Abstract
Objective
Acute myeloid leukemia (AML) is primarily a disease of older adults. These patients may not be candidates for intensive treatment, and there has been an ongoing need for treatment options for this group. We review the use of glasdegib, a hedgehog-pathway inhibitor available for use in combination with low-dose cytarabine (LDAC).
Data Sources: PubMed and relevant congress abstracts were searched using the term “glasdegib”. In addition, based on our experience with glasdegib, we considered treatment aspects of particular relevance to pharmacists and advanced practitioners.
Data Summary: In a randomized phase II study, the combination of glasdegib plus LDAC demonstrated superior overall survival versus LDAC alone (hazard ratio 0.51, 80% confidence interval 0.39–0.67, p = 0.0004). The trial reported adverse events (AEs) of special relevance for older patients, such as hematologic events, gastrointestinal toxicity, and fatigue, as well as AEs associated with Hh-pathway inhibitors (alopecia, muscle spasms, dysgeusia). Educating patients about typical AEs can facilitate adherence as well as early AE identification and proactive management. For LDAC, which is a long-established therapy in AML, various stages of delivery need consideration, with attention to individual circumstances. Practical measures such as dispensing a longer supply can reduce the number of return clinic visits, providing a meaningful difference for many patients.
Conclusions
Pharmacists and advanced practitioners play important roles in treatment with glasdegib plus LDAC. Ultimately, framing plans for treatment delivery within the individual circumstances of each patient may enable them to stay on therapy longer, giving them the greatest potential to achieve benefit.
Keywords: Acute myeloid leukemia, elderly, glasdegib, hedgehog pathway inhibitor, low-dose cytarabine
Introduction
Acute myeloid leukemia (AML) is the most common adult acute leukemia, with approximately 20,000 new cases in the United States (US) every year.1 AML is characterized by proliferation and accumulation of abnormal myeloid blasts in bone marrow and peripheral blood and, if untreated, is typically rapidly progressing and deadly. Generally, AML is a disease of older adults. More than half of patients diagnosed each year are ≥65 years, and around one-third are ≥75 years. The poorest outcomes are seen in those ≥75 years.1
For patients with newly diagnosed AML, intensive induction chemotherapy offers the best chance for curative outcomes. Unfortunately, the risk of treatment-related mortality often outweighs the benefit of response in older patients or those who present with significant comorbidities or functional impairment, as indicated by a score of >2 on Eastern Cooperative Oncology Group (ECOG) performance status.2 In addition, a long stay in the hospital is usually required, with potential for significant effects on quality of life.3
Health-care professionals (HCPs) use a combination of factors to determine whether intensive induction chemotherapy will be appropriate for an individual patient. The most significant predictor is the patient’s age – clinical outcomes in response to intensive induction therapy in patients ≥60 years are historically poorer compared with younger counterparts.2 However, age alone should not be the primary factor driving the decision whether or not to use intensive induction therapy. Comorbid conditions, organ function, and other factors affecting a patient’s ability to tolerate treatment or undergo bone marrow transplant must also be taken into account. Geriatric screening tools may be used for a more comprehensive assessment of the likely risks with chemotherapy.4 The presence of unfavorable prognostic factors such as molecular or cytogenetic abnormalities that confer resistance to intensive induction chemotherapy require consideration. Individual preferences also drive decisions, as patients considered “fit” for intensive induction chemotherapy may decline in favor of less intensive therapies that may be more consistent with their desired quality of life.
In “unfit” patients, less intensive frontline treatment reduces treatment-related toxicity but at the cost of potentially reduced response and survival. Until recently, established options have been hypomethylating agents (HMAs; azacitidine or decitabine) or low-dose cytarabine (LDAC). In this patient group, these agents have demonstrated efficacy in clinical trials, yet response rates remain low at around 16–24% for HMAs and 7–18% for LDAC and median overall survival of 8–10 months with HMAs and 4–8 months with LDAC.5–9 Despite availability of these options in the non-intensive setting, clinical practice surveys have highlighted the need for more effective treatment options. Studies from 2001 to 2013 found more than half of older patients (>65 years) diagnosed with AML in the US did not receive any active leukemia chemotherapy and in 2013 alone, nearly 43% received no active treatment.10 Since these surveys were conducted, novel therapies have been made available in the US (Table 1).
Table 1.
Therapeutic agents for newly diagnosed patients with AML who are not suitable for intensive induction chemotherapy.
| Hypomethylating agents | AzacitidineDecitabine |
| Cytotoxic chemotherapy | Low-dose cytarabine |
| BCL-2 inhibitors | Venetoclaxa |
| Hh pathway inhibitors | Glasdegibb |
| Agents for patients with a specific mutationc,d | Ivosidenib (IDH1 mutation)Enasidenib (IDH2 mutation) |
| Agents for patients with CD33-positive AML | Gemtuzumab ozogamicin (CD33-directed antibody–drug conjugate) |
AML: acute myeloid leukemia; BCL-2: B-cell lymphoma 2 protein; Hh: hedgehog. Based on agents approved in the United States in October 2019. Clinical trial enrollment or best supportive care (hydroxyurea/transfusion support) should also be considered.
aVenetoclax is currently approved in combination with low-dose cytarabine or hypomethylating agents.
bGlasdegib is currently approved in combination with low-dose cytarabine.
cPatients with these mutations are also eligible for therapies that are not mutation-specific.
dGilteritinib is also available for relapsed or refractory AML with FLT3 mutation, but is not currently approved for newly diagnosed AML.
In November 2018, the US Food and Drug Administration (FDA) approved glasdegib (DAURISMO™) for use in combination with LDAC. For optimal use of the glasdegib plus LDAC combination, HCPs will require detailed knowledge of LDAC as well as glasdegib. In light of this, we have reviewed practical considerations for glasdegib plus LDAC, including all aspects of LDAC use from shipping to follow-up, and a checklist for LDAC use. In addition, we have summarized recommendations for counseling patients about potential adverse events (AEs) associated with glasdegib plus LDAC as well as monitoring and managing AEs in practice.
Recommendations are based on the available literature as well as our experience of using glasdegib and LDAC in a variety of roles, as our multidisciplinary group includes pharmacists and nurse practitioners. Careful consideration of quality of life and proactive management of AEs is needed to enable patients to stay on therapy. Pharmacists and advanced practitioners will be involved in these aspects of treatment, and we discuss practical points from their perspective.
