Abstract
Background
Cumulative evidence suggests that neuronal death including autophagy, apoptosis, and necrosis is closely related to the occurrence and development of cerebral ischemia–reperfusion (I/R) injury. Moreover, vagal nerve stimulation (VNS) is involved in many different neuroprotective and neuroplasticity pathways. Thus, VNS may be a novel approach for treating various neurodegenerative diseases. The present study aims to determine whether VNS protects against cerebral I/R injury in rats by inhibiting autophagy and apoptosis.
Methods
Cerebral I/R injury is induced by middle cerebral artery occlusion (MCAO) and VNS is carried out. Infarct volume, neurological deficit, autophagy, and apoptosis are examined 24 h after reperfusion.
Results
Vagal nerve stimulation decreases infarct volume and suppresses neurological deficit. Moreover, obvious autophagy and apoptosis are detected in rats that have undergone I/R, and VNS inhibits autophagy and apoptosis.
Conclusion
Vagal nerve stimulation exerts neuroprotective effects following I/R injury by inhibiting autophagy and apoptosis.
Keywords: vagal nerve stimulation, cerebral ischemia–reperfusion injury, autophagy, apoptosis
Introduction
Ischemic stroke is a highly disabling and fatal disease caused by interrupted cerebral blood flow.1 Presently, the only way to treat ischemic stroke is to rapidly restore blood flow. Tissue plasminogen activator is recognized as an effective method for the treatment of acute ischemic stroke but its wide application in clinical practice is limited due to its inadequate therapeutic time window. This approach can also initiate various pathological processes including oxidative stress, apoptosis, inflammation, and autophagy, which includes cerebral ischemia–reperfusion (I/R) injury.2–4
There is increasing evidence that cerebral I/R injury causes autophagy and apoptosis.5,6 Autophagy is a catabolic cellular process that subjects macromolecules and organelles to lysosomal degradation. Autophagy is considered a process with both positive and negative qualities, as it can potentially promote both survival and death in cases of cerebral I/R.7 Selected reports have shown that autophagy promotes ischemic neuronal death.8,9 However, others indicate that autophagy has a neuroprotective effect in cerebral I/R injury.10
The primary form of cell death in the ischemic penumbra is apoptosis, which occurs 0.5–4 h after focal cerebral ischemia, and reaches its maximum value at 24–48 h.11 Therefore, regulating autophagy and apoptosis may represent a promising treatment strategy for cerebral I/R injury.
Vagal nerve stimulation (VNS) is considered a safe and effective adjunctive treatment for intractable epilepsy12 and treatment-resistant depression.13 Recent studies have shown that VNS is involved in neuroprotection after brain I/R injury.14,15 Our previous studies indicate that VNS prevents neuronal apoptosis in rats following cerebral I/R.16,17 In many cases, autophagy and apoptosis can occur either simultaneously or sequentially.18 Nevertheless, whether VNS regulates autophagy and apoptosis in cerebral I/R injury is unclear. Thus, the present study aims to evaluate the effects of VNS on autophagy and apoptosis after cerebral I/R injury in rats.
Materials and Methods
Animals
Male Sprague–Dawley rats weighing 230–280 g and aged 7–8 weeks were sourced from the Experimental Animal Center at Chongqing Medical University, China. Rats were fed in a 12-h light/dark cycle at a temperature ranging from 21°C–22°C with 60% humidity and were allowed ad libitum access to food and water. All experiments were carried out in strict accordance with protocols approved by the Institutional Animal Care and Use Committee and the Institutional Ethics Committee at Chongqing Medical University (Permit No. SCXK (Chongqing) 2007–0001) and the State Science and Technology Commission of China. All animals were treated in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (1996). Only 92 rats met the standards for the experiment and were randomly divided into four groups: the sham I/R group (n = 16), sham I/R + VNS group (n = 16), I/R group (n = 30), and the I/R + VNS group (n = 30).
