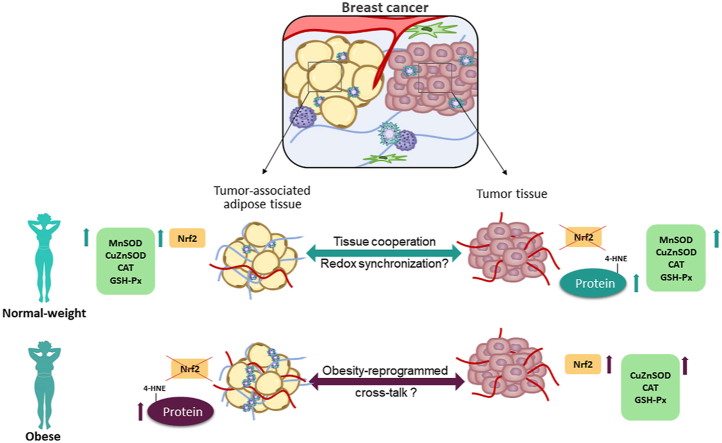
Abstract
One of the underlying mechanisms that could link breast cancer and obesity is shifted redox homeostasis in the tumor microenvironment. To reveal the relationship between the malignant phenotype and obesity, we compared redox profiles of breast tumor and tumor-associated adipose tissue from premenopausal women: normal-weight with benign tumors, overweight/obese with benign tumors, normal-weight with malignant tumors, and overweight/obese with malignant tumors. Namely, we examined the protein expression of nuclear factor erythroid 2-related factor 2 (Nrf2), protein expression and activity of main antioxidant defense (AD) enzymes: copper, zinc- and manganese superoxide dismutase, catalase, and glutathione peroxidase, as well as the level of 4-hydroxy-2-nonenal (4-HNE) modified proteins. Higher protein expression and activity of AD enzymes were found in malignant tumor tissue than benign tumor tissue, irrespective of obesity. Nevertheless, malignant tumor tissue of overweight/obese women was characterized by higher protein expression of Nrf2 and weaker immunopositivity for 4-HNE modified proteins. In malignant tumor-associated adipose tissue, the redox profile was clearly related to obesity. Higher Nrf2 protein expression and higher AD enzyme levels were observed in normal-weight women, while stronger immunopositivity for 4-HNE modified proteins was found in overweight/obese women. The results suggest that the complex interplay between obesity and malignancy involves redox-sensitive pathways in breast tumor and tumor-associated adipose tissue.
Keywords: Nrf2, 4-HNE, Premenopausal breast cancer, Cancer-associated adipose tissue, Redox regulation, Obesity
Graphical abstract
Highlights
-
•
In malignant breast tumor tissue, antioxidant defense enzyme levels are not related to obesity.
-
•
In malignant tumor-associated adipose tissue, redox profile is related to obesity.
-
•
Nrf2 contributes to the “activated” phenotype of adipose tissue in malignancy.
1. Introduction
Breast cancer is the most prevalent malignancy in women, with the highest mortality rate worldwide. Excess body weight is a known risk factor for breast cancer in postmenopausal women, contributing to more severe disease progression [[1], [2], [3]]. However, evidence linking obesity to breast cancer in premenopausal women remains inconclusive [[4], [5], [6], [7]].
One of the underlying mechanisms that could link obesity and breast cancer is shifted redox homeostasis in breast tissues. Since the pioneering work of Warburg and Oberley, metabolic reprogramming and underlying changes in redox regulation have been recognized as hallmarks of neoplastic transformation [8]. The chief characteristics of cancer cells are mediated by redox-sensitive cellular processes that serve to sustain the malignant phenotype, i.e., genomic instability, proliferation, migration, and apoptosis [9]. Accordingly, malignant cells are often characterized by atypical redox signaling, different reactive species generation rates, and altered levels of antioxidant defense (AD) enzymes. This is evident as increased production of reactive oxygen species (ROS), presence of oxidative stress biomarkers (e.g., 8-hydroxy-2′-deoxyguanosine, protein carbonyls, 4-hydroxy-2-nonenal protein adducts, malondialdehyde), as well as tumor-specific overexpression or underexpression of several redox proteins [[10], [11], [12]]. A delicate balance between ROS production and neutralization could reflect the metabolic blueprint of every cancer, determining its invasiveness, metastatic potential, and resistance to conventional therapies. It is recognized that obesity, as a chronic state of altered energy homeostasis, can affect the metabolism of different tissues, including cancer [13,14]. Our recent study showed obesity-related changes to the lactate metabolism in the breast cancer microenvironment [15]; however, the impact of obesity on cancer tissue redox homeostasis has not been studied thoroughly.
On the local level, dysfunction of resident adipose tissue in obesity may be one of the critical features that contribute to cancer initiation and progression. Fat accumulation leads to systemic and adipose tissue (AT) localized prooxidant state [16], mainly attributed to stimulated ROS generation and decreased activity of superoxide dismutase (SOD) isoforms [17]. Moreover, adipose tissue possesses unique morpho-functional plasticity that comes to light in obesity. Adipogenesis, adipose hypertrophy, and hyperplasia are supported by redox-driven alterations in glucose homeostasis, oxidative metabolism, and antioxidant defense [18]. Such processes of local tissue remodeling are potentially responsible for establishing and sustaining the tumor microenvironment. The relationship between malignancy and obesity deepens by our increasing understanding of the importance of cancer-adipose tissue cross-talk. This complex communication, recently termed adipocyte cancer cell paracrine loop, leads to excessive mutual remodeling and promotes overall cancer aggressiveness [[19], [20], [21], [22]]. However, cancer-adipose tissue cross-talk is mostly studied in vitro, in the light of paracrine functions of growth factors, adipokines, and proinflammatory cytokines. Human studies considering the morpho-functional specificity of the mammary adipose depot, especially in complex physiological states such as obesity, are still scarce [17,23,24].
This study aimed to reveal the redox profile related to the malignant phenotype and its relationship with obesity in premenopausal women. To this end, we examined the protein expression of transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), the activity and protein expression of first-line AD enzymes: copper, zinc- and manganese superoxide dismutase (CuZnSOD and MnSOD, respectively), catalase (CAT), glutathione peroxidase (GSH-Px) and level of 4-hydroxy-2-nonenal (4-HNE) modified proteins in paired biopsies of tumor tissue and associated adipose tissue from normal-weight and overweight/obese women with breast cancer. As respective controls, paired biopsies of tumor tissue and associated adipose tissue from normal-weight and overweight/obese women with non-malignant (benign) breast changes were used.