Relevant studies were identified by searching PubMed (in November 2019) with the term “glasdegib” and reviewing the results for studies where glasdegib was used in combination with LDAC. Very few publications were retrieved, presumably because glasdegib is a new agent. In fact, only one study of glasdegib in combination with LDAC was identified (the BRIGHT AML 1003 study,11 discussed in detail below); therefore, to gather as much information as possible, abstracts from relevant hematology congresses in 2018 or 2019 were also searched, and identified 2 further abstracts related to this treatment.12,13
Glasdegib
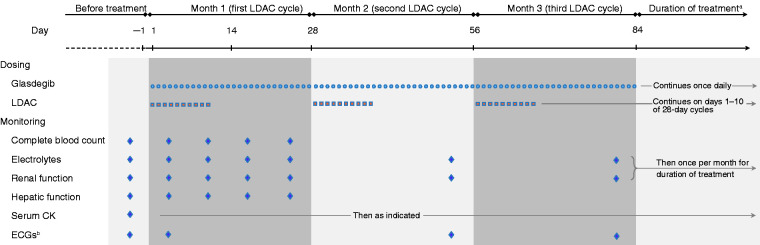
Glasdegib is a small-molecule drug taken orally once daily.14 Currently, glasdegib is approved by the FDA for use in combination with LDAC for treatment of newly diagnosed AML in adults aged ≥75 years or who have comorbidities that preclude use of intensive induction chemotherapy. According to current US labeling, the recommended dosing is glasdegib 100 mg orally once daily continuously in combination with cytarabine 20 mg subcutaneously twice daily on days 1–10 of a 28-day cycle.14 Figure 1 shows the schedule for dosing and monitoring of glasdegib plus LDAC.
Figure 1.
Glasdegib plus LDAC dosing and monitoring. CK: creatine kinase; ECG: electrocardiogram; LDAC: low-dose cytarabine; QTc: corrected QT interval.
aThis schedule shows 3 cycles for illustration; current US labeling recommends patients without unacceptable toxicity should be treated for a minimum of 6 cycles to allow time for clinical response.14
bRepeat ECG if abnormal. Some patients may need more frequent ECG monitoring (e.g. patients with congenital long QT syndrome, congestive heart failure, or electrolyte abnormalities; or who are taking QTc-prolonging medications).
Glasdegib acts by inhibiting the hedgehog (Hh) signaling pathway, and is the first approved agent to use this mechanism in AML therapy. Specifically, glasdegib binds the Smoothened protein, a key protein in this pathway.15 Over-expression of the Hh pathway has been observed in various cancer types, including AML and basal cell carcinoma, and other Hh pathway inhibitors (vismodegib and sonidegib) have been approved for use in the latter.16 In AML, the Hh pathway is implicated in the dormancy of leukemic stem cells – inhibiting over-expression prevents stem cells being maintained in the quiescent state and, as they differentiate, stem cells become a target for chemotherapy.17 Theoretically, therefore, glasdegib acts as a chemosensitizer of leukemic stem cells. Preclinical studies for glasdegib have supported this hypothesis,18,19 and it follows that glasdegib should be used in combination with a cytotoxic agent.
Overview of the BRIGHT AML 1003 study
In 2018, the FDA approved glasdegib based on the randomized phase II portion of BRIGHT AML 1003,20 which included patients considered unsuitable for intensive induction chemotherapy. All patients were aged ≥55 years and had newly diagnosed AML or high-risk myelodysplastic syndrome (MDS); patients with high-risk MDS were required to have 10–19% bone marrow blasts.11 In addition, based on characteristics associated with poor outcomes following intensive therapy,21 patients were required to have ≥1 of the following criteria: aged ≥75 years, ECOG score of 2, serum creatinine >1.3 mg/dL, or severe cardiac disease (for example, left-ventricular ejection fraction <45%).
In total, 132 patients were randomized 2:1 to glasdegib plus LDAC (n = 88) or LDAC alone (n = 44). For the primary endpoint, glasdegib plus LDAC demonstrated superior overall survival versus LDAC alone (hazard ratio 0.51, 80% confidence interval [CI] 0.39–0.67, p = 0.0004).11 Safety analyses included all patients who received study treatments: 84 received glasdegib plus LDAC and 41 received LDAC alone, with median treatment durations of 2.7 months (range 0.1–31.9) for glasdegib plus LDAC and 1.5 months (range 0.2–7.9) for LDAC alone.11 Addition of glasdegib to LDAC was generally well tolerated. During the study, 9/84 (11%) and 3/41 (7%) patients in the respective treatment arms discontinued due to treatment-related AEs.11 Of those starting glasdegib plus LDAC, 47 patients (56%) temporarily discontinued glasdegib and/or LDAC and 22 (26%) had a dose reduction owing to AEs.11 Table 2 summarizes dose modifications for AEs, as recommended by current US labeling, except for QTc-prolongation AEs, which are discussed separately below.
Table 2.
Dose modifications for adverse events.
| Adverse event | Recommended action |
|---|---|
| Hematologic toxicity (in the absence of disease) | |
| Platelets <10 × 109/L (<10 Gi/L) for >42 days |
|
| Neutrophil count <500/µL (<0.5 Gi/L) for >42 days |
|
| Non-hematologic toxicity | |
| Grade 3 |
|
| Grade 4 |
|
Adding glasdegib: Our clinical experience
The glasdegib plus LDAC regimen is self-administered, making it critical for providers and caregivers to facilitate adherence to treatment. A key consideration will be managing patients’ expectations, and it will be essential to communicate the most common AEs and those that may need particular attention. Comprehensive and consistent education for patients and caregivers, early identification and proactive management of AEs, and encouragement of adherence to self-administered therapy and follow-up visits can allow patients to stay on therapy to maximize potential benefit.
Common laboratory abnormalities in BRIGHT AML 1003 are shown in Table 3. Electrolyte changes were among common laboratory abnormalities seen more often in the glasdegib plus LDAC arm, although few were grade ≥3. Common AEs are shown in Table 4. Some of these AEs are familiar to HCPs treating patients with AML, and these are not further discussed here. Instead, we discuss AEs of special relevance for older patients, such as hematologic events, gastrointestinal (GI) toxicity, and fatigue, as well as AEs unique to Hh pathway inhibitors. Events associated with the Hh pathway inhibitors vismodegib or sonidegib are alopecia, muscle spasms, and dysgeusia, and all have been reported in approximately 40–60% of patients when these agents were studied in basal cell carcinoma.22 With glasdegib, these AEs were observed at lower rates as described below; the reasons underlying lower rates with glasdegib are unclear, but the shorter half-life of glasdegib compared with vismodegib and sonidegib may play a role.22
Table 3.