Focal Cerebral Ischemia–Reperfusion Model in Rats
According to a previous description,18 I/R injury can be induced by middle cerebral artery occlusion (MCAO). Briefly, rats were deeply anesthetized using chloral hydrate (i.p., 350 mg/kg body weight) and body temperature was maintained at approximately 37°C using a heating lamp. The right common carotid arteries (CCA), the external carotid artery (ECA), and the internal carotid artery (ICA) were sequentially exposed via a midline neck incision. The right CCA was distally ligated and the ECA was proximally ligated to the bifurcation of the ICA and ECA. A silicone-coated nylon filament (diameter, 0.34 ± 0.02 mm) was gently inserted from the ECA into the lumen of the ICA to obstruct the middle cerebral artery for 2 h. Following on, rats were reperfused by the withdrawal of the nylon filament. Rats in the sham I/R group underwent surgical exposure of the CCA and the ECA but the nylon filament was not inserted. During the surgical procedure, heart rate (HR), tail arterial pressure, and blood gas concentrations were monitored as described in.17
Electrical Stimulation of the Right Vagus Nerve
After 30 min of MCAO, rats underwent right cervical VNS delivered by a Grass Stimulator S48 as described in.19 The stimulating electrodes were self-constructed according to a design by Smith20 and comprised two polyethylene-coated curved silver wires that were fixed 1.5 mm apart by a solid bar. Under a microscope, the electrodes were twined around the right cervical vagus nerve and sutured to the sternocleidomastoid muscle.16,17 Vagal nerve stimulation comprised 0.5 ms of 30-s square pulses (0.5 mA, 20 Hz) every 5 min for 1 h.19 Rats in the sham I/R + VNS group received stimulation without MCAO.
Neurological Scoring
Rats subjected to MCAO underwent neurological functioning evaluation 24 h after reperfusion. A neurological evaluation was performed using a modified five-point scoring system.21
Measurement of Infarct Volume
Rats were deeply anesthetized and decapitated 24 h after reperfusion. Brains were rapidly removed and sectioned into 2-mm-thick sections using a blade. The sections were immersed in 2% 2,3,5-triphenyltetrazolium chloride at 37°C for 30 min in the dark and fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS). The stained slices were imaged and analyzed using Image J (v.*) software (National Institute of Health). Infarction volume was calculated as described in.22
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Assay
Twenty-four hours after cerebral I/R injury, cell apoptosis was detected in 10-μm frozen coronal sections using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using the In Situ Cell Death Detection Kit, POD (Roche) according to the manufacturer’s instructions as described in.22 Five randomly selected high-power fields (×400) in the ischemic penumbra region were imaged using a laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany). The TUNEL-positive cell numbers were totaled and averaged by an investigator who was blinded to the experimental conditions.
Immunohistochemistry
Rats were anesthetized using chloral hydrate 24 h after MCAO. Paraffinized sections were prepared as described in.23 After deparaffinization with xylene, sections were dehydrated by a graded series of ethanol and boiled in a 0.01 M citrate buffer for antigen retrieval. After blocking with hydrogen peroxide (3%, 15 min) and normal goat serum (10%, 20 min), sections were incubated with a rabbit polyclonal anti-cleaved caspase-3 antibody (1:500, Cell Signaling Technology) overnight at 4°C. Then, the samples were incubated with goat anti-rabbit immunoglobulin G (IgG) antibody for 30 min. Specific staining was revealed using diaminobenzidine. The number of positive cells in five visual fields within the ischemic penumbra was counted using a light microscope under high-power magnification (×400). Cell counting was performed by an investigator who was blinded to the study.
Double Immunofluorescence for the LC3B Gene and NeuN Protein
Twenty-four hours after reperfusion, rats were deeply anesthetized and perfused with 0.9% normal saline and 4% PFA. Frozen brains were cut into 10-µm-thick coronal sections. The sections were permeabilized with Triton X-100 (0.4%, 20 min), blocked in normal donkey serum (0.4%, 1.5 h), and incubated with primary antibodies (rabbit anti-LC3B antibody [1:50, Merck Millipore] and mouse anti-NeuN antibody [1:50, Merck Millipore]) at 4°C overnight. The following day, sections were treated with Alexa Fluor-555 donkey anti-rabbit IgG antibody (H + L, 1:100; Beyotime Institute of Biotechnology) and fluorescein isothiocyanate-conjugated donkey anti-mouse IgG antibody (H + L, 1:100; Proteintech) for 1.5 h at 37°C, washed with PBS, and incubated with a 4,6-diamidino-2-phenylindole nuclear stain. Three areas in the peri-infarct cortex were imaged using a laser scanning confocal microscope (Nikon). Digital images were analyzed using Image-Pro Plus (v.*) software.
