Abstract
Background:
Caffeic acid phenethyl ester (CAPE) is one of the major components of honeybee propolis and its structure is similar to flavonoids. CAPE has been shown to possess anti-inflammatory, immunomodulatory, and antioxidant properties. Despite a wide range of biological activities of CAPE, detailed biochemical mechanisms of its action are poorly described. The aim of the present study was to investigate the in vitro effect of CAPE on isolated rat trachea.
Materials and Methods:
A 20 mm long portion of rat tracheal spiral was submerged in 20 ml Krebs solution in an isolated organ bath at 37°C. Changes in tracheal contractility in response to the application of agonist agents were measured using an isometric transducer connected to a Harvard polygraph.
Results:
Acetylcholine (ACH), histamine (HIS), and CaCl2 caused the trachea to contract in a dose-dependent manner. Incubation of trachea with 10-7 M and 10-6M of CAPE induced a significant reduction in contraction induced by ACH and HIS. The degree of drug-induced tracheal contraction or relaxation was dose-dependent.
Conclusion:
The CAPE potential to relax the trachea may antagonize cholinergic and histaminergic receptors of the trachea. The findings provide new insight into the effectiveness of CAPE in the control of asthma and the possible use of propolis for its treatment. The results highlight the anti-muscarinic, anti-histaminic, anti-inflammatory, and relaxant activities of CAPE and critically show its potential therapeutic effects.
Keywords: Caffeic Acid Phenethyl Ester (CAPE), Rat, Trachea, Asthma, Pharmacological response
INTRODUCTION
Asthma is a common chronic inflammatory disease of the airways. It is characterized by airway obstruction, hyperresponsiveness, and remodeling (1). The rate and prevalence of asthma episodes have climbed dramatically among developing countries. Despite the increased use of medications that suppress airway inflammation and repress contraction of smooth muscle that encircles the airways, there is only a small decrease in total asthma exacerbation (2). Corticoids are the currently used medications as anti-inflammatory agents in combination with β2-adrenergic agonists as bronchodilators (3). These drugs cause serious side effects even in those with mild to moderate disease. Therefore, there has been an increase in demand for finding new medicines in asthma therapy. Natural products are novel products for treating asthma and offer a remarkable pharmacological perspective for drug development with regard to their widespread acceptability and broad-spectrum activities.
Propolis is a honey-bee hive product, which has long been used due to its several beneficial medical properties (4). Caffeic acid phenethyl ester (CAPE) found in propolis (bee glue) is a derivative of a honeybee product (5). CAPE has anti-inflammatory, immunomodulatory, and antioxidant properties (6). CAPE may pass easier through the cell membrane and has greater pharmacologic effects than caffeic acid (7). The activation of nuclear factor kappa B (NF-KB) is induced by various agents producing reactive oxygen species (ROS) in human histiocytic cells and coronary artery endothelial cells. This induction was inhibited by CAPE (8, 9). The inhibition of NF-KB is crucial for the development of novel and safe anti-inflammatory agents (10). The current treatment of chronic inflammatory diseases, such as asthma is based on pharmacological interventions. Due to numerous pharmacological effects, such as anti-inflammatory, immunomodulatory, and antioxidant properties, the aim of the present study was to evaluate the effect of CAPE on pharmacological responses of isolated rat trachea in vitro.
