Abstract
Background
Previous studies have reported a relationship between upper limb motor function and activities of daily living. However, their relationship after removing the influence of lower limb motor function has not been clarified.
Objective
This study aimed to investigate the relationship between Fugl-Meyer assessment upper limb and total Functional Independence Measure motor score and between Fugl-Meyer assessment upper limb and each item contained in Functional Independence Measure motor score after eliminating the influence of the motor function of the affected lower limb.
Methods
This retrospective cross-sectional study included 58 subacute stroke patients. To investigate the relationship between the Fugl-Meyer assessment upper limb and total Functional Independence Measure motor score before and after removing the influence of Fugl-Meyer assessment lower limb, Spearman’s rank correlation coefficient and partial correlation analysis were used. Additionally, the relationship between Fugl-Meyer assessment upper limb and each item of Functional Independence Measure motor score after removing the influence was assessed.
Results
Before removing the influence of Fugl-Meyer assessment lower limb, Fugl-Meyer assessment upper limb was strongly correlated with total Functional Independence Measure motor score (r = 0.74, p < 0.001). However, it became weak after removing the influence (r = 0.27, p = 0.04). Regarding each item of Functional Independence Measure motor score, Fugl-Meyer assessment upper limb was correlated with grooming (r = 0.27, p = 0.04), bathing (r = 0.28, p = 0.03), dressing upper body (r = 0.33, p = 0.01), dressing lower body (r = 0.31, p = 0.02), and stair-climbing (r = 0.31, p = 0.02) after removing the influence.
Conclusion
These findings suggest that the relationship between the upper limb motor function and activities of daily living is strongly influenced by lower limb motor function.
Keywords: Upper limb, activities of daily living, stroke, partial correlation analysis, occupational therapy
Introduction
Stroke causes various impairments such as motor paralysis, muscle weakness, sensory disorder, and cognitive dysfunction, and is known as one of the leading causes of long-term disability (Dobkin, 2004; Hankey et al., 2001; Patel et al., 2006; Takeda et al., 2018). Because many of these impairments affect independence in activities of daily living (ADLs) (Mercier et al., 2001; Oros et al., 2016; Perry et al., 1995), they are major targets of rehabilitation therapy for stroke patients.
One of the factors influencing independence in ADLs in patients with stroke is motor dysfunction of the affected upper limb (Chanubol et al., 2012; Filiatrault et al., 1991; Lee et al., 2015; Rabadi & Rabadi, 2006). For example, Filiatrault et al. (1991) showed that the Fugl-Meyer assessment upper limb (FMA-UL) section was positively correlated with the total Barthel Index (BI) score in patients with subacute stroke (r = 0.60, p < 0.01). Chanubol et al. (2012) showed that scores of other evaluation methods for assessing upper limb motor function (Action Research Arm Test, and Box and Block Test) were also positively correlated with extended BI in patients with subacute stroke (r = 0.56, p < 0.001; and r = 0.47, p < 0.001, respectively). In addition, a previous study suggested that the motor function of the affected upper limb has a notable effect on independence in ADLs compared with perceptual and cognitive dysfunction (Mercier et al., 2001).
Although the aforementioned studies reported a relationship between affected upper limb motor function and ADLs, they did not eliminate the possibility of a spurious correlation caused by the level of lower limb motor function, which is associated with the motor function of the affected upper limb and ADLs (Fong et al., 2001; Fujita et al., 2015). In particular, Fong et al. (2001) showed that Fugl-Meyer assessment lower limb (FMA-LL) section was positively correlated with the total Functional Independence Measure motor (FIM-M) score in patients with subacute stroke (r = 0.89, p < 0.01). In addition, Fujita et al. (2015) indicated that the motor function of the affected lower limb was positively correlated with the motor function of the affected upper limb in patients with subacute stroke (r = 0.68, p < 0.01). However, it is not sufficiently clear whether there is a relationship between the motor function of the affected upper limb and ADLs after removing the influence of the level of lower limb motor function.
This study aimed to investigate the relationship (1) between FMA-UL and total FIM-M score after removing the influence of the motor function of the affected lower limb from both variables, and (2) between FMA-UL and each item contained in FIM-M score after eliminating the influence of the motor function of the affected lower limb. If the presence or absence of a relationship between the motor function of the affected upper limb and ADLs can be identified after removing the influence of lower limb motor function, this would have important clinical implications regarding the clarification of factors influencing independence in ADLs and the development of effective interventions intended to improve ADLs.
