearity assumption was not met, and a quadratic term was not appropriate to model, the variable was categorized using cut points observed on the lowess curve.
All significant variables from the univariable analysis, as well as the a priori variables of interest, were offered to a multivariable logistic regression model with random effects for barn, visit, and floor. A manual backwards elimination method was used to build the model, with a P value ≤ 0.05 (Wald's test for dichotomous and continuous variables, likelihood ratio test for categorical variables) indicating significance. If, upon removal, a variable changed the coefficient of any significant variable by ≥ |20%|, it was considered to be a confounding variable and retained in the model regardless of statistical significance. All biologically plausible 2-way interactions with our a priori variables were generated and assessed using the likelihood ratio test. Using the lincom command in STATA, contrasts were built between interacting variables that included any of our a priori variables.
Sample-level Pearson residuals that were ≥ |3.0| SDs were considered to be outliers, and the raw data were inspected for possible errors, corrected accordingly, and the model refit. If there were no errors in the raw data, the outliers were kept in the model. To assess the fit of the model, the best linear unbiased predictors (BLUPS) for the barn-level residuals were plotted to assess normality and homoscedasticity. Finally, intraclass correlation coefficients (ICC) were estimated using the latent variable technique; this was done for each level of clustering.
Two additional multilevel, logistic regression models were built in a similar manner: 1) a S. enterica disinfection model that only included data from barns that had been disinfected (to identify additional factors for S. enterica specific to the disinfection step) and 2) a qacEΔ1 model that included a subset of E. coli samples (to identify factors associated with the presence of the qacEΔ1 gene). For the qacEΔ1 model, samples that were culture-negative for E. coli, E. coli-positive samples that were not tested for the qacEΔ1 gene, and samples from barns that had been wet-cleaned (due to low numbers in this group) were excluded from the analysis.
Statistical Analysis: Multilevel Linear Regression Models
Owing to a large number of zeros and the lack of normality in our data, a value of 2.5 was added to each raw E. coli concentration (cfu/g), and then the data were natural log transformed. The value of 2.5 came from taking one-quarter of the lowest nonzero value (William Sears, Statistical Consultant, Department of Population Medicine, University of Guelph, Guelph, ON, Canada, personal communication). Using data from all 3 sanitation groups, univariable linear regression models, with random effects for barn, visit, and floor, were created to screen independent variables in a manner similar to that described for the S. enterica data. Lowess curves were used to assess the linearity between the outcome (natural log transformed E. coli concentration) and continuous variables that met the screening criterion.
All significant variables from the univariable analysis, as well as our a priori variables of interest, were offered to a multivariable linear regression model with random effects for barn, visit, and floor. A manual backward elimination method was used to build the model, with a P-value ≤ 0.05 (t test for dichotomous and continuous variables, partial F-test for categorical variables) indicating significance. Confounding and interactions were assessed as described previously.
Sample-level standardized residuals that were ≥ |3.0| SDs were considered to be outliers, and the raw data were inspected for possible errors. To assess the fit of the model, the barn-level BLUPS were plotted to assess normality and homoscedasticity. Finally, ICC were estimated for each level of clustering.
An additional multilevel, linear regression model was built in a similar manner: an E. coli disinfection model that only included data from barns that had been disinfected (to identify additional factors for E. coli concentration specific to the disinfection step).
Results
A total of 696 samples were collected from the 36 commercial broiler chicken barns included in this study. Of the 36 barns, 16 (44.4%) were 1 story, 18 (50.0%) were 2 stories, and 2 (5.6%) were 3 stories. Sixteen (44.4%) of the barns were dry-cleaned only, 3 (8.3%) were wet-cleaned, and 17 (47.2%) were disinfected. A total of 312 samples were from barns that had been dry-cleaned only, 48 were from barns that used wet cleaning for their final sanitation procedure, and 336 were from barns that used disinfection as their final sanitation procedure.
There were 432 samples from concrete floors and 264 from wooden floors. All 36 barns had 1 concrete floor; thus, 16 concrete floors were dry-cleaned, 3 were wet-cleaned, and 17 were disinfected. All the 2-story and 3-story barns (20 barns in total) had upper wooden floors; thus, 10 wooden floors were dry-cleaned, 1 was wet-cleaned, and 11 were disinfected. Summary statistics of the categorical and continuous variables investigated are presented in Tables 1 and 2, respectively.
Table 1.
Descriptive characteristics of categorical variables in a study that investigated factors associated with the presence of S. enterica and the concentration of E. coli in samples collected from the floor(s) of commercial broiler chicken barns in Ontario during the rest period.
| Variable | Category | No. of observations |
|---|---|---|
| Sanitation procedure (n = 696)1 | Dry cleaning | 312 |
| Wet cleaning | 48 | |
| Disinfection | 336 | |
| Sampling point (n = 696) | Baseline | 232 |
| 2 d after sanitation | 232 | |
| 6 d after sanitation | 232 | |
| Flooring type (n = 696) | Concrete | 432 |
| Wood | 264 | |
| Season (n = 696)2 | Fall | 216 |
| Winter | 276 | |
| Spring | 48 | |
| Summer | 156 | |
| Floor number within barn (n = 696)3 | Bottom | 432 |
| Middle | 240 | |
| Top | 24 | |
| Total number of stories (n = 696) | One | 192 |
| Two | 432 | |
| Three | 72 | |
| Personnel entered barn after sanitation was completed (n = 696) | No | 96 |
| Yes | 600 | |
| Method of dry cleaning (n = 696) | Blower only | 420 |
| Blow and sweep | 240 | |
| Did not dry-clean | 36 | |
| Who completed dry cleaning (n = 696) | Producer | 492 |
| Farm employee | 168 | |
| Did not dry-clean | 36 | |
| Product used for wet cleaning (n = 384) | Water | 216 |
| Biosolve Plus4 | 12 | |
| Basic H5 | 24 | |
| Did not wet-clean before disinfection | 132 | |
| Method of wet cleaning (n = 384) | Spray | 216 |
| Not specified | 36 | |
| Did not wet-clean before disinfection | 132 | |
| Temperature of water for wet cleaning (n = 384) | Cold | 36 |
| Warm or hot | 216 | |
| Did not wet-clean before disinfection | 132 | |
| Pressure washer used for wet cleaning (n = 384) | No | 12 |
| Yes | 240 | |
| Did not wet-clean before disinfection | 132 | |
| Who completed the wet cleaning (n = 384) | Producer | 156 |
| Farm employee | 48 | |
| Contracted company | 48 | |
| Did not wet-clean before disinfection | 132 | |
| Product used for disinfection (n = 336) | QAC | 24 |
| Aldehyde | 48 | |
| Phenol | 12 | |
| Chlorine | 48 | |
| Peroxygen | 120 | |
| QAC/Aldehyde | 84 | |
| Method of disinfection (n = 336) | Spray | 180 |
| Foam | 60 | |
| Fog | 96 | |
| Temperature of disinfection (n = 336) | Cold | 132 |
| Warm or hot | 108 | |
| Not relevant to method | 96 | |
| Pressure washer used for disinfection (n = 336) | No | 48 |
| Yes | 192 | |
| Not relevant to method | 96 | |
| Who completed disinfection (n = 336) | Producer | 180 |
| Farm employee | 72 | |
| Contracted company | 84 | |
| Was the wet cleaning and disinfection done back-to-back (i.e., without allowing time for the surfaces to dry before applying the disinfectant) (n = 336) | No | 84 |
| Yes | 144 | |
| Did not wet-clean before disinfection | 108 | |
| Sanitation procedure used with previous flock (n = 696) | Dry clean only | 240 |
| Wet clean | 96 | |
| Disinfection | 360 | |
| No. of times per yr the barn is wet-cleaned (n = 696) | 0 | 240 |
| 1 | 324 | |
| 2 | 60 | |
| 3 | 24 | |
| 4 | 0 | |
| 5 | 24 | |
| 6 | 24 | |
| No. of times per yr the barn is disinfected (n = 696) | 0 | 120 |
| 1 | 120 | |
| 2 | 108 | |
| 3 | 48 | |
| 4 | 12 | |
| 5 | 48 | |
| 6 | 240 | |
| Cycle length (n = 696) | 8 wk | 228 |
| 9 wk | 300 | |
| 10 wk | 168 |
QAC, quaternary ammonium compound.
Dry cleaning: dry cleaning only; wet cleaning: dry cleaning followed by wet cleaning; disinfection: dry cleaning followed by wet cleaning followed by disinfection.
Fall: September 21st to December 20th; winter: December 21st to March 20th; spring: March 21st to June 20th; summer: June 21st to September 20th.
Variable created to determine if the location of the floor within the barn had a significant impact.