MATERIALS AND METHODS
All chemical reagents were obtained from Sigma (St Louis, MO, USA). This study was approved by the animal ethics committee of Ahvaz Jundishapur University of Medical Sciences (AJUMS). Albino rats of either sex were anesthetized by intraperitoneal administration of pentobarbital (45 mg/kg) and two sections of trachea about 20 millimeters in length were removed from each rat and cut into a spiral strip. The tracheal specimen was mounted using two steel plates and submersed in a 20ml organ bath at 37°C. The bath was filled with ml Krebs solution consisting of 118 mmol/l NaCl, KCl (4.7 mmol/l), CaCl2 (2.5 mmol/l), MgSO4·7H2O (1.2 mmol/l), KH2PO4 (1.2 mmol/l), NaHCO3 (25.0 mmol/l); and glucose (10.0 mmol/l). Continuous aeration with a mixture of 95% O2 and 5% CO2 was applied. The upper side of the tracheal strip was attached to a UF1 isometric transducer another side of the strip was fixed to a steel tissue holder. A resting tension of 500mg was applied to the tissue and allowed for equilibration in the bath solution for 30 min prior to the main tests. Preliminary tests showed that a tracheal strip immersed in the bath solution used for subsequent experiments did not contract when basal tension was applied. Stepwise increases in the number of drugs were employed to study the contraction or relaxation responses of tracheal strips. All drugs were administered by adding a defined volume of stock solution to the organ bath to attain molar concentration. A cumulative dose-response curve was attained using acetylcholine (ACH) and histamine (HIS). The addition of CAPE into the tissue bath could not cause any contraction or change in the baseline tissue tension. However, incubation of trachea with 10−7 M and 10−6M of CAPE induced a significant reduction in contractions induced by ACH and HIS, which was dose-dependent. To assess the possible roles of extracellular Ca2+ on the CAPE effect, the preparation was allowed to stabilize in the normal Krebs solution, then replaced with Ca-free and high-K (60mM) Krebs solution. Dose-response curve for CaCl2 (10−6M to 10−3M) was obtained in the absence of CAPE (control group), or after a 20 min incubation with 10−7 M and 10−6M of CAPE, the procedure was repeated. Finally, the contractile effect induced by CaCl2 was compared in the absence (control group) and the presence of CAPE.
To evaluate the involvement of relaxing factors in the CAPE-induced bronchodilator activity, the effect of N omega-nitro-L-arginine methyl ester (L-NAME (0.1 mM); a non-selective inhibitor of nitric oxide synthase), as a nitric oxide synthase (NOS) inhibitor, was examined. Tracheal relaxant effects of CAPE on ACH contractile activity significantly reduced after the treatment of tracheal tissue with L-NAME prior to CAPE incubation.
Results
Effects of ACH and HIS
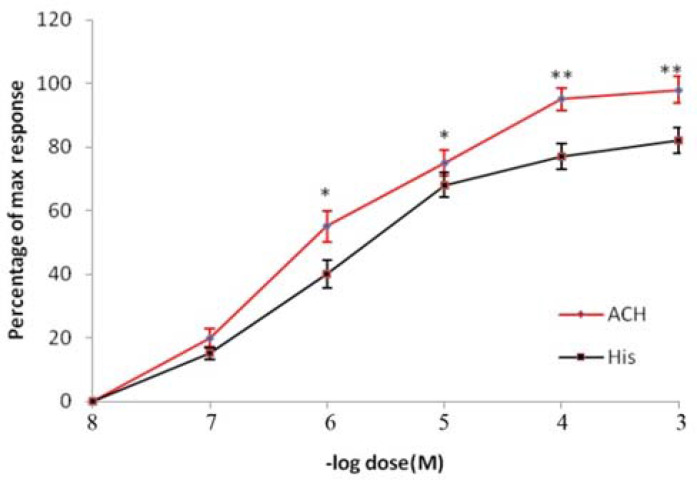
As shown in Figure 1, ACH and HIS could cause the contraction of the tracheal strip in a dose-dependent manner. The potency of ACH in this preparation seems to be higher than HIS because the ED50 for ACH is 7.6x10−7M and for HIS is 6.3x10−6M.
Figure 1.
Comparative dose-response curves of ACH and HIS on rat tracheal spiral strips. Symbols and vertical bars represent means and SEM. Value significantly different from HIS are presented *(p<0.05) and ** (p<0.01).