2. Materials and methods
2.1. Subjects and sample collection
This study followed the standards set by the latest version of the Declaration of Helsinki. The ethics committee of the Clinical Center of Vojvodina approved all the procedures. Patients volunteered for the study and signed an informed consent form. The study group consisted of 36 women who were hospitalized for breast surgery. All subjects were premenopausal (with regular menses for the last six months) with an average age of 39.8 ± 8.77 years. Indications for surgical intervention were benign cases of breast fibroadenoma or malignant cases of luminal type A (ER+/PR+/HER2-) invasive ductal carcinoma. At the beginning of the surgical procedure under general balanced anesthesia, samples of breast tumor and tumor-associated adipose tissue (in the immediate vicinity to the tumor mass) were obtained. From each patient, one piece of the tumor and the adipose tissue sample was snap-frozen in liquid nitrogen and stored at −80 °C until subsequent protein isolation by TRI Regent procedure (Ambion, USA) for protein expression analysis by Western blot. The remaining piece was homogenized (Heidolph DIAX 600) at 0–4 °C in 0.25 M sucrose, 0.1 mM EDTA, and 50 mM Tris buffer, pH 7.4 for enzyme activity measurements. Body mass index (BMI) was used to classify samples as normal-weight (BMI < 25 kg/m2) or overweight/obese (BMI ≥ 25 kg/m2) [25]. According to the tumor type and BMI, samples were further classified into four groups (n = 9); normal-weight (non-obese) with benign tumors, overweight/obese with benign tumors, normal-weight (non-obese) with malignant tumors, and overweight/obese with malignant tumors.
2.2. Western blot analysis
Western blot analysis was conducted as described previously [26] using antibodies against: CuZnSOD (0.2 μg ml−1; ab13498), MnSOD (0.2 μg ml−1, ab13533), CAT (1 μg ml−1; ab1877), GSH-Px (1 μg ml−1; ab17926-500), Nrf2 (1 μg ml−1; ab31163), 4-HNE (1 μg ml−1; ab46545) and β-actin (0.5 μg ml−1; ab8226) (all purchased from Abcam, Cambridge, UK). Quantitative analysis of immunoreactive bands was performed with ImageJ software (National Institute of Health, USA). Total band density was calculated as the sum of pixel intensities within a band. The ratio of dots per band for the target protein was averaged against β-actin (gel loading control) from three independent experiments, and the levels of protein expression were expressed in arbitrary units (AU).
2.3. Immunofluorescence analysis
Standard immunolabeling procedure we conducted as previously described [27] using primary antibodies against 4-HNE (5 μg ml−1; ab46545, Abcam, UK) and with the appropriate fluorochrome-conjugated secondary antibody (1:400; Alexa Fluor® 633 goat anti-rabbit, A2070, Thermo Fisher Scientific, USA). For counterstaining of the nuclei, Sytox Orange (1 μL ml−1, Thermo Fisher Scientific) was used. Slides were mounted with Mowiol (Polysciences, Eppelheim, Germany), and confocal images were acquired with a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems, Austria) in sequential mode to avoid cross-talk between channels. The specificity of immunofluorescence was tested by the omission of primary antibodies. Image processing and quantification were performed with NIH Image J software.
2.4. AD enzyme activity
The activity of superoxide dismutase isoforms was determined using the method of Misra and Fridovich [28] and expressed in U mg−1 protein. Catalase activity was assayed according to the method of Beutler, and the activity was expressed in U mg−1 protein [29]. Glutathione peroxidase was determined using the method of Paglia and Valentine [30], and the activity was expressed in nmol of reduced NADPH min−1 mg−1 protein.
2.5. Statistics
