Abstract
Background/Objectives:
Geriatric guidelines recommend against statin use in older adults with limited life expectancy (LLE) or advanced dementia (AD). This study examined resident and facility factors predicting statin discontinuation after nursing home (NH) admission in Veterans with LLE/AD taking statins for secondary prevention.
Design:
Retrospective cohort study of Veterans Affairs (VA) bar code medication administration (BCMA) records, Minimum Data Set (MDS) assessments, and utilization records linked to Medicare claims.
Setting:
VA NHs, known as Community Living Centers (CLCs).
Participants:
Veterans ≥65 years with coronary artery disease, stroke, or diabetes admitted in fiscal years 2009-15, who met criteria for LLE/AD on their admission MDS and received statins in the week after admission (n=13,110).
Measurements:
Residents were followed until statin discontinuation (i.e., gap in statin use ≥14 days), death, or censoring due to discharge, day 91 of the stay, or end of study period. Competing risk models assessed cumulative incidence and predictors of discontinuation, stratified by whether the resident had their end-of-life (EOL) status designated or used hospice at admission.
Results:
Overall cumulative incidence of statin discontinuation was 31% (95% CI = 30%- 32%) by day 91, and was markedly higher in those with (52%, 95% CI=50%-55%) vs. without (25%, 95% CI=24%-26%) EOL designation/hospice. In patients with EOL designation/hospice (n=2,374), obesity, congestive heart failure, and admission from non-hospital settings predicted decreased likelihood of discontinuation; advanced dementia, dependency in activities of daily living, greater number of medications, and geographic region predicted increased likelihood of discontinuation. In patients without EOL designation/hospice (n=10,736), older age and several specific markers of poor prognosis predicted greater discontinuation, while obesity/overweight predicted decreased discontinuation.
Conclusion:
Most Veterans with LLE/AD taking statins for secondary prevention do not discontinue statins following CLC admission. Designating residents as EOL, hospice use, and individual clinical factors indicating poor prognosis may prompt deprescribing.
Keywords: Deprescribing, nursing homes, statins, Veterans Affairs
INTRODUCTION
The delivery of high quality care to older adults with limited life expectancy (LLE), including those with advanced dementia (AD), necessitates careful attention to prescribed medications in context of their goals of care.1 This includes the deprescribing (discontinuation or de-intensification) of medications that were originally indicated to prevent complications of chronic conditions, but have reduced benefits and increased risks in the context of LLE.1,2
Statins are one of the most commonly prescribed medications of questionable benefit in patients with LLE,3-6 possibly due to differing recommendations across clinical practice guidelines.7 Major cardiovascular professional societies recommend statin use in adults aged 40-75 with atherosclerotic cardiovascular disease (ASCVD) or diabetes and other cardiovascular risk factors, stating it is “reasonable” to initiate statins in patients >75 years and continue use in patients tolerating them, after considering benefits, risks, and patient frailty and preferences.8 However, several geriatric groups explicitly recommend against using statins in older adults with LLE (<2 years) and/or AD.9-12 As patients approach the end of life, many choose to limit life-sustaining treatments and other therapies, and focus on comfort.13-15 Continuing statins is not well-aligned with these goals, especially in light of the lack of survival benefits16 and heightened potential for adverse effects17,18 (e.g., muscle pain) in this population. Although little research has examined outcomes of statin discontinuation in adults with LLE/AD, a recent trial found that patients with <1 year life expectancy randomized to discontinue statins reported higher quality of life with no increased risk of mortality,16 lending support to discontinuation.
There have been few studies examining real-world patterns of statin discontinuation in older adults with LLE/AD.19-22 Admission to the nursing home (NH) represents an opportunity for medications of questionable benefit, including statins, to be reviewed and discontinued.23 Although a recent study showed that 34% of U.S. long-stay NH residents with life-limiting conditions received statins in 2016,6 little is known about how often statins are discontinued, or the demographic, clinical, and environmental circumstances that may prompt or impede discontinuation in this setting. To our knowledge, there has been only one population-based U.S. study examining statin discontinuation near the end-of-life in the NH, finding that 37% of primarily female residents in 2007-2008 discontinued statins in the six months after declining to advanced dementia.19
This study sought to determine incidence and predictors of statin discontinuation in a national cohort of older NH residents with LLE/AD diagnosed with coronary artery disease, stroke/transient ischemic attack (TIA), and/or diabetes, who received statins at admission to Veterans Affairs (VA) NHs, known as Community Living Centers (CLCs). Specifically examining discontinuation in VA CLCs allows for elucidation of more recent statin deprescribing patterns across a national, integrated network and robust examination of resident and environmental factors that may influence deprescribing. We focused on discontinuation of statins for secondary prevention, given that clinicians may be more hesitant to deprescribe in residents at greater risk for cardiovascular events
METHODS
This was a retrospective cohort study using administrative and clinical data from the VA and Centers for Medicare & Medicaid Services for a national cohort of Veterans admitted to CLCs in fiscal years (FY) 2009-2015. The Institutional Review Boards at the [removed to maintain anonymity] approved this study.
Data Sources
Data sources included the VA Residential History File,24,25 Minimum Data Set (MDS) for VA CLCs, VA utilization and bar-coded medication administration (BCMA) records through the VA Corporate Data Warehouse (CDW), and Medicare claims for Veterans.26 The Residential History File provides information on Veterans’ transitions across care settings within and outside of VA including CLC admission and discharge dates.24,25 The MDS is a standardized assessment of the functional, psychosocial, and health status of NH residents designed to guide care planning,27,28 and is required at CLC admission and quarterly thereafter. CLCs transitioned from MDS version 2.0 to 3.0 in July 2012. We used MDS admission assessments to identify newly admitted Veterans with LLE/AD, and capture demographic and clinical characteristics. VA utilization records and Medicare claims provided diagnosis and procedure codes for variables related to past medical history. BCMA records provided information on every medication administered in the CLC, including medication name, dose, and time of administration. The VHA Support Service Center databases provided information on facility characteristics.