Effect of CAPE on ACH response
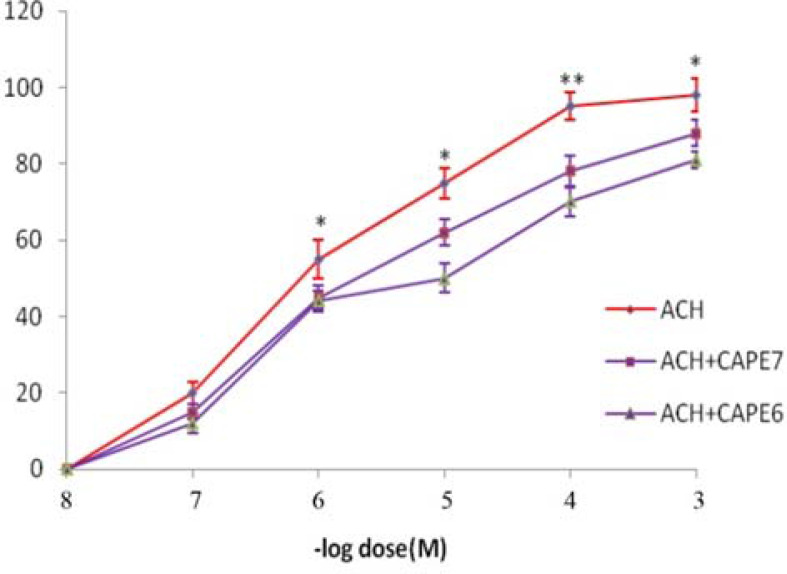
Figure 2 displays the inhibitory effect of CAPE on the contractile efficacy of ACH. Incubation of the tissue with CAPE could shift the dose-response curve of ACH to the right and increase the ED50 of ACH from 9x10−7M to 3x10−6M and 9x10−6M in the presence of CAPE (10−7M and 10−6M), respectively.
Figure 2.
Dose-effect curves of ACH on tracheal spiral strips of rat in the absence and in the presence of CAPE (10-7 and 10-6 M). Symbols and vertical bars represent means and SEM. Value significantly different from CAPE are presented *(p<0.05) and **(p<0.01).
Effect of CAPE on HIS response
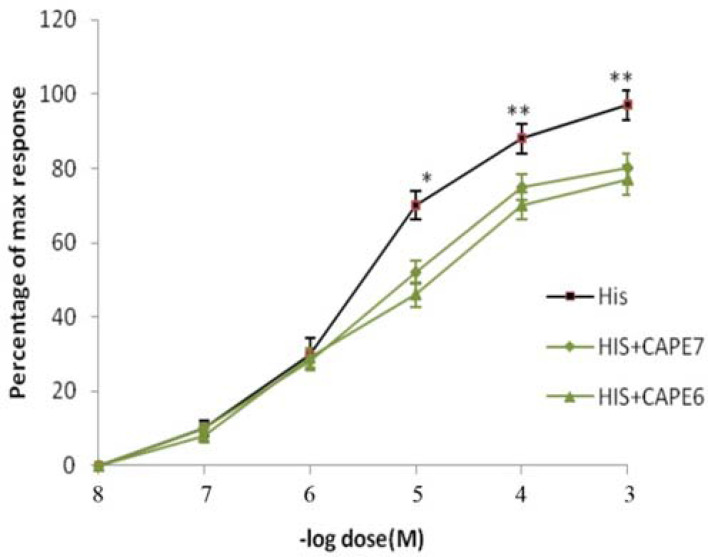
The inhibitory effect of CAPE on the contractile efficacy of HIS was observed in this experiment. Incubation of the tissue with CAPE could reduce the response of HIS and increase the ED50 of HIS 6.x10−6M to 9x10−6M and 2x10−5M in the presence of CAPE (10−7M and 10−6M), respectively (Figure 3).
Figure 3.
