Abstract
Brain sex differences are programmed largely by sex hormone secretions and direct sex chromosome effects in early life, and are subsequently modulated by early life experiences. The brain’s resident immune cells, called microglia, actively contribute to brain development. Recent research has shown that microglia are sexually dimorphic, especially during early life, and may participate in sex-specific organization of the brain and behavior. Likewise, sex differences in immune cells and their signaling in the adult brain have been found, although in most cases their function remains unclear. Additionally, immune cells and their signaling have been implicated in many disorders in which brain development or plasticity is altered, including autism, schizophrenia, pain disorders, major depression, and postpartum depression. This review summarizes what is currently known about sex differences in neuroimmune function in development and during other major phases of brain plasticity, as well as the current state of knowledge regarding sex-specific neuroimmune function in psychiatric disorders.
Introduction
How sex differences in the brain are established and maintained:
Gonadal hormones:
Gonadal hormones, also called sex hormones, belong to the class of steroid hormones and include three major subclasses: androgens, estrogens, and progestins. Gonadal hormones are all synthesized from the common precursor, cholesterol, in several different endocrine tissues. These include the ovaries, which largely but not exclusively synthesize estrogens and progestins, the testes, which largely but not exclusively synthesize androgens, and the adrenal glands, which largely produce the closely-related stress and electrolyte regulating hormones, glucocorticoids and mineralocorticoids, but also produce sex hormones. These steroid hormones are hydrophobic, and when released into the bloodstream, they are carried by soluble steroid hormone binding globulins. Given their hydrophobic nature, steroid hormones readily cross the blood brain barrier and bind to their receptors to influence brain development and function (Stumpf & Sar, 1976). The brain, too, synthesizes steroid hormones, called ‘neurosteroids’. The brain possesses all of the necessary enzymes to synthesize steroid hormones de novo from cholesterol (Kimoto et al., 2001; Hojo et al., 2004). Thus, gonadally-derived and locally-derived hormones can modulate brain function in concert or independently.
Steroid hormones largely exert their effects on target cells by crossing the plasma membranes of cells via diffusion and binding to intracellular receptors located largely in the nucleus. Classical steroid hormone receptors include the androgen receptor, estrogen receptor alpha, estrogen receptor beta, and the progesterone receptor. Upon hormone binding, these intracellular receptors dimerize and undergo a transformational change that allows the hormone-receptor dimer to bind to hormone response elements on the DNA to regulate gene transcription (King & Greene, 1984; O’Malley & Tsai, 1992). This mode of steroid hormone-receptor action is a relatively slow process since it depends upon gene transcription and translation of new proteins. Beyond their role as transcription factors, steroid hormone-receptor complexes can have more rapid, non-genomic effects, often via activation of second messenger cascades (reviewed in Woolley, 2007). Hormones also act on non-nuclear receptors that are membrane-bound, including the G-protein coupled estrogen receptor (e.g., GPR30), estrogen receptor X, membrane progestin receptors and possibly others (Toran-Allerand, 2004; Thomas Pang, 2012). Membrane-associated estrogen receptors and progestin receptors can influence neural physiology within seconds to minutes (e.g., Kelly & Levin, 2001; Mermelstein & Micevych, 2008). For the purposes of this review on sex differences in the neuroimmune system, it is important to note that both neurons as well as immunocompetent cells in the brain, including microglia and astrocytes, have been shown to express receptors for all three major classes of hormones (e.g., Brinton et al., 2008; Garcia-Ovejero et al., 2002; Sierra et al., 2008; Spense & Voskul, 2012; see Table 1).
Table 1:
Immunocompetent cell types in the brain: Location, function, sex differences and hormonal responsiveness.
| Cell type | Location in CNS |
Neuroimmune function in CNS |
Known sex differences |
Hormone receptor expression |
|---|---|---|---|---|
| Microglia | Parenchyma | Secretion of cytokines, chemokines, growth factors; phagocytosis; antigen presentation; injury response; autoimmune/degenerative conditions |
Yes: number, signaling and phenotype across life |
ERα,β; AR; PR |
| Astrocytes | Parenchyma | Secretion of cytokines, chemokines, growth factors; phagocytosis; antigen presentation |
Yes: morphology and inflammatory signaling across life |
ERα; AR; PR |
|
Perivascular macrophages |
Parenchyma | BBB function; humoral communication across BBB; injury response; autoimmune/degenerative conditions |
Unknown | Unknown, but hormones influence their function |
| Mast cells | Parenchyma; meninges; choroid plexus |
Immune surveillance, host defense; Behavioral effects; contribute to injury response; autoimmune/degenerative conditions |
Yes: mast cell protease 2 |
ERα |
|
T & B cells, granulocytes; monocytes; dendritic cells |
Meninges; choroid plexus; parenchyma? |
Immune surveillance, antigen presentation; host defense; Behavioral effects; contribute to injury response; autoimmune/degenerative conditions |
Unknown | ERα (T & B cells); ERβ (dendritic cells) |
The Organizational/Activational model of hormonally-regulated behavior:
Sex-typical hormone exposure regulates the display of sex-typical physiological and behavioral outcomes throughout the lifespan, including during development and during the period in which the outcome is observed, usually in adulthood. This two-pronged role for steroid hormones was defined by Phoenix et al., (1959) in a series of experiments showing that both early life as well as adult hormones was crucial to the display of sexual behavior, which has since come to be known as the “Organizational/Activational” model of hormone action. During particular windows of development, dubbed ‘critical’ or ‘sensitive’ periods, hormone exposure leads to sex-typical “organization” of the brain, including the establishment of sexually-dimorphic gene expression, cell birth, death, migration, differentiation and circuit wiring and refinement. Critical or sensitive periods are so named because hormones cannot induce such organizational effects if the timing of exposure is outside of that period. There is now also evidence to suggest that puberty is a second “mini-organizational” period for completing and/or refining the sexual differentiation process (Sisk, 2017; Sisk & Zehr, 2005). In the case of both male- and female-typical behaviors, adult concentrations of circulating hormones are the second necessary component for the full complement of sex-typical physiological or behavioral phenotypes to manifest. These “activational” hormones act on the sex-typical brain that resulted from the organizational effects established during ontogeny.
Sexual differentiation
Sexual differentiation is the name for the organizational process by which the brain develops sex-biased characteristics during a critical period of early life. Brain sexual differentiation is a largely permanent process: Sex differences in the size, shape, and function of the brain are established early in development and remain relatively stable throughout life. Sexual differentiation begins with sex determination, which depends first upon sex hormone complement— in mammals, either XX in genetic females or XY in genetic males. The gene sex determining region of the Y chromosome (SRY) initiates the sex determination process by coding for the protein testis determining factor (TDF). TDF is a secreted signal that programs the differentiation of the bipotential gonadal tissue into testes in males. In females, the lack of TDF allows differentiation of the bipotential gonad into ovaries along a default program in the absence of any intervening signals.
Male-typical sexual differentiation depends upon secretion of androgens during the perinatal window in males. The testes secrete androgens, which in turn act to differentiate the internal and external genitalia as well as the brain. In humans, male androgen levels are elevated in utero, and the process of brain sexual differentiation is largely complete by birth. In contrast, rodent sexual differentiation begins during the late prenatal period, around embryonic day (E) 18.5, and extends into the early postnatal period (Reviewed in McCarthy et al., 2008). Moreover, in rodents but not primates, the dominant effects of sexual differentiation are accomplished by the actions of the hormone estradiol (Bakker & Baum, 2008), which is locally metabolized in the brain from testosterone by the enzyme p450 aromatase (Naftolin et al., 1971; Roselli & Resko, 1993).
Early life androgen exposure promotes a process called masculinization, wherein a male typical phenotype is programmed. Behavioral outcomes of this masculinization process include a strong motivation to mount sexually receptive females, intromit, and ejaculate, and additionally to engage in male-male aggressive behaviors (Fig. 1; Reviewed in Yang & Shah, 2016; Hull & Dominguez, 2007). Genetic females (both humans and rodents) can be shunted toward a masculinized path of sexual differentiation by exogenous or atypical androgen exposure. In rodents, females can be masculinized by exogenous testosterone or estradiol administered during the prenatal period or first 10 postnatal days (McCarthy, 2008). In humans, this role for androgens in disrupting female-typical development is best evidenced by the condition congenital adrenal hyperplasia (CAH), in which an enzyme deficiency leads the adrenal glands to produce high levels of androgens. Girls and women with CAH show partial masculinization of the genitalia, as well as masculinized play behavior in childhood and masculinization of spatial reasoning (Berenbaum et al., 2012; Berenbaum and Hines, 1992; Pasterski et al., 2011).
Figure 1: Sexual differentiation of the brain and behavior.
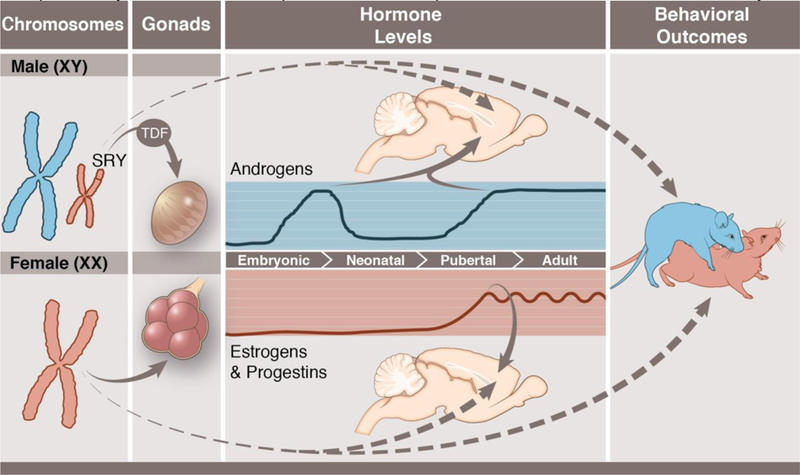
Male typical (top; blue) and female typical (bottom; pink) sexual differentiation. Sex chromosome complement (XX in females; XY in males) leads to sex determination. The Y-chromosome gene, SRY, codes for the protein testicular determining factor (TDF), which programs the bi-potential gonads to differentiate into testes (left). In rodents, the testes begin secreting androgens during the late embryonic period, and these androgens lead to sexual differentiation of the brain and other tissues (middle). In the rodent brain, androgens are converted into estrogens, and estrogens masculinize the brain. In genetic females, the gonads differentiate into ovaries, and the ovaries are largely quiescent in the perinatal period (middle), thus brain feminization occurs largely in the absence of active hormonal signals. At puberty, both male and female gonads produce sex-typical hormone secretions, namely high androgen levels and high but pulsatile estrogen and progestin levels in females. Both early life (organizational) and post-pubertal (activational) hormones contribute to sex differences in behavior, most notably male mounting behavior (blue rat) and female lordosis behavior (pink rat) (right). Sex chromosomes can also have independent effects on brain organization as well as adult sex differences in behaviors (dashed lines). Anthony S. Baker, CMI, Reproduced with the permission of The Ohio State University.
Additionally, early life androgen exposure in males leads to a parallel, but mechanistically distinct process, called defeminization, whereby the male brain is rendered incapable of engaging in female-typical sexual behaviors. The process of defeminization has been shown to be distinct from masculinization, because steroid hormones induce different downstream effectors to induce the two processes and can be dissociated by manipulating these effectors (Todd et al., 2005). In the absence of androgen exposure, the female brain undergoes feminization. In females, the ovaries are largely quiescent during the perinatal period, and thus feminization has been considered a default process (Jost et al., 1973). However, at least in mice, females lacking functional aromatase activity to synthesize estrogens do not show entirely wildtype female sexual behavior, and pre-pubertal estrogen exposure rescues sexual behavior deficits, indicating a possible role for estrogens in the feminization process (Bakker & Baum, 2008; Brock et al., 2011). The nature of this ‘feminization’ process by estrogens remains unelucidated. Outcomes in females that are programmed in the absence of androgen exposure include strong motivation to perform sexual solicitation and take the reflexive sexually receptive posture known as lordosis, as well as the motivation to engage in maternal behavior following pregnancy and parturition (Lonstein & De Vries, 2000; Beach et al., 1976; Zemlan & Adler, 1977).
Recent work from Nugent et al. (2015) suggests that, although the female differentiation process proceeds along a default program without the necessity of an active hormonal signal, the female brain is actively prevented from undergoing masculinization via epigenetic means. In the rat medial preoptic area, females have higher levels of DNA methylation than males, and this methylation acts to silence male-typical patterns of gene expression (Nugent et al., 2015). When methylation is inhibited in the female POA, masculinization of the nucleus and adult sexual behavior results. Additionally, methylation appears to be crucial to maintaining the organizational period for sex differences: Interfering with DNA methylation after the critical period for sexual differentiation has occurred can “reopen” the organizational capacity of steroid hormones on the brain (Nugent et al., 2015). There are other examples in which permanent sexual differentiation appears reversible via relatively limited genetic manipulations (e.g., Kimchi et al., 2007), thus there may be many active signals that repress a feminine phenotype and/or masculine phenotype outside the critical period for sexual differentiation.
Despite the dominance of the “organizational” hormonal theory of sexual differentiation, there is increasing evidence that some sex differences established during ontogeny are also programmed by other contributing factors, including sex chromosome effects and maternal-fetal interactions. For example, studies using the four core genotype mouse model, in which sex chromosome complement and gonadal hormone status are dissociated, certain sexually dimorphic phenotypes (e.g., pain sensitivity, alcohol consumption, metabolic status) appear to be more programmed by sex chromosome status than hormonal status (reviewed in Arnold, 2017). The placenta is another key contributor to sex differences in brain development, both at baseline and in response to stressors, and sex differences in placental function emerge prior to the onset of gonadal hormone secretion (Nugent and Bale, 2015). This review will not exhaustively describe these sex differences or the mechanisms downstream of hormones for establishing these sex differences, but we point interested readers to other excellent reviews on these topics (Arnold, 2017; McCarthy et al., 2017; Nugent and Bale, 2015). Herein, we will narrow in on sex differences in the neuroimmune system across the developmental, pubertal and adult windows. Recently emerging evidence suggests that there are significant sex differences in neuroimmune function that may mediate the establishment and maintenance of sex differences in brain function and behavioral outcomes later in life. These sex differences may also confer sex-biased risk for brain-based disorders, particularly those related to mental health.
A primer on immune cells in the developing brain:
Before we explore what is known (and still unknown) about neuroimmune cells as both sexually differentiated and sexual differentiator, we must briefly describe the origin and development of immunocompetent cells within the CNS. Immunocompetent cells within the brain include microglia, which are tissue resident macrophages of the CNS (Kettenmann et al., 2011); mast cells, which are also innate immune cells that, in the periphery, are associated with allergy, atopy, tissue edema, and itch (Silver & Curley, 2013); astrocytes, which are derived from neural stem cells, but are capable of inflammatory signaling, antigen presentation, and phagocytosis (Dong & Benveniste, 2001); perivascular macrophages, which have a similar developmental origin to microglia and communicate peripheral information to endothelial cells of the blood brain barrier (Faraco et al., 2017); and meningeal and choroid plexus immune cells, including monocytes, mast cells, neutrophils, dendritic cells, and T cells, and which can influence brain physiology and behavior across the blood brain barrier (Herz et al., 2017; Meeker et al., 2012). Each of these other cell types has been implicated in normal CNS function the response to injury, and the pathophysiology of autoimmune and neurodegenerative conditions, and in many cases gonadal hormones modulate their function in these contexts (Brinton et al., 2008; Garcia-Ovejero et al., 2002; Spense & Voskuhl, 2012; Wu et al., 2013) see table 1 for a summary). Because most work on central immune cells has focused on microglia, the bulk of this review will focus on microglia and known sex differences in their function.
