Abstract
Background:
The current study was done to evaluate the validity and reliability of the Vitalograph COPD-6 portable device for detecting chronic obstructive pulmonary disease (COPD) in high-risk individuals in Iran.
Materials and Methods:
This research was a cross-sectional descriptive study. Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and FEV1/FVC using standard spirometer and FEV1, FEV6, and FEV1/FEV6 with COPD- 6 device were measured and recorded. Descriptive analysis was done.
Results:
19 of 122 patients (15.6%) were diagnosed with COPD. The COPD-6 had an acceptable performance for detecting COPD as assessed by the area under the receiver operating characteristic (ROC) curve (0.72 ; 95% CI: 0.42–0.86), with an average sensitivity of 84% and specificity of 98%, positive predictive value of 89%, and negative predictive value of 97%. The positive likelihood ratio resulted was 42 and the negative likelihood ratio was 0.16.
Conclusion:
COPD-6 is a validate and reliable device for detecting COPD in non-specialized health care settings and the best cut-off point for FEV1/FEV6 ratio is 0.72.
Keywords: Chronic Obstructive Pulmonary Disease, COPD, COPD-6, FEV1/FEV6 Ratio, Portal Spirometer
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a common disease which is both preventable and treatable (1). By 2020, it will be the third leading cause of mortality worldwide (2–4). Prevalence of COPD in general population is 4% - 10% (1,4). Its prevalence in Iran is 6–9% (5,6). Unfortunately despite relatively high prevalence of COPD in the world, about 80% of cases remain underdiagnosed until advanced stages (1,7,8). Early diagnosis of COPD and interventions which motivate smoking cessation can preserve lung function, reduce burden of COPD and improve quality of life (1,3,9).
Although spirometry as a safe, reliable and noninvasive technique, is the essential tool for screening, diagnosis and fallow-up of COPD, various studies reported several problems in using spirometry such as lack of spirometer as a routine device in primary care centers, underuse and poor quality of performing spirometry, especially in non-specialized settings (4,7,9,10). Obtaining forced vital capacity (FVC) is difficult and affects the quality and accuracy of spirometry especially in primary care settings with non-expert staff in performing spirometry, so forced expiratory volume in 6 seconds (FEV6) has been accepted as an appropriate alternative to FVC. Using FEV6 simplifies spirometry and improves its accuracy in detecting air way obstruction especially in primary care and non-specialized health-care settings (4,9,11).
Vitalograph COPD-6 device is a novel portable spirometer which has already been validated in detection of COPD in developed countries, but not in developing countries (4,9,12). So we designed this study for the first time in Iran, to assess validity and reliability of COPD-6 in screening for COPD among high risk individuals in Isfahan, Iran. On the other hand, as previous studies on COPD-6 have been conducted in European countries (4,9,12), it seems necessary to check validity and reliability of this device in other parts of the world including Iran.
MATERIALS AND METHODS
Current study was a cross- sectional descriptive study on 122 patients with high risk for COPD who referred to pulmonary disease clinic of Al-Zahra teaching hospital, Isfahan, Iran. Inclusion criteria were: age above 40 years, smokers with more than 10 pack-years, symptoms suggestive of COPD (cough, sputum, and dyspnea) and willingness to participate. Exclusion criteria were: Unwillingness to participate in the study, having any of the absolute contraindications for spirometry (such as respiratory infection in previous two weeks, using bronchodilators in past 24 hours, surgery in thorax or abdomen and hemoptysis) and patients with previously diagnosed respiratory diseases. The study was approved by the Ethics Committee of Isfahan University of Medical Sciences and participants were provided with informed written consent. A questionnaire including sociodemographic data (name, age, gender and smoking), pulmonary symptoms, previous diseases and medications was filled out for each patient by a trained health-care staff.
COPD-6 is a portable device which is easy to use and can measure FEV1, FEV6 and the FEV1/FEV6 ratio. It uses the reference values and shows for each parameter the percentage of the value obtained versus its theoretical value. It has a comfortable design that allows it to be easily held by the patient. At the beginning the user must enter some patient data including age, sex and size. The maneuver that must be performed is similar to that of a spirometry; the patient must take a deep breath, then insert the mouthpiece into their mouth and then exhale vigorously and continuously for six seconds. It can detect errors such as the premature ending of the maneuver or coughing.