Methods
Study design
A retrospective cross-sectional study was used to analyse the relationship between FMA-UL and FIM-M score after removing the influence of the motor function of the affected lower limb. This study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology statement (Von et al., 2014).
Participant
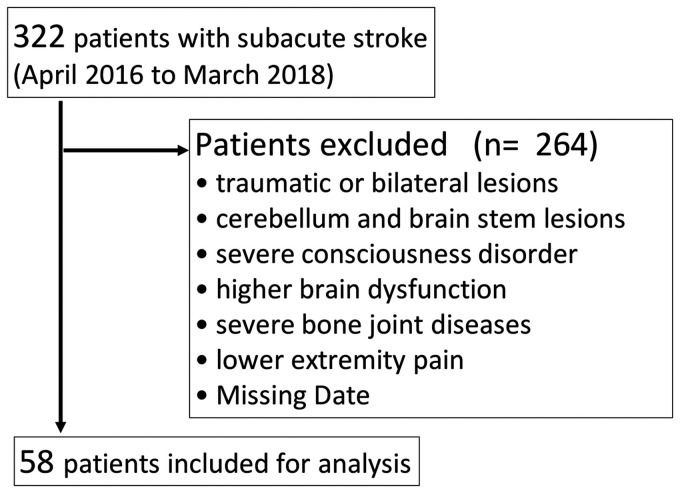
This study recruited a convenience sample of subacute stroke patients between April 2016 and March 2018. We evaluated 322 stroke patients who were hospitalised and discharged from the convalescent rehabilitation ward of our hospital. The study included patients who were admitted to our hospital with a first episode of stroke. Exclusion criteria were traumatic or bilateral lesions, cerebellar and brain stem lesions, severe consciousness disorder, severe bone joint diseases, lower extremity pain, and missing data. All assessments were performed by the primary therapist. All patients were informed of the purpose of the study and gave informed consent to participate in the treatment programme. This study was approved by the human ethics committee of Kawamura hospital (approval number 01-001). This study was performed in accordance with the Declaration of Helsinki.
Measurement
FIM-M score at discharge was used for the assessment of ADLs. FIM-M score is commonly used to quantify functional independence and has high inter-rater reliability (Ottenbacher, Hsu, et al., 1996; Ottenbacher, Mann, et al., 1994). FIM-M score consists of 13 items for daily living, which are graded on a 7-point scale: 1 = total assistance, 2 = maximal assistance, 3 = moderate assistance, 4 = minimal contact assistance, 5 = supervision or set-up, 6 = modified independence, and 7 = complete independence (Linacre et al., 1994). FIM-M score is divided into four parts as follows: Part 1, self-care (including eating, grooming, bathing, dressing upper body, dressing lower body, toileting); Part 2, sphincter control (including bladder management and bowel management); Part 3, mobility (including transfers to bed/chair/wheelchair, transfers to toilet, transfers to tub/shower); and Part 4, locomotion (including walking or wheelchair propulsion, stair-climbing). The total FIM-M score was 91 points.
FMA at discharge was used to assess the motor function of the affected upper and lower limb. FMA is commonly used to quantify upper and lower limb motor function and has high inter-rater reliability (Duncan et al., 1983; Michaelsen et al., 2011; Sanford et al., 1993). In the FMA-UL, a quantitative measure comprising 33 items was used. Since each item is rated on a 3-point ordinal scale (0 = cannot perform, 1 = can perform partially, 2 = can perform fully), the maximum score was 66 points. In the FMA-LL, a quantitative measure comprising 17 items was used. Since each item is rated on a 3-point ordinal scale (0 = cannot perform, 1 = can perform partially, 2 = can perform fully), the maximum score was 34 points.
Statistical analysis
The total FMA-UL and FIM-M score were calculated for each subject. The Shapiro–Wilk normality test was used to test data distribution. With regard to objective 1, Pearson correlation coefficient was used to test the relationship between FMA-UL and FIM-M score if the homoscedasticity and normality of all data could be assumed. However, if they could not be assumed, Spearman’s rank correlation was used to test the relationship. Spearman’s rank correlation coefficient (rs) was interpreted as low (less than 0.25), fair (0.25–0.5), moderate to good (0.5–0.75), and good to excellent (greater than 0.75) (Chanubol et al., 2012).
