Abstract
Malaria vector control in Mali relies heavily on the use of long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) in selected districts. As part of strengthening vector control strategies in Koulikoro district, the National Malaria Control Programme (NMCP) through the support from the US President's Malaria Initiative (PMI) has strategically driven the implementation of IRS, with the LLINs coverage also rising from 93.3% and 98.2%. Due to the increased reports of vector resistance to both pyrethroid and carbamates, there was a campaign for the use of pirimiphos-methyl, an organophosphate at Koulikoro between 2015 and 2016. In this study, the effect of IRS on malaria transmission was assessed, by comparing some key entomological indices between Koulikoro, where IRS was implemented and its neighboring district, Banamba that has never received IRS as vector control intervention. The study was conducted in two villages of each district (Koulikoro and Banamba). Pyrethrum spray catches and entry window trapping were used to collect mosquitoes on a monthly basis. WHO tube tests were carried out to assess mosquito susceptibility to insecticides. Mosquitoes were identified to species level by PCR and their infection to P. falciparum was detected by Enzyme Linked-Immuno-Sorbent Assay (ELISA). Of the 527 specimens identified, An. coluzzii was the most frequent species (95%) followed by An. gambiae (4%) and An. arabiensis (1%). Its density was rainfall dependent in the no-IRS area, and almost independent in the IRS area. The infection rate (IR) in the no-IRS area was 0.96%, while it was null in the IRS area. In the no-IRS area, the entomological inoculation rate (EIR) was 0.21 infective bites /person month with a peak in September. High resistance to pyrethroids and carbamates and susceptibility to organophosphates was observed at all sites. The introduction of pirimiphos-methyl based IRS for vector control resulted in a significant decrease in malaria transmission. An. gambiae s.l., the main malaria vector in the area, was resistant to pyrethroids and carbamates but remained susceptible to the organophosphate pirimiphos-methyl.
Keywords: infection rate, entomological inoculation rate, insecticide resistance, transmission
Introduction
Long-lasting insecticidal nets (LLINs) and Indoor Residual Spraying (IRS) are actively promoted as the main prevention tools for malaria control and elimination (WHO, 2006). The recent wide deployment of these two control tools is considered to be responsible for the substantial reduction of the incidence and deaths related to the disease in Africa (Bhatt et al., 2015; WHO, 2016b) but they are generally not sufficient to eliminate transmission (PNLP, 2016).
Malaria affects about 40% of the population in Mali (SLIS, 2014). The health services registered 2,345,481 cases of malaria including 750,973 severe cases in 2018(Direction Générale de la Santé et de l’Hygiène Publique, 2019). Children under 5 years are the most affected by the disease with 33.70% of cases, followed by pregnant women (4.77%). The number of recorded deaths was 1001, with a case fatality rate of 1.33 ‰ (Direction Générale de la Santé et de l’Hygiène Publique, 2019).
The national strategy for malaria control in Mali is based on four main interventions: i) Early diagnosis and treatment by ACTs, ii.) Seasonal Malaria Chemoprevention (SMC) in children aged 3 to 59 months (SMC), iii) Intermittent Preventive Treatment (IPT) with sulfadoxine-pyrimethamin (SP) in pregnant women and, iv) Vector control. Vector control relies heavily on the use of LLINs at a country level and IRS in selected districts. After a mass distribution of LLINs in 2014 to achieve universal coverage of all populations at risk in 2014, demographic health surveillance study reported 93.3% coverage, 90.5% usage, and 3.6 LLINs per household in the region of Koulikoro, including Koulikoro and Banamba districts (PNLP, 2016). As part of the strengthening of vector control strategies in the district of Koulikoro, IRS was initiated by the National Malaria Control Program (NMCP) with the support of the US President's Malaria Initiative (PMI) since 2008. The IRS coverage was 98.2% in Koulikoro, the only district of the region where the IRS was supported by the US President malaria initiative (AIRS, 2015). Pyrethroid insecticides (deltamethrin and lambda-cyhalothrin) were used for the IRS during the first 4 years (from 2008 to 2011) (Cisse et al., 2015). Due to the resistance to these insecticides, they were replaced by a carbamate insecticide (Bendiocarb) from 2012 to 2014 campaigns. In 2015, given the short residual life of Bendiocarb on mud walls, the Mali NMCP, as well as its partners, have decided to switch to pirimiphos-methyl, an organophosphate insecticide.
With the emergence and expansion of the resistance of Anopheles gambiae sensu lato (An. gambiae s.l.), to insecticides, the fragile progress made in malaria control can be compromised (Keita et al., 2016). The wide and long term use of pyrethroid insecticides in LLINs and the agricultural sector seems to be one of the main causes of the increasing resistance of malaria vector to these products (Namountougou et al., 2012; Reid and McKenzie, 2016).
So far, no study was performed in Mali to assess the IRS effect on malaria transmission expressed by the entomological inoculation rate in a context of vector resistance to insecticides. Therefore, the objective of this study was to assess the effect of IRS on malaria transmission by comparing some entomological indices between two localities of the district of Koulikoro; villages where IRS was implemented for about 9 consecutive years and to similar villages in a neighbored district, Banamba, where IRS had never been implemented.
