Abstract
The atrioventricular heart valve (AHV) leaflets are critical to the facilitation of proper unidirectional blood flow through the heart. Previously, studies have been conducted to understand the tissue mechanics of the healthy AHV leaflets to inform the development of valve-specific computational models and replacement materials for use in diagnosing and treating valvular heart disease. Generally, these studies involved biaxial mechanical testing of the leaflet tissue specimens to extract relevant mechanical properties. Most of those researchers used freezing-based storage systems based on previous findings for other connective tissues such as aortic tissue or skin. However, there remains no study which specifically examines the effect of freezing storage on the characterized mechanical properties of the AHV leaflets. In this study, we aimed to address this gap in knowledge by performing biaxial mechanical characterizations of the tricuspid valve anterior leaflet (TVAL) tissue both before and after a 48-hour freezing period. Primary findings of this study include: (i) a statistically insignificant change in the tissue extensibilities, with the frozen tissues being slightly stiffer and more anisotropic than the fresh tissues; and (ii) minimal variation in the stress relaxation behaviors between the fresh and frozen tissues, with the frozen tissues demonstrating slightly lessened relaxation. The findings from this study suggested that freezing-based storage does not significantly impact the observed mechanical properties of one of the five AHV leaflets—the TVAL. The results from this study are useful for reaffirming the previous researchers’ methodologies, as well as informing the tissue preservation methods of future investigations of AHV leaflet mechanics.
Keywords: the tricuspid valve, heart valve biomechanics, freezing storage effects, biaxial mechanical testing, stress relaxation
1. Introduction
The atrioventricular heart valves (AHVs) are critical in ensuring unidirectional flow of blood from the atria into the ventricles. The AHVs include the mitral valve (MV) and the tricuspid valve (TV), which are composed of soft tissue leaflets that facilitate proper blood flow by cyclically opening and closing throughout the cardiac cycle. When these valves fail to properly close, a regurgitant jet of blood occurs from the ventricles into the atria during systole, leading to insufficient blood in the ventricles and long-term health problems, such as an increased possibility of heart failure (Waller et al., 1994; Waller et al., 1995). Currently, research has been focused on the development of computational models and biomimetic materials for better understanding and treatment of valvular heart disease, such as valve regurgitation (Arzani and Mofrad, 2017; Kidane et al., 2009; Kunzelman et al., 1997; Lee et al., 2015; Sun et al., 2014). These studies were based on an understanding of the mechanical properties and behaviors of the heart valve leaflets. Determinations of the valve leaflets’ properties are generally performed using a mechanical testing procedure, most primarily in the form of cyclic biaxial mechanical loading. Many of these prior studies of the AHV leaflets have utilized freezing-based storage systems to preserve the freshness of the leaflet tissues for extended periods of time (Eckert et al., 2013; Grashow et al., 2006; Huang et al., 2012; Jett et al., 2018; Laurence et al., 2019; Liao et al., 2007; Pham et al., 2017; Pham and Sun, 2014; Pokutta-Paskaleva et al., 2019; Stella et al., 2007; Stella and Sacks, 2007) based on the findings in the previous literature for mechanical testing of other biological materials.
Previously, studies have had mixed or contradictory results regarding the effect of freezing on the mechanics of biological tissues. Some researchers have examined the efficacy of freezer storage for bone tissue (Kaye et al., 2012; Linde and Sørensen, 1993; Pelker et al., 1983) or cartilage (Changoor et al., 2010), by performing mechanical characterizations of the structures both before and after freezing of the tissue. These researchers found that there was no significant change in the mechanical properties of these tissues influenced by freezing storage. Those researchers investigating tendons (Clavert et al., 2001; Giannini et al., 2008; Matthews and Ellis, 1968; Smith et al., 1996), on the other hand, did find significant changes in the mechanical properties following freezing. Other researchers have looked specifically at soft biological tissues such as arteries (Delgadillo et al., 2010; Venkatasubramanian et al., 2006), aortic tissue (O’Leary et al., 2014; Stemper et al., 2007), skin (Foutz et al., 1992) and liver (Santago et al., 2009). Researchers analyzing freezing effects on soft tissues have mixed results, where some studies found no significant changes in the observed mechanical properties due to freezing (Delgadillo et al., 2010; O’Leary et al., 2014; Stemper et al., 2007), while others observed notable changes in the tissue’s mechanical properties, such as the elastic modulus or failure strain (Santago et al., 2009; Venkatasubramanian et al., 2006). Furthermore, the storage methods for the tissues vary greatly from study to study, including refrigeration at 4 °C, freezing at temperature levels ranging from −20 °C to −80 °C (or even as low as −196 °C), several freeze-thaw cycles or rates of freezing, and the use of liquid nitrogen.
To elaborate on these inter-study variations, for example, Delgadillo et al. used cryoprotective solutions when freezing arterial tissue, which may explain why they did not observe a change in the tissue mechanical response as compared to Venkatasubramanian et al., who observed a change in the elastic modulus using the same tissue type and storage temperature as Delgadillo et al. Stemper et al. noticed a change in mechanical response of porcine aortas due to refrigerator storage (4 °C) but not freezer storage (−20 °C or −80°C). Additionally, Santago et al. found a difference in failure strain for liver tissue, which they explained was due to the microstructure of the tissue. Santago et al. proposed that the liver tissue has a greater proportion of cells compared to other tissues, such as tendon, and that the cells sustained damage due to the freezer storage. The differences in the freezing storage methods or type of biological tissue may explain the variety of results from these researchers in the soft tissue biomechanics literature.
Although the effect of freezing on the mechanical properties of many other soft biological tissues has been investigated, there still exists a gap in literature on the freezing-induced effects on the observed mechanical properties of the atrioventricular heart valve leaflets. Previous researchers of the AHV leaflets employed a freezing-based storage system for their studies based on the assumption that results from studies of other soft biological tissues, such as arteries, skin or aortic tissue—analogous for the heart valve leaflets, which may not necessarily be valid. Therefore, our primary goal in this study is to fill this gap in knowledge by examining the effects of freezing-based storage on the characterized tissue mechanics of one of the five AHV leaflets—the tricuspid valve anterior leaflet (TVAL). This investigation was done by performing biaxial mechanical testing and stress relaxation testing of the selected AHV leaflet tissue specimens before and after freezer storage. Our findings in this study will provide helpful insight into the AHV leaflet-specific storage methods, which will be informative to the future use of freezing storage in AHV leaflet tissue experiments.
2. Methods
2.1. Tissue acquisition and preparation
Healthy, adult porcine hearts (80–140 kg, 1–1.5 years of age, a female-to-male ratio of 1:1, n=10) were obtained from a local USDA-approved slaughterhouse (Chickasha Meat Co., Chickasha, OK). Then, the tricuspid valve anterior leaflet was excised from the heart within 3 hours of animal death. The TVAL was selected due to its large size relative to the other TV leaflets (Rogers and Bolling, 2009). Once excised, the leaflet was sectioned into a 10 × 10 mm square (Fig. 1a), and the tissue’s circumferential and radial directions noted by labelling the top-right corner of the square tissue specimen using a surgical pen. The tissue thickness was measured with digital calipers (Westward 1AAU4 – 0.01 mm resolution) three times per sectioned leaflet. The three thickness measurements were then averaged to account for regional variations in tissue thickness. The tissue specimen was then mounted to a commercial biaxial mechanical testing system (BioTester, CellScale, Canada) using five-tined BioRakes. The fresh tissue specimens were mounted with an effective edge length of 6.5–8.5 mm, and the frozen tissues were mounted with a smaller effective edge length of 6.0–7.0 mm. Next, using a surgical pen, four fiducial markers were applied in a square configuration in the middle third of the tissue for later digital image correlation (DIC) methods (cf. Section 2.3). The tissue was submerged in a phosphate-buffered saline (PBS) solution bath heated to 37 °C to emulate the physiological conditions for the biaxial mechanical testing as described in the next subsection.