Microglia are the most abundant innate immune cell of the brain, comprising approximately 8–12% of total brain cells (Kettenmann et al., 2011). Unlike other brain cells, however, microglia are not derived from the ectoderm. Instead, microglia progenitors are peripheral in origin and invade the brain early in ontogeny. These microglia become entrapped behind the developing blood brain barrier and constitute a locally sustaining and long-lived cell population within the brain (Ajami et al., 2007; Ginhoux et al., 2010). Starting at E8.5–9.5 in rodents, microglia progenitor cells derived from the yolk-sac begin to infiltrate the fetal neural tissue through the nascent vasculature (Ginhoux et al., 2010; Stremmel et al., 2018). Similarly to microglia in rodents, microglia in humans appear early in neural development, around 4.5 weeks of gestation (Verney et al., 2010). Microglia in the immature brain have recently been phenotyped in several studies and shown to have distinct gene expression signatures during ontogeny. Thus, microglia can be classified as belonging to one of several stages of development, including the progenitor phase (~E9.5-E14), embryonic phase (~E14-E18), the perinatal phase (~E18-Postnatal day (PN) 9), and the more mature phase that begins to appear at PN28 and endures to adulthood (Bennett et al., 2016; Matcovitch-Natan et al., 2016; Thion et al., 2017).
The pre-microglia progenitor is developmentally distinct from most other resident tissue macrophages in terms of gene expression (Bennett et al., 2018; Hoeffel et al., 2015; Sheng et al., 2015). Once microglia progenitors infiltrate the brain, they differentiate into early microglia and colonize the entirety of the brain through migration and local proliferation (Bennett et al., 2016; Matcovitch-Natan et al., 2016). Colonizing microglia are typically amoeboid in shape, and over the course of the first weeks of postnatal life, microglia become fully ramified in shape. In rodents, this transition to a ramified morphology is complete around the end of the 2nd-3rd postnatal week, which is also accompanied by increased expression of Tmem119 (Bennett et al., 2016; Dalmau et al., 1998, 1997). Microglia density peaks approximately 3 weeks after birth; microglia number decreases to adult levels 4–5 weeks after birth (Kim et al., 2015; Nikodemova et al., 2015). Microglia appear to use local microenvironment signals to determine the density at which they seed the brain, as cultured microglia transplanted into a brain genetically depleted of microglia via cell survival factor (CSF) 1 receptor knockout rapidly assume a wild-type distribution of cells (Bennett et al., 2018). With the exception of the initial infiltration of the pre-microglia yolk-sac derived progenitors, the microglia population is largely sustained by proliferation throughout life, with little to no contribution of peripheral macrophages under basal conditions (Ajami et al., 2007). Across the remainder of the lifespan, microglia completely turn over approximately twice, with apoptotic cell death and new cell genesis occurring in a ‘coupled’ process (Askew et al., 2016).
Microglia function during development:
Microglia sample their local microenvironment by extending and retracting their many processes (Nimmerjahn, 2005). In doing so, microglia sense debris, dead or dying cells, and pathogens in the brain; engulf deleterious or foreign materials in phagosomes; and digest and eliminate this debris within phagolysosomes (reviewed in (Kettenmann et al., 2011). Within the context of brain injury or infection, microglia home to sites of inflammation and contribute to brain repair or host defense and recruit other immune cells to the site of active infection or degeneration (reviewed in Kettenmann et al., 2011). As such, microglia had long been dismissed as “garbage collectors” or defenders against rare viral or bacterial infections of the brain. And yet, a recent surge of evidence also suggests that microglia actively regulate brain development and physiology by a) sculpting the developing brain early in life and into adulthood, and b) regulating normal developmental processes in the brain by secretion of cytokines, chemokines, and other canonical immune molecules.
Microglia contribute to brain function as soon as they infiltrate the neural tissue, engulfing apoptotic cells as early as the second prenatal week (Kierdorf et al., 2013). During brain development, microglia also engulf and digest (phagocytose) cells and synapses, release diffusible molecules that stimulate synapse formation, cell genesis, and myelin production, and mediate neurite outgrowth (Cunningham et al., 2013; Hagemeyer et al., 2017; Miyamoto et al., 2016; Parkhurst et al., 2013; Pont-Lezica et al., 2014; Schafer et al., 2012; Ueno et al., 2013; Wakselman et al., 2008). Through activation of the complement cascade, microglia phagocytose presynaptic elements in the dorsolateral geniculate nucleus of thalamus in an activity-dependent manner (Schafer et al., 2012). Microglia also phagocytose postsynaptic elements in the developing brain, and recent work has identified astroglia-derived interleukin (IL) 33 as a factor that regulates microglial synaptic phagocytosis (Vainchtein et al., 2018). However, the strength of past studies that examined microglia synaptic engulfment has recently been questioned. During development, a recent study shows that microglia phagocytized presynaptic, but not postsynaptic elements, largely by engaging in ‘trogocytosis’, or nibbling away small bits of presynaptic elements (Weinhard et al., 2018a). Weinhard et al. demonstrated that, while under light sheet microscopy, postsynaptic densities appeared to co-localize with microglia however, when examined using serial electron microscopy microglia were not engulfing the post-synaptic element. These data suggest that standard microscopy techniques may be inadequate to definitively determine whether microglia are pruning post-synaptic elements under homeostatic or diseased conditions.
Microglia also support developmental cell genesis. Microglia support neurogenesis and oligodendrogenesis by producing IL-1β, tumor necrosis factor alpha (TNFα), interferon gamma (IFNγ), IL-6 and insulin-like growth factor 1 (IGF1) (Hagemeyer et al., 2017; Shigemoto-Mogami et al., 2014). Recent research has found that a Cd11c+ population of microglia, previously thought to be dendritic cells, may be primarily responsible for supporting oligodendrogenesis (Hagemeyer et al., 2017; Wlodarczyk et al., 2017). Microglia also regulate neurogenesis by mediating progenitor pool size in the subventricular area: Microglia phagocytose healthy neural progenitor cells at the end of the period during which robust neurogenesis occurs, and depleting microglia during this window increases the size of the progenitor pool (Cunningham et al.,2013). In the case of the Cunningham et al. study, it would appear that microglia eliminate healthy living cells, because many engulfed cells do not express apoptotic markers (Cunningham et al., 2013). However, the mechanism or “tags” that regulate microglia phagocytosis of progenitor cells remains unknown.
In the young adult brain, microglia also clear apoptotic cells: 42% of these cells were PSA-NCAM+, a marker of neural progenitor cells, and 28% were POMC+, a marker of post-mitotic differentiating newborn neurons (Abiega et al., 2016; Sierra et al., 2010). Within the hippocampus, developmental cell death is at least partly mediated by microglia through Cd11b and DAP12 (Wakselman et al., 2008). Microglia also contribute actively to the cell death process by delivering free radicals to apoptosis-committed cells to complete the cell death process (Marin-Teva et al., 2004). Last, microglia support spinogenesis and synaptogenesis. Microglial physical contact with developing synapses, as well as microglial-derived prostaglandin E2 (PGE2), brain-derived neurotrophic factor (BDNF), IL-1β and IL-10 all regulate spinogenesis and synaptogenesis across several brain regions during development (Lenz et al., 2013; Lim et al., 2013; Miyamoto et al., 2016; Parkhurst et al., 2013).
Perinatal sex differences in neuroimmune parameters:
Microglia:
A growing body of research documents sex differences in microglia during development that are dynamic across the critical period for sexual differentiation. One of the first studies to compare male and female microglia found that females have more microglia in the hypothalamus, amygdala, and hippocampus shortly after the prenatal testosterone surge (Schwarz et al., 2012). However, in the early postnatal period, males have more microglia than females. Others have found that females have more microglia in the somatosensory area throughout perinatal development (Thion et al., 2017), though the function of this sex difference is unknown.
Males also have more microglia than females overall and more deramified, ameboid-shaped microglia in the developing preoptic area (POA). The POA is a brain a region that is crucial to the expression of male-typical sexual behavior in adulthood, as well as female-typical maternal behavior (reviewed in Lenz & McCarthy, 2010). Sex differences in POA microglia number and morphology are organized by exposure to estradiol, such that females treated neonatally with estradiol show rapid increases in the number of ameboid microglia in the POA (Lenz et al., 2013). This study is one of few cases in which the function of a sex difference in microglia has been determined. Male-typical synaptic patterning, namely an increase of dendritic spine synapses, depends on microglial release of the immune mediator PGE2 in the POA (Lenz et al., 2013). This synaptic patterning correlates strongly with masculine patterns of sexual motivation and sexual performance in adulthood (Amateau and McCarthy, 2004, 2002; Lenz et al., 2013; Wright and McCarthy, 2009).
We have also recently found that microglia phagocytose more cells in the neonatal female hippocampus than in the neonatal male hippocampus, but not before the prenatal testosterone surge (Nelson et al., 2017). This sex difference, too, is hormone-driven— estradiol treatment of females reduces phagocytic microglia to male-typical numbers (Nelson et al.,2017). Compared with males, females also show higher gene expression related to microglia phagocytosis, and the sex difference in microglia phagocytosis is directed at neural progenitor cells. Thus females could have lower levels of cell proliferation due to increased phagocytosis of progenitor cells. Others have found the rate of neurogenesis in the developing hippocampus is hormone-dependent and higher in males than it is in females (Bowers et al., 2010). However, it is unknown if there is a sex difference in the rest of the brain or later in development. Interestingly, although there was no difference in the number of phagocytic cups that contained pyknotic nuclei, there was a sex difference in the number of engulfed non-pyknotic nuclei (Nelson et al., 2017). However, we have not yet determined if the sex difference in phagocytosis of progenitor cells included apoptotic or healthy cells. Future studies are necessary to test for a mechanistic link between sex differences in microglia phagocytosis of progenitor cells and sex differences in neurogenesis. Additionally, how healthy cells are “tagged” for removal and if there are sex differences in such “tags” remains unknown.
In line with increased phagocytic capacity in females during development, another recent study has demonstrated that, in the neonatal hippocampus, female microglia express more CD68, a lysosomal marker used into indicate phagocytic capacity, in the second week postnatal that diminishes by puberty (Weinhard et al., 2018b). Moreover, this female-biased phagocytic capacity correlates with a transient sex difference in synapse number in the neonatal hippocampus, though the two phenomena have yet to be mechanistically linked (Weinhard et al., 2018b). Several labs have found that a) female microglia are more developmentally mature than male microglia across development and b) females have higher expression of inflammatory, phagocytic, and immune genes than males (Hanamsagar et al., 2017; Thion et al., 2017), though it is unknown whether this in maturity alters the rate of any normal developmental processes, such as cell genesis, death, synaptic patterning, etc., between the sexes.
Considering the differences between the main hormonal effector of sex differentiation of the brain between rodents and humans (i.e. estradiol vs testosterone), it is important to determine whether sex differences in human microglia development bear similarity to that seen in rodents. A landmark study of human microglial maturation found that while microglia gene expression was distinct across developmental stages, in the mid-gestation human brain, microglia gene expression did not differ by sex (Thion et al., 2017). However, another study, using a much larger cohort than Thion et al., 2017, found higher expression of microglia markers in males compared with females in whole brain tissue over development (Werling et al., 2016). Further research is needed to determine whether sex differences found in rodents map onto sex differences found in humans. Additionally, it remains unknown whether microglia are sexually differentiated throughout the brain or only in specific brain areas during development.
Lastly, it is important to determine how sex differences in microglia arise. Sex steroids could have direct or indirect effects on microglia depending on whether microglia themselves express sex steroid receptors (see Table 1). This may also be important for understanding differences in development sex differences of microglia in humans compared to rodents (i.e. testosterone vs estradiol driven). Some groups have found that microglia enriched from the whole brain express the gene for estrogen receptor alpha (ESR1) at low levels early in life and higher levels in adulthood (Crain et al., 2013). However, other groups found that neonatal microglia in the hippocampus do not express ESR1 or ESR2, the gene for estrogen receptor estrogen receptor beta, or androgen receptor; rather, other cells express sex steroid receptors (Turano et al., 2017). Similarly, estrogen receptor alpha staining was not seen in microglia in the neonatal POA, where strong sex differences in microglia properties have been demonstrated (Lenz et al., 2013). Others have found that microglia express several steroid hormone receptors in adulthood (Sierra et al., 2008). Thus, microglia likely express sex steroid receptors in a region-specific, time-dependent, and/or context dependent manner (such as after injury, stress, etc), and more work is needed to appreciate the subtleties of hormone sensitivity in microglia. Figure 2 summarizes what is currently known (or hypothesized) about sex differences in microglia during development.
Figure 2: Sex differences in microglia across different developmental windows and conditions.
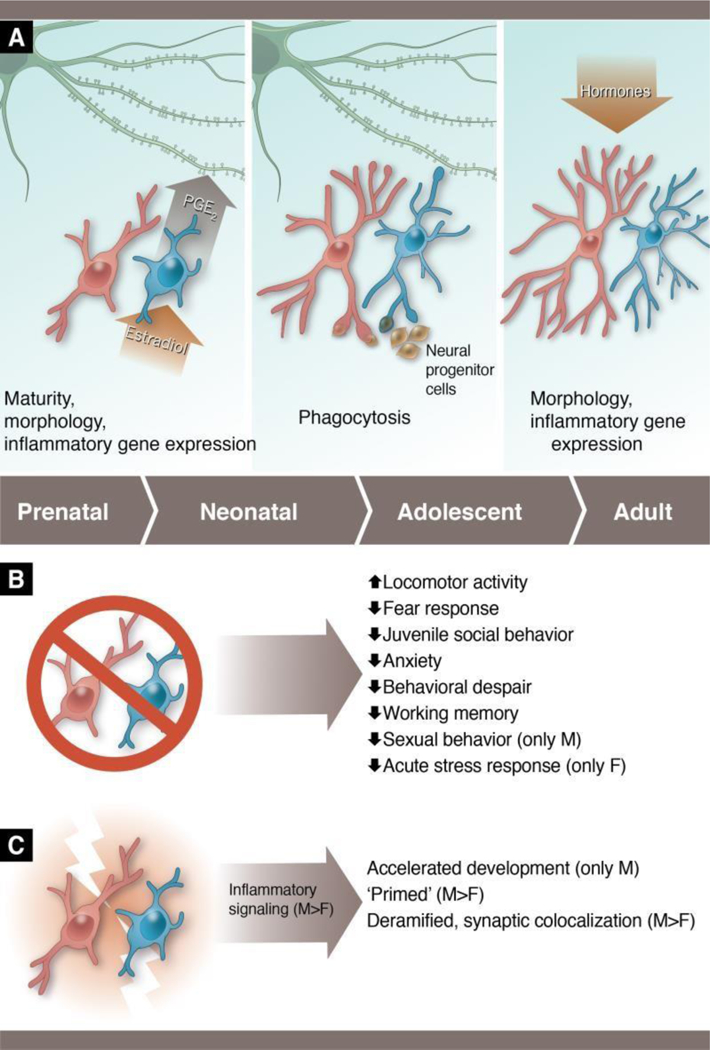
(A) During the perinatal period (left panel), males have more microglia than females, and the microglia are in a more activated and immature form. Microglia in the male preoptic area respond to the masculinizing hormone, estradiol, by releasing the inflammatory lipid, prostaglandin E2 (PGE2), and PGE2 is responsible for programming male-typical dendritic spine genesis/maintenance that is necessary to display adult copulatory behavior. During the perinatal-juvenile period (middle panel), female microglia appear to show a higher level of phagocytic activity in the hippocampus than male microglia. Female microglia target neural progenitor cells at a higher rate than males, and higher phagocytosis in females is also correlated with reduced dendritic spines in the hippocampus at this time, though they have not been causally linked to date. In adulthood (right), female microglia are hormone responsive and show hyper-ramification relative to males. (B) Studies in which microglia have been depleted early in life demonstrate lifelong behavioral changes in both males and females, with sex-specific effects being seen in some outcomes. (C) Studies in which microglia are stimulated early in life show that male microglia are more responsive than female microglia. Male microglia depicted in blue and female microglia in pink throughout. Figure based on data from Bollinger et al., 2016; 2017; Hanamsagar et al., 2017; Hui et al., 2018; Lenz et al., 2013; Makinson et al., 2017; Nelson & Lenz, 2016; Nelson et al., 2017; Schwarz et al., 2011; Van Ryzin et al., 2016, Weinhard et al., 2018b). Anthony S. Baker, CMI, Reproduced with the permission of The Ohio State University.