Material & Methods:
Study areas
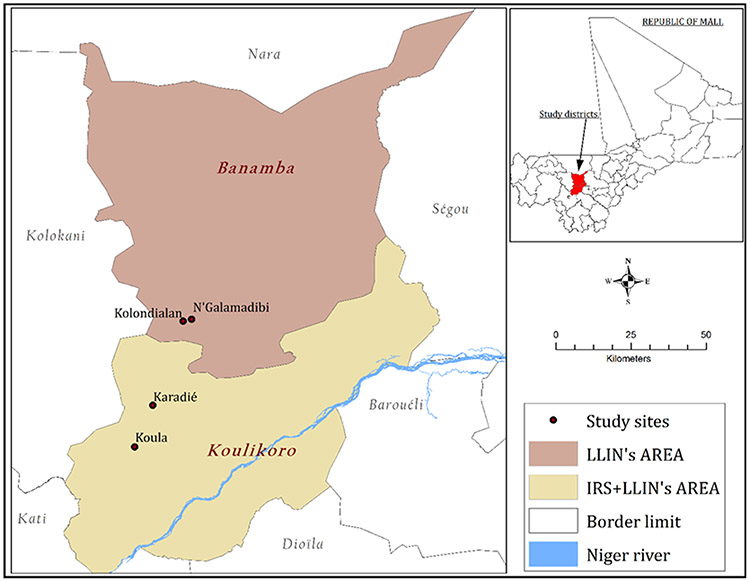
Cross-sectional surveys were undertaken in two health districts, located in North Savanna area of Mali, to assess the effects of pirimiphos-methyl based IRS in the context of vector resistance to pyrethroids, on entomologic parameters of malaria transmission.The study sites were selected after performing a World Health Organization standard bioassay test in many villages of Koulikoro and Banamba health districts to determine malaria vector resistance status to pyrethroids. Koula (7.65W, 13.12N) and Karadié (7.60W, 13.24N) in Koulikoro health district, and Kolondialan (7.51W, 13.49N) and N'Galamadibi (7.48W, 13.48N) in Banamba health district (Fig. 1) presenting comparable pyrethroid resistance status were then selected to represent the areas with IRS and no-IRS area, respectively. Table 1 shows the characteristics of the different selected sites. The climate in both districts is a typical Sudano-sahelian with two seasons: a long dry season (November to May) and a wet season (June to October) with a mean annual rainfall of 900–1200 mm. The monthly mean temperature during the rainy season varies between 29 and 33°C. Anopheles gambiae sensu lato (s.l.) is the major malaria vector (> 98%) in all the villages and malaria control core intervention are mainly LLINs, IRS, and SMC. Besides, PMI has supported nine IRS campaigns in Koulikoro (2008 to 2016), while it has never been implemented in Banamba. The coverage rate for the 2016 IRS campaign was 97.14%. Agriculture, livestock, and trade are the main economic activities in the two districts. Malaria transmission occurs mostly during the rainy season (June to October) with a mean monthly mosquito man-biting rate reaching its peak in August/September.
Figure 1:
Map of Mali showing the different study sites
Table 1:
Characteristics of the selected study sites in the health districts of Koulikoro and Banamba.
| Villages | Districts | Population | Resistance mechanisms | Control interventions |
|---|---|---|---|---|
| Koula | Koulikoro | 9003 | KdrW (29.6%) | LLINs, IRS, SMC |
| Karadié | Koulikoro | 4854 | KdrW (49.0%) | LLINs, IRS, SMC |
| N’Galamadibi | Banamba | 5074 | KdrW (48.0%) | LLINs, SMC |
| Kolondialan | Banamba | 4311 | KdrW (49.0%) | LLINs, SMC |
Implementation of the IRS and LLINs distribution
Since 2008, the US President’s Malaria Initiative (PMI) has supported IRS in three health districts in Mali, including the district of Koulikoro. The IRS was performed by the team of the Africa Indoor Residual Spraying (AIRS) project in Mali in collaboration with the NMCP. Every year only one IRS was done to ensure that the sprayed surfaces would retain their efficacy through the peak of malaria transmission season (September and October). The 2016 IRS campaign was done in July using the pirimiphos methyl. For the purpose of this study, a mass distribution of LLINs was performed in June 2016 to scale up the coverage rate to 100% in both IRS and no-IRS areas with the support of the NMCP. A household survey was conducted in October to determine net usage rates.
Mosquito vector sampling:
In each selected village, vector populations were sampled monthly from June to November 2016 by pyrethrum spray catch (PSC) at day time and entry windows traps (EWT) during the night.
Pyrethrum spray catches (PSC)
In each study village, 30 sentinel houses were randomly selected from the list of households’ census for indoor-resting mosquitoes sampling by PSC. The sampling was done taking into account the proportion of the housing type (e.g. straw roof, mud roof, and metal roof) in each village. The PSC consisted of spraying pyrethrum in the selected houses (from 8:00 h to 11:00 h) in the morning. Two teams of 2 entomologists (a total of 4) were operating simultaneously and sampling 10-15 houses per day. The insecticide used was a mixture of a pyrethroid (Tetramethrin 0.15%) and organophosphates (Dichlorvos, 1.20%) and Fenitrothion, 0.40%) marketed under the name "Premium®".
Entry window trapping (EWT).
Ten (10) other houses were randomly selected in each village as sentinel sites for EWT sampling. The EWT consisted of mounting traps on windows, which collected the mosquitoes trying to enter the rooms (Figure 2). The catches were done for three successive nights per month per village. One of the local guides of the different villages was responsible for closing the traps very early in the morning (around 6:00 am) with a small curtain placed at their opening to prevent the trapped mosquitoes from escaping. The trapped mosquitoes were removed from the cages using a mouth aspirator, transferred into paper cups, and the number recorded on a data sheet.
Figure 2:
Field technician and local guide mounting an entry window trap
Insecticide susceptibility bioassay
Anopheles gambiae s.l. larvae were collected in different types of breeding habitats in and around each study site and reared to adulthood at the Malaria Research and Training Center (MRTC) insectary at Bamako. Female adult mosquitoes of 2-5 day-olds were exposed to impregnated papers with deltamethrin [0.05%], bendiocarb [0.1%], and pirimiphos-methyl [0.25%]. Approximately 20-25 mosquitoes per tube with 2-6 replicates were exposed to impregnated filter papers at the diagnostic concentrations for 1 hour and then transferred to a clean holding tube supplied with a 10% sugar solution. The post-exposure mortality rate was determined after 24 hours according to WHO standard operating procedures(WHO, 2016a). All the tests were carried out at 27 ± 1°C and relative humidity of 70-80%.