Figure 1 –
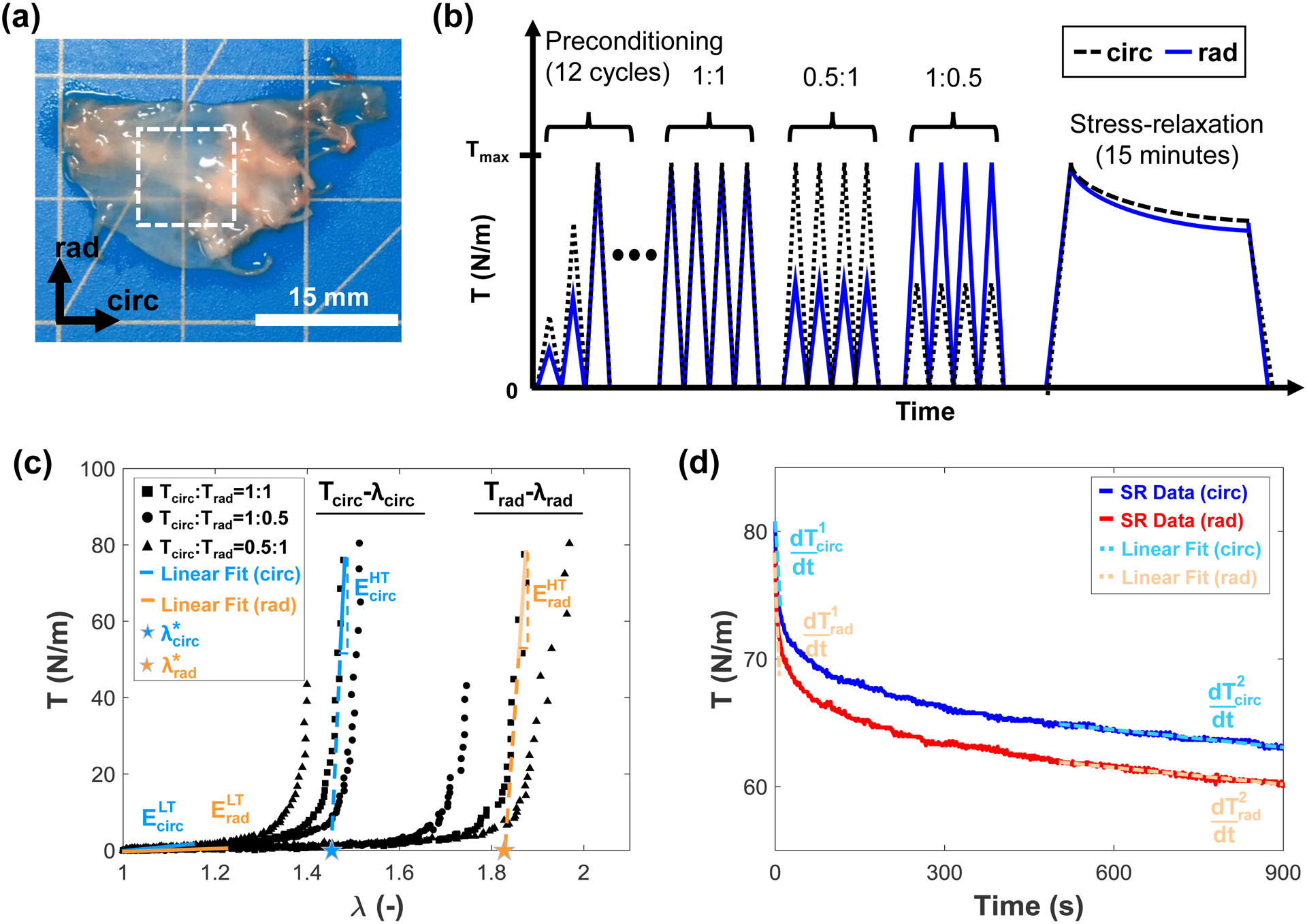
(a) The tricuspid valve anterior leaflet was excised and sectioned into a central 10mm × 10mm region before (b) biaxial mechanical testing involving a preconditioning protocol, biaxial loading to ratios of the peak membrane tension (Tcirc:Trad=1:1, 1:0.5, 0.5:1), and stress-relaxation testing. (c) Parameters analyzed from the biaxial loading include the index of extensibility (λ*), tangent modulus at the low-tension (ELT) and high-tension regimes (EHT), and the stretches (λ). (d) Parameters analyzed from the stress relaxation include the membrane tension decay, and the initial and saturated membrane tension rates of change ( and , respectively).
A range of edge lengths were used in this study due to limitations based on the retrievable sizes of the tissue specimens. Additionally, different effective edge lengths were used between the fresh and frozen tissue samples because the same tissues were used both before and after freezer storage (cf. Section 2.2) and overlapping holes from the previous BioRake mounting could result in tissue tearing during testing. Additionally, preliminary results of an investigation in our lab suggest that the various edge lengths employed have a negligible effect on the measured tissue mechanical properties.
2.2. Biaxial mechanical testing
To compare the mechanical response of the fresh tissue specimen to the response after freezer storage, a sequential testing procedure was performed: (1) biaxial mechanical testing of a fresh (control) specimen, (2) freezing of the tissue for 48 hours, and (3) thawing and biaxial mechanical testing of the previously-frozen tissue using the same protocols as in Step (1). First, for biaxial mechanical testing of the fresh TVAL tissue specimen, a 12-cycle preconditioning protocol was performed that stretched the tissue specimen to a targeted membrane tension of 75 N/m (Fig. 1b). Tissues were tested to a membrane tension of 75 N/m to emulate both the physiological loading and the stress-overloading conditions of the TVAL (Ayoub et al., 2017; Khoiy and Amini, 2016; Pant et al., 2018). After preconditioning, mechanical testing protocols were performed as developed in our prior work for the TVAL tissues (Jett et al., 2018) with some minor modifications. These modifications included reducing the number of repetitions from 10 cycles to 4 cycles for each loading ratio and reducing the number of loading ratios from Tcirc:Trad=1:1, 1:0.75, 0.75:1, 1:0.5, and 0.5:1 to Tcirc:Trad=1:1, 1:0.5, and 0.5:1 (Fig. 1b), where Tcirc and Trad are the applied membrane tensions in the tissue’s circumferential and radial directions, respectively. Data was collected from the last unloading cycle of each protocol.
Next, a stress-relaxation protocol was performed during which the tissue was stretched to the displacement associated with the peak membrane tension, held at the same displacement for 15 minutes, and the force decrease over time monitored (Fig. 1b). Throughout the biaxial testing and stress-relaxation 1280×960 images were captured at a rate of 15 Hz using a CCD camera mounted to the biaxial tester. In the meantime, load cell force and tine displacement readings were recorded by the biaxial tester. All protocols were performed on both fresh and frozen tissues. For freezer storage, the fresh tissue samples at room temperature were placed in plastic containers immediately after testing, and the containers stored in the freezer of a standard refrigerator (Frigidaire FRT18L4JW7) at −14°C for two days before further biaxial mechanical testing.