At first step, each participant underwent both conventional spirometry and spirometry via COPD-6 by a trained experienced health-care personnel in Al-Zahra hospital, using the portable COPD-6 device ((model 4000, Vitalograph Ltd., Ennis, Co.Clare, Ireland) and spirometer (Jaeger Ltd. Hochberg, Germany). Measurements were repeated until obtaining at least three reliable amounts and best values were recorded for each patient. Then salbutamol spray 400 μg (4 puffs) was administrated for each participant and both conventional spirometry and spirometry via COPD-6, were repeated after 15 minutes until obtaining three reliable amounts and best values were recorded. The same technician, who performed spirometry using COPD-6, performed conventional spirometry for each patient according to ATS/ERS guidelines (13). FEV1 (S-FEV1), FVC and FEV1/FVC, with standard spirometer and FEV1 (co- FEV1), FEV6, FEV1/FEV6 with COPD- 6 device were measured and recorded. Patients with post bronchodilator FEV1/FVC ratio less than 0.7 were considered as COPD.
Data were analyzed using the Statistical Package for Social Sciences version 22 (SPSS, Illinois, USA). Descriptive analysis was done. The quantitative variables are expressed as means and standard deviations and the qualitative variables were expressed by their absolute value and their percentage. We used the mean of the difference and its 95% CI to express the differences between the parameters studied. The comparison of the quantitative variables was carried out by applying the Student’s t test for paired samples, and a p-value equal or less than 0.05 was considered statistically significant. The relation between the values of FEV1 and FVC in absolute value and the FEV1/ FVC ratio measured by both devices were analyzed by calculating the Pearson correlation coefficient (r) and were plotted using correlation plots.
Sensitivity, specificity, positive and negative predictive values (PPV) and positive and negative likelihood ratios (PLR/NLR) were calculated for the different cut-off points of the FEV1/FEV6 ratio. P value<0.05 considered statistically significant. The area under the receiver operating characteristic (ROC) curve, was measured for FEV1/FEV6 ratio, calculated by COPD-6 device. FEV1/FVC ratio<0.7 which was obtained via spirometry after the bronchodilator test, was considered as the gold standard for COPD diagnosis. Chi-square test was used for comparison of the areas under the ROC curves.
RESULTS
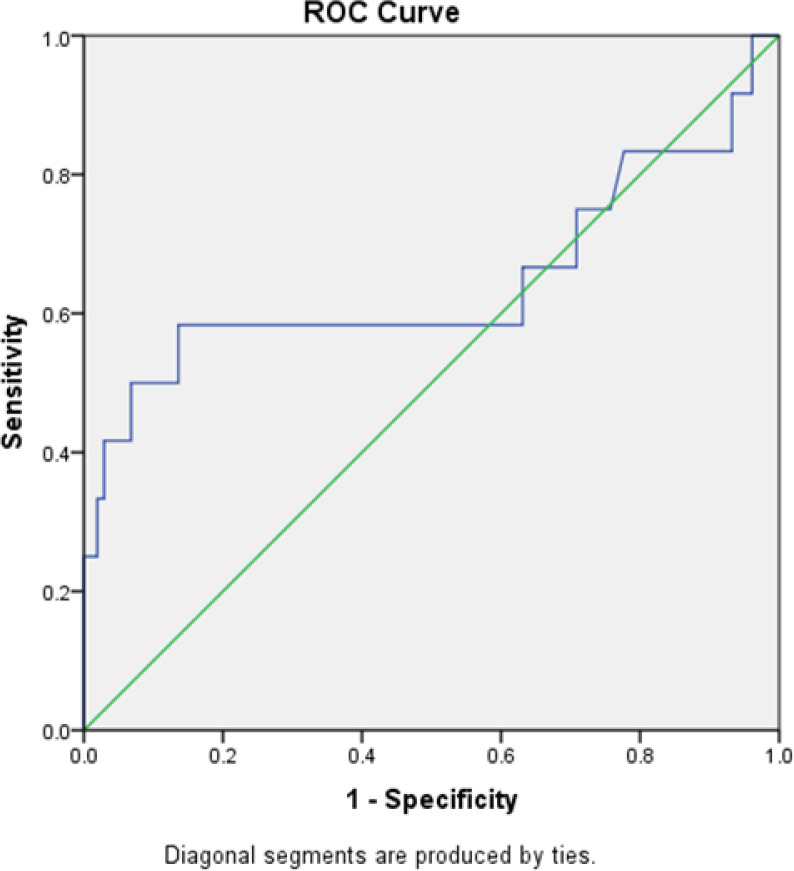
A total of 122 participants were included in this study. The mean age was 53.2± 9.0 years and the mean number of smoking was 32.9± 19.8 pack- year. Nineteen patients (15.6%) were diagnosed with COPD. Most of the patients with COPD diagnosis (N =10, 66.7%), were in stage II of Global Initiative for Chronic Obstructive Lung Disease (GOLD). The area under the receiver operating characteristic (ROC) curve of calculated FEV1/FEV6 ratio using COPD-6 for detecting COPD, is shown in Figure 1. FEV1/FVC ratio<0.7 considered as reference pattern. The area under the curve (AUC) was 0.72(95% CI: 0.42–0.86)
Figure 1.