Sample and Design
Sample construction details are shown in Supplemental Figure S-1. We used the Residential History File to identify admission and discharge dates for CLC episodes beginning in FY2009-2015 that were linkable to an MDS admission assessment occurring within the first 30 days of the stay (n=200,333). Our episode definition allowed for temporary leaves from the CLC setting to community or acute care settings for <7 consecutive days. We used MDS assessments to identify Veterans with LLE/AD according to at least one of three criteria (n=81,273, 41%): 1) endorsement of the MDS item for “end-stage disease, <6 months to live”;29,30 2) score of ≥36 on the MDS Mortality Risk Index-Revised, which has good predictive validity for 6-month mortality in NH residents assessed with both MDS v2.03 and v3.0;30,31 and/or 3) advanced dementia, defined as a score of ≤7 on the Brief Interview for Mental Status or ≥4 on the Cognitive Performance Scale.32,33 These cutoffs were based on published validity data.32,33
We then limited to CLC stays in which residents were aged ≥65 years at admission (n=61,137, 75%) that lasted for ≥7 days (n=58,782, 96%). Because of our focus on statin use for secondary prevention, we required residents to have coronary artery disease, stroke/transient ischemic attack (TIA), and/or diabetes (n=43,968, 75%), identified through established, ICD-9-based algorithms,34 or MDS indicators35 (Supplemental Table S1 for details). Finally, residents had to receive a statin medication at least once during the first week of their stay (n=16,473, 38%) and remain in the CLC for ≥14 days after this index statin medication (n=13,110, 80%), to ensure sufficient time for observing discontinuation. A total of 13,110 admissions for 12,353 unique Veterans met inclusion criteria, with 6% contributing >1 episode.
Dependent Variable: Statin Discontinuation
Using BCMA records, statin discontinuation was defined as ≥14 consecutive days after the statin index date in which no statins were administered. Using a gap length of ≥14 days ensured that apparent gaps in use were not due to temporarily leaving the CLC setting for <7 days. The date of discontinuation was defined as the first day of the 14-day gap within 90 days after admission. Residents were assessed for statin discontinuation from the index date until death or censoring due to CLC discharge, day 91 of the CLC stay, or end of available data (Sept. 30, 2015). For residents experiencing a censoring event prior to meeting criteria for discontinuation, the censoring date was set to 14 days prior to the censoring event, because the last 14 days of available follow-up in such cases represented time in which the outcome of statin discontinuation could not be observed.36,37 We also conducted sensitivity analyses requiring a 30-day gap in therapy (see Analytic Approach).
Independent Variables
Selection of independent variables was driven by our conceptual model, which hypothesized that discontinuation may be affected by resident socio-demographics, the environment of care, facility characteristics, and clinical characteristics. Specifically, we hypothesized that cardiovascular risk factors may reduce likelihood of statin discontinuation, while designation of a resident as end-of-life or hospice use at admission, as well as specific markers of poor prognosis (e.g., cancer, weight loss,), may increase likelihood of discontinuation.
Socio-demographics from the MDS included age, sex, race/ethnicity, and marital status. Environment of care factors included fiscal year of admission (2009-2011 vs. 2012-2015), admission source (acute hospital, home/assisted living, NH, other), and characteristics of the next-of-kin recorded in the electronic health record, including relationship to the resident and distance from the next-of-kin’s ZIP code to the CLC, categorized into quartiles. We were interested in next-of-kin factors due to mixed evidence in prior studies regarding the influence of next-of-kin characteristics on NH and end-of-life care decisions.38,39 Facility characteristics included U.S. census region, urban influence code,40 bed size, and complexity level of the VA parent station (Supplemental Table S-2), based on prior research showing associations between these factors and discontinuation of other medications of questionable benefit.37,41.
Cardiovascular risk variables were constructed using validated ICD-9-based algorithms applied to VA utilization data and Medicare claims34,42 and MDS assessments.35 These included number of cardiovascular risk conditions qualifying patients for inclusion (range 1-3: coronary artery disease, stroke/TIA, diabetes), congestive heart failure, hypertension, hyperlipidemia, venous thromboembolism, myocardial infarction in the past year, and stroke/TIA in the past year. The MDS provided current smoking status as well as height/weight; the latter were used to calculate body mass index and categorize residents as underweight, normal weight, overweight, or obese.43 Use of other non-statin antilipemic agents was assessed during the first 7 days of the CLC stay.
Although the entire sample met at least one of the three criteria for LLE/AD, only some had their <6 month life expectancy explicitly designated by healthcare providers at admission, either on the MDS or via receipt of hospice services. Therefore, we created an indicator for end-of-life (EOL) designation or hospice use at admission, defined as 1) endorsement of the single MDS item for “end-stage disease, less than 6 months to live”; 2) MDS documentation of hospice use within the past 14 days; and/or 3) admission to a VA hospice specialty for the CLC stay. We also created an indicator for whether the resident had AD, described above. We computed the number of Elixhauser comorbidities44 using VA utilization records and Medicare claims for the year prior to admission. We used single MDS items to capture weight loss, poor appetite, renal failure, dehydration, acute change in mental status, shortness of breath, cancer, presence of pain, and infection. The Activities of Daily Living Self-Performance Hierarchy was used to classify residents’ physical functioning.45 Aggressive behavior was measured with the Aggressive Behavior Scale.46 We used three MDS items (swallowing disorder, parenteral diet or tube feeding, mechanically altered diet) to create an overall indicator for potential difficulty with swallowing medications. We created an indicator for recent fall history using a validated claims-based algorithm for identifying serious falls/fractures along with the MDS indicator for a fall within the past 180 days.47,48 Finally, we assessed total number of medication classes used in the first 7 days of the stay.
Analytic Approach
Analyses were conducted using StataMP v15 (StataCorp, College Station, TX). Missing values (≤5% on any individual variable) were imputed using multiple imputation with chained equations, with 10 imputed datasets.49,50 We then estimated the crude, 90-day cumulative incidence of statin discontinuation treating death as a competing risk,51,52 overall and by whether or not the resident was designated as EOL and/or used hospice at admission. We used multivariable Fine and Gray competing risk models to estimate subdistribution hazard ratios (SDHRs) for each independent variable, controlling for all other variables in our conceptual model. Because cumulative incidence of discontinuation was revealed to be much greater for residents with EOL designation/hospice use at admission (HR=3.08, 95% CI=2.73-3.48), we estimated models stratifying by this variable. We used robust standard errors clustered at the facility level to account for both facility-level correlation and individual-level correlation among residents contributing ≥1 CLC stay. We assessed multicollinearity by examining variance inflation factors (VIF); no VIF exceeded 5.0, a commonly used threshold for problematic multicollinearity. Finally, we re-ran competing risk models using an alternative definition of statin discontinuation that required a 30-day gap in use and a minimum 30-day CLC stay after the index statin date (n=8,968).