2.3. Tissue stress-stretch analysis
For more details on the stress and stretch calculations, refer to our previous publication (Jett et al., 2018). Briefly, the DIC function of the BioTesting system software was used to determine the time-dependent pixel coordinates of the four fiducial markers. These marker positions were then used to calculate the deformation gradient F using a four-node bilinear finite element (Sacks, 2000; Tadmor et al., 2012). Next, principal stretches in the circumferential and radial directions (λcirc and λrad) were calculated by taking the square roots of the principal values of the Cauchy-Green deformation tensor C=FTF. Tissue stretches were calculated with respect to different deformation states, including the peak stretch (), which was the tissue stretch at the peak membrane tension with respect to the mounted tissue configuration (Ω0), the preconditioning stretch (), defined as the tissue stretch observed at the end of the preconditioning protocol determined between configuration Ω0 and the post-preconditioning configuration (Ω1), and the mechanical stretch , which was the tissue stretch at the peak membrane tension with respect to configuration Ω1. The tissue stretches can be related by .
As for the tissue stress calculation, the membrane tensions in both tissue directions (Tcirc and Trad) were determined by dividing the load cell’s force reading by the effective edge length of the tissue specimen. Other stress measures, such as the Cauchy stress σ, the first Piola-Kirchhoff stress P and the second Piola-Kirchhoff (2nd-PK) stress S, can be computed using the thickness t of the tissue specimen (Reddy, 2013; Tadmor et al., 2012). In addition, we also calculated the low-tension modulus (ELT), the high-tension elastic modulus (EHT), the index of extensibility (λ*), and the anisotropy index (AI), which are described in more detail in previous studies (May-Newman and Yin, 1995; Pham et al., 2017; Wells et al., 2012). As illustrated in Figure 1c, ELT refers to the tangent modulus at the pre-transitional region, EHT denotes the tangent modulus at the post-transitional region, λ* is the stretch as the intercept of the extended line of the post-transitional tangent, and AI represents the level of anisotropy of the tissue defined as the ratio .
The stress-relaxation behavior of the tissue was also analyzed by calculating the initial slope () and the saturated slope () of the 900-second stress relaxation data. The initial slope of stress-relaxation was determined by using the first 5-second stress relaxation data, whereas the saturated slopes were considered as the stress relaxation between 500 seconds and 900 seconds (Fig. 1d). Here, the over-dot operator denotes the time derivative, i.e., .
2.4. Statistical analysis
To determine statistically significant differences in the mechanical responses of the paired fresh and frozen tissue samples, a nonparametric Mann-Whitney U test was performed using an in-house MATLAB (MathWorks, Natick, MA) program. This nonparametric test was chosen after analyses of the normality of the data using the quantile-quantile (Q-Q) plots, which showed that the data did not follow a normal distribution (Figs. S1–S2). Next, in our statistical analyses, the null hypothesis was that the difference in the mean between the control (fresh) tissue group and the frozen tissue group was equal to zero, meaning that the freezing storage had no statistically significant effect on the mechanics of the TVAL tissue sample. All quantities presented in this study are reported as mean ± standard error of the mean (SEM).
The quantities that were analyzed include data from biaxial mechanical testing (Fig. 1c): the peak tissue stretches ( and ), the preconditioning stretches ( and ), the mechanical stretches ( and ), the elastic moduli at the high tension regime ( and ), the elastic moduli at the low tension regime ( and ), and the indices of extensibility ( and ). Quantities from biaxial stress relaxation testing were also analyzed (Fig. 1d): the percentage reductions in the membrane tensions, the time history of the membrane tensions (Tcirc(t) and Trad(t)), the initial slopes of stress relaxation ( and ), and the saturated slopes of stress relaxation ( and ). Significance was considered as statistically significant (p<0.05), nearly statistically significant (0.05≤p<0.1), and statistically not significant (p≥0.1).
3. Results
3.1. Thickness measurements
Measurements of the tissue thickness before and after freezing showed only a minimal change (<1%) in the tissue thickness: fresh TVAL, 0.35±0.04 mm; frozen TVAL, 0.35±0.04 mm. The thickness measurements and the effective testing edge lengths for all specimens are summarized in Table 1.
Table 1 –
Tissue thickness, effective edge length, and peak stretches for all the investigated TVAL tissue specimens.
| TVAL Specimen ID | Fresh (Control) | Frozen | ||||||
|---|---|---|---|---|---|---|---|---|
| Thickness (mm) | Effective Edge Length (mm) | Thickness (mm) | Effective Edge Length (mm) | |||||
| 1 | 0.30 | 7.5 | 1.17 | 1.81 | 0.25 | 6.0 | 1.20 | 2.21 |
| 2 | 0.18 | 6.5 | 1.15 | 1.58 | 0.35 | 6.0 | 1.14 | 1.19 |
| 3 | 0.16 | 8.5 | 1.45 | 1.64 | 0.19 | 7.0 | 1.05 | 1.09 |
| 4 | 0.47 | 8.5 | 1.40 | 1.80 | 0.47 | 7.0 | 1.18 | 1.49 |
| 5 | 0.40 | 8.5 | 1.33 | 1.34 | 0.31 | 7.0 | 1.36 | 1.54 |
| 6 | 0.30 | 8.5 | 1.37 | 2.10 | 0.35 | 7.0 | 1.47 | 1.87 |
| 7 | 0.32 | 8.5 | 1.20 | 1.80 | 0.30 | 7.0 | 1.19 | 1.65 |
| 8 | 0.51 | 7.5 | 1.34 | 2.01 | 0.53 | 6.5 | 1.30 | 1.84 |
| 9 | 0.41 | 8.0 | 1.18 | 1.87 | 0.49 | 7.0 | 1.12 | 1.86 |
| 10 | 0.48 | 8.5 | 1.29 | 1.86 | 0.20 | 7.0 | 1.44 | 2.20 |
| Mean | 0.35 | 8.1 | 1.29 | 1.78 | 0.35 | 6.8 | 1.25 | 1.69 |
| SEM | 0.04 | 0.2 | 0.03 | 0.07 | 0.04 | 0.1 | 0.05 | 0.12 |
3.2. Biaxial mechanical testing results
The results from biaxial mechanical testing are summarized in Figures 2–4, the peak stretch values are summarized in Table 1, and the p-values from statistical comparisons are summarized in Table 2, which showed statistically insignificant differences in most parameters between the fresh and frozen tissue responses (Fig. 2a–c). Specifically, under equibiaxial loading (Tcirc:Trad=1:1), there was only a 3.4% difference observed in the circumferential peak stretch () between the fresh and frozen tissues (p=0.427) (Table 1). Similarly, the circumferential preconditioning stretch () had a 2.8% difference (p=0.308) (Fig, 3a), and the mechanical stretch () had a 0.6% difference (p=0.970) (Fig. 3b). As for the radial direction, similar trends were observed: , 4.8% difference (p=0.791); , 7.1% difference (p=0.241) (Fig. 3a); and , 1.4% difference (p=0.734) (Fig. 3b). Values for the peak tissue stretches (Fig. 2b and Fig. 2c), the preconditioning stretches (Fig. 3c), and the mechanical stretches (Fig. 3d) associated with the other two biaxial loading protocols (Tcirc:Trad=0.5:1 and Tcirc:Trad=1:0.5) were comparable, and none demonstrated statistically significant differences.