Laboratory abnormalities in the randomized phase II BRIGHT AML 1003 study.
| Glasdegib plus LDAC |
LDAC alone |
|||||
|---|---|---|---|---|---|---|
| Laboratory abnormality | n | All grades, % | Grade ≥3, % | n | All grades, % | Grade ≥3, % |
| Creatinine increased | 81 | 96 | 1 | 40 | 80 | 5 |
| Hyponatremia | 81 | 54 | 7 | 39 | 41 | 8 |
| Hypomagnesemia | 81 | 33 | 0 | 39 | 23 | 0 |
| AST increased | 80 | 28 | 1 | 40 | 23 | 0 |
| Blood bilirubin increased | 80 | 25 | 4 | 39 | 33 | 3 |
| ALT increased | 80 | 24 | 0 | 40 | 28 | 3 |
| ALP increased | 80 | 23 | 0 | 40 | 28 | 3 |
| Hyperkalemia | 81 | 16 | 1 | 40 | 8 | 3 |
| CK increased | 38 | 16 | 0 | 17 | 6 | 0 |
| Hypokalemia | 81 | 15 | 0 | 40 | 23 | 0 |
ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CK: creatine kinase; LDAC: low-dose cytarabine. Showing the most common laboratory abnormalities in either study arm within the first 90 days of treatment, in patients (n) with data available during this timeframe. Information from Pfizer (2018).14
Table 4.
Adverse events in the randomized phase II BRIGHT AML 1003 study.
| Glasdegib plus LDAC (n = 84) |
LDAC alone (n = 41) |
|||
|---|---|---|---|---|
| Adverse event | All grades | Grade ≥3 | All grades | Grade ≥3 |
| Anemia | 43 | 41 | 42 | 37 |
| Fatigue | 36 | 14 | 32 | 7 |
| Hemorrhage | 36 | 6 | 42 | 12 |
| Febrile neutropenia | 31 | 31 | 22 | 22 |
| Edema | 30 | 0 | 20 | 2 |
| Musculoskeletal pain | 30 | 2 | 17 | 2 |
| Thrombocytopenia | 30 | 30 | 27 | 24 |
| Nausea | 29 | 1 | 12 | 2 |
| Dyspnea | 23 | 11 | 24 | 7 |
| Decreased appetite | 21 | 1 | 7 | 2 |
| Dysgeusia | 21 | 0 | 2 | 0 |
| Mucositis | 21 | 1 | 12 | 0 |
| Constipation | 20 | 1 | 12 | 0 |
| Rash | 20 | 2 | 7 | 2 |
| Abdominal pain | 19 | 0 | 12 | 0 |
| Pneumonia | 19 | 15 | 24 | 22 |
| Renal insufficiency | 19 | 5 | 10 | 0 |
| Cough | 18 | 0 | 15 | 2 |
| Diarrhea | 18 | 4 | 22 | 0 |
| Dizziness | 18 | 1 | 7 | 0 |
| Pyrexia | 18 | 1 | 22 | 2 |
| Vomiting | 18 | 2 | 10 | 2 |
| Muscle spasms | 15 | 0 | 5 | 0 |
| Platelet count decreased | 15 | 15 | 10 | 10 |
| Atrial arrhythmia | 13 | 4 | 7 | 2 |
| Weight decreased | 13 | 0 | 2 | 0 |
| Chest pain | 12 | 1 | 2 | 0 |
| Headache | 12 | 0 | 10 | 2 |
| Hyponatremia | 11 | 6 | 0 | 0 |
| White blood cell count decreased | 11 | 11 | 5 | 2 |
AE: adverse event; LDAC: low-dose cytarabine. Showing common AEs in either study arm within the first 90 days of treatment. None of these common AEs were grade 5. Information from Pfizer (2018).14
Patients and caregivers should be counseled on these AEs, alongside information related to dosing, storage, handling, and disposal. Based on our clinical experience, the AEs discussed below have the potential to affect quality of life and possibly adherence. In older or frail patients, some of these AEs could contribute to further decline in performance status. Education is essential to ensure patients are aware of what they may experience, and it should also be explained that proactive reporting by the patient is crucial, as many of these AEs can be prevented with prophylactic medications or managed with early intervention.
The following recommendations are based on current clinical trial evidence complemented by our experience with glasdegib. Although the recommendations may not be applicable to every patient, we hope they will provide a frame of reference for this new therapy.
Hematologic events
In BRIGHT AML 1003, hematologic events were some of the most common AEs in both treatment arms (Table 4). During the first 90 days of the study, anemia was reported in 43% of the glasdegib plus LDAC arm and 42% of the LDAC-alone arm, while thrombocytopenia occurred in 30% and 27% of the respective groups (Table 4). Patient counseling for hematologic events is recommended, including advising that monitoring will be done via blood tests, and on practical measures to avoid cuts and bruises. In the patient population for which glasdegib plus LDAC is approved in the US (patients aged ≥75 years or with comorbidities that preclude the use of intensive chemotherapy), cytopenias are an important consideration as these patients may find it hard to recover from infectious or bleeding complications.
In BRIGHT AML 1003, febrile neutropenia was reported in 31% with glasdegib plus LDAC and 22% with LDAC-alone; all patients in both groups had grade ≥3 events (Table 4). It is noteworthy that there was no increase in infectious or bleeding complications, with lower rates of pneumonia and hemorrhage with glasdegib plus LDAC versus LDAC alone (Table 4). Exploratory analyses found bone marrow recovery within 1–2 months of starting treatment,13 and the majority of patients (65%) on glasdegib plus LDAC achieved absolute neutrophil counts of ≥500/μL during the study (after a median of 16 days) versus 53% receiving LDAC alone (median 11 days). Recovery within 1–2 cycles was seen consistently for platelets and hemoglobin.13 Although this provides some reassurance that cytopenia risk is not increased over LDAC alone, it is critical to ensure that patients are educated about reducing the risk of infection (such as avoiding people with a flu or fever) and contacting their HCP if they have symptoms.
Muscle spasms
In BRIGHT AML 1003, muscle spasms were more common with glasdegib plus LDAC than with LDAC alone (Table 4). All events reported during the first 90 days of treatment were grade 1–2,20 although during longer-term treatment, one patient had muscle spasms deemed a serious AE.11 In addition, worsening of laboratory abnormalities potentially related to muscle spasms was sometimes seen after 90 days.14 In a pooled analysis of 89 patients with AML receiving glasdegib plus LDAC in phases Ib and II of the study, 21% had muscle spasms.12 The median time to onset was 128 days but there was considerable variation (range 1–508 days), with 18 patients having onset before 6 months, four during 6–12 months, and four after 12 months.12
Before treatment starts, patients should be advised of the importance of adequate fluid intake to maintain hydration, and that they might feel achy, or have flu-like body aches or cramping while on glasdegib. It is worthwhile explaining that symptoms will be reviewed at every visit, so that patients are prepared to report any new symptoms at the stage when they can be more easily managed.