Western Blot Analysis
The ischemic penumbra cortex was dissected 24 h after reperfusion. Western blot analysis was carried out as described in.16 Briefly, protein was extracted, and its concentration was measured according to the kit instructions. Protein samples were placed in the polyacrylamide gel electrophoresis system. A Bio-Rad instrument was used for electrophoresis and electrotransfer, and the protein was successfully transferred to the polyvinylidene fluoride membrane. The membranes were blocked with 5% non-fat dried milk at room temperature for 2 h and incubated with rabbit anti-LC3B (1:1000; Merck Millipore), rabbit anti-Beclin-1 (1:1000; Proteintech), rabbit anti-Bcl-2 (1:500; Proteintech), rabbit anti-Bax (1:600; Proteintech), and rabbit anti-GAPDH (1:4000; Proteintech) antibodies at 4°C overnight. After washing, membranes were incubated with anti-rabbit secondary antibodies. Blots were examined using the Bio Image Analysis System and analyzed using Image J software.
Statistical Analysis
Experimental data are expressed in this paper as mean ± standard deviation. All data were evaluated for normal distribution prior to analysis. Data were analyzed using a one-way analysis of variance followed by Tukey’s multiple comparison test. A p-value of <0.05 was considered statistically significant.
Results
Physiological Parameters
Table 1 shows that the blood pressure (BP) and HR in the sham I/R and I/R groups were maintained within the normal range during MCAO surgery. In the sham I/R + VNS and I/R + VNS groups, HR and BP declined over time for the 30-s stimulation period but rapidly recovered near the baseline levels as soon as stimulation was terminated. During the experimental period, pH and blood gas concentrations (pO2, and pCO2) in all groups remained within the normal range. Mean BP, HR, and blood gas concentrations were within the normal ranges and were not significantly different between groups, which is consistent with the results presented in.17
Table 1.
The Physiological Parameters During the Experiment
| Group | Mean Blood Pressure (mmHg) | Heart Rate (bp/min) | PH | PCO 2 (mmHg) | PO 2 (mmHg) |
|---|---|---|---|---|---|
| sham I/R | 90±4.1 | 376±9 | 7.39±0.02 | 46.9±1.2 | 112.7±8.9 |
| sham I/R+VNS | 87±7.2 | 368±11 | 7.39±0.01 | 46.4±1.0 | 114.8±10.7 |
| I/R | 89±4.3 | 372±10 | 7.38±0.02 | 46.6±0.8 | 109.9±9.6 |
| I/R+VNS | 85±5.5 | 365±12 | 7.38±0.02 | 46.0±0.9 | 111.7±11.8 |
Note: All data are shown as the mean±SD.
Vagal Nerve Stimulation Improved Neurological Scores and Decreased Infarct Volume After Ischemia–Reperfusion Injury
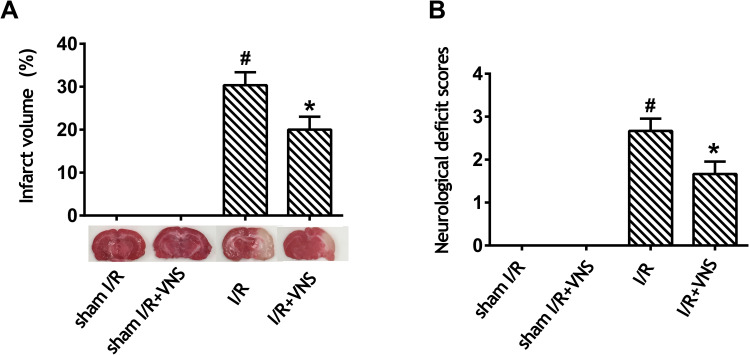
As shown in Figure 1, we evaluated cerebral infarct size and neurological deficit scores in rats 24 h after reperfusion. No infarction was observed in the sham I/R and sham I/R + VNS groups, whereas VNS significantly decreased infarct volume when compared with the I/R group (Figure 1A; P < 0.05). No neurological deficit was observed in either the sham I/R or the sham I/R + VNS groups. Furthermore, VNS caused a significant decrease in the neurological score when compared with the I/R group (Figure 1B; P < 0.05), indicating that VNS improved the neurological deficit. The data indicated that VNS reversed neurological deficit and reduced infarct volume compared with rats with ischemic stroke.
Figure 1.
Vagal nerve stimulation improves neurological scores and decreases infarct volume after ischemia–reperfusion injury. (A) Infarct volume was measured by 2,3,5-triphenyltetrazolium chloride staining. Obvious infarction was detected in rats who had undergone ischemia–reperfusion (I/R), and infarction was improved after vagal nerve stimulation. (B) A neurological evaluation was performed using a modified five-point scoring system. Obvious neurological deficit was detected in rats who had undergone I/R and neurological deficit improved after vagal nerve stimulation; #P < 0.05 vs sham I/R group, *P < 0.05 vs I/R group.