In addition, in any of these cases (assumption or not), partial correlation analysis (r) was used to test the relationship between FMA-UL and FIM-M score without the influence of the motor function of the affected lower limb represented by FMA-LL. With regard to objective 2, partial correlation analysis (r) was used to test the relationship between FMA-UL and each item of FIM-M score without the influence of the motor function of the affected lower limb. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, Illinois).
Results
In this study, subjects were eligible 58 stroke patients out of 322 (Figure 1). The subject characteristics are shown in Table 1.
Figure 1.
Subject enrolment flowchart.
Table 1.
Characteristics of the subjects (N = 58).
| Variable | Mean | Range |
|---|---|---|
| Age (y) | 78.6 (11.4) | 44–96 |
| MMSE | 16.2 (9.9) | 0–30 |
| FMA-UL | 37.2 (25.3) | 0–66 |
| FMA-LL | 19.3 (12.1) | 0–34 |
| FIM-M score | 52.3 (26) | 13–91 |
| Sex (male/female) | 34/24 | |
| Time since stroke (d) | 156.9 (58) | 18–264 |
| Type of stroke (haemorrhage/infarction) | 20/38 |
FIM-M: Functional Independence Measure motor; FMA-LL: Fugl-Meyer assessment lower limb; FMA-UL: Fugl-Meyer assessment upper limb; MMSE: Mini Mental State Examination.
Values are presented as mean (standard deviation) or as counts.
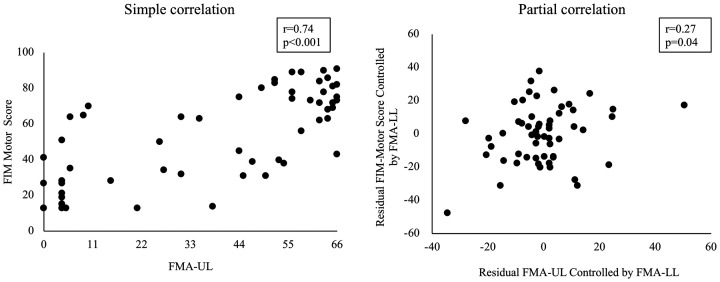
The result of simple and partial correlation analyses of the relationship between FMA-UL and total FIM-M score is shown in Figure 2. In the simple correlation analysis, the relationship between FMA-UL and total FIM-M score became moderate to good (r = 0.74, p < 0.001). However, in the partial correlation analysis, the relationship between FMA-UL and total FIM-M score became fair after the influence of FMA-LL was removed (r = 0.27, p = 0.046).
Figure 2.
The effect of removing the influence of FMA-LL on the relationship between FMA-UL and FIM-M score. The left figure shows the relationship between FMA-UL and FIM-M score before the influence of FMA-LL was removed (simple correlation). The X axis indicates the FMA-UL score. The Y axis indicates the FIM-M score. The right figure shows the relationship between FMA-UL and FIM-M score after the influence of FMA-LL was removed (partial correlation). The X axis indicates residual FMA-UL controlled by FMA-LL. The Y axis indicates residual FIM-M score controlled by FMA-LL. FIM: Functional Independence Measure; FMA-LL: Fugl-Meyer assessment lower limb; FMA-UL: Fugl-Meyer assessment upper limb.