Immune molecules:
There are also sex differences in several immune molecules in the developing brain, and some of these sex differences have been shown to actively contribute to the sexual differentiation process. Above, we have discussed the case of PGE2 in the medial preoptic area, which is derived from microglia and also from neurons, and leads to male-typical dendritic spine patterning that underlies sexual behavior (Lenz et al., 2013). Another such example is a case in the developing anteroperiventricular nucleus of the preoptic area (AVPV), which is a sexually dimorphic nucleus that controls female hormonal cyclicity. During the perinatal period, increased cell death in the male AVPV leads to a smaller AVPV in males compared with females (Sumida et al., 1993). A study by Krishnan et al., (2009) demonstrated that an immune-related molecule, tumor necrosis factor (TNF) family member repressor protein (TRIP), is necessary for this developmental sex difference in cell death of GABAergic cells to occur. TRIP prevents the TNFα-activated cell survival cascade in males, leading to higher rates of cell death, whereas in females, TRIP is not expressed and thus cell death is lower (Krishnan et al., 2009). It is unknown which cell type produces this signal, though microglia may be the most likely candidate.
Beyond these examples, there are several other studies that document sex differences in immune molecules, though the functional significance of these sex differences is still unknown. Within the hippocampus and cortex, female rats have higher concentrations of IL-1β and IL-1 receptor antagonist, and IL-1 receptor through life (Schwarz et al., 2012). On the other hand, male rats produce higher concentrations of CCL4 and CCL20 at PN0 than females, but not at other time points. Some chemokines and chemokine receptors, such as CCL22 and CCR4, are higher in females at P4, but higher in males at P60. Although these data were not collected from isolated microglia, several studies have found sex differences in immune gene expression in isolated microglia. Microglia-specific gene expression studies have found that whole brain microglia from females express more IL-1β, TNFα, IL-6, IL-10, P2X5, and P2Y4 at PN3, but not at later time-points (Crain et al., 2013, 2009). The consequences of these sex differences may be highly relevent for the physiological responses of microglia and their regulation of brain development or in response to early life perturbations, but have not been investigated to date.
Astrocytes:
There are also sex differences in astrocyte number and morphology in the developing brain. In the rat POA, male astrocytes are more morphologically complex than female astrocytes; estradiol drives this effect (Amateau and McCarthy, 2002). In the nearby arcuate nucleus, a hypothalamic region that regulates pituitary function, male astrocytes are more stellate than female astrocytes (Mong and McCarthy, 2002). This sex difference is apparent at birth, remains so throughout life, and is also driven by organizational gonadal hormone effects (Mong and McCarthy, 2002). Extensive work by Garcia-Segura, Barreto, and colleagues has assessed sex differences in astrocytes and their inflammatory capacity throughout life, including in the context of healthy adulthood, injury, or animal models of neurodegenerative diseases (reviewed in Acaz-Fonseca et al., 2016). In the context of development, primary astrocytes derived from the neonatal cerebral cortex of males, females, or females treated with male-typical androgen exposure showed differential responses to immune challenge, with male and masculinized female astrocytes producing more inflammatory molecules, such as IL-6, TNFα, and IL-1β (Santos-Galindo et al., 2011). Such differences in the astroglial response to immune challenge may indicate that astrocytes participate in early life programming of the brain and behavior by stress, infection, or similar insults. A recent study has shown that astrocyte-derived IL-33 directs microglial synaptic pruning during development (Vainchtein et al., 2018), but it has not yet been determined whether there are sex differences in astroglial function in this context that could be relevant to sex-specific synaptic patterning. However, this study highlights the importance investigating crosstalk between different immunocompetent cell types in the developing brains of both sexes rather than studying one cell type in isolation.
Mast cells:
Mast cells are peripherally-derived innate immune cells of hematopoetic origin that reside in various tissues, including the gut, skin, airway, and brain (reviewed in Silver Curley & Silver). They are best appreciated as contributors to type I hypersensitivity conditions, including food allergy, atopy, and asthma, as well as the response to parasitic infections (Abraham and St John, 2010). Mast cells within the brain are most abundant during early life in both rodents and humans (Dropp, 1979; Khalil et al., 2007; Masliniska et al., 2001). Like microglia, mast cells rapidly respond to inflammatory stimuli by releasing cytokines, prostaglandins, and proteases (Silver and Curley, 2013), but mast cells also engage in bolus release of granules containing histamine and serotonin (Wernersson and Pejler, 2014). Mast cells are also known to regulate microglia function in response to inflammation. For example, mast cell degranulation, when triggered by immune challenge or a mast cell secretagogue, activates microglia release of pro-inflammatory cytokines, effects that can be blocked in vitro by co-administration of mast cell inhibitors (Dong et al., 2017; Zhang et al., 2016). Males express a mast cell specific gene, mast cell protease 2, in the developing POA, whereas females do not (Nugent et al., 2015), suggesting that sex differences in mast cells could contribute to sexual differentiation in this brain area. Additionally, mast cell numbers in the brain have been shown to increase during courting and reproduction in birds (Silverman et al., 2002) and to be a source of gonadotropin-releasing hormone (GnRH) (Khalil et al., 2003). Thus, mast cell dynamics may contribute to sex-specific brain function in ways that have not yet been reported.
Overall, these studies show that males generally appear to have higher basal microglia and neuroimmune tone in the developing brain, but in very few cases have these sex differences been linked to sex differences in brain development, physiology, or later life behavior. This is one area in which research on sex differences in neuroimmune function needs to expand and focus future inquiry. The following section focused on sex differences in neurodevelopmental disorders is arguably the best reason to focus on developmental sex differences in neuroimmune parameters, as they may give tremendous insight into the pathophysiology of these complex and often debilitating disorders.
Relevance to sex differences in developmental disorders:
Almost every mental health disorder has a neurodevelopmental component. Environmental factors such as prenatal stress, early life adversity, prenatal inflammation/immune activation, altered prenatal steroid environment, famine, and maternal obesity all increase the risk for developing a mental health disorder (Andersen, 2015; Baron-Cohen et al., 2015; Brown, 2011; Brown et al., 2014; Canetta et al., 2014; Godfrey et al., 2017; Kosidou et al., 2015; Zeanah et al., 2009). Furthermore, there are sex differences in many mental health disorders that have a neurodevelopmental origin. More males are diagnosed with Autism Spectrum Disorder (ASD) and Tourette Disorder than females, whereas more females than males are diagnosed with anxiety, mood disorders, and Rett Syndrome (Bandelow and Michaelis, 2015; Kessler et al., 2012; Kim et al., 2011; Robertson, 2008; Wingate et al., 2014). Additionally, it is more common for males to be diagnosed with schizophrenia (1.4:1) and males often have more severe negative symptoms (Abel et al., 2010). Interestingly, perturbations that increase the risk for these disorders also alter microglia function, which may mean that microglia mediate some of the effects of perinatal perturbations.
Neurodevelopmental disorders have a multifactorial origin that includes many contributors beyond the immune system or neuroimmune dysfunction, which are beyond the scope of this review. However, a large body of research has shown that there are significant alterations in the microglia and immune system in many disorders with a neurodevelopmental origin. Some studies have found increased microglia number and/or density in post-mortem brain samples from patients with schizophrenia, autism, and Tourette disorder. In living human patients, PET scans using the ligand that binds translocator protein (TSPO) have been used to infer microglial (and astroglial) activation in various neuropathological conditions. TSPO is likely indicative of both increases in microglia proinflammatory tone as well as phagocytosis (Karlstetter et al., 2014). In several studies of people with neurodevelopmental disorders, TSPO binding is elevated relative to non-affected control participants (Bloomfield et al., 2016; Fillman et al., 2013; Laskaris et al., 2016; Lennington et al., 2016; Morgan et al., 2012, 2010; Suzuki et al., 2013). However, several studies have also found no change in TPSO binding or even decreased binding in schizophrenia (Collste et al., 2017; Coughlin et al., 2016; Hafizi et al., 2016). TPSO binding density must be interpreted cautiously because changes in binding are context-dependent, because other cell types express TPSO, and because there is high individual variability within healthy populations (see Notter et al., 2017) for an in-depth review). Transcriptomic analysis of brain tissue from people with ASD and Tourette Disorder shows a strong microglia and immune component in the altered transcriptome (Gandal et al., 2018; Lennington et al., 2016; Werling et al., 2016). Several mouse studies have shown some microglia involvement in Rett Syndrome, an X linked disorder with a similar phenotype to ASD, though the centrality of the microglia involvement in the disorder has not proved consistent across studies (Derecki et al., 2012; Maezawa and Jin, 2010; Schafer et al., 2016; Wang et al., 2015). Yet no studies to date have analyzed human tissue from Rett Syndrome patients. All these studies analyzed adults because many symptoms of these disorders are diagnosed later in life (with the exception of ASD and Tourette Disorder, which show symptoms early in life). Many mental health disorders with a developmental component are also associated with peripheral immune changes. These changes include altered immune responses and increased levels of circulating cytokines (Ashwood et al., 2011; Careaga et al., 2017; Goldsmith et al., 2016; Miller et al., 2011; Pramparo et al., 2015).
Perinatal immune activation
Several epidemiological studies noted that mothers that experienced infection during pregnancy had a higher risk of their offspring developing schizophrenia (Mednick et al., 1988). These findings gave rise to the maternal immune activation hypothesis of neurodevelopmental disorders. It has since become clear that maternal infection and general maternal immune activation/inflammation can increase risk of offspring developing schizophrenia as well as ASD (Brown, 2012; Brown et al., 2014). For example, maternal infection with influenza or Poly I:C immune challenge altered pre-pulse inhibition of the acoustic startle response in offspring (a behavior associated with schizophrenia) and decreased offspring exploratory behavior and social interaction (Shi et al., 2003). Many studies have replicated these findings in rodents using systemic poly I:C (a viral mimetic); lipopolysaccharide (LPS), a bacterial endotoxin; exogenous cytokines; live bacterial infection; intact viruses (Bilbo and Schwarz, 2012; Meyer, 2014); or intrauterine LPS (Makinson et al., 2017) as immune challenges. Many of these studies also found that both male and female offspring show behavioral changes following prenatal maternal immune challenge (Lin et al., 2012; Meyer et al., 2008; Smith et al., 2007; Van den Eynde et al., 2014). One study found that males only show decreased social play behavior following prenatal immune challenge with LPS (Taylor et al., 2012). A recent prenatal poly I:C study has shown sex-specific effects on microglia development and function, as well as sex-specific behavioral impairments that bear relevance to schizophrenia and autism (Hui et al., 2018). Makinson et al. (2017) showed that intrauterine inflammation increased microglia numbers, TNFα expression, and induced anxiety-like behavior equally in male and female offspring, but led to greater secondary inflammatory response to adult immune challenge in males, suggesting that male microglia were more robustly “primed” by prenatal inflammation. Similarly, two studies in humans noted that high maternal inflammation during pregnancy (as measured by c-reactive protein levels) increased risk for ASD and schizophrenia, but the risk was increased in both males and females (Brown et al., 2014; Canetta et al., 2014). Several factors in rodents influence prenatal immune activation: for example, timing of challenge, presence of specific gut microbiota, and maternal production of IL-6 and IL-17 (Choi et al., 2016; Kim et al., 2017; Meyer, 2006, 2006; Smith et al., 2007). Due to the variability in challenges used, it is difficult to determine whether perinatal immune challenges have sex-specific effects.
How do microglia fit in to the picture of early life inflammation? Since microglia are the primary resident immune cell of the brain, it makes sense to hypothesize that microglia mediate maternal or neonatal immune activation effects on development. Recent research based on comprehensive transcriptome analysis has shown that maternal immune activation affects the maturation of microglia (Matcovitch-Natan et al., 2016). Furthermore, neonatal immune challenge alters maturation of microglia only in males, not in females (Hanamsagar et al., 2017). Hui et al. (2018) recently showed that adult male offspring of poly I:C challenged dams showed more deramified microglia compared to controls, increased physical interaction of microglia with synapses, increased cellular stress, and elevated inflammatory gene expression in the hippocampus, whereas females did not. In contrast, female microglia showed increased association with myelinated axons compared with male microglia following maternal immune challenge. In addition, while prenatally poly I:C-challenged males and females both showed decreased sociability in adulthood compared with control mice, only males showed increased anxiety and perturbations in pre-pulse inhibition of the acoustic startle response (Hui et al., 2018). Perinatal immune activation can also change microglial function by ‘priming’ microglia. When previous inflammation “primes” microglia, (Bilbo and Schwarz, 2012; Frick et al., 2013), a second hit of inflammation later in life can elicit exaggerated pro-inflammatory signaling that, amongst other effects, disrupts learning and memory and increases anxiety in adulthood (Niraula et al., 2017; Williamson et al., 2011; Wohleb et al., 2014). However, questions remain as to whether these changes in microglia alter behavior in a sex-specific manner and whether gene expression changes in microglia represent changes in microglia function. Figure 2 summarizes what is currently known (or hypothesized) about sex differences in microglia in response to early life perturbations.