Dose-effect curves of HIS on tracheal spiral strips of rat in the absence and in the presence of CAPE (10-7 and 10-6 M). Symbols and vertical bars represent means and SEM. Value significantly different from CAPE are presented *(p<0.05) and ** (p<0.01).
Effect of CAPE on CaCl2 response
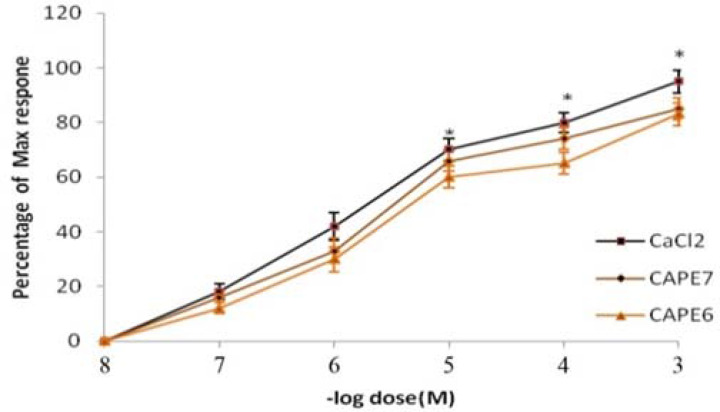
Incubation of the tissue with CAPE in Ca2+-free Krebs could reduce the contractile response of CaCl2 and increase the ED50 of CaCl2 from 2x10−6M to 4 x10−6M and 6x10−6M in the presence of CAPE (10−7M and 10−6M), respectively (Figure 4).
Figure 4.
Dose-effect curves of CaCl2 on rat tracheal spiral strips in the absence and in the presence of CAPE (10-7 and 10-6 M). Symbols and vertical bars represent means and SEM. Value significantly different from CAPE are presented *(p<0.05)
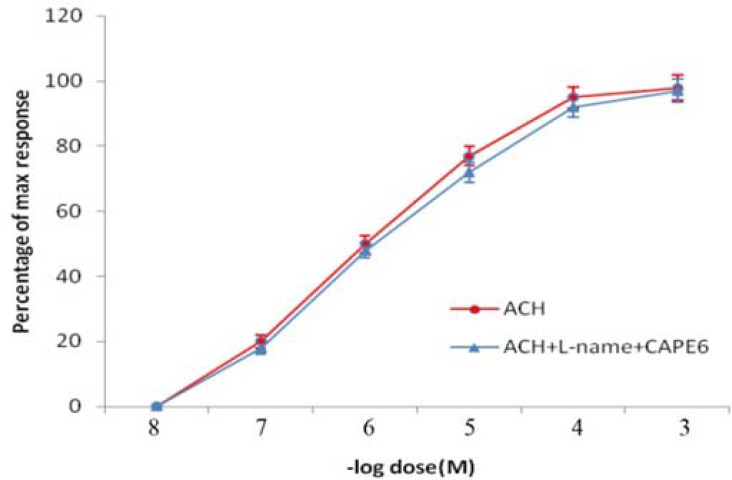
Effect of L-NAME on relaxant effect of CAPE
Figure 6 shows the inhibitory effect of L-NAME on the relaxant efficacy of CAPE. Incubation of the tissue with L-NAME prior to CAPE could abolish the relaxant activity of CAPE on ACH-induced contraction of tracheal smooth muscle (Figure 5).
Figure 5.
Dose-effect curves of ACH on rat tracheal spiral strips in the presence of L-NAME (10-4M) + CAPE (10-6 M). Symbols and vertical bars represent means and SEM. No significant differences are seen between to curves.
DISCUSSION
Asthma is one of the most important diseases of the respiratory system. It is a long-term inflammatory disease of the airways of the lungs, which is characterized by variable and recurring symptoms, reversible airflow obstruction, and bronchospasm (8). Asthma is thought to be caused by a combination of environmental factors and genetic (11). Many environmental factors are associated with the formation and exacerbation of asthma, including allergens, air pollution, and other environmental chemicals. Other potential triggers include medications, such as aspirin and beta-blockers (12).
