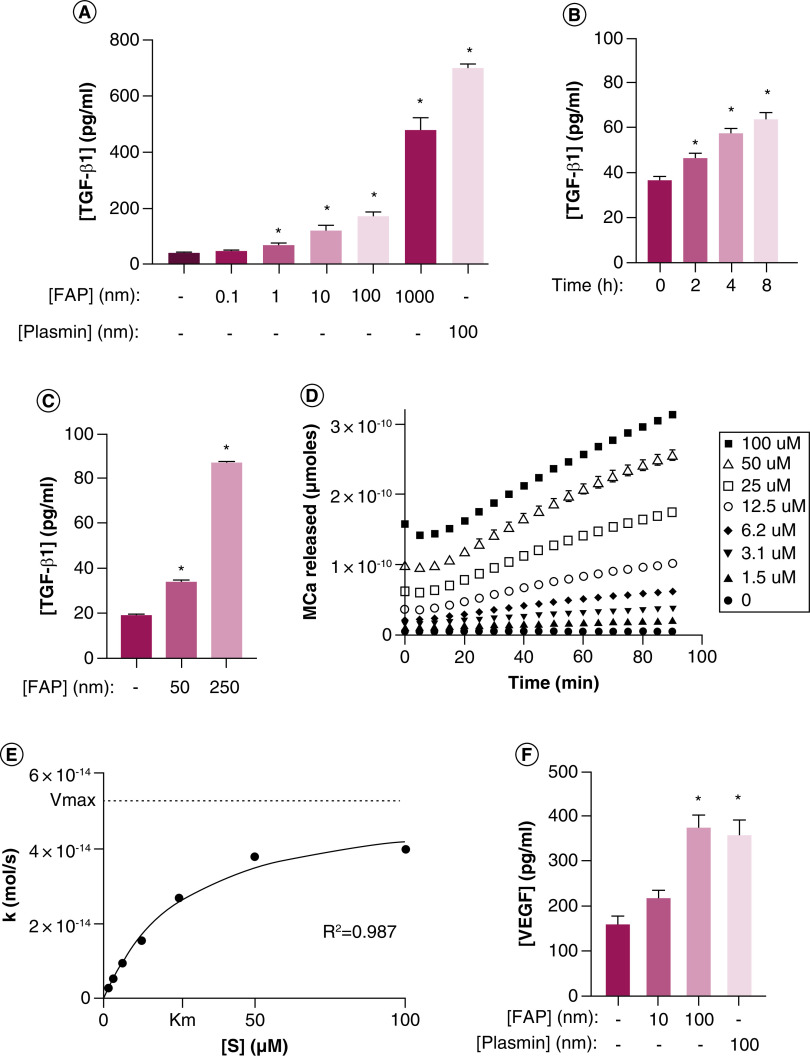
Figure 2. . Fibroblast activation protein activates latent reservoirs of TGF-β sequestered in the basement membrane and extracellular matrix.
(A) HUVEC treated with increasing concentrations of exogenous recombinant human FAP (rhFAP) for 2.5 h at 37°C demonstrates a dose-dependent increase in TGF-β1 in the supernatant compared with vehicle-treated controls as measured by ELISA from two independent experiments performed in duplicate. Plasmin used as a positive control. (B) rhFAP (50 nM) releases TGF-β1 from HUVEC-derived basement membrane denuded of cells in a time-dependent manner as determined by ELISA of conditioned media at the indicated time points. Experiment performed in duplicate. (C) FAP releases TGF-β1 from human fibroblast (WPMY-1) derived ECM denuded of cells in a concentration-dependent manner over 24 h at 37°C. Three independent experiments performed in duplicate. (D) FAP-dependent cleavage of a fluorescence-quenched peptide substrate (Mca-EIPESGPSSG-Dnp) designed from the hinge region of LTBP-4 in the presence of FAP (100 nM) at 37°C. No Mca released in the absence of FAP. All experiments performed in triplicate with a representative plot shown here. (E) Representative plot of hydrolysis rate (k) versus substrate concentration ([S]) for the peptide presented in panel D. (F) Increasing concentrations of VEGF detected in conditioned media from human prostate cancer (PC3) cells treated with increasing concentrations of exogenous rhFAP release as measured by ELISA and measured in triplicate. Plasmin used as a positive control. All error bars represent standard error (SE). p-values < 0.05 (*) relative to the untreated controls.
ECM: Extracellular matrix; FAP: Fibroblast activation protein.