Sample processing
Collected mosquitoes were identified to species level using polymerase chain reaction (PCR) technique (Fanello et al., 2002). The infection rate and human blood index (HBI) were established using the Enzyme Linked-Immuno-Sorbent Assay (ELISA) techniques according to Burkot et al(Burkot et al., 1984) and Beier et al (Beier et al., 1988), respectively.
Statistical analysis.
The data were entered in Microsoft Excel and analyzed in SPSS version 22, STATA version 10, and GraphPad Prism 8.3.0. The following entomological parameters were calculated: vector density per room, human biting rate (HBR), infection rate (IR), entomological inoculation rate (EIR), human blood index (HBI), and parity rate (PR). The density of malaria vectors was calculated as the average number of indoor resting mosquitoes per room per day; the HBR as the average number of mosquito bites received by a sleeper per time unit (blood-fed and half gravid mosquito/number of room sleepers); the IR corresponds to the proportion of An. gambiae s.l. carrying Plasmodium falciparum sporozoites; the HBI as the proportion of female mosquitoes having human blood in their guts; the PR was calculated as the percentage of parous females relative to the total number of mosquitoes dissected (parous + nulliparous), and the EIR was calculated as the total number of infectious bites per person per time unit. Density, infection and entomological inoculation rates were calculated using a Bayesian model in WinBug (version 1.4.1) and the Bayesian Credible Interval (BCI), corresponding to the 95% CI, was used to compare the means. The Pearson correlation test was used to determine the correlation between density and rainfall. The Chi-square test was used to compare the vector composition, HBR, HBI, and PR.
Mosquito mortality rates 24 hours after exposure to the insecticide-impregnated filter papers were calculated by dividing the number of dead mosquito by the number exposed to the insecticide.
Mortality ≥ 98 indicate mosquitoes were susceptible,
Mortality between 90 and 97% indicate mosquitoes were suspected to be resistant,
Mortality < 90% indicate mosquitoes were resistant.
Results
IRS coverage and LLINs usage
The coverage rate of the IRS was 97.1%. During the distribution, LLINs ownership was scale up to 100% on each study site. A household survey carried out in October showed an overall usage rates of 91.7% in the IRS area and 89.2% in the no-IRS area. In both areas, the main reasons for not using the LLINs includes issues related to room space and hot temperature. The new LLINs were provided in June and were found in good shape during the surveys.
Molecular species composition of An. gambiae s.l.
A total of 2270 female An. gambiae s.l. were collected over the study period, 2000 by PSC, and 270 mosquitoes by EWT. An. gambiae s.l. was the only malaria vector collected in both areas. Molecular identification of An. gambiae s.l. by PCR showed that An. gambiae s.l. was composed of three species in all the study sites except for N’Galamadibi where we did not find An. arabiensis (Table 2). In the IRS area, An. gambiae s.l. consisted of An. coluzzii 95.88% (93/97), An. gambiae 2.06% (2/97) and An. arabiensis 2.06% (2/97) and in the no-IRS area, it consisted of An. coluzzii 94.12% (409/430), An. gambiae 4.42% (19/430) and An. arabiensis 0.47% (2/430) (Table 2). However, there was no significant difference between the proportions of the three species found in the two areas (Fisher x2 = 3.767, P=0.1521).
Table 2:
Species composition of three members of the Anopheles gambiae s.l. in four localities in Mali in 2016.
| Areas | Localities | An. arabiensis | An. coluzzii | An. gambiae | Total | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| IRS area | Koula | 1 | 1.72 | 55 | 94.83 | 2 | 3.45 | 58 |
| Karadié | 1 | 2.56 | 38 | 97.44 | 0 | 0.00 | 39 | |
| Total | 2 | 2.06 | 93 | 95.88 | 2 | 2.06 | 97 | |
| no-IRS area | Kolondialan | 2 | 0.68 | 278 | 95.21 | 12 | 4.11 | 292 |
| N’Galamadibi | 0 | 0.00 | 131 | 94.93 | 7 | 5.07 | 138 | |
| Total | 2 | 0.47 | 409 | 95.12 | 19 | 4.42 | 430 | |
The density of Anopheles gambiae s.l. per room per day
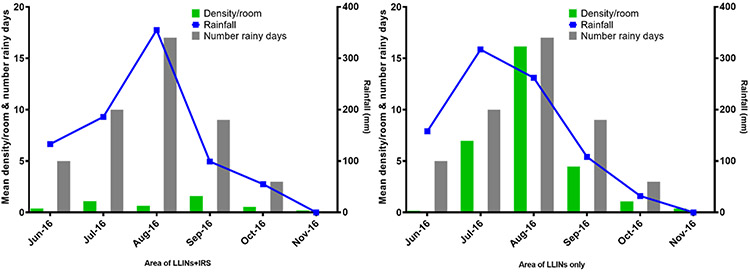
The mean density of An. gambiae s.l. per house over the study period was 7.18 times higher (4.88/0.68) in the no-IRS area (4.88, 1756/360) than in the IRS area (0.68, 244/360). However, there were monthly variations in An. gambiae s.l. density (Table 3). It increased with rainfall in the areas with no IRS usage, while it remained low regardless of the variations in rainfall in the IRS area (Figure 3). The highest density was observed in August (16.27, 968/60) in the no-IRS area. In the IRS area, the peak was observed in September (1.27, 95/60) two months after the IRS was done. There was a strong correlation between rainfall and An. gambiae s.l. density, with 1-month lag, and rainfall in both the IRS (R = 0.888, P = 0.018) and the no-IRS (R = 0.806, P = 0.053) areas. The mean annual rainfall in 2016 (June to October) was 904 mm over 47 rainy days in the IRS area, and 907 mm over 54 rainy days in the no-IRS area.