There is no cure for asthma and the currently used therapy includes inhaled corticosteroids and short- and long-acting beta-agonists. Anti-leukotriene agents can be also used in addition to classic drugs (13, 14). However, numerous studies are currently trying to develop more appropriate drugs with higher efficacy and lower adverse effects; however, the use of agents with natural sources has attained great attention. Natural products provide a remarkable pharmacological perspective for drug development with regard to their widespread acceptability and broad-spectrum activities. Positive effects of CAPE have been investigated in a variety of diseases, such as pulmonary fibrosis (15, 16), heart disease (17, 18), and rheumatoid arthritis (19). Its effectiveness has not yet been studied in all aspects of asthma. Therefore, it is valuable to search for effective medicines for treating asthma using natural products.
In the present study, we investigated the effectiveness of CAPE on bronchial contractile activity, which is a major factor in bronchospasm and asthma. The cholinergic system affects the lungs was through the release of ACH and activation of muscarinic receptors, which causes bronchospasm and asthma (20). Such activity of ACH was diminished by CAPE in isolated tracheal preparation as indicated in Figure 3. CAPE caused its effect in a dose-dependent manner with the doses of 10−7 and 10−6 M and increasing the EC50 of ACH (Figure 2). Therefore, CAPE may at least in part inhibit muscarinic receptors in lung tissue.
HIS is one of the oldest mediators causing bronchospasm and asthma. It is usually released in response to allergens and mast cell stimulants (21). Therefore, its role in asthma should be considered in all studies dealing with asthma. In the present study, we compared the contractile activity of the trachea in response to ACH and HIS. HIS showed less potency than ACH to elicit the contraction of the isolated trachea as exhibited in Figure 2. HIS has a higher EC50 than ACH. In order to elucidate whether CAPE is able to interact with HIS response, trachea tissue was incubated with 10−7 and 10−6 M of CAPE. CAPE could significantly reduce the HIS response in a dose-dependent manner and increase the EC50 of HIS (Fig. 3). Such finding is consistent with a previous study, which showed the inhibitory activity of CAPE in HIS--induced itching in mice (22). The results showed the therapeutic potential of CAPE and its anti-histaminic effects on the lung. Because ACH and HIS play a crucial role in the onset of lung diseases, the inhibition of CAPE seems appropriate for the development of novel and safe drugs for lung diseases.
Various spasmolytic constituents mediate their relaxant effects through calcium (Ca2+) antagonists (23). We also found that CAPE remarkably blocked CaCl2-induced contraction, in Ca2+-free medium containing 60 mM KCl, supporting the idea that CAPE possesses a Ca2+ entry blocking activity (Figure 4). These results indicate that CAPE may be able to obstruct Ca2+ channels. This finding is consistent with earlier studies, which showed that CAPE is able to inhibit Ca2+ released from intracellular stores and block extracellular Ca2+ entry, which suggests that CAPE cab act as a CA antagonist (24). Our further experiment to elucidate the mechanism involved in CAPE effect showed that L-NAME can abolish the relaxing effect of CAPE on ACH response in trachea. This means that the relaxing effect of CAPE may partly be due to the release of nitric oxide (NO). NO has an important physiologic role in lung and acts as a neurotransmitter for the non-adrenergic and non-cholinergic nerves. NO is produced through an enzyme called NOS and modulates the airway tone via a bronchodilator action (25). Additionally, it is widely known that both inflammation and bronchospasm play an essential role in asthma (26). The regular use of CAPE in honey can help control asthma attacks because it acts as a bronchodilator. It is likely that bronchodilation is mediated through an inhibitory effect on Ca2+ movement across cell membrane (27). Also, the CAPE reduces oxidative stress through the levels of trace elements, such as zinc and copper. These elements are important for the control and treatment of asthma (28). Therefore, we propose that propolis contains CAPE that can be used as an adjuvant treatment to treat patients with mild to moderate asthma. The results showed that asthma attacks due to HIS and ACH may be significantly decreased and lung function is improved in patients receiving CAPE. The diverse effects of CAPE might be attributed to its ability to pass through the cell membranes more easily than caffeic acid. These results show that honey has antiasthma activities and confirm its use in traditional medicine. Indeed, clinical effectiveness of propolis in the treatment of asthma should be more investigated in animal studies and human clinical trials.