Figure 2 –
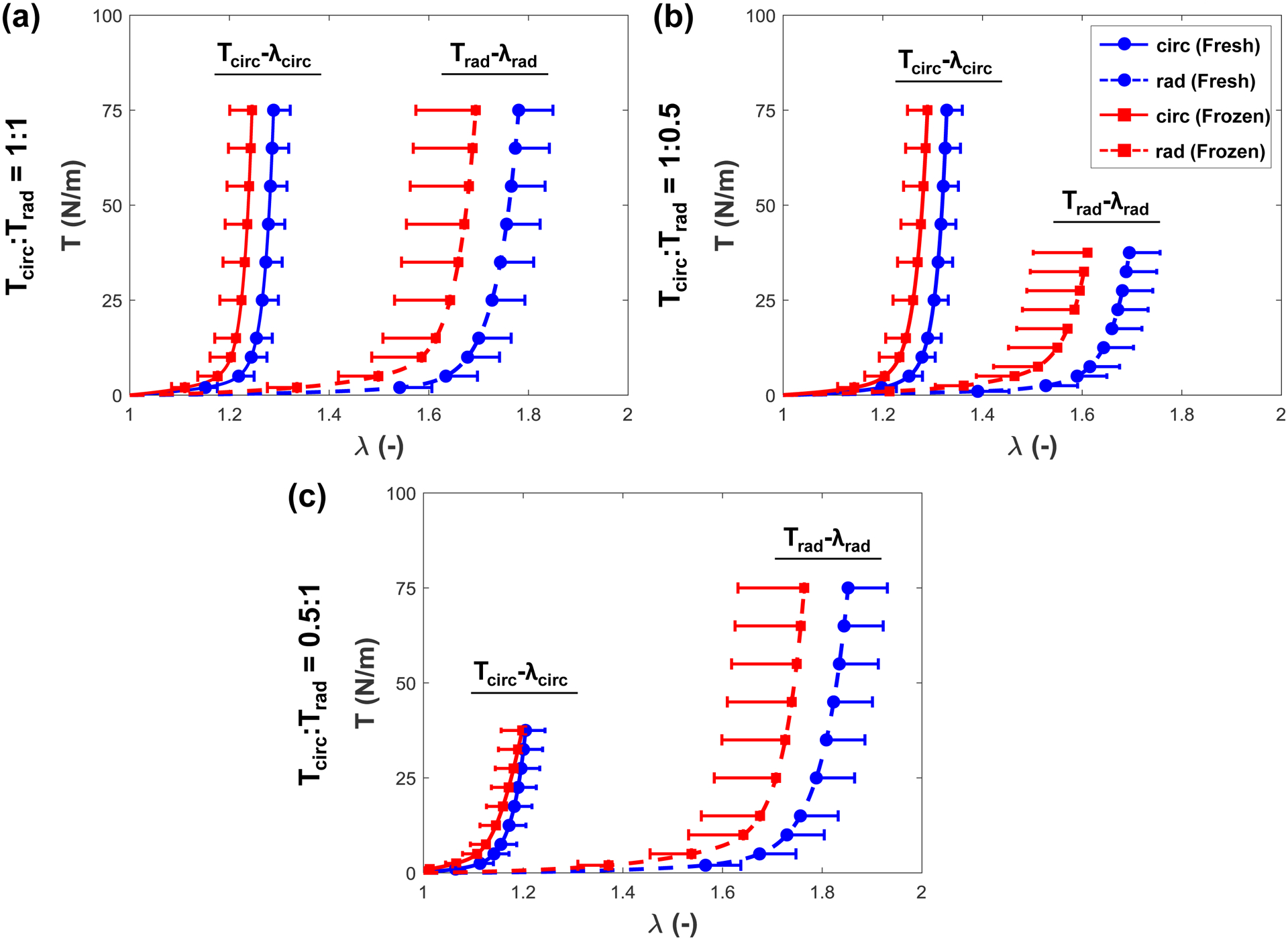
Peak stretches () of the fresh and frozen tricuspid valve anterior leaflet tissues under the (a) Tcirc:Trad=1:1, (b) Tcirc:Trad=1:0.5, and (c) Tcirc:Trad=0.5:1 loading ratios.
Figure 4 –
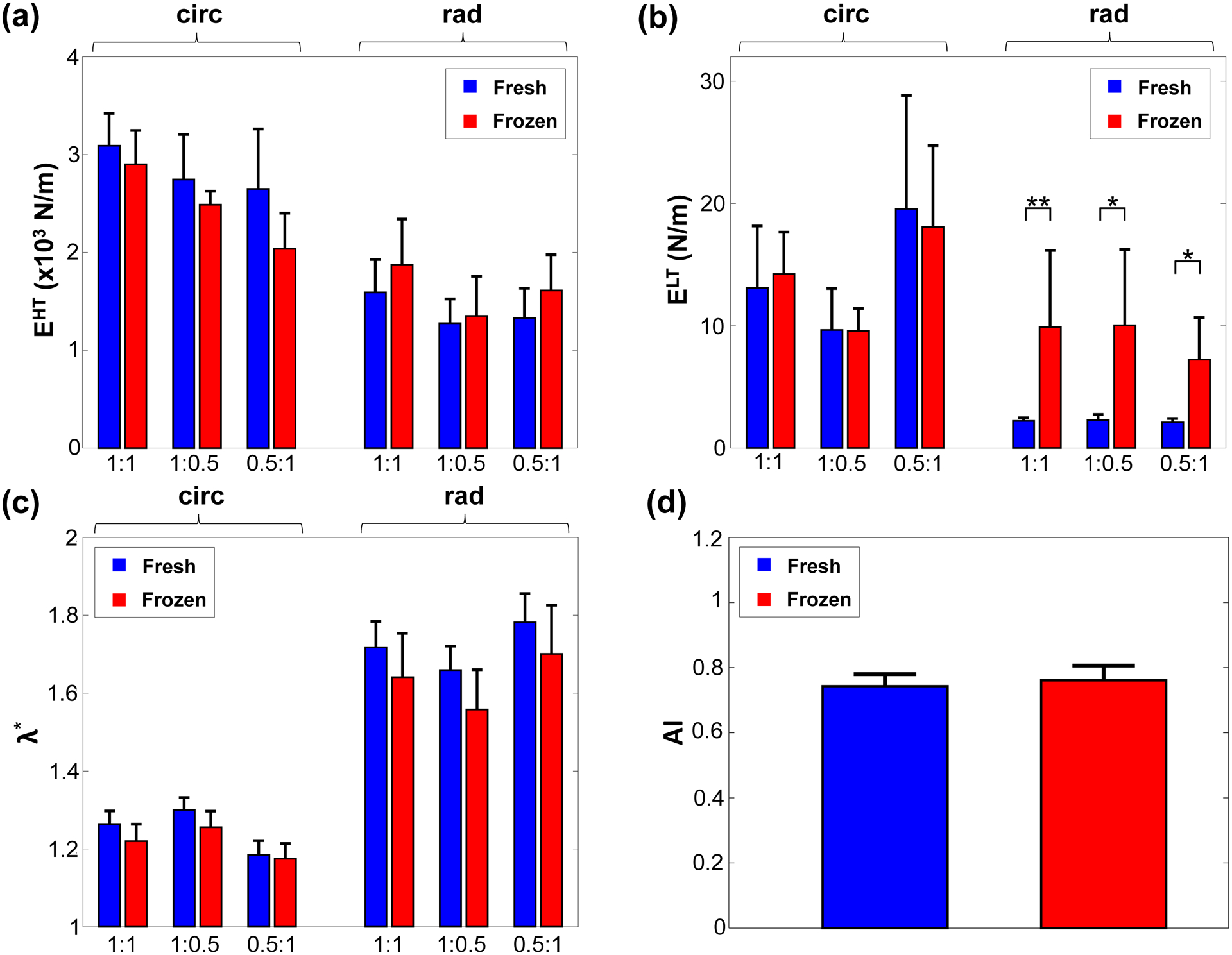
Comparison of the (a) low-tension modulus (ELT), (b) high-tension modulus (EHT), (c) index of extensibility (λ*), and (d) anisotropy indices (AI) between the fresh and frozen tissues under 3 different biaxial loading protocols. *, statistically significant (p<0.05); **, nearly statistically significant (0.05≤p<0.1).