Table 3:
Variation of mean monthly density of An. gambiae s.l. per room in IRS and no-IRS areas from June to November 2016
| Months | IRS area | no-IRS area | ||||
|---|---|---|---|---|---|---|
| # collection rooms |
Mean density |
95% CI | # collection rooms |
Mean density |
95% CI | |
| June 16 | 60 | 0.38 | 0.20—0.57 | 60 | 0.15 | 0.05—0.25 |
| July 16 | 60 | 1.10 | 0.60—1.60 | 60 | 7.02 | 4.67—9.36 |
| August 16 | 60 | 0.62 | 0.10—1.14 | 60 | 16.27 | 12.60—19.93 |
| September 16 | 60 | 1.27 | 0.79—1.75 | 60 | 4.43 | 3.07—5.79 |
| October 16 | 60 | 0.52 | 0.25—0.78 | 60 | 1.07 | 0.58—1.56 |
| November 16 | 60 | 0.18 | 0.07—0.29 | 60 | 0.33 | 0.08—0.58 |
| TOTAL | 360 | 0.68 | 0.52—0.84 | 360 | 4.88 | 3.92—5.84 |
Figure 3:
Variation in An. gambiae s.l. density (green bars) and rainfall (blue line) in areas of IRS and no-IRS from June to November 2016.
Parity rate of Anopheles gambiae s.l.
The ovaries of females of An. gambiae s.l. were dissected for the vector population’s age grading from samples collected by EWTs (Table 4). Surprisingly, the mean parity rate in the IRS area (96.42%, 54/56) was not different from the one in the no-IRS area (98.36%, 120/122) (P = 0.4190).
Table 4:
Variation of the parity rate in IRS area and no-IRS area from June to November 2016.
| Month | IRS area | No-IRS area | ||||
|---|---|---|---|---|---|---|
| Dissected | Parous | % PR* | Dissested | Parous | % PR | |
| June 16 | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| July 16 | 16 | 16 | 100 | 25 | 25 | 100 |
| August 16 | 8 | 7 | 87.50 | 87 | 85 | 97.70 |
| September 16 | 20 | 19 | 95.00 | 7 | 7 | 100 |
| October 16 | 7 | 7 | 100 | 0 | 0 | 0.00 |
| November 16 | 5 | 5 | 100 | 3 | 3 | 100 |
| Mean rate | 56 | 54 | 96.42 | 122 | 120 | 98.36 |
PR: Parity rate
Human biting rate of Anopheles gambiae s.l.
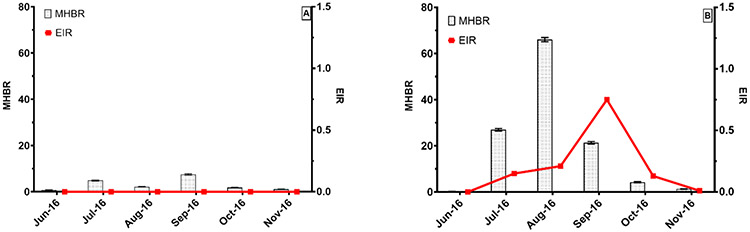
Using PSC, the overall mean monthly human biting rate (MHBR) of An. gambiae s.l. was significantly higher in the no-IRS area (20.41, 95%CI = 20.20—20.62) than in the IRS area (3.03, 95%CI = 2.95—3.12). There were monthly variations in both areas (Figure 4). In the IRS area, the peak of the MHBR (7.47, 95% CI = 7.15—7.80) was observed in September (> 2 months after IRS), while it was observed in August in the no-IRS area (66.00, 95% CI = 65.09—66.92). In both areas, the lowest MHBR was observed in June (Figure 4).
Figure 4:
Monthly variation in An. gambiae s.l. man biting rates (gray bars) and entomological inoculation rates (Red line) in areas of IRS (A) and no-IRS (B) from June to November 2016.
Infection rate (IR) and Entomological inoculation rates (EIR) in An. gambiae s.l.
An. gambiae s.l. sample collected from PSC and EWT were screened for P. falciparum circumsporozoite protein (CSP). The mean infection rate of An. gambiae s.l. in the no-IRS area was 0.96% (16/1670) whereas no mosquito sample was infected in the IRS area. In the no-IRS area, the mean EIR was 0.21 infective bites/person/month with a peak in September at 0.75 infective bites/person/ month (Figure 4 and Table 5).
Table 5:
Variation of the infection rate in IRS area and no-IRS area from June to November 2016.
| Month | IRS area | No-IRS area | ||||
|---|---|---|---|---|---|---|
| N* tested | N positive | % IR* | N tested | N positive | % IR | |
| June 16 | 21 | 0 | 0.00 | 8 | 0 | 0.00 |
| July 16 | 64 | 0 | 0.00 | 374 | 2 | 0.53 |
| August 16 | 35 | 0 | 0.00 | 945 | 3 | 0.31 |
| September 16 | 92 | 0 | 0.00 | 256 | 9 | 3.52 |
| October 16 | 31 | 0 | 0.00 | 65 | 2 | 3.08 |
| November 16 | 10 | 0 | 0.00 | 22 | 0 | 0.00 |
| Mean rate | 171 | 0 | 0.00 | 1670 | 16 | 0.96 |
N: number,
IR: infection rate
Human blood index
The HBI (PSC) shows that the mosquitoes were highly anthropophilic in both areas. The average HBI in the IRS area was 74.27% (127/171) and was significantly lower (χ2 = 19.09; P < 0.001) compared to the no-IRS area 86.90% (1042/1199). However, monthly variations in these rates were observed in both areas (Table 6 It is only in October that significant-high HBI was recorded in the no-IRS area (97.62%). In August, the HBI almost looked similar in both areas (88.51 vs 88.89%).