Biosolve Plus is an alkaline cleaner and degreaser sold by Vétoquinol.
Basic H is an organic cleaner sold by Shaklee.
Table 2.
Descriptive characteristics of continuous variables in a study that investigated factors associated with the presence of S. enterica and the concentration of E. coli in samples collected from the floor(s) of commercial broiler chicken barns in Ontario during the rest period.
| Variable | Mean (d) | Median (d) | Range (d) |
|---|---|---|---|
| Time between end of shipping and end of litter removal (n = 696) | 2.1 | 1.1 | 0.1 - 9.6 |
| Time between end of shipping and end of baseline sampling (n = 696) | 3.3 | 2.9 | 0.2 - 11.3 |
| Time between end of shipping and dry cleaning1 (n = 660) | 5.3 | 4.3 | 0.5 - 14.4 |
| Time between end of shipping and wet cleaning1 (n = 252) | 8.5 | 7.0 | 5.3 - 25.2 |
| Time between end of shipping and disinfection1 (n = 336) | 7.9 | 7.0 | 2.5 - 25.3 |
| Time between end of shipping and end of sanitation procedure (n = 696) | 7.2 | 6.5 | 1.5 - 25.3 |
| Time between end of shipping and end of 2-day postsanitation sampling (n = 696) | 9.0 | 8.0 | 2.2 - 26.4 |
| Time between end of shipping and end of 6-day postsanitation sampling (n = 696) | 12.7 | 12.1 | 6.6 - 29.4 |
| Time between end of litter removal and end of baseline sampling (n = 696) | 1.3 | 0.8 | -0.2 - 5.8 |
| Time between end of litter removal and end of dry cleaning (n = 660) | 3.1 | 2.0 | 0.1 - 9.8 |
| Time between end of litter removal and end of wet cleaning (n = 252) | 6.7 | 5.7 | 2.3 - 22.8 |
| Time between end of litter removal and end of disinfection (n = 336) | 6.0 | 4.1 | 1.0 - 22.8 |
| Time between end of litter removal and end of sanitation procedure (n = 696) | 4.8 | 4.3 | 0.2 - 22.8 |
| Time between end of litter removal and end of 2-day postsanitation sampling (n = 696) | 7.3 | 6.6 | 3.0 - 24.0 |
| Time between end of litter removal and end of 6-day postsanitation sampling (n = 696) | 10.7 | 9.7 | 5.2 - 27.0 |
| Time between end of baseline sampling and end of dry cleaning (n = 660) | 2.2 | 1.6 | -1.8 - 12.4 |
| Time between end of baseline sampling and end of wet cleaning (n = 252) | 5.0 | 4.0 | 2.2 - 17.0 |
| Time between end of baseline sampling and end of disinfection (n = 336) | 4.6 | 3.0 | 1.1 - 17.1 |
| Time between end of baseline sampling and end of sanitation procedure (n = 696) | 4.0 | 2.9 | 0.1 - 17.1 |
| Time between end of baseline sampling and end of 2-day postsanitation sampling (n = 696) | 6.0 | 5.1 | 3.0 - 18.2 |
| Time between end of baseline sampling and end of 6-day postsanitation sampling (n = 696) | 9.4 | 8.0 | 5.2 - 21.7 |
| Time between end of dry cleaning and end of wet cleaning (n = 228) | 3.8 | 3.8 | 0.2 - 15.5 |
| Time between end of dry cleaning and end of disinfection (n = 288) | 3.0 | 2.5 | 0.2 - 15.5 |
| Time between end of dry cleaning and end of 2-day postsanitation sampling (n = 660) | 4.0 | 3.1 | 1.3 - 17.1 |
| Time between end of dry cleaning and end of 6-day postsanitation sampling (n = 660) | 7.4 | 6.9 | 4.3 - 20.1 |
| Time between end of wet cleaning and end disinfection (n = 204) | 0.3 | 0.2 | 0.01 - 0.8 |
| Time between end of wet cleaning and end of 2-day postsanitation sampling (n = 252) | 2.4 | 2.1 | 1.2 - 3.9 |
| Time between end of wet cleaning and end of 6-day postsanitation sampling (n = 252) | 6.0 | 6.1 | 4.2 - 7.7 |
| Time between end of disinfection and end of 2-day postsanitation sampling (n = 336) | 2.2 | 2.0 | 1.0 - 3.1 |
| Time between end of disinfection and end of 6-day postsanitation sampling (n = 336) | 5.8 | 5.9 | 4.1 - 7.0 |
| Time between end of sanitation procedure and end of 2-day postsanitation sampling (n = 696) | 2.4 | 2.1 | 1.0 - 4.8 |
| Time between end of sanitation procedure and end of 6-day postsanitation sampling (n = 696) | 5.8 | 5.9 | 4.1 - 8.1 |
| Time between and end of 2-day postsanitation sampling and end of 6-day postsanitation sampling (n = 696) | 3.4 | 3.0 | 1.9 - 5.9 |
Dry cleaning: dry cleaning only; wet cleaning: dry cleaning followed by wet cleaning; disinfection: dry cleaning followed by wet cleaning followed by disinfection.
S. enterica: All Sanitation Procedures Model
At each sampling point, 232 samples were collected; thus, a total of 696 samples were tested for the presence of S. enterica. A total of 132 (19.0%) samples tested positive over the course of the study. Fifty-six of 232 (24.1%) baseline samples, 40 (17.2%) 2-day postsanitation samples, and 36 (15.5%) 6-day postsanitation samples tested positive. Forty-seven of 312 (15.1%) samples from dry-cleaned barns, 13 of 48 (27.1%) samples from wet-cleaned barns, and 72 of 336 (21.4%) samples from disinfected barns tested positive. Furthermore, 72 of 432 (16.7%) samples from concrete floors and 60 of 264 (22.7%) samples from wooden floors tested positive.
The final multivariable model included sanitation procedure, sampling point, flooring type, season, and an interaction between sanitation procedure and flooring type (Table 3). The odds of detecting S. enterica were lower if the sample was collected 2 d after sanitation (odds ratio [OR] = 0.36, P = 0.032) or 6 d after sanitation (OR = 0.28, P = 0.008) than at baseline and if the sample was collected during the winter/spring (OR = 0.02, P = 0.008) than at fall. The effect of flooring type on the presence of S. enterica depended on sanitation procedure: Wooden floors that had been wet-cleaned had 53 times higher odds (P = 0.001) of testing positive than concrete floors that had been dry-cleaned. Statistically significant (P ≤ 0.05) contrasts for the interaction between sanitation procedure and flooring type are presented in Supplementary File 1.
Table 3.
Logistic regression model of variables associated with the presence of S. enterica in samples collected from the floor(s) of commercial broiler chicken barns in Ontario during the rest period (n = 696 samples from 36 b; random effects for barn, visit, and floor; α = 0.05).
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Sanitation procedure1,2 | |||
| Dry cleaning (n = 312) | Referent | ||
| Wet cleaning (n = 48) | 0.33 | 0.002–59.53 | 0.674 |
| Disinfection (n = 336) | 5.67 | 0.35–91.46 | 0.221 |
| Sampling point1 | |||
| Baseline (n = 232) | Referent | ||
| 2 d After sanitation (n = 232) | 0.36 | 0.14–0.92 | 0.032 |
| 6 d After sanitation (n = 232) | 0.28 | 0.11–0.72 | 0.008 |
| Flooring type1 | |||
| Concrete (n = 432) | Referent | ||
| Wood (n = 264) | 0.41 | 0.15–1.11 | 0.078 |
| Sanitation procedure ∗ Floor type | |||
| Dry ∗ Concrete (n = 192) | Referent | ||
| Wet ∗ Wood (n = 12) | 53.00 | 4.96–566.75 | 0.001 |
| Disinfection ∗ Wood (n = 132) | 3.76 | 0.95–14.87 | 0.059 |
| Season3 | |||
| Fall (n = 216) | Referent | ||
| Winter/Spring (n = 324) | 0.02 | 0.001–0.35 | 0.008 |
| Summer (n = 156) | 0.30 | 0.009–9.36 | 0.489 |
| Variance components | Variance | SE | ICC4 |
|---|---|---|---|
| Barn | 12.10 | 6.17 | 0.76 |
| Visit | 0.54 | 0.54 | 0.79 |
| Floor | 3.61 × 10−34 | 2.38 × 10−17 | 0.79 |
Overall P value for the model: 0.0022.
Abbreviations: CI, confidence interval; OR, odds ratio; SE, significance of error.
Included regardless of significance because it was an a priori variable of interest.
Dry cleaning: dry cleaning only; wet cleaning: dry cleaning followed by wet cleaning; disinfection: dry cleaning followed by wet cleaning followed by disinfection.