Table 2 –
p-values from the statistical comparison of the quantities between the control (C) and frozen (F) TVAL tissue specimens (n=10). All values are reported as mean ± SEM.
| Loading | Quantity | p-value (Fresh vs. Frozen) | |
|---|---|---|---|
| Preconditioning | circ | 0.308 | |
| rad | 0.241 | ||
| Tcirc:Trad=1:1 | circ | 0.427 | |
| rad | 0.791 | ||
| circ | 0.969 | ||
| rad | 0.734 | ||
| EHT (N/m) | circ | 0.678 | |
| rad | 0.571 | ||
| ELT (N/m) | circ | 0.162 | |
| rad | 0.054* | ||
| λ* | circ | 0.473 | |
| rad | 0.678 | ||
| AI | 0.570 | ||
| Tcirc:Trad=0.5:1 | circ | 0.909 | |
| rad | 0.678 | ||
| circ | 0.427 | ||
| rad | 0.791 | ||
| EHT (N/m) | circ | 0.791 | |
| rad | 0.571 | ||
| ELT (N/m) | circ | 0.186 | |
| rad | 0.038* | ||
| λ* | circ | 0.791 | |
| rad | 0.623 | ||
| Tcirc:Trad=1:0.5 | circ | 0.345 | |
| rad | 0.521 | ||
| circ | 0.850 | ||
| rad | 0.850 | ||
| EHT (N/m) | circ | 0.623 | |
| rad | 0.678 | ||
| ELT (N/m) | circ | 0.308 | |
| rad | 0.089* | ||
| λ* | circ | 0.385 | |
| rad | 0.385 | ||
statistically significant (p<0.05)
nearly statistically significant (0.05≤p<0.1)
Figure 3 –
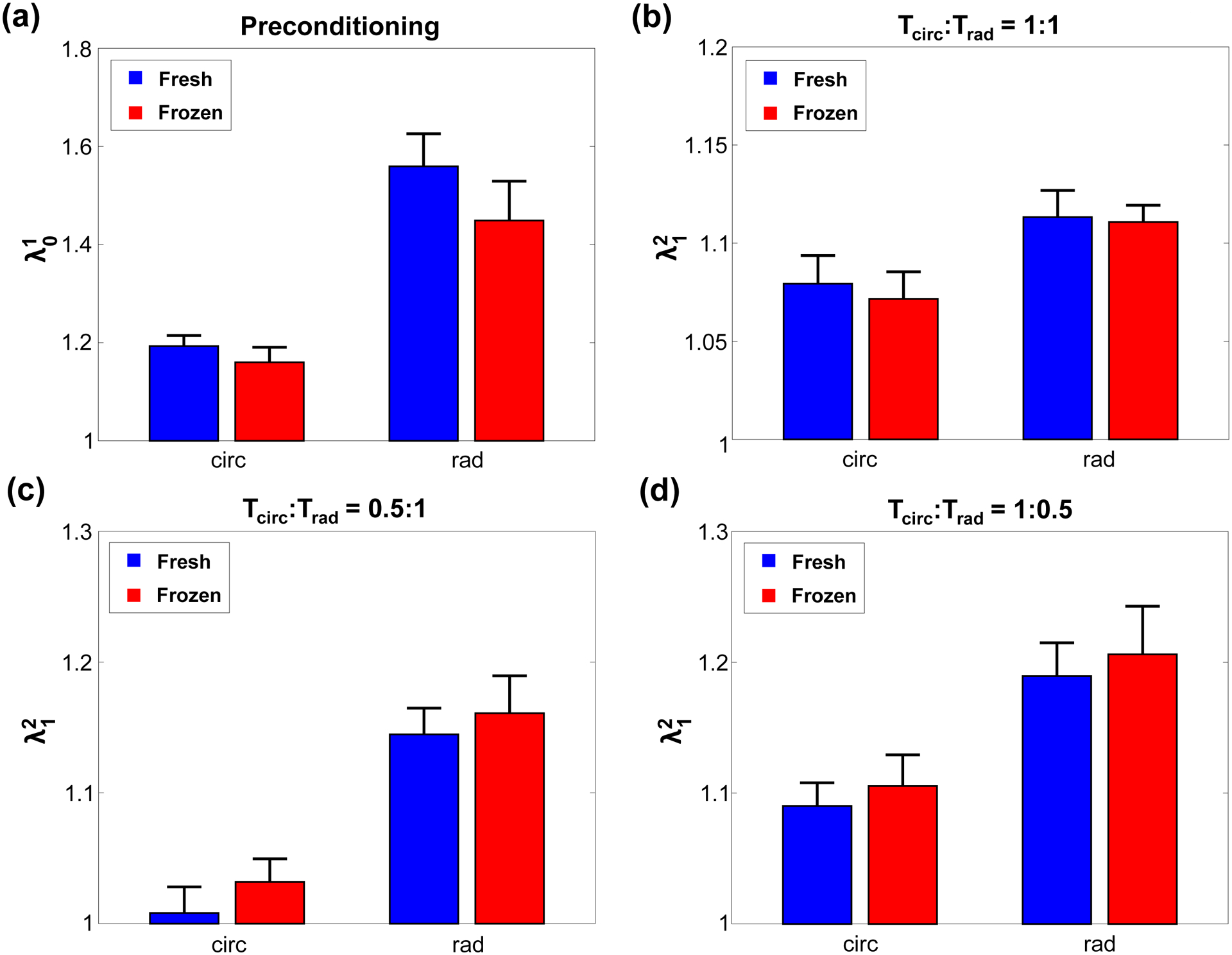
(a) Preconditioning stretches (), and mechanical stretches () at the 3 biaxial loading protocols: (b) Tcirc:Trad=1:1, (c) Tcirc:Trad=1:0.5, and (d) Tcirc:Trad=0.5:1 for the fresh and frozen tricuspid valve anterior leaflet tissues.
In addition, the radial elastic modulus of the low-tension regime () was noted to be statistically significant or nearly statistically significant at each of the three loading ratios (Fig. 4b and Table 2). At the Tcirc:Trad=0.5:1 loading ratio, statistical significance was observed (p=0.038), whereas nearly statistical significance was observed under the loading protocols Tcirc:Trad=1:1 (p=0.054) and Tcirc:Trad=1:0.5 (p=0.089). As for the circumferential direction, ELT was observed not to have a statistically significant difference (p=0.162). The elastic modulus of the high-tension regime (EHT) was not statistically significant in etiher tissue’s direction: udner Tcirc:Trad=1:1 loading, the circumferential direction had a 6.1% difference (p=0.678) and the radial direction had a 17.8% difference (p=0.571). Other biaxial loading protocols had similar, statistically insignificant trends. The indices of extensibility ( and ) and the anisotropy index (AI) followed similarly statistically insignificant trends across the three loading protocols (Table 2), with the fresh TVAL tissues demonstrating slightly greater extensibility and a lessened material anisotropy than the frozen tissues.
