To the Editor: Emergency use authorizations for two messenger RNA (mRNA) vaccines — the BNT162b2 vaccine (Pfizer–BioNTech) and the mRNA-1273 vaccine (Moderna) — marked important milestones in efforts to respond to the coronavirus disease 2019 (Covid-19) pandemic. These Food and Drug Administration actions were based on impressive results of clinical trials, but only in the weeks since the authorizations has real-world evidence been available to shed light on the overall effect of the vaccines on transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and related illness.
Here, we report data from the University of Texas Southwestern Medical Center (UTSW), which initiated a program on December 15, 2020, to offer vaccine against SARS-CoV-2 to its frontline employees in phase 1a of vaccination, as directed by the Texas Department of State Health Services. The launch of the vaccination effort coincided with a rapidly escalating number of new SARS-CoV-2 infections in North Texas. This escalation led to the largest surge to date in the region and strained health systems.
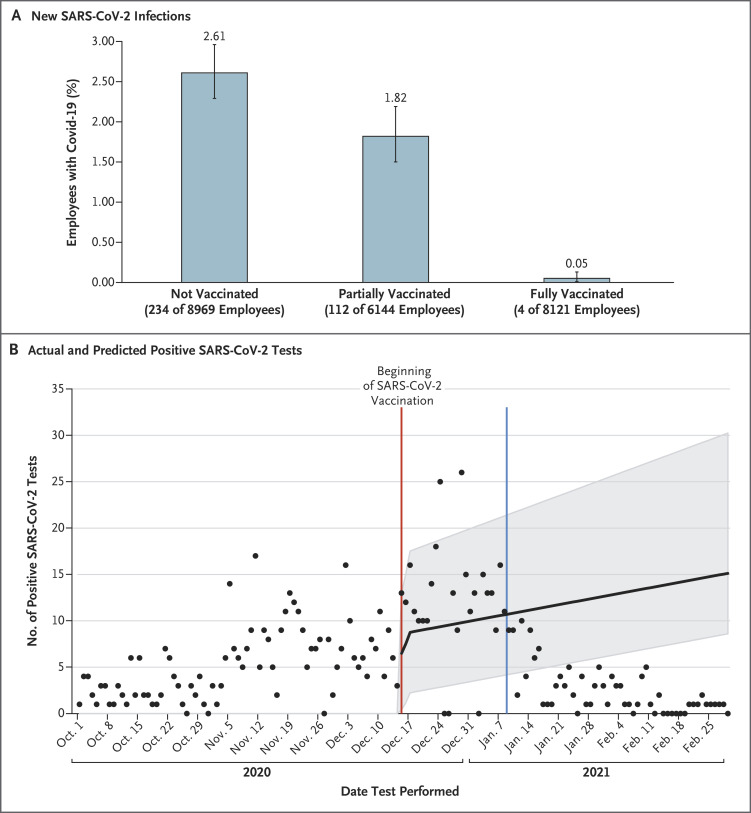
In the initial 31 days of the vaccination campaign, 59% of 23,234 UTSW employees received a first dose of either one of the mRNA vaccines and 30% received a second dose. Between December 15, 2020, and January 28, 2021, a total of 350 of the 23,234 employees (1.5%) who were eligible to receive the vaccine were identified as being newly infected with SARS-CoV-2. As shown in Figure 1A, the percentages of persons who became infected differed according to vaccination status, with infections in 234 of 8969 nonvaccinated employees (2.61%; 95% confidence interval [CI], 2.29 to 2.96), 112 of 6144 partially vaccinated employees (1.82%; 95% CI, 1.50 to 2.19), and 4 of 8121 fully vaccinated employees (0.05%; 95% CI, 0.01 to 0.13) (P<0.01 for all pairwise comparisons).
Figure 1. Early Results of SARS-CoV-2 Vaccination.
Panel A shows the percentage of persons with new SARS-CoV-2 infection among 23,234 employees of the University of Texas Southwestern Medical Center (UTSW) who were eligible to receive SARS-CoV-2 vaccine, stratified according to vaccination status from December 15, 2020, through January 28, 2021. Vaccination status was determined at the time of the first SARS-CoV-2–positive test on or after December 15; if no infection was detected between December 15 and January 28, the vaccination status on January 28 was used. Nonvaccinated persons were those for whom there was no record of vaccine received at UTSW, partially vaccinated persons were those who had received one dose or who had received the second dose of the BNT162b2 vaccine less than 7 days before the index date or the second dose of the mRNA-1273 vaccine less than 14 days before the index date, and fully vaccinated persons were those who had received the second dose of the BNT162b2 vaccine at least 7 days before the index date or the second dose of the mRNA-1273 vaccine at least 14 days before the index date. 𝙸 bars denote 95% confidence intervals. Panel B shows that the number of positive tests was projected to increase without vaccination (black line). The shaded area denotes the 95% confidence interval. The actual number of positive tests (black dots) decreased from January 9 (blue line) onward (25 days after the initiation of employee vaccinations).
As shown in Figure 1B, from January 9 onward, the actual number of positive tests among all UTSW employees was consistently lower than the number projected on the basis of the actual increasing SARS-CoV-2 positivity rate among patients who presented to our emergency department during this same period and had samples that were tested on polymerase-chain-reaction assay.1 Additional information is provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
The effect of vaccination on the preservation of our workforce has been dramatic. We observed a greater than 90% decrease in the number of employees who are either in isolation or quarantine (Fig. S2 in the Supplementary Appendix). Real-world experience with SARS-CoV-2 vaccination at UTSW has shown a marked reduction in the incidence of infections among employees. This decrease has preserved the workforce when it was most needed.
Approximately 70% of our employees were vaccinated in phase 1a by February 4, and 78% were vaccinated in phase 1a by March 5. It is clear that vaccine hesitancy presents an important challenge, even when access is not an obstacle. Addressing the factors underlying this reluctance through insights gained from experience will be essential if the full potential benefit of vaccination in creating a path to normalcy is to be achieved.
Supplementary Appendix
Disclosure Forms
This letter was published on March 23, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Reference
- 1.Ceylan Z. Estimation of COVID-19 prevalence in Italy, Spain, and France. Sci Total Environ 2020;729:138817-138817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.