Abstract
Aim:
To investigate whether genes implicated in dementia pathogenesis are differently methylated in peripheral blood.
Materials & methods:
Participants included 160 cognitively healthy individuals aged 70+ years: 73 who were subsequently diagnosed with dementia and 87 controls matched on age, gender, education, smoking and baseline cognition. A total of 49 participants also provided blood samples at diagnosis. Blood DNA methylation of APOE, APP, BDNF, PIN1, SNCA and TOMM40 was examined.
Results:
A total of 56 of 299 probes were differentially methylated in dementia compared with controls and 39 probes prior to diagnosis. The greatest effect size was in APP (cg19423170, Δ-8.32%, adjusted p = 0.009 at diagnosis; cg19933173, Δ-4.18%, adjusted p < 0.0001 prediagnosis).
Conclusion:
Genes implicated in dementia pathogenesis show differential blood methylation in dementia, even prior to diagnosis.
Keywords: : APOE, APP, BDNF, biomarker, blood, dementia, DNA methylation, PIN1, SNCA, TOMM40
Late-onset dementia likely results from a complex interplay of genetic factors and gene–environment interactions, potentially mediated by epigenetic mechanisms [1]. It is well known that the APOE gene ε4 allele is the single strongest genetic risk factor [2]. APOE is involved in brain repair and amyloid-β (Aβ) metabolism. The build-up of Aβ causes senile plaques in the brain, which is one of the primary pathologies of dementia, particularly the most common cause, Alzheimer’s disease (AD) [3].
Other candidate genes implicated in dementia are the APP, encoding a signaling and intracellular transport transmembrane protein, of which Aβ is a constituent [4] and TOMM40, responsible for the formation of pores for translocation of proteins into the mitochondria [5]. TOMM40 lies in close genomic proximity to the APOE gene [6] and dysregulation causes mitochondrial neurotoxicity and oxidative stress in AD [7].
PIN1 is a protein involved in the maintenance of neuronal health, the dysregulation of which is thought to lead to over production of Aβ and tau [8] and is linked to genetic variation within the gene [9]. SNCA is a protein expressed highly in neurons that is involved in synaptic transmission. SNCA protein aggregation is a primary pathology of Parkinson’s disease as well as a major component of Lewy bodies involved in dementia with Lewy bodies [10]. Genetic variation within SNCA has been shown to be associated with increased risk of pathology associated with dementia with Lewy bodies, as well as a risk factor for Parkinson’s disease [11].
Another well studied gene is BDNF, which incodes a neurotrophin that promotes the development of neurons, involved in cognition and memory. In dementia, particularly AD, genetic variation of BDNF is associated with an increased risk of depression and serum levels of BDNF have been found to be lower in later stages of dementia, compared with those with mild cognitive impairment (MCI) [12,13].
Some preliminary evidence exists that differential DNA methylation in these six genes in blood cells may be associated with dementia or cognitive impairment (Table 1) [14–26]. However, due to the limited studies and a lack of consistent findings, robust evidence that peripheral blood based differential methylation is associated with dementia risk remains elusive. Currently reported findings are thus not appropriate for use as a biomarker for preclinical detection or diagnosis of dementia.
Table 1. . Candidate gene regions examined in previous DNA methylation studies reporting significant associations and the corresponding EPIC probes.
| Candidate gene | Study | Outcome of interest | n | Measurement method | Genomic region (hg19) | Findings in individuals with outcome | Corresponding epic probes associated with dementia/memory deficits | Ref. |
|---|---|---|---|---|---|---|---|---|
| APOE | Liu et al. | Delayed recall | 289 | 450k | chr19:45407868-45412648 | Higher methylation with lower delayed recall score (FDR q <0.1, effect size not shown) |
cg04406254, CpG19:45407945 cg18768621, CpG19:45409440 |
[1] |
| Shao et al. | AD, MCI | 67 | 450k | chr19:45407868-45412599 | Difference between groups in raw analysis (p < 0.5, effect size and direction not shown) |
cg26190885, CpG19:45409005 cg12049787, CpG19:45409080 cg19514613, CpG19:45409713 cg05501958, CpG19:45411873 cg18799241, CpG19:45412599 cg14123992, CpG19:45407868 cg04406254, CpG19:45407945 cg18768621, CpG19:45409440 |
[2] | |
| Karlsson et al. | AD | 447 | 450k | chr19:45407868-45412648 | Higher promoter methylation | cg14123992, CpG19:45407868 cg04406254, CpG19:45407945 cg26190885, CpG19:45409005 |
[3] | |
| Mancera-Paez et al. | MCI | 100 | BS PCR | chr19:45412445-45412605 | Generally higher methylation, in four sig. CpGs. | NA, no sig. EPIC probes | [4] | |
| APP | D'Addario et al. | AD (twins) | 2 | MSP | chr21:27543426-27543579 | Average promoter methylation higher (0.5%, no p-value) | cg27158854, CpG21:27543469 cg10253538, CpG21:27543504 cg08866780, CpG21:27543523 cg00542846, CpG21:27543545 |
[5] |
| Hou et al. | AD | 12 | MSP | chr21:27542923-27543318 | Lower methylation at ten CpGs (p = 0.05, effect size not shown) | NA, no sig. EPIC probes | [6] | |
| BDNF | Chang et al. | AD | 160 | PS | chr11:27743841-27743870 | Average methylation higher (2.05%; p = 0.004) |
NA, no EPIC probes in region | [7] |
| Nagata et al. | AD | 40 | BS PCR | chr11:27722101-27722210 | Average methylation higher (2.99%; p = 0.04) |
NA, no EPIC probes in region | [8] | |
| Xie et al. | aMCI, AD | 458 | PS | Promoter I (P1) chr11:27743543-27743744 Promoter IV (P4) chr11:27723021-27723252 |
Average methylation at four CpGs across both promoters higher at BL (2.99%; p < 0.001) FU (2.49%; p < 0.001) P1: Not sig. at aligned EPIC probe cg16257091, CpG11:27743580 P4: Sig. at aligned EPIC probe cg11241206, CpG11:27723128 at BL: 0.86%; p < 0.001, MCI vs CT FU: 1.76%; p < 0.001, AD vs MCI |
cg11241206, CpG11:27723128 lines up with ‘P4: CpG 6’ | [9] | |
| PIN1 | Arosio et al. | LOAD | 60 | MSP | chr19:9945760-9945910 | Average methylation across region lower (LOAD vs CT) (-7.69%; p = 0.001) |
cg18744802, CpG19:9945810 cg10998950, CpG19:9945815 cg26231243, CpG19:9945826 cg01731038, CpG19:9945838 cg13388992, CpG19:9945906 cg12082325, CpG19:9945909 |
[10] |
| D'Addario et al. | AD (twins) | 2 | MSP | chr19:9945625-9945826 | Average methylation across region lower (-2.0%, no p-value) | cg22728790, CpG19:9945654 cg06539622, CpG19:9945677 cg04699474, CpG19:9945708 cg18744802, CpG19:9945810 cg10998950, CpG19:9945815 cg26231243, CpG19:9945826 |
[5] | |
| Ferri et al. | LOAD, FTD | 317 | PS | chr19:9945616-9945965 | Higher methylation at CpGs (FTD vs CT/AD) (≤0.57%; p ≤ 0.03). |
cg18744802, CpG19:9945810 cg26231243, CpG19:9945826 cg01731038, CpG19:9945838 |
[11] | |
| SNCA | Funahashi et al. | DLB | 40 | PS | chr4:90757391-90757463 | Lower average methylation across region (2.3%; p < 0.001, DLB, vs CT) as well as at seven CpGs | cg14346243, CpG4:90757452 | [12] |
| Yoshino et al. | AD | 100 | PS | chr4:90757391-90757463 | Lower average methylation across region (0.7%; p = 0.027, AD vs CT) as well as at seven CpGs | cg20003494, CpG4:90757398 cg14346243, CpG4:90757452 |
[13] | |
| TOMM40 | Liu et al. | Delayed recall | 289 | 450k | chr19:45393621-45406887 | Increased methylation associated with lower delayed recall score (effect size not shown) | cg22024783, CpG19:45393916 cg12271581, CpG19:45394330 |
[1] |
| Shao et al. | AD, MCI | Pilot: 67 Rep :57 |
450k | chr19:45392813-45407871 | Difference (p < 0.05) between groups pre-adjustment for multiple testing (effect size and direction not shown) | cg22024783, CpG19:45393916 (CT vs MCR, CT vs MCI/AD) cg12271581, CpG19:45394330 (MCI vs AD) cg06632829, CpG19: 45394476 (MCI vs AD) Replication Cohort: (AD vs CT) cg19375044, CpG19:45394343 cg02613937, CpG19: 45395297 cg13447416, CpG19: 45398091 |
[2] |
450K: Illumina 450k array; AD: Alzheimer’s disease; aMCI: Acute mild cognitive impartment; BL: Baseline; BS: Bisulphite; CpG: Cytosine-phosphate-guanine; CT: Controls; DLB: Dementia with Lewy bodies; EPIC: Illumina MethylEPIC array; FDR: False discovery rate; FU: Follow up; FTD: Frontotemporal dementia; LOAD: Late onset Alzheimer’s disease; MCI: Mild cognitive impairment; MSP: Methylation specific PCR; NA: Not available; PS: Pyrosequencing; pval: p-value; Rep: Replication; Sig: Significant.