Early life stress
In humans, prenatal stress increases the risk of the offspring developing a neurodevelopmental disorder such as schizophrenia, autism, and generalized externalizing symptoms (Bale et al., 2011). Male mice are particularly susceptible to the deleterious effects of early prenatal stress: They show altered emotionality, stress-induced corticosterone release, and locomotor hyperactivity later in life (Bronson and Bale, 2014; Mueller and Bale, 2008). Little work has been done to identify acute effects of prenatal stress on microglia. Microglia isolated from prenatally stressed animals had higher expression of several “pro-inflammatory” cytokines than microglia isolated from control animals (Ślusarczyk et al., 2015). Prenatal stress potentially alters microglial colonization. Prenatal stress decreased the total number of microglia and amoeboid microglia most markedly in the corpus callosum (Gómez-González and Escobar, 2010) . In other areas, prenatal stress decreased amoeboid microglia and increased ramified microglia at PN1, but no changes in microglia were observed by PN10. Interestingly, these data suggest that microglia maturation is altered by prenatal stress, similarly to what is seen with maternal or postnatal immune challenge, in germ free mice, or following neonatal stress exposure (Delpech et al., 2016; Hanamsagar et al., 2017; Matcovitch-Natan et al., 2016; Thion et al., 2017). Further research is needed to determine whether microglia mediate the programming effects of maternal stress on behavior, or whether they simply react to an altered neural microenvironment. Several studies have analyzed the long-term effects of prenatal stress on microglia. Prenatal stress alters microglia morphology and increases the inflammatory response to LPS in adult males and females, suggesting that prenatal stress might “prime” microglia (Diz-Chaves et al., 2013, 2012). Another group found that, following maternal treatment with dexamethasone, a synthetic glucocorticoid, during pregnancy, microglia ramification in the medial prefrontal cortex was increased in males but decreased in females (Caetano et al., 2016). Thus, stress may impact microglia through mediating effects on the stress system and release of stress hormones, such as glucocorticoids.
Childhood adversities are estimated to cause ~40% of all childhood onset mental health disorders and ~25% of adult onset disorders (Green et al., 2010). There have been several reviews outlining how early life adversity “programs” behavior (see Andersen, 2015; Chen and Baram, 2016). Here, we will focus on how the immune system may moderate the early life programming effects of exposure to adverse experiences and whether there are sex specific interactions of early life adversity and the neuroimmune system.
Adversity during infancy and childhood has a long-term impact on internalizing and externalizing behaviors, as well as other cognitive behaviors in both rodents and humans (Andersen, 2015; Chen and Baram, 2016; Teicher and Samson, 2016). Early postnatal stress in rodents increases microglia density in the hippocampus (Delpech et al., 2016; Roque et al., 2015). Like other challenges that alter immune function, postnatal stress alters microglia development and function (Delpech et al., 2016). Such stress exposure can also increase the number of circulating peripheral monocytes; however, these monocytes do not appear to infiltrate the brain (Delpech et al., 2016). In addition to the acute developmental effects of early postnatal adversity, there are long-term effects on the immune system. Maternal separation stress in newborn rats increases the concentration of circulating IL-1β and IL-6 and decreases the number of parvalbumin interneurons in adolescent rats, as discussed further below (Brenhouse and Andersen, 2011; Wieck et al., 2013). Interestingly, maternal separation seems to disrupt social behavior and decrease parvalbumin expression earlier in females than it does in males (Holland et al., 2014), which may stem from the aforementioned basal sex differences in microglia maturation in the brain (Hanamsagar et al., 2017). However, little work has been done to assess sex differences in how early life stress programs the immune system. The sensitive period of sex-specific programming in the brain overlaps with the period of early life adversity, however, suggesting effects of early life stress may be sexually dimorphic.
Maternal diet
Because 36% of adults in the US are obese and many more are overweight, interest in how maternal obesity affects neurodevelopment is growing. Obesity increases adverse outcomes of pregnancy such as maternal infection, preterm birth, inflammation, and gestational diabetes (Marchi et al., 2015; Norman and Reynolds, 2011). Adverse effects related to obesity during pregnancy are also known risk factors for neurodevelopmental disorders (see Perinatal Immune Activation). However, how obesity affects fetal neurodevelopment and later-life neurological function in humans is still unclear. Godfrey et al. (2018) conclude that, at this stage, the evidence is mixed: Some studies find that obesity increases the risk of the offspring developing ADHD, ASD, and cerebral palsy, whereas others find no correlations. Many confounding factors need to be assessed to determine the true effect of obesity on offspring, such as presence or absence of comorbid conditions, such as diabetes, high blood pressure, preeclampsia, or autoimmune disorders.
Many studies in rodents have shown that maternal weight gain induced by a high-fat diet altered neurodevelopment and behavior in offspring. High-fat diets have been found to increase circulating and hippocampal IL-1β neonatally, concomitant with an increase in microglia density as well as decreased spatial memory performance and increased anxiety (Bilbo and Tsang, 2010). Similarly, data in non-human primates showed that high-fat diet early in pregnancy increased anxiety responses in females (Sullivan et al., 2010). In rodents, dietary intervention during lactation is capable of preventing brain inflammation and social behavior deficits and anxiety following gestational high fat diet (Kang et al., 2014). Some of fetal and sex effects of high-fat diet may be mediated by exposure to maternal glucocorticoids: Male fetuses of rodent dams on a high-fat diet had higher levels of corticosterone and females had lower levels compared with respective controls (Chin et al., 2017). Maternal high-fat diet has also been shown to alter spine density, dendritic remodeling, and myelination in offspring (Graf et al., 2016; Janthakhin et al., 2017; Rincel et al., 2018), but future work is needed to mechanistically link microglia to high-fat diet-induced neurodevelopmental and behavioral outcomes.
Juvenile and adolescent sex differences in neuroimmune parameters:
Markedly less experimental attention has been directed at microglia function and sex differences in microglia during the peripubertal and adolescent period, yet this topic is likely an important one to investigate. The adolescent period is gaining increasing attention as an overlooked mini critical period during which sex-specific organization of the CNS and resulting behavior is refined (reviewed in (Lenroot and Giedd, 2010; Sisk, 2017; Walker et al., 2017)). Additionally, many neuropsychiatric disorders typically manifest during puberty (for example, schizophrenia, depression, eating disorders, substance abuse, and migraine). Thus, adolescents and young adults may be especially vulnerable to exogenous perturbations that are associated with risk for neuropsychiatric or neurological disorders (Lenroot and Giedd, 2010; Walker et al., 2017).
What little work has been devoted to microglia during this period suggests that sex differences are apparent. In the aforementioned article by Schwarz et al (2012), male and female microglia were compared in the early pubertal period (PN30). Schwarz et al. found that females had a greater number of microglia with thick branches (which indicate a “reactive” phenotype) in several brain regions, including the parietal cortex, amygdala, and CA3 region of the hippocampus. Also, in the AVPV nucleus, a robustly sexually differentiated brain region (discussed above in “Perinatal sex differences in neuroimmune parameters”), more new cells are born in females than in males during the peripubertal period, when this region becomes functional and responsible for controlling pulsatile hormone release in females (Ahmed et al., 2008). Interestingly, of the new cells added to the AVPV during adolescence, almost half are either astrocytes or microglia (Mohr et al., 2016). Together, these two studies suggest that there are sex differences in adolescent immunocompetent cells that could contribute to sex-specific brain function later in life.
Several recent studies have shown adolescent sex differences in the neuroimmune response to previous early life perturbations. One such perturbation is a two-hit model of inflammation: neonatal E. coli infection and juvenile endotoxin challenge. Using this protocol, Osborne et al. (2017) found that elevated concentrations of IL-1β persist in the hippocampus and prefrontal cortex of juveniles at PN24. In response to a second hit of inflammation with LPS, both males and females show an exaggerated IL-1β response in the hippocampus if previously infected with E. coli, whereas only females show increases in the prefrontal cortex (Osborne et al., 2017). However, this exaggerated inflammatory response was unaccompanied by deficits in hippocampal-dependent learning during the juvenile period, Thus, the behavioral consequences of this persistent and sex-specific inflammation remain unknown.
Maternal separation stress has also been used to assess sex-specific immune function (as mentioned above). Following 3 weeks of daily maternal separation stress in rats, Grassi-Oliveria et al. (2016) found evidence of persistent peripheral inflammation, with males but not females showing decreases in anti-inflammatory IL-10 concentrations in the early juvenile period (PN25) (Grassi-Oliveira et al., 2016). Moreover, the authors found significant correlations between behavioral performance on a win-shift cognitive task assessed in adulthood and juvenile peripheral cytokine levels in females but not males. These data suggest that sex-specific responses to systemic inflammation during the juvenile period may predict adolescent behavioral outcomes. However, it should be noted that central levels of cytokines or microglia function were not reported in this study, thus future research is needed to address whether early life stress impacts the neuroimmune systems of males and females differentially.
Though much less researched, there is also evidence that males and females show differential neuroinflammatory priming effects in response to juvenile stress (Pyter et al., 2013). Following adolescent stress coupled with adult immune challenge with LPS, males and females show elevated proinflammatory gene expression in the hippocampus following LPS. Yet only males showed evidence of stress-induced priming of the neuroinflammatory response, with greater TNFα, IL-1β, iNOS, and NFkB elevations to LPS following previous adolescent stress exposure (Pyter et al., 2013). Interestingly, estradiol concentrations in females were correlated with gene expression for the microglia marker Cd11b. Estradiol and Cd11b expression were not correlated in stress-exposed animals, however, implying dysregulation in the relationship between female-typical hormones and microglia in response to stress.
We have recently found that adolescent rats show behavioral changes as a result of early life microglia loss. Specifically, we depleted microglia from the brains of male and female rats during the early postnatal period (PN1–10), and subsequently allowed microglia to repopulate the brain. When assessed between PN28–29, we found that both males and females that experienced early life microglial loss showed changes in social play behaviors, with microglia-depleted males and females displaying reduced social chasing compared with vehicle rats, and depletion female rats showing increased social exploration (Nelson and Lenz, 2016). In the open field test on PN30–32, microglia-depleted males and females both showed evidence of locomotor hyperactivity, and only females showed increases in center entries, indicating a reduced anxiety-like response (Nelson and Lenz, 2016). A similar study by Van Ryzin et al. (2016) also found anti-anxiety and hyperactive phenotypes in juvenile males and females that experienced early life microglia depletion (Van Ryzin et al., 2016). This study also showed that microglia-depleted males and females exhibit impaired performance on a T-maze alternation task as well as decreased fear behavior and increased risk-assessing behaviors in a predator odor exposure during the juvenile period (Van Ryzin et al., 2016). These data indicate that juvenile behaviors are sensitive to the effects of microglia programming in the developing brain, and that some but not all behavioral outcomes show sex-specific effects. Future work is necessary to determine which aspects of juvenile brain function are altered in microglia-depleted animals. Nevertheless, it is clear that microglia contribute to sex-specific programming of behavior observed in proximal developmental periods, such as puberty, and thus future studies focused on microglia and development should strive to connect neuroanatomical effects of microglia to behavioral outcomes throughout the lifespan.
Adult sex differences in neuroimmune parameters:
Few studies, either in the healthy brain or following perturbations, have assessed microglia and neuroimmune signaling in both males and females and compared the sexes. Yet some studies that compare males and females show notable adult sex differences in microglia or neuroimmune function. In general, the data suggest that microglia within the adult brain have a more activated or basally primed phenotype in females. The hallmark sex differences survey experiment by Schwarz et al. (2011) found that adult females showed evidence of more primed microglia than males in the hippocampus, parietal cortex, and amygdala. Additionally, females have increased cortical/hippocampal levels of IL-10, the IL-10 receptor antagonist, IL-16, IL-1α, CD11b, and TOLLIP genes and IL-1β protein in adulthood, whereas males have higher expression of the genes for IL-18, chemokine receptor CCR4, and the chemokine CCL22 in adulthood (Schwarz et al., 2012). Two studies by Bollinger et al. investigated sex differences on the effects of acute versus chronic stress on microglia in limbic areas of the rat brain, as will be discussed below (Bollinger et al., 2017, 2016).,These studies also documented several notable baseline sex differences in microglia. In the medial prefrontal cortex, Bollinger et al. (2016) showed females have a higher baseline ratio of primed to ramified microglia as well as higher baseline levels of fractalkine (CX3CL1), which is a microglia regulator released by neurons, compared with males. In a follow-up study, Bollinger et al., (2017) investigated additional brain regions, including the amygdala, orbitofrontal cortex, and hippocampus, and found evidence of mild basal sex differences in neuroimmune parameters, including higher baseline expression of CD40, CD200, and arginine genes in the amygdala in females than in males. Correlation matrices of gene expression and microglia morphology suggested that microglial activation is more correlated across corticolimbic brain regions in males under basal conditions than in females (Bollinger et al., 2017). Another recent transcriptomic study compared male and female mouse hippocampus at 3 (young adult), 12 (‘middle age’) and 24 months of age (old) and found that sex differences were detectable at each age examined (Mangold et al., 2017). Interestingly, the particular inflammatory genes that showed sex differences in expression were age specific, and that male and female gene expression become increasingly divergent as mice age (Mangold et al., 2017). The functional significance of these basal sex differences remains uninterrogated, but such studies are certainly warranted given sex differences in stress-related psychiatric disorders (and likely underway).
Relevance to sex differences in brain-based disorders with adolescent or adult onset:
Pain
Sex differences in neuroimmune parameters in the adult CNS may be relevant for pain-related disorders. In the periaqueductal gray of the rat, females have a more reactive microglia morphology than males at baseline (Doyle et al., 2017). In response to LPS, female microglia show an exaggerated activation response relative to males as well as a greater elevation in IL-1β concentration (Doyle et al., 2017). Moreover, this sex difference in microglia predicted a reduced female response to morphine via the toll-like receptor 4, which may have implications for sex difference in pain perception, pain treatment, or susceptibility to opioid abuse. Not only do sex differences in microglia correlate with pain-related neurobiology, but a recent study has implicated differing immune cell types in the spinal cord on the induction of pain hypersensitivity following nerve injury in male and female mice. Sorge et al (2015) found that nerve injury was associated with microglial activation in both males and females, but that microglia-inhibiting interventions prevented only the induction of allodynia in males and had no effect on females (Sorge et al., 2015). Rather, in females, T cells of the adaptive immune system appeared to drive the development of pain hypersensitivity. These data illustrate why it is important to study both males and females, even when their end phenotypes (e.g., mechanical pain sensitivity) are the same. Different biological mechanisms may drive a similar behavioral or functional outcome, which would mean that different intervention or treatment strategies may be necessary for males and females in a variety of brain-based disorders.
Microglia in the developing brain are also implicated in sex-specific behavioral outcomes in adult rodents. Following transient early postnatal microglia depletion, both male and female rats showed hyperactivity and evidence of decreased anxiety-like behavior (Nelson and Lenz, 2016; VanRyzin et al., 2016) and decreased behavioral despair (Nelson and Lenz, 2016). In contrast, only microglial-depletion females showed a blunting of the acute stress response in adulthood (Nelson and Lenz, 2016), whereas only males showed evidence of impaired sexual behavior (VanRyzin et al., 2016), similar to inhibiting microglia with minocycline during the early neonatal period (Lenz et al., 2013). Together, these data show that sex differences in how microglia shape brain development and long-term function may have previously unappreciated implications for sex differences in behavior throughout life.
Anixety and mood disorders:
Anxiety and mood disorders are more common in females than in males, and inflammatory processes have been linked to mood dysregulation and anxiety and depression. This section will summarize what is known about the mechanisms underlying sex differences in anxiety and depression with a focus on potential crosstalk between biological sex and inflammation as contributing factors.
Major depressive disorder (MDD) has been associated with high concentrations of peripheral cytokines in humans (e.g., morning IL-6) (Alesci et al., 2005) and diminished natural killer activity relative to those observed in healthy controls (reviewed in Irwin and Miller, 2007; Réus et al., 2015)). People who are diagnosed with immune disorders (such as allergy, asthma, and multiple sclerosis) are also diagnosed with depression at higher rates than control populations (Himmerich et al., 2008; Ortega et al., 2004; Siegert, 2005). MDD also appears to worsen the course of non-psychiatric diseases such as HIV (reviewed in Arseniou et al., 2014; Irwin and Miller, 2007). Allergic rhinitis, too, may confer vulnerability to MDD (Chen et al., 2013).