Vagal Nerve Stimulation Downregulated the Expression of Autophagy-Related Proteins After Ischemia–Reperfusion Injury
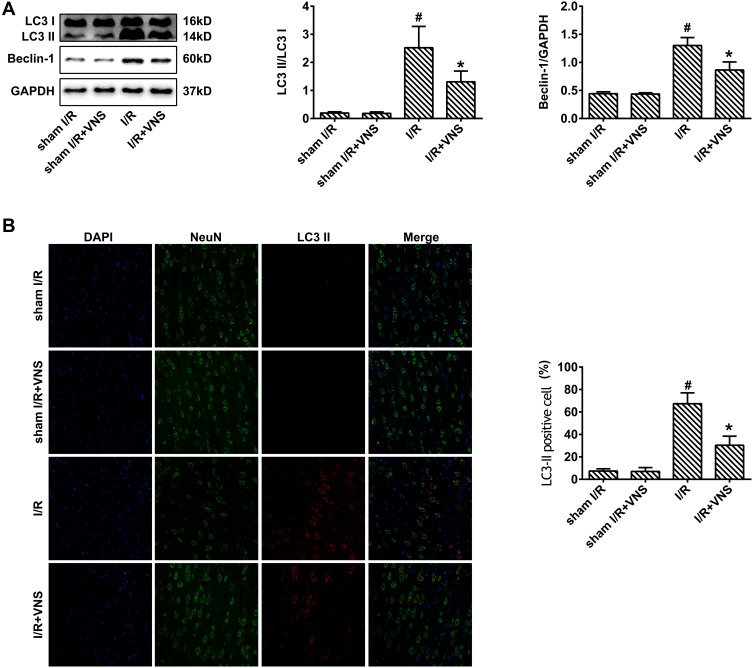
To confirm the influence of VNS on autophagy, we first observed the expression of autophagy-related proteins during I/R. Microtubule-associated protein 1 light chain 3 (LC3)-II was used to detect autophagic activity.23 Beclin-1 is also an important protein that regulates neuronal autophagy.23 The expression of LC3-II and Beclin-1 in the cortex after 24 h was measured by Western blot analysis (Figure 2A). Compared with the sham group, the LC3-II/LC3-I ratio exhibited obvious elevation in the I/R group (Figure 2A; P < 0.05) but VNS significantly decreased the LC3-II/LC3-I ratio (Figure 2A; P < 0.05). Beclin-1 expression showed significant elevation in rats that had undergone I/R (Figure 2A; P < 0.05). However, VNS significantly attenuated Beclin-1 expression compared with the I/R group (Figure 2A; P < 0.05). Double immunofluorescence with LC3-II and NeuN antibodies was performed to determine the effect of VNS on neuronal autophagic activities after I/R injury. As shown in Figure 2B, LC3-II-positive cells were rarely observed in the sham I/R group, while co-expression of LC3-II and NeuN was observed in the ischemic penumbra in the I/R group. Compared with the I/R group, the number of LC3-II-positive neurons was significantly decreased after VNS (Figure 2B; P < 0.05).
Figure 2.
Vagal nerve stimulation downregulates the expression of autophagy-related proteins after ischemia–reperfusion injury. (A) The expression of LC3-II and Beclin-1 was measured by Western blot analysis. The expression of LC3-II and Beclin-1 was increased in rats who had undergone ischemia–reperfusion (I/R) and decreased after vagal nerve stimulation. (B) Double immunofluorescence for LC3-II and NeuN indicated that the number of LC3-II-positive cells was increased in rats who had undergone I/R and decreased after vagal nerve stimulation; #P < 0.05 vs sham I/R group, *P < 0.05 vs I/R group.