Here, we measured DNA methylation changes across these six candidate genes in blood samples collected from participants when they were cognitively healthy and who were then followed up to determine dementia status. We assessed whether methylation changes across these genes were associated with dementia diagnosis and whether methylation changes occur in blood cells prior to the manifestation of clinical dementia symptoms.
Materials & methods
Study sample
This study involved participants from the ASPirin in Reducing Events in the Elderly (ASPREE) cohort, a study of low dose aspirin and its effect on disability-free survival in an older population [27]. Participants recruited to ASPREE were relatively healthy and free from severe cognitive impairments (Modified Mini-Mental State Examination [3MS] >77) and dementia diagnosis. Participants provided peripheral blood samples at recruitment and most participants provided additional blood samples 3 years after inclusion.
Neurocognitive assessments performed at baseline and over follow-up included the 3MS [28,29], Symbol Digit Modalities Test (SDMT) [30], Controlled Oral Word Association Test (COWAT) [31] and the Hopkins Verbal Learning Test Revised (HVLT-R) [32,33]. Baseline and follow-up cognitive scores are shown in Supplementary Table 1. When dementia was suspected, additional cognitive and functional assessment was administered. Dementia diagnosis was adjudicated by the specialist panel of neuropsychologists, neurologists and geriatricians after comprehensive review of all available information, based on Diagnostic and Statistical Manual for Mental Disorders, American Psychiatric Association (DSM-IV) criteria [34,35]. In addition to the cognitive and functional assessments, information used included medical records, specialists' reports, blood samples and neuroimaging (when available).
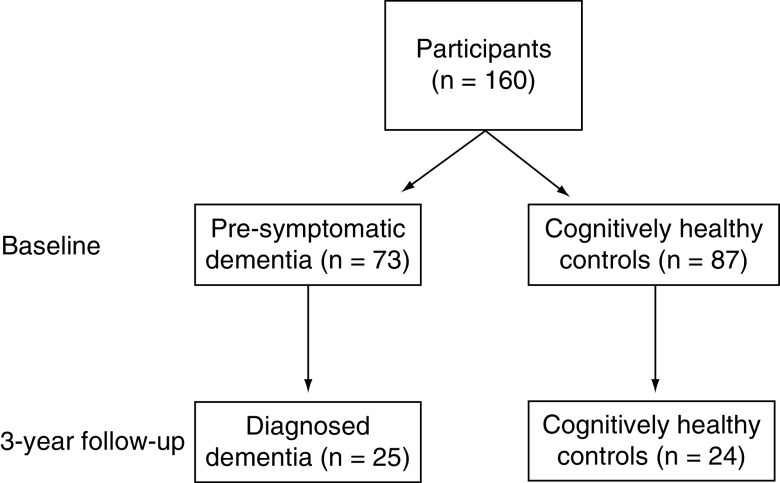
The current substudy used a case-control design to select dementia cases and cognitively healthy controls based on their status at the 3-year follow-up. All participants self-identified as white Australians. DNA methylation was measured in 160 participants, all of whom were cognitively normal at baseline and included 87 participants who remained cognitively healthy at the 3-year follow-up, referred to as ‘controls’ (Figure 1). These controls were matched on age, self-reported gender, education, smoking status and baseline cognitive function to 73 participants who received an adjudicated dementia diagnosis at least 1 year after the baseline (referred to as ‘presymptomatic dementia cases’). Of these 160 participants, we also analyzed blood samples from 24 controls at the 3-year follow-up and 25 dementia cases (who provided blood samples within 9 months of their dementia diagnosis).
Figure 1. . Participants included in candidate gene analyses.
Candidate gene selection
Candidate genes were selected based on those that have been previously implicated in dementia and where significant associations have been found between differential DNA methylation in peripheral blood in previous studies [36] and where at least one methylation site lined up with Infinium MethylationEPIC BeadChip probes (Illumina, CA, USA). Genes included APOE [14–17], APP [18,19], BDNF [20–22], PIN1 [18,23,24], SNCA [25,26] and TOMM40 [14,15]. Regions and probes from each study were compared with EPIC annotation using the UCSC genome browser [37]. BiSearch was used where papers did not specify exact genomic location but instead reported bisulfite converted primers [38]. Full details of previous studies that identified associations with these genes are given in Table 1.
Generation of DNA methylation data
DNA was extracted from peripheral blood (buffy coat) using Qiagen DNeasy Blood & Tissue Kits (Qiagen, Hilden, Germany) [39]. DNA methylation was measured at the Australian Genome Research Facility (Melbourne, Victoria) using Illumina Infinium MethylationEPIC BeadChips (EPIC) [40]. Where mentioned in text, probes are labelled as EPIC probe name, followed by standardized methylation site nomenclature [41]. R version 3.5.1 was used to normalize EPIC data (subset quantile normalization method [42]) and standard quality control was carried out [43]. A total of 299 probes were selected for analysis in this study, which were mapped to ‘hg19’ human genome assembly GRCh37. Methylation measures are derived from average DNA methylation at each probe within a sample, as a measure between 0 and 100% methylated (known as β-values). Blood cell type proportion estimation, originally proposed by Houseman et al. [44], was carried out using ‘estimateCellCounts2’, (R package FlowSorted.Blood.EPIC) [45,46]. This was considered based on the premise that each cell type has its own methylation profile [47] and blood contains varying proportions of different cell types but the exact composition varies between individuals.
Candidate gene analysis
Methylation data were extracted for all probes lying within a region of interest, spanning the gene body, nearby CpG islands (regions of the genome densely packed with CpGs) and probes within upstream/downstream proximal regions (from 1 to 180 kbp depending on the size of the gene of interest). STATA 14 was used to compare average DNA methylation across each gene, as well as DNA methylation at each probe, between individuals with and without dementia using t-tests. To assess the correlation between probes within each gene, correlation matrices were determined using Pearson’s method. Initial t-test analysis underwent Benjamini Hochberg (BH) adjustment for multiple comparisons [48]. Non-BH adjusted significant probes were further investigated using two regression models adjusting for possible confounding factors. Model 1 adjusted for age, gender and methylation assay batch. Model 2 also adjusted for age, gender and methylation assay batch, as well as estimated blood cell proportions of monocytes, neutrophils, natural killer cells, B cells, CD8+ T and CD4+ T cells. This analysis was also carried out to compare DNA methylation between presymptomatic cases of dementia and controls.