RESULTS
The sample consisted of 13,110 Veterans with LLE/AD and coronary artery disease, stroke/TIA, or diabetes, receiving a statin within the first week after admission to a VA CLC. Only 18% (n=2,374) had an EOL designation or hospice use at admission (Table 1). Most residents were aged ≥75 years (40% 75-84 years, 28% ≥85 years); male (99%); white, non-Hispanic (79%); and were admitted from an acute hospital setting (73%). Most residents had two (46%) or all three (15%) of the qualifying cardiovascular risk conditions, hypertension (94%), and hyperlipidemia (79%) (Table 2). Twenty-nine percent had advanced dementia.
Table 1.
Socio-demographic, Environment of Care, and Facility Characteristics of Older Veterans with Limited Life Expectancy and/or Advanced Dementia Taking Statins at Admission to a Veterans Affairs (VA) Community Living Center (CLC), n=13,110.
| Full Sample (n=13,110) n(%) |
End-of-Life Designation/ Hospice Use (n=2,374) n (%) |
No End-of-Life Designation/ Hospice Use (n=10,736) n (%) |
p-value for comparison by End-of-Life Designation/ Hospice Use |
|
|---|---|---|---|---|
| Socio-demographics | ||||
| Age at admission | 0.76 | |||
| 65-74 | 4,220 (32.2) | 766 (32.3) | 3,454 (32.2) | |
| 75-84 | 5,264 (40.2) | 939 (39.6) | 4,325 (40.3) | |
| ≥85 | 3,626 (27.7) | 669 (28.2) | 2,957 (27.5) | |
| Sex | 0.009 | |||
| Male | 12,971 (98.9) | 2,337 (98.4) | 10,634 (99.1) | |
| Female | 139 (1.1) | 37 (1.6) | 102 (1.0) | |
| Race/ethnicity | <0.001 | |||
| White, non-Hispanic | 10,346 (78.9) | 1,980 (83.4) | 8,366 (77.9) | |
| Black, non-Hispanic | 1,909 (14.6) | 291 (12.3) | 1,618 (15.1) | |
| Hispanic | 557 (4.3) | 58 (2.4) | 499 (4.7) | |
| Other race/ethnicity | 203 (1.6) | 26 (1.1) | 177 (1.7) | |
| Missing* | 95 (0.7) | 19 (0.8) | 76 (0.7) | |
| Marital status | <0.001 | |||
| Not married | 6,583 (50.2) | 1,309 (55.1) | 5,274 (49.1) | |
| Married | 6,515 (49.7) | 1,063 (44.8) | 5,452 (50.8) | |
| Missing | 12 (0.1) | 2 (0.1) | 10 (0.1) | |
| Environment of Care Factors | ||||
| Fiscal year of admission | 0.53 | |||
| 2009-2011 | 5,210 (39.7) | 957 (40.3) | 4,253 (39.6) | |
| 2012-2015 | 7,900 (60.3) | 1,417 (59.7) | 6,483 (60.4) | |
| Living arrangement before admission | 0.36 | |||
| Acute hospital | 9,554 (72.9) | 1,765 (74.4) | 7,789 (72.6) | |
| Home/assisted living | 2,662 (20.3) | 454 (19.1) | 2,208 (20.6) | |
| Nursing home | 606 (4.6) | 104 (4.4) | 502 (4.7) | |
| Other | 288 (2.2) | 51 (2.2) | 237 (2.2) | |
| Next-of-kin relationship to the Veteran | 0.02 | |||
| Spouse | 5,306 (40.5) | 915 (38.5) | 4,391 (40.9) | |
| Child | 5,290 (40.4) | 972 (40.9) | 4,318 (40.2) | |
| Sibling | 1,252 (9.6) | 256 (10.8) | 996 (9.3) | |
| Other relative | 546 (4.2) | 113 (4.8) | 433 (4.0) | |
| Friend or other specified person of unknown relation | 705 (5.4) | 118 (5.0) | 587 (5.5) | |
| Missing* | 11 (0.1) | 0 (0.0) | 11 (0.1) | |
| Distance from next of kin ZIP code centroid to the CLC | <0.001 | |||
| Quartile 1 ≤ 12.9 miles | 3,164 (24.1) | 635 (26.8) | 2,529 (23.6) | |
| Quartile 2 (12.9-33.4) miles | 3,160 (24.1) | 611 (25.7) | 2,549 (23.7) | |
| Quartile 3 (33.5-88.9) miles | 3,165 (24.1) | 548 (23.1) | 2,617 (24.4) | |
| Quartile 4 >88.9 miles | 3,157 (24.1) | 505 (21.3) | 2,652 (24.7) | |
| Missing* | 464 (3.5) | 75 (3.2) | 389 (3.6) | |
| Facility Characteristics* | ||||
| US Census region of the CLC | <0.001 | |||
| Northeast | 2,096 (16.0) | 489 (20.6) | 1,607 (15.0) | |
| Midwest | 4,449 (34.0) | 627 (26.4) | 3,822 (35.6) | |
| South | 4,218 (32.2) | 854 (36.0) | 3,364 (31.3) | |
| West | 2,347 (17.9) | 404 (17.0) | 1,943 (18.1) | |
| Urban Influence Code for the CLC | <0.001 | |||
| Large metro | 6,377 (48.6) | 1,223 (51.5) | 5,154 (48.0) | |
| Small metro | 5,463 (41.7) | 982 (41.4) | 4,481 (41.7) | |
| Micropolitan | 949 (7.2) | 134 (5.6) | 815 (7.6) | |
| Noncore rural | 321 (2.5) | 35 (1.5) | 286 (2.7) | |
| Complexity Level of the VA parent station | 0.30 | |||
| 1a (Highest) | 5,520 (42.1) | 952 (40.1) | 4,568 (42.6) | |
| 1b | 1,810 (13.8) | 341 (14.4) | 1,469 (13.7) | |
| 1c | 2,066 (15.8) | 383 (16.1) | 1,683 (15.7) | |
| 2 | 1,457 (11.1) | 272 (11.5) | 1,185 (11.0) | |
| 3 (Least Complex) | 2,257 (17.2) | 426 (17.9) | 1,831 (17.1) | |
| Bed Size of CLC | <0.001 | |||
| <60 beds | 1,733 (13.2) | 415 (17.5) | 1,.318 (12.3) | |
| 60-120 beds | 4,802 (36.6) | 836 (35.2) | 3,966 (36.9) | |
| >120 beds | 6,575 (50.2) | 1,123 (47.3) | 5,452 (50.8) |
86% of CLC episodes had no missing data on any study variable (i.e., were complete cases); 13% were missing on only one variable, and 1% were missing on 2-4 variables.
Table 2.