Vagal Nerve Stimulation Attenuated Apoptosis Following Ischemia–Reperfusion Injury
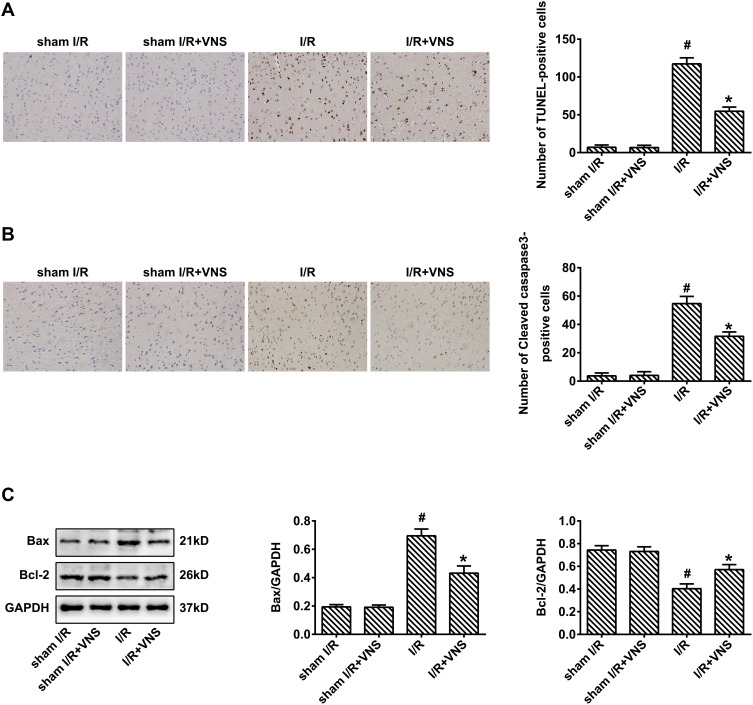
Few apoptotic cells were found in the cortex in the sham I/R group. In contrast, there was a large number of TUNEL-positive cells in the infarct area of the right cortex in the I/R group. Intervention with VNS reduced the number of TUNEL-positive cells compared with the I/R group (Figure 3A; P < 0.05), indicating that VNS attenuated cerebral I/R injury in the cortex. We found that the expression of cleaved caspase-3 was significantly decreased following VNS, 24 h after reperfusion (Figure 3B; P < 0.05). Moreover, treatment with VNS significantly downregulated the expression of the pro-apoptotic protein, Bax, but upregulated the expression of the anti-apoptotic protein, Bcl-2, 24 h after I/R injury (Figure 3C; P < 0.05). These results indicate that VNS suppressed the apoptotic response after I/R injury.
Figure 3.
Vagal nerve stimulation attenuates apoptosis following ischemia–reperfusion injury. (A) Staining using terminal deoxynucleotidyl transferase dUTP nick end labeling indicated that obvious apoptosis was detected in rats who had undergone ischemia–reperfusion (I/R), and apoptosis was decreased after vagal nerve stimulation (VNS). (B) Immunohistochemical staining indicated that the expression of cleaved caspase-3 was increased in rats who had undergone I/R and decreased after VNS. (C) Western blot analysis indicated that the expression of Bax was increased in rats who had undergone I/R and decreased after VNS. The expression of Bcl-2 was decreased in rats who had undergone I/R and increased after VNS; #P < 0.05 vs sham I/R group, *P < 0.05 vs I/R group.
Discussion
Cumulative evidence suggests that neuronal death, including autophagy, apoptosis, and necrosis, plays a pivotal role in the occurrence and development of I/R injury.18,24,25 Autophagy is a process that subjects cytoplasmic macromolecules and organelles in mammalian cells to lysosomal degradation. Furthermore, autophagy also plays an important role in maintaining internal environmental balance and survival.26,27 In the central nervous system, autophagy can trigger programmed neuronal death (type-II death), which is different from apoptosis and is involved in the progression of neurodegenerative diseases, such as epilepsy, stroke, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease.28–33 In I/R injury, it is not surprising that autophagy is activated to degrade toxic metabolites induced by ischemic preconditioning. However, autophagy, which is supposed to be suppressed, is also activated during reperfusion.34
In the present study, we found an increased expression of autophagy-related proteins LC3-II and Beclin-1, indicating that autophagy had been activated in I/R injury.
Apoptosis, another type of cell death, is a biomolecular process used by organisms to kill cells that endanger neighboring cells, and also kills cells that undergo genetic damage or endure other types of stress.25 Apoptosis is also involved in various neurodegenerative diseases, such as brain trauma, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease.35–38 In I/R injury, manipulation of various molecular reactions that participate in apoptotic pathways is activated, thereby inhibiting cell/organ function and survival.25
We found that the number of TUNEL-positive cells and the expression of cleaved caspase-3 and Bax were increased, and the expression of Bcl-2 was decreased in rats who had experienced I/R. This suggests that apoptosis is activated in I/R injury.