Results
Participant characteristics
The characteristics of dementia cases (presymptomatic and at diagnosis) and controls are listed in Table 2. There were more females and most participants had never smoked.
Table 2. . Participant characteristics at baseline and 3-year follow-up.
| Baseline (n = 160) | Follow-up (n = 49)† | |||||
|---|---|---|---|---|---|---|
| Characteristic | Controls (n = 87) | Presymptomatic‡ dementia (n = 73) | p-value | Controls (n = 24) | Dementia (n = 25) | p-value |
| Age, mean (SD) | 76.4 (4.6) | 77.6 (5.1) | 0.11 | 80.7 (4.7) | 80.7 (4.7) | 0.97 |
| Gender n (% female) | 50 (57.5) | 42 (57.5) | 0.99 | 15 (62.5) | 17 (68.0) | 0.67 |
| n (%) | n (%) | |||||
| Smoking: | ||||||
| Current | 2 (2.3) | 0 (0) | 0.42 | 0 (0) | 0 (0) | 0.91 |
| Past | 36 (41.4) | 32 (43.8) | 9 (37.5) | 9 (36.0) | ||
| Never | 49 (56.3) | 41 (56.2) | 15 (62.5) | 16 (64.0) | ||
| Education: | ||||||
| ≤12 years | 60 (69) | 43 (58.9) | 0.19 | 19 (79.2) | 10 (40) | 0.005 |
| >12 years | 27 (31) | 30 (41.1) | 5 (20.8) | 15 (60) | ||
All 49 participants also gave samples included in baseline analysis.
Presymptomatic dementia participants are defined as participants who gave blood samples when cognitively healthy, who received an adjudicated dementia diagnosis at least 1 year after the baseline.
SD: Standard deviation.
Candidate gene DNA methylation in adjudicated dementia cases versus controls
Average methylation across each gene did not differ significantly between the dementia and control groups (Table 3). Of the 299 probes (otherwise known as CpG dinucleotides) investigated across the six gene regions, 18.7% of probes (n = 56) were found to be differentially methylated in association with dementia status at the 5% significance level (Table 3). Results of probe wise methylation comparisons between dementia cases and controls can be seen in Table 4. No probes passed adjustment for multiple comparisons (BH Adj.p < 0.05). The SNCA gene region had the greatest proportion of differentially methylated probes (12/40, 30%), followed by APP (27/133, 23.9%). The greatest methylation difference seen across the six gene regions was at cg19423170, CpG21:27472122 in the APP gene. Average methylation at this probe was 8.31% lower in dementia cases (45.5%) compared with controls (53.8%) (p = 0.009). There was no association between APOE or PIN1 region probe methylation and dementia status.
Table 3. . Candidate genes and findings of differential methylation identified in the current study.
| Gene | Genomic region | n EPIC probes† | Dementia (n = 25) versus controls (n = 24) |
Presymptomatic dementia (n = 73) versus controls (n = 87) |
Dementia versus controls |
Presymptomatic dementia versus controls |
Common probes across two analyses |
|---|---|---|---|---|---|---|---|
| Mean gene region methylation Δ (%) | Number of differential probes‡ (% of total) | ||||||
| APOE | Chr19:45407810-45412718 | 13 | +0.43, SE: 0.29; p = 0.14 | +0.41, SE: 0.14; p = 0.004 | 0 (0%) | 2 (15.4%) | 0 |
| APP | Chr21:27152684-27736037 | 113 | +0.01, SE: 0.22; p = 0.93 | +0.21, SE: 0.13; p = 0.12 | 27 (23.9%) | 16 (14.2%) | 4 |
| BDNF | Chr11:27664568-27754772 | 93 | -0.20, SE: 0.16; p = 0.21 | -0.08, SE: 0.10; p = 0.4 | 15 (16.1%) | 10 (10.8%) | 3 |
| PIN1 | Chr19:9942630-9963080 | 24 | +0.16, SE: 0.14; p = 0.25 | -0.12, SE: 0.10; p = 0.25 | 0 (0%) | 1 (0.42%) | 0 |
| SNCA | Chr4:90627859-90762694 | 40 | -0.24, SE: 0.25; p = 0.34 | -0.26, SE: 0.14; p = 0.06 | 12 (30%) | 5 (12.5%) | 3 |
| TOMM40 | Chr19:45392810-45407809 | 16 | +0.17, SE: 0.21; p = 0.41 | +0.39, SE: 0.12; p = 002 | 2 (12.5%) | 5 (31.3%) | 0 |
| Total | – | 299 | – | – | 56 | 39 | 10 |
Probes available from dataset after removal of cross-reactive probes, as well as probes that failed in more than one sample or were located at a known site for single nucleotide polymorphism.
p < 0.05.
EPIC: Illumina MethylEPIC array; SE: Standard error.
Table 4. . Differentially methylated probes between dementia cases and cognitively healthy controls.
| Gene | Probe | Hg19 location | Case mean | Control mean | Δ | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| APP | cg23311364 | chr21:27163832 | 81.85% | 80.18% | 1.66% | 0.18–3.14% | 0.03 |
| cg13823477 | chr21:27217655 | 73.52% | 70.39% | 3.12% | 0.80–5.45% | 0.01 | |
| cg17555382 | chr21:27259976 | 83.17% | 81.46% | 1.71% | 0.26–3.17% | 0.02 | |
| cg23877117 | chr21:27298484 | 22.09% | 25.28% | -3.19% | -0.16 to -6.22% | 0.04 | |
| cg25306719 | chr21:27298698 | 29.00% | 32.80% | -3.80% | -0.67 to -6.91% | 0.02 | |
| cg08542030 | chr21:27306498 | 77.75% | 75.36% | 2.39% | 0.51–4.26% | 0.01 | |
| cg01825010 | chr21:27335716 | 79.01% | 75.33% | 3.68% | 1.12–6.24% | 0.01 | |
| cg19013695 | chr21:27340093 | 5.11% | 5.85% | -0.75% | -0.05 to -1.44% | 0.04 | |
| cg12827812 | chr21:27352541 | 77.19% | 76.09% | 1.11% | 0.01–2.21% | 0.05 | |
| cg25314245 | chr21:27354743 | 73.88% | 71.55% | 2.33% | 0.12–4.45% | 0.04 | |
| cg17478810 | chr21:27370617 | 88.44% | 87.42% | 1.03% | 0.20–1.86% | 0.02 | |