Table 6:
Variation of the human blood index in IRS area and no-IRS area from June to November 2016.
| Month | IRS area | No-IRS area | P-Value | ||||
|---|---|---|---|---|---|---|---|
| N* tested |
N positive |
% HBI* (95%CI) |
N tested |
N positive |
% HBI* (95%CI) |
||
| June 16 | 15 | 8 | 53.33 | 2 | 0 | 0.00 | 0.169 |
| July 16 | 46 | 29 | 63.04 | 266 | 212 | 79.70 | 0.015 |
| August 16 | 18 | 16 | 88.89 | 670 | 593 | 88.51 | 0.960 |
| September 16 | 66 | 58 | 87.88 | 205 | 186 | 90.73 | 0.502 |
| October 16 | 17 | 12 | 70.59 | 42 | 41 | 97.62 | 0.002 |
| November 16 | 9 | 4 | 44.44 | 14 | 10 | 71.43 | 0.206 |
| Mean rate | 171 | 127 | 74.27 | 1199 | 1042 | 86.91 | 0.0001 |
N* = Number; HBI* = human blood index
The resistance of An. gambiae s.l. to insecticides
Bioassay results showed a high resistance to deltamethrin in the four study sites (Figure 5) with mortality rates of 15, 20, 24.7, and 41 % in Karadié (N = 100), N'Galamadibi (N = 150), Kolondialan (N = 150) and Koula (N = 200), respectively. A significant difference between deltamethrin mortalities was observed at the different study sites (χ2 = 31.06; P < 0.000). An. gambiae s.l. was also resistant to bendiocarb (Figure 5) in all study sites with mortality rates of 56, 73, 79, 69 % in Koula (N = 150), Karadié (N=100), N'Galamadibi (N = 200) and Kolondialan (N = 200), respectively. However, there was a significant difference between the mortality observed after exposure to bendiocarb in the four study sites (χ2 = 22.11; P < 0.000). However, there mosquito populations were susceptible pirimiphos-methyl in all the study sites.
Figure 5:
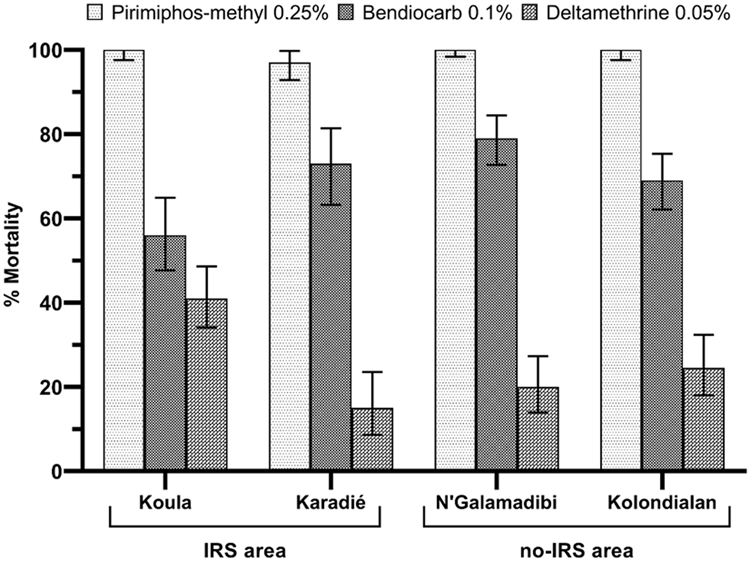
Observed 24 h mortality (%) of An. gambiae s.l. following 60 mn exposition to Pyrethroids (Deltamethrin), Carbamates (Bendiocarb) and Organophosphates (Pirimiphos-methyl) in the selected study sites using WHO standard bioassay test.
Discussion
In this study, there were comparisons of several entomological indices of malaria transmission in mosquitoes from Koulikoro district where IRS was implemented for about 9 consecutive years to the Banamba district, where the IRS has never been conducted.
Our results showed that Anopheles gambiae s.l. was the only malaria vector encountered in both study areas and consisted of three species: An. coluzzii, An. arabiensis and An. gambiae. This is consistent with findings of several studies carried out in Mali, which also showed that the mosquito species live in sympatry in these geographical areas (Keita et al., 2014; Toure et al., 1998b). Anopheles coluzzii was the most abundant species in both areas as previously found in several other parts of the country (Adamou et al., 2011; Cisse et al., 2015; Keita et al., 2014; Toure et al., 1994).
The mean densities of Anopheles gambiae s.l. per room per day over the study period were lower in the IRS area compared to the no-IRS area with monthly variations. We did not observe a significant difference in the densities between the two areas before the application of the IRS. Just after the IRS application, densities were significantly increasing through the rainiest period (July to September) in no-IRS area compared to the IRS area, to then become comparable at the end of the rainy season (October to November). Many studies have demonstrated the association between An. gambiae s.l. abundance and rainfall (Kelly-Hope et al., 2009; Koenraadt et al., 2004; Zhou et al., 2004) in dry areas where its breeding is almost exclusively rain-dependent. Therefore, the observed difference in An. gambiae s.l. densities between the two areas could be explained by the residual effect of the IRS campaign (Mashauri et al., 2017; Sy et al., 2018) as An. gambiae s.l, was fully susceptible to the pirimiphos-methyl in all sites of both areas..