Fall: September 21st to December 20th; winter: December 21st to March 20th; spring: March 21st to June 20th; summer: June 21st to September 20th.
ICC: intraclass correlation coefficient calculated using the latent variable technique (variance at sample-level = π2/3 = 3.29).
In the final model, there were 8 samples that were considered to be outliers. The removal of all these at once resulted in the main effect for flooring type becoming significant, and the interaction between disinfection and wooden floors becoming significant; however, there was no valid reason to remove any of these outliers from the model. The barn-level BLUPS were determined to be homoscedastic and fairly normally distributed. The ICC for barn-level, visit-level, and floor-level were 0.76, 0.79, and 0.79, respectively.
S. enterica: Disinfection Model
A total of 336 samples were collected from barns that had been disinfected. Of these, 72 (21.4%) samples tested positive for S. enterica. Thirty-five of 112 (31.3%) baseline samples, 20 (17.9%) 2-day postsanitation samples, and 17 (15.2%) 6-day postsanitation samples tested positive. Furthermore, 41 of 204 (20.1%) samples from concrete floors and 31 of 132 (23.5%) samples from wooden floors tested positive.
The final multivariable model included sampling point, flooring type (although not statistically significant), and season (Table 4). The odds of detecting S. enterica were lower if the sample was collected 2 d after sanitation (OR = 0.17, P = 0.007) or 6 d after sanitation (OR = 0.09, P = 0.002) than at baseline and if the sample was collected during the winter/spring (OR = 0.005, P = 0.005) than during summer/fall.
Table 4.
Logistic regression model of variables associated with the presence of S. enterica in samples collected from the floor(s) of commercial broiler chicken barns in Ontario that were disinfected during the rest period (n = 336 samples from 17 b; random effects for barn, visit, and floor; α = 0.05).
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Sampling point1 | |||
| Baseline (n = 112) | Referent | ||
| 2 d After sanitation (n = 112) | 0.17 | 0.04–0.61 | 0.007 |
| 6 d After sanitation (n = 112) | 0.09 | 0.02–0.40 | 0.002 |
| Flooring type1 | |||
| Concrete (n = 204) | Referent | ||
| Wood (n = 132) | 1.58 | 0.60–4.17 | 0.356 |
| Season2 | |||
| Summer/Fall (n = 144) | Referent | ||
| Winter/Spring (n = 192) | 0.005 | 0.0001–0.20 | 0.005 |
| Variance components | Variance | SE | ICC3 |
|---|---|---|---|
| Barn | 10.16 | 7.14 | 0.73 |
| Visit | 0.42 | 0.90 | 0.76 |
| Floor | 3.57 × 10−33 | 9.45 × 10−17 | 0.76 |
Overall P value for the model: 0.0021.
Abbreviations: CI, confidence interval; OR, odds ratio; SE, significance of error.
Included regardless of significance because it was an a priori variable of interest.
Fall: September 21st to December 20th; winter: December 21st to March 20th; spring: March 21st to June 20th; summer: June 21st to September 20th.
ICC: intraclass correlation coefficient calculated using the latent variable technique (variance at sample-level = π2/3 = 3.29).
In the final model, there were 3 samples that were considered to be outliers. The removal of all these at once or individually did not result in any major changes to any of the variables. The barn-level BLUPS were determined to be homoscedastic and fairly normally distributed. The ICC for barn-level, visit-level, and floor-level were 0.73, 0.76, and 0.76, respectively.
S. enterica: Serovars and Phagetypes
A total of 16 serovars were identified (Table 5); the 2 most common serovars—S. Kentucky and S. Heidelberg—were found in approximately equal proportions in dry-cleaned and disinfected barns. Phagetypes (PT) included S. Enteritidis PT 8 (n = 1); S. Heidelberg PT 5 (n = 12), 19 (n = 11), 41 (n = 1), and 47 (n = 6); and S. Typhimurium PT 193 (n = 1).
Table 5.
Descriptive summary of S. enterica serovars isolated from samples collected from the floor(s) of commercial broiler chicken barns in Ontario during the rest period, stratified by barn sanitation procedure (n = 139 isolates from 132 samples).
| Serovar | Total no. of isolates | Dry cleaning1 | Wet cleaning1 | Disinfection1 |
|---|---|---|---|---|
| Braenderup | 9 | 0 | 0 | 9 |
| Enteritidis | 1 | 0 | 0 | 1 |
| Heidelberg | 30 | 17 | 0 | 13 |
| I:4,12:r:- | 1 | 0 | 0 | 1 |
| I:6,7,14:k:- | 1 | 0 | 0 | 1 |
| I:8,20:-:- | 1 | 1 | 0 | 0 |
| I:ROUGH-O:i:z6 | 1 | 0 | 0 | 1 |
| I:Rough-O:z10:e,n,z15 | 1 | 0 | 0 | 1 |
| Infantis | 3 | 3 | 0 | 0 |
| Kentucky | 56 | 31 | 0 | 25 |
| Liverpool | 5 | 0 | 0 | 5 |
| Livingstone | 3 | 0 | 0 | 3 |
| Mbandaka | 13 | 1 | 0 | 12 |
| Schwarzengrund | 11 | 0 | 11 | 0 |
| Thompson Var. 14+ | 1 | 0 | 0 | 1 |
| Typhimurium | 1 | 0 | 1 | 0 |
| No growth | 1 | 0 | 1 | 0 |
7 samples were positive for 2 serovars; S. Kentucky and S. Heidelberg (n = 6), S. Kentucky and I:ROUGH-O:i:z6 (n = 1).
Dry cleaning: dry cleaning only; wet cleaning: dry cleaning followed by wet cleaning; disinfection: dry cleaning followed by wet cleaning followed by disinfection.
E. coli: All Sanitation Procedures Model
Raw E. coli values ranged from 0 to 700,000 cfu/g, with 109 of 696 (15.7%) samples having zero counts. Of the samples with a zero count, none were collected at baseline, 49 (45.0%) were collected 2 d after sanitation, and 60 (55.0%) were collected 6 d after sanitation. The mean concentration was 8,575.1, and the 25th, 50th, and 75th percentiles were 55, 735, and 4,250, respectively.
The final multivariable model included sanitation procedure, sampling point, flooring type, season, and an interaction between sanitation procedure and sampling point (Table 6). The effect of sampling point on the E. coli concentration depended on the sanitation procedure used: Compared with samples collected at baseline from barns that had been dry-cleaned, the concentration was lower in samples collected 2 d after sanitation (β = −2.49, P < 0.001) or 6 d after sanitation (β = −3.20, P < 0.001) from barns that had been disinfected (Table 6). Statistically significant (P ≤ 0.05) contrasts for the interaction between sanitation procedure and sampling point are presented in Supplementary File 2. The E. coli concentration was higher on wooden floors (β = 0.53, P = 0.008) than on concrete floors and in summer (β = 1.31, P = 0.023) than in fall (Table 6). The E. coli concentration was lower in winter (β = −1.06, P = 0.022) than in fall.
Table 6.
Linear regression model of variables associated with the concentration of E. coli (natural log cfu/g) in samples collected from the floor(s) of commercial broiler chicken barns in Ontario during the rest period (n = 696 samples from 36 b; random effects for barn, visit, and floor; α = 0.05).
| Variable | Coefficient | 95% CI | P value |
|---|---|---|---|
| Sanitation procedure1,2 | |||
| Dry cleaning (n = 312) | Referent | ||
| Wet cleaning (n = 48) | −0.86 | −2.78–1.05 | 0.375 |
| Disinfection (n = 336) | 0.54 | −0.53–1.61 | 0.325 |
| Sampling point1 | |||
| Baseline (n = 232) | Referent | ||
| 2 d After sanitation (n = 232) | −2.26 | −3.07–−1.45 | <0.001 |
| 6 d After sanitation (n = 232) | −2.10 | −2.91–−1.29 | <0.001 |
| Flooring type1 | |||
| Concrete (n = 432) | Referent | ||
| Wood (n = 264) | 0.53 | 0.14–0.91 | 0.008 |
| Sanitation procedure ∗ Sampling point | |||
| Dry ∗ baseline (n = 104) | Referent | ||
| Wet ∗ 2 d after sanitation (n = 16) | 0.49 | −1.65–2.63 | 0.656 |
| Wet ∗ 6 d after sanitation (n = 16) | −0.23 | −2.37–1.91 | 0.832 |
| Disinfection ∗ 2 d after sanitation (n = 112) | −2.49 | −3.62–-1.37 | <0.001 |
| Disinfection ∗ 6 d after sanitation (n = 112) | −3.20 | −4.33–−2.08 | <0.001 |
| Season3 | |||
| Fall (n = 216) | Referent | ||
| Winter (n = 276) | −1.06 | −1.97–−0.15 | 0.022 |
| Spring (n = 48) | −0.07 | −1.47–1.33 | 0.923 |
| Summer (n = 156) | 1.31 | 0.18–2.44 | 0.023 |
| Variance components | Variance | SE | ICC |
|---|---|---|---|
| Barn | 0.84 | 0.32 | 0.19 |
| Visit | 0.53 | 0.24 | 0.31 |
| Floor | 0.69 | 0.21 | 0.47 |
| Sample | 2.35 | 0.15 |
Overall P value for the model: < 0.0001.