As recommended in current US labeling, all patients should have serum creatine kinase (CK) measured before starting glasdegib and again if muscle symptoms are reported.14 If CK results are normal, these events can usually be managed with symptomatic care (acetaminophen, muscle relaxants). As a proactive measure, levels of potassium should be maintained above 3.8 mEq/L and magnesium above 1.8 mg/dL. Patients with muscle spasms may be advised to try stretching and/or an oral electrolyte replacement solution; quinine water or over-the-counter topical preparations may also help. For some patients, vitamin B12 supplementation might be considered, although the patient should first be evaluated by an HCP, including measurement of the vitamin B12 level, as deficiency typically causes other symptoms before muscular effects.
Severe muscle spasms are rare; nevertheless, patients need to seek help if cramp worsens or does not respond to supportive care measures. If muscle spasms are significantly impacting the patient’s activities of daily living (ADLs), they should contact their physician, who can determine whether the patient needs to seek urgent care. Urgent help would certainly be required if patients were unable to move any extremities (arms, legs) due to pain. In such cases, CK should be checked as it may be elevated and indicate a more serious condition.
For troublesome muscle spasms, a dose reduction might be considered, and in BRIGHT AML 1003, muscle spasms were the AE most commonly associated with glasdegib dose reductions – reported for 5% of patients.14 Muscle spasms also showed the potential to worsen with longer treatment, as 4 of 12 patients progressed from grade ≤2 to grade ≥3 after 90 days of therapy.14 For severe muscle spasms, as for any grade 3 non-hematologic AEs (Table 2), current US labeling recommends interruption of glasdegib, with re-introduction at either the same dose or 50 mg once daily when symptoms have returned to the baseline level or grade 1.14 For patients with grade 4 AEs, glasdegib and LDAC should be permanently discontinued.14
Dysgeusia
During the first 90 days of BRIGHT AML 1003, dysgeusia was reported for 21% of the glasdegib plus LDAC group and 2% of the LDAC-alone group (Table 4). All events in both groups were grade 1–2.20 In the phase Ib/II pooled analysis, 25% of patients treated with glasdegib plus LDAC had dysgeusia, with median onset after 29 days (range 2–560), and 21 patients having onset before 6 months, two during 6–12 months, and one after 12 months.12
Patients should be advised that some foods may not taste the same as they did before treatment. Sour candy or Synsepalum dulcificum tablets may help some patients manage dysgeusia.23
Alopecia
Alopecia is an important issue for patients and they may have questions about it before beginning treatment. During the first 90 days of therapy in BRIGHT AML 1003, alopecia occurred in 6% of patients in the glasdegib plus LDAC arm (all events grade 1) versus none in the LDAC-alone arm.20 In the phase Ib/II pooled analysis, median onset of alopecia was 101 days (range 49–226), with seven patients having onset before 6 months, one during 6–12 months, and none after 12 months.12 The counseling, monitoring, and management of alopecia with glasdegib would be essentially similar to alopecia associated with other anti-cancer treatments. However, in discussions before treatment, it should be borne in mind that alopecia may be a particular side effect patients are trying to avoid when opting for a less intensive regimen – and here it is noteworthy that no alopecia events were reported with LDAC alone.20
Gastrointestinal events
The emetogenic potential of glasdegib has been classified as minimal to low risk;24 however, in BRIGHT AML 1003, GI events occurred more commonly with glasdegib plus LDAC than with LDAC alone, although most events were grade 1–2 (Table 4). Nausea of any grade was reported in 29% of patients (1% grade ≥3), diarrhea in 18% (4% grade ≥3), constipation in 20% (1% grade ≥3), and vomiting in 18% (2% grade ≥3) during the first 90 days of treatment.20
Because GI AEs are relatively common, they must be discussed with patients. Most events will likely be mild, usually with little intervention needed, but patients must be advised of the specific symptoms that should prompt them to call their HCP. For example, abdominal pain or nausea that interferes with ability to eat or maintain oral hydration is a cause for concern, and dizziness or lightheadedness and low urine output suggest dehydration. Patients may need hospitalization if they have diarrhea symptoms meeting grade 3 criteria (increase from baseline of ≥7 bowel movements per day or severe increase in ostomy output, with limitation of self-care ADLs).25 In general, patients should be asked to make their HCP aware of any change in bowel habits; in particular, changes since starting therapy in the number of stools per day or stool consistency, and whether a fever is present. If the patient has fever and/or watery stools, infectious workup is recommended.
For some GI events, practical considerations may be helpful and it is usually preferable to consider non-medical approaches before adding to pill burden (with corresponding medication costs). Concomitant medications should always be reviewed: even without changing a regimen, it may be possible to improve GI symptoms by changing the timing of concomitant medications. Glasdegib can be taken at any time of day, provided a regular schedule is followed. It can also be taken with or without food;26 however, for patients having problems with GI events, it is worth suggesting they first try taking glasdegib with food and/or adjusting the time of day before starting anti-emetic regimens. If patients do vomit their glasdegib dose, per current US labeling, they should take their next dose at the regular time, and not take an extra dose.14 When medications are needed to control GI events, the choice of agent should be individualized taking into account the patient’s current medications, including glasdegib. Anti-emetics, such as the 5HT3-receptor antagonist ondansetron, do have QT-prolonging potential, therefore care must be used when prescribing these agents, as discussed in the QT section.
Fatigue
Within the first 90 days of treatment in BRIGHT AML 1003, fatigue AEs (including asthenia) were reported in 36% of the glasdegib plus LDAC arm and 32% of the LDAC-alone arm (Table 4). Of the patients on glasdegib, 4% had a dose reduction due to fatigue.14
Fatigue may be of particular relevance in the population likely to receive glasdegib plus LDAC, given their age and comorbidities.27 Fatigue can be assessed with one or more of a variety of validated questionnaires to capture details including characteristics and manifestations of fatigue, as well as its impact on function. Fatigue is associated with frailty,28 which can be assessed using the Timed Up & Go test or other frailty scales.29 If geriatric services or support from a palliative care team are available, they may be able to help provide individualized care. Overall, fatigue is a challenging AE to manage, with a lack of pharmacologic strategies, but patients should be advised to stay active, eat well, stay hydrated, practice good sleep habits, and avoid caffeine in the afternoons.