| cg17092246 | chr21:27374319 | 79.31% | 80.90% | -1.59% | -0.40 to -2.78% | 0.01 | |
| cg17728373 | chr21:27404776 | 60.92% | 57.07% | 3.86% | 0.95–6.76% | 0.01 | |
| cg06085525 | chr21:27414888 | 76.78% | 74.18% | 2.59% | 0.14–5.05% | 0.04 | |
| cg19423170 | chr21:27472122 | 45.50% | 53.81% | -8.31% | -2.20 to -14.43% | 0.009 | |
| cg22592725 | chr21:27484586 | 73.90% | 71.09% | 2.80% | 0.24–5.37% | 0.03 | |
| cg22552084 | chr21:27497496 | 63.84% | 61.16% | 2.68% | 0.30–5.05% | 0.03 | |
| cg24675442 | chr21:27509220 | 6.41% | 7.99% | -1.58% | -0.17 to -2.99% | 0.03 | |
| cg03881418 | chr21:27512686 | 28.21% | 32.01% | -3.80% | -1.01 to -6.59% | 0.004 | |
| cg27160886 | chr21:27520639 | 63.67% | 59.49% | 4.18% | 1.39–6.96% | 0.004 | |
| cg14414154 | chr21:27538021 | 33.38% | 39.68% | -6.30% | -1.61 to -10.99% | 0.01 | |
| cg01286133 | chr21:27540106 | 75.03% | 77.56% | -2.53% | -0.08 to -4.99% | 0.04 | |
| cg27372898 | chr21:27543410 | 7.29% | 9.10% | -1.81% | -0.33 to -3.29% | 0.02 | |
| cg15835366 | chr21:27543683 | 9.68% | 10.86% | -1.18% | -0.32 to -2.04% | 0.008 | |
| cg08164005 | chr21:27544052 | 67.54% | 64.04% | 3.51% | 0.54–6.47% | 0.02 | |
| cg03015479 | chr21:27544797 | 69.96% | 66.64% | 3.32% | 0.94–5.70% | 0.007 | |
| cg07195338 | chr21:27562500 | 59.91% | 57.79% | 2.12% | 0.24–4.00% | 0.03 | |
| BDNF | cg02386994 | chr11:27679976 | 63.15% | 60.71% | 2.44% | 0.27–4.62% | 0.03 |
| cg12296752 | chr11:27681211 | 75.06% | 71.24% | 3.82% | 0.97–6.67% | 0.01 | |
| cg08760147 | chr11:27685307 | 67.68% | 64.99% | 2.69% | 0.22–5.15% | 0.03 | |
| cg18595174 | chr11:27701991 | 63.71% | 58.31% | 5.40% | 0.96–9.84% | 0.02 | |
| cg27193031 | chr11:27721088 | 16.37% | 18.25% | -1.88% | -0.17 to - 3.57% | 0.03 | |
| cg09505801 | chr11:27722009 | 5.68% | 6.73% | -1.06% | -0.07 to -2.04% | 0.04 | |
| cg17882499 | chr11:27722048 | 6.11% | 8.24% | -2.13% | -0.06 to -4.19% | 0.04 | |
| cg24377657 | chr11:27723245 | 8.98% | 10.86% | -1.88% | -0.09 to -3.67% | 0.04 | |
| cg26949694 | chr11:27742060 | 16.69% | 19.00% | -2.30% | -0.29 to -4.34% | 0.03 | |
| cg01225698 | chr11:27742355 | 10.47% | 12.29% | -1.83% | -0.27 to -3.40% | 0.02 | |
| cg10635145 | chr11:27742435 | 35.40% | 41.28% | -5.88% | -0.43 to -11.34% | 0.04 | |
| cg27351358 | chr11:27743258 | 6.94% | 8.33% | -1.39% | -0.32 to -2.46% | 0.01 | |
| cg03167496 | chr11:27743619 | 7.43% | 8.55% | -1.12% | -0.15 to -2.08% | 0.02 | |
| cg11718030 | chr11:27744363 | 11.02% | 12.39% | -1.37% | -0.003 to -2.74% | 0.05 | |
| cg06046431 | chr11:27744675 | 5.88% | 6.92% | -1.04% | -0.03 to -2.04% | 0.04 | |
| SNCA | cg06176111 | chr4:90674837 | 64.34% | 61.87% | 2.48% | 0.18–4.77% | 0.04 |
| cg06632027 | chr4:90757378 | 12.37% | 16.19% | -3.81% | -0.54 to -7.09% | 0.02 | |
| cg00193021 | chr4:90758120 | 6.87% | 8.17% | -1.30% | -0.27 to -2.33% | 0.01 | |
| cg17045024 | chr4:90758207 | 16.36% | 19.72% | -3.36% | -0.33 to -6.39% | 0.03 | |
| cg08708229 | chr4:90758216 | 13.10% | 17.37% | -4.28% | -1.63 to -6.92% | 0.002 | |
| cg02192967 | chr4:90758406 | 12.27% | 14.95% | -2.68% | -0.41 to -4.94% | 0.02 | |
| cg00119181 | chr4:90758537 | 7.20% | 9.53% | -2.34% | -0.40 to -4.27% | 0.02 | |
| cg23396644 | chr4:90758777 | 4.99% | 5.57% | -0.58% | -0.01 to -1.14% | 0.05 | |
| cg20776829 | chr4:90758797 | 5.08% | 7.59% | -2.51% | -0.80 to -4.22% | 0.005 | |
| cg00869039 | chr4:90759188 | 4.75% | 5.43% | -0.68% | -0.05 to -1.30% | 0.03 | |
| cg12030690 | chr4:90759203 | 7.09% | 8.25% | -1.16% | -0.37 to -1.95% | 0.005 | |
| cg14372885 | chr4:90760483 | 73.73% | 69.02% | 4.71% | 1.56–7.86% | 0.004 | |
| TOMM40 | cg25093158 | chr19:45394327 | 4.68% | 4.44% | 0.26% | 0.04–0.48% | 0.02 |
| cg12271581 | chr19:45394330 | 6.40% | 5.94% | 0.45% | 0.08–0.82% | 0.02 |
APOE and PIN1 not present in table as no significant results were found.
Presymptomatic dementia cases versus controls
Average APOE (+0.41%, standard error [SE]: 0.14; p = 0.004) and TOMM40 (+0.39%, SE: 0.12; p = 0.002) methylation differed between presymptomatic and control groups (Table 3). A lower number of differentially methylated probes were identified in presymptomatic dementia cases compared with controls (n = 39, 13%; Table 5). Two probes passed BH adjustment for multiple comparisons, one of which showed the largest effect size, a 4.31% higher methylation at cg19933173, CpG21:27562920, approximately 20 kbp upstream of the APP transcription site (65.04 vs 60.73%; p < 0.0001, BH Adj.p = 0.015), this was almost half the magnitude of the largest effect size seen in the analysis of dementia cases and controls. The other BH Adj. significant probe was also within APP, cg15407086, CpG21:27543045, also showing a higher methylation in presymptomatic dementia (+2.09%, 17.08 vs 14.99%; p < 0.0001, BH Adj.p = 0.015). TOMM40 had the highest proportion of differential probes (5/16, 31.3%), comparing presymptomatic cases and controls.
Table 5. . Differentially methylated probes in pre-symptomatic dementia versus controls at baseline.