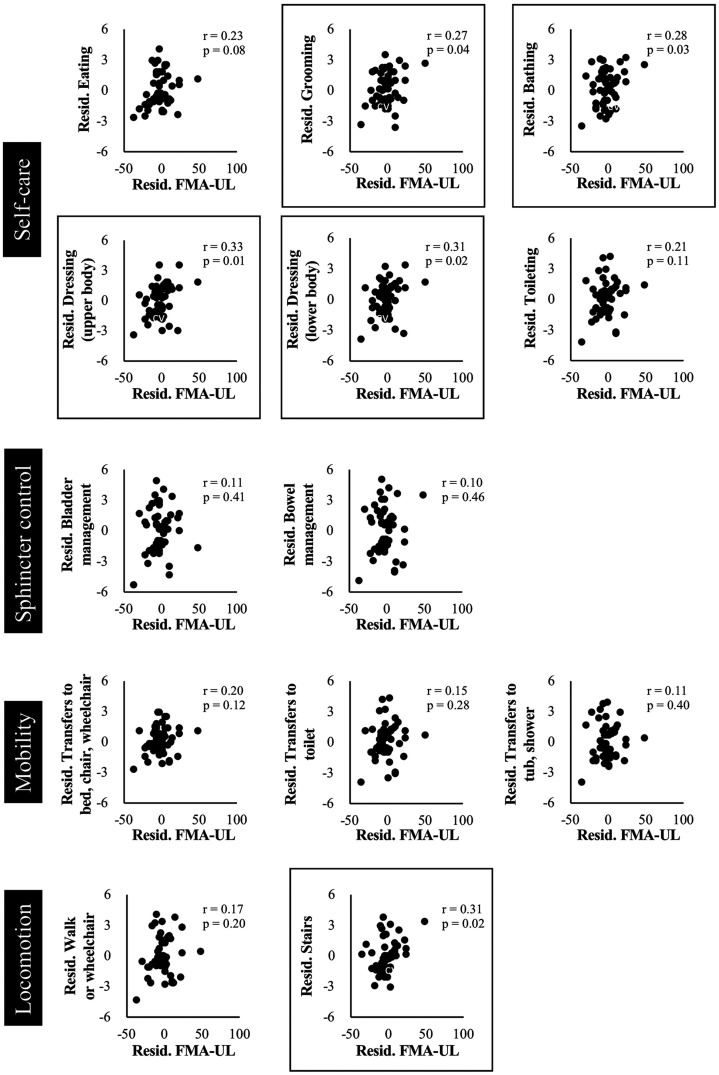
The result of a partial correlation analysis between the FMA-UL and each item contained in FIM-M score is shown in Figure 3. FMA-UL was correlated with grooming (r = 0.27, p = 0.04), bathing (r = 0.28, p = 0.03), dressing upper body (r = 0.33, p = 0.01), dressing lower body (r = 0.31, p = 0.02), and stair-climbing (r = 0.31, p = 0.02) even after the influence of FMA-LL was removed.
Figure 3.
Relationship between FMA-UL and each item of FIM-M score after the influence of FMA-LL was removed. In all graphs, the X axis indicates residual FMA-UL controlled by FMA-LL. The Y axis indicates the residual score of each item in FIM-M score controlled by FMA-LL. Boxed graphs show statistically significant differences (P < 0.05). FMA-UL: Fugl-Meyer assessment upper limb.
Discussion
This study investigated whether the relationship between FMA-UL and total FIM-M score was changed by removing the influence of the motor function of the affected lower limb from both variables. Moreover, this study attempted to clarify the relationship between FMA-UL and every item contained in FIM-M score without the influence of lower limb motor function. The results showed that FMA-UL was strongly correlated with total FIM-M score, but the relationship became very weak after removing the influence of lower limb motor function. In addition, FMA-UL was correlated with grooming, bathing, dressing (upper and lower body), and stair-climbing items of FIM-M score, when the influence of lower limb motor function was excluded.
Considering the relationship between FMA-UL and total FIM-M score, the present result showed almost the same degree of correlation reported in previous studies (Chanubol et al., 2012; Filiatrault et al., 1991). However, these relationships became very weak with partial correlation analysis, which was used to remove the influence of lower limb motor function. The present result suggests that the relationship was changed by the influence of the motor function of the affected lower limb. Similarly, Fujita et al. (2015) reported that the motor function of the affected upper limb was not significantly correlated with the total score of FIM-M when removing the influence of other body parts including the affected lower limb.
With regard to the items in FIM-M score that were correlated with FMA-UL, although it is not fully clear why those relationships were observed, the degree of upper limb movement might have affected the relationships. Specifically, the ‘grooming’ item is used to assess the degree of independence of oral care, hair grooming, washing of hands and face, and shaving or applying makeup (Granger & Hamilton, 1992). ‘Bathing’ is used to assess the degree of independence when washing and drying off the body from the neck down excluding the back (Granger & Hamilton, 1992). Further, ‘dressing’ (upper and lower body) is used to assess the degree of independence when putting on and off upper and lower wears (e.g. putting arm through a sleeve using hands and pulling up pants using hands, etc.) (Granger & Hamilton, 1992). Among the items in FIM-M, these items frequently require upper limb movement. For dressing alone, Fujita et al. (2015) suggest that most patients require the use of both hands to pass their arms through sleeves, close buttons and fasteners, and raise or lower undergarments. Notably, there was a correlation between FMA-UL and ‘stair-climbing’, which is used to assess the degree of independence when going up and down a stairs of 12–14 steps (Granger & Hamilton, 1992). Although many studies have focused on lower limb motor function in stair-climbing (Flansbjer et al., 2006; Novak & Brouwer, 2012; Vallabhajosula et al., 2015), it appears that not only lower limb motor function but also upper limb motor function is important for stair-climbing. Previous studies showed that the stance time of the affected side and the velocity of the centre of pressure during stair-climbing increased with handrail use compared to not using the handrail (Novak & Brouwer, 2013; Reid et al., 2011).