Corrected QT interval prolongation
Corrected QT (QTc) interval prolongation is of interest with glasdegib, as dose-escalation studies in patients with various myeloid malignancies showed transient QTc prolongation with supratherapeutic doses, establishing 400 mg once daily as the maximum-tolerated dose.30 In this patient group, QTc events resolved after correction of reversible causes such as electrolyte abnormalities. In healthy volunteers without these confounding factors, a thorough QT study with placebo and moxifloxacin controls showed no clinical effect with glasdegib 100 or 200 mg.31 While this suggests these confounding factors may have increased risk, such factors are likely to be present in many patients in clinical practice.
In the randomized phase II BRIGHT AML 1003 study, the therapeutic glasdegib dose of 100 mg once daily was used.11 During the study, abnormal electrocardiogram (ECG) findings were reported in both treatment arms (Table 5), although not all these findings were symptomatic AEs. In the glasdegib plus LDAC group, five patients had AEs of ECG QTc prolongation, with onset between 7 and 57 days after starting glasdegib.32 As a result of these AEs, two patients had a permanent glasdegib dose reduction and two temporarily discontinued treatment.11 All five patients were receiving concomitant therapy that could have been relevant (each patient received ≥2 of citalopram, ciprofloxacin, fluconazole, levofloxacin, moxifloxacin, ondansetron, and voriconazole) and one also had significant cardiac disease history.32 While this provides some insight into these AEs, it is worth remembering that in this trial, many patients who did not have ECG events also had factors predisposing to QTc prolongation.11 In particular, more than half of patients had a history of severe cardiac disease (66% of the glasdegib plus LDAC arm and 53% of the LDAC-alone arm).14 For concomitant medications, 58% of patients in the glasdegib plus LDAC arm received concomitant cytochrome P450 (CYP) 3A4 inhibitors, most commonly ciprofloxacin (23 patients) and fluconazole (22 patients), and 75% received medication with QTc-prolongation potential, most commonly levofloxacin (37 patients) and ondansetron (36 patients).32 These medications were also given to patients in the LDAC-alone arm (39% for CYP3A4 inhibitors and 71% for QTc-prolonging medications) but are not expected to increase QTc prolongation with LDAC.32
Table 5.
QTc interval prolongation.
| Glasdegib plus LDAC | LDAC alone | |
|---|---|---|
| ECG findings, n (%)a | n = 83 | n = 17 |
| QTcF >480 ms and/or increase >60 ms from baseline | 9 (10.8) | 5 (29.4) |
| Subset with QTcF prolongation >500 ms | 5 (6.0) | 2 (11.8) |
ECG: electrocardiogram; LDAC: low-dose cytarabine; ms: milliseconds; QTcF: QT interval corrected for heart rate using Fridericia’s formula. Information from Cortes et al (2019).11
aIncludes patients with a baseline and ≥1 post-baseline ECG; a lower proportion of patients in the LDAC-alone arm were included as the protocol did not require ECG for this group.32
Regardless of etiology, we recognize the importance of QTc prolongation, and recommend that all patients beginning glasdegib be told about signs and symptoms that might suggest QTc interval prolongation (syncope, pre-syncopal symptoms such as feeling light-headed, or cardiac palpitations) and advised to contact their HCP immediately. Patients should also be advised that other medications can cause QTc interval prolongation, so their medication list will be evaluated and some medications may be changed to an alternative.
According to current US labeling, most patients should have ECGs before treatment starts, then ∼1 week after starting, and then once a month for the next 2 months (Figure 1). Serum electrolytes should be assessed before starting treatment, weekly for the first month, and once a month thereafter while glasdegib treatment continues.14 The randomized phase II study BRIGHT AML 1003 excluded patients with congenital long QT syndrome, torsades de pointes, clinically significant ventricular arrhythmias within 6 months before the study, or QTc interval >470 milliseconds.11 Our consensus is that it might be reasonable to avoid treatment with glasdegib for patients such as these, although the balance of risks and benefits will need to be weighed for each patient. Current US labeling does not specifically contraindicate use in these cases, but recommends more frequent ECG monitoring for patients with congenital long QT syndrome, congestive heart failure, or electrolyte abnormalities, and for patients taking medications known to prolong the QTc interval, such as ketoconazole.14
During glasdegib treatment, if QTc prolongation >480 milliseconds is found, current US labeling recommends repeating the ECG and, if prolongation is confirmed, patient management depends on the level of prolongation (Table 6).14 Glasdegib does not always need to be interrupted: supplementing electrolyte levels and adjusting any concomitant medications with known effects may return the QTc interval to ≤480 milliseconds (Table 6). If a patient has life-threatening arrhythmia accompanying QTc interval prolongation, referral to a cardiologist or cardio-oncologist may be of value; however, in these cases the US labeling recommends that glasdegib should be stopped and not resumed, regardless of subsequent ECG results.
Table 6.
QTc interval prolongation: Management and dose adjustments.
| QTc prolongation on ≥2 separate ECGsa | Management considerations | Discontinuation and/or therapy adjustment |
|---|---|---|
| >480–500 ms | • Correct abnormalities of electrolytes (e.g. potassium and magnesium)• Review and adjust concomitant medications with known QTc-prolongation effects• Continue to monitor: after QTc interval returns to ≤480 ms, patients should have a weekly ECG for 2 further weeks | • No changes required |
| >500 ms | • Correct electrolyte abnormalities• Review and adjust concomitant medications with known QTc-prolongation effects• Continue to monitor: after QTc interval returns to ≤480 ms, patients should have a weekly ECG for 2 further weeks | • Interrupt glasdegib• When QTc interval falls either to ≤480 ms or within 30 ms of the patient’s baseline measurement, resume glasdegib at 50 mg once daily• Maintain glasdegib dose at 50 mg once daily unless an alternative cause of QTc prolongation is found, when 100 mg once daily can be reconsidered |
| Accompanied by life-threatening arrhythmia | • Discontinue glasdegib• Do not resume glasdegib, regardless of further ECG results |
ECG: electrocardiogram; ms: milliseconds; QTc: corrected QT interval. Showing management and dose adjustments are according to current US labeling.14
aIf QTc prolongation is found on a routine ECG, the ECG should be repeated before changing treatment.