Abbreviations: CI, confidence interval; ICC, intraclass correlation coefficient; SE, significance of error.
Included regardless of significance because it was an a priori variable of interest.
Dry cleaning: dry cleaning only; wet cleaning: dry cleaning followed by wet cleaning; disinfection: dry cleaning followed by wet cleaning followed by disinfection.
Fall: September 21st to December 20th; winter: December 21st to March 20th; spring: March 21st to June 20th; summer: June 21st to September 20th.
In the final model, there were 3 samples that were considered to be outliers. The removal of these collectively or individually resulted in no major changes to the model. The barn-level BLUPS were determined to be homoscedastic and normally distributed. The ICC for barn-level, visit-level, and floor-level were 0.19, 0.31, and 0.47, respectively.
E. coli: Disinfection Model
Raw E. coli values for disinfected barns ranged from 0 to 202,000 cfu/g, with 99 of 336 (29.5%) samples having zero counts. The mean concentration was 7,082.3, and the 25th, 50th, and 75th percentiles were 0, 200, and 2,950, respectively.
The final multivariable model included sampling point and flooring type (Table 7). In disinfected barns, the E. coli concentration was lower if the sample was collected 2 d after sanitation (β = −4.74, P < 0.001) or 6 d after sanitation (β = −5.30, P < 0.001) than at baseline and higher on wooden floors (β = 0.69, P = 0.043) than on concrete floors.
Table 7.
Linear regression model of variables associated with the concentration of E. coli (natural log cfu/g) in samples collected from the floor(s) of commercial broiler chicken barns in Ontario that were disinfected during the rest period (n = 336 samples from 17 b; random effects for barn, visit, and floor; α = 0.05).
| Variable | Coefficient | 95% CI | P value |
|---|---|---|---|
| Sampling point1 | |||
| Baseline (n = 112) | Referent | ||
| 2 d After sanitation (n = 112) | -4.74 | −5.62–−3.86 | <0.001 |
| 6 d After sanitation (n = 112) | -5.29 | −6.17–−4.41 | <0.001 |
| Flooring type1 | |||
| Concrete (n = 204) | Referent | ||
| Wood (n = 132) | 0.69 | 0.02–1.36 | 0.043 |
| Variance components | Variance | SE | ICC |
|---|---|---|---|
| Barn | 1.65 | 0.78 | 0.27 |
| Visit | 0.51 | 0.42 | 0.35 |
| Floor | 1.21 | 0.43 | 0.55 |
| Sample | 2.78 | 0.25 |
Overall P value for the model: < 0.0001.
Abbreviations: CI, confidence interval; ICC, intraclass correlation coefficient; SE, significance of error.
Included regardless of significance because it was an a priori variable of interest.
In the final model, there was 1 sample that was considered to be an outlier. The removal of this observation resulted in no major changes to the model. The barn-level BLUPS were determined to be homoscedastic and normally distributed. The ICC for barn-level, visit-level, and floor-level were 0.27, 0.35, and 0.55, respectively.
E. coli: qacEΔ1 Model
A total of 342 E. coli isolates were tested for the presence of qacEΔ1, of which 69 (20.2%) tested positive. Twenty-five of 135 (18.5%) baseline isolates, 21 of 99 (21.2%) 2-day postsanitation isolates, and 23 of 108 (21.3%) 6-day postsanitation isolates tested positive. Forty-two of 214 (19.6%) isolates from dry-cleaned barns, 2 of 4 (50.0%) isolates from wet-cleaned barns, and 25 of 124 (20.2%) isolates from disinfected barns tested positive. Furthermore, 33 of 202 (16.3%) isolates from concrete floors and 36 of 140 (25.7%) isolates from wooden floors tested positive.
The final multivariable model included sanitation procedure (dry-cleaned and disinfected barns only), sampling point (although not statistically significant), flooring type, cycle length, the number of times per yr the barn is disinfected, and an interaction between flooring type and the number of times per yr the barn is disinfected (Table 8). The odds of having a qacEΔ1-positive isolate, among the tested E. coli, were lower if the barn was disinfected (OR = 0.40, P = 0.044) than when dry-cleaned and higher if the cycle length was 8 wk (OR = 6.03, P = 0.001) than if 10 wk. The effect of flooring type on the presence of qacEΔ1 depended on the number of times per yr the barn was disinfected: Wooden floors in barns that are disinfected after every flock had lower odds of having a qacEΔ1-positive isolate among the tested E. coli (OR = 0.22, P = 0.047) than concrete floors in barns that are disinfected 1 or fewer times per yr.
Table 8.
Logistic regression model of variables associated with the presence of qacEΔ1 in E. coli isolates from samples collected from the floor(s) of commercial broiler chicken barns in Ontario during the rest period (n = 338 isolates from 24 b; random effects for barn, visit, and floor; α = 0.05).
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Sanitation procedure1,2 | |||
| Dry cleaning (n = 214) | Referent | ||
| Disinfection (n = 124) | 0.40 | 0.16–0.98 | 0.044 |
| Sampling point1 | |||
| Baseline (n = 133) | Referent | ||
| 2 d After sanitation (n = 98) | 0.94 | 0.43–2.01 | 0.864 |
| 6 d After sanitation (n = 107) | 0.91 | 0.43–1.94 | 0.815 |
| Flooring type1 | |||
| Concrete (n = 198) | Referent | ||
| Wood (n = 140) | 7.23 | 2.13–24.47 | 0.001 |
| No. of times per yr the barn is disinfected | |||
| 0 or 1 (n = 116) | Referent | ||
| 2 to 4 (n = 84) | 1.67 | 0.44–6.42 | 0.452 |
| Every (n = 138) | 6.57 | 1.94–22.23 | 0.002 |
| No. of times per yr the barn is disinfected ∗ Flooring type | |||
| 0 or 1 ∗ Concrete (n = 78) | Referent | ||
| 2 to 4 ∗ Wood (n = 42) | 0.20 | 0.03–1.34 | 0.098 |
| Every ∗ Wood (n = 60) | 0.22 | 0.05–0.98 | 0.047 |
| Cycle length | |||
| 10 wk (n = 104) | Referent | ||
| 8 wk (n = 98) | 6.03 | 2.14–17.00 | 0.001 |
| 9 wk (n = 136) | 1.59 | 0.53–4.79 | 0.408 |
| Variance components | Variance | SE | ICC3 |
|---|---|---|---|
| Barn | 2.41 × 10−34 | 5.20 × 10−18 | 7.09 × 10−35 |
| Visit | 1.26 × 10−33 | 1.57 × 10−17 | 4.42 × 10−34 |
| Floor | 0.12 | 0.47 | 0.03 |
Overall P value for the model: <0.0001.
Abbreviations: CI, confidence interval; ICC, intraclass correlation coefficient; OR, odds ratio; SE, significance of error.
Included regardless of significance because it was an a priori variable of interest.
Dry cleaning: dry cleaning only; disinfection: dry cleaning followed by wet cleaning followed by disinfection.
ICC: intraclass correlations calculated using the latent variable technique (variance at sample level = π2/3 = 3.29).
In the final model, there were 9 samples that were considered to be outliers. The removal of all these at once resulted in sanitation procedure becoming nonsignificant, and the interaction between wooden floor and disinfecting the barn 2 to 4 times per yr becoming significant. However, there was no valid reason to remove any of these outliers from the model. The barn-level BLUPS were determined to be homoscedastic and normally distributed. The ICC for barn-level, visit-level, and floor-level were 7.09 × 10−35, 4.42 × 10−34, and 0.03, respectively.