3.3. Stress relaxation testing results
Analysis of the stress relaxation parameters showed mostly statistically insignificant differences, and these statistical analysis results are summarized in Table 3. For the initial slope (), there was a difference of 0.01 between the fresh and frozen tissues in the circumferential direction (p=0.909) and a difference of 0.04 in the radial direction (p=0.677). As for the saturated slope (), the differences between the fresh and frozen tissues were found to be 0.9×10−3 N/m in the circumferential direction (p=0.069) and 0.3×10−3 N/m in the radial direction (p=0.791) respectively.
Table 3 –
Comparison of the stress-relaxation parameters between the control (C) and frozen (F) TVAL tissue samples (n=10). All values are reported as mean ± SEM.
| Quantity | Fresh (Control) | Frozen | p-value | |
|---|---|---|---|---|
| (N/m) | circ | 1.01 ± 0.04 | 1.02 ± 0.05 | 0.909 |
| rad | 1.07 ± 0.02 | 1.03 ± 0.03 | 0.677 | |
| (x10−3 N/m) | circ | 5.50 ± 0.20 | 6.40 ± 0.30 | 0.069** |
| rad | 5.20 ± 0.20 | 5.50 ± 0.40 | 0.791 | |
nearly statistically significant (0.05≤p<0.1)
4. Discussion
4.1. Overall findings
Comparison of the mechanical parameters between the fresh and the frozen TVAL showed minimal differences between the fresh and frozen tissues. Specifically, differences in the peak, preconditioning, and mechanical stretches between the two groups were statistically insignificant. Additionally, the elastic modulus of the high-tension regime and the index of extensibility were not significantly different. While the elastic modulus of the low-tension regime was found to have a statistically significant difference, this difference was only significant in the radial direction and was only nearly significant for most of the biaxial mechanical testing protocols. Finally, the analysis of the initial and saturated slopes of stress relaxation also showed no statistically significant differences. These findings suggest that freezing-based storage does not have a significant effect on the mechanical properties of the investigated atrioventricular heart valve leaflet tissue.
Although changes in the observed mechanical properties were statistically insignificant, the slight variations from the freezing storage observed in this study could impact the biaxial mechanical testing results of our previous studies (Jett et al., 2018; Laurence et al., 2019; Ross et al., 2019). Specifically, the frozen tissues were found to be slightly stiffer and more anisotropic than the fresh tissues (Fig. 2), and the frozen tissues had a slightly lessened stress relaxation behavior than the fresh tissues (Fig. 5). This trend could have bearing on the tissue mechanics observed in previous studies of the AHV leaflets, and possibly obscure the results. For example, in another study performed by our lab, glycosaminoglycans (GAGs) were removed from previously-frozen AHV leaflets and the mechanical responses were characterized (Ross et al., Under review). It was observed that the fully intact tissues were stiffer than tissues with a majority (>50%) of the GAGs removed, but part of this response could be partially attributed to the freezing storage rather than the effect of the GAG removal. Overall, it is important to consider the method of tissue storage, when designing a study of soft biological materials such as atrioventricular heart valve leaflets. However, the effect of freezing on AHV leaflet mechanical properties was observed to be minimal, and our results suggest the mechanical responses of the tissues could be mostly preserved by freezing.
Figure 5 –
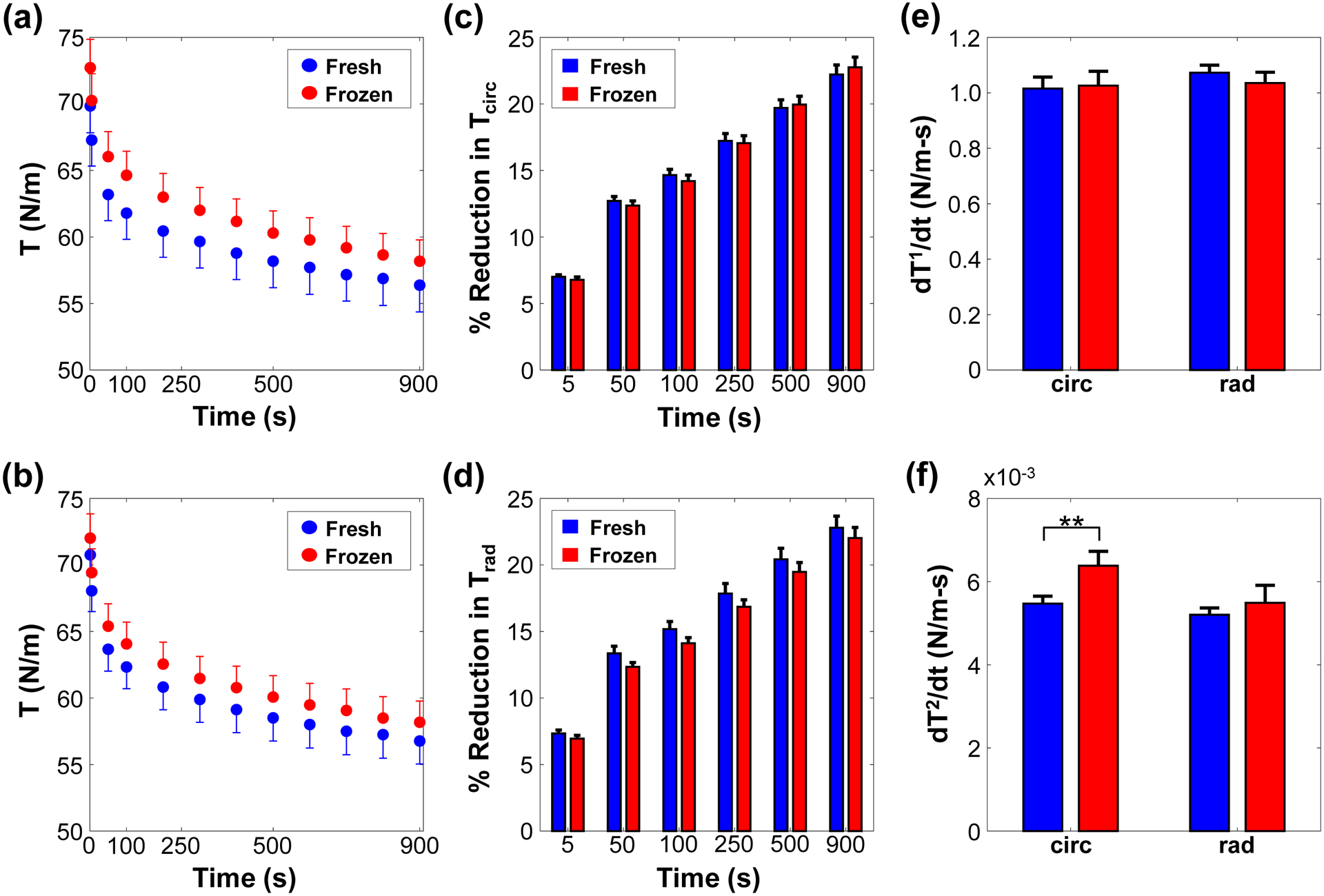
The stress-relaxation of the fresh and frozen tricuspid valve anterior leaflet in the (a) circumferential and (b) radial directions, the percent reduction of the stress-relaxation in the (c) circumferential and (d) radial directions, and the (e) initial () and (f) saturated () slopes of the stress-relaxation reduction behavior. **, nearly statistically significant (0.05≤p<0.1).