Clinical characteristics of the sample, including cardiovascular risk factors and markers of poor prognosis (n=13,110).
| Full Sample (n=13,110) n (%) |
End-of-Life Designation/ Hospice Use (n=2,374) n (%) |
No End-of-Life Designation/ Hospice Use (n=10,736) n (%) |
p-value for comparison by End-of-Life Designation/ Hospice Use |
|
|---|---|---|---|---|
| Cardiovascular Risk Factors | ||||
| Number of qualifying conditions (coronary artery disease, stroke/transient ischemic attack, and/or diabetes) | <0.001 | |||
| One | 5,125 (39.1) | 1,060 (44.7) | 4,065 (37.9) | |
| Two | 6,019 (45.9) | 1,051 (44.3) | 4,968 (46.3) | |
| Three | 1,966 (15.0) | 263 (11.1) | 1,703 (15.9) | |
| Congestive heart failure | 6,389 (48.7) | 1,145 (48.2) | 5,244 (48.9) | 0.59 |
| Hypertension | 12,409 (94.7) | 2,212 (93.2) | 10,197 (95.0) | <0.001 |
| Hyperlipidemia | 10,413 (79.4) | 1,901 (80.1) | 8,512 (79.3) | 0.39 |
| Venous thromboembolism | 1,740 (13.3) | 321 (13.5) | 1,419 (13.2) | 0.69 |
| Recent myocardial infarction (past year) | 934 (7.1) | 201 (8.5) | 733 (6.8) | 0.005 |
| Recent stroke/transient ischemic attack (past year) | 3,441 (26.3) | 482 (20.3) | 2,959 (27.6) | <0.001 |
| Current smoker | <0.001 | |||
| No | 11,802 (90.0) | 2,069 (87.2) | 9,733 (90.7) | |
| Yes | 1,140 (8.7) | 250 (10.5) | 890 (8.3) | |
| Missing* | 168 (1.3) | 55 (2.3) | 113 (1.1) | |
| Body Mass Index | <0.001 | |||
| Underweight (<18.5) | 733 (5.6) | 187 (7.9) | 546 (5.1) | |
| Normal or healthy weight (18.5 to <25.0) | 4,959 (37.8) | 1,027 (43.3) | 3,932 (36.6) | |
| Overweight (25.0 to <30.0) | 4,041 (30.8) | 693 (29.2) | 3,348 (31.2) | |
| Obese (≥30) | 3,190 (24.3) | 424 (17.9) | 2,766 (25.8) | |
| Missing* | 187 (1.4) | 43 (1.8) | 144 (1.3) | |
| Use of non-statin antilipemic medications | 945 (7.2) | 143 (6.0) | 802 (7.5) | 0.014 |
| Markers of Poor Prognosis | ||||
| Limited prognosis explicitly documented at admission | 2,374 (18.1) | (N/A) | (N/A) | |
| Advanced dementia | 3,780 (28.8) | 515 (21.7) | 3,265 (30.4) | <0.001 |
| Number of Elixhauser comorbidities | <0.001 | |||
| 0-1 | 1,126 (8.6) | 139 (5.9) | 987 (9.2) | |
| 2-3 | 3,193 (24.4) | 576 (24.3) | 2,617 (24.4) | |
| 4-5 | 3,892 (29.7) | 707 (29.8) | 3,185 (29.7) | |
| >5 | 4,899 (37.4) | 952 (40.1) | 3,947 (36.8) | |
| Recent weight loss | 5,378 (41.0) | 919 (38.7) | 4,459 (41.5) | 0.011 |
| Poor appetite | 4,732 (36.1) | 1,123 (47.3) | 3,609 (33.6) | <0.001 |
| Renal failure | 2,788 (21.3) | 382 (16.1) | 2,406 (22.4) | <0.001 |
| Dehydration | 135 (1.0) | 35 (1.5) | 100 (0.9) | 0.018 |
| Acute change in mental status | 1,122 (8.6) | 240 (10.1) | 882 (8.2) | 0.003 |
| Shortness of breath | 5,079 (38.7) | 1,035 (43.6) | 4,044 (37.7) | <0.001 |
| Cancer | 4,605 (35.1) | 1,104 (46.5) | 3,501 (32.6) | <0.001 |
| Activities of Daily Living (ADL) | <0.001 | |||
| Independent, requires supervision, or requires limited assistance | 3,726 (28.4) | 639 (26.9) | 3,087 (28.8) | |
| Requires extensive assistance | 5,092 (38.8) | 669 (28.2) | 4,423 (41.2) | |
| Dependent or totally dependent | 4,292 (32.7) | 1,066 (44.9) | 3,226 (30.1) | |
| Aggressive Behavior Symptoms | <0.001 | |||
| None | 10,936 (83.4) | 2,019 (85.1) | 8,917 (83.1) | |
| Moderate | 1,421 (10.9) | 223 (9.4) | 1,198 (11.2) | |
| Severe | 535 (4.1) | 91 (3.8) | 444 (4.1) | |
| Very severe | 173 (1.3) | 20 (0.8) | 153 (1.4) | |
| Missing* | 45 (0.3) | 21 (0.9) | 24 (0.2) | |
| Presence of any pain | <0.001 | |||
| Yes | 3,912 (29.8) | 1,683 (70.9) | 6,796 (63.3) | |
| No | 8,479 (64.7) | 579 (24.4) | 3,333 (31.1) | |
| Missing* | 719 (5.5) | 112 (4.7) | 607 (5.7) | |
| Infection | 0.001 | |||
| Yes | 3,504 (26.7) | 561 (23.6) | 2,943 (27.4) | |
| No | 9,602 (73.2) | 1,812 (76.3) | 7,790 (72.6) | |
| Missing* | 4 (0.03) | 1 (0.0) | 3 (0.0) | |
| Any swallowing difficulty or altered diet | 5,868 (44.8) | 1,131 (47.6) | 4,737 (44.1) | 0.002 |
| History of falls, hip fracture, and other fractures in past 180 days | <0.001 | |||
| Yes | 6,323 (48.2) | 1,047 (44.1) | 5,276 (49.1) | |
| No | 6,508 (49.6) | 1,258 (53.0) | 5,250 (48.9) | |
| Missing* | 279 (2.1) | 69 (2.9) | 210 (2.0) | |
| Total Number of Medication Classes | ||||
| <10 | 2,459 (18.8) | 396 (16.7) | 2,063 (19.2) | <0.001 |
| 10 to <15 | 6,054 (46.2) | 1,060 (44.7) | 4,994 (46.5) | |
| ≥15 | 4,597 (35.1) | 918 (38.7) | 3,679 (34.3) |
86% of CLC episodes had no missing data on any study variable (i.e., were complete cases); 13% were missing on only one variable, and 1% were missing on 2-4 variables.