E. coli: sugE(p)
A total of 342 E. coli isolates were tested for the presence of sugE(p), of which 17 (5.0%) tested positive. Three of 135 (2.2%) baseline isolates, 8 of 99 (8.1%) 2-day postsanitation isolates, and 6 of 108 (5.6%) 6-day postsanitation isolates tested positive. Seventeen of 214 (7.9%) isolates from dry-cleaned barns, 0 of 4 isolates from wet-cleaned barns, and 0 of 124 isolates from disinfected barns tested positive. Of 17 positive isolates, 11 (64.7%) were from concrete floors, 13 (76.5%) were from 2-story barns, 11 (64.7%) were from barns on an 8-week cycle, and 11 (64.7%) were from barns that were disinfected after the flock prior to our study flock. Winter had the highest number of positive isolates (11), whereas fall had the lowest (0). Most positive isolates (11) were from barns that are wet-cleaned more than 2 times per yr, whereas no positive isolates were from barns that are never wet-cleaned. Barns that are disinfected after every crop also yielded the highest number of positive isolates (10), whereas barns that are disinfected 0 or 1 time per yr had the lowest number of positive isolates (3).
Of the 342 isolates that were tested for both resistance genes, 58 (17.0%) were positive for qacEΔ1 only, 6 (1.8%) were positive for sugE(p) only, and 11 (3.2%) were positive for both resistance genes.
Discussion
One of our 3 main variables of interest was the sanitation procedure performed by the producer. Our findings indicate that for S. enterica, the effect of the sanitation procedure depended on the flooring type, in that the risk of S. enterica contamination was higher on wet-cleaned wooden floors than on dry-cleaned concrete floors. For E. coli, the effect of the sanitation procedure depended on the sampling point, in that compared with presanitation samples collected from dry-cleaned barns, the E. coli concentration was lower in postsanitation samples collected from disinfected barns. Davies and Wray (1995) showed that S. enterica and coliform populations increase during the pressure washing process often used before disinfection, whereas others have shown that using a high-pressure rinse before disinfection was more effective against E. coli (Berrang and Northcutt, 2005) and S. enterica (Hinojosa et al., 2018) than a low-pressure rinse. Gibson et al. (1999) showed that high-pressure water sprayers increase the number of tiny aerosol droplets in the air for a longer duration than low-pressure sprayers, potentially spreading organisms throughout the barn rather than washing them away. It has been suggested that water use be limited for controlling S. enterica because of the possibility of reactivating dormant organisms that have been left behind (Rose et al., 2000). Of note, of the 17 disinfected barns in our study, 2 (11.8%) were not dry-cleaned and 4 (23.5%) were not wet-cleaned before disinfection. This highlights the reality of how broiler barns are cleaned within the framework of the On-Farm Food Safety Assurance Program (Chicken Farmers of Canada, 2018), and although the proportion of producers varying from expected practices was relatively low in our study, the effect of omitting 1 or both of these steps on pathogen prevalence and concentration on different flooring types warrants further research. Among the E. coli isolates, the risk of carrying qacEΔ1 was lower in isolates from disinfected barns than in those from dry-cleaned barns. The protective effect of disinfection on the presence of this gene is an interesting finding, given that qacEΔ1 is associated with QAC resistance (Zou et al., 2014). Possible explanations include removal of organic material before disinfection (Payne et al., 2005; Ward et al., 2006), application of the disinfectant according to the manufacturer's instructions, and use of non-QAC disinfectants. The use of quaternary ammonium alone was relatively uncommon among the producers who disinfected their barn, although the use of a quaternary ammonium-aldehyde combination was somewhat common. However, we were unable to investigate disinfectants further through modeling because of a large variety of products with relatively low numbers of observations per disinfectant type. Additional research on the effects of short- and long-term use of QACs for barn disinfection on qacEΔ1 in E. coli isolates would help to support or refute our finding.
Although we did not have a detailed variable that represented the whole time between flock shipment and placement of the subsequent flock, we did assess multiple time points within the rest period: after litter removal and before cleaning began (baseline); 2 d after the sanitation procedure was completed; and 6 d after the sanitation procedure was completed. The time of sample collection was significantly associated with the presence of S. enterica and the quantity of E. coli on barn floors during the rest period, yet not with the presence of qacEΔ1 in E. coli isolates. For S. enterica, the risk was lower if the sample was collected after sanitation than before sanitation (i.e., baseline), and this effect was consistent for both S. enterica models. For E. coli, when data from all 3 sanitation groups were considered, the effect of the sampling point depended on the sanitation procedure used (as described previously). However, when the analysis was limited to barns that had been disinfected, a direct effect was identified, in that the E. coli concentration was lower if the sample was collected after sanitation than before sanitation. These findings suggest that, independent of the method of sanitation carried out by the producer, both S. enterica and E. coli are less likely to be present in the barn after sanitation than before sanitation, which highlights the importance of cleaning after litter removal. Research on the impact of downtime between flocks suggests that shorter downtimes increase the occurrence of pathogens in broiler flocks (Berndtson et al., 1996; Hald et al., 2000; Wedderkopp et al., 2000) and that 2 wk of clean downtime is ideal (Nespeca et al., 1997), although Voss-Rech et al. (2019) found that the length of downtime between flocks did not influence the prevalence of S. enterica. From our qacEΔ1 model, we found that the risk of carrying qacEΔ1, among E. coli isolates tested, was higher in isolates from barns on an 8-wk cycle than in those on a 10-week cycle. Although the reason for this finding is unknown, we initially considered that shorter cycles might have shorter downtimes, which could affect the thoroughness of cleaning or prevent the barn from drying completely, thereby trapping moisture under the fresh bedding placed for the next flock. When stratified by cycle length, the maximum time between the end of shipping and the end of the 6-day postsanitation sampling for barns on an 8-week, 9-week, and 10-week cycle was 18.2, 29.4, and 17.4 d, respectively. However, as these represent our sample collection methodology rather than the full rest period, this premise is not testable using the data from this study.
The flooring material was associated with both the presence of S. enterica and the quantity of E. coli on barn floors, as well as the presence of qacEΔ1 in E. coli isolates. For S. enterica, when data from all 3 sanitation groups were considered, the effect of the flooring type depended on the sanitation procedure used, with wet-cleaned wooden floors having the highest risk of S. enterica (as described previously). However, when the analysis was limited to barns that had been disinfected, wooden floors did not carry a significantly higher risk of S. enterica than concrete floors. For E. coli, the concentration was higher on wooden floors than on concrete floors, and this effect was consistent for both E. coli models. Our findings are consistent with previous research, which has shown that rough surfaces tend to be more contaminated than smooth ones (Madec et al., 1999) and older wooden structures that have organic material ingrained in them are more difficult to clean (Berchieri and Barrow, 1996). Volkova et al. (2011) showed that using wood to cover the foundation or the base of the walls in a barn increased the probability of detecting S. enterica in the litter. Similar to S. enterica and E. coli, the risk of qacEΔ1 was higher in E. coli isolates from wooden floors than in those from concrete floors, although the effect depended on the number of times per yr the barn was disinfected; the risk was reduced if the wooden floors were disinfected frequently (i.e., after every flock). The latter finding was unexpected, given the known link between qacEΔ1 and resistance to QACs (Zou et al., 2014), and could be related to the use of non-QAC products for disinfection. However, it could also indicate that repeated disinfection has merit, as Singer et al. (2000) showed that repeated cleaning and disinfection is needed to diminish or eliminate specific cellulitis-associated E. coli. Research investigating the long-term effect of disinfection (e.g., over a 1- to 2-yr period), including the disinfectants used and the disinfection process itself, would be beneficial to understand this finding.
We deemed it was important to include season in the model-building process to account for the cyclical fluctuation in weather patterns in southern Ontario that could affect pathogen presence or concentration. Although there were 48 to 276 samples collected per season, each season was repeated only once because of the relatively short study period (11 mo); thus, our ability to interpret the effect of season is limited. Notwithstanding, we found that the season of grow-out was significantly associated with the presence of S. enterica and the quantity of E. coli, yet not with the presence of qacEΔ1. For S. enterica, the risk was lower during the winter or spring than during the fall in both of our models. Prior research has indicated that summer/fall has the highest prevalence of S. enterica (Angen et al., 1996; van der Fels-Klerx et al., 2008; Valcheva et al., 2011; Zdragas et al., 2012), although some research has shown that there is no seasonal effect (Meldrum et al., 2004; Jordan et al., 2006; van de Giessen et al., 2006). For E. coli, the concentration was lower during the winter and higher in the summer than in the fall; however, when the analysis was limited to barns that had been disinfected, season was no longer a significant explanatory variable. There is limited research on the seasonality of E. coli prevalence or concentration in poultry barns; however, it has been stated that both biotic (competition) and abiotic (pH, temperature) factors likely impact the ability of E. coli to survive in its secondary environment (i.e., outside the intestinal tract of the animal) (Franz et al., 2014). Research into Campylobacter, another gram-negative bacterium, has consistently shown that intestinal colonization of broiler chickens peaks in the summer; this finding has been attributed to the humidity and higher temperatures and to increased rodent and insect activity (Hald et al., 2004, 2007; Nichols, 2005; Meerburg et al., 2006; Guerin et al., 2008; Zweifel et al., 2008).