To summarize, the prevention or reduction of autophagy and apoptosis may be the first choice of treatment for I/R injury. For example, tetrahydroxystilbene glucoside may reduce the neurological score and cerebral infarct volume and improve neuronal damage in the ischemic cortex and hippocampus in mice that have undergone MCAO by suppressing apoptosis and autophagy.39 In addition, Li et al found that 002C-3 had significant protective effects against cerebral I/R injury by inhibiting autophagy and apoptosis.40 Therefore, finding a novel way to inhibit autophagy and apoptosis is a relatively effective strategy that can be used to suppress I/R damage.
The vagus nerve, the longest nerve in the human autonomous nervous system, originates in the medulla, in the brainstem. The fundamental function of the vagus nerve is to provide parasympathetic innervation to many organs, which in turn affects the function of the relevant nerve branches and target tissues/organs.41 In the central nervous system, VNS drives neural activity in the locus coeruleus, contributing to an increase in the concentration of norepinephrine in the hippocampus and cortex, and actives downstream proteins involved in many different neuroprotective and neuroplasticity pathways. Thus, VNS could be a potential adjuvant to behavioral therapy for neurodegenerative diseases.42–44 Recently, VNS was reported to reduce infarct size and neurological deficit and improve memory and cognition in experimental stroke models.45 However, the mechanism of the protective effect of VNS in the context of stroke remains unclear.
In the present study, we found that VNS improved the neurological score, decreased the infarct volume, and suppressed autophagy and apoptosis following I/R injury. These results suggest that VNS protects against I/R injury by minimizing neuronal death. An existing study indicated that recovery from ischemic stroke could be summarized by the following two mechanisms: 1) a reduction in neuronal death in the ischemic penumbra; 2) enhancement of neuroplasticity in a relatively late phase of stroke occurrence to improve memory, cognition, and limb function.45 Thus, we believe that VNS improves neurological scores and decreases infarct volume in rats with cerebral I/R by inhibiting neuronal death, including autophagy and apoptosis. However, whether VNS can enhance neuroplasticity in the case of I/R injury remains undetermined.
In conclusion, we investigated the effect of VNS in rats with cerebral I/R injury. We found that VNS decreased infarct volume and suppressed neurological deficit. Moreover, obvious autophagy and apoptosis were detected in rats with cerebral I/R injury. Additionally, VNS inhibited autophagy and apoptosis in our study. Thus, our results suggest that VNS exerts neuroprotective effects against I/R injury by inhibiting autophagy and apoptosis. However, the specific molecular mechanisms by which VNS inhibits autophagy and apoptosis in cerebral I/R injury remain unclear and must be further explored. Moreover, whether VNS can affect nerve regeneration after cerebral I/R injury also requires further investigation. It is also anticipated that VNS technology will be applicable to the clinic at a subsequent stage to further explore its protective effects on stroke patients.
Ethics Approval
All experiments were carried out in strict accordance with protocols approved by the Institutional Animal Care and Use Committee and the Institutional Ethics Committee at Chongqing Medical University (Permit No. SCXK (Chongqing) 2007-0001) and the State Science and Technology Commission of China. All animals were treated in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (1996).
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Poustchi F, Amani H, Hmadian Z, et al. Combination therapy of killing diseases by injectable hydrogels: from concept to medical applications. Adv Healthc Mater. 2021;10(3):e2001571. doi: 10.1002/adhm.202001571 [DOI] [PubMed] [Google Scholar]
- 2.Zhou L, Ao LY, Yan YY, et al. JLX001 ameliorates ischemia/reperfusion injury by reducing neuronal apoptosis via down-regulating JNK signaling pathway. Neuroscience. 2019;418:189–204. doi: 10.1016/j.neuroscience.2019.08.053 [DOI] [PubMed] [Google Scholar]