Statistical analysis was performed in GraphPad Prism software (GraphPad Prism, Version 6.01). Normality of distribution for all data sets was assessed with D'Agostino and Pearson's omnibus normality test. One-way two-tailed analysis of variance (ANOVA) was applied for within-group comparison of the data from molecular analysis. If the F test showed an overall difference, multiple comparison Tukey's post hoc test was used to evaluate the significance of the among group differences. Statistical significance was accepted at p < .05.
3. Results
Protein expression of AD enzymes, Nrf2, and level of 4-HNE modified proteins in benign and malignant tumor tissue of normal-weight and overweight/obese women.
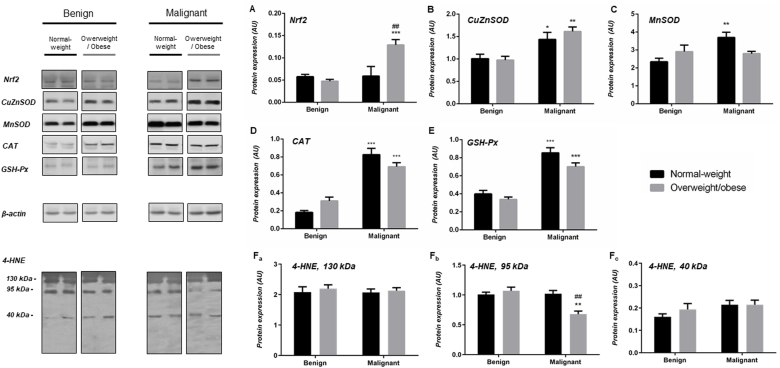
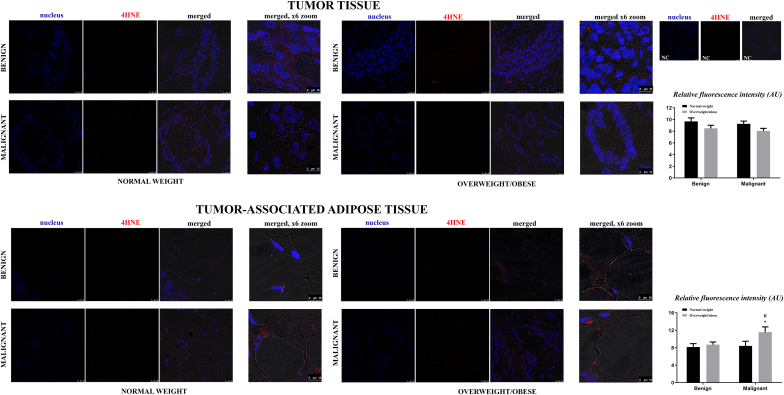
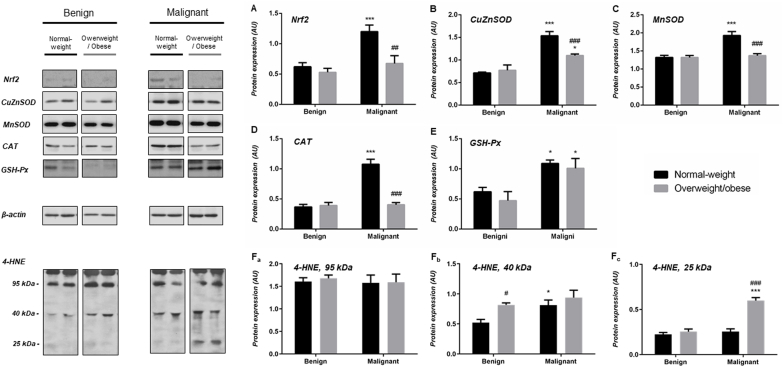
In general, malignant tumor tissue of normal weight and overweight/obese women displays increased protein expression of AD enzymes in comparison to benign tumor tissue of weight-matched women. Namely, a clear difference in protein expression of CuZnSOD, CAT and GSH-Px was observed (CuZnSOD (normal-weight) p < .05, CuZnSOD (obese) p < .01; CAT(normal-weight) p < .001, CAT(obese) < 0.001; GSH-Px (normal-weight) p < .001, GSH-Px (obese) < 0.001). Besides, there were no differences in protein expression of CuZnSOD, CAT, and GSH-Px between normal-weight and overweight/obese pairs of benign and malignant tumors. Higher protein expression of MnSOD was found only in malignant tumor tissue of normal-weight women (p < .01). Interestingly, Nrf2 showed higher protein expression in malignant tumor tissue of overweight/obese women, compared to both its corresponding benign counterpart (p < .001) and to the malignant tumor tissue of normal-weight women (p < .01) (Fig. 1A–E). Semi-quantitative analysis of 4-HNE immunofluorescence intensity showed no significant differences between analyzed groups. However, Western blot analyses showed multiple prominent immunopositive bands for 4-HNE, where a weaker positivity corresponding to the 95 kDa band was found in malignant tumor tissue of overweight/obese women compared to their overweight/obese benign counterparts and malignant tumor tissue of normal-weight women (Figs. 1F and 5).
Fig. 1.
Protein expression of Nrf2 (A), AD enzymes (CuZnSOD (B), MnSOD (C), CAT (D), GSH-Px (E)), and level of 4-HNE modified proteins (130 kDa (Fa), 95 kDa (Fb), 40 kDa (Fc)) in benign and malignant breast tumor tissue of normal-weight (black) and overweight/obese (gray) women. The protein content is expressed in arbitrary units (AU). Band images from a representative blot of three trials are shown. Bars represent the mean ± S.E.M. *Compared to respective benign counterpart, *p < .05, **p < .01, ***p < .001; # compared to normal-weight malignant counterpart, ##p < .01.
Fig. 5.
Immunofluorescence staining and confocal microscopy of 4-HNE modified proteins presence and localization in breast tumor tissue and tumor-associated adipose tissue of normal-weight (black bars) and overweight/obese (gray bars) women with benign and malignant breast tumors. 4-HNE- (red) and nuclei Sytox Orange staining (false blue). Merged images are an overlay of two channels on phase-contrast tissue images. NC-negative control. Scale bars: 25 μm and 10 μm on merged x6 zoom.
AD enzyme activity in benign and malignant tumor tissue of normal-weight and overweight/obese women.
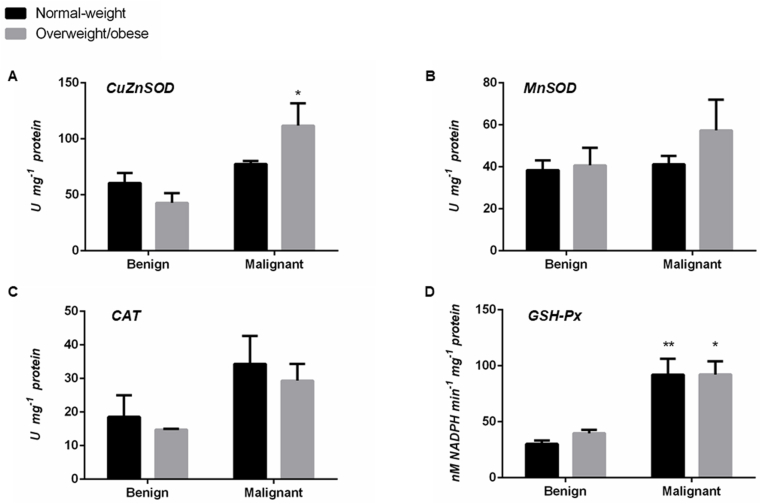
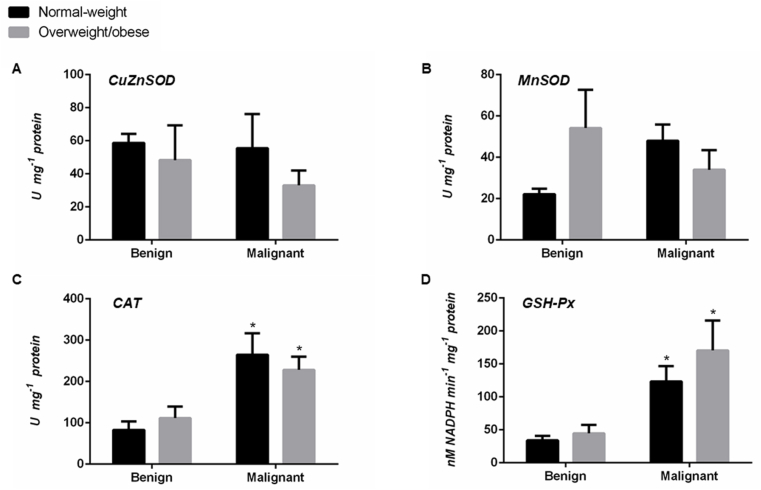
There were no significant differences in CuZnSOD and MnSOD activity in the tumor tissue when weight-matched benign and malignant counterparts were compared, except for slightly higher CuZnSOD activity (p < .05) in overweight/obese women with malignant tumors. Clearly higher GSH-Px activity characterized malignant tumor tissue of both normal-weight (p < .01) and overweight/obese women (p < .05), compared to benign weight-matched counterparts. Similarly, a trend towards the higher activity of CAT was present in malignant tumor tissue in comparison to benign, irrespective of the BMI (Fig. 2).