CONCLUSION
This study showed the protective effect of CAPE on drug-induced contractility, allergic airway inflammation, and hyperresponsiveness in asthma. Thus, it will be of great significance if the CAPE has bronchodilator effects as well as anti-inflammatory function. Many studies have proved that CAPE has anti-inflammatory effects, which can be associated with its therapeutic effect on asthma (29). Accordingly, the beneficial effects of CAPE can be used in the management of asthma. Our results indicated that CAPE may be utilized as adjuvant therapy for bronchial asthma patients.
REFERENCES
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161(5):1720–45. [DOI] [PubMed] [Google Scholar]
- 2.Khan M, Shah AJ, Gilani AH. Insight into the bronchodilator activity of Vitex negundo. Pharm Biol 2015;53(3):340–4. [DOI] [PubMed] [Google Scholar]
- 3.Chung KF, Caramori G, Adcock IM. Inhaled corticosteroids as combination therapy with beta-adrenergic agonists in airways disease: present and future. Eur J Clin Pharmacol 2009;65(9):853–71. [DOI] [PubMed] [Google Scholar]
- 4.Borrelli F, Maffia P, Pinto L, Ianaro A, Russo A, Capasso F, Ialenti A. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia 2002;73 Suppl 1:S53–63. [DOI] [PubMed] [Google Scholar]
- 5.Russo A, Longo R, Vanella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia 2002;73 Suppl 1:S21–9. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Lee JK, Kim HS, Chung ST, Eom JH, Kim KA, Chung SJ, Paik SY, Oh HY. Immunomodulatory effect of caffeic acid phenethyl ester in Balb/c mice. Int Immunopharmacol 2004;4(3):429–36. [DOI] [PubMed] [Google Scholar]
- 7.Fesen MR, Pommier Y, Leteurtre F, Hiroguchi S, Yung J, Kohn KW. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem Pharmacol 1994;48(3):595–608. [DOI] [PubMed] [Google Scholar]
- 8.Martinez FD. Genes, environments, development and asthma: a reappraisal. Eur Respir J 2007;29(1):179–84. [DOI] [PubMed] [Google Scholar]
- 9.Jung WK, Lee DY, Choi YH, Yea SS, Choi I, Park SG, Seo SK, Lee SW, Lee CM, Kim SK, Jeon YJ, Choi IW. Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced asthma. Life Sci 2008;82(13–14):797–805. [DOI] [PubMed] [Google Scholar]
- 10.Armutcu F, Akyol S, Ustunsoy S, Turan FF. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review). Exp Ther Med 2015;9(5):1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr TF, Bleecker E. Asthma heterogeneity and severity. World Allergy Organ J. 2016. November 29;9(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc 2014;11(3):404–6. [DOI] [PubMed] [Google Scholar]
- 13.Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S, International Study of Asthma and Allergies in Childhood Phase Three Study Group . Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009;64(6):476–83. [DOI] [PubMed] [Google Scholar]
- 14.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43(2):343–73. [DOI] [PubMed] [Google Scholar]
- 15.Zaeemzadeh N, Hemmati A, Arzi A, Jalali M, Rashidi I. Protective Effect of Caffeic Acid Phenethyl Ester (CAPE) on Amiodarone-Induced Pulmonary Fibrosisin Rat. Iran J Pharm Res 2011;10(2):321–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Larki A, Hemmati AA, Arzi A, Borujerdnia MG, Esmaeilzadeh S, Zad Karami MR. Regulatory effect of caffeic acid phenethyl ester on type I collagen and interferon-gamma in bleomycin-induced pulmonary fibrosis in rat. Res Pharm Sci 2013;8(4):243–52. [PMC free article] [PubMed] [Google Scholar]