Drug–drug interactions
Older patients with AML are likely to be receiving multiple medications. Before starting therapy, all medications, including over-the-counter and herbal supplements, should be reviewed for drug–drug interactions. Patients should be counseled to discuss with their HCPs before beginning any new medications. Glasdegib is primarily metabolized via the CYP3A4 pathway, so strong CYP3A4 inhibitors or inducers need special attention.33 Concomitant use of glasdegib with strong CYP3A4 inducers such as rifampin is not recommended, as it is expected to decrease glasdegib exposure.34 Concomitant use with moderate CYP3A4 inducers such as efavirenz should also be avoided if possible; if concomitant use is essential, the glasdegib dose should be increased.14 Concomitant use with rabeprazole (a proton pump inhibitor) had little effect on glasdegib exposure26 and administration with ketoconazole (a strong CYP3A4 inhibitor) in healthy volunteers increased glasdegib exposure.35 Current US labeling recommends avoiding concomitant use of strong CYP3A4 inhibitors as well as other drugs that can prolong QTc.14 If QTc prolongation is observed, medications with known QTc-prolongation effects should be reviewed (Table 6). However, concomitant use is not contraindicated and may be unavoidable given agents such as voriconazole and posaconazole are frequently used to prevent and treat fungal infections in patients with AML.
Other aspects of counseling
In clinical practice, access to anti-cancer agents often presents financial challenges. In the US, financial assistance programs are available for glasdegib.36 Making patients aware of these programs is worthwhile, as knowing they are available will likely be a comfort to the patient and family. Advanced practitioners and pharmacists should discuss these programs with patients in need of assistance.
Future glasdegib research
Given the critical need for more options for patients with newly diagnosed AML who are not candidates for intensive therapy, results of clinical trials of glasdegib in combination with HMAs are eagerly awaited.37 A single-arm phase II study is evaluating glasdegib plus azacitidine in patients with AML or higher-risk MDS.38 In addition, a phase III trial is evaluating glasdegib in addition to intensive or non-intensive induction chemotherapy in patients with newly diagnosed AML.39 These trials will generate extensive safety and pharmacokinetics data and patient-reported outcomes, all of which will be useful for counseling patients and optimizing AE management. Naturally, AEs associated with Hh pathway inhibition (muscle spasms, dysgeusia, and alopecia) will be of most interest as we gain further experience with glasdegib. In addition, while BRIGHT AML 1003 suggested little impact on QTc prolongation at the therapeutic dose, more data from clinical practice will be reassuring.
In terms of counseling patients, clinical data showing whether efficacy is maintained after reducing the glasdegib dose will be useful. In BRIGHT AML 1003, dose reductions occurred in 26% of patients due to AEs, but because this was a relatively small group it was not possible to analyze any outcome effect.11 Pooled data with future studies might also provide insight into any potential association between AEs and outcomes, such as whether diarrhea may affect efficacy by decreasing absorption – a long-term effect that is impossible to measure in pharmacokinetic analyses.
Low-dose cytarabine
For many years, LDAC was the standard low-intensity therapy for AML.7 For older patients, studies suggest that while the initial response rate with LDAC is lower than with intensive induction chemotherapy, overall survival is similar with fewer toxicities, reduced antibiotic requirements, and less time in hospital.40 Not only is LDAC relatively well tolerated, it can also be given in an outpatient setting and allows for patients to self-administer. However, use has generally declined with the availability of HMAs, with a recent US clinical practice survey finding LDAC accounted for 31% of first-line low-intensity regimens, while HMAs accounted for 45%.41
At many institutions, LDAC may be reserved for patients who have cycled through other options, such as an elderly patient with refractory AML previously treated with azacitidine, with no targetable mutations and multiple comorbidities, but who desires continued treatment. The general preference for HMAs is not the only reason LDAC holds this place among treatment options: in addition, cytarabine shortages have occurred intermittently over the past 10 years.2 Major cancer centers are likely to have supplies, but this is not the case at all institutions, and not all pharmacies will supply cytarabine. Occasionally, only certain concentrations are available, which may be problematic for pharmacists and might require new order sets within the electronic medical record systems. Over time, physicians have received less training on LDAC and, being less familiar with it, they may be less likely to use it in clinical practice. Therefore, LDAC is not without challenges that might be considered potential barriers to using the glasdegib plus LDAC combination, but these challenges can be successfully managed, as discussed below.
LDAC clinical practices and approaches: Our clinical experience
In this section, we consider the accessibility and availability of LDAC. Once the decision has been made to use LDAC – whether alone or in combination with another agent such as glasdegib or venetoclax – the care team must consider various stages of delivery within the context of their own institution. This “LDAC journey” may vary between practices and understanding the process within each practice is essential; however, all practices need to consider the following: shipment of cytarabine, preparation of prefilled syringes, relevant patient education, how LDAC is supplied to the patient and how they will store it, administration of LDAC, and follow-up (Table 7). Key points are discussed below, and Table 8 provides an LDAC checklist of items to be covered. Note that most stages are not unique to LDAC and this process is similar for other therapies; for example, HMAs are also given subcutaneously so patient education will be similar.
Table 7.
The LDAC journey: Clinical experiences.
| Cytarabine shipped | Prefilled syringes prepared | Patient receives product | Administration | Follow-up | |
|---|---|---|---|---|---|
| Example considerations |
|
|
|
|
|
| Care team members involved |
|
|
|
|
|
| Challenges/barriers |
|
|
|
|
|
| Gaps/needs |
|
|
|
|
|
| Institutional practices |
|
|
|
|
|
| Patient considerations |
|
|
|
|
AE: adverse event; AP: advanced practitioner; CBC: complete blood count; LDAC: low-dose cytarabine; USP: United States Pharmacopeia.
Table 8.
The LDAC checklist.
| Preparation | • At least 1 week prior: if patient is to receive first dose in the clinic, start the insurance approval process to ensure LDAC is available |
| • Determine if a home health-care service is involved | |
| • Prepare doses within 24 hours prior to pick-up by patient | |
| • Verify that cytarabine concentration is correct | |
| • Identify the correct syringe size and quantity | |
| • Ensure labeling includes beyond-use date | |
| • Verify correct dose is ordered | |
| • Refrigerate doses until pick-up by patient | |
| • Gather patient supplies: chemotherapy kit, sharps container, alcohol pads, needles, syringe caps | |
| Administration | • Ensure patient is able to self-administer or has a health-care provider/caregiver to help them |
| • Discuss storage: educate patient on refrigeration requirements | |
| • Provide needles (and extras in case of mishaps with safety needles) and alcohol preps | |
| • Discuss safe handling and what to do in event of exposure | |
| • Discuss needle disposal | |
| • Advise patient to allow syringe to warm to room temperature prior to injection | |
| • Rotate the injection site | |
| • Educate on solution appearance | |
| • Educate on volume displacement with needle in place | |
| Post-administration considerations | |
| • Provide checklist of when to call health-care provider | |
| Follow-up | • Discuss injection-site reactions |
| • Schedule next follow-up, provide calendar | |
| • Provide patient education materials | |
| • Communicate with PCP to keep them abreast of patient’s treatment |
LDAC: low-dose cytarabine; PCP: primary care practitioner.