Fig. 2.
The activity of CuZnSOD (A), MnSOD (B), CAT (C), and GSH-Px (D) in benign and malignant breast tumor tissue of normal-weight (black) and overweight/obese (gray) women. Enzyme activity is expressed in absolute units in U mg−1 protein (A, B, C) and nM NADPH min−1mg−1 protein (D). Bars represent the mean ± S.E.M. *Compared to respective benign counterpart, *p < .05, **p < .01.
Protein expression of AD enzymes, Nrf2, and level of 4-HNE modified proteins in tumor-associated adipose tissue of normal-weight and overweight/obese women with benign and malignant breast tumors.
Expression patterns of AD enzymes in tumor-associated adipose tissue were mainly consistent. Significantly higher protein expression of CuZnSOD, MnSOD, and CAT was found in adipose tissue of normal-weight women with malignant tumors, as compared to weight-matched women with benign breast tumors (p < .001) and to overweight/obese women with malignant tumors (p < .001). Higher protein expression of GSH-Px was found in tumor-associated adipose tissue of women with malignant tumors (p < .05), compared to their benign counterparts, regardless of BMI. In addition, protein expression of Nrf2 was found to be higher in adipose tissue of normal-weight women with malignant breast tumors, in comparison to weight-matched women with benign tumors (p < .001) and overweight/obese women with malignant tumors (p < .01) (Fig. 3A–E). Semi-quantitative analysis of 4-HNE immunofluorescence intensity showed the strongest immunopositivity for 4-HNE modified proteins in adipose tissue of overweight/obese women with malignant tumors. Interestingly, Western blot analysis showed that the intensity of immunoreactive bands for 4-HNE at 40 kDa was higher in adipose tissue of overweight/obese women with benign tumors as well as in adipose tissue of normal-weight women with malignant tumors compared to adipose tissue of normal-weight women with benign tumors. However, additional immunoreactive bands for 4-HNE at 25 kDa were markedly visible only in adipose tissue of overweight/obese women with malignant tumors (Figs. 3F and 5).
Fig. 3.
Protein expression of Nrf2 (A), AD enzymes (CuZnSOD (B), MnSOD (C), CAT (D), GSH-Px (E)), and level of 4-HNE modified proteins (95 kDa (Fa), 40 kDa (Fb), 25 kDa (Fc)) in tumor-associated adipose tissue of normal-weight (black) and overweight/obese (gray) women with benign and malignant breast tumors. The protein content is expressed in arbitrary units (AU). Band images from a representative blot of three trials are shown. Bars represent the mean ± S.E.M. *Compared to respective benign counterpart, *p < .05, ***p < .001; # compared to normal-weight malignant counterpart, #p < .05, ##p < .01, ###p < .001.
AD enzyme activity in tumor-associated adipose tissue of normal-weight and overweight/obese women with benign and malignant breast tumors.
There were no differences in adipose tissue CuZnSOD and MnSOD activity between examined groups of women. Higher CAT and GSH-Px activity in adipose tissue of normal-weight (p < .05) and overweight/obese (p < .05) women with malignant tumors was observed, in comparison to respective adipose tissue of women with benign tumors (Fig. 4).
Fig. 4.
The activity of CuZnSOD (A), MnSOD (B), CAT (C), and GSH-Px (D) in tumor-associated adipose tissue of normal-weight (black) and overweight/obese (gray) women with benign and malignant breast tumors. Enzyme activity is expressed in absolute units in U mg−1 protein (A, B, C) and nM NADPH min−1mg−1 protein (D). Bars represent the mean ± S.E.M. *Compared to respective benign counterpart, *p < .05, **p < .01.
4. Discussion
This study evaluated the redox profile of breast tumor tissue and tumor-associated adipose tissue and its relationship with malignancy and obesity in premenopausal women. Cross-examination of malignant tumor tissue biopsies revealed higher protein expression and activity of investigated AD enzymes regardless of BMI compared to benign tumor tissue. Nevertheless, protein expression of Nrf2 in malignancy was associated with obesity. Interestingly, the redox profile of malignant tumor-associated adipose tissue was clearly BMI-related. Significantly higher protein expression of AD enzymes was found in normal-weight women, where activation of the Nrf2 pathway seems to play a role in establishing such “activated” phenotype in cancer-associated adipose tissue. The results suggest a specific redox-sensitive relationship between neoplastic transformation, mammary adipose tissue, and obesity in premenopausal women.
Redox profile represents a blueprint of every cancer, reflecting the stage of progression [31,32], metabolic demands [33,34], or selective pressures imposed by the tumor microenvironment [8,35,36]. Accordingly, data obtained for different types of tumors and at different stages of tumor progression in vitro and in vivo are inconsistent. Increased or decreased levels of ROS, oxidative stress biomarkers, and redox-related proteins were associated with the malignant phenotype [[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]]. Furthermore, a shift towards oxidative or peroxidative state, evident as the relative disproportion in the expression of O2•- and H2O2 eliminating enzymes, has been previously described [[51], [52], [53]]. We show higher CuZnSOD, MnSOD, CAT, and GSH-Px protein expression and CAT and GSH-Px activity in malignant tumor tissue than in benign tumor tissue, irrespective of BMI. This is in agreement with the higher redox homeostasis threshold hypothesis [54,55] and indicates a well-balanced capacity of malignant tumor tissue to metabolize ROS [44,52]. In an indirect assessment of ROS levels and lipid peroxidation rate, stronger immunopositivity for 4-HNE modified proteins was found in normal-weight women than in overweight/obese. In contrast, protein expression of transcription factor Nrf2 was higher in overweight/obese women with malignant tumors. Consistent with overall metabolic changes caused by systemic effects of obesity, cancer tissue metabolism has been previously shown to differ between normal-weight and overweight/obese women [15,56]. Differential protein expression of Nrf2 and level of 4-HNE modified proteins could reflect such obesity-related intricate metabolic differences, especially in the light of new evidence for the pleiotropic role of Nrf2 in metabolic reprogramming [[57], [58], [59], [60]] and a complex signaling role of 4-HNE in cancer [[61], [62], [63], [64]]. Moreover, there is evidence to support a worse prognosis, shorter disease-free interval, and higher mortality in breast cancer patients with high Nrf2 expression [59,65].