The ultimate goal of the IRS is to reduce the lifespan of mosquitoes, hence the parity rate. However, despite of the monthly variations in the parity rate, our results did not show a significant difference in the overall parity rate between the two areas. It is generally expected to observe higher mosquito parity rates in unsprayed control areas compared to IRS0 area as reported in the neighbor country of Senegal (Sy et al., 2018). Our unexpected observation could be due to the low number of mosquitoes dissected, and also the collection method (EWT) we used as alternative to the human landing collection (HLC). Indeed, there is yet no mosquito collection method more accurate or representative than the human landing catch in measuring entomological parameters of malaria transmission (Lima et al., 2014) . Other factors such as temperature and humidity vector longevity and abundance of parous females (Ribeiro et al., 2013) between study sites.
The deployment of LLIN and IRS to both areas were observed to limit mosquitoes’ access to human blood sources. However, An. gambiae s.l. was found to be highly anthropophilic in both areas even though the overall HBI was significantly higher in the no-IRS area. Several studies conducted across Africa have shown that the long-term use of LLIN or IRS alone or combined could lead to changes in the biting behavior of An. gambiae s.l. both in- and outdoor. In addition, based on our own observation in the field, a lot of people were staying longer outside (up to midnight) watching television without any personal protection during the night due to the houses structure and household activities. Therefore, they may get mosquito bites before going to bed under their nets. This may reduce the impact of vector control measures such as LLNS and IRS. Thus, in both areas, mosquitoes may have feed outside before getting inside to rest. In the IRS area, non-sprayed resting places are limited explaining the lower densities and HBR in this area compared to the no-IRS area.
In the IRS area, we could not detect transmission as measured by IR because none of the tested specimens of An. gambiae s.l. by ELISA was infected, while in the no-IRS area, the transmission was typically seasonal with the peak observed at the end of the rainy season. The non-detection of transmission in the IRS area can be attributed to the low number of mosquitoes we collected and probably also because of the effects of the IRS. IRS may have shortened the life expectancy of the mosquito population to complete parasite development in mosquito as shown by the parity rate.
A limitation of this study has been that we could not use human landing collection (HLC) to estimate indoor and outdoor transmission. Nevertheless, the PSC and EWT were consistently used across all sites. The HBR calculated from PSC collections should also be interpreted cautiously as this could have been underestimated. Not all the mosquitoes could have recovered and some could have also left the house before the PSC. The vector is also known to be resistant to pyrethroids and not all mosquitoes may have been knocked down during PSC.
Susceptibility testing performed show high and moderate phenotypic resistance respectively to deltamethrin and bendiocarb in all four study sites. Insecticide selection pressure exerted on vector populations may explain this resistance. Indeed, in addition to the mass distribution of the LLINs and agricultural pesticides use, the area has been subjected to 9 consecutive years of IRS during which pyrethroids (lambda-cyhalothrin), carbamate (bendiocarb) and organophosphate (pirimiphos-methyl) have been successively used. The similarity in the resistance status of populations of An. gambiae s.l. in the two areas could be due to their closeness (separated by a 7 km distance). Indeed, mosquitoes can actively migrate up to 2-7 km (Baber et al., 2010; Toure et al., 1998a). Moreover, malaria vectors can migrate at long-distance in the Sahel (up to 300 km) by the wind as reported by a recent study in the same region of Koulikoro where this study was conducted (Huestis et al., 2019). Also, previous studies have shown the same trend of resistance in An. gambiae s.l. population, low resistance to bendiocarb in some from sentinel sites of the NMCP far away from the IRS areas (Cisse et al., 2015; Keita et al., 2014). The emergence and spread of resistance of An. gambiae s.l. to different classes of insecticides used in vector control could be due to their massive use as pesticides and herbicides in agriculture for the protection of crops (cotton, cereals, oilseeds, and market gardening) and the use of bendiocarb in IRS campaigns between 2012 and 2014 (Akogbeto et al., 2006; Antonio-Nkondjio et al., 2016; Diabate et al., 2002; Olatunbosun-Oduola et al., 2019; Yadouleton et al., 2009). We observed a difference in resistance level between the two families (carbamates and organophosphates) of insecticide. Resistance to bendiocarb does not imply systematic resistance to pirimiphos-methyl even though they share the same resistance gene. These results confirm those observed in several African countries which had shown that the presence of G119S does not systematically confer resistance to organophosphates (Asidi et al., 2005; Salako et al., 2018).
Despite the lack of baseline data and the use of human landing collection, coupled with the low number of study sites and mosquitoes collected and tested in the IRS area, the differences in entomological parameters of malaria transmission observed between the two areas can be attributed to the effects of the pirimiphos-methyl based IRS in the context of pyrethroids resistance. Indeed, except IRS, both areas located in the same eco-climatic conditions were subject to the same control interventions over 10 years (Table 1). Also, ours results are in line with findings of a previous article which reported a 4-times greater increase of the prevalence of infection in children < 5 years from June to October in no-IRS area compared to the IRS area. The incidence rate was 2.7 per 100 person-months in the IRS area compared with 6.8 per 100 person-month in the no-IRS area, and children living in the no-IRS area were 5-times more likely to have malaria than those living in the IRS area (Kane et al., 2020).
Conclusion.
IRS using pirimiphos-methyl has been successful in reducing substantially entomological parameters of malaria transmission in areas with pyrethroids resistance in Koulikoro, Mali. However, further studies by extending these findings to other districts is needed for a better impact assessment of pirimiphos-methyl-based IRS on malaria transmission. As the IRS intervention ended after the 2016 campaign, further studies are needed to determine the rebound effect and monitor the potential emergence of organophosphate resistance for better management of insecticide resistance.
Acknowledgement.
We thank the NMCP for their support and the population of the study sites for their collaboration. We also thank all MRTC staff who supported for the field work.
Funding: This work was funded by TDR/WHO [grant number B20388] with a support from the National Institutes of Health (NIH) through grant numbers [U19 AI 129387 and D43TW008652].