Similar to qacEΔ1, sugE(p) also coexists with other resistance genes and is associated with resistance to QACs (Zou et al., 2014). There were too few sugE(p)-positive isolates to build a regression model; therefore, we could not explore potential explanatory variables for the presence of this resistance gene. All the sugE(p)-positive isolates were from barns that conducted a dry cleaning. However, approximately two-third of the positive isolates were from dry-cleaned barns that had been disinfected, with an unknown product, before the study. Previous research has shown that QAC resistance genes that are on mobile genetic elements, including sugE(p), were relatively low in frequency in E. coli isolates from retail meat (Zou et al., 2014). Future research would benefit from investigating a larger number of isolates from barns that were disinfected to increase the likelihood of detecting the gene, as well as looking into possible reasons why the gene might be isolated from barns that had conducted a disinfection for the flock prior and not for the flock being sampled.
Several of the time intervals investigated were significant on univariable analysis, yet could not be transformed in a meaningful way to become linear with the outcome, or were highly collinear with each other and were therefore excluded from the multivariable models. This suggests that the time between each step in the sanitation process might be important with respect to pathogen prevalence or concentration and should therefore be a focus of future research. The impact of wet cleaning on S. enterica and E. coli in poultry barns should also be a focus of future research, as there were only a small number of barns enrolled in our study in which wet cleaning was conducted. Such studies should include a comparison of barns that are wet-cleaned with detergent to those that are cleaned solely with water, as we noted that some producers did not use a detergent for washing.
For the S. enterica models, the majority of the variation was at the barn level, suggesting that barn-level interventions could have the most impact at reducing the prevalence of S. enterica under commercial conditions. Our findings are in line with a Danish study on risk factors for S. enterica serovar 4, 12:b:- in broiler chickens, in which random effects at the house and/or farm level were statistically significant (Chadfield et al., 2001). One such barn-level factor could be bedding type, as there is some evidence to suggest that S. enterica is detected frequently in wood shavings (Völkel et al., 2011; Volkova et al., 2011). In Ontario, approximately 70% of broiler farms use chopped straw as bedding, with the remainder using pine shavings (∼20%) or peat moss (∼10%). However, as our research focused on factors during the rest period between flocks rather than during the grow-out period, the composition of the litter was not included in the questionnaire; thus, we do not know whether it was the same in all barns. For the E. coli models, the relatively high correlation (0.47–0.55) between samples from the same floor likely reflects the highly dynamic state of the intestinal flora of growing birds (Pleydell et al., 2007) and reinforces the need for repeated samples when investigating E. coli in poultry barns. There was also moderate correlation (0.31–0.35) between samples from different floors during the same visit, indicating that the inclusion of sampling point in the E. coli models did not account for all the visit-to-visit variations in concentration. This finding is consistent with a study that investigated antimicrobial-resistant E. coli on dairy farms in Ohio, in which there was a relatively high correlation among fecal samples with resistant E. coli at the time of a visit (Medhanie et al., 2016). The researchers suggested that this could be indicative of farm management or environmental factors that vary by visit or season (Medhanie et al., 2016), although these could be hard to identify.
Although our study was conducted under commercial conditions in Ontario, there were limitations. First, involvement was entirely voluntary; therefore, it is possible that producers who use stricter cleaning protocols or were sure of their cleaning procedures, as outlined in the On-Farm Food Safety Assurance Program (Chicken Farmers of Canada, 2018), agreed to participate, leading to selection bias. Second, interviews were conducted at least 2 d after the sanitation procedure was completed; this was a potential cause of recall bias, as some of the producers could not recall specific details of the procedures or products or the specific times at which they finished each step of the sanitation procedure.
In summary, this study has shown that risk factors for S. enterica and E. coli differ; disinfection appears to be more favorable for the reduction of both E. coli and the presence of the qacEΔ1 gene found in E. coli isolates, whereas dry cleaning appears to be more favorable for the reduction of S. enterica. Our study has also shown that both S. enterica and E. coli are less likely to be present in the barn after sanitation than before sanitation, which highlights the importance of cleaning after litter removal, although no sanitation procedure will remove all these bacteria from the broiler chicken environment. Other factors that were found to impact both S. enterica and E. coli were flooring type and season; for new barns, concrete floors, rather than wooden floors, are recommended. It was also found that the majority of the variations in S. enterica presence and E. coli concentration could be explained by differences between barns and between samples, respectively. A surprising finding was that none of the explanatory variables related to the wet cleaning and disinfection procedures were significant in any of the final models. Thus, future research should consider strategies to address the considerable variability in cleaning methods to investigate specific aspects of barn sanitation. Future research should include other economically important bacteria and viruses to guide the development of comprehensive cleaning and disinfection standards to control the wide array of pathogens that infect poultry.
Acknowledgments
The authors gratefully acknowledge the producers who participated in this study, as well as Gabhan Chalmers (Research Associate, Department of Pathobiology, University of Guelph), who conducted the PCRs for detection of the quaternary ammonium resistance genes in the E. coli isolates. Funding was provided by the Canadian Poultry Research Council—Agriculture and Agri-Food Canada Poultry Science Cluster (AIP-CL11), under the Growing Forward 2 program; and Ontario Ministry of Agriculture, Food and Rural Affairs under the Food Safety Research Program (FS2012-1469).
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2021.101065.
Disclosures
The authors declare no conflicts of interest.
Supplementary data
References
- Angen Ø., Skov M.N., Chriél M., Agger J.F., Bisgaard M. A retrospective study on Salmonella infection in Danish broiler flocks. Prev. Vet. Med. 1996;26:223–237. [Google Scholar]
- Berchieri A., Barrow P.A. The antibacterial effects for Salmonella Enteritidis phage type 4 of different chemical disinfectants and cleaning agents tested under different conditions. Avian Pathol. 1996;25:663–673. doi: 10.1080/03079459608419173. [DOI] [PubMed] [Google Scholar]
- Berrang M.E., Northcutt J.K. Water spray and immersion in chemical sanitizer to lower bacterial numbers on the broiler transport coop flooring. J. Appl. Poult. Res. 2005;14:315–321. doi: 10.1093/ps/84.11.1797. [DOI] [PubMed] [Google Scholar]
- Berndtson E., Emanuelson U., Engvall A., Danielsson-Tham M.L. A 1-year epidemiological study of campylobacters in 18 Swedish chicken farms. Prev. Vet. Med. 1996;26:167–185. [Google Scholar]
- Buffet-Bataillon S., Tattevin P., Bonnaure-Mallet M., Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds - a critical review. Int. J. Antimicrob. Agents. 2012;39:381–389. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Cardinale E., Tall F., Guèye E.F., Cisse M., Salvat G. Risk factors for Salmonella enterica subsp. enterica infection in Senegalese broiler-chicken flocks. Prev. Vet. Med. 2004;63:151–161. doi: 10.1016/j.prevetmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Chadfield M., Skov M., Christensen J., Madsen M., Bisgaard M. An epidemiological study of Salmonella enterica serovar 4, 12:b:- in broiler chickens in Denmark. Vet. Microbiol. 2001;82:233–247. doi: 10.1016/s0378-1135(01)00347-9. [DOI] [PubMed] [Google Scholar]