Local interaction between adipose tissue and cancer tissue has been recently shown to play an important role in cancer development and progression [20,22,66]. Cancer cells have been shown to communicate with adipocytes and “activate” their phenotype towards dedifferentiation, deregulated secretory activity, increased lipolysis, and β-oxidation [67]. In turn, adipocytes secrete free fatty acids, adipokines, proinflammatory cytokines, proteases, and components of the extracellular matrix to promote cancer invasion. It has been proposed that obesity could enhance two-way communication between these tissues [21,[68], [69], [70], [71]]. However, this has mostly been addressed in cell culture and co-culture studies, not fully considering the morpho-functional diversity of adipose tissue depots. Here, our data on paired human biopsies indicate that described cross-talk also affects redox-sensitive pathways in vivo. Initial assessment of adipose tissue from women with benign breast changes showed no significant obesity-related differences in Nrf2 and AD enzyme expression. However, cross-examination of malignant tumor-associated adipose tissue revealed a clear association between the redox profile and BMI. Higher protein expression of AD enzymes (CuZnSOD, MnSOD, and CAT) was found in normal-weight women, compared to overweight/obese. This could be related to the increased metabolic demands of cancer tissue, which favor oxidative metabolism [22,72]. Indeed, AD enzyme levels in adipose tissue were parallel to those observed in malignant tumor tissue, suggesting a coordinated redox response of breast tissues in a normal-weight state. A similar mirror image was previously shown for cancer-associated fibroblasts. Activated fibroblasts exhibit a slight increase in antioxidant defense, following phenotypic change that promotes cancer aggressiveness [33,35,73,74]. Such “redox coupling” could serve to sustain metabolic cooperation between cancer and its associated stromal tissues [36,74]. There is evidence for metabolic cooperation between adipocytes and cancer cells, but this was not further addressed so far in the redox-dependent context. To the best of our knowledge, we show the redox profile of cancer-associated adipose tissue for the first time and propose that the Nrf2 signaling pathway plays a role in establishing such “activated” phenotype.
Compelling differences in redox profile between normal-weight and overweight/obese women suggest that malignancy-related redox response of adipose tissue differs in obesity. If the increase in AD enzyme expression, found in breast adipose tissue of normal-weight women, is due to pressure imposed by cancer cells, the question of what happens in overweight/obese women remains. Are adipocytes in obesity immune to this induced prooxidant state, or is their ability to respond to it impaired? Malignant tumor-associated adipose tissue in overweight/obese women showed significantly reduced antioxidant capacity and stronger immunopositivity for 4-HNE modified proteins compared to normal-weight women. An increase in 4-HNE was reported as a biomarker of localized prooxidant state [17,[75], [76], [77]] and an inter- and intracellular redox signaling mediator of metabolic reprogramming in adipose tissue of obese individuals [78,79]. Recently, increased 4-HNE release from obese adipose tissue and Nrf2-dependent proliferation of breast cancer cells upon 4-HNE treatment was shown in two independent studies [80,81]. Here, in paired biopsies from obese women, we observed high Nrf2 expression in malignant tumor tissue, concomitantly with high immunopositivity for 4-HNE modified proteins in malignant tumor-associated adipose tissue.
This study's cross-sectional nature does not permit us to discuss temporal relations between obesity and breast cancer or whether described redox differences in tumor and adipose tissue are related to the risk of cancer development or severity of disease progression. Multiple studies showed that obesity reduces breast cancer risk in premenopausal women, contrary to the increased risk in postmenopausal women [[1], [2], [3], [4], [5]]. Still, there is substantial evidence that obesity leads to higher mortality, shorter disease-free intervals, and increased chemotherapy resistance in pre- and postmenopausal women [6,82]. Our future efforts will be directed towards studying redox-related protein expression and localization in the context of breast cancer tissue architecture and cancer cell and adipocyte ultrastructure. Studies regarding metabolic reprograming of breast tumor tissue and its associated adipose tissue, especially in the context of metabolic cooperation between cancer cells and adipocytes, are needed to expand our current understanding of the redox-driven relationship between mammary adipose tissue, neoplastic transformation, and obesity in premenopausal women.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia [grant numbers 451-03-9/2021-14/200007 and 451-03-9/2021-14/200178 and the Science Fund of the Republic of Serbia, PROMIS [grant number 6066747].
Abbreviations
- Antioxidant defense
AD
- Copper, zinc superoxide dismutase
CuZnSOD
- Manganese superoxide dismutase
MnSOD
- Catalase
CAT
- Glutathione peroxidase
GSH-Px
- Reactive oxygen species
ROS
- White adipose tissue
WAT
- Body mass index
BMI
- Superoxide anion
O2•
- Hydrogen peroxide
H2O2, Nuclear factor erythroid 2-related factor 2 (Nrf2), 4-hydroxy-2-nonenal (4-HNE)
References
- 1.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 2.Cheraghi Z., Poorolajal J., Hashem T., Esmailnasab N., Doosti Irani A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PloS One. 2012;7:1–9. doi: 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White K.K., Park S.Y., Kolonel L.N., Henderson B.E., Wilkens L.R. Body size and breast cancer risk: the Multiethnic Cohort. Int. J. Canc. 2012;131:1–12. doi: 10.1002/ijc.27373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia X., Chen W., Li J., Chen X., Rui R., Liu C., Sun Y., Liu L., Gong J., Yuan P. Body mass index and risk of breast cancer: a nonlinear dose-response meta-analysis of prospective studies. Sci. Rep. 2014;4:1–5. doi: 10.1038/srep07480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose D.P., Vona-Davis L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas. 2010;66:33–38. doi: 10.1016/j.maturitas.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Lee J.D., Cai Q., Shu X.O., Nechuta S.J. The role of biomarkers of oxidative stress in breast cancer risk and prognosis: a systematic review of the epidemiologic literature. J. Wom. Health. 2017;26:467–482. doi: 10.1089/jwh.2016.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colditz G.A., Lindsay L. Obesity and cancer: evidence, impact, and future directions. Clin. Chem. 2018;64:154–162. doi: 10.1373/clinchem.2017.277376. [DOI] [PubMed] [Google Scholar]