The approved LDAC regimen in combination with glasdegib is cytarabine 20 mg subcutaneously twice daily for days 1–10 of a 28-day cycle.14 Before treatment starts, it should be determined whether patients are likely to comply with twice-daily injections and, as with any subcutaneous medicine, the patient’s dexterity must be assessed, including whether they can attach the needle to the syringe.
Typically, LDAC is compounded and dispensed from a pharmacy in patient-specific, ready-to-use syringes. The pharmacy could be a hospital pharmacy, specialty pharmacy, or other compounding pharmacy; if the syringes are prepared by a hospital pharmacy, they are given to the patient, otherwise, the syringes will be shipped to the hospital. LDAC-prepared syringes require refrigeration, so consideration must be given to practical matters, such as an ice pack or cooler for patients to transport the LDAC home from the pharmacy. The beyond-use date depends on the local pharmacy and its preparation, but can be extended beyond the labeled duration with additional testing – in some cases to 14 days under refrigeration.42
Education for the patient or caregiver on how to self-inject is critical. For teaching purposes, the first dose should be given in the infusion center, after which the remainder of the doses are usually administered at home. Most centers provide patients with a handout including instructions for proper storage and how to discard used syringes. Patients should also be provided with the necessary supplies for LDAC administration, such as prescriptions for needles and alcohol pads, chemotherapy handling materials (gloves, sharps disposal container, and spill kit), as well as instruction around universal precautions. Practices for disposal vary between institutions, and patients or caregivers should consult their pharmacy or HCP regarding proper disposal of chemotherapy waste, including used needles and syringes. In some cases, home-care agencies may be able to provide delivery of LDAC and patient support.
The AE profile associated with LDAC is well established, including hematologic events and infections. Patients need counseling on potential side effects and how and when to contact the health-care team. Patient monitoring requires clinic visits once- or twice-weekly for complete blood count checks. Supportive care visits, usually 3 times per week during the first cycle and twice-weekly thereafter, are particularly important for AE management. As with any treatment regimen, a plan that is practical for the patient is essential, and for patients who have long journeys to the clinic, follow-up with a local provider may be arranged. Many patients will also still be seen by their primary care practitioner (PCP), and communication to keep the PCP aware of treatment will be useful, especially if the patient reaches out to the PCP in the case of AEs.
Conclusions
Patients with AML who are not eligible for intensive induction chemotherapy have a poor prognosis, with few treatment options. In this group, LDAC is an established agent that is relatively well tolerated and can be used in an outpatient setting, given appropriate planning. Glasdegib in combination with LDAC offers clinically meaningful improvement in overall survival versus LDAC alone, and has a manageable safety profile. This is especially important in this patient group, where life expectancy is limited, and maintaining quality of life is a priority. Generally, these patients are older and use of self-administered injectable medication may be challenging, requiring extensive education and coordination of support. In our experience, advanced practitioners can further support patients by becoming more familiar with this regimen to ensure adherence, monitor for AEs, and provide management strategies.
Supplemental Material
Supplemental material, sj-pdf-1-opp-10.1177_1078155220973737 for Glasdegib plus low-dose cytarabine for acute myeloid leukemia: Practical considerations from advanced practitioners and pharmacists by Valerie Relias, Ali McBride, Matthew J Newman, Shilpa Paul, Seyyedeh Saneeymehri, Genique Stanislaus, Jennifer Tobin, Caroline J Hoang, Joanne C Ryan and Ilene Galinsky in Journal of Oncology Pharmacy Practice
Acknowledgements
Medical writing support was provided by Geraldine Thompson of Engage Scientific Solutions, and was funded by Pfizer.
Data accessibility: Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Relias serves on Speakers Bureau for AbbVie and Genentech. Dr McBride is a consultant for Sandoz, Sanofi Genzyme, and Teva. Dr Newman has no potential disclosures to report. Dr Paul has no potential disclosures to report. Dr Saneeymehri has no potential disclosures to report. Ms Stanislaus discloses potential conflicts of interest with Celgene, Jazz, Novartis, and Takeda. Dr Tobin is a consultant for KaryoPharm. Drs Hoang and Ryan are employees of Pfizer. Ms Galinsky is a consultant/advisory board member for AbbVie, Merus Pharmaceuticals, and Pfizer.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The BRIGHT AML 1003 trial reviewed in this article is a clinical study sponsored by Pfizer. Pfizer funded a meeting for discussion of glasdegib, and provided a formal review of the publication, including for medical accuracy, but the authors had final authority, including choice of journal. The authors were compensated for expenses for their attendance at the meeting, but were not compensated for manuscript preparation.
ORCID iDs: Valerie Relias https://orcid.org/0000-0002-1476-5747
Matthew J Newman https://orcid.org/0000-0002-1402-5554
Supplemental material: Supplemental material is available for this article online.
References
- 1.National Cancer Institute. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia − acute myeloid leukemia (AML), https://seer.cancer.gov/statfacts/html/amyl.html (accessed 24 July 2019).
- 2.National Comprehensive Cancer Network. Acute myeloid leukemia (version 2.2020), www.nccn.org/professionals/physician_gls/pdf/aml.pdf (password protected, accessed 16 October 2019).
- 3.Vaughn JE, Buckley SA, Walter RB. Outpatient care of patients with acute myeloid leukemia: benefits, barriers, and future considerations. Leuk Res 2016; 45: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loh KP, Klepin HD. Geriatric assessment in older patients with acute myeloid leukemia. Cancers (Basel) 2018; 10: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015; 126: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cashen AF, Schiller GJ, O'Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol 2010; 28: 556–561. [DOI] [PubMed] [Google Scholar]
- 7.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 2007; 109: 1114–1124. [DOI] [PubMed] [Google Scholar]
- 8.Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood 2020; 135: 2137–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012; 30: 2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeidan AM, Podoltsev NA, Wang X, et al. Temporal patterns and predictors of receiving no active treatment among older patients with acute myeloid leukemia in the United States: a population-level analysis. Cancer 2019; 125: 4241–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 2019; 33: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes JE, Papayannidis C, Jamieson C, et al. Glasdegib in addition to intensive or non-intensive chemotherapy in patients with acute myeloid leukemia: Safety analysis of glasdegib ‘on target' adverse events. Blood 2018; 132: 2732–2732. [Google Scholar]
- 13.Wang ES, Heuser M, Montesinos P, et al. Glasdegib with LDAC in newly diagnosed patients with acute myeloid leukemia (AML) unsuitable for intensive chemotherapy: effects on transfusions and marrow recovery vs LDAC alone. HemaSphere 2019; 3: 87. [Google Scholar]
- 14.Pfizer. DAURISMO™ (glasdegib) tablets, for oral use. US prescribing information revised March 2020. Initial US approval November 2018, www.accessdata.fda.gov/drugsatfda_docs/label/2020/210656s002s004lbl.pdf (accessed 11 June 2020).