- Chen H.M., Wang Y., Su L.H., Chiu C.H. Nontyphoid Salmonella infection: Microbiology, clinical features, and antimicrobial therapy. Pediatr. Neonatol. 2013;54:147–152. doi: 10.1016/j.pedneo.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Chicken Farmers of Canada Chicken Farmers of Canada On-Farm Food Safety Assurance Program Manual. 2018. https://www.chickenfarmers.ca/wp-content/uploads/2014/07/OFFSAP-Manual-2014-with-2018-update.pdf
- Davies R.H., Wray C. Observations on disinfection regimens used on Salmonella Enteritidis infected poultry units. Poult. Sci. 1995;74:637–647. doi: 10.3382/ps.0740638. [DOI] [PubMed] [Google Scholar]
- Davies R.H., Wray C. Persistence of Salmonella Enteritidis in poultry units and poultry food. Br. Poult. Sci. 1996;37:589–596. doi: 10.1080/00071669608417889. [DOI] [PubMed] [Google Scholar]
- Ewers C., Antão E.M., Diehl I., Philipp H.C., Wieler L.H. Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential. Appl. Environ. Microbiol. 2009;75:184–192. doi: 10.1128/AEM.01324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley S.L., Lynne A.M., Nayak R. Salmonella challenges: prevalence in swine and poultry and potential pathogenicity of such isolates. J. Anim. Sci. 2008;86:146–162. doi: 10.2527/jas.2007-0464. [DOI] [PubMed] [Google Scholar]
- Franz E., Schijven J., De Roda Husman A.M., Blaak H. Meta-regression analysis of commensal and pathogenic Escherichia coli survival in soil and water. Environ. Sci. Technol. 2014;48:6763–6771. doi: 10.1021/es501677c. [DOI] [PubMed] [Google Scholar]
- Garber L., Smeltzer M., Fedorka-Cray P., Ladely S., Ferris K. Salmonella enterica serotype Enteritidis in table egg layer house environments and in mice in US layer houses and associated risk factors. Avian Dis. 2003;47:134–142. doi: 10.1637/0005-2086(2003)047[0134:SESEIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Gast R.K. Serotype-specific and serotype-independent strategies for preharvest control of food-borne Salmonella in poultry. Avian Dis. 2007;51:817–828. doi: 10.1637/8090-081807.1. [DOI] [PubMed] [Google Scholar]
- Gibson H., Taylor J.H., Hall K.E., Holah J.T. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J. Appl. Microbiol. 1999;84:41–48. doi: 10.1046/j.1365-2672.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- Gradel K.O., Jørgensen J.C., Andersen J.S., Corry J.E.L. Monitoring the efficacy of steam and formaldehyde treatment of naturally Salmonella-infected layer houses. J. Appl. Microbiol. 2004;96:613–622. doi: 10.1111/j.1365-2672.2004.02198.x. [DOI] [PubMed] [Google Scholar]
- Guerin M.T., Martin S.W., Reiersen J., Berke O., McEwen S.A., Fridriksdóttir V., Bisaillon J.R., Lowman R. Temperature-related risk factors associated with the colonization of broiler-chicken flocks with Campylobacter spp. in Iceland, 2001-2004. Prev. Vet. Med. 2008;86:14–29. doi: 10.1016/j.prevetmed.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Hald B., Wedderkopp A., Madsen M. Thermophilic Campylobacter spp. in Danish broiler production: a cross-sectional survey and a retrospective analysis of risk factors for occurrence in broiler flocks. Avian Pathol. 2000;29:123–131. doi: 10.1080/03079450094153. [DOI] [PubMed] [Google Scholar]
- Hald B., Skovgård H., Bang D.D., Pedersen K., Dybdahl J., Jespersen J.B., Madsen M. Flies and Campylobacter infection of broiler flocks. Emerg. Infect. Dis. 2004;10:1490–1492. doi: 10.3201/eid1008.040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald B., Sommer H.M., Skovgård H. Use of fly screens to reduce Campylobacter spp. introduction in broiler houses. Emerg. Infect. Dis. 2007;13:1951–1953. doi: 10.3201/eid1312.070488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegstad K., Langsrud S., Lunestad B.T., Scheie A.A., Sunde M., Yazdankhah S.P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010;16:91–104. doi: 10.1089/mdr.2009.0120. [DOI] [PubMed] [Google Scholar]
- Hinojosa C., Caldwell D., Byrd J., Droleskey R., Lee J., Stayer P., Resendiz E., Garcia J., Klein S., Caldwell D., Pineda M., Farnell M. Use of foaming disinfectants and cleaners to reduce aerobic bacteria and Salmonella on poultry transport coops. Animals. 2018;8:195. doi: 10.3390/ani8110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan E., Egan J., Dullea C., Ward J., McGillicuddy K., Murray G., Murphy A., Bradshaw B., Leonard N., Rafter P., McDowell S. Salmonella surveillance in raw and cooked meat and meat products in the Republic of Ireland from 2002 to 2004. Int. J. Food Microbiol. 2006;112:66–70. doi: 10.1016/j.ijfoodmicro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Karatzas K.A.G., Webber M.A., Jorgensen F., Woodward M.J., V Piddock L.J., Humphrey T.J. Prolonged treatment of Salmonella enterica serovar Typhimurium with commercial disinfectants selects for multiple antibiotic resistance, increased efflux and reduced invasiveness. J. Antimicrob. Chemother. 2007;60:947–955. doi: 10.1093/jac/dkm314. [DOI] [PubMed] [Google Scholar]
- Kemmett K., Humphrey T., Rushton S., Close A., Wigley P., Williams N.J. A longitudinal study simultaneously exploring the carriage of APEC virulence associated genes and the molecular epidemiology of faecal and systemic E. coli in commercial broiler chickens. PLoS One. 2013;8:e67749. doi: 10.1371/journal.pone.0067749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kücken D., Feucht H.H., Kaulfers P.M. Association of qacE and qacEΔ1 with multiple resistance to antibiotics and antiseptics in clinical isolates of Gram-negative bacteria. FEMS Microbiol. Lett. 2000;183:95–98. doi: 10.1111/j.1574-6968.2000.tb08939.x. [DOI] [PubMed] [Google Scholar]
- Langsrud S., Sundheim G., Borgmann-Strahsen R. Intrinsic and acquired resistance to quaternary ammonium compounds in food-related Pseudomonas spp. J. Appl. Microbiol. 2003;95:874–882. doi: 10.1046/j.1365-2672.2003.02064.x. [DOI] [PubMed] [Google Scholar]
- Lapidot A., Romling U., Yaron S. Biofilm formation and the survival of Salmonella Typhimurium on parsley. Int. J. Food Microbiol. 2006;109:229–233. doi: 10.1016/j.ijfoodmicro.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Li D., Yu T., Zhang Y., Yang M., Li Z., Liu M., Qi R. Antibiotic resistance characteristics of environmental bacteria from an oxytetracycline production wastewater treatment plant and the receiving river. Appl. Environ. Microbiol. 2010;76:3444–3451. doi: 10.1128/AEM.02964-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljebjelke K.A., Hofacre C.L., Liu T., White D.G., Ayers S., Young S. Vertical and horizontal transmission of Salmonella within integrated broiler production system. Foodborne Pathog. Dis. 2005;2:90–102. doi: 10.1089/fpd.2005.2.90. [DOI] [PubMed] [Google Scholar]
- Madec F., Humbert F., Salvat G., Maris P. Measurement of the residual contamination of post-weaning facilities for pigs and related risk factors. J. Vet. Med. 1999;46:47–56. doi: 10.1046/j.1439-0450.1999.00207.x. [DOI] [PubMed] [Google Scholar]
- Marcus R. New information about pediatric foodborne infections: the view from FoodNet. Curr. Opin. Pediatr. 2008;20:79–84. doi: 10.1097/MOP.0b013e3282f43067. [DOI] [PubMed] [Google Scholar]
- McLaren I., Wales A., Breslin M., Davies R. Evaluation of commonly-used farm disinfectants in wet and dry models of Salmonella farm contamination. Avian Pathol. 2011;40:33–42. doi: 10.1080/03079457.2010.537303. [DOI] [PubMed] [Google Scholar]
- Medhanie G.A., Pearl D.L., McEwen S.A., Guerin M.T., Jardine C.M., Schrock J., LeJeune J.T. On-farm starling populations and other environmental and management factors associated with the presence of cefotaxime and ciprofloxacin resistant E. coli among dairy cattle in Ohio. Prev. Vet. Med. 2016;134:122–127. doi: 10.1016/j.prevetmed.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Meerburg B.G., Jacobs-Reitsma W.F., Wagenaar J.A., Kijlstra A. Presence of Salmonella and Campylobacter spp. in wild small mammals on organic farms. Appl. Environ. Microbiol. 2006;72:960–962. doi: 10.1128/AEM.72.1.960-962.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum R.J., Tucker D., Edwards C. Baseline rates of Campylobacter and Salmonella in raw chicken in Wales, United Kingdom, in 2002. J. Food Prot. 2004;67:1226–1228. doi: 10.4315/0362-028x-67.6.1226. [DOI] [PubMed] [Google Scholar]