- 8.Panieri E., Santoro M.M. Ros homeostasis and metabolism: a dangerous liaison in cancer cells. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.105. e2253-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Díaz B., Courtneidge S.A. Redox signaling at invasive microdomains in cancer cells. Free Radic. Biol. Med. 2012;52:247–256. doi: 10.1016/j.freeradbiomed.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korac B., Kalezic A., Pekovic-Vaughan V., Korac A., Jankovic A. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol. 2021 doi: 10.1016/j.redox.2021.101887. 101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nourazarian A.R., Kangari P., Salmaninejad A. Roles of oxidative stress in the development and progression of breast cancer. Asian Pac. J. Cancer Prev. APJCP. 2014;15:4745–4751. doi: 10.7314/APJCP.2014.15.12.4745. [DOI] [PubMed] [Google Scholar]
- 12.Hornsveld M., Dansen T.B. The hallmarks of cancer from a redox perspective. Antioxidants Redox Signal. 2016;25:300–325. doi: 10.1089/ars.2015.6580. [DOI] [PubMed] [Google Scholar]
- 13.Incio J., Ligibel J.A., McManus D.T., Suboj P., Jung K., Kawaguchi K., Pinter M., Babykutty S., Chin S.M., Vardam T.D., Huang Y., Rahbari N.N., Roberge S., Wang D., Gomes-Santos I.L., Puchner S.B., Schlett C.L., Hoffmman U., Ancukiewicz M. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aag0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyengar N.M., Gucalp A., Dannenberg A.J., Hudis C.A. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J. Clin. Oncol. 2016;34:4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalezic A., Udicki M., Galic B.S., Aleksic M., Korac A., Jankovic A., Korac B. Lactate metabolism in breast cancer microenvironment: contribution focused on associated adipose tissue and obesity. Int. J. Mol. Sci. 2020;21:1–13. doi: 10.3390/ijms21249676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankovic A., Korac A., Srdic-Galic B., Buzadzic B., Otasevic V., Stancic A., Vucetic M., Markelic M., Velickovic K., Golic I., Korac B. Differences in the redox status of human visceral and subcutaneous adipose tissues - relationships to obesity and metabolic risk. Metabolism. 2014;63:661–671. doi: 10.1016/j.metabol.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Jankovic A., Korac A., Buzadzic B., Otasevic V., Stancic A., Daiber A., Korac B. Redox implications in adipose tissue (dys)function-A new look at old acquaintances. Redox Biol. 2015;6:19–32. doi: 10.1016/j.redox.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dirat B., Bochet L., Dabek M., Daviaud D., Dauvillier S., Majed B., Wang Y.Y., Meulle A., Salles B., Le Gonidec S., Garrido I., Escourrou G., Valet P., Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Canc. Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 20.Duong M.N., Geneste A., Fallone F., Li X., Dumontet C., Muller C. The fat and the bad: mature adipocytes, key actors in tumor progression and resistance. Oncotarget. 2017;8:57622–57641. doi: 10.18632/oncotarget.18038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rio M.C., Dali-Youcef N., Tomasetto C. Local adipocyte cancer cell paracrine loop: can "sick fat" be more detrimental? Horm. Mol. Biol. Clin. Invest. 2015;21:43–56. doi: 10.1515/hmbci-2014-0044. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y.Y., Attané C., Milhas D., Dirat B., Dauvillier S., Guerard A., Gilhodes J., Lazar I., Alet N., Laurent V., Le Gonidec S., Biard D., Hervé C., Bost F., Ren G.S., Bono F., Escourrou G., Prentki M., Nieto L. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2 doi: 10.1172/jci.insight.87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchkonia T., Thomou T., Zhu Y., Karagiannides I., Pothoulakis C., Jensen M.D., Kirkland J.L. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metabol. 2013;17:644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giordano A., Smorlesi A., Frontini A., Barbatelli G., Cint S. White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur. J. Endocrinol. 2014;170 doi: 10.1530/EJE-13-0945. [DOI] [PubMed] [Google Scholar]
- 25.Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. World Heal Organ - Tech Rep Ser; 2000. p. 894. [PubMed] [Google Scholar]
- 26.Petrović V., Korać A., Buzadžić B., Korać B. The effects of L-arginine and L-NAME supplementation on redox-regulation and thermogenesis in interscapular brown adipose tissue. J. Exp. Biol. 2005;208:4263–4271. doi: 10.1242/jeb.01895. [DOI] [PubMed] [Google Scholar]
- 27.Jankovic A., Golic I., Markelic M., Stancic A., Otasevic V., Buzadzic B., Korac A., Korac B. Two key temporally distinguishable molecular and cellular components of white adipose tissue browning during cold acclimation. J. Physiol. 2015;593:3267–3280. doi: 10.1113/JP270805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. doi: 10.1016/s0021-9258(19)45228-9. [DOI] [PubMed] [Google Scholar]
- 29.Ateş N.A., Yildirim Ö., Tamer L., Ünlü A., Ercan B., Muşlu N., Kanik A., Hatungil R., Atik U. Plasma catalase activity and malondialdehyde level in patients with cataract. Eye. 2004;18:785–788. doi: 10.1038/sj.eye.6700718. [DOI] [PubMed] [Google Scholar]
- 30.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. doi: 10.5555/uri:pii:0022214367900765. [DOI] [PubMed] [Google Scholar]
- 31.Tsanou E., Ioachim E., Briasoulis E., Damala K., Charchanti A., Karavasilis V., Pavlidis N., Agnantis N.J. Immunohistochemical expression of superoxide dismutase (MnSOD) anti-oxidant enzyme in invasive breast carcinoma. Histol. Histopathol. 2004;19:807–813. doi: 10.14670/HH-19.807. [DOI] [PubMed] [Google Scholar]