MDD is hypothesized to have a large immune and/or microglia component to the disorder, and these neuroimmune parameters may be sex-specific. Studies have found increased TSPO binding in the patients with depression as well as increased microglial density and increased peripheral macrophage infiltration in depressed suicides (Holmes et al., 2018; Setiawan et al., 2015; Torres-Platas et al., 2014). Depression severity correlated positively with TSPO density in the anterior cingulate cortex, a crucial mood- and emotion-regulating brain region (Setiawan et al., 2015). Surprisingly, serum inflammatory markers did not correlate with TSPO density in the brain; these data suggest that peripheral and central immune activation are somewhat independent (citation here? Or is citation in previous sentence sufficient?). In humans, IL-6 concentrations are elevated in patients with MDD relative to controls, and in individuals with “treatment-resistant” MDD , pharmacological antidepressants do not effectively reduce IL-6 levels (Hodes et al., 2014).
Women are more susceptible than men to developing depressed mood following immune activation (Moieni et al., 2015). Recent studies of brain tissue from people with MDD showed that males and females have dramatically different transcriptomic signatures associated with the disorder (Labonté et al., 2017; Seney et al., 2018). Interestingly, both men and women with depression showed dramatic shifts in gene sets associated with microglia within the brain, but in opposite directions: Men with MDD show increases and women with MDD show decreases in microglia-related gene expression (Seney et al., 2018). Further, similar transcriptional changes are seen in a rodent model of depression induced by chronic variable stress (Labonté et al., 2017).
In animal studies, chronic stress paradigms have been used to investigate environmentally-driven onset of mood dysregulation. Most studies of microglial involvement in mood-related behavior have used only males, yet the female bias in depressive disorder prevalence warrants the addition of female animals to psychoneuroimmunological research. A series of studies by Bollinger et al. (2016; 2017) compared the microglial response to acute and chronic restraint stress in males and females. Results showed complex region- and sex-dependent relationships between stress and microglia/neuroimmune function. Highlights of Bollinger et al.’s findings include that chronic stress had opposite effects on cross-correlations between measures of microglia morphological changes in corticolimbic regions, including the amygdala, hippocampus, and orbitofrontal cortex. Stress decreased correlations in males and increased them in females (Bollinger et al., 2017). In the medial prefrontal cortex, female microglia (both microglia-related gene expression and morphology) were impacted by chronic stress, whereas male microglia were not affected (Bollinger et al., 2016). In a different study with a similar foundational question, chronic variable stress elicited sex specific effects on microglia function, such that stress in males more dramatically altered microglia morphology, increased microglia expression of the CSF receptor, Csf1r, and the fracktalkine receptor (Cx3cr1), and more putative phagocytosis of dendritic inclusions compared to microglia in females (Wohleb et al, 2017). These studies underscore how complicated studies of sex by environment interactions become, especially once multiple brain regions are considered. We have only just begun to appreciate the complex dynamics underlying stress-induced dysfunction of the neuroimmune system across sexes, but the question is of clear importance given the prominent sex differences in depression incidence, symptomology, and severity.
Repeated social defeat stress (RSDS) is another rodent model of environmentally-induced anxiety and depression. RSDS has been carried out mostly in male mice to date, because the model relies upon the sexually dimorphic display of intra-male aggression which females do not endogenously display. Despite the downside of not being able to easily utilize the model in females, RSDS has given fantastic insight into the neuroimmune contributions to mood dysregulation. Experimenters place a male C57BL/6J mouse in an enclosure with a larger, aggressive male CD-1 mouse who attacks the first mouse; this encounter is repeated daily for 7–10 days (Golden et al., 2011). RSDS resembles the psychosocial stress of repeated bullying and abuse, which are associated with the development of depression and anxiety in humans (Brunstein Klomek et al., 2007; Campbell, 2002; Huhman, 2006). Although RSDS elicits depressive-like and anxiety-like behavior from most mice, a substantial minority of mice are resilient to social defeat stress (Golden et al., 2011), making RSDS an excellent model to study the mechanisms of stress susceptibility and resilience.
RSDS has dramatic effects upon both the peripheral and central immune system. Adoptive transfer of the immune system from mice exposed to RSDS to naïve controls induces a depressive and anxiety-like phenotype in the naïve animals (Hodes et al., 2014). Both central and peripheral immune function appear to contribute to the onset and maintenance of mood dysregulation following RSDS. Peripheral monocytes respond to RSDS by proliferating, adopting a more pro-inflammatory (and glucocorticoid-resistant) phenotype, and traveling to the brain (McKim et al., 2017, 2016; Wohleb et al., 2013, 2011). There, peripheral monocytes engage with blood-brain barrier endothelial cells, promote microglial morphological and inflammatory gene expression changes, and induce anxiety behavior by releasing IL1β that acts on endothelial cells (McKim et al., 2017; Sawicki et al., 2015; Wohleb et al., 2013; 2014). Communication between the central and peripheral branches of the immune system may contribute to stress-related psychological disorders, such as depression and anxiety (Weber et al., 2017; Yirmiya et al., 2015). The cytokine IL-6 participates in this crosstalk and acts on various CNS cells, including microglia (Hodes et al., 2014; Menard et al., 2017). The intensity of the leukocyte IL-6 response to the initial bout of RSDS predicts later stress susceptibility or resilience: Stress-susceptible mice tend to secrete more IL-6 following social stress than stress resilient mice (Menard et al., 2017). Depleting leukocyte-derived IL-6 or sequestering IL-6 from entering the brain similarly promotes resilience to RSDS, implicating peripheral to central signaling by this cytokine in depression susceptibility. Stress-induced catecholamine activity at the β-adrenoceptor also (at least partly) mediates microglial proinflammatory changes and adoption of an anxiety-like phenotype following RSDS (Wohleb et al., 2011).
A downside of the RSDS model is that it does not allow for studies of biological sex, as adult male mice typically cannot be induced to attack females, and females do not show intra-sex aggression. But several modifications of the RSDS have recently been validated to adapt the task for use in female mice (Harris et al., 2017; Takahashi et al., 2017). Chemogenetic manipulations using designer receptors exclusively activated by designer drugs (DREADDs) enable researchers to induce male mice to attack adult female conspecifics by activating the ventrolateral portion of the ventromedial hypothalamus, a pro-aggression node in the brain (Lee et al., 2014; Lin et al., 2011; Takahashi et al., 2017). Another recently identified approach to elicit female-directed aggression from male mice is the application of male odorants to female mice (Harris et al., 2017). These adaptations of RSDS might allow neuroscientists to determine the neurological bases of sex differences in mood and anxiety disorder course and prevalence. Additionally, adaption of RSDS to female mice will allow researchers to determine similarities and differences between RSDS induced- and chronic variable stress induced-depression and anxiety.
Although these RSDS models have not been implemented in any yet-published mechanistic studies in females, a recent study in mice using RSDS in males alongside chronic variable stress manipulations in females has shown that resilience to chronic stress in males is linked to estrogen receptor alpha-associated gene networks in the nucleus accumbens (Lorsch et al., 2018). This study showed that chronic stress alters estrogen receptor alpha in the nucleus accumbens, that overexpression of estrogen receptor alpha promotes resilience to chronic stress, and that males and females show differential transcriptional networks related to estrogen receptor alpha following chronic stress. Interestingly, in addition to estrogen receptor genes, several immune genes were uncovered as differentially expressed in resilient versus susceptible mice following stress (Lorsch et al., 2018). Additionally, gene ontology analyses showed that overexpression of estrogen receptor alpha in both males and females led primarily to decreases in expression of genes associated with the immune response and inflammation in the brain, but that increases in gene expression were different in males and females (Lorsch et al., 2018). This study shows that sex-specific hormone receptor interactions can both promote resilience to mood-related dysfunction as well as contribute to stress-induced mood dysregulation.
Neuroimmune parameters in the maternal brain:
Pregnancy and the postpartum period are associated with dramatic sex-specific plasticity in the brain, yet this association is often overlooked. During pregnancy and immediately following parturition, maternal hormones induce plasticity in the brain to promote the onset of maternal behavior (Bridges, 2015; Dulac et al., 2014; Numan, 2007). Many of these same hormones, including estrogens, progesterone, oxytocin, and glucocorticoids are known to exert effects on microglia and inflammatory signaling in other contexts (Garcia-Ovejero et al., 2005; Lenz & McCarthy, 2015). We recently profiled microglia number and morphology over the course of normal, healthy pregnancy in rats and found significant shifts in microglia properties in limbic regions of the brain (Haim et al., 2017). In late pregnancy and extending into the early postpartum period, we found significant decreases in the overall number of microglia in the prefrontal cortex, hippocampus, nucleus accumbens, and amygdala (Haim et al., 2017). This decrease in microglia number was driven by a reduction in microglia proliferation but no change in microglia death. Interestingly, these effects on microglia were not observed in the motor cortex, suggesting regional specificity, potentially as a result of differential sensitivity of limbic and non-limbic brain regions to hormones. Finally, we observed elevations in IL-6 and IL-10 concentrations in the postpartum hippocampus. Another research group has documented similar pregnancy-related changes in neuroimmune parameters, including increases in expression of the CD11b, IL-4, BDNF, and IL-6 in the postpartum prefrontal cortex and decreased microglia staining in the hippocampus (Sherer et al., 2017). This same group has shown that the inflammatory gene expression in the brain in response to immune challenge with LPS during pregnancy is also blunted, suggesting central immunosuppression (Sherer et al., 2017).
Relevance to postpartum depression:
As discussed above in the section on major depression, there are clear links between stress, depression and anxiety and neuroimmune dysregulation. As with major depression, stress during pregnancy is a major risk factor for postpartum depression (PPD) (Beck, 2001). In women with postpartum depression, peripheral immune changes have been documented, including mild changes in serum IL-6 and IL-10 (Simpson et al., 2016), which are cytokines we observed to be altered in the rodent brain over the course of healthy pregnancy (Haim et al., 2017). Another study found a host of inflammatory mediators to be suppressed during late pregnancy in women who went on to develop postpartum depression (Bränn et al., 2017). Peripheral immune cells have different gene expression signatures in women with postpartum depression and those without (Segman et al., 2010). Yet other studies have found little relationship between immune mediators in women and postpartum depression (Blackmore et al., 2014, 2011). Thus, at this stage, studies have been somewhat contradictory and hard to compare due to the trimester in which women were studied, the demographics of the sample population, and level of stress exposure in women (see review by (Osborne and Monk, 2013)). An additional caveat is that imaging of possible microglia “activation” as measured by TSPO binding in the context of major depression has showed little correlation between peripheral inflammatory markers and central markers of inflammation (Setiawan et al., 2015), therefore peripheral immune changes may not be a good marker for neuroimmune changes occurring in the peripartum brain. Future studies in which TSPO imaging of brain inflammatory changes in new mothers with PPD or measuring CSF cytokine levels in samples obtained from mothers undergoing epidural anesthesia may be more informative.
Hormonal imbalances following childbirth, including rapid declinations in estrogen and progesterone levels, blunted oxytocin levels, and elevated corticotropin releasing factor levels have also been associated with risk for postpartum depressive symptoms (reviewed in (Schiller et al., 2015)). Microglia are known to be responsive to estrogens in the context of development (Lenz et al., 2013; Nelson et al., 2017)) and in adulthood (Loram et al., 2012). Further, as discussed above, resilience to stress-induced mood dysregulation is conferred by higher levels of estrogen receptor tone in the brain, and increased estrogen receptor function downregulates immune-related gene expression in females (Lorsch et al., 2018). Together, these data suggest that crosstalk between hormones of pregnancy and neuroimmune parameters may contribute to the onset of postpartum depression and/or resilience to the disorder, and they warrant future inquiry in both animal models and human patient populations.
Conclusions:
where do we go from here?
To date, most rodent studies that investigate neuroimmune contributions to brain development and plasticity have only focused on males. The small subset of studies that has compared the sexes is clear proof that such studies are warranted, because in many cases, sex differences in behavioral outcomes or underlying biochemical processes are found. Microglia have been shown to be hormone sensitive during brain development and throughout life, and clearly show phenotypic differences both at baseline and in response to perturbations. Sometimes these sex differences in neuroimmune function are complicated and not easy to interpret, but this complexity does not mean they should be ignored or dismissed. The data published to date on sex differences in microglia and neuroimmune signaling suggest that microglia could be actively playing a role in setting up sex-specific neural circuitry and resulting brain physiology. In doing so, microglia may contribute to sex differences in psychiatric disorder risk or symptomology. It is time to include males and females in all studies as a matter of course, and report findings so that consistencies and converging evidence can accrue and show that sex-specific mechanisms are at play during brain development, plasticity, and in brain-based disease states.
Highlights.
Sex differences in the brain are programmed by early life exposure to hormones and sex chromosome genes, and sex differences in brain and behavior are also modulated by hormones in the pubertal and adult period.
There are normal sex differences in immunocompetent cells in the brain, such as microglia, astrocytes, and others, that contribute to normal, sex specific brain function and sex differences in the brain’s response to perturbations.