Residents with an EOL designation/hospice use exhibited differences from those without an EOL designation/hospice use on most covariates. Of note, residents with an EOL designation/hospice were more likely to be white, non-Hispanic (83% vs. 78%), less likely to have advanced dementia (22% vs. 30%), and more likely to exhibit other individual markers of poor prognosis (Table 2).
Cumulative incidence of statin discontinuation in the first 90 days after CLC admission in the overall sample was 31% (95% CI=30%-32%; Figure S-2 in Supplemental Material). Eleven percent of residents died before discontinuing statins, including 26% with EOL designation/hospice use and 8% without EOL designation/hospice. Among remaining residents who were censored (n=8,912), 72% were discharged from the CLC, 25.6% reached 91 days of follow-up, and 2.4% reached the end of available data. Overall mortality over the entire 90-day follow-up period was 23%, including 57% of those with EOL designation/hospice and 16% without EOL designation/hospice. In stratified analyses (Figure 1), cumulative incidence of discontinuation was markedly higher in residents with an EOL designation/hospice use at admission (52%, 95% CI=50%-55%) compared to those without (25%, 95% CI=24%-26%).
Figure 1.
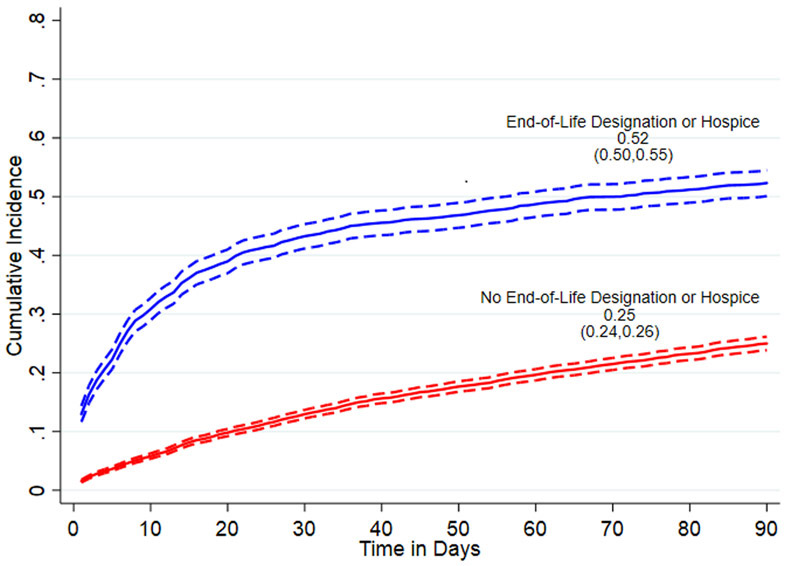
Cumulative incidence of statin discontinuation, using primary statin discontinuation definition requiring a 14-day gap in statin use, stratified by end-of-life designation/hospice use at admission.
Complete results of the Fine and Gray subdistribution hazard models are shown in Supplemental Table S-3. Figure 2 highlights factors which demonstrated statistically significant (p<.05) associations with statin discontinuation in residents with an EOL designation/hospice use at admission. Of the environment of care and facility factors, being admitted from home or assisted living (SDHR=0.76, 95% CI=0.64-0.91) or another NH (SDHR=0.75, 95% CI=0.57-0.99) versus the hospital was associated with decreased likelihood of discontinuation, while factors associated with increased likelihood of discontinuation included having a sibling versus spouse next-of-kin (SDHR=1.29, 95% CI=1.02, 1.63), and receiving care in a CLC in the southern (SDHR=1.41, 95% CI=1.05-1.89) or western (SDHR=1.39, 95% CI=1.01-1.92) vs. northeast census region. Of the cardiovascular risk factors, only obesity vs. normal weight (SDHR=0.72, 95% CI=0.60-0.86) and congestive heart failure (SDHR=0.85, 95% CI=0.75-0.97) were associated with a decreased likelihood of discontinuation. Three markers of poor prognosis were significantly associated with increased likelihood of discontinuation: advanced dementia SDHR=1.25, 95% CI=1.05-1.48), dependency in ADLs (SDHR=1.22, 95% CI=1.03-1.44, and total number of medications taken at admission (SDHR=1.23, 95% CI=1.01, 1.49).
Figure 2.
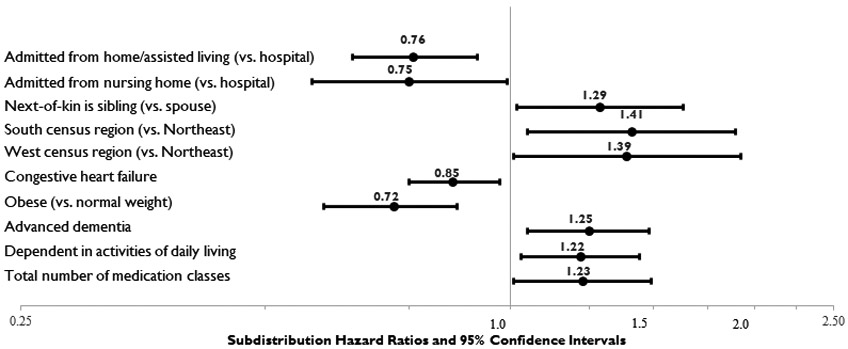
Factors with Statistically Significant (p<.05) Associations with Statin Discontinuation in Residents with End-of-Life Designation or Hospice Use at Admission (n=2,374).*
*The Fine and Gray competing risk model included all covariates included in Tables 1 and 2. Only factors significantly associated with statin discontinuation in this model are shown in this figure. Complete model results are available in Supplemental Material (Table S-3).
Figure 3 shows factors that were significantly associated with discontinuation in residents without EOL designation/hospice use at admission. In this group, being aged 75-84 (SDHR=1.13, 95% CI=1.00-1.27) or ≥85 years (SDHR=1.24, 95% CI=1.06-1.45) versus 65-74 was associated with increased likelihood of discontinuation, along with several individual markers of poor prognosis, including advanced dementia (SDHR=1.28, 95% CI=1.10-1.49), recent weight loss (SDHR=1.14, 95% CI=1.02-1.28), poor appetite (SDHR=1.25, 95% CI=1.12-1.40), dehydration (SDHR=2.54, 95% CI=1.82-3.55), cancer (SDHR=1.42, 95% CI=1.25-1.61), ADL dependence (SDHR=1.30, 95% CI=1.11-1.52), moderate (SDHR=1.19, 95% CI=1.01, 1.39) or very severe aggressive behavior (SDHR=2.05, 95% CI=1.53-2.74), and infection (SDHR=1.13, 95% CI=1.02-1.26). Being overweight (SDHR=0.89, 95% CI=0.80, 0.996) or obese (SDHR=0.82, 95% CI=0.69, 0.97) was associated with lower likelihood of discontinuation.