The present study has some limitations. The number of participants was limited. In addition, the study was a single-centre retrospective study. These factors could decrease the generalisability of the present results. Thus, studies should be conducted with more participants in a prospective multicentre study to verify our findings.
Conclusion
This study clarified the relationship between the motor function of the affected upper limb and ADLs after removing the influence of lower limb motor function. This finding has important clinical implications on the factors influencing independence in ADLs and the development of effective interventions intended to improve ADLs. To improve the self-care items in functional measures, occupational therapists should focus on both upper limb and low limb motor functions together in the ADL training for patients with subacute stroke.
Acknowledgements
We appreciate the staff of the Department of Rehabilitation at Kawamura Hospital for their helpful technical assistance.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Soichiro Koyama https://orcid.org/0000-0001-5837-8477
Shigeo Tanabe https://orcid.org/0000-0003-1993-5896
References
- Chanubol R., Wongphaet P., Ot N. C., Chira-Adisai W., Kuptniratsaikul P., Jitpraphai C. (2012). Correlation between the action research arm test and the box and block test of upper extremity function in stroke patients. Journal of the Medical Association of Thailand, 95(4), 590–597. 10.1016/S1474-4422(04)00851-8 [DOI] [PubMed] [Google Scholar]
- Dobkin B. H. (2004). Strategies for stroke rehabilitation. The Lancet Neurology, 3(9), 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan P. W., Propst M., Nelson S. G. (1983). Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Physical Therapy, 63(10), 1606–1610. [DOI] [PubMed] [Google Scholar]
- Filiatrault J., Arsenault A. B., Dutil E., Bourbonnais D. (1991). Motor function and activities of daily living assessments: A study of three tests for persons with hemiplegia. The American Journal of Occupational Therapy, 45(9), 806–810. [DOI] [PubMed] [Google Scholar]
- Flansbjer U. B., Downham D., Lexell J. (2006). Knee muscle strength, gait performance, and perceived participation after stroke. Archives of Physical Medicine and Rehabilitation, 87(7), 974–980. 10.1016/j.apmr.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Fong K. N., Chan C. C., Au D. K. (2001). Relationship of motor and cognitive abilities to functional performance in stroke rehabilitation. Brain Injury, 15(5), 443–453. 10.1080/02699050118772 [DOI] [PubMed] [Google Scholar]
- Fujita T., Sato A., Togashi Y., Kasahara R., Ohashi T., Tsuchiya K., Otsuki K. (2015). Identification of the affected lower limb and unaffected side motor functions as determinants of activities of daily living performance in stroke patients using partial correlation analysis. Journal of Physical Therapy Science, 27(7), 2217–2220. 10.1589/jpts.27.2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger C. V., Hamilton B. B. (1992). UDS report. The Uniform Data System for Medical Rehabilitation Report of First Admissions for 1990. American Journal of Physical Medicine & Rehabilitation, 71(2), 108–113. [PubMed] [Google Scholar]
- Hankey G. J., Jamrozik K., Broadhurst R. J., Forbes S., Anderson C. S. (2001). Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989–1990. Stroke, 33(4), 1034–1040. 10.1161/01.STR.0000012515.66889.24 [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Yi E. S., Choi W. H., Lee B. M., Cho S. B., Kim J. Y. (2015). A study on the effect of self bedside exercise program on resilience and activities of daily living for patients with hemiplegia. Journal of Exercise Rehabilitation, 11(1), 30–35. 10.12965/jer.140159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linacre J. M., Heinemann A. W., Wright B. D., Granger C. V., Hamilton B. B. (1994). The structure and stability of the Functional Independence Measure. Archives of Physical Medicine and Rehabilitation, 75(2), 127–132. [PubMed] [Google Scholar]