List of abbreviations
- (IRS)
Indoor residual spraying
- (LLINs)
Long-lasting insecticidal nets
- (IPT)
Intermittent Preventive Treatment
- (SMC)
Seasonal Malaria Chemoprevention
- (MOH)
The Ministry of Health
- (NMCP)
The National Malaria Control Program
- (IPT)
Preventive Intermittent Therapy
- (CRFs)
Case report forms
- (RDT)
Rapid diagnostic test
- (USTTB)
University of Sciences, Techniques and Technologies of Bamako
- (HBR)
Human biting rate
- (IR)
Infection rate
- (EIR)
Entomological inoculation rate
- (HBI)
Human blood index
- (PR)
Parity rate
Footnotes
Ethical considerations
The protocol of this project has been approved by the Ethics Committee of FMPOS/USTTB under the letter N°2014/51/CE/FMPOS. The research activities related to this protocol were carried out in accordance with good clinical research practice in humans and good laboratory practice as set out in the international conventions (Helsinki Declaration; International Conference on the Harmonisation of Good Practice in Biomedical Research). All our researchers were trained in good clinical and laboratory practice during the research. In the field, the community (administrative, customary authorities) was informed of all aspects of the study.
Consent for publication: Not applicable
Availability of data and materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests: The authors declare that they have no competing interests
References:
- PMI ∣ Africa IRS (AIRS) Project Indoor Residual Spraying (IRS 2) Task Order Six. 2016 Mali End of Spray Report. Bethesda, MD: Abt Associates. [Google Scholar]
- Adamou A, Dao A, Timbine S, Kassogue Y, Yaro AS, Diallo M, Traore SF, Huestis DL, Lehmann T, 2011. The contribution of aestivating mosquitoes to the persistence of Anopheles gambiae in the Sahel. Malar J 10, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AIRS, 2015. Africa Indoor Residual Spraying project / President’s Malaria Initiative /Report 2015 http://www.africairs.net/where-we-work/mali/. [DOI] [PMC free article] [PubMed]
- Akogbeto MC, Djouaka RF, Kinde-Gazard DA, 2006. Screening of pesticide residues in soil and water samples from agricultural settings. Malar J 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio-Nkondjio C, Poupardin R, Tene BF, Kopya E, Costantini C, Awono-Ambene P, Wondji CS, 2016. Investigation of mechanisms of bendiocarb resistance in Anopheles gambiae populations from the city of Yaounde, Cameroon. Malar J 15, 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asidi AN, N'Guessan R, Koffi AA, Curtis CF, Hougard JM, Chandre F, Corbel V, Darriet F, Zaim M, Rowland MW, 2005. Experimental hut evaluation of bednets treated with an organophosphate (chlorpyrifos-methyl) or a pyrethroid (lambdacyhalothrin) alone and in combination against insecticide-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes. Malar J 4, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baber I, Keita M, Sogoba N, Konate M, Diallo M, Doumbia S, Traore SF, Ribeiro JM, Manoukis NC, 2010. Population size and migration of Anopheles gambiae in the Bancoumana Region of Mali and their significance for efficient vector control. PLoS One 5, e10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JC, Perkins PV, Wirtz RA, Koros J, Diggs D, Gargan TP 2nd, Koech DK, 1988. Bloodmeal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. J Med Entomol 25, 9–16. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle K, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CLJ, Smith DL, Hay SI, Cibulskis RE, Gething PW, 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkot TR, Zavala F, Gwadz RW, Collins FH, Nussenzweig RS, Roberts DR, 1984. Identification of malaria-infected mosquitoes by a two-site enzyme-linked immunosorbent assay. Am J Trop Med Hyg 33, 227–231. [DOI] [PubMed] [Google Scholar]
- Cisse MB, Keita C, Dicko A, Dengela D, Coleman J, Lucas B, Mihigo J, Sadou A, Belemvire A, George K, Fornadel C, Beach R, 2015. Characterizing the insecticide resistance of Anopheles gambiae in Mali. Malar J 14, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabate A, Baldet T, Chandre F, Akoobeto M, Guiguemde TR, Darriet F, Brengues C, Guillet P, Hemingway J, Small GJ, Hougard JM, 2002. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg 67, 617–622. [DOI] [PubMed] [Google Scholar]
- Direction Générale de la Santé et de l’Hygiène Publique, 2019. Annuaire statistique 2018 du Système Local d'Information Sanitaire du Mali Bamako, p. 195.