- 15.Queiroz KC, Ruela-de-Sousa RR, Fuhler GM, et al. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene 2010; 29: 6314–6322. [DOI] [PubMed] [Google Scholar]
- 16.Tay EY, Teoh YL, Yeo MS. Hedgehog pathway inhibitors and their utility in basal cell carcinoma: a comprehensive review of current evidence. Dermatol Ther (Heidelb) 2019; 9: 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giordani G, Barraco M, Giangrande A, et al. The human smoothened inhibitor PF-04449913 induces exit from quiescence and loss of multipotent drosophila hematopoietic progenitor cells. Oncotarget 2016; 7: 55313–55327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tauchi T, Okabe S, Katagiri S, et al. Targeting the hedgehog signaling pathway by glasdegib limits the self-renewal of MDS-derived induced potent stem cells (iPSC). J Cancer Sci Ther 2017; 9: 479–484. [Google Scholar]
- 19.Fukushima N, Minami Y, Kakiuchi S, et al. Small-molecule hedgehog inhibitor attenuates the leukemia-initiation potential of acute myeloid leukemia cells. Cancer Sci 2016; 107: 1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norsworthy KJ, By K, Subramaniam S, et al. FDA approval summary: glasdegib for newly diagnosed acute myeloid leukemia. Clin Cancer Res 2019; 25: 6021–6025. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer 2006; 106: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 22.Lacouture ME, Dreno B, Ascierto PA, et al. Characterization and management of hedgehog pathway inhibitor-related adverse events in patients with advanced basal cell carcinoma. Oncologist 2016; 21: 1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilken MK, Satiroff BA. Pilot study of “miracle fruit” to improve food palatability for patients receiving chemotherapy. Clin J Oncol Nurs 2012; 16: E173–177. [DOI] [PubMed] [Google Scholar]
- 24.National Comprehensive Cancer Network. Antiemesis (version 2.2020), www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf (password protected, accessed 8 June 2020).
- 25.National Institutes of Health. U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE). Version 5.0, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (2017, accessed 19 July 2019).
- 26.Shaik N, Hee B, Wei H, et al. Evaluation of the effects of formulation, food, or a proton-pump inhibitor on the pharmacokinetics of glasdegib (PF-04449913) in healthy volunteers: a randomized phase I study. Cancer Chemother Pharmacol 2019; 83: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsythe A, Kwon CS, Bell T, et al. Health-related quality of life in acute myeloid leukemia patients not eligible for intensive chemotherapy: results of a systematic literature review. Clinicoecon Outcomes Res 2019; 11: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofer F, Koinig KA, Nagl L, et al. Fatigue at baseline is associated with geriatric impairments and represents an adverse prognostic factor in older patients with a hematological malignancy. Ann Hematol 2018; 97: 2235–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood 2018; 131: 515–524. [DOI] [PubMed] [Google Scholar]
- 30.Martinelli G, Oehler VG, Papayannidis C, et al. Treatment with PF-04449913, an oral smoothened antagonist, in patients with myeloid malignancies: a phase 1 safety and pharmacokinetics study. Lancet Haematology 2015; 2: e339-346–e346. [DOI] [PubMed] [Google Scholar]
- 31.Shaik N, Mendes da Costa L, Hee B, et al. A thorough QT study to evaluate the effect of glasdegib on cardiac repolarization in healthy adult subjects. J Pharmacokinet Pharmacodyn 2018; 45: 3–134.28884259 [Google Scholar]
- 32.Pfizer. Data on file. BRIGHT AML study unpublished data.
- 33.Indiana University. Drug interactions flockhart table™, https://drug-interactions.medicine.iu.edu/Main-Table.aspx (accessed 30 August 2019).
- 34.Shaik MN, Hee B, Wei H, et al. Evaluation of the effect of rifampin on the pharmacokinetics of the smoothened inhibitor glasdegib in healthy volunteers. Br J Clin Pharmacol 2018; 84: 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaik MN, LaBadie RR, Rudin D, et al. Evaluation of the effect of food and ketoconazole on the pharmacokinetics of the smoothened inhibitor PF-04449913 in healthy volunteers. Cancer Chemother Pharmacol 2014; 74: 411–418. [DOI] [PubMed] [Google Scholar]
- 36.NeedyMeds. HealthWell foundation copay program/patient access network foundation (PAN)/Pfizer oncology together, www.needymeds.org/generic-drug/name/glasdegib (accessed 6 February 2019).
- 37.Cortes JE, Dombret H, Merchant A, et al. Glasdegib plus intensive/nonintensive chemotherapy in untreated acute myeloid leukemia: BRIGHT AML 1019 phase III trials. Future Oncol 2019; 15: 3531–3545. [DOI] [PubMed] [Google Scholar]
- 38.BRIGHT MDS & AML 1012; NCT02367456 A combination study of PF-04449913 (glasdegib) and azacitidine in untreated MDS, AML and CMML patients (BRIGHT 1012), https://clinicaltrials.gov/ct2/show/NCT02367456 (accessed 8 February 2019).
- 39.BRIGHT AML 1019; NCT03416179. A study evaluating intensive chemotherapy with or without glasdegib or azacitidine with or without glasdegib in patients with previously untreated acute myeloid leukemia (BRIGHT AML1019), https://clinicaltrials.gov/ct2/show/NCT03416179 (accessed 16 September 2019).
- 40.Burnett AK, Russell NH, Hunter AE, et al. Clofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood 2013; 122: 1384–1394. [DOI] [PubMed] [Google Scholar]
- 41.Medeiros BC, Pandya BJ, Hadfield A, et al. Treatment patterns in patients with acute myeloid leukemia in the United States: a cross-sectional, real-world survey. Curr Med Res Opin 2019; 35: 927–935. [DOI] [PubMed] [Google Scholar]
- 42.Wier P, Ireland D. Chemical stability of cytarabine and vinblastine injections. Br J Pharm Pract 1990; 12: 53–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-opp-10.1177_1078155220973737 for Glasdegib plus low-dose cytarabine for acute myeloid leukemia: Practical considerations from advanced practitioners and pharmacists by Valerie Relias, Ali McBride, Matthew J Newman, Shilpa Paul, Seyyedeh Saneeymehri, Genique Stanislaus, Jennifer Tobin, Caroline J Hoang, Joanne C Ryan and Ilene Galinsky in Journal of Oncology Pharmacy Practice