4.2. Study Limitations and Future Extensions
There are several limitations in this study. First, it is unknown whether the specimen size has a significant effect on the observed mechanical properties of the leaflets; however, preliminary investigations in our lab suggest this to have a minimal impact. There are also some limitations of our biaxial testing procedures, including: (i) the possibility for shear stress in the specimen using the tissue mounting hardware BioRake, while the shear deformations were found to be negligible if the tissue’s directions were aligned with the primary axes of the biaxial testing system; and (ii) the stress relaxation procedure (15 minutes) was relatively short to capture the entire stress relaxation behavior of the TVAL tissue; however, it has been shown that the majority of relaxation occurs in the first 1000 seconds (Grashow et al., 2006; Huang and Huang, 2015). There is also a potential limitation with the contact-based thickness measurement approach due to the possibility for slight tissue compression when performing the measurements. In our previous investigation we have shown reasonable agreement between our dial caliper-based approach and histological measurements (Jett et al., 2018). Additionally, the exact freezing rate for the tissue specimen was unknown, acting as another potential limitation. Finally, the investigation on porcine heart valves may not automatically extend to the human heart valve leaflets and further research using human valves may need to be performed to fully evaluate the effect of freezing on human heart valves.
Future extensions of this study of the freezing effect include an examination of the effect of freezing over varied periods of time. In this study, tissues were frozen for 48 hours, but tissues have been frozen for a week or more before testing in the previous literature. Furthermore, freezing times may have varied from specimen-to-specimen with the assumption that there would be no effect on the observed mechanical behaviors. Although this experiment showed no significant changes due to freezing, longer storage times may show some effect in the quantified mechanical properties of the tissue. Additionally, the effects could be examined from varying freezing temperature, freezing methods, number of freeze-thaw cycles, or the freezing rates. Another future work could include analyzing the effect of freezer storage on the tissue microstructure, which may be linked to the differences in the observed mechanical properties such as the radial low-tension moduli. The effects of freezing storage on the mechanical properties of other atrioventricular valve leaflets not included in this study could also be investigated.
4.3. Conclusion
Our results from biaxial mechanical testing and stress relaxation testing of the tricuspid valve anterior leaflet suggested that the commonly adopted freezing storage procedure does not significantly affect the observed mechanical properties of the examined atrioventricular heart valve leaflet. Most parameters from the testing did not demonstrate a significant difference between the fresh and frozen tissues. From this study, greater confidence can be had in the continued use of a freezing-based storage system for the preservation of AHV leaflet tissues.
The presented work has a broader impact to the field of AHV leaflet mechanical characterizations because it demonstrates the effect of a highly-employed storage method on the observed mechanical properties of the tissues. The finding that the storage method has minimal effect on the mechanics is useful as it provides a confirmation for those researchers that their reported mechanical parameters of the AHV leaflets are not skewed by their storage method. As an extension, this also reassures those researchers performing computational studies utilizing mechanical data from studies with a freezer-based leaflet storage method.
Supplementary Material
Figure S1 – Quantile-quantile (Q-Q) plots for the peak stretches ( and ), preconditioning stretches ( and ), mechanical stretches ( and ), indices of extensibility ( and ), low-tension elastic moduli ( and ), high-tension elastic moduli ( and ), tissue thicknesses (t), and the anisotropy index (AI) of the fresh TVAL tissue specimens.
Figure 2 – Quantile-quantile (Q-Q) plots for the peak stretches ( and ), preconditioning stretches ( and ), mechanical stretches ( and ), indices of extensibility ( and ), low-tension elastic moduli ( and ), high-tension elastic moduli ( and ), tissue thicknesses (t), and the anisotropy index (AI) of the frozen TVAL tissue specimens.
Acknowledgments
Support from the American Heart Association Scientist Development Grant (SDG) Award (16SDG27760143) is gratefully acknowledged. CHL was in part supported by the institutional start-up funds from the School of Aerospace and Mechanical Engineering (AME) and the research funding through the Faculty Investment Program from the Research Council at the University of Oklahoma (OU). CJ and GD were supported by the Mentored Research Fellowship (MRF) from the Office of Undergraduate Research and the Undergraduate Research Opportunity Program (UROP) sponsored by the VPR Office at OU. We also acknowledge undergraduate researchers Allyson Echols, Lauren Evans, Brennan Mullins, and Katherine Casey for their assistance with the biaxial mechanical testing.
Footnotes
Conflicts of Interest
The authors of this paper have no financial or personal relationships with other people or organizations that could inappropriately influence (bias) our work.
References
- Arzani A, Mofrad MRK, 2017. A strain-based finite element model for calcification progression in aortic valves. Journal of Biomechanics 65, 216–220. [DOI] [PubMed] [Google Scholar]
- Ayoub S, Lee C-H, Driesbaugh KH, Anselmo W, Hughes CT, Ferrari G, Gorman RC, Gorman JH III, Sacks MS, 2017. Regulation of valve interstitial cell homeostasis by mechanical deformation: Implications for heart valve disease and surgical repair. Journal of the Royal Society Interface 14, 20170580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changoor A, Fereydoonzad L, Yaroshinsky A, Buschmann MD, 2010. Effects of refrigeration and freezing on the electromechanical and biomechanical properties of articular cartilage. Journal of biomechanical engineering 132, 064502. [DOI] [PubMed] [Google Scholar]
- Clavert P, Kempf J-F, Bonnomet F, Boutemy P, Marcelin L, Kahn J-L, 2001. Effects of freezing/thawing on the biomechanical properties of human tendons. Surgical and Radiologic Anatomy 23, 259–262. [DOI] [PubMed] [Google Scholar]
- Delgadillo JOV, Delorme S, El-Ayoubi R, DiRaddo R, Hatzikiriakos SG, 2010. Effect of freezing on the passive mechanical properties of arterial samples. Journal of Biomedical Science and Engineering 3, 645. [Google Scholar]
- Eckert CE, Fan R, Mikulis B, Barron M, Carruthers CA, Friebe VM, Vyavahare NR, Sacks MS, 2013. On the biomechanical role of glycosaminoglycans in the aortic heart valve leaflet. Acta Biomaterialia 9, 4653–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foutz TL, Stone EA, Abrams CF III, 1992. Effects of freezing on mechanical properties of rat skin. American Journal of Veterinary Research 53, 788–792. [PubMed] [Google Scholar]
- Giannini S, Buda R, Di Caprio F, Agati P, Bigi A, De Pasquale V, Ruggeri A, 2008. Effects of freezing on the biomechanical and structural properties of human posterior tibial tendons. International orthopaedics 32, 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashow JS, Sacks MS, Liao J, Yoganathan AP, 2006. Planar biaxial creep and stress relaxation of the mitral valve anterior leaflet. Annals of Biomedical Engineering 34, 1509–1518. [DOI] [PubMed] [Google Scholar]
- Huang H-YS, Balhouse BN, Huang S, 2012. Application of simple biomechanical and biochemical tests to heart valve leaflets: implications for heart valve characterization and tissue engineering. Proceedings of the Institution of Mechanical Engineers. Part H: Journal of Engineering in Medicine 226, 868–876. [DOI] [PubMed] [Google Scholar]