ROC curves of the FEV1/FEV6 ratio measured using the COPD-6 device to screen for chronic obstructive pulmonary disease.
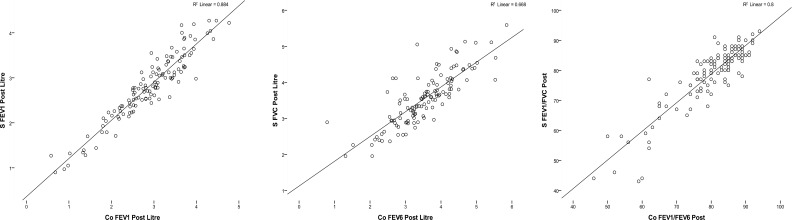
Table 1 demonstrates the comparison between measured parameters using conventional spirometer and COPD-6. Although there was no statistically significant difference between FEV6 and FVC, the value of FEV6 was less than FVC. Mean of FEV1/FEV6 was higher than FEV1/FVC, but There is no significant difference between FEV1/FVC in conventional spirometer and FEV1/FEV6 ratios in COPD-6 (P value >0.001 in both pre and post bronchodilator test). Value of FEV1 in COPD-6 is as same as conventional spirometer too (P value <0.001 in pre and post bronchodilator test). The correlation between the parameters of the two devices was S-FEV1vs. CO- FEV1: r = 0.864, r=0.940 (p <0.001) (in pre and post bronchodilator test respectively), FVC vs. FEV6: r = 0.791, r= 0.817 (p <0.001) (in pre and post bronchodilator test respectively), FEV1/FVC vs. FEV1/FEV6: r = 0.846, r= 0.895 (p <0.001) (in pre and post bronchodilator test respectively).
Table 1.
Mean values and correlation between the parameters measured by the COPD -6 and the conventional spirometer
| Conventional Spirometer | COPD-6 | 95% Confidence Interval of the Difference | correlation | P- value | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Pre bronchodilator | FEV1 (lit) | 2.7±0.77 | 2.8±0.87 | −0.16 | −0.01 | 0.864 | <0.001 |
| FVC vs FEV6 (lit) | 3.46±0.8 | 3.40±0.91 | −0.07 | 0.12 | 0.791 | <0.001 | |
| FEV1/FVC % vs FEV1/FEV6 % | 76.5±10.2 | 78.3±13.8 | −4.01 | −2.03 | 0.846 | <0.001 | |
| Post bronchodilator | FEV1 (lit) | 2.8±0.74 | 2.8±0.82 | −0.13 | −0.02 | 0.940 | <0.001 |
| FVC vs FEV6 (lit) | 3.59±0.72 | 3.48±0.86 | −0.07 | 0.10 | 0.817 | <0.001 | |
| FEV1/FVC % vs FEV1/FEV6 % | 78.3 ± 10.3 | 79.5 ± 9.7 | −2.06 | −0.41 | 0.895 | <0.001 | |
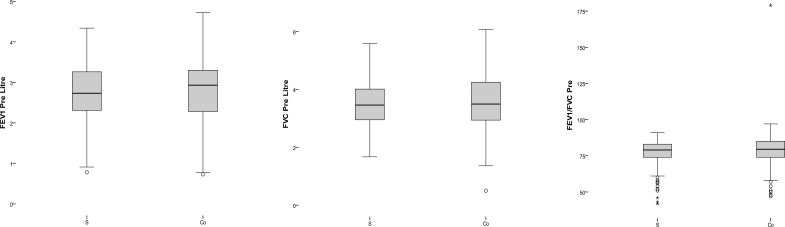
A contingency table (Table 2) presents patients diagnosed with airway obstruction with conventional spirometry and COPD- 6 in two level of FEV1/FEV6 (<70 as standard and <0.72 as resulted from ROC curve). Table 3 shows the values for sensitivity, specificity, PPV, NPV, PLR and NLR for determining obstructions (using the value FEV1/FVC < 0.7 obtained by spirometry as the gold standard) for the two different cutoff points of the FEV1/FEV6 quotient measured by COPD-6. There is not a significant difference between results of two cutoff points. Figure 2 presents box plot graphs, for values of pre bronchodilator test and Figure 3 shows the correlation graphs of the values of post bronchodilator test in which an excellent correlation can be observed for all parameters.