- 3.Amani H, Habibey R, Shokri F, et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep. 2019;9(1):6044. doi: 10.1038/s41598-019-42633-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan J, Li X, Guo F, et al. Ginkgetin attenuates cerebral ischemia-reperfusion induced autophagy and cell death via modulation of the NF-κB/p53 signaling pathway. Biosci Rep. 2019;39(9):BSR20191452. doi: 10.1042/BSR20191452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YG, Tao W, Yang SB, et al. Autophagy: novel insights into therapeutic target of electroacupuncture against cerebral ischemia/reperfusion injury. Neural Regen Res. 2019;14(6):954–961. doi: 10.4103/1673-5374.250569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang WW, Huang BS, Han Y, et al. Sodium hydrosulfide attenuates cerebral ischemia/reperfusion injury by suppressing overactivated autophagy in rats. FEBS Open Bio. 2017;7(11):1686–1695. doi: 10.1002/2211-5463.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei K, Wang P, Miao CY. A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury. CNS Neurosci Ther. 2012;18:879–886. doi: 10.1111/cns.12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Yue F. Suppression of REDD1 attenuates oxygen glucose deprivation/reoxygenation-evoked ischemic injury in neuron by suppressing mTOR-mediated excessive autophagy. J Cell Biochem. 2019;120:14771–14779. doi: 10.1002/jcb.28737 [DOI] [PubMed] [Google Scholar]
- 9.Tao J, Shen C, Sun Y, et al. Neuroprotective effects of pinocembrin on ischemia/reperfusion-induced brain injury by inhibiting autophagy. Biomed Pharmacother. 2018;106:1003–1010. doi: 10.1016/j.biopha.2018.07.026 [DOI] [PubMed] [Google Scholar]
- 10.Peng C, Rao W, Zhang L, et al. Mitofusin 2 exerts a protective role in ischemia reperfusion injury through increasing autophagy. Cell Physiol Biochem. 2018;46:2311–2324. doi: 10.1159/000489621 [DOI] [PubMed] [Google Scholar]
- 11.Uzdensky AB. Apoptosis regulation in the penumbra after ischemic stroke: expression of pro- and antiapoptotic proteins. Apoptosis. 2019;24:687–702. doi: 10.1007/s10495-019-01556-6 [DOI] [PubMed] [Google Scholar]
- 12.Morris GL, Gloss D, Buchhalter J, et al. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(16):1453–1459. doi: 10.1212/WNL.0b013e3182a393d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conway CR, Sheline YI, Chibnall JT, et al. Brain blood-flow change with acute vagus nerve stimulation in treatment-refractory major depressive disorder. Brain Stimul. 2012;5:163–171. doi: 10.1016/j.brs.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao XP, Zhao Y, Qin XY, et al. Non-invasive vagus nerve stimulation protects against cerebral ischemia/reperfusion injury and promotes microglial M2 polarization via interleukin-17A inhibition. J Mol Neurosci. 2019;67:217–226. doi: 10.1007/s12031-018-1227-7 [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Ma J, Jin X, et al. L-PGDS mediates vagus nerve stimulation-induced neuroprotection in a rat model of ischemic stroke by suppressing the apoptotic response. Neurochem Res. 2017;42:644–655. doi: 10.1007/s11064-016-2121-8 [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Li L, Tan X, et al. miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J Neurochem. 2015;134:173–181. doi: 10.1111/jnc.13097 [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Li L, Liu B, et al. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS One. 2014;9:e102342. doi: 10.1371/journal.pone.0102342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabryel B, Kost A, Kasprowska D. Neuronal autophagy in cerebral ischemia–a potential target for neuroprotective strategies. Pharmacol Rep. 2012;64:1–15. doi: 10.1016/S1734-1140(12)70725-9 [DOI] [PubMed] [Google Scholar]
- 19.Ay I, Sorensen AG, Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: an unlikely role for cerebral blood flow. Brain Res. 2011;1392:110–115. doi: 10.1016/j.brainres.2011.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith DC, Modglin AA, Roosevelt RW, et al. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005;22:1485–1502. doi: 10.1089/neu.2005.22.1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schäbitz WR, Weber J, Takano K, et al. The effects of prolonged treatment with citicoline in temporary experimental focal ischemia. J Neurol Sci. 1996;138:21–25. doi: 10.1016/0022-510X(95)00341-X [DOI] [PubMed] [Google Scholar]
- 22.Li P, Shen M, Gao F, et al. An antagomir to microRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol. 2017;54:2901–2921. doi: 10.1007/s12035-016-9842-1 [DOI] [PubMed] [Google Scholar]