Neuroimmune cells and their activity are implicated in many psychiatric and neurological disorders with sex biases in prevalence, and sex differences in these neuroimmune cells may underlie these sex biases in disorder risk or resilience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel KM, Drake R, Goldstein JM, 2010. Sex differences in schizophrenia. Int. Rev. Psychiatry 22, 417–428. 10.3109/09540261.2010.515205 [DOI] [PubMed] [Google Scholar]
- Abiega O, Beccari S, Diaz-Aparicio I, Nadjar A, Layé S, Leyrolle Q, Gómez-Nicola D, Domercq M, Pérez-Samartín A, Sánchez-Zafra V, Paris I, Valero J, Savage JC, Hui C-W, Tremblay M-È, Deudero JJP, Brewster AL, Anderson AE, Zaldumbide L, Galbarriatu L, Marinas A, Vivanco M, Matute C, Maletic-Savatic M, Encinas JM, Sierra A, 2016. Neuronal Hyperactivity Disturbs ATP Microgradients, Impairs Microglial Motility, and Reduces Phagocytic Receptor Expression Triggering Apoptosis/Microglial Phagocytosis Uncoupling. PLOS Biol 14, e1002466. 10.1371/journal.pbio.1002466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham SN, St John AL, 2010. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol 10, 440–52. 10.1038/nri2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acaz-Fonseca E, Avila-Rodriguez M, Garcia-Segura LM, Barreto GE, 2016. Regulation of astroglia by gonadal steroid hormones under physiological and pathological conditions. Prog. Neurobiol 144, 5–26. 10.1016/j.pneurobio.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL, 2008. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat. Neurosci 11, 995–997. 10.1038/nn.2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV, 2007. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci 10, 1538–1543. 10.1038/nn2014 [DOI] [PubMed] [Google Scholar]
- Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, Ayala AR, Licinio J, Gold HK, Kling MA, Chrousos GP, Gold PW, 2005. Major Depression Is Associated with Significant Diurnal Elevations in Plasma Interleukin-6 Levels, a Shift of Its Circadian Rhythm, and Loss of Physiological Complexity in Its Secretion: Clinical Implications. J. Clin. Endocrinol. Metab 90, 2522–2530. 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM, 2004. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat. Neurosci 7, 643–650. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM, 2002. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J. Neurosci 22, 8586–8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, 2015. Exposure to early adversity: Points of cross-species translation that can lead to improved understanding of depression. Dev. Psychopathol 27, 477–491. 10.1017/S0954579415000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, 2017. A general theory of sexual differentiation: A General Theory of Sexual Differentiation. J. Neurosci. Res 95, 291–300. 10.1002/jnr.23884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseniou S, Arvaniti A, Samakouri M, 2014. HIV infection and depression: HIV infection and depression. Psychiatry Clin. Neurosci 68, 96–109. 10.1111/pcn.12097 [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J, 2011. Altered T cell responses in children with autism. Brain. Behav. Immun 25, 840–849. 10.1016/j.bbi.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, Sierra A, Molnár Z, Cragg MS, Garaschuk O, Perry VH, Gomez-Nicola D, 2017. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Reports 18, 391–405. 10.1016/j.celrep.2016.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Baum MJ, 2008. Role for estradiol in female-typical brain and behavioral sexual differentiation. Frontiers in Neuroendocrinology 29, 1–16. 10.1016/j.yfrne.2007.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Michaelis S, 2015. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci 17, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, Cohen AS, Chakrabarti B, Ruta L, Lombardo MV, 2015. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 20, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach FA, 1976. Sexual Attractivity, Proceptivity, and Receptivity in Female Mammals. Hormones and Behavior 7, 105–138. [DOI] [PubMed] [Google Scholar]
- Bennett FC, Bennett ML, Yaqoob F, Mulinyawe SB, Grant GA, Hayden Gephart M, Plowey ED, Barres BA, 2018. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 10.1016/j.neuron.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA, 2016. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci 201525528. 10.1073/pnas.1525528113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum SA, Bryk KLK, Beltz AM, 2012. Early androgen effects on spatial and mechanical abilities: Evidence from congenital adrenal hyperplasia. Behav. Neurosci 126, 86–96. 10.1037/a0026652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum SA, Hines M, 1992. Early Androgens Are Related to Childhood Sex-Typed Toy Preferences. Psychol. Sci 3, 203–206. 10.1111/j.1467-9280.1992.tb00028.x [DOI] [Google Scholar]
- Bilbo SD, Schwarz JM, 2012. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol 33, 267–286. 10.1016/j.yfrne.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V, 2010. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 24, 2104–2115. 10.1096/fj.09-144014 [DOI] [PubMed] [Google Scholar]
- Blackmore ER, Côté-Arsenault D, Tang W, Glover V, Evans J, Golding J, O’Connor TG, 2011. Previous prenatal loss as a predictor of perinatal depression and anxiety. Br. J. Psychiatry 198, 373–378. 10.1192/bjp.bp.110.083105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore ER, Groth SW, (Din) Chen D-G, Gilchrist MA, O’Connor TG, Moynihan JA, 2014. Depressive symptoms and proinflammatory cytokines across the perinatal period in African American women. J. Psychosom. Obstet. Gynecol 35, 8–15. 10.3109/0167482X.2013.868879 [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MAP, Bonoldi I, Kalk N, Turkheimer F, McGuire P, de Paola V, Howes OD, 2016. Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [ 11 C]PBR28 PET Brain Imaging Study. Am. J. Psychiatry 173, 44–52. 10.1176/appi.ajp.2015.14101358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JL, Bergeon Burns CM, Wellman CL, 2016. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain. Behav. Immun 52, 88–97. 10.1016/j.bbi.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JL, Collins KE, Patel R, Wellman CL, 2017. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PLOS ONE 12, e0187631. 10.1371/journal.pone.0187631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JM, Waddell J, McCarthy MM, 2010. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ 1, 10–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bränn E, Papadopoulos F, Fransson E, White R, Edvinsson Å, Hellgren C, Kamali-Moghaddam M, Boström A, Schiöth HB, Sundström-Poromaa I, Skalkidou A, 2017. Inflammatory markers in late pregnancy in association with postpartum depression—A nested case-control study. Psychoneuroendocrinology 79, 146–159. 10.1016/j.psyneuen.2017.02.029 [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL, 2011. Nonsteroidal Anti-Inflammatory Treatment Prevents Delayed Effects of Early Life Stress in Rats. Biol. Psychiatry 70, 434–440. 10.1016/j.biopsych.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, 2015. Neuroendocrine Regulation of Maternal Behavior. Front. Endocrinol 36, 178–196. 10.1007/978-1-59259-707-9_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J, 2008. Progesterone receptors: Form and function in brain. Frontiers in Neuroendocrinology 29, 313–339. 10.1016/j.yfrne.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock O, Baum MJ, Bakker J, 2011. The Development of Female Sexual Behavior Requires Prepubertal Estradiol. Journal of Neuroscience 31, 5574–5578. 10.1523/JNEUROSCI.0209-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Bale TL, 2014. Prenatal Stress-Induced Increases in Placental Inflammation and Offspring Hyperactivity Are Male-Specific and Ameliorated by Maternal Antiinflammatory Treatment. Endocrinology 155, 2635–2646. 10.1210/en.2014-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, 2012. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol 72, 1272–1276. 10.1002/dneu.22024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, 2011. The environment and susceptibility to schizophrenia. Prog. Neurobiol 93, 23–58. 10.1016/j.pneurobio.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, Surcel H-M, 2014. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol. Psychiatry 19, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein Klomek A, Marrocco F, Kleinman M, Schonfeld IS, Gould MS, 2007. Bullying, Depression, and Suicidality in Adolescents. J. Am. Acad. Child Adolesc. Psychiatry 46, 40–49. 10.1097/01.chi.0000242237.84925.18 [DOI] [PubMed] [Google Scholar]
- Caetano L, Pinheiro H, Patrício P, Mateus-Pinheiro A, Alves ND, Coimbra B, Baptista FI, Henriques SN, Cunha C, Santos AR, Ferreira SG, Sardinha VM, Oliveira JF, Ambrósio AF, Sousa N, Cunha RA, Rodrigues AJ, Pinto L, Gomes CA, 2016. Adenosine A2A receptor regulation of microglia morphological remodeling-gender bias in physiology and in a model of chronic anxiety. Mol. Psychiatry 10.1038/mp.2016.173 [DOI] [PubMed] [Google Scholar]
- Campbell JC, 2002. Health consequences of intimate partner violence. THE LANCET 359, 6. [DOI] [PubMed] [Google Scholar]
- Canetta S, Sourander A, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, McKeague IW, Brown AS, 2014. Elevated Maternal C-Reactive Protein and Increased Risk of Schizophrenia in a National Birth Cohort.pdf. Am. J. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Rogers S, Hansen RL, Amaral DG, Van de Water J, Ashwood P, 2017. Immune Endophenotypes in Children With Autism Spectrum Disorder. Biol. Psychiatry 81, 434–441. 10.1016/j.biopsych.2015.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M-H, Su T-P, Chen Y-S, Hsu J-W, Huang K-L, Chang W-H, Bai Y-M, 2013. Allergic rhinitis in adolescence increases the risk of depression in later life: A nationwide population-based prospective cohort study. J. Affect. Disord 145, 49–53. 10.1016/j.jad.2012.07.011 [DOI] [PubMed] [Google Scholar]
- Chen Y, Baram TZ, 2016. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology 41, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin EH, Schmidt KL, Martel KM, Wong CK, Hamden JE, Gibson WT, Soma KK, Christians JK, 2017. A maternal high-fat, high-sucrose diet has sex-specific effects on fetal glucocorticoids with little consequence for offspring metabolism and voluntary locomotor activity in mice. PLOS ONE 12, e0174030. 10.1371/journal.pone.0174030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR, 2016. The maternal interleukin-17a pathway in mice promotes autismlike phenotypes in offspring. Science 10.1126/science.aad0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collste K, Plav?n-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A, Amini N, Aeinehband SL, Farde L, Flyckt G, E., S, E., H, F.-B., S, C., L, S., F, P., I, A., K, C., P, V., A, M., M, H., F, O., Erhardt, Halldin C, Flyckt L, Farde L, Cervenka S, 2017. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Mol. Psychiatry 22, 850–856. 10.1038/mp.2016.247 [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C, Hayes LN, Schretlen DJ, Dannals RF, Kassiou M, Sawa A, Pomper MG, 2016. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [11C]DPA-713 PET and analysis of CSF and plasma. Transl. Psychiatry 6, e777. 10.1038/tp.2016.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ, 2013. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice: Microglial Gene Expression in Healthy Brain. J. Neurosci. Res 91, 1143–1151. 10.1002/jnr.23242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ, 2009. Expression of P2 nucleotide receptors varies with age and sex in murine brain microglia. J. Neuroinflammation 6, 24. 10.1186/1742-2094-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain JM, Watters JJ, 2015. Microglial P2 Purinergic Receptor and Immunomodulatory Gene Transcripts Vary By Region, Sex, and Age in the Healthy Mouse CNS. Transcr. Open Access 03 10.4172/2329-8936.1000124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Martinez-Cerdeno V, Noctor SC, 2013. Microglia Regulate the Number of Neural Precursor Cells in the Developing Cerebral Cortex. J. Neurosci 33, 4216–4233. 10.1523/JNEUROSCI.3441-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Tønder N, Zimmer J, Gonzalez B, Castellano B, 1997. Development of microglia in the prenatal rat hippocampus. J. Comp. Neurol 377, 70–84. [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Zimmer J, González B, Castellano B, 1998. Development of microglia in the postnatal rat hippocampus. Hippocampus 8, 458–474. [DOI] [PubMed] [Google Scholar]
- Delpech J-C, Wei L, Hao J, Yu X, Madore C, Butovsky O, Kaffman A, 2016. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain. Behav. Immun 10.1016/j.bbi.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SBG, Guyenet PG, Kipnis J, 2012. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature 484, 105–109. 10.1038/nature10907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Chaves Y, Astiz M, Bellini MJ, Garcia-Segura LM, 2013. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain. Behav. Immun 28, 196–206. 10.1016/j.bbi.2012.11.013 [DOI] [PubMed] [Google Scholar]
- Diz-Chaves Y, Pernía O, Carrero P, Garcia-Segura LM, others, 2012. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J Neuroinflammation 9, 2094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN, 2001. Immune function of astrocytes. Glia 36, 180–190. 10.1002/glia.1107 [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang X, Wang Y, Zhou X, Qian Y, Zhang S, 2017. Suppression of Brain Mast Cells Degranulation Inhibits Microglial Activation and Central Nervous System Inflammation. Mol. Neurobiol 54, 997–1007. [DOI] [PubMed] [Google Scholar]
- Doyle HH, Eidson LN, Sinkiewicz DM, Murphy AZ, 2017. Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine. J. Neurosci 37, 3202–3214. 10.1523/JNEUROSCI.2906-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dropp JJ, 1979. Mast cells in the human brain. Acta Anat. (Basel) 105, 505–513. [DOI] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA, Wu Z, 2014. Neural control of maternal and paternal behaviors. Science 345, 765–770. 10.1126/science.1253291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Park L, Anrather J, Iadecola C, 2017. Brain perivascular macrophages: characterization and functional roles in health and disease. Journal of Molecular Medicine 95, 1143–1152. 10.1007/s00109-017-1573-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman S, Cloonan N, Catt V, Miller L, Wong J, McCrossin T, Cairns M, Weickert C, 2013. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol. Psychiatry 206–214. [DOI] [PubMed] [Google Scholar]
- Frick LR, Williams K, Pittenger C, 2013. Microglial Dysregulation in Psychiatric Disease. Clin. Dev. Immunol 2013, 1–10. 10.1155/2013/608654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, Schork AJ, Appadurai V, Buil A, Werge TM, Liu C, White KP, Horvath S, Geschwind DH, 2018. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359, 693. 10.1126/science.aad6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ovejero D, Veiga S, García-Segura LM, Doncarlos LL, 2002. Glial expression of estrogen and androgen receptors after rat brain injury: Reactive Glia Express Gonadal Hormone Receptors. Journal of Comparative Neurology 450, 256–271. 10.1002/cne.10325 [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M, 2010. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science 330, 841–845. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VWV, Eriksson JG, Broekman BFP, 2017. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol 5, 53–64. 10.1016/S2213-8587(16)30107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, Berton O, Russo SJ, 2011. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc 6, 1183–1191. 10.1038/nprot.2011.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ, 2016. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 21, 1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-González B, Escobar A, 2010. Prenatal stress alters microglial development and distribution in postnatal rat brain. Acta Neuropathol. (Berl.) 119, 303–315. 10.1007/s00401-009-0590-4 [DOI] [PubMed] [Google Scholar]