Table 2.
Contingency table of the number of patients diagnosed with obstruction by conventional spirometry and by the COPD-6 in different two level of FEV1/FEV6
| Obstruction in COPD 6 (FEV1/FEV6) | Total | |||||
|---|---|---|---|---|---|---|
| <0.7 | <0.72 | |||||
| Yes | No | Yes | No | |||
| Obstruction in spirometer FEV1/FEVC | Yes | 16 | 3 | 16 | 3 | 19 (15.6%) |
| No | 2 | 101 | 3 | 100 | 103 (84.4%) | |
| Total | 18 | 104 | 19 | 103 | 122 | |
Table 3.
Values for sensitivity, specificity, predictive values and likelihood ratio for detecting obstructions (FEV1/FVC < 0.7 by spirometry) for two cutoff points of the FEV1/FEV6 ratio measured with the Vitalograph COPD-6
| FEV1/FEV6 | Sensitivity% | Specificity % | PPV% | NPV% | + LR | −LR |
|---|---|---|---|---|---|---|
| <0.7 | 88% | 98 | 89 | 97 | 42 | 0.16 |
| <0.72 | 84 | 97 | 84 | 97 | 28 | 0.16 |
Figure 2.
box plot of values in pre bronchodilator test
Figure 3.
Correlation graphs of values in post bronchodilator test
Of the 19 patients with COPD pattern determined with conventional spirometer, 3 (15.8%) would not have been detected with the COPD-6 in both level of FEV1/FEV6. All of them were heavy smoker, had reversibility pattern in both test, and pre bronchodilator FEV1/FEV6 was lower than 0.7. For the detection of airflow obstruction, the COPD-6 (with cutoff point < 0.72) had a sensitivity of (88%), specificity of (98%), PPV of (89%) and NPV of (97%). The positive likelihood ratio resulted in 42 and the negative likelihood ratio was 0.16.
DISCUSSION
Current study is the first to assess validity and reliability of COPD-6 in screening for COPD in Middle East. We showed that COPD-6 device, is a valid and sensitive tool for COPD screening in high risk patients. According to the current study, the best cut-off point for FEV1/FEV6 ratio for COPD screening is 0.72.
For proper screening and early diagnosis of COPD, it is necessary to perform spirometry in primary care centers as a routine testb (1). Conventional spirometry which is the gold standard for diagnosis of COPD, is rarely performed in primary care centers due to lack of time, spirometers and trained experienced health staff which is more important in developing countries because of economic issues (4,9,11). Obtaining proper FVC in spirometry maneuver is difficult and leads to very different results according to experience and training of health-care staff. Besides, sometimes the maneuver which is performed to obtain FVC in spirometry, causes dyspnea, dizziness and syncope. The substitution of FVC with FEV6 which is obtained more easily, can improve performing spirometry especially in primary care settings (9,14,15). Wang et al. considered FEV1/FEV6 <0.72 as a valid alternative to FEV1/FVC < 0.70 and could be used as a fixed cutoff point for detection of COPD in primary care settings (2). Therefore using portable, simple and easy-to –use spirometers such as Piko-6, COPD-6 and Air-Smart Spirometer combined with obtaining FEV6 instead of FCV, could be useful in screening and early diagnosis of COPD in non-specialized health care settings (4,7,9).