| Gene | Probe | Hg19 location | Case mean | Control mean | Δ | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| APOE | cg16471933 | chr19:45411802 | 69.18% | 67.14% | 2.04% | 0.51–3.57% | 0.009 |
| cg18799241 | chr19:45412599 | 80.15% | 79.21% | 0.91% | 0.12–1.70% | 0.02 | |
| APP | cg11278459 | chr21:27210355 | 52.43% | 56.18% | 3.75% | 1.53–5.98% | 0.001 |
| cg17660372 | chr21:27305727 | 83.42% | 82.38% | 1.04% | 0.22–1.86% | 0.01 | |
| cg04424048 | chr21:27306084 | 68.42% | 66.97% | 1.44% | 0.12–2.77% | 0.03 | |
| cg08542030 | chr21:27306498 | 78.07% | 76.66% | 1.41% | 0.21–2.61% | 0.02 | |
| cg07896369 | chr21:27326987 | 83.77% | 84.51% | -0.75% | -0.02 to -1.47% | 0.04 | |
| cg17728373 | chr21:27404776 | 60.51% | 58.34% | 2.16% | 0.23–4.10% | 0.03 | |
| cg23830184 | chr21:27425841 | 77.84% | 78.93% | -1.09% | -0.09 to 2.09% | 0.03 | |
| cg22552084 | chr21:27497496 | 63.69% | 61.80% | 1.90% | 0.25–3.55% | 0.03 | |
| cg19591392 | chr21:27513218 | 64.04% | 66.42% | -2.38% | -0.16 to -4.59% | 0.04 | |
| cg15407086 | chr21:27543045 | 17.08% | 14.99% | 2.09% | 1.05–3.12% | 0.0001 | |
| cg27158854 | chr21:27543469 | 8.77% | 8.34% | 0.43% | 0.15–0.71% | 0.003 | |
| cg08866780 | chr21:27543523 | 11.80% | 10.65% | 1.15% | 0.26–2.05% | 0.01 | |
| cg01148198 | chr21:27544373 | 75.19% | 74.33% | 0.87% | 0.09–1.68% | 0.03 | |
| cg03015479 | chr21:27544797 | 69.91% | 68.09% | 1.82% | 0.10–3.53% | 0.04 | |
| cg23393368 | chr21:27561643 | 69.52% | 67.75% | 1.77% | 0.26–3.28% | 0.02 | |
| cg19933173 | chr21:27562920 | 65.04% | 60.73% | 4.31% | 2.28–6.33% | <0.0001 | |
| BDNF | cg23330212 | chr11:27672697 | 57.91% | 56.07% | 1.83% | 0.05–3.62% | 0.04 |
| cg14291693 | chr11:27683959 | 64.07% | 61.92% | 2.15% | 0.48–3.81% | 0.01 | |
| cg08362738 | chr11:27722636 | 5.53% | 6.03% | -0.49% | -0.07 to -0.91% | 0.02 | |
| cg25328597 | chr11:27722638 | 5.80% | 6.19% | -0.39% | -0.00001 to -0.78% | 0.05 | |
| cg04672351 | chr11:27722889 | 5.81% | 5.53% | 0.28% | 0.03–0.54% | 0.03 | |
| cg05733135 | chr11:27740876 | 24.75% | 27.37% | -2.62% | -0.31 to -4.92% | 0.03 | |
| cg22043168 | chr11:27741077 | 28.96% | 30.27% | -1.31% | -0.25 to -2.37% | 0.02 | |
| cg26949694 | chr11:27742060 | 16.82% | 17.86% | -1.05% | -0.12 to -1.97% | 0.03 | |
| cg01225698 | chr11:27742355 | 10.66% | 11.70% | -1.05% | -0.14 to -1.96% | 0.02 | |
| cg27351358 | chr11:27743258 | 7.21% | 8.13% | -0.92% | -0.13 to -1.71% | 0.02 | |
| PIN1 | cg06539622 | chr19:9945676 | 4.85% | 5.29% | -0.43% | -0.06 to -0.81% | 0.03 |
| SNCA | cg01681236 | chr4:90647041 | 79.33% | 77.96% | 1.37% | 0.33–2.41% | 0.01 |
| cg06176111 | chr4:90674837 | 63.72% | 62.15% | 1.57% | 0.03–3.11% | 0.05 | |
| cg17045024 | chr4:90758207 | 17.00% | 18.89% | -1.89% | -0.14 to -3.64% | 0.04 | |
| cg01035160 | chr4:90758529 | 5.46% | 6.10% | -0.62% | -0.12 to -1.11% | 0.01 | |
| cg00119181 | chr4:90758537 | 7.21% | 8.40% | -1.20% | -0.01 to -2.38% | 0.05 | |
| TOMM40 | cg08267701 | chr19:45393621 | 5.19% | 4.94% | 0.24% | 0.02–0.47% | 0.04 |
| cg22024783 | chr19:45393916 | 17.89% | 16.36% | 1.53% | 0.53–2.53% | 0.003 | |
| cg27534894 | chr19:45393925 | 13.11% | 11.77% | 1.34% | 0.48–2.20% | 0.002 | |
| cg21549639 | chr19:45394156 | 6.81% | 6.40% | 0.43% | 0.13–0.72% | 0.004 | |
| cg27443666 | chr19:45394427 | 2.85% | 3.00% | -0.15% | -0.002 to -0.29% | 0.05 |
Differently methylated probes in both diagnosed dementia & presymptomatic cases
Differential methylation at ten probes were associated with both pre-symptomatic and diagnosed dementia, across APP (n = 4), BDNF (n = 3) and SNCA (n = 3) (Table 6). In all cases, there was concordance in the direction of methylation difference, in other words, either higher or lower methylation at both timepoints at a particular probe. All had a greater effect size in dementia cases compared with presymptomatic cases.
Table 6. . Significant probes common between diagnosed and presymptomatic dementia groups.
| Probe | Probe | Location | Timepoint | Status | Mean (%) | Δ Case versus controls | p-value |
|---|---|---|---|---|---|---|---|
| APP | cg08542030 | chr21:27306498 | Baseline | Control | 76.66% | 1.41% | 0.02 |
| Presymptomatic | 78.07% | ||||||
| Follow-up | Control | 75.36% | 2.39% | 0.01 | |||
| Dementia | 77.75% | ||||||
| cg17728373 | chr21:27404776 | Baseline | Control | 58.34% | 2.16% | 0.03 | |
| Presymptomatic | 60.51% | ||||||
| Follow-up | Control | 57.07% | 3.86% | 0.01 | |||
| Dementia | 60.92% | ||||||
| cg22552084 | chr21:27497496 | Baseline | Control | 61.80% | 1.90% | 0.03 | |
| Presymptomatic | 63.69% | ||||||
| Follow-up | Control | 61.16% | 2.68% | 0.03 | |||
| Dementia | 63.84% | ||||||
| cg03015479 | chr21:27544797 | Baseline | Control | 68.09% | 1.82% | 0.04 | |
| Presymptomatic | 69.91% | ||||||
| Follow-up | Control | 66.64% | 3.32% | 0.007 | |||
| Dementia | 69.96% | ||||||
| BDNF | cg26949694 | chr11:27742060 | Baseline | Control | 17.86% | -1.05% | 0.03 |
| Presymptomatic | 16.82% | ||||||
| Follow-up | Control | 19.00% | -2.30% | 0.03 | |||
| Dementia | 16.69% | ||||||
| cg01225698 | chr11:27742355 | Baseline | Control | 11.70% | -1.05% | 0.02 | |
| Presymptomatic | 10.66% | ||||||
| Follow-up | Control | 12.29% | -1.83% | 0.02 | |||
| Dementia | 10.47% | ||||||
| cg27351358 | chr11:27743258 | Baseline | Control | 8.13% | -0.92% | 0.02 | |
| Presymptomatic | 7.21% | ||||||
| Follow-up | Control | 8.33% | -1.39% | 0.01 | |||
| Dementia | 6.94% | ||||||
| SNCA | cg06176111 | chr4:90674837 | Baseline | Control | 62.15% | 1.57% | 0.05 |
| Presymptomatic | 63.72% | ||||||
| Follow-up | Control | 61.87% | 2.48% | 0.04 | |||
| Dementia | 64.34% | ||||||
| cg17045024 | chr4:90758207 | Baseline | Control | 18.89% | -1.89% | 0.04 | |
| Presymptomatic | 17.00% | ||||||
| Follow-up | Control | 19.72% | -3.36% | 0.03 | |||
| Dementia | 16.36% | ||||||
| cg00119181 | chr4:90758537 | Baseline | Control | 8.40% | -1.20% | 0.05 | |
| Presymptomatic | 7.21% | ||||||
| Follow-up | Control | 9.53% | -2.34% | 0.02 | |||
| Dementia | 7.20% |
Intragenic Pearson’s correlation between probes
DNA methylation at individual probes within each of the genes showed weak to no correlation with each other (r <0.5), with only a few showing moderate to strong correlations (r >0.5) (Supplementary Files 1 & 2). In pre-symptomatic dementia analysis, the APP probe cg19933173 was moderately correlated (r = 0.5 to 0.7) with 21% of all probes in the region, but was otherwise largely independent from the other probes. Further the APP probe cg15407086 showed little to no correlation with any other probe in the region and is only weakly correlated with APP cg19933173.