- Nayak R., Kenney P.B., Keswani J., Ritz C. Isolation and characterisation of Salmonella in a Turkey production facility. Br. Poult. Sci. 2003;44:192–202. doi: 10.1080/0007166031000088370. [DOI] [PubMed] [Google Scholar]
- Nespeca R., Vaillancourt J.-P., Morrow W.E.M. Validation of a poultry biosecurity survey. Prev. Vet. Med. 1997;31:73–86. doi: 10.1016/s0167-5877(96)01122-1. [DOI] [PubMed] [Google Scholar]
- Nichols G.L. Fly transmission of Campylobacter. Emerg. Infect. Dis. 2005;11:361–364. doi: 10.3201/eid1103.040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Jarquin R., Hanning I., Almeida G., Ricke S.C. Detection of Salmonella spp. survival and virulence in poultry feed by targeting the hilA gene. J. Appl. Microbiol. 2011;111:426–432. doi: 10.1111/j.1365-2672.2011.05054.x. [DOI] [PubMed] [Google Scholar]
- Payne J.B., Kroger E.C., Watkins S.E. Evaluation of disinfectant efficacy when applied to the floor of poultry grow-out facilities. J. Appl. Poult. Res. 2005;14:322–329. [Google Scholar]
- Pleydell E.J., Brown P.E., Woodward M.J., Davies R.H., French N.P. Sources of variation in the ampicillin-resistant Escherichia coli concentration in the feces of organic broiler chickens. Appl. Environ. Microbiol. 2007;73:203–210. doi: 10.1128/AEM.01482-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploy M.C., Courvalin P., Lambert T. Characterization of In40 of Enterobacter aerogenes BM2688, a class 1 integron with two new gene cassettes, cmlA2 and qacF. Antimicrob. Agents Chemother. 1998;42:2557–2563. doi: 10.1128/aac.42.10.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R., Mini M. Outbreaks of salmonellosis in three different poultry farms of Kerala, India. Asian Pac. J. Trop. Biomed. 2013;3:496–500. doi: 10.1016/S2221-1691(13)60103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron E.Z. Host specificity of septicemic Escherichia coli: Human and avian pathogens. Curr. Opin. Microbiol. 2006;9:28–32. doi: 10.1016/j.mib.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Rose N., Beaudeau F., Drouin P., Toux J.Y., Rose V., Colin P. Risk factors for Salmonella enterica subsp. enterica contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 1999;39:265–277. doi: 10.1016/s0167-5877(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Rose N., Beaudeau F., Drouin P., Toux J.Y., Rose V., Colin P. Risk factors for Salmonella persistence after cleansing and disinfection in French broiler-chicken houses. Prev. Vet. Med. 2000;44:9–20. doi: 10.1016/s0167-5877(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Rose N., Mariani J.P., Drouin P., Toux J.Y., Rose V., Colin P. A decision-support system for Salmonella in broiler-chicken flocks. Prev. Vet. Med. 2003;59:27–42. doi: 10.1016/s0167-5877(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Singer R.S., Jeffrey J.S., Carpenter T.E., Cooke C.L., Atwill E.R., Johnson W.O., Hirsh D.C. Persistence of cellulitis-associated Escherichia coli DNA fingerprints in successive broiler chicken flocks. Vet. Microbiol. 2000;75:59–71. doi: 10.1016/s0378-1135(00)00205-4. [DOI] [PubMed] [Google Scholar]
- Stacy A.K., Mitchell N.M., Maddux J.T., De La Cruz M.A., Durán L., Girón J.A., Curtiss R., 3rd, Mellata M. Evaluation of the prevalence and production of Escherichia coli common pilus among avian pathogenic E. coli and its role in virulence. PLoS One. 2014;9:e86565. doi: 10.1371/journal.pone.0086565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata A., Nagamune H., Maeda T., Murakami K., Miyake Y., Kourai H. Correlation between resistance of Pseudomonas aeruginosa to quaternary ammonium compounds and expression of outer membrane protein OprR. Antimicrob. Agents Chemother. 2003;47:2093–2099. doi: 10.1128/AAC.47.7.2093-2099.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabo D.A., Diguimbaye C.D., Granier S.A., Moury F., Brisabois A., Elgroud R., Millemann Y. Prevalence and antimicrobial resistance of non-typhoidal Salmonella serotypes isolated from laying hens and broiler chicken farms in N’Djamena, Chad. Vet. Microbiol. 2013;166:293–298. doi: 10.1016/j.vetmic.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Taylor J.H., Holah J.T. A comparative evaluation with respect to the bacterial cleanability of a range of wall and floor surface materials used in the food industry. J. Appl. Bacteriol. 1996;81:262–266. [Google Scholar]
- Valcheva R., Belopopska P., Mateva G., Hristova T., Daskalov H. Distribution and serological typing of Salmonella spp. isolates from broiler carcasses in Bulgaria. Bulg. J. Vet. Med. 2011;14:31–38. [Google Scholar]
- van de Giessen A.W., Bouwknegt M., Dam-Deisz W.D.C., van Pelt W., Wannet W.J.B., Visser G. Surveillance of Salmonella spp. and Campylobacter spp. in poultry production flocks in The Netherlands. Epidemiol. Infect. 2006;134:1266–1275. doi: 10.1017/S0950268806005905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fels-Klerx H.J., Jacobs-Reitsma W.F., Van Brakel R., Van Der Voet H., Van Asselt E.D. Prevalence of Salmonella in the broiler supply chain in The Netherlands. J. Food Prot. 2008;71:1974–1980. doi: 10.4315/0362-028x-71.10.1974. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., De Zutter L., Houf K., Pasmans F., Haesebrouck F., Ducatelle R. Strategies to control Salmonella in the broiler production chain. Worlds. Poult. Sci. J. 2009;65:367–392. [Google Scholar]
- Velge P., Cloeckaert A., Barrow P. Emergence of Salmonella epidemics: the problems related to Salmonella enterica serotype Enteritidis and multiple antibiotic resistance in other major serotypes. Vet. Res. 2005;36:267–288. doi: 10.1051/vetres:2005005. [DOI] [PubMed] [Google Scholar]
- Völkel I., Schmitz C., Moors E., Gauly M., Czerny C.-P. Frequency of Salmonella detection in a broiler flock depending on different litter materials--a field study. Berl. Munch. Tierarztl. Wochenschr. 2011;124:71–77. [PubMed] [Google Scholar]
- Volkova V.V., Bailey R.H., Rybolt M.L., Dazo-Galarneau K., Hubbard S.A., Magee D., Byrd J.A., Wills R.W. Inter-relationships of Salmonella status of flock and grow-out environment at sequential segments in broiler production and processing. Zoonoses Public Health. 2010;57:463–475. doi: 10.1111/j.1863-2378.2009.01263.x. [DOI] [PubMed] [Google Scholar]
- Volkova V.V., Wills R.W., Hubbard S.A., Magee D.L., Byrd J.A., Bailey R.H. Risk factors associated with detection of Salmonella in broiler litter at the time of new flock placement. Zoonoses Public Health. 2011;58:158–168. doi: 10.1111/j.1863-2378.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- Voss-Rech D., Kramer B., Silva V.S., Rebelatto R., Abreu P.G., Coldebella A., Vaz C.S.L. Longitudinal study reveals persistent environmental Salmonella Heidelberg in Brazilian broiler farms. Vet. Microbiol. 2019;233:118–123. doi: 10.1016/j.vetmic.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Voss-Rech D., Vaz C.S.L., Alves L., Coldebella A., Leao J.A., Rodrigues D.P., Back A. A temporal study of Salmonella enterica serotypes from broiler farms in Brazil. Poult. Sci. 2015;94:433–441. doi: 10.3382/ps/peu081. [DOI] [PubMed] [Google Scholar]
- Ward P.J., Fasenko G.M., Gibson S., McMullen L.M. A microbiological assessment of on-farm food safety cleaning methods in broiler barns. J. Appl. Poult. Res. 2006;15:326–332. [Google Scholar]
- Wedderkopp A., Rattenborg E., Madsen M. National surveillance of Campylobacter in broilers at slaughter in Denmark in 1998. Avian Dis. 2000;44:993–999. [PubMed] [Google Scholar]
- Williams J.E., Benson S.T. Survival of Salmonella Typhimurium in poultry feed and litter at three temperatures. Avian Dis. 1978;22:742–747. [PubMed] [Google Scholar]
- Zdragas A., Mazaraki K., Vafeas G., Giantzi V., Papadopoulos T., Ekateriniadou L. Prevalence, seasonal occurrence and antimicrobial resistance of Salmonella in poultry retail products in Greece. Lett. Appl. Microbiol. 2012;55:308–313. doi: 10.1111/j.1472-765X.2012.03298.x. [DOI] [PubMed] [Google Scholar]
- Zou L., Meng J., McDermott P.F., Wang F., Yang Q., Cao G., Hoffmann M., Zhao S. Presence of disinfectant resistance genes in Escherichia coli isolated from retail meats in the USA. J. Antimicrob. Chemother. 2014;69:2644–2649. doi: 10.1093/jac/dku197. [DOI] [PubMed] [Google Scholar]
- Zweifel C., Scheu K.D., Keel M., Renggli F., Stephan R. Occurrence and genotypes of Campylobacter in broiler flocks, other farm animals, and the environment during several rearing periods on selected poultry farms. Int. J. Food Microbiol. 2008;125:182–187. doi: 10.1016/j.ijfoodmicro.2008.03.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.