- Mercier L., Audet T., Hébert R., Rochette A., Dubois M. F. (2001). Impact of motor, cognitive, and perceptual disorders on ability to perform activities of daily living after stroke. Stroke, 32(11), 2602–2608. 10.1161/hs1101.098154 [DOI] [PubMed] [Google Scholar]
- Michaelsen S. M., Rocha A. S., Knabben R. J., Rodrigues L. P., Fernandes C. G. (2011). Translation, adaptation and inter-rater reliability of the administration manual for the Fugl-Meyer assessment. Revista Brasileira de Fisioterapia, 15(1), 80–88. 10.1590/S1413-35552011000100013 [DOI] [PubMed] [Google Scholar]
- Novak A. C., Brouwer B. (2012). Strength and aerobic requirements during stair ambulation in persons with chronic stroke and healthy adults. Archives of Physical Medicine and Rehabilitation, 93(4), 683–689. 10.1016/j.apmr.2011.10.009 [DOI] [PubMed] [Google Scholar]
- Novak A. C., Brouwer B. (2013). Kinematic and kinetic evaluation of the stance phase of stair ambulation in persons with stroke and healthy adults: A pilot study. Journal of Applied Biomechanics, 29(4), 443–452. 10.1123/jab.29.4.443 [DOI] [PubMed] [Google Scholar]
- Oros R. I., Popescu C. A., Iova C. A., Mihancea P., Iova S. O. (2016). The impact of cognitive impairment after stroke on activities of daily living. Human & Veterinary Medicine, 8(1), 41–44. [Google Scholar]
- Ottenbacher K. J., Hsu Y., Granger C. V., Fiedler R. C. (1996). The reliability of the functional independence measure: A quantitative review. Archives of Physical Medicine and Rehabilitation, 77(12), 1226–1232. [DOI] [PubMed] [Google Scholar]
- Ottenbacher K. J., Mann W. C., Granger C. V., Tomita M., Hurren D., Charvat B. (1994). Inter-rater agreement and stability of functional assessment in the community-based elderly. Archives of Physical Medicine and Rehabilitation, 75(12), 1297–1301. [PubMed] [Google Scholar]
- Patel M. D., Tilling K., Lawrence E., Rudd A. G., Wolfe C. D., McKevitt C. (2006). Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing, 35(3), 273–279. 10.1093/ageing/afj074 [DOI] [PubMed] [Google Scholar]
- Perry J., Garrett M., Gronley J. K., Mulroy S. J. (1995). Classification of walking handicap in the stroke population. Stroke, 26(6), 982–989. [DOI] [PubMed] [Google Scholar]
- Rabadi M. H., Rabadi F. M. (2006). Comparison of the action research arm test and the Fugl-Meyer assessment as measures of upper-extremity motor weakness after stroke. Archives of Physical Medicine and Rehabilitation, 87(7), 962–966. 10.1016/j.apmr.2006.02.036 [DOI] [PubMed] [Google Scholar]
- Reid S. M., Novak A. C., Brouwer B., Costigan P. A. (2011). Relationship between stair ambulation with and without a handrail and centre of pressure velocities during stair ascent and descent. Gait & Posture, 34(4), 529–532. 10.1016/j.gaitpost.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Sanford J., Moreland J., Swanson L. R., Stratford P. W., Gowland C. (1993). Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Physical Therapy, 73(7), 447–454. [DOI] [PubMed] [Google Scholar]
- Takeda K., Tanabe S., Koyama S., Shomoto K., Naoi Y., Sakurai H., Kanada Y. (2018). Relationship between the rate of force development in knee extensor muscles and gait speed in patients with chronic stroke: A cross-sectional study. NeuroRehabilitation, 43(4), 425–430. 10.3233/NRE-182455 [DOI] [PubMed] [Google Scholar]
- Vallabhajosula S., Tan C. W., Mukherjee M., Davidson A. J., Stergiou N. (2015). Biomechanical analyses of stair-climbing while dual-tasking. Journal of Biomechanics, 48(6), 921–929. 10.1016/j.jbiomech.2015.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von E. E., Altman D. G., Egger M., Pocock S. J., Gøtzsche P. C., Vandenbroucke J. P. (2014). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. International Journal of Surgery, 12(12), 1495–1499. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]