Linear regression of significant observations
Probes with significant differences between dementia cases versus controls or pre-symptomatic dementia cases versus controls were investigated further in linear regression analysis to consider the potential influence of covariates such as age, self-reported gender and batch effects, as well as estimated blood-cell proportions. Evidence for all but four of the associations remained after adjustment for age, gender and assay batch (Model 1, Supplementary Tables 2 & 3). Those that were no longer significant at the 5% level (p > 0.05 but all with <0.10) were APP cg01286133 and BDNF cg24377657 in dementia cases versus controls and APP cg08866780 and SNCA cg00119181 in presymptomatic analysis. After further adjustment for cell type proportions of B cells, CD8+ T and CD4+ T cells, monocytes, neutrophils and natural killer cells, only two associations remained significant in association with diagnosed dementia (Model 2, Supplementary Table 4); however, for presymptomatic dementia, 15 of the original 39 probes remained significant after further adjustment (p < 0.05) (Model 2, Supplementary Table 4).
Discussion
This study identified compelling evidence of differential methylation of several genes implicated in dementia pathology in the blood of both those diagnosed with dementia and presymptomatic cases. Of particular note is the differential DNA methylation observed in dementia cases compared with controls for the SNCA and APP gene regions, with over 20% of measured probes being significantly differentially methylated between the sample groups in each gene region. Differential DNA methylation at almost a third of measured probes in TOMM40 was seen in cases of pre-symptomatic dementia.
Several of these differentially methylated probes were identified at both timepoints to have the same direction of effect compared with controls, also having a greater effect size in diagnosed dementia in contrast to presymptomatic dementia. This could suggest that methylation at these probes may be involved in the progression of disease pathology, as well as having utility as an early biomarker of disease. APP is possibly the best candidate gene for a methylation-based dementia biomarker in peripheral blood. Not only did APP contain the greatest number of probes significant at both time points, but it also showed the greatest effect size in any one probe across common probes, as well as in both individual analyses. Further, methylation at cg19933173 and cg15407086, which were associated with presymptomatic dementia, passed BH adjustment and were largely independent from methylation of all other probes in the region. This suggests that these two probes may be possible standalone early biomarkers of the disease. All common probes in APP were increased compared with controls at both time points, suggesting that increasing DNA methylation at these probes could be associated with disease progression.
From the 299 probes examined in this study, direct comparisons could be made with 32 identical CpGs from previous studies (Supplementary Table 5). This includes ten probes within APOE, four within APP, one within BDNF, nine within PIN1, two within SNCA and six within TOMM40.
When comparing probes within APOE, only one within our dataset (cg18799241) showed a small increased methylation in presymptomatic dementia compared with controls (+0.91%; p = 0.0243). A previous study (n = 67) had shown differential methylation in a group combining both MCI and AD participants, compared with controls, although the effect size (and direction) were not given [15]. The three other studies to examine APOE methylation [14,16,17] found higher methylation across the region assessed, which aligns with the small but significant higher methylation across the APOE gene region that we observed in presymptomatic dementia (+0.41; p = 0.004).
For two APP probes identified in our study (cg27158854, +0.43%; p = 0.0027 and cg08866780, +1.15; p = 0.012, where higher methylation was observed in dementia cases) findings were similar to a previous study of AD (n = 2) [18]; however, they only reported average higher methylation over four CpG’s (+0.5%). The same study reported AD was associated with an average lower methylation in PIN1. Of the six CpG in common with our study, we found one was negatively associated with presymptomatic dementia (cg06539622, -0.44%; p = 0.025).
The findings of our study that higher methylation at cg22024783 in TOMM40 was found in presymptomatic cases compared with controls (+1.53; p = 0.0029), aligns with the two previous studies that included this probe. One study of 289 individuals found higher methylation was associated with lower delayed recall score [14] and the other study of 67 individuals reported a significant difference in methylation between grouped AD/MCI and controls [15]. Impairments in delayed recall is often a feature of pre-symptomatic dementia. Additionally, a small increase in cg12271581 methylation in TOMM40 was associated with diagnosed dementia in our study (+0.46%; p = 0.0178). Methylation of this probe was previously found to be higher in individuals with lower delayed recall scores (n = 289) [14] and significantly different between MCI and AD (n = 67) [15]. We also observed an average higher methylation of TOMM40 in presymptomatic dementia (+0.41; p = 0.004); however, neither of the aforementioned studies reported on average methylation across the region.
Previously reported findings concerning BDNF (only one CpG in common) and SNCA (two CpGs) could not be replicated. The primary factors limiting exact replication of findings are the differing methods used to measure methylation and the poor reporting of genomic regions assayed. For example, previous studies used multiple methods of measuring DNA methylation, including methylation specific PCR, pyrosequencing and epigenome wide array-based measures such as the Illumina 450K [49]. Further, often studies reported regions based on different human genome assembly builds, or did not report specific regions at all, thus, insufficient information was provided to ascertain the exact gene region. Another limitation is that in some cases when associations were reported, the effect size or degree of methylation difference between cases and controls was not reported and surprisingly, sometimes even the direction of association (higher or lower methylation) was not given. Finally, discordant results between ours and previous findings could be due in part to a lack of power in previous studies, with most having a smaller sample size in comparison (eight out of 13 studies) and several studies failing to account for multiple comparisons (six out of 13 studies), which would have increased the risk of false positive findings. These variations and lack of accurate reporting of results make it difficult to directly compare methylation findings, resulting in a lack of clear replication and discordant findings across some reported genes. Our study has used the most recently available technology for measuring genome-wide methylation changes and provided clear genomic locations for each of the CpGs.
Strengths & limitations
The main strength of this study was the ability to analyze methylation across previously implicated genes in those with a dementia diagnosis and to investigate differential methylation at these same genes in presymptomatic cases. Differential methylation at specific probes in the presymptomatic group increased in effect size and in the same direction when compared with the analysis of diagnosed dementia. A limitation of the study is the moderate sample size; however, this is comparable to other studies published in this field to date (ranging from two to 458 participants) and the inclusion of presymptomatic dementia cases is a strength over previous studies. Given this, only two probes within our analysis passed adjustment for multiple testing. There is thus a possibility of an increased risk of type 1 error and that some of the other reported findings could be false positives. However, particularly in the dementia versus controls analysis, there are more significant findings in this study than we would expect based on chance alone. Another limitation is that we only attempted to replicate genes where significant findings had already been identified from prior studies in the field. While this was a conservative approach, focusing on strong a priori genes, it also means that there may be other important genes that have not been considered here. For example, Mise et al. measured methylation over the TOMM40 gene region and found no differences in methylation between dementia and controls [50]. When comparing probe locations, we were able to directly compare cg06632829, chr19:45,394,476, which in our study also showed no association between methylation and dementia. The measurements of the Illumina Infinium MethylationEPIC BeadChip used in this study have been shown to have high reproducibility when using biological and technical replicates [51]. Regardless, before any findings progress further for true biomarker development, technical validation by using separate methylation measurement methods, such as pyrosequencing would be required [52].
Conclusion
Findings in this study were partially concordant with previous methylation studies of candidate genes in dementia. Further, we found good evidence that differential methylation at some novel sites within these genes were associated with dementia and that some of these could be detected prior to the appearance of clinical dementia symptoms. Methylation at several sites within the APP gene have the potential to be a biomarker of presymptomatic dementia, dementia diagnosis and of the progression of the disease. Further studies of APP, SNCA and TOMM40, including a focus on presymptomatic dementia, that include genotype and gene expression analysis, are required to strengthen this evidence.
Future perspective
Genetic variation within the genes assessed in this study has been associated with dementia risk. Genetic variation is also known to influence DNA methylation [53,54], but it remains unknown to which degree the genetic variation of dementia risk genotypes influences DNA methylation. Linking DNA methylation data to genotypic data in the same gene region should be investigated further. It has the potential to help identify novel dementia risk related SNPs, but also to determine how changes in genotype may influence methylation associated with the disease. It also remains unclear whether the differential methylation observed here is functionally relevant. Not only could methylation at these genes have utility as a biomarker for the disease, but differential methylation may lead to differential gene expression and thus could contribute to dementia pathology.