Figure 3.
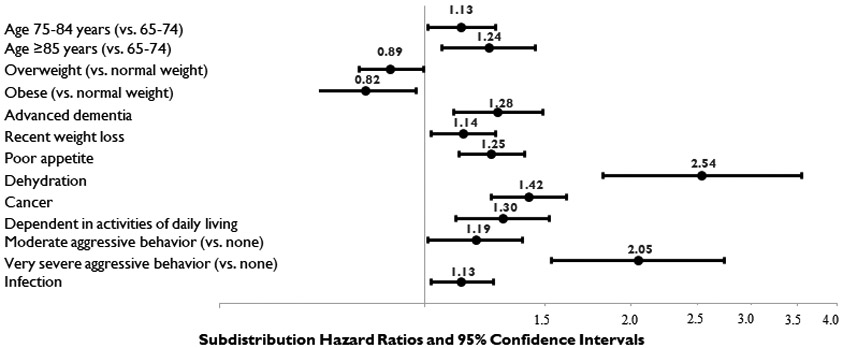
Factors with Statistically Significant (p<.05) Associations with Statin Discontinuation in Residents without End-of-Life Designation or Hospice Use at Admission (n=10,736).*
*The Fine and Gray competing risk model included all covariates included in Tables 1 and 2. Only factors significantly associated with statin discontinuation in this model are shown in this figure. Complete model results are available in Supplemental Material (Table S-3).
Sensitivity Analyses
Sensitivity analyses requiring a 30-day gap in statin use to define discontinuation resulted in an overall cumulative incidence of 26% (95% CI=25%-28%), including 48% (95% CI=45%-51%) in residents with EOL designation/hospice use at admission, and 21% (95% CI=20%-23%) in residents without (Supplemental Figures S-3, S-4). Supplemental Table S-4 shows full hazard model results when using a 30-day gap to define discontinuation.
DISCUSSION
In this national sample of Veterans with LLE/AD taking statins for secondary prevention at admission to a VA nursing home, the cumulative incidence of statin discontinuation within the subsequent 90 days was just under one-third. We found that residents who received hospice services or whose end-of-life status was designated on the admission MDS were over twice as likely as other residents with LLE/AD to discontinue statins. At the same time, only about half of these patients with an EOL designation or hospice use discontinued statins after admission. In those without EOL designation/hospice, individual markers of poor prognosis (e.g., weight loss, cancer, dehydration) exhibited stronger associations with discontinuation, while environmental/facility characteristics, congestive heart failure, and total number of medications at admission exhibited stronger associations in those with EOL designation/hospice use. Obesity was consistently associated with decreased discontinuation in both groups.
To our knowledge, this is only the second study to provide insight into real-world patterns of statin deprescribing in U.S. NH residents with LLE/AD. Tjia et al found that 37% of their sample of primary female, non-VA NH residents studied in 2007-2008 discontinued statins in the 6 months following progression to advanced dementia.19 This slightly higher rate of discontinuation is also aligned with our finding that advanced dementia was associated with greater hazard of discontinuation in both residents with and without an EOL designation/hospice use, and suggests that advanced dementia may be a marker of poor prognosis that consistently encourages statin deprescribing. However, overall our results suggest that, at least through 2015, significant barriers to statin discontinuation persisted, despite recommendations from geriatric and long-term care groups supporting discontinuation,9-12 heightened potential for drug interactions leading to myopathy,17 and positive attitudes about statin discontinuation in patients near the end-of-life.53 Whether results from a 2015 randomized trial showing no increased mortality risk and positive effects of quality of life associated with statin discontinuation in patients with <1 year life expectancy16 have since increased statin deprescribing remains an area for future research.
Our results suggest that continued use of statins in NH residents with LLE/AD may be, in part, due to a failure to recognize residents as being at the end-of-life. Efforts to increase identification of a resident as having limited prognosis at admission might be one key method to overcome clinical inertia, encourage medication reviews, and discuss statin deprescribing. Several measures have been developed and validated to assist with identifying individuals with limited life expectancy or advanced dementia, including the MDS Mortality Risk Index-Revised30,31 and MDS-based cognitive functioning measures32,33 that we used in this study. These measures have the advantage of utilizing MDS items that are routinely collected at repeated intervals at all Medicare and Medicaid-certified and VA NHs, and thus can potentially be used to automate identification of residents with LLE/AD. Decision-support tools could flag these residents for review54 and engagement in shared decision-making about deprescribing of statins and other medications.
Although the need to improve recognition of EOL status is one important implication of our results, our finding that almost half of residents with an EOL designation or hospice use continued statins suggests that interventions will need to go beyond this. In this group, congestive heart failure and obesity were associated with decreased discontinuation, suggesting that providers, patients, and/or informal caregivers may be hesitant to remove statins when cardiovascular risk factors are present, even in patients who are recognized as being near the end-of-life or are receiving hospice services. This may be due to a lack of available data on patient-centered outcomes of statin discontinuation in patients near the end-of-life with higher versus lower cardiovascular risk, another important direction for future research. Furthermore, interventions designed to change clinician behaviors require multiple strategies that go beyond prescriber and staff education. These strategies may include academic detailing, reminders that are embedded in the electronic health records, audit and feedback, as well as attention to the myriad barriers to changing clinical practice.
Our results should be interpreted in light of several limitations. First, the optimal method for defining discontinuation in BCMA records is unknown. Most prior studies have defined discontinuation using outpatient prescription refill records, considering a gap in days’ supply of 30 days as indicative of discontinuation. Given the availability of more detailed medication administration records recording every statin dose actually administered to CLC residents – a strength of our approach – we used a shorter gap length of 14 days in primary analyses. As the sensitivity analysis using a longer gap of 30 days reduced the cumulative incidence of deprescribing by 5%, our primary results may be a slightly conservative estimate of the true cumulative incidence. It is also possible that lower incidence of discontinuation using the 30-day definition may reflect less deprescribing in patients in the subsample who lived more than 30 days after admission and met criteria for inclusion in this sensitivity analysis. Second, generalizability of our results to non-VA NH residents, who are majority female, is unknown, as is generalizability to patients with LLE/AD living in non-NH settings. Third, our available data ended in late 2015; therefore, our results may not reflect more recent statin discontinuation patterns that may have changed due to additional evidence of its safety in LLE populations.16 Fourth, we were not able to assess the effect of advanced directives or other palliative care interventions on statin deprescribing, or the effect of time-varying factors during the CLC stay, such as adverse drug events, emergency department visits, or hospitalizations, which are important directions for future research. Fifth, we did not correct for multiple comparisons given the exploratory nature of our study and prioritization of avoiding Type II errors over Type I errors.55,56 Finally, due to the study design, the reported associations should not be interpreted as causal.