Represas et al. assessed validity and safety of COPD-6 device for detecting obstructive airway diseases for the first time (9). In their study the prevalence of COPD, detected using COPD-6, was about 18% They reported for cut-off point of FEV1/FEV6 ratio<0.7,the sensitivity, specificity, PPV and NPV were about 58%,100%,100% and 73% and for a cut-off point<0.8 were 96%,76%,78% and 96%.They concluded that the cut-off point for FEV1/FEV6 ratio between 0.75–0.76 has the most sensitivity and specificity in detection of COPD and increasing cut-off point up to 0.79–0.80 is more sensitive but less specific which is helpful in screening for obstructive airway diseases. Also in 2016, Represas et al. published the results of a prospective multi-cohort study which was the first study to validate COPD-6 device in screening for COPD in different health-care settings. The overall prevalence of COPD was reported 31.5% and majority of the patients (about 84.2%) were in stage I or II of Global Initiative for Chronic Obstructive Lung Disease (GOLD) (4). AUC of ROC curve, calculated for FEV1/FEV6 ratio in overall sample of three cohorts, was estimated 0.8 (95% confidence interval, 0.75–0.84). They remarked, as FEV1/FEV6 ratio is used for screening of COPD, higher sensitivity is needed so increasing the cut-off point to 0.8 for achieving higher sensitivity and negative predictive value, is desirable although it decreases the specificity (4). Our results are close to the results of these two previous studies by Represas et al. in 2010 and 2016 which both reported COPD-6 as a valid and reliable device for detection and screening of COPD6 with cut-off point =0.72 for FEV1/FEV6 ratio (4,9). In a study in 2012 Vial et al. reported that FEV1/FEV6 ratio<0.8 is a valid cut-off point for COPD screening in emergency department via Neo 6 device which is a portable spirometer (16).Their results are in concordance with our results and Represas et al. studies, although devices are different (4,9,16). Miravitlles et al. concluded that the best cut-off point of FEV1/FEV6 ratio for screening COPD is 0.75 (17) which is close to the cut-off point we reported for screening of COPD (0.72).
Prevalence of COPD in our study is 15.6% which is close to Represas et al. 2010 study (18%) but is about half of COPD prevalence in Represas et al. multi-cohort study (2016) which is reported 31.5%. This difference between current study and multi-cohort study may be because of difference in sample sizes and studied patients (120 versus 362) (4,9). Majority of our patients with COPD (66.7%) were in GOLD Stage II. Our study as well as other above mentioned studies fit within the category of proposed COPD researches by the official declaration of the ATS/ERS which recommend finding new COPD screening strategies using portable spirometers (18).
CONCLUSION
Our study showed that COPD-6 is a validated and reliable device for detecting COPD and the best cut-off point for FEV1/FEV6 ratio is 0.72 (95% CI, 0.42–0.86).Higher results than this range could be reliable for ruling out the COPD and lower results confirm the indication for conventional spirometry.
Limitation
In the current study an experienced trained personnel performed spirometry via COPD-6 that could limit the assessment of device validity in primary care settings where non-experienced staff will work with COPD-6. As the training and experience of the practitioner who performs spirometry with COPD-6 affects the results, we suggest health-care staff with brief training and less experience, cooperate in COPD-6 assessments for screening COPD in primary-care setting, in future studies.
Ethics Committee Approval
This research is approved by Ethics committee of Isfahan University of Medical Sciences. Approval number: IR.mui.REC.1396.3.795, Date: 2017/11/07
Informed Consent
A written consent has obtained from the patient to publish this article.
Acknowledgements
We gratefully acknowledge the spirometry team in Al-Zahra hospital.