- Graf AE, Lallier SW, Waidyaratne G, Thompson MD, Tipple TE, Hester ME, Trask AJ, Rogers LK, 2016. Maternal high fat diet exposure is associated with increased hepcidin levels, decreased myelination, and neurobehavioral changes in male offspring. Brain. Behav. Immun 58, 369–378. 10.1016/j.bbi.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, Brenhouse HC, 2016. Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology 71, 19–30. 10.1016/j.psyneuen.2016.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC, 2010. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry 67, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S, Tseng H-H, Rao N, Selvanathan T, Kenk M, Bazinet RP, Suridjan I, Wilson AA, Meyer JH, Remington G, others, 2016. Imaging Microglial Activation in Untreated First-Episode Psychosis: A PET Study With [18F] FEPPA. Am. J. Psychiatry appi–ajp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeyer N, Hanft K-M, Akriditou M-A, Unger N, Park ES, Stanley ER, Staszewski O, Dimou L, Prinz M, 2017. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. (Berl.) 10.1007/s00401-017-1747-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim A, Julian D, Albin-Brooks C, Brothers HM, Lenz KM, Leuner B, 2017. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain. Behav. Immun 59, 67–78. 10.1016/j.bbi.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, Bilbo SD, 2017. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 10.1002/glia.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, Abbas AI, Leonardo ED, Bolkan SS, Hen R, Gordon JA, 2017. A Novel Method for Chronic Social Defeat Stress in Female Mice. Neuropsychopharmacology 10.1038/npp.2017.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Filiano AJ, Smith A, Yogev N, Kipnis J, 2017. Myeloid Cells in the Central Nervous System. Immunity 46, 943–956. 10.1016/j.immuni.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, Gedrich K, Kloiber S, Lucae S, Ising M, Uhr M, Holsboer F, Pollmächer T, 2008. Depression, comorbidities and the TNF-α system. Eur. Psychiatry 23, 421–429. 10.1016/j.eurpsy.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Labonté B, Horn S, Lapidus KA, Stelzhammer V, Wong EHF, Bahn S, Krishnan V, Bolaños-Guzman CA, Murrough JW, Merad M, Russo SJ, 2014. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci 111, 16136–16141. 10.1073/pnas.1415191111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JKY, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F, 2015. C-Myb+ Erythro-Myeloid Progenitor-Derived Fetal Monocytes Give Rise to Adult Tissue-Resident Macrophages. Immunity 42, 665–678. 10.1016/j.immuni.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori T. -a, Enami T, Furukawa A, Suzuki K, Ishii H. -t., Mukai H, Morrison JH, Janssen WGM, Kominami S, Harada N, Kimoto T, Kawato S, 2004. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017 and P450 aromatase localized in neurons. Proceedings of the National Academy of Sciences 101, 865–870. 10.1073/pnas.2630225100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland FH, Ganguly P, Potter DN, Chartoff EH, Brenhouse HC, 2014. Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neurosci. Lett 566, 131–136. 10.1016/j.neulet.2014.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, Gerhard A, Talbot PS, 2018. Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biol. Psychiatry 83, 61–69. 10.1016/j.biopsych.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Huhman KL, 2006. Social conflict models: Can they inform us about human psychopathology? Horm. Behav 50, 640–646. 10.1016/j.yhbeh.2006.06.022 [DOI] [PubMed] [Google Scholar]
- Hui CW, St-Pierre A, El Hajj H, Remy Y, Hébert SS, Luheshi GN, Srivastava LK, Tremblay M-È, 2018. Prenatal Immune Challenge in Mice Leads to Partly Sex-Dependent Behavioral, Microglial, and Molecular Abnormalities Associated with Schizophrenia. Front. Mol. Neurosci 11. 10.3389/fnmol.2018.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM, 2007. Sexual behavior in male rodents. Hormones and Behavior 52, 45–55. 10.1016/j.yhbeh.2007.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH, 2007. Depressive disorders and immunity: 20 years of progress and discovery. Brain. Behav. Immun 21, 374–383. 10.1016/j.bbi.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Janthakhin Y, Rincel M, Costa A-M, Darnaudéry M, Ferreira G, 2017. Maternal high-fat diet leads to hippocampal and amygdala dendritic remodeling in adult male offspring. Psychoneuroendocrinology 83, 49–57. 10.1016/j.psyneuen.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Kang SS, Kurti A, Fair DA, Fryer JD, 2014. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Nothdurfter C, Aslanidis A, Moeller K, Horn F, Scholz R, Neumann H, Weber BH, Rupprecht R, Langmann T 2014. Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J. Neuroinflammation 11:3 10.1186/1742-2094-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER, 2001. Rapid actions of plasma membrane estrogen receptors. Trends in Endocrinology & Metabolism 12, 152–156. 10.1016/S1043-2760(01)00377-0 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U, 2012. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States: Anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res 21, 169–184. 10.1002/mpr.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U-K, Noda M, Verkhratsky A, 2011. Physiology of Microglia. Physiol. Rev 91, 461–553. 10.1093/med/9780199794591.003.0019 [DOI] [PubMed] [Google Scholar]
- Khalil M, Ronda J, Weintraub M, Jain K, Silver R, Silverman A-J, 2007. Brain mast cell relationship to neurovasculature during development. Brain Res 1171, 18–29. 10.1016/j.brainres.2007.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil MH, Silverman A-J, Silver R, 2003. Mast cells in the rat brain synthesize gonadotropin-releasing hormone. J. Neurobiol 56, 113–124. 10.1002/neu.10220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Hölscher C, Müller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M, 2013. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci 16, 273–280. 10.1038/nn.3318 [DOI] [PubMed] [Google Scholar]
- Kim I, Mlsna LM, Yoon S, Le B, Yu S, Xu D, Koh S, 2015. A postnatal peak in microglial development in the mouse hippocampus is correlated with heightened sensitivity to seizure triggers. Brain Behav 5, n/a-n/a. 10.1002/brb3.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, Huh JR, 2017. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532. 10.1038/nature23910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh Y-J, Fombonne E, Laska E, Lim E-C, Cheon K-A, Kim S-J, Kim Y-K, Lee H, others, 2011. Prevalence of autism spectrum disorders in a total population sample. Am. J. Psychiatry [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C, 2007. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448, 1009–1014. 10.1038/nature06089 [DOI] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N, Kawato S, 2001. Neurosteroid Synthesis by Cytochrome P450-Containing Systems Localized in the Rat Brain Hippocampal Neurons: N-Methyl-D-Aspartate and Calcium-Dependent Synthesis. Endocrinology 142, 3578–3589. [DOI] [PubMed] [Google Scholar]
- King WJ, Greene GL, 1984. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature 307, 745–747. [DOI] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL, 2016. Sex differences in immune responses. Nat. Rev. Immunol 16, 626–638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C, Gardner RM, 2015. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol. Psychiatry 10.1038/mp.2015.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh Y-HE, Cahill M, Lorsch ZS, Hamilton PJ, Calipari ES, Hodes GE, Issler O, Kronman H, Pfau M, Obradovic ALJ, Dong Y, Neve RL, Russo S, Kazarskis A, Tamminga C, Mechawar N, Turecki G, Zhang B, Shen L, Nestler EJ, 2017. Sex-specific transcriptional signatures in human depression. Nat. Med 23, 1102–1111. 10.1038/nm.4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskaris LE, Di Biase MA, Everall I, Chana G, Christopoulos A, Skafidas E, Cropley VL, Pantelis C, 2016. Microglial activation and progressive brain changes in schizophrenia: Microglial activation and brain changes in schizophrenia. Br. J. Pharmacol 173, 666–680. 10.1111/bph.13364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim D-W, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, Anderson DJ, 2014. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632. 10.1038/nature13169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, Huttner A, Pletikos M, Sestan N, Leckman JF, Vaccarino FM, 2016. Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biol. Psychiatry 79, 372–382. 10.1016/j.biopsych.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN, 2010. Sex differences in the adolescent brain. Brain Cogn 72, 46–55. 10.1016/j.bandc.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, McCarthy MM, 2010. Organized for sex - steroid hormones and the developing hypothalamus: Steroids and the developing hypothalamus. Eur. J. Neurosci 32, 2096–2104. 10.1111/j.1460-9568.2010.07511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, McCarthy MM, 2013. Microglia Are Essential to Masculinization of Brain and Behavior. J. Neurosci 33, 2761–2772. 10.1523/JNEUROSCI.1268-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, McCarthy MM, 2012. Sexual Differentiation of the Rodent Brain: Dogma and Beyond. Front. Neurosci 6. 10.3389/fnins.2012.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S-H, Park E, You B, Jung Y, Park A-R, Park SG, Lee J-R, 2013. Neuronal Synapse Formation Induced by Microglia and Interleukin 10. PLoS ONE 8, e81218. 10.1371/journal.pone.0081218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ, 2011. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226. 10.1038/nature09736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-L, Lin S-Y, Wang S, 2012. Prenatal lipopolysaccharide exposure increases anxiety-like behaviors and enhances stress-induced corticosterone responses in adult rats. Brain. Behav. Immun 26, 459–468. 10.1016/j.bbi.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Lonstein J, De Vries G, 2000. Maternal behaviour in lactating rats stimulates c-fos in glutamate decarboxylase-synthesizing neurons of the medial preoptic area, ventral bed nucleus of the stria terminalis, and ventrocaudal periaqueductal gray. Neuroscience 100, 557–568. 10.1016/S0306-4522(00)00287-6 [DOI] [PubMed] [Google Scholar]
- Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HEW, Maier SF, Watkins LR, 2012. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 37, 1688–1699. 10.1016/j.psyneuen.2012.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch ZS, Loh Y-HE, Purushothaman I, Walker DM, Parise EM, Salery M, Cahill ME, Hodes GE, Pfau ML, Kronman H, Hamilton PJ, Issler O, Labonté B, Symonds AE, Zucker M, Zhang TY, Meaney MJ, Russo SJ, Shen L, Bagot RC, Nestler EJ, 2018. Estrogen receptor α drives pro-resilient transcription in mouse models of depression. Nat. Commun 9. 10.1038/s41467-018-03567-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Jin LW, 2010. Rett Syndrome Microglia Damage Dendrites and Synapses by the Elevated Release of Glutamate. J. Neurosci 30, 5346–5356. 10.1523/JNEUROSCI.5966-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinson R, Lloyd K, Rayasam A, McKee S, Brown A, Barila G, Grissom N, George R, Marini M, Fabry Z, Elovitz M, Reyes TM, 2017. Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain. Behav. Immun 66, 277–288. 10.1016/j.bbi.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi J, Berg M, Dencker A, Olander EK, Begley C, 2015. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews: Obesity in pregnancy - a review of reviews. Obes. Rev 16, 621–638. 10.1111/obr.12288 [DOI] [PubMed] [Google Scholar]
- Masliniska D, Dambska M, Kaliszek A, Maslinski S, 2001. Accumulation, distribution and phenotype heterogeneity of mast cells (MC) in human brains with neurocysticercosis. Folia Neuropathol 39, 7–13. [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada Gonzalez F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, Itzkovitz S, Elinav E, Sieweke MH, Schwartz M, Amit I, 2016. Microglia development follows a stepwise program to regulate brain homeostasis. Science 10.1126/science.aad8670 [DOI] [PubMed] [Google Scholar]
- McCarthy MM, 2008. Estradiol and the Developing Brain. Physiol. Rev 88, 91–134. 10.1152/physrev.00010.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM, Lenz KM, 2017. Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat. Rev. Neurosci 10.1038/nrn.2017.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, Sheridan JF, 2016. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol. Psychiatry 79, 803–813. 10.1016/j.biopsych.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, Sobol CG, Quan N, Sheridan JF, Godbout JP, 2017. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 10.1038/mp.2017.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D, 1988. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry 45, 189–192. [DOI] [PubMed] [Google Scholar]
- Meeker RB, Williams K, Killebrew DA, Hudson LC, 2012. Cell trafficking through the choroid plexus. Cell Adhesion & Migration 6, 390–396. 10.4161/cam.21054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, Janssen WG, Labonté B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, Fayad ZA, Tang CY, Merad M, Russo SJ, 2017. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci 20, 1752–1760. 10.1038/s41593-017-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Micevych PE, 2008. Nervous System Physiology Regulated by Membrane Estrogen Receptors. Reviews in the Neurosciences 19 10.1515/REVNEURO.2008.19.6.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, 2014. Prenatal Poly(I:C) Exposure and Other Developmental Immune Activation Models in Rodent Systems. Biol. Psychiatry 75, 307–315. 10.1016/j.biopsych.2013.07.011 [DOI] [PubMed] [Google Scholar]
- Meyer U, 2006. The Time of Prenatal Immune Challenge Determines the Specificity of Inflammation-Mediated Brain and Behavioral Pathology. J. Neurosci 26, 4752–4762. 10.1523/JNEUROSCI.0099-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J, 2008. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol. Psychiatry 13, 208–221. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B, 2011. Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol. Psychiatry 70, 663–671. 10.1016/j.biopsych.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J, 2016. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun 7, 12540. 10.1038/ncomms12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr MA, Garcia FL, DonCarlos LL, Sisk CL, 2016. Neurons and Glial Cells Are Added to the Female Rat Anteroventral Periventricular Nucleus During Puberty. Endocrinology 157, 2393–2402. 10.1210/en.2015-2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI, 2015. Sex Differences in Depressive and Socioemotional Responses to an Inflammatory Challenge: Implications for Sex Differences in Depression. Neuropsychopharmacology 40, 1709–1716. 10.1038/npp.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM, 2002. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Dev. Brain Res 139, 151–158. 10.1016/S0165-3806(02)00541-2 [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, Everall IP, 2012. Abnormal microglial–neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res 1456, 72–81. 10.1016/j.brainres.2012.03.036 [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP, 2010. Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biol. Psychiatry 68, 368–376. 10.1016/j.biopsych.2010.05.024 [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL, 2008. Sex-Specific Programming of Offspring Emotionality after Stress Early in Pregnancy. J. Neurosci 28, 9055–9065. 10.1523/JNEUROSCI.1424-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z, 1971. Aromatization of androstenedione by the diencephalon. The Journal of Clinical Endocrinology & Metabolism 33, 3. [DOI] [PubMed] [Google Scholar]
- Nelson LH, Lenz KM, 2016. Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behav. Brain Res 10.1016/j.bbr.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, Warden S, Lenz KM, 2017. Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain. Behav. Immun 64, 11–22. 10.1016/j.bbi.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Kimyon RS, De I, Small AL, Collier LS, Watters JJ, 2015. Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J. Neuroimmunol 278, 280–288. 10.1016/j.jneuroim.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, 2005. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 308, 1314–1318. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Niraula A, Sheridan JF, Godbout JP, 2017. Microglia Priming with Aging and Stress. Neuropsychopharmacology 42, 318–333. 10.1038/npp.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JE, Reynolds R, 2011. The consequences of obesity and excess weight gain in pregnancy. Proc. Nutr. Soc 70, 450–456. 10.1017/S0029665111003077 [DOI] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Sawa A, Meyer U, 2017. Reconceptualization of translocator protein as a biomarker of neuroinflammation in psychiatry. Mol. Psychiatry 10.1038/mp.2017.232 [DOI] [PubMed] [Google Scholar]
- Nugent BM, Bale TL, 2015. The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Front. Neuroendocrinol 39, 28–37. 10.1016/j.yfrne.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM, 2015. Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci 18, 690–697. 10.1038/nn.3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, 2007. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol 49, 12–21. 10.1002/dev.20198 [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Tsai M-J, 1992. Molecular Pathways of Steroid Receptor Action. Biology of Reproduction 46, 163–167. 10.1095/biolreprod46.2.163 [DOI] [PubMed] [Google Scholar]
- Ortega AN, McQuaid EL, Canino G, Goodwin RD, Fritz GK, 2004. Comorbidity of Asthma and Anxiety and Depression in Puerto Rican Children. Psychosomatics 45, 93–99. 10.1176/appi.psy.45.2.93 [DOI] [PubMed] [Google Scholar]
- Osborne BF, Caulfield JI, Solomotis SA, Schwarz JM, 2017. Neonatal infection produces significant changes in immune function with no associated learning deficits in juvenile rats: Developmental Neurobiology. Dev. Neurobiol 77, 1221–1236. 10.1002/dneu.22512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LM, Monk C, 2013. Perinatal depression—The fourth inflammatory morbidity of pregnancy? Psychoneuroendocrinology 38, 1929–1952. 10.1016/j.psyneuen.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, Hempstead BL, Littman DR, Gan W-B, 2013. Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell 155, 1596–1609. 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterski V, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M, 2011. Prenatal hormones and childhood sex segregation: Playmate and play style preferences in girls with congenital adrenal hyperplasia. Horm. Behav 59, 549–555. 10.1016/j.yhbeh.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pheonix CH, Goy RW, Gerall AA, Young WC, 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 14. [DOI] [PubMed] [Google Scholar]