- 32.Loo S.Y., Hirpara J.L., Pandey V., Tan T.Z., Yap C.T., Lobie P.E., Thiery J.P., Goh B.C., Pervaiz S., Clément M.V., Kumar A.P. Manganese superoxide dismutase expression regulates the switch between an epithelial and a mesenchymal-like phenotype in breast carcinoma. Antioxidants Redox Signal. 2016;25:283–299. doi: 10.1089/ars.2015.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart P.C., Mao M., De Abreu A.L.P., Ansenberger-Fricano K., Ekoue D.N., Ganini D., Kajdacsy-Balla A., Diamond A.M., Minshall R.D., Consolaro M.E.L., Santos J.H., Bonini M.G. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat. Commun. 2015;6:1–14. doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li P., Zhang D., Shen L., Dong K., Wu M., Ou Z., Shi D. Redox homeostasis protects mitochondria through accelerating ROS conversion to enhance hypoxia resistance in cancer cells. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Outschoorn U.E., Balliet R.M., Rivadeneira D.B., Chiavarina B., Pavlides S., Wang C., Whitaker-Menezes D., Daumer K.M., Lin Z., Witkiewicz A.K., Flomenberg N., Howell A., Pestell R.G., Knudsen E.S., Sotgia F., Lisanti M.P. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–3276. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balliet R.M., Capparelli C., Guido C., Pestell T.G., Martinez-Outschoorn U.E., Lin Z., Whitaker-Menezes D., Chiavarina B., Pestell R.G., Howell A., Sotgia F., Lisanti M.P. Mitochondrial oxidative stress in cancer-associated fibroblasts drives lactate production, promoting breast cancer tumor growth: understanding the aging and cancer connection. Cell Cycle. 2011;10:4065–4073. doi: 10.4161/cc.10.23.18254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambrosone C.B., Ahn J., Singh K.K., Rezaishiraz H., Furberg H., Sweeney C., Coles B., Trovato A. Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Canc. Res. 2005;65:1105–1111. [PubMed] [Google Scholar]
- 38.Er T.K., Hou M.F., Tsa E.M., Lee J.N., Tsai L.Y. Differential expression of manganese containing superoxide dismutase in patients with breast cancer in Taiwan. Ann. Clin. Lab. Sci. 2004;34:159–164. [PubMed] [Google Scholar]
- 39.Ray G., Batra S., Shukla N.K., Deo S., Raina V., Ashok S., Husain S.A. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Canc. Res. Treat. 2000;59:163–170. doi: 10.1023/A:1006357330486. [DOI] [PubMed] [Google Scholar]
- 40.Sander C.S., Hamm F., Elsner P., Thiele J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003;148:913–922. doi: 10.1046/j.1365-2133.2003.05303.x. [DOI] [PubMed] [Google Scholar]
- 41.Tas F., Hansel H., Belce A., Ilvan S., Argon A., Camlica H., Topuz E. Oxidative stress in breast cancer. Med. Oncol. 2005;22:11–15. doi: 10.1385/mo:22:1:011. [DOI] [PubMed] [Google Scholar]
- 42.Kim A. Modulation of MnSOD in cancer: epidemiological and experimental evidences. Toxicol Res. 2013;26:83–93. doi: 10.5487/TR.2010.26.2.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gönenç A., Tokgöz D., Aslan S., Torun M. Oxidative stress in relation to lipid profiles in different stages of breast cancer. Indian J. Biochem. Biophys. 2005;42:190–194. [PubMed] [Google Scholar]
- 44.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem. J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 45.Hwang T.S., Choi H.K., Han H.S. Differential expression of manganese superoxide dismutase, copper/zinc superoxide dismutase, and catalase in gastric adenocarcinoma and normal gastric mucosa. Eur. J. Surg. Oncol. 2007;33:474–479. doi: 10.1016/j.ejso.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Kumaraguruparan R., Subapriya R., Kabalimoorthy J., Nagini S. Antioxidant profile in the circulation of patients with fibroadenoma and adenocarcinoma of the breast. Clin. Biochem. 2002;35:275–279. doi: 10.1016/S0009-9120(02)00310-7. [DOI] [PubMed] [Google Scholar]
- 47.Kumaraguruparan R., Subapriya R., Viswanathan P., Nagini S. Tissue lipid peroxidation and antioxidant status in patients with adenocarcinoma of the breast. Clin. Chim. Acta. 2002;325:165–170. doi: 10.1016/S0009-8981(02)00292-9. [DOI] [PubMed] [Google Scholar]
- 48.Marklund S.L., Westman N.G., Lundgren E., Roos G. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Canc. Res. 1982;42:1955–1961. [PubMed] [Google Scholar]
- 49.Oberley T.D. Antioxidant enzyme levels in cancer. Histol. Histopathol. 1997;12:525–535. [PubMed] [Google Scholar]
- 50.Punnonen K., Ahotupa M., Asaishi K., Hyöty M., Kudo R., Punnonen R. Antioxidant enzyme activities and oxidative stress in human breast cancer. J. Canc. Res. Clin. Oncol. 1994;120:374–377. doi: 10.1007/BF01247464. [DOI] [PubMed] [Google Scholar]
- 51.Doskey C.M., Buranasudja V., Wagner B.A., Wilkes J.G., Du J., Cullen J.J., Buettner G.R. Tumor cells have decreased ability to metabolize H2O2: implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016;10:274–284. doi: 10.1016/j.redox.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miar A., Hevia D., Muñoz-Cimadevilla H., Astudillo A., Velasco J., Sainz R.M., Mayo J.C. Manganese superoxide dismutase (SOD2/MnSOD)/catalase and SOD2/GPx 1 ratios as biomarkers for tumor progression and metastasis in prostate, colon, and lung cancer. Free Radic. Biol. Med. 2015;85:45–55. doi: 10.1016/j.freeradbiomed.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Kim D., Koo J.S., Lee S. Overexpression of reactive oxygen species scavenger enzymes is associated with a good prognosis in triple-negative breast cancer. Oncol. 2015;88:9–17. doi: 10.1159/000358365. [DOI] [PubMed] [Google Scholar]
- 54.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 55.Nogueira V., Hay N. Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Canc. Res. 2013;19:4309–4314. doi: 10.1158/1078-0432.CCR-12-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao H., Wang J., Fang D., Lee O., Chatterton R.T., Stearns V., Khan S.A., Bulun S.E. Adiposity results in metabolic and inflammation differences in premenopausal and postmenopausal women consistent with the difference in breast cancer risk. Horm Cancer. 2018;9:229–239. doi: 10.1007/s12672-018-0329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y.Y., Chen J., Liu X.M., Zhao R., Zhe H. Nrf2-mediated metabolic reprogramming in cancer. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/9304091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu S., Lu H., Bai Y. Nrf2 in cancers: a double-edged sword. Cancer Med. 2019;8:2252–2267. doi: 10.1002/cam4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Onodera Y., Motohashi H., Takagi K., Miki Y., Shibahara Y., Watanabe M., Ishida T., Hirakawa H., Sasano H., Yamamoto M., Suzuki T. NRF2 immunolocalization in human breast cancer patients as a prognostic factor. Endocr. Relat. Canc. 2014;21:241–252. doi: 10.1530/ERC-13-0234. [DOI] [PubMed] [Google Scholar]