Footnotes
Conflict of Interest
All authors declare no conflicts of interest
Financial Disclosure
This research is financially supported by Isfahan University of Medical Sciences. Fund code: 396795
REFERENCES
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195(5):557–582. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Gong W, Tian Y, Zhou J. FEV1/FEV6 in Primary Care Is a Reliable and Easy Method for the Diagnosis of COPD. Respir Care 2016;61(3):349–53. [DOI] [PubMed] [Google Scholar]
- 3.Fu SN, Yu WC, Wong CK, Lam MC. Prevalence of undiagnosed airflow obstruction among people with a history of smoking in a primary care setting. Int J Chron Obstruct Pulmon Dis 2016;11:2391–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Represas-Represas C, Fernández-Villar A, Ruano-Raviña A, Priegue-Carrera A, Botana-Rial M, study group of “Validity of COPD-6 in non-specialized healthcare settings” . Screening for Chronic Obstructive Pulmonary Disease: Validity and Reliability of a Portable Device in Non-Specialized Healthcare Settings. PLoS One 2016;11(1):e0145571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amra B, Golshan M, Fietze I, Penzel T, Welte T. Correlation between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome in a general population in Iran. Journal of Research in Medical Sciences 2011;16(7):885. [PMC free article] [PubMed] [Google Scholar]
- 6.Sharifi H, Masjedi MR, Emami H, Ghanei M, Eslaminejad A, Radmand G, et al. Burden of obstructive lung disease study in Tehran: Prevalence and risk factors of chronic obstructive pulmonary disease. Lung India 2015;32(6):572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos Hernández C, Núñez Fernández M, Pallares Sanmartín A, Mouronte Roibas C, Cerdeira Domínguez L, Botana Rial MI, et al. Validation of the portable Air-Smart Spirometer. PLoS One 2018;13(2):e0192789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker AB, Abrams EM. Asthma guidelines: the Global Initiative for Asthma in relation to national guidelines. Curr Opin Allergy Clin Immunol 2017;17(2):99–103. [DOI] [PubMed] [Google Scholar]
- 9.Represas CR, Rial MB, Fernández VL, Silva AI, del Campo Pérez V, Fernández-Villar A. Assessment of the portable COPD-6 device for detecting obstructive airway diseases. Archivos de Bronconeumología ((English Edition)) 2010;46(8):426–32. [DOI] [PubMed] [Google Scholar]
- 10.Arne M, Lisspers K, Ställberg B, Boman G, Hedenström H, Janson C, et al. How often is diagnosis of COPD confirmed with spirometry? Respir Med 2010;104(4):550–6. [DOI] [PubMed] [Google Scholar]
- 11.Jing JY, Huang TC, Cui W, Xu F, Shen HH. Should FEV1/FEV6 replace FEV1/FVC ratio to detect airway obstruction? A metaanalysis. Chest 2009;135(4):991–998. [DOI] [PubMed] [Google Scholar]
- 12.Vitalograph . Date last update: December 19 2011; Available from: https://vitalograph.ie/products/monitors-screeners/copd/copd-6
- 13.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017;50(2):1602426. [DOI] [PubMed] [Google Scholar]
- 14.Toda R, Hoshino T, Kawayama T, Imaoka H, Sakazaki Y, Tsuda T, et al. Validation of “lung age” measured by spirometry and handy electronic FEV1/FEV6 meter in pulmonary diseases. Intern Med 2009;48(7):513–21. [DOI] [PubMed] [Google Scholar]
- 15.Duong-Quy S, Hua-Huy T, Mai-Huu-Thanh B, Doan-Thi-Quynh N, Le-Quang K, Nguyen-Van H, et al. Détection précoce de la bronchopneumopathie chronique obstructive post-tabagique au Viet Nam [Early detection of smoking related chronic obstructive pulmonary disease in Vietnam]. Rev Mal Respir 2009;26(3):267–74. [DOI] [PubMed] [Google Scholar]
- 16.Vial M, Apelt P, Cavalli P, Guerin T, Vercherin P, Vergnon JM. Faisabilité d’un dépistage de la BPCO aux urgences [Possibility to screen COPD in emergency department]. Rev Pneumol Clin 2012;68(1):10–6. [DOI] [PubMed] [Google Scholar]
- 17.Miravitlles M, Llor C, Calvo E, Diaz S, Díaz-Cuervo H, Gonzalez-Rojas N. Validación de la versión traducida del Chronic Obstructive Pulmonary Disease-Population Screener (COPD-PS). Su utilidad y la del FEV1/FEV6 para el diagnóstico de enfermedad pulmonar obstructiva crónica [Validation of the Spanish version of the Chronic Obstructive Pulmonary Disease-Population Screener (COPD-PS). Its usefulness and that of FEV1/FEV6 for the diagnosis of COPD]. Med Clin (Barc) 2012;139(12):522–30. [DOI] [PubMed] [Google Scholar]
- 18.Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agustí A, Criner GJ, et al. An Official American Thoracic Society/European Respiratory Society Statement: Research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;191(7):e4–e27. [DOI] [PubMed] [Google Scholar]