- 17.Ozer MK, Parlakpinar H, Acet A. Reduction of ischemia--reperfusion induced myocardial infarct size in rats by caffeic acid phenethyl ester (CAPE). Clin Biochem 2004;37(8):702–5. [DOI] [PubMed] [Google Scholar]
- 18.Ozyurt H, Irmak MK, Akyol O, Söğüt S. Caffeic acid phenethyl ester changes the indices of oxidative stress in serum of rats with renal ischaemia-reperfusion injury. Cell Biochem Funct 2001;19(4):259–63. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Sun W, Jin L. Caffeic acid alleviates inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes by inhibiting phosphorylation of IκB kinase α/β and IκBα. Int Immunopharmacol 2017;48:61–66. [DOI] [PubMed] [Google Scholar]
- 20.Albertson TE, Chenoweth JA, Adams JY, Sutter ME. Muscarinic antagonists in early stage clinical development for the treatment of asthma. Expert Opin Investig Drugs 2017;26(1):35–49. [DOI] [PubMed] [Google Scholar]
- 21.Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol 2017;140(2):335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pradhananga S, Shim WS. Caffeic acid exhibits anti-pruritic effects by inhibition of multiple itch transmission pathways in mice. Eur J Pharmacol 2015;762:313–21. [DOI] [PubMed] [Google Scholar]
- 23.Guo H, Zhang J, Gao W, Qu Z, Liu C. Gastrointestinal effect of methanol extract of Radix Aucklandiae and selected active substances on the transit activity of rat isolated intestinal strips. Pharm Biol 2014;52(9):1141–9. [DOI] [PubMed] [Google Scholar]
- 24.Trumbeckaite S, Pauziene N, Trumbeckas D, Jievaltas M, Baniene R. Caffeic Acid Phenethyl Ester Reduces Ischemia-Induced Kidney Mitochondrial Injury in Rats. Oxid Med Cell Longev 2017;2017:1697018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aviello G, Scalisi C, Fileccia R, Capasso R, Romano B, Izzo AA, Borrelli F. Inhibitory effect of caffeic acid phenethyl ester, a plant-derived polyphenolic compound, on rat intestinal contractility. Eur J Pharmacol 2010;640(1–3):163–7. [DOI] [PubMed] [Google Scholar]
- 26.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161(5):1720–45. [DOI] [PubMed] [Google Scholar]
- 27.Cicala C, Morello S, Iorio C, Capasso R, Borrelli F, Mascolo N. Vascular effects of caffeic acid phenethyl ester (CAPE) on isolated rat thoracic aorta. Life Sci 2003;73(1):73–80. [DOI] [PubMed] [Google Scholar]
- 28.Aliosmanoglu C, Erbiş H, Aliosmanoglu I, Türkoglu MA, Ulger BV, Türkoglu A, Yüksel H. Protective effect of caffeic acid phenethyl ester on antituberculosis drug-induced hepatotoxicity in rats. International Surgery 2018;102(9):473–8. [Google Scholar]
- 29.Mirzoeva OK, Sud’ina GF, Pushkareva MA, Korshunova GA, Sumbatian NV, Varfolomeev SD. Lipofil’nye proizvodnye kofeĭnoĭ kisloty--lipoksigenaznye ingibitory s antioksidantnymi svoĭstvami [Lipophilic derivatives of caffeic acid as lipoxygenase inhibitors with antioxidant properties]. Bioorg Khim 1995;21(2):143–51. [PubMed] [Google Scholar]