Blood is a heterogeneous mix of multiple cell types and differing cell types have different methylation profiles [55]. Here we adjusted for estimated blood–cell proportions, which was shown to affect the association between methylation and pre-diagnosed/dementia status. It should be noted that cell type estimation of epigenome data may introduce a source of unwanted variation to the study and differing cell types may be a result of the disease itself, as has been seen previously in Alzheimer’s disease [56]. Should an easy to obtain biomarker for dementia be found using DNA methylation measurable in blood, it should be detectable regardless of cell composition. That said, a greater understanding of the relationship between dementia and blood-based methylation could come from specific cell type analyses in blood. Thus, future studies may consider using methylation profiles built off specific blood cell types, rather than whole blood which gives an average methylation value across all blood cell types.
Finally, direct comparisons between studies and the pooling of data for meta-analyses, imperative to advance the field, requires the proper reporting of gene regions including genome build, array probe name and exact genomic locations as well as the full reporting of results, including the direction and magnitude of effect size.
Summary points.
Blood DNA methylation could be a useful biomarker – which may aid in diagnosis and could predict future risk of disease.
Candidate genes targeted in this study are those that have been previously implicated in dementia pathology, including APOE, APP, BDNF, PIN1, SNCA and TOMM40.
DNA methylation was measured in the peripheral blood of 160 cognitively healthy individuals, 73 (presymptomatic for dementia) who were subsequently diagnosed and 87 controls matched for age gender, education, smoking and baseline cognition.
DNA methylation was also measured in 49 of these participants at follow-up, including 24 with diagnosed dementia and 25 who remained cognitively healthy.
Analysis included comparisons of average methylation across each of the six genes and at 299 specific methylation probes across the genes, between cases (presymptomatic and diagnosed dementia) and controls.
Linear regression models were used to adjust for age, sex and assay batch, as well as estimated blood cell proportions.
A total of 56 probes were found to be differentially methylated between diagnosed dementia participants and matched controls in adjusted analysis, though none passed Benjamini-Hochberg adjustment.
A total of 39 probes were associated with presymptomatic dementia, two of which also passed Benjamini-Hochberg adjustment.
cg19933173, CpG21:27562920 upstream of the APP transcription site (+4.31% in presymptomatic dementia; p < 0.0001, BH Adj.p = 0.015) and cg15407086, CpG21:27543045, within the APP gene, (+2.09%; p < 0.0001, BH Adj.p = 0.015).
We found good evidence of differential methylation of several genes implicated in dementia and that some of these can be detected prior to the appearance of clinical dementia symptoms.
Supplementary Material
Acknowledgments
The authors acknowledge the dedicated and skilled staff in Australia and the USA involved in the ASPREE cohort. The authors also are most grateful to the ASPREE participants, who so willingly volunteered for this study and the general practitioners and staff of the medical clinics who cared for the participants.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/epi-2020-0236
Financial & competing interests disclosure
The work was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (U01AG029824); the National Health and Medical Research Council (NHMRC) of Australia (334047 and 1127060); Monash University and the Victorian Cancer Agency. J Ryan is funded by an NHMRC Dementia Research Leader Fellowship (APP1135727). PD Fransquet is gratefully funded by RTP stipend PhD scholarship, awarded by Monash University and the Australian Government. A Murray reports receiving consulting fees from Alkahest, Inc. and reports grants from National Institute on Aging. RC Shah reports grants for clinical research regarding dementia and Alzheimer’s disease from National Institutes of Health, the Centers for Medicare and Medicaid Services, the Department of Defense and the Illinois Department of Public Health; being a noncompensated board member of the Alzheimer’s Association – Illinois Chapter; and, being the site principal investigator or subinvestigator for clinical trials for which his institution (Rush University Medical Center) is compensated (Amylyx Pharmaceuticals, Inc., Eli Lilly & Co., Inc., Genentech, Inc., Merck & Co, Inc., Navidea Biopharmaceuticals, Novartis Pharmaceuticals, Inc., Roche Holdings AG and Takeda Development Center Americas, Inc.). The funders had no role in the study design; collection, analysis and interpretation of data; writing of the report; and decision to submit the article for publication. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
The authors certify that this manuscript reports original clinical trial data. The data that support the findings of this study are available from the ASPREE principle investigators, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the ASPREE principle investigators through the web site (www.ASPREE.org). Data will be shared with researchers assessed upon request, for use in epigenetic analysis. If approved data will be made available through a web-based data portal safe haven at Monash University, Australia.
Ethical conduct of research
The ASPREE study was approved by Monash Human Ethics Committee (2006/745M), all participants gave informed consent. This DNA methylation substudy was approved by The Alfred Human Ethics Committee (Project 448/16). The study was conducted in accordance with the Declaration of Helsinki 2008 revision, NHMRC Guidelines on Human Experimentation, the federal patient privacy (HIPAA) law, the International Conference of Harmonisation Guidelines for Good Clinical Practice and the Code of Federal Regulations.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Fenoglio C, Scarpini E, Serpente M, Galimberti D. Role of genetics and epigenetics in the pathogenesis of Alzheimer's disease and frontotemporal dementia. J. Alzheimers Dis. 62(3), 913–932 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamazaki Y, Zhao N, Caulfield TR, Liu C-C, Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15(9), 501–518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9(2), 106–118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van der kant R, Goldstein Lawrence SB. Cellular functions of the amyloid precursor protein from development to dementia. Dev. Cell 32(4), 502–515 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Ferencz B, Laukka EJ, Lövdén M et al. The influence of APOE and TOMM40 polymorphisms on hippocampal volume and episodic memory in old age. Front. Hum. Neurosci. 7, 198–198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyall DM, Harris SE, Bastin ME et al. Alzheimer's disease susceptibility genes APOE and TOMM40 and brain white matter integrity in the Lothian Birth Cohort 1936. Neurobiol. Aging 35(6), 1513.e1525–1513.e1511.1513E1533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prendecki M, Florczak-Wyspianska J, Kowalska M et al. Biothiols and oxidative stress markers and polymorphisms of TOMM40 and APOC1 genes in Alzheimer's disease patients. Oncotarget 9(81), 35207–35225 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driver JA, Lu KP. Pin1: a new genetic link between Alzheimer's disease, cancer and aging. Curr. Aging Sci. 3(3), 158–165 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Park JS, Lee J, Jung ES et al. Brain somatic mutations observed in Alzheimer's disease associated with aging and dysregulation of tau phosphorylation. Nat. Commun. 10(1), 3090 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villar-Pique A, Lopes Da Fonseca T, Outeiro TF. Structure, function and toxicity of alpha-synuclein: the Bermuda triangle in synucleinopathies. J. Neurochem. 139(Suppl. 1), 240–255 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Linnertz C, Lutz MW, Ervin JF et al. The genetic contributions of SNCA and LRRK2 genes to lewy body pathology in Alzheimer's disease. Hum. Mol. Genet. 23(18), 4814–4821 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borroni B, Grassi M, Archetti S et al. BDNF genetic variations increase the risk of Alzheimer's disease-related depression. J. Alzheimers Dis. 18(4), 867–875 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Ng TKS, Ho CSH, Tam WWS, Kua EH, Ho RC-M. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer's disease (AD): a systematic review and meta-analysis. Int. J. Mol. Sci. 20(2), 257 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Zhao W, Ware EB, Turner ST, Mosley TH, Smith JA. DNA methylation in the APOE genomic region is associated with cognitive function in African Americans. BMC Med. Genomics 11, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao Y, Shaw M, Todd K et al. DNA methylation of TOMM40-APOE-APOC2 in Alzheimer's disease. J. Hum. Genet. 