- Fanello C, Santolamazza F, della Torre A, 2002. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol 16, 461–464. [DOI] [PubMed] [Google Scholar]
- Huestis DL, Dao A, Diallo M, Sanogo ZL, Samake D, Yaro AS, Ousman Y, Linton YM, Krishna A, Veru L, Krajacich BJ, Faiman R, Florio J, Chapman JW, Reynolds DR, Weetman D, Mitchell R, Donnelly MJ, Talamas E, Chamorro L, Strobach E, Lehmann T, 2019. Windborne long-distance migration of malaria mosquitoes in the Sahel. Nature 574, 404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane F, Keita M, Traore B, Diawara SI, Bane S, Diarra S, Sogoba N, Doumbia S, 2020. Performance of IRS on malaria prevalence and incidence using pirimiphos-methyl in the context of pyrethroid resistance in Koulikoro region, Mali. Malar J 19, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita M, Baber I, Sogoba N, Maiga HM, Diallo M, Doumbia S, Traore SF, 2014. [Vectorial transmission of malaria in a village along the Niger River and its fishing hamlet (Kenieroba and Fourda, Mali)]. Bull Soc Pathol Exot 107, 356–368. [DOI] [PubMed] [Google Scholar]
- Keita M, Traore S, Sogoba N, Dicko AM, Coulibaly B, Sacko A, Doumbia S, Traore SF, 2016. [Susceptibility status of Anopheles gambiae sensu lato to insecticides commonly used for malaria control in Mali]. Bull Soc Pathol Exot 109, 39–45. [DOI] [PubMed] [Google Scholar]
- Kelly-Hope LA, Hemingway J, McKenzie FE, 2009. Environmental factors associated with the malaria vectors Anopheles gambiae and Anopheles funestus in Kenya. Malar J 8, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenraadt CJ, Githeko AK, Takken W, 2004. The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae s.s. and Anopheles arabiensis in a Kenyan village. Acta Trop 90, 141–153. [DOI] [PubMed] [Google Scholar]
- Lima JB, Rosa-Freitas MG, Rodovalho CM, Santos F, Lourenco-de-Oliveira R, 2014. Is there an efficient trap or collection method for sampling Anopheles darlingi and other malaria vectors that can describe the essential parameters affecting transmission dynamics as effectively as human landing catches? - A Review. Mem Inst Oswaldo Cruz 109, 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashauri FM, Manjurano A, Kinung'hi S, Martine J, Lyimo E, Kishamawe C, Ndege C, Ramsan MM, Chan A, Mwalimu CD, Changalucha J, Magesa S, 2017. Indoor residual spraying with micro-encapsulated pirimiphos-methyl (Actellic(R) 300CS) against malaria vectors in the Lake Victoria basin, Tanzania. PLoS One 12, e0176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namountougou M, Simard F, Baldet T, Diabate A, Ouedraogo JB, Martin T, Dabire RK, 2012. Multiple insecticide resistance in Anopheles gambiae s.l. populations from Burkina Faso, West Africa. PLoS One 7, e48412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunbosun-Oduola A, Abba E, Adelaja O, Taiwo-Ande A, Poloma-Yoriyo K, Samson-Awolola T, 2019. Widespread Report of Multiple Insecticide Resistance in Anopheles gambiae s.l. Mosquitoes in Eight Communities in Southern Gombe, North-Eastern Nigeria. J Arthropod Borne Dis 13, 50–61. [PMC free article] [PubMed] [Google Scholar]
- PNLP, Programme National de Lutte contre le Paludisme-PNLP/Mali, Institut National de la Statistique - INSTAT/Mali, INFO-STAT/Mali, Institut National de la Recherche en Santé Publique - INRSP/Mali, and ICF International. 2016. République du Mali Enquête sur les Indicateurs du Paludisme (EIPM) 2015. Bamako, Mali: PNLP, INSTAT, INFO-STAT, INRSP, and ICF Internationl. Available at http://dhsprogram.com/pubs/pdf/MIS24/MIS24.pdf. . [Google Scholar]
- PNLP, 2016. Programme National de Lutte contre le Paludisme (PNLP), Institut National de la Statistique (INSTAT), INFO-STAT, Institut National de la Recherche en Santé Publique (INRSP) et ICF International, 2016. Enquête sur les Indicateurs du Paludisme au Mali (EIPM) 2015. Rockville, Maryland, USA: : INSTAT, INFO-STAT et ICF International. [Google Scholar]
- Reid MC, McKenzie FE, 2016. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar J 15, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AL, Miyazaki RD, Rodrigues JS, Campelo Junior JH, 2013. Parity and influence of abiotic factors on Anopheles in the Manso dam, State of Mato Grosso, Brazil. Rev Soc Bras Med Trop 46, 498–501. [DOI] [PubMed] [Google Scholar]
- Salako AS, Ahogni I, Aikpon R, Sidick A, Dagnon F, Sovi A, Sominahouin AA, Agossa F, Iyikirenga L, Akogbeto MC, 2018. Insecticide resistance status, frequency of L1014F Kdr and G119S Ace-1 mutations, and expression of detoxification enzymes in Anopheles gambiae (s.l.) in two regions of northern Benin in preparation for indoor residual spraying. Parasit Vectors 11, 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLIS, 2014. Evaluation du Système Local d'Information Sanitaire (SLIS) avec les Outils- PRISM. 2014
- Sy O, Niang EHA, Ndiaye M, Konate L, Diallo A, Ba ECC, Tairou F, Diouf E, Cisse B, Gaye O, Faye O, 2018. Entomological impact of indoor residual spraying with pirimiphos-methyl: a pilot study in an area of low malaria transmission in Senegal. Malar J 17, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toure YT, Dolo G, Petrarca V, Traore SF, Bouare M, Dao A, Carnahan J, Taylor CE, 1998a. Mark-release-recapture experiments with Anopheles gambiae s.l. in Banambani Village, Mali, to determine population size and structure. Med Vet Entomol 12, 74–83. [DOI] [PubMed] [Google Scholar]
- Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di Deco MA, Coluzzi M, 1994. Ecological genetic studies in the chromosomal form Mopti of Anopheles gambiae s.str. in Mali, west Africa. Genetica 94, 213–223. [DOI] [PubMed] [Google Scholar]
- Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di Deco MA, Coluzzi M, 1998b. The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parassitologia 40, 477–511. [PubMed] [Google Scholar]
- WHO, 2006. Global Malaria Programme & World Health Organization. Malaria Unit. (2006). Indoor residual spraying : use of indoor residual spraying for scaling up global malaria control and elimination : WHO position statement. World Health Organization. https://apps.who.int/iris/handle/10665/69386 [Google Scholar]
- WHO, 2016a. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes (Second edition), 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; bookorders@who.int). [Google Scholar]
- WHO, 2016b. World Malaria Report 2016. Geneva: World Health Organization; 2016. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Yadouleton AW, Asidi A, Djouaka RF, Braima J, Agossou CD, Akogbeto MC, 2009. Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar J 8, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Minakawa N, Githeko AK, Yan G, 2004. Association between climate variability and malaria epidemics in the East African highlands. Proc Natl Acad Sci U S A 101, 2375–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]