CONCLUSION
Despite geriatric consensus recommendations supporting the discontinuation of statins in older adults at the end of life, we found that most Veterans admitted to VA NHs with LLE/AD taking statins for secondary prevention did not discontinue these medications after admission. Our results suggest that improving recognition and documentation of residents’ end-of-life status, and additional interventions to encourage greater attention to medications of questionable benefit in the context of hospice services, may encourage discontinuation. Additional investigation into barriers and facilitators to discontinuing statins in this setting are needed to further inform intervention design, along with studies to improve the evidence base and consistency of treatment guidelines regarding patient-centered outcomes of statin deprescribing.
Supplementary Material
ACKNOWLEDGEMENTS
Funding Source: This study was funded by a grant from the U.S. Department of Veterans Affairs (IIR 14-306, PI: Carolyn T. Thorpe). Dr. Niznik was funded by a T32 award from the National Institutes on Aging (T32AG021885) and Drs. Hunnicutt, Springer, and Vu were funded by postdoctoral fellowships through the Veterans Affairs Office of Academic Affiliations. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Prior presentations: Parts of this manuscript were presented at the 2019 American Geriatrics Society Annual Meeting and the 2019 Department of Veterans Affairs HSR&D/QUERI National Conference.
Conflict of Interest: JNH is now a full-time employee at GlaxoSmithKline; however, he was a VA postdoctoral fellow during the conduct of this study. All other authors have no conflicts of interest to disclose.
Sponsor’s Role: The VA had no role in the study design, data collection, analysis, or manuscript preparation. The views expressed in this paper are those of the authors, and do not necessarily represent the U.S. Department of Veterans Affairs.
REFERENCES
- 1.Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166(6):605–609. [DOI] [PubMed] [Google Scholar]
- 2.Bain KT, Holmes HM, Beers MH, Maio V, Handler SM, Pauker SG. Discontinuing medications: a novel approach for revising the prescribing stage of the medication-use process. J Am Geriatr Soc. 2008;56(10):1946–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poudel A, Yates P, Rowett D, Nissen LM. Use of Preventive Medication in Patients With Limited Life Expectancy: A Systematic Review. J Pain Symptom Manage. 2017;53(6):1097–1110 e1091. [DOI] [PubMed] [Google Scholar]
- 4.Matlow JN, Bronskill SE, Gruneir A, et al. Use of Medications of Questionable Benefit at the End of Life in Nursing Home Residents with Advanced Dementia. J Am Geriatr Soc. 2017;65(7):1535–1542. [DOI] [PubMed] [Google Scholar]
- 5.Hanlon JT, Aspinall SL, Handler SM, et al. Potentially suboptimal prescribing for older veteran nursing home patients with dementia. Ann Pharmacother. 2015;49(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack DS, Tjia J, Hume AL, Lapane KL. Prevalent Statin Use in Long-Stay Nursing Home Residents with Life-Limiting Illness. J Am Geriatr Soc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Ploeg MA, Floriani C, Achterberg WP, et al. Recommendations for (Discontinuation of) Statin Treatment in Older Adults: Review of Guidelines. J Am Geriatr Soc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundy SM, Stone NJ, Bailey AL, et al. 2019 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Journal of the American College of Cardiology. 2019;73(24):e285–e350. [DOI] [PubMed] [Google Scholar]
- 9.AMDA. Ten Things Physicians and Patients Should Question. 2013.
- 10.Holmes HM, Sachs GA, Shega JW, Hougham GW, Cox Hayley D, Dale W. Integrating palliative medicine into the care of persons with advanced dementia: identifying appropriate medication use. J Am Geriatr Soc. 2008;56(7):1306–1311. [DOI] [PubMed] [Google Scholar]
- 11.Onder G, Landi F, Fusco D, et al. Recommendations to prescribe in complex older adults: results of the CRIteria to assess appropriate Medication use among Elderly complex patients (CRIME) project. Drugs Aging. 2014;31(1):33–45. [DOI] [PubMed] [Google Scholar]
- 12.Lavan AH, Gallagher P, Parsons C, O'Mahony D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age Ageing. 2017;46(4):600–607. [DOI] [PubMed] [Google Scholar]
- 13.Levy C, Ersek M, Scott W, Carpenter JG, Foglia M. Life-sustaining treatment decisions initiative: Early implementation results of a national Veterans Affairs program to honor veterans' care preferences. Journal of General Internal Medicine. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng CW, Cheong SK, Govinda Raj A, Teo W, Leong I. End-of-life care preferences of nursing home residents: Results of a cross-sectional study. Palliat Med. 2016;30(9):843–853. [DOI] [PubMed] [Google Scholar]
- 15.Fromme EK, Zive D, Schmidt TA, Olszewski E, Tolle SW. POLST Registry do-not-resuscitate orders and other patient treatment preferences. JAMA. 2012;307(1):34–35. [DOI] [PubMed] [Google Scholar]
- 16.Kutner JS, Blatchford PJ, Taylor DH Jr., et al. Safety and Benefit of Discontinuing Statin Therapy in the Setting of Advanced, Life-Limiting Illness: A Randomized Clinical Trial. JAMA internal medicine. 2015;175(5):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thai M, Hilmer S, Pearson SA, Reeve E, Gnjidic D. Prevalence of Potential and Clinically Relevant Statin-Drug Interactions in Frail and Robust Older Inpatients. Drugs Aging. 2015;32(10):849–856. [DOI] [PubMed] [Google Scholar]
- 18.Akgun KM, Krishnan S, Feder SL, Tate J, Kutner JS, Crothers K. Polypharmacy Increases Risk of Dyspnea Among Adults With Serious, Life-Limiting Diseases. The American journal of hospice & palliative care. 2019:1049909119877512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tjia J, Cutrona SL, Peterson D, Reed G, Andrade SE, Mitchell SL. Statin discontinuation in nursing home residents with advanced dementia. J Am Geriatr Soc. 2014;62(11):2095–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullvain JA, Kozak KR, Moody JS, Campbell TC. Statin use in cancer patients with brain metastases: a missed communication opportunity at the end of life. Support Care Cancer. 2015;23(9):2643–2648. [DOI] [PubMed] [Google Scholar]