63(4), 459–471 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson IK, Ploner A, Wang Y, Gatz M, Pedersen NL, Hägg S. Apolipoprotein E DNA methylation and late-life disease. Int. J. Epidemiol. 47(3), 899–907 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancera-Paez O, Estrada-Orozco K, Mahecha MF et al. Differential methylation in APOE (Chr19; Exon four; from 44,909,188 to 44,909,373/hg38) and increased Apolipoprotein E plasma levels in subjects with mild cognitive impairment. Int. J. Mol. Sci. 20(6), 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'addario C, Candia SB, Arosio B et al. Transcriptional and epigenetic phenomena in peripheral blood cells of monozygotic twins discordant for Alzheimer's disease, a case report. J. Neurol. Sci. 372, 211–216 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Hou Y, Chen H, He Q et al. Changes in methylation patterns of multiple genes from peripheral blood leucocytes of Alzheimer's disease patients. Acta Neuropsychiatr. 25(2), 66–76 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Chang L, Wang Y, Ji H et al. Elevation of peripheral BDNF promoter methylation links to the risk of Alzheimer's disease. PLoS ONE 9(11), e110773 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagata T, Kobayashi N, Ishii J et al. Association between DNA Methylation of the BDNF promoter region and clinical presentation in Alzheimer's disease. Dement. Geriatr. Cogn. Dis. Extra 5(1), 64–73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie B, Xu Y, Liu Z et al. Elevation of peripheral BDNF promoter methylation predicts conversion from Amnestic Mild Cognitive Impairment to Alzheimer's disease: a 5-Year longitudinal study. J. Alzheimers Dis. 56(1), 391–401 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Arosio B, Bulbarelli A, Bastias Candia S et al. Pin1 contribution to Alzheimer's disease: transcriptional and epigenetic mechanisms in patients with Late-Onset Alzheimer's disease. Neurodegener. Dis. 17, 207–211 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Ferri E, Arosio B, D'addario C et al. Gene promoter methylation and expression of Pin1 differ between patients with frontotemporal dementia and Alzheimer's disease. J. Neurol. Sci. 362, 283–286 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Funahashi Y, Yoshino Y, Yamazaki K et al. DNA methylation changes at SNCA intron 1 in patients with dementia with Lewy bodies. Psychiatry Clin. Neurosci. 71(1), 28–35 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Yoshino Y, Mori T, Yoshida T et al. Elevated mRNA expression and low methylation of SNCA in Japanese Alzheimer's disease subjects. J. Alzheimers Dis. 54(4), 1349–1357 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Mcneil JJ, Woods RL, Nelson MR et al. Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study. J. Gerontol. A Biol. Sci. Med. Sci. 72(11), 1586–1593 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Full details of the ASPREE study and participant characteristics
- 28.Ryan J, Woods RL, Britt C et al. Normative performance of healthy older individuals on the Modified Mini-Mental State (3MS) examination according to ethno-racial group, gender, age and education level. Clin. Neuropsychol. 33(4), 779–797 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones TG, Schinka JA, Vanderploeg RD, Small BJ, Graves AB, Mortimer JA. 3MS normative data for the elderly. Arch. Clin. Neuropsychol. 17(2), 171–177 (2002). [PubMed] [Google Scholar]
- 30.Smith A. Symbol digit modalities test: manual. Los Angeles: Western Psychological Services (1982). [Google Scholar]
- 31.Ruff RM, Light RH, Parker SB, Levin HS. Benton controlled oral word association test: reliability and updated norms. Arch. Clin. Neuropsychol. 11(4), 329–338 (1996). [PubMed] [Google Scholar]
- 32.Ryan J, Woods RL, Murray AM et al. Normative performance of older individuals on the Hopkins Verbal Learning Test-Revised (HVLT-R) according to ethno-racial group, gender, age and education level. Clin. Neuropsychol. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 12(1), 43–55 (1998). [Google Scholar]
- 34.First MB, Frances A, Pincus HA. DSM-IV-TR Handbook of Differential Diagnosis. American Psychiatric Publishing, Inc, Arlington, VA, USA: (2002). [Google Scholar]
- 35.Ryan J, Storey E, Murray AM et al. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology 95(3), e320–e331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fransquet PD, Ryan J. The current status of blood epigenetic biomarkers for dementia. Crit. Rev. Clin. Lab. Sci. 56(7), 435–457 (2019). [DOI] [PubMed] [Google Scholar]; • Summarizes recent studies of blood based epigenetic dementia biomarkers.
- 37.Haeussler M, Zweig AS, Tyner C et al. The UCSC Genome Browser database: 2019 update. Nucleic Acids Res. 47(D1), D853–d858 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An extremely useful set of tools for visualizing and interrogating the human genome.
- 38.Tusnády GE, Simon I, Váradi A, Arányi T. BiSearch: primer-design and search tool for PCR on bisulfite-treated genomes. Nucleic Acids Res. 33(1), e9–e9 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]; • A useful online tool for reverse engineering bisulphite converted primers to their original code.
- 39.Qiagen. DNeasy blood & tissue kits. (2019). www.qiagen.com/au/products/top-sellers/dneasy-blood-and-tissue-kit/#orderinginformation
- 40.Illumina Inc. “Illumina Infinium MethylationEPIC Array”. (2019). https://sapac.illumina.com/products/by-type/microarray-kits/infinium-methylation-epic.html
- 41.Saffery R, Gordon L. Time for a standardized system of reporting sites of genomic methylation. Genome Biol. 16(1), 85 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Touleimat N, Tost J. Complete pipeline for Infinium((R)) Human Methylation 450K BeadChip data processing using subset quantile normalization for accurate DNA methylation estimation. Epigenomics 4(3), 325–341 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Maksimovic J, Phipson B, Oshlack A. A cross-package Bioconductor workflow for analysing methylation array data. F1000Res 5, 1281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • A great resource detailing an analysis pipeline for an epigenome wide association study
- 44.Houseman EA, Accomando WP, Koestler DC et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 13(1), 86 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salas L, Koestler D. FlowSorted.Blood.EPIC: illumina EPIC data on immunomagnetic sorted peripheral adult blood cells. www.bioconductor.org/packages/release/data/experiment/html/FlowSorted.Blood.EPIC.html (2018).
- 46.Salas LA, Koestler DC, Butler RA et al. An optimized library for reference-based deconvolution of whole-blood biospecimens assayed using the Illumina HumanMethylationEPIC BeadArray. Genome Biol. 19(1), 64 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaffe AE, Irizarry RA. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biol. 15(2), R31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc. 57(1), 289–300 (1995). [Google Scholar]
- 49.Dedeurwaerder S, Defrance M, Bizet M, Calonne E, Bontempi G, Fuks F. A comprehensive overview of Infinium HumanMethylation450 data processing. Brief. Bioinform. 15(6), 929–941 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mise A, Yoshino Y, Yamazaki K et al. TOMM40 and APOE gene expression and cognitive decline in Japanese Alzheimer's disease subjects. J. Alzheimers Dis. 60(3), 1107–1117 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Pidsley R, Zotenko E, Peters TJ et al. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 17(1), 208–208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delaney C, Garg SK, Yung R. Analysis of DNA methylation by pyrosequencing. Methods Mol. Biol. 1343, 249–264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Lou D, Wang Z. Crosstalk of genetic variants, allele-specific DNA methylation and environmental factors for complex disease risk. Front. Genet. 9(695), 1–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith AK, Kilaru V, Kocak M et al. Methylation quantitative trait loci (meQTLs) are consistently detected across ancestry, developmental stage and tissue type. BMC Genomics 15(1), 145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bauer M. Cell-type-specific disturbance of DNA methylation pattern: a chance to get more benefit from and to minimize cohorts for epigenome-wide association studies. Int. J. Epidemiol. 47(3), 917–927 (2018). [DOI] [PubMed] [Google Scholar]; • Describes how methylation profiles of specific blood cell types is an important consideration for epigenome wide association studies
- 56.Lunnon K, Ibrahim Z, Proitsi P et al. Mitochondrial dysfunction and immune activation are detectable in early Alzheimer's disease blood. J. Alzheimers Dis. 30, 685–710 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.