- 60.Mitsuishi Y., Taguchi K., Kawatani Y., Shibata T., Nukiwa T., Aburatani H., Yamamoto M., Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Canc. Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Zhong H., Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 2015;4:193–199. doi: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao M., Zhong H., Xia L., Tao Y., Yin H. Pathophysiology of mitochondrial lipid oxidation: role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Radic. Biol. Med. 2017;111:316–327. doi: 10.1016/j.freeradbiomed.2017.04.363. [DOI] [PubMed] [Google Scholar]
- 63.Siow R.C.M., Ishii T., Mann G.E. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–15. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- 64.Weisz J., Shearer D.A., Murata E., Patrick S.D., Han B., Berg A., Clawson G.A. Identification of mammary epithelial cells subject to chronic oxidative stress in mammary epithelium of young women and teenagers living in USA: implication for breast carcinogenesis. Canc. Biol. Ther. 2012;13:101–113. doi: 10.4161/cbt.13.2.18873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Urpilainen E., Kangaskokko J., Puistola U., Karihtala P. Metformin diminishes the unfavourable impact of Nrf2 in breast cancer patients with type 2 diabetes. Tumor Biol. 2019;41:1–10. doi: 10.1177/1010428318815413. [DOI] [PubMed] [Google Scholar]
- 66.Lazar I., Clement E., Dauvillier S., Milhas D., Ducoux-Petit M., LeGonidec S., Moro C., Soldan V., Dalle S., Balor S., Golzio M., Burlet-Schiltz O., Valet P., Muller C., Nieto L. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Canc. Res. 2016;76:4051–4057. doi: 10.1158/0008-5472.CAN-16-0651. [DOI] [PubMed] [Google Scholar]
- 67.Balaban S., Shearer R.F., Lee L.S., van Geldermalsen M., Schreuder M., Shtein H.C., Cairns R., Thomas K.C., Fazakerley D.J., Grewal T., Holst J., Saunders D.N., Hoy A.J. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Canc. Metabol. 2017;5:1–14. doi: 10.1186/s40170-016-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang F., Gao S., Chen F., Fu Z., Yin H., Lu X., Yu J., Lu C. Mammary fat of breast cancer: gene expression profiling and functional characterization. PloS One. 2014;9 doi: 10.1371/journal.pone.0109742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Picon-Ruiz M., Pan C., Drews-Elger K., Jang K., Besser A.H., Zhao D., Morata-Tarifa C., Kim M., Ince T.A., Azzam D.J., Wander S.A., Wang B., Ergonul B., Datar R.H., Cote R.J., Howard G.A., El-Ashry D., Torné-Poyatos P., Marchal J.A. Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox 2/miR-302b-mediated malignant progression. Canc. Res. 2016;76:491–504. doi: 10.1158/0008-5472.CAN-15-0927. [DOI] [PubMed] [Google Scholar]
- 70.Cai Q., Lin T., Kamarajugadda S., Lu J. Regulation of glycolysis and the Warburg effect by estrogen-related receptors. Oncogene. 2013;32:2079–2086. doi: 10.1038/onc.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prieto-Hontoria P.L., Pérez-Matute P., Fernández-Galilea M., Bustos M., Martínez J.A., Moreno-Aliaga M.J. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim. Biophys. Acta Bioenerg. 2011;1807:664–678. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Sotgia F., Whitaker-Menezes D., Martinez-Outschoorn U.E., Flomenberg N., Birbe R.C., Witkiewicz A.K., Howell A., Philp N.J., Pestell R.G., Lisanti M.P. Mitochondrial metabolism in cancer metastasis: visualizing tumor cell mitochondria and the "reverse Warburg effect" in positive lymph node tissue. Cell Cycle. 2012;11:1445–1454. doi: 10.4161/cc.19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ludtmann M.H.R., Angelova P.R., Zhang Y., Abramov A.Y., Dinkova-Kostova A.T. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem. J. 2014;457:415–424. doi: 10.1042/BJ20130863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinez-Outschoorn U., Sotgia F., Lisanti M.P. Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function. Semin. Oncol. 2014;41:195–216. doi: 10.1053/j.seminoncol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Cohen G., Riahi Y., Sasson S. Lipid peroxidation of poly-unsaturated fatty acids in normal and obese adipose tissues. Arch. Physiol. Biochem. 2011;117:131–139. doi: 10.3109/13813455.2011.557387. [DOI] [PubMed] [Google Scholar]
- 76.Long E.K., Olson D.M., Bernlohr D.A. High-fat diet induces changes in adipose tissue trans-4-oxo-2-nonenal and trans-4-hydroxy-2-nonenal levels in a depot-specific manner. Free Radic. Biol. Med. 2013;63:390–398. doi: 10.1016/j.freeradbiomed.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castro J.P., Grune T., Speckmann B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 2016;397:709–724. doi: 10.1515/hsz-2015-0305. [DOI] [PubMed] [Google Scholar]
- 78.Cai J., Pires K.M., Ferhat M., Chaurasia B., Buffolo M.A., Smalling R., Sargsyan A., Atkinson D.L., Summers S.A., Graham T.E., Boudina S. Autophagy ablation in adipocytes induces insulin resistance and reveals roles for lipid peroxide and Nrf2 signaling in adipose-liver crosstalk. Cell Rep. 2018;25:1708–1717. doi: 10.1016/j.celrep.2018.10.040. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X., Wang Z., Li J., Gu D., Li S., Shen C., Song Z. Increased 4-hydroxynonenal formation contributes to obesity-related lipolytic activation in adipocytes. PloS One. 2013;8 doi: 10.1371/journal.pone.0070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crujeiras A.B., Cabia B., Carreira M.C., Amil M., Cueva J., Andrade S., Seoane L.M., Pardo M., Sueiro A., Baltar J., Morais T., Monteiro M.P., Lopez-Lopez R., Casanueva F.F. Secreted factors derived from obese visceral adipose tissue regulate the expression of breast malignant transformation genes. Int. J. Obes. 2016;40:514–523. doi: 10.1038/ijo.2015.208. [DOI] [PubMed] [Google Scholar]
- 81.Li Y.P., Tian F.G., Shi P.C., Guo L.Y., Wu H.M., Chen R.Q., Xue J.M. 4-Hydroxynonenal promotes growth and angiogenesis of breast cancer cells through HIF-1α stabilization. Asian Pac. J. Cancer Prev. APJCP. 2014;15:10151–10156. doi: 10.7314/APJCP.2014.15.23.10151. [DOI] [PubMed] [Google Scholar]
- 82.Kroenke C.H., Chen W.Y., Rosner B., Holmes M.D. Weight, weight gain, and survival after breast cancer diagnosis. J. Clin. Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]