- Huang S, Huang H-YS, 2015. Biaxial stress relaxation of semilunar heart valve leaflets during simulated collagen catabolism: Effects of collagenase concentration and equibiaxial strain state. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine 229, 721–731. [DOI] [PubMed] [Google Scholar]
- Jett S, Laurence D, Kunkel R, Babu AR, Kramer K, Baumwart R, Towner R, Wu Y, Lee C-H, 2018. An investigation of the anisotropic mechanical properties and anatomical structure of porcine atrioventricular heart valves. J. Mech. Behav. Biomed. Mater 87, 155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye B, Randall C, Walsh D, Hansma P, 2012. The effects of freezing on the mechanical properties of bone. The Open Bone Journal 4. [Google Scholar]
- Khoiy KA, Amini R, 2016. On the biaxial mechanical response of porcine tricuspid valve leaflets. Journal of Biomechanical Engineering 138, 104504-104504-104506. [DOI] [PubMed] [Google Scholar]
- Kidane AG, Burriesci G, Cornejo P, Dooley A, Sarkar S, Bonhoeffer P, Edirisinghe M, Seifalian AM, 2009. Current developments and future prospects for heart valve replacement therapy. Journal of Biomedical Materials Research Part B: Applied Biomaterials 88B, 290–303. [DOI] [PubMed] [Google Scholar]
- Kunzelman KS, Reimink MS, Cochran RP, 1997. Annular dilatation increases stress in the mitral valve and delays coaptation: A finite element computer model. Cardiovascular Surgery 5, 427–434. [DOI] [PubMed] [Google Scholar]
- Laurence D, Ross C, Jett S, Johns C, Echols A, Baumwart R, Towner R, Liao J, Bajona P, Wu Y, Lee C-H, 2019. An investigation of regional variations in the biaxial mechanical properties and stress relaxation behaviors of porcine atrioventricular heart valve leaflets. J. Biomech 83, 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-H, Rabbah J-P, Yoganathan AP, Gorman RC, Gorman JH III, Sacks MS, 2015. On the effects of leaflet microstructure and constitutive model on the closing behavior of the mitral valve. Biomechanics and Modeling in Mechanobiology 14, 1281–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Yang L, Grashow J, Sacks MS, 2007. The relation between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. Journal of Biomechanical Engineering 129, 78–87. [DOI] [PubMed] [Google Scholar]
- Linde F, Sørensen HCF, 1993. The effect of different storage methods on the mechanical properties of trabecular bone. Journal of biomechanics 26, 1249–1252. [DOI] [PubMed] [Google Scholar]
- Matthews LS, Ellis D, 1968. Viscoelastic properties of cat tendon: effects of time after death and preservation by freezing. Journal of biomechanics 1, 65–71. [DOI] [PubMed] [Google Scholar]
- May-Newman K, Yin FC, 1995. Biaxial mechanical behavior of excised porcine mitral valve leaflets. Am. J. Physiol. Heart Circ. Physiol 269, H1319–H1327. [DOI] [PubMed] [Google Scholar]
- O’Leary SA, Doyle BJ, McGloughlin TM, 2014. The impact of long term freezing on the mechanical properties of porcine aortic tissue. Journal of the mechanical behavior of biomedical materials 37, 165–173. [DOI] [PubMed] [Google Scholar]
- Pant AD, Thomas VS, Black AL, Verba T, Lesicko JG, Amini R, 2018. Pressure-induced microstructural changes in porcine tricuspid valve leaflets. Acta Biomaterialia 67, 248–258. [DOI] [PubMed] [Google Scholar]
- Pelker RR, Friedlaender GE, Markham TC, Panjabi MM, Moen CJ, 1983. Effects of freezing and freeze‐drying on the biomechanical properties of rat bone. Journal of Orthopaedic Research 1, 405–411. [DOI] [PubMed] [Google Scholar]
- Pham T, Sulejmani F, Shin E, Wang D, Sun W, 2017. Quantification and comparison of the mechanical properties of four human cardiac valves. Acta Biomaterialia 54, 345–355. [DOI] [PubMed] [Google Scholar]
- Pham T, Sun W, 2014. Material properties of aged human mitral valve leaflets. Journal of Biomedical Materials Research, Part A 102, 2692–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta-Paskaleva A, Sulejmani F, DelRocini M, Sun W, 2019. Comparative mechanical, morphological, and microstructural characterization of porcine mitral and tricuspid leaflets and chordae tendineae. Acta Biomaterialia 85, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy JN, 2013. An Introduction to Continuum Mechanics. Cambridge University Press. [Google Scholar]
- Rogers JH, Bolling SF, 2009. The tricuspid valve: Current perspective and evolving management of tricuspid regurgitation. Circulation 119, 2718–2725. [DOI] [PubMed] [Google Scholar]
- Ross C, Laurence D, Richardson J, Babu A, Evans L, Beyer E, Wu Y, Towner R, Fung K-M, Mir A, Burkhart HM, Holzapfel GA, Lee C-H, Under review. An investigation of the glycosaminoglycan contribution to biaxial mechanical behaviors of porcine atrioventricular heart valve leaflets. J R Soc Interface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C, Laurence D, Wu Y, Lee C-H, 2019. Biaxial mechanical characterizations of atrioventricular heart valves. J. Vis. Exp 146, e59170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks MS, 2000. Biaxial mechanical evaluation of planar biological materials. Journal of Elasticity 61, 199. [Google Scholar]
- Santago A, Kemper A, McNally C, Sparks J, Duma S, 2009. Freezing affects the mechanical properties of bovine liver. Biomed. Sci. Instrum 45, 24–29. [PubMed] [Google Scholar]
- Smith C, Young I, Kearney J, 1996. Mechanical properties of tendons: changes with sterilization and preservation. Journal of biomechanical engineering 118, 56–61. [DOI] [PubMed] [Google Scholar]
- Stella JA, Liao J, Sacks MS, 2007. Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. Journal of Biomechanics 40, 3169–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella JA, Sacks MS, 2007. On the biaxial mechanical properties of the layers of the aortic valve leaflet. Journal of Biomechanical Engineering 129, 757–766. [DOI] [PubMed] [Google Scholar]
- Stemper BD, Yoganandan N, Stineman MR, Gennarelli TA, Baisden JL, Pintar FA, 2007. Mechanics of fresh, refrigerated, and frozen arterial tissue. Journal of Surgical Research 139, 236–242. [DOI] [PubMed] [Google Scholar]
- Sun W, Martin C, Pham T, 2014. Computational modeling of cardiac valve function and intervention. Annual Review of Biomedical Engineering 16, 53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadmor EB, Miller RE, Elliott RS, 2012. Continuum Mechanics and Thermodynamics: From Fundamental Concepts to Governing Equations. Cambridge University Press. [Google Scholar]
- Venkatasubramanian RT, Grassl ED, Barocas VH, Lafontaine D, Bischof JC, 2006. Effects of freezing and cryopreservation on the mechanical properties of arteries. Annals of Biomedical engineering 34, 823–832. [DOI] [PubMed] [Google Scholar]
- Waller BF, Howard J, Fess S, 1994. Pathology of mitral valve stenosis and pure mitral regurgitation—Part I. Clinical Cardiology 17, 330–336. [DOI] [PubMed] [Google Scholar]
- Waller BF, Howard J, Fess S, 1995. Pathology of tricuspid valve stenosis and pure tricuspid regurgitation—Part I. Clinical Cardiology 18, 97–102. [DOI] [PubMed] [Google Scholar]
- Wells SM, Pierlot CM, Moeller AD, 2012. Physiological remodeling of the mitral valve during pregnancy. American Journal of Physiology-Heart and Circulatory Physiology. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 – Quantile-quantile (Q-Q) plots for the peak stretches ( and ), preconditioning stretches ( and ), mechanical stretches ( and ), indices of extensibility ( and ), low-tension elastic moduli ( and ), high-tension elastic moduli ( and ), tissue thicknesses (t), and the anisotropy index (AI) of the fresh TVAL tissue specimens.
Figure 2 – Quantile-quantile (Q-Q) plots for the peak stretches ( and ), preconditioning stretches ( and ), mechanical stretches ( and ), indices of extensibility ( and ), low-tension elastic moduli ( and ), high-tension elastic moduli ( and ), tissue thicknesses (t), and the anisotropy index (AI) of the frozen TVAL tissue specimens.