- Pont-Lezica L, Beumer W, Colasse S, Drexhage H, Versnel M, Bessis A, 2014. Microglia shape corpus callosum axon tract fasciculation: functional impact of prenatal inflammation. Eur. J. Neurosci 39, 1551–1557. 10.1111/ejn.12508 [DOI] [PubMed] [Google Scholar]
- Pramparo T, Pierce K, Lombardo MV, Carter Barnes C, Marinero S, Ahrens-Barbeau C, Murray SS, Lopez L, Xu R, Courchesne E, 2015. Prediction of Autism by Translation and Immune/Inflammation Coexpressed Genes in Toddlers From Pediatric Community Practices. JAMA Psychiatry 72, 386. 10.1001/jamapsychiatry.2014.3008 [DOI] [PubMed] [Google Scholar]
- Pyter LM, Kelly SD, Harrell CS, Neigh GN, 2013. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain. Behav. Immun 30, 88–94. 10.1016/j.bbi.2013.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, Kapczinski F, Quevedo J, 2015. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300, 141–154. 10.1016/j.neuroscience.2015.05.018 [DOI] [PubMed] [Google Scholar]
- Rincel M, Lépinay AL, Janthakhin Y, Soudain G, Yvon S, Da Silva S, Joffre C, Aubert A, Séré A, Layé S, Theodorou V, Ferreira G, Darnaudéry M, 2018. Maternal high-fat diet and early life stress differentially modulate spine density and dendritic morphology in the medial prefrontal cortex of juvenile and adult rats. Brain Struct. Funct 223, 883–895. 10.1007/s00429-017-1526-8 [DOI] [PubMed] [Google Scholar]
- Robertson MM, 2008. The prevalence and epidemiology of Gilles de la Tourette syndrome. J. Psychosom. Res 65, 461–472. 10.1016/j.jpsychores.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Roque A, Ochoa-Zarzosa A, Torner L, 2015. Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain. Behav. Immun 10.1016/j.bbi.2015.09.017 [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA, 1993. Aromatase activity in the rat brain: Hormonal regulation and sex differences. The Journal of Steroid Biochemistry and Molecular Biology 44, 499–508. 10.1016/0960-0760(93)90254-T [DOI] [PubMed] [Google Scholar]
- Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM, 2011. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol. Sex Differ 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki CM, McKim DB, Wohleb ES, Jarrett BL, Reader BF, Norden DM, Godbout JP, Sheridan JF, 2015. Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience 302, 151–164. 10.1016/j.neuroscience.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Heller CT, Gunner G, Heller M, Gordon C, Hammond T, Wolf Y, Jung S, Stevens B, 2016. Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression. Elife 5, e15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B, 2012. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 74, 691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, Rubinow DR, 2015. The role of reproductive hormones in postpartum depression. CNS Spectr 20, 48–59. 10.1017/S1092852914000480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD, 2012. Sex differences in microglial colonization of the developing rat brain. J. Neurochem no-no. 10.1111/j.1471-4159.2011.07630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segman RH, Goltser-Dubner T, Weiner I, Canetti L, Galili-Weisstub E, Milwidsky A, Pablov V, Friedman N, Hochner-Celnikier D, 2010. Blood mononuclear cell gene expression signature of postpartum depression. Mol. Psychiatry 15, 93–100. 10.1038/mp.2009.65 [DOI] [PubMed] [Google Scholar]
- Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, Logan RW, Tseng G, Lewis DA, Sibille E, 2018. Opposite Molecular Signatures of Depression in Men and Women. Biol. Psychiatry 10.1016/j.biopsych.2018.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH, 2015. Role of Translocator Protein Density, a Marker of Neuroinflammation, in the Brain During Major Depressive Episodes. JAMA Psychiatry 72, 268. 10.1001/jamapsychiatry.2014.2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J, Ruedl C, Karjalainen K, 2015. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity 43, 382–393. 10.1016/j.immuni.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Sherer ML, Posillico CK, Schwarz JM, 2017. An examination of changes in maternal neuroimmune function during pregnancy and the postpartum period. Brain. Behav. Immun 66, 201–209. 10.1016/j.bbi.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH, 2003. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci 23, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K, 2014. Microglia Enhance Neurogenesis and Oligodendrogenesis in the Early Postnatal Subventricular Zone. J. Neurosci 34, 2231–2243. 10.1523/JNEUROSCI.1619-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert RJ, 2005. Depression in multiple sclerosis: a review. J. Neurol. Neurosurg. Psychiatry 76, 469–475. 10.1136/jnnp.2004.054635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJP, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M, 2010. Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell 7, 483–495. 10.1016/j.stem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K, 2008. Steroid hormone receptor expression and function in microglia. Glia 56, 659–674. 10.1002/glia.20644 [DOI] [PubMed] [Google Scholar]
- Silver R, Curley JP, 2013. Mast cells on the mind: new insights and opportunities. Trends Neurosci 36, 513–521. 10.1016/j.tins.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Silverman A-J, Asarian L, Khalil M, Silver R, 2002. GnRH, brain mast cells and behavior, in: Progress in Brain Research Elsevier, pp. 315–325. 10.1016/S0079-6123(02)41102-8 [DOI] [PubMed] [Google Scholar]
- Simpson W, Steiner M, Coote M, Frey BN, 2016. Relationship between inflammatory biomarkers and depressive symptoms during late pregnancy and the early postpartum period: a longitudinal study. Rev. Bras. Psiquiatr 38, 190–196. 10.1590/1516-4446-2015-1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, 2017. Development: Pubertal Hormones Meet the Adolescent Brain. Curr. Biol 27, R706–R708. 10.1016/j.cub.2017.05.092 [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL, 2005. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology 26, 163–174. 10.1016/j.yfrne.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Ślusarczyk J, Trojan E, Głombik K, Budziszewska B, Kubera M, Lasoń W, Popiołek-Barczyk K, Mika J, Wędzony K, Basta-Kaim A, 2015. Prenatal stress is a vulnerability factor for altered morphology and biological activity of microglia cells. Front. Cell. Neurosci 9. 10.3389/fncel.2015.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH, 2007. Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. J. Neurosci 27, 10695–10702. 10.1523/JNEUROSCI.2178-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin J-S, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji R-R, Zhang J, Salter MW, Mogil JS, 2015. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci 10.1038/nn.4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Voskuhl RR, 2012. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Frontiers in Neuroendocrinology 33, 105–115. 10.1016/j.yfrne.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel C, Schuchert R, Wagner F, Thaler R, Weinberger T, Pick R, Mass E, Ishikawa-Ankerhold HC, Margraf A, Hutter S, Vagnozzi R, Klapproth S, Frampton J, Yona S, Scheiermann C, Molkentin JD, Jeschke U, Moser M, Sperandio M, Massberg S, Geissmann F, Schulz C 2018. Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nat. Commun 9, 75. 10.1038/s41467-017-02492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf WE, Sar M, 1976. Steroid hormone target sites in the brain: The differential distribution of estrogen, progestin, androgen and glucocorticosteroid. Journal of Steroid Biochemistry 7, 1163–1170. 10.1016/0022-4731(76)90050-9 [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Smith MS, Coleman K, Grove KL, 2010. Chronic Consumption of a High-Fat Diet during Pregnancy Causes Perturbations in the Serotonergic System and Increased Anxiety-Like Behavior in Nonhuman Primate Offspring. J. Neurosci 30, 3826–3830. 10.1523/JNEUROSCI.5560-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, Yoshihara Yujiro, Omata K, Matsumoto K, Tsuchiya KJ, Iwata Y, Tsujii M, Sugiyama T, Mori N, 2013. Microglial Activation in Young Adults With Autism Spectrum Disorder. JAMA Psychiatry 70, 49. 10.1001/jamapsychiatry.2013.272 [DOI] [PubMed] [Google Scholar]
- Takahashi A, Chung J-R, Zhang S, Zhang H, Grossman Y, Aleyasin H, Flanigan ME, Pfau ML, Menard C, Dumitriu D, Hodes GE, McEwen BS, Nestler EJ, Han M-H, Russo SJ, 2017. Establishment of a repeated social defeat stress model in female mice. Sci. Rep 7. 10.1038/s41598-017-12811-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, De Vries GJ, 2012. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, 2016. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry 57, 241–266. 10.1111/jcpp.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, Lledo P-M, Greter M, Chan JKY, Amit I, Beyer M, Schultze JL, Schlitzer A, Pettersson S, Ginhoux F, Garel S, 2017. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell 10.1016/j.cell.2017.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, 2012. Membrane Progesterone Receptors: Evidence for Neuroprotective, Neurosteroid Signaling and Neuroendocrine Functions in Neuronal Cells. Neuroendocrinology 96, 162–171. 10.1159/000339822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd B, Schwarz J, Mccarthy M, 2005. Prostaglandin-E2: A point of divergence in estradiol-mediated sexual differentiation. Hormones and Behavior 48, 512–521. 10.1016/j.yhbeh.2005.07.011 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, 2004. Minireview: A Plethora of Estrogen Receptors in the Brain: Where Will It End? Endocrinology 145, 1069–1074. 10.1210/en.2003-1462 [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N, 2014. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain. Behav. Immun 42, 50–59. 10.1016/j.bbi.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Tsai M-J, O’Malley BW, 1994. Molecular Mechanisms of Action of Steroid/Thyroid Receptor Superfamily Members. Annu. Rev. Biochem 63, 451–486. [DOI] [PubMed] [Google Scholar]
- Turano A, Lawrence JH, Schwarz JM, 2017. Activation of neonatal microglia can be influenced by other neural cells. Neurosci. Lett 657, 32–37. 10.1016/j.neulet.2017.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T, 2013. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci 16, 543–551. 10.1038/nn.3358 [DOI] [PubMed] [Google Scholar]
- Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, Liddelow SA, Nguyen PT, Nakao-Inoue H, Dorman LC, Akil O, Joshita S, Barres BA, Paz JT, Molofsky AB, Molofsky AV, 2018. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science eaal3589. 10.1126/science.aal3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eynde K, Missault S, Fransen E, Raeymaekers L, Willems R, Drinkenburg W, Kumar-Singh S, Dedeurwaerdere S, 2014. Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav. Brain Res 258, 179–186. 10.1016/j.bbr.2013.10.005 [DOI] [PubMed] [Google Scholar]
- VanRyzin JW, Yu SJ, Perez-Pouchoulen M, McCarthy MM, 2016. Temporary Depletion of Microglia during the Early Postnatal Period Induces Lasting Sex-Dependent and Sex-Independent Effects on Behavior in Rats. eNeuro 3 10.1523/ENEURO.0297-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney C, Monier A, Fallet-Bianco C, Gressens P, 2010. Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants: Microglial colonization of the human forebrain. J. Anat 217, 436–448. 10.1111/j.1469-7580.2010.01245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A, 2008. Developmental Neuronal Death in Hippocampus Requires the Microglial CD11b Integrin and DAP12 Immunoreceptor. J. Neurosci 28, 8138–8143. 10.1523/JNEUROSCI.1006-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Bell MR, Flores C, Gulley JM, Willing J, Paul MJ, 2017. Adolescence and Reward: Making Sense of Neural and Behavioral Changes Amid the Chaos. J. Neurosci 37, 10855–10866. 10.1523/JNEUROSCI.1834-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wegener JE, Huang T-W, Sripathy S, De Jesus-Cortes H, Xu P, Tran S, Knobbe W, Leko V, Britt J, Starwalt R, McDaniel L, Ward CS, Parra D, Newcomb B, Lao U, Nourigat C, Flowers DA, Cullen S, Jorstad NL, Yang Y, Glaskova L, Vigneau S, Kozlitina J, Yetman MJ, Jankowsky JL, Reichardt SD, Reichardt HM, Gartner J, Bartolomei MS, Fang M, Loeb K, Keene CD, Bernstein I, Goodell M, Brat DJ, Huppke P, Neul JL, Bedalov A, Pieper AA, 2015. Wild-type microglia do not reverse pathology in mouse models of Rett syndrome. Nature 521, E1–E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Godbout JP, Sheridan JF, 2017. Repeated Social Defeat, Neuroinflammation and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology 42, 46–61. 10.1038/npp.2016.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y, Gross CT, 2018a. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun 9. 10.1038/s41467-018-03566-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L, Neniskyte U, Vadisiute A, di Bartolomei G, Aygün N, Riviere L, Zonfrillo F, Dymecki S, Gross C, 2018b. Sexual dimorphism of microglia and synapses during mouse postnatal development: Sexual Dimorphism in Microglia and Synapses. Dev. Neurobiol 10.1002/dneu.22568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling DM, Parikshak NN, Geschwind DH, 2016. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat. Commun 7, 10717. 10.1038/ncomms10717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernersson S, Pejler G, 2014. Mast cell secretory granules: armed for battle. Nat. Rev. Immunol 14, 478–94. 10.1038/nri3690 [DOI] [PubMed] [Google Scholar]
- Wieck A, Andersen SL, Brenhouse HC, 2013. Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: Relationship to cortical NMDA receptor expression. Brain. Behav. Immun 28, 218–226. 10.1016/j.bbi.2012.11.012 [DOI] [PubMed] [Google Scholar]
- Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD, 2011. Microglia and Memory: Modulation by Early-Life Infection. J. Neurosci 31, 15511–15521. 10.1523/JNEUROSCI.3688-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate M, Kirby RS, Pettygrove S, Cunniff C, Schulz E, Ghosh T, Robinson C, Lee L-C, Landa R, Constantino J, others, 2014. Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ 63. [PubMed] [Google Scholar]
- Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R, Benmamar‐ Badel A, de Boer‐Bergsma JJ, Martin NA, Karram K, Kramer I, Boddeke EW, Waisman A, Eggen BJ, Owens T, 2017. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J e201696056. 10.15252/embj.201696056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF, 2011. Beta-Adrenergic Receptor Antagonism Prevents Anxiety-Like Behavior and Microglial Reactivity Induced by Repeated Social Defeat. J. Neurosci 31, 6277–6288. 10.1523/JNEUROSCI.0450-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb Eric S., McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP, 2014. Re-establishment of Anxiety in Stress-Sensitized Mice Is Caused by Monocyte Trafficking from the Spleen to the Brain. Biol. Psychiatry 75, 970–981. 10.1016/j.biopsych.2013.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF, 2014. Knockdown of Interleukin-1 Receptor Type-1 on Endothelial Cells Attenuated Stress-Induced Neuroinflammation and Prevented Anxiety-Like Behavior. J. Neurosci 34, 2583–2591. 10.1523/JNEUROSCI.3723-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF, 2013. Stress-Induced Recruitment of Bone Marrow-Derived Monocytes to the Brain Promotes Anxiety-Like Behavior. J. Neurosci 33, 13820–13833. 10.1523/JNEUROSCI.1671-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Terwilliger R, Duman CH, Duman RS, 2017. Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior. Biological Psychiatry 10.1016/j.biopsych.2017.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, 2007. Acute Effects of Estrogen on Neuronal Physiology. Annual Review of Pharmacology and Toxicology 47, 657–680. 10.1146/annurev.pharmtox.47.120505.105219 [DOI] [PubMed] [Google Scholar]
- Wright CL, McCarthy MM, 2009. Prostaglandin E2-Induced Masculinization of Brain and Behavior Requires Protein Kinase A, AMPA/Kainate, and Metabotropic Glutamate Receptor Signaling. J. Neurosci 29, 13274–13282. 10.1523/JNEUROSCI.3603-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Tan X, Dai Y, Krishnan V, Warner M, Gustafsson J-Å, 2013. Targeting estrogen receptor β in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences 110, 3543–3548. 10.1073/pnas.1300313110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Shah NM, 2016. Molecular and neural control of sexually dimorphic social behaviors. Current Opinion in Neurobiology 38, 89–95. 10.1016/j.conb.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, Reshef R, 2015. Depression as a Microglial Disease. Trends Neurosci 38, 637–658. 10.1016/j.tins.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, Guthrie D, 2009. Institutional rearing and psychiatric disorders in Romanian preschool children. Am. J. Psychiatry [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Adler NT, 1977. Hormonal control of female sexual behavior in the rat. Hormones and Behavior 9, 345–357. 10.1016/0018-506X(77)90069-1 [DOI] [PubMed] [Google Scholar]
- Zhang X, Yao H, Qian Q, Li N, Jin W, Qian Y, 2016. Cerebral Mast Cells Participate In Postoperative Cognitive Dysfunction by Promoting Astrocyte Activation. Cellular Physiology and Biochemistry 40, 104–116. 10.1159/000452528 [DOI] [PubMed] [Google Scholar]