- 23.Liu B, Zhang YH, Jiang Y, et al. Gadd45b is a novel mediator of neuronal apoptosis in ischemic stroke. Int J Biol Sci. 2015;11:353–360. doi: 10.7150/ijbs.9813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugawara T, Fujimura M, Noshita N, et al. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRx. 2004;1:17–25. doi: 10.1602/neurorx.1.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakka VP, Gusain A, Mehta SL, et al. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol. 2008;37:7–38. [DOI] [PubMed] [Google Scholar]
- 26.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol. 1990;181:195–213. doi: 10.1007/BF00174615 [DOI] [PubMed] [Google Scholar]
- 28.Xu M, Zhang HL. Death and survival of neuronal and astrocytic cells in ischemic brain injury: a role of autophagy. Acta Pharmacol Sin. 2011;32:1089–1099. doi: 10.1038/aps.2011.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali SO, Shahin NN, Safar MM, et al. Therapeutic potential of endothelial progenitor cells in a rat model of epilepsy: role of autophagy. J Adv Res. 2019;18:101–112. doi: 10.1016/j.jare.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han B, Zhang Y, Zhang Y, et al. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke. Autophagy. 2018;14:1164–1184. doi: 10.1080/15548627.2018.1458173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kizilarslanoğlu MC, Ülger Z. Role of autophagy in the pathogenesis of Alzheimer disease. Turk J Med Sci. 2015;45:998–1003. doi: 10.3906/sag-1407-75 [DOI] [PubMed] [Google Scholar]
- 32.Campbell P, Morris H, Schapira A. Chaperone-mediated autophagy as a therapeutic target for Parkinson disease. Expert Opin Ther Targets. 2018;22:823–832. doi: 10.1080/14728222.2018.1517156 [DOI] [PubMed] [Google Scholar]
- 33.Martin DD, Ladha S, Ehrnhoefer DE, et al. Autophagy in Huntington disease and huntingtin in autophagy. Trends Neurosci. 2015;38:26–35. doi: 10.1016/j.tins.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 34.Wang YC, Zhang S, Du TY, et al. Hyperbaric oxygen preconditioning reduces ischemia-reperfusion injury by stimulating autophagy in neurocyte. Brain Res. 2010;1323:149–151. doi: 10.1016/j.brainres.2010.01.074 [DOI] [PubMed] [Google Scholar]
- 35.Mikrogianakis A, Shaye RE, Griffin P, et al. Hypoxia alters the expression of inhibitor of apoptosis proteins after brain trauma in the mouse. J Neurotrauma. 2007;24:338–353. doi: 10.1089/neu.2006.003615 [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Raina AK, Perry G, et al. Apoptosis in Alzheimer disease: a mathematical improbability. Curr Alzheimer Res. 2006;3:393–396. doi: 10.2174/156720506778249470 [DOI] [PubMed] [Google Scholar]
- 37.Zhou W, Chen L, Hu X, et al. Effects and mechanism of epigallocatechin-3-gallate on apoptosis and mTOR/AKT/GSK-3β pathway in substantia nigra neurons in Parkinson rats. Neuroreport. 2019;30:60–65. doi: 10.1097/WNR.0000000000001149 [DOI] [PubMed] [Google Scholar]
- 38.Cho KJ, Lee BI, Cheon SY, et al. Inhibition of apoptosis signal-regulating kinase 1 reduces endoplasmic reticulum stress and nuclear huntingtin fragments in a mouse model of Huntington disease. Neuroscience. 2009;163:1128–1134. doi: 10.1016/j.neuroscience.2009.07.048 [DOI] [PubMed] [Google Scholar]
- 39.Yu F, Xue W, Dong L, et al. Tetrahydroxystilbene glucoside suppresses NAPDH oxidative stress to mitigate apoptosis and autophagy induced by cerebral ischemia/reperfusion injury in mice. Evid Based Complement Alternat Med. 2019;2019:3913981. doi: 10.1155/2019/3913981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Liu X, Zhu Y, et al. Magnolol derivative 002C-3 protects brain against ischemia-reperfusion injury via inhibiting apoptosis and autophagy. Neurosci Lett. 2015;588:178–183. doi: 10.1016/j.neulet.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 41.Ekmekçi H, Kaptan H. Vagus nerve stimulation. Open Access Maced J Med Sci. 2017;5:391–394. doi: 10.3889/oamjms.2017.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79 [DOI] [PubMed] [Google Scholar]
- 43.Engineer CT, Hays SA, Kilgard MP. Vagus nerve stimulation as a potential adjuvant to behavioral therapy for autism and other neurodevelopmental disorders. J Neurodev Disord. 2017;9:20. doi: 10.1186/s11689-017-9203-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roosevelt RW, Smith DC, Clough RW, et al. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006;1119:124–132. doi: 10.1016/j.brainres.2006.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma J, Qiao P, Li Q, et al. Vagus nerve stimulation as a promising adjunctive treatment for ischemic stroke. Neurochem Int. 2019;131:104539. doi: 10.1016/j.neuint.2019.104539 [DOI] [PubMed] [Google Scholar]