- 21.Campitelli MA, Maxwell CJ, Giannakeas V, et al. The Variation of Statin Use Among Nursing Home Residents and Physicians: A Cross-Sectional Analysis. J Am Geriatr Soc. 2017;65(9):2044–2051. [DOI] [PubMed] [Google Scholar]
- 22.Gulliford M, Ravindrarajah R, Hamada S, Jackson S, Charlton J. Inception and deprescribing of statins in people aged over 80 years: cohort study. Age Ageing. 2017;46(6):1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morin L, Vetrano DL, Grande G, Fratiglioni L, Fastbom J, Johnell K. Use of Medications of Questionable Benefit During the Last Year of Life of Older Adults With Dementia. J Am Med Dir Assoc. 2017;18(6):551 e551–551 e557. [DOI] [PubMed] [Google Scholar]
- 24.Intrator O, Hiris J, Berg K, Miller SC, Mor V. The residential history file: studying nursing home residents' long-term care histories(*). Health Serv Res. 2011;46(1 Pt 1):120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Intrator OM R; Cai S; Miller SC. . The Veterans Health Adminstration Residential History File: A Resource for Research and Operations. In. 2015 HSR&D/QUERI National Conference2015. [Google Scholar]
- 26.Hynes DM, Koelling K, Stroupe K, et al. Veterans' access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45(3):214–223. [DOI] [PubMed] [Google Scholar]
- 27.Mor V A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Med Care. 2004;42(4 Suppl):III50–59. [DOI] [PubMed] [Google Scholar]
- 28.Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13(7):595–601. [DOI] [PubMed] [Google Scholar]
- 29.Porock D, Oliver DP, Zweig S, et al. Predicting death in the nursing home: development and validation of the 6-month Minimum Data Set mortality risk index. J Gerontol A Biol Sci Med Sci. 2005;60(4):491–498. [DOI] [PubMed] [Google Scholar]
- 30.Porock D, Parker-Oliver D, Petroski GF, Rantz M. The MDS Mortality Risk Index: The evolution of a method for predicting 6-month mortality in nursing home residents. BMC research notes. 2010;3:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niznik JD, Zhang S, Mor MK, et al. Adaptation and Initial Validation of Minimum Data Set (MDS) Mortality Risk Index to MDS Version 3.0. J Am Geriatr Soc. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. Journal of Gerontology. 1994;49(4):M174–182. [DOI] [PubMed] [Google Scholar]
- 33.Saliba D, Buchanan J, Edelen MO, et al. MDS 3.0: brief interview for mental status. J Am Med Dir Assoc. 2012;13(7):611–617. [DOI] [PubMed] [Google Scholar]
- 34.Buccaneer Computer Systems & Service I. Chronic Condition Data Warehouse Medicare Administrative Data User Guide. 2013.
- 35.Berlowitz DR, Hickey EC, Saliba D. Can administrative data identify active diagnoses for long-term care resident assessment? J Rehabil Res Dev. 2010;47(8):719–724. [DOI] [PubMed] [Google Scholar]
- 36.Thorpe CT, Fowler NR, Harrigan K, et al. Racial and Ethnic Differences in Initiation and Discontinuation of Antidementia Drugs by Medicare Beneficiaries. J Am Geriatr Soc. 2016;64(9):1806–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niznik JD, Zhao X, He M, et al. Factors Associated With Deprescribing Acetylcholinesterase Inhibitors in Older Nursing Home Residents With Severe Dementia. J Am Geriatr Soc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maust DT, Blass DM, Black BS, Rabins PV. Treatment decisions regarding hospitalization and surgery for nursing home residents with advanced dementia: the CareAD Study. International psychogeriatrics / IPA. 2008;20(2):406–418. [DOI] [PubMed] [Google Scholar]
- 39.Boucher A, Haesebaert J, Freitas A, et al. Time to move? Factors associated with burden of care among informal caregivers of cognitively impaired older people facing housing decisions: secondary analysis of a cluster randomized trial. BMC Geriatr. 2019;19(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Department of Agriculture Economic Research Service. Measuring Rurality: Urban Influence Codes. http://www.ers.usda.gov/Briefing/Rurality/urbaninf/. Updated August 8, 2007. Accessed October 12, 2010.
- 41.Springer SP, Mor MK, Sileanu F, et al. Incidence and Predictors of Aspirin Discontinuation in Older Adult Veteran Nursing Home Residents at End of Life. J Am Geriatr Soc. 2020;68(4):725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21Suppl 1:154–162. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. Mean Body Mass Index (BMI): Situation and trends. https://www.who.int/gho/ncd/risk_factors/bmi_text/en/. Published 2019. Accessed June 24, 2019.
- 44.Elixhauser A, Steiner C, Harris DR, Coffey RN. Comorbidity measures for use with administrative data. Medical Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 45.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546–553. [DOI] [PubMed] [Google Scholar]
- 46.Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56(12):2298–2303. [DOI] [PubMed] [Google Scholar]
- 47.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45(7):703–714. [DOI] [PubMed] [Google Scholar]
- 48.Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53(2):183–194. [DOI] [PubMed] [Google Scholar]
- 49.Creating imputed variables through single hotdeck imputation [computer program]. College Station, TX: StataCorp; 2017. [Google Scholar]
- 50.StataCorp L. Stata Multiple Imputation Reference Manual, Release 15. College Station, TX: 2017. [Google Scholar]
- 51.Coviello V, boggess M. Cumulative incidence estimation in the presence of competing risks. The Stata Journal. 2004;4(2):103–112. [Google Scholar]
- 52.Cleves M, Gould W, Gutierrez R, Marchenko Y. An introduction to survival analysis using Stata, 3rd edition. 3 ed: Taylor & Francis; 2010. [Google Scholar]
- 53.Tjia J, Kutner JS, Ritchie CS, et al. Perceptions of Statin Discontinuation among Patients with Life-Limiting Illness. J Palliat Med. 2017;20(10):1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monteiro L, Maricoto T, Solha I, Ribeiro-Vaz I, Martins C, Monteiro-Soares M. Reducing Potentially Inappropriate Prescriptions for Older Patients Using Computerized Decision Support Tools: Systematic Review. J Med Internet Res. 2019;21(